
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 11 June 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1402316
This article is part of the Research Topic Vitamin D: From Pathophysiology to Clinical Impact, volume II View all 24 articles
 Yanfei Chen1†
Yanfei Chen1† Xingzhu Liu1†
Xingzhu Liu1† Bin Li1
Bin Li1 Jun Li2
Jun Li2 Lijuan Meng1
Lijuan Meng1 Caixia Ye3
Caixia Ye3 Linfei Han1
Linfei Han1 Hong Li1
Hong Li1 Li Li Deng1
Li Li Deng1 Zhongjian Su1*
Zhongjian Su1* Xing Zhang1*
Xing Zhang1*Background: Kawasaki Disease (KD) is a pediatric vasculitic disorder characterized by systemic small vasculitis, notably coronary arteritis, with unclear pathogenesis. This explorative case-control study investigated the association between folic acid (FA), vitamin D3 (VD3), and vitamin B12 (VB12) levels and the different types of Kawasaki Disease, as well as the incidence of coronary artery lesions (CALs).
Methods: In this explorative case control study, 365 KD children admitted to our hospital from January 1, 2022 to June 30, 2023 were included as the KD group. Simultaneously, 365 healthy children who received physical examination during the same period were included as the control group. The KD group was divided into typical KD group and incomplete KD group (IKD group), CALs group and non-CALS group, and IVIG sensitive group and IVIG resistant group. The children with CALs were divided into small tumor group, medium tumor group and large tumor group. Serum levels of FA, VB12, and VD3 were compared across all groups.
Results: Serum levels of FA and VD3 were significantly decreased in both the KD and CALs groups (p < 0.05), and both factors were identified as independent risk factors for KD and CALs. Similarly, reduced serum VD3 levels were observed in the IKD and IVIG-resistant groups (p < 0.05), with VD3 also being an independent risk factor for both IKD and IVIG resistance. Additionally, lower serum FA levels were noted in the group with large aneurysms (p < 0.05), establishing FA as an independent risk factor for aneurysm size.
Conclusion: Serum levels of folic FA and vitamin VD3 were significantly reduced in children with KD. Furthermore, these reductions were more pronounced in children with IKD and CALs. This pattern suggests that lower FA and VD3 levels may increase the risk of more severe coronary lesions in KD patients. Therefore, monitoring these biomarkers could provide valuable insights for early clinical diagnosis and intervention.
Kawasaki disease (KD) is a systemic vasculitic disorder commonly seen in pediatric clinics, primarily affecting children aged 6 months to 5 years (1). The primary pathological manifestation of KD is systemic vasculitis, with coronary arteritis being the predominant form. Notably, approximately 20% of KD patients develop complications involving coronary artery lesions (CALs) (2, 3). CALs secondary to KD are linked to alterations in specific biomarkers. These include counts of platelets and neutrophils, measures of plateletcrit and platelet distribution width, along with mean platelet volume, erythrocyte sedimentation rate, cardiac troponin I, endothelin-1, albumin, and hemoglobin levels (4). In a retrospective study involving 113 Chinese children, the Kobayashi score was applied to predict CALs in KD. Although the score demonstrated predictive capabilities, its accuracy was not sufficiently high. Therefore, there is a great need for reliable laboratory markers to accurately predict the subsequent development of CAL in the acute early stages of KD (5). The pathogenesis of KD is still not completely clear. It is currently believed that KD results from abnormal immune activation, which is induced by infections, especially in individuals with genetic susceptibility (4, 6). Previous study has shown that hyperhomocysteinemia can induce subclinical endothelial dysfunction, leading to advanced coronary arteritis in patients with KD (7). 5-methyltetrahydrofolate (5-MTHF) is the main circulating form of FA and is a methyl donor for the remethylation of homocysteine (Hcy) to methionine. FA other study has indicated that 10-methylenetetrahydrofolate reductase (MTHFR) is a candidate gene involved in the process of arteriosclerosis, and MTHFR is an enzyme that catalyzes the reduction of 5, 10-methyltetrahydrofolate to 5-MTHF (8). Therefore, it can be hypothesized that folic acid (FA) levels in the body may influence the development of arteriosclerosis. However, the specific role of FA in the progression of arteriosclerosis in KD patients remains unclear. Vitamin D3 (VD3) is an essential fat-soluble vitamin and a crucial steroid hormone. Its deficiency is widespread globally. Chen et al. (9) discovered that, besides regulating calcium and phosphorus metabolism, VD3 also influences immune disorders through the modulation of inflammatory signaling pathways. Reports suggested that adjunct therapy with 1-alpha, 25-dihydroxyvitamin D3, known for its anti-inflammatory and immunomodulatory properties, is beneficial in managing KD-induced vasculitis (10, 11). Comparatively, KD patients exhibit higher Vitamin D Receptor (VDR) expression in T cells than children with febrile respiratory infections or healthy individuals, suggesting that overactivated T cells may trigger the release of 25-(OH) D3. As a result, the enhanced inflammatory response observed in patients with CALs could result in an increased expression of VDR and consequently, elevated levels of 25-(OH) D3. Moreover, vitamin D deficiency has been recognized as an independent risk factor for arterial diseases (12). Therefore, the correlation between KD and VD3 has garneredincreasing attention. One study demonstrated that circulating 25-hydroxyvitamin D3 [25-(OH) D3] could predict CALs secondary to KD in children; however, the study was limited by a small sample size and low statistical power (13). VB12 is one of the most important B vitamins and serves as the primary coenzyme of Hcy forming methionine. The reduction of elevated homocysteine levels in the body depends on both FA and VB12, which provide methyl groups enabling the remethylation of homocysteine back to methionine, thus lowering Hcy levels (14). Many researchers’ studies have demonstrated relations of high Hcy levels with low intake of FA and VB12 (15–18). FA and VB12 play important roles in remethylation and transsulfuration pathway of Hcy metabolism. A defect in any of these can lead to increased levels of homocysteine in circulation. Therefore, we hypothesized that FA, VD3, and VB12 levels are involved in the pathogenesis of KD and CALs. In this study, we used our data to conduct a preliminary exploration of the associations of FA, VB12, and VD3 on different types of KD and CALs.
In this prospective case-control study, 365 children with KD admitted to our hospital from January 1, 2022, to June 30, 2023 were selected as the KD group. Simultaneously, a total of 365 children from the same period were selected as the control group. The KD group was divided into typical KD (n = 293) and incomplete KD (IKD) (n = 72) groups. Based on their sensitivity to IVIG, they were divided into IVIG-sensitive (n = 303) and IVIG resistance groups (n = 62). Additionally, they were divided into CALs (n = 18) and non-CALs (n = 347) groups according to the presence of CALs, and the CALs group was further divided into small, medium, and large tumor groups according to the aneurysm size (see Table 1).
The inclusion criteria were as follows: (1) a diagnosis of KD according to the 2017 American Heart Association (AHA) criteria; (2) all children in the KD group were diagnosed for the first time. (3) diagnostic criteria for coronary artery lesions (12): The Z-score was adopted as the diagnostic criterion for coronary artery lesions. (i) Normal: Z values < 2. (ii) Coronary artery dilation: 2 ≤ Z value <2.5. (iii) Small coronary aneurysm: 2.5 ≤ Z value < 5. (iv) Medium coronary aneurysm: 5 ≤ Z value < 10, absolute diameter < 8 mm. (v) Large coronary aneurysm: Z-value < 10 or internal diameter > 8 mm. Z-value ≥ 2.0 indicates CAL+. (4) IVIG resistant KD is defined as patients with KD develop recrudescent or persistent fever (T ≥ 38°C) at least 36 h after the end of IVIG infusion (2 g/kg).
The exclusion criteria for this study were as follows: (1) combined bacterial and viral infections; (2) combined cardiovascular and respiratory diseases; (3) use of glucocorticoids, immunosuppressants, and gamma globulin in the past 2 weeks. (4) Incomplete clinical data and unwillingness to participate with treatment.
Fasting venous blood (2 mL) was collected from all children and allowed to stand for 30 min before undergoing low-speed centrifugation at 2,500 rpm. After 15 min of centrifugation, supernatants were collected. Serum levels of FA (ng/ml), VD3 (VD3 refers to 1,25-dihydroxyvitamin D3, ng/ml), and VB12 (μg/l) FA were measured using an enzyme-linked immunosorbent assay (ELISA) purchased from Sigma. The levels of serum FA, VB12, and VD3 were also measured using a test kit from Shenzhen Teled Medical Co., Ltd., with an automatic chemiluminescence analyzer, VIT700.
Statistical analyses were performed using SPSS software version 26.0. Measurement data that conformed to a normal distribution were presented as mean ± standard deviation, and analyzed using independent T-tests. Data not following a normal distribution were described using the median and interquartile range, and analyzed with the Mann–Whitney U test. Categorical data were expressed as percentages and assessed using the chi-square (χ2) test or Fisher’s exact test, as appropriate. Statistical significance was established at p < 0.05.
The KD group was divided into typical KD group and IKD group, CALs group and non-CALS group, IVIG sensitive group and IVIG resistant group, small tumor group, medium and large tumor group. Serum levels of FA, VB12, and VD3 were compared among all groups (see Table 2).
There were no significant differences in sex or age between the two groups (p > 0.05). The levels of serum FA and VD3 indices in the KD group were significantly lower compared those in the healthy control group (p < 0.05), while there was no difference in the VB12 indices between the two groups, as shown in Figure 1. Multifactor regression analysis revealed that lower FA and VD3 indices are independent risk factors for KD, as detailed in Table 3.
There were no significant differences in sex or age between the two groups (p > 0.05). Serum VD3 levels were significantly lower in the KD group compared to controls (p < 0.05); while there was no difference in the VB12 indices between the two groups, as shown in Figure 2. Multifactor regression analysis showed that the VD3 levels is an independent risk factor for IKD (Table 4).
There were no significant differences in the sex ratio, serum FA levels and VB12 levels between the two groups (p > 0.05). Serum vitamin D3 (VD3) levels were significantly higher in the IVIG-sensitive group compared to the IVIG-resistant group (p < 0.05), as shown in Figure 3. Multifactor regression analysis showed that decreased serum VD3 level is an independent risk factor for IVIG resistance (Table 5).
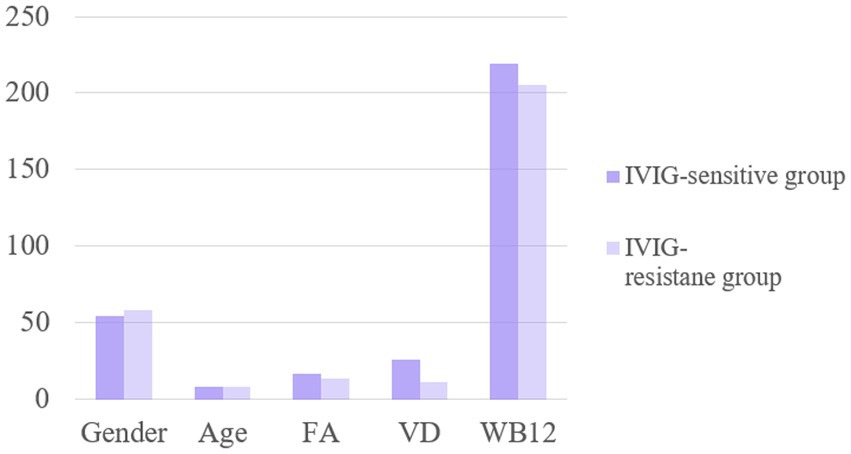
Figure 3. The levels of FA, VD and WB12 indexes were compared between IVIG-sensitive group and resistance group.
There were no significant differences in sex, age or VB12 levels between the two groups (p > 0.05). The serum FA and VD3 levels in the CALs group were significantly lower than those in the non-CALs group (p < 0.05), as shown in Figure 4. Multivariate regression analysis showed that FA and VD3 levels are independent risk factors for CALs (Table 6).
There were no significant differences in sex or age between the two groups (p > 0.05). The serum FA levels in the small tumor group were significantly higher than those in the medium and large tumor groups (p < 0.05), while there was no difference in the VD3 and VB12 levels among these groups (p > 0.05), as shown in Figure 5. Multivariate regression analysis showed that the FA level is an independent risk factor for aneurysm size (Table 7).
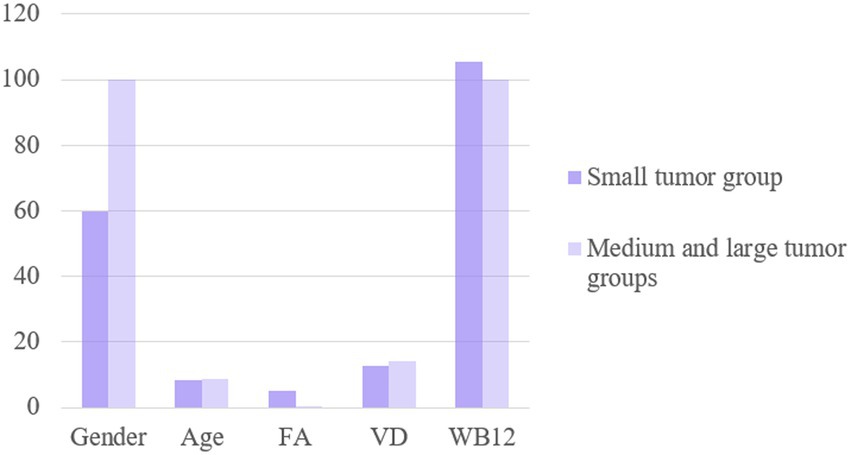
Figure 5. The levels of FA, VD and WB12 were compared between small tumor group and medium and large tumor groups.
Consistent with previous studies, we observed that serum FA levels in the KD group were significantly lower than those of the healthy group. Specifically, a study conducted by the Japan Environment and Child Research Group (18) showed that insufficient FA intake in pregnant mothers is associated with the occurrence of KD in infants and that FA supplementation in pregnant mothers could reduce the risk of KD. FA serves an essential role as a cofactor in both nucleotide biosynthesis and methylation processes, emphasizing its importance in cellular function and development (19). In addition, Amarasekera et al. (20) reported a positive correlation between FA concentrations in neonatal cord blood and maternal blood. Maternal FA supplementation is associated with upregulation of DNA methylation and genomic imprinting in CD4+ progenitor cells. Furthermore, another study (21) showed that FA inhibited gene expression of inflammatory cytokines such as interleukin-1β, tumor necrosis factor-α, and monocyte chemotactic protein-1 in lipopolysaccharide-activated macrophages. Considering the significant role of these inflammatory cytokines in the pathophysiology of KD, we hypothesize that sufficient FA exposure in utero could influence the development of immune functions in offspring, potentially reducing the risk of developing KD. As there was no difference in the estimated daily dietary FA between the KD and healthy groups, further researches are needed to elucidate the mechanism by which FA prevents the onset of KD.
This study additionally revealed that serum FA levels in the IKD group were significantly lower than those in the KD group, and levels in the CALs group were significantly lower than in the non-CALs group. This has not been explicitly mentioned in previous studies, suggesting that FA levels are involved in the occurrence of CALs by influencing Hcy levels. Recent epidemiological studies indicated that Hcy levels are independent risk factors for coronary heart disease. Homocysteine, a sulfur-containing amino acid derived from methionine after demethylation, is synthesized with the assistance of methionine synthase, which requires VB12 as a cofactor (22). This study demonstrated that the serum FA levels in the small tumor group was significantly higher than that in the medium and large tumor groups, while VD3 and VB12 levels did not differ from the other two groups. This deviation from previous studies may be attributed to the limited sample size used in this study. The product of tetrahydrofolate catalyzed by N-5 N-10-methylene tetrahydrofolate reductase (NTHFR) is the most important methyl donor in vivo. In addition, in vivo Hcy can condense with serine to form cystathione under the catalysis of cystathionine β-synthase (CBS) (23). When FA is deficient, the activities of NTHFR and CBS are significantly reduced, which leads to the obstruction of methionine regeneration and the excessive accumulation of Hcy. In conclusion, FA is an essential component for the conversion of Hcy to methionine and exhibits a significant negative correlation with Hcy levels. A deficiency in FA not only hampers the function of FA reductase but also disrupts the production of methyltetrahydrofolate, contributing to elevated Hcy levels.
The mechanisms by which homocysteine induces coronary artery lesions (CLAs) are complex, involving damage to the vascular endothelium, enhanced platelet activity, increased platelet aggregation, elevated fibrinogen production, and the promotion of smooth muscle cell migration, layering, and other pathophysiological processes (24, 25). These mechanisms bear a resemblance to the pathophysiological processes of CALs in KD; thus, it is hypothesized that homocysteine may also contribute to coronary artery injury in children with KD. Further studies (26) have demonstrated that homocysteine activates protein kinase C, which in turn promotes the expression of c-Fos and c-Myb in vascular endothelial and smooth muscle cells. Fundamental studies (27) have established a strong link between FA, a key factor in Hcy metabolism, and the development of CALs. In the realm of clinical research (28), findings indicated that Hcy levels were significantly higher in CHD patients than in non-CHD patients. Additionally, there is a negative correlation between FA levels and the incidence of CHD, with Hcy levels also showing a negative association with FA levels. However, FA is not an independent risk factor for CHD (29). It has been hypothesized that FA may influence homocysteine (Hcy) metabolism. Furthermore, a deficiency in FA leads to elevated Hcy levels, which, in turn, contributes to the development of CALs and CHD. This study confirmed significant differences in serum VD3 levels across groups: patients in the KD group exhibited lower VD3 levels compared to the healthy control group; within the KD cohort, those with IKD had significantly lower VD3 levels than those with complete KD; and patients with CALs had lower VD3 levels than those without CALs. Additionally, substantial evidence indicated that a deficiency in VD3 elevates the risk of cardiovascular diseases by impairing vascular endothelial function. Recent studies have shown that VD3 levels are related to KD, particularly KD combined with CALs. Research has demonstrated that serum levels of 25-(OH) D3 are significantly lower in children diagnosed with KD and that these levels are positively associated with the incidence of CALs and IKD (30). Furthermore, Stagi et al. (31) also found that a deficiency of serum 25 (OH) D3 in children with KD is closely related to an increased risk of cardiovascular events, and its low level may play an important role in the occurrence of KD combined with coronary complications. In addition, previous studies have found that the serum VD3 level of the population exhibit a seasonal pattern of being lower in winter and higher in summer (32), which is consistent with the seasonal variation trend of KD incidence. Thus, it is hypothesized that the seasonal pattern of KD incidence may be linked to the seasonal fluctuations in serum VD3 levels (33). However, the mechanism underlying the relationship between serum VD3 levels and the pathogenesis of KD remains unclear. Activation of immune factors and the release of inflammatory factors are increasingly recognized as critical elements in KD’s pathogenesis. Experimental studies involving KD mice models have demonstrated overexpression of the transcription factor kappa B (κB) and matrix metalloproteinase-9 (MMP-9) (34). Other literatures also found that interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and other inflammatory factors are related to KD. Furthermore, cytokines such as TNF-α, IL-6, and IL-1β have been found to induce endothelial cells to increase the production of matrix metalloproteinases (MMPs) This action accelerates the breakdown of extracellular matrix proteins and basement membrane components, leading to the deterioration of vascular walls and promoting the occurrence of CALs (35). Studies in animal models and cell cultures have revealed that the loss of the VD3R gene can lead to higher levels of pro-inflammatory factors (including TNF-α, IL-6, and IL-β1) produced by bone marine-derived macrophages. Additionally, the signaling pathway generated by the combination of VD3 and VD3R can inhibit the activation of NF-κB, which enhances the negative feedback regulation of the inflammatory response (36). VD3 deficiency leads to an unregulated and persistent inflammatory response, which may also explain the relationship between VD3 deficiency and inflammatory disorders, including KD, to a certain extent (37).
Our study also had some limitations. We measured only plasma folate levels, not its active form, 5-methyltetrahydrofolate (5-MTHF), which we plan to investigate further.
In summary, serum levels of FA and VD3 were reduced in children with KD. At the same time, it was found that the serum levels of FA and VD3 were particularly decreased in children with CALs, suggesting that the levels of FA and VD3 were involved in the occurrence and development of KD and CALs, providing a new research direction for the pathogenesis of KD and reducing the risk of CALs.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Kunming Children’s Hospital, Kunming, Yunnan, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
YC: Writing – original draft, Writing – review & editing. XL: Writing – original draft, Writing – review & editing. BL: Formal Analysis, Investigation, Writing – review & editing. JL: Methodology, Writing – review & editing. LM: Conceptualization, Writing – review & editing. CY: Conceptualization, Writing – review & editing. LH: Conceptualization, Writing – review & editing. HL: Project administration, Writing – review & editing. LD: Methodology, Writing – review & editing. ZS: Writing – original draft, Writing – review & editing. XZ: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant from Kunming “Spring City Plan” High-level talent introduced by the Engineering Young Talents Special Project and Health Research Project of Kunming Municipal Health Commission, Project (2023-06-01-013), Kunming Health Science and Technology Talent Training Project (2022-SW-0014), Kunming Health Science and Technology Talent Training Project (2023-SW-50).
We thank all of our patients and their families for participating in this study. The authors thank Huifeng Han for her contribution to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dajani, AS, Taubert, KA, Gerber, MA, Shulman, ST, Ferrieri, P, Freed, M, et al. Committee on rheumatic fever, endocarditis, and Kawasaki disease, council on cardiovascular disease in the young, American Heart Association. Diagnosis and therapy of Kawasaki disease in children. Circulation. (1993) 87:1776–80. doi: 10.1161/01.CIR.87.5.1776
2. Hartiala, O, Magnussen, CG, Bucci, M, Kajander, S, Knuuti, J, Ukkonen, H, et al. Coronary heart disease risk factors, coronary artery calcification and epicardial fat volume in the young Finns study. Eur Heart J Cardiovasc Imaging. (2015) 16:1256–63. doi: 10.1093/ehjci/jev085
3. McCrindle, BW, and Cifra, B. The role of echocardiography in Kawasaki disease. Int J Rheum Dis. (2018) 21:50–5. doi: 10.1111/1756-185X.13216
4. Chen, J, Liu, Y, Liu, W, and Wu, Z. A meta-analysis of the biomarkers associated with coronary artery lesions secondary to Kawasaki disease in Chinese children. J Huazhong Univ Sci Technolog Med Sci. (2011) 31:705–11. doi: 10.1007/s11596-011-0587-9
5. Chen, JJ, and Liu, YL. Clinical values of Kobayashi scoring system in Chinese children with Kawasaki disease. Acta Med Univ Sci Technol Huazhong. (2011) 40:746–9. doi: 10.1038/cdd.2010.68
6. Kudo, K, Hasegawa, S, Suzuki, Y, Hirano, R, Wakiguchi, H, Kittaka, S, et al. 1α,25-Dihydroxyvitamin D(3) inhibits vascular cellular adhesion molecule-1 expression and interleukin-8 production in human coronary arterial endothelial cells. J Steroid Biochem Mol Biol. (2012) 132:290–4. doi: 10.1016/j.jsbmb.2012.07.003
7. Valtueña, J, Breidenassel, C, Folle, J, and González-Gross, M. Retinol, β-carotene, α-tocopherol and vitamin D status in European adolescents; regional differences an variability: a review. Nutr Hosp. (2011) 26:280–8. doi: 10.1590/S0212-16112011000200006
8. van de Luijtgaarden, KM, Voûte, MT, Hoeks, SE, Bakker, EJ, Chonchol, M, Stolker, RJ, et al. Vitamin D deficiency may be an independent risk factor for arterial disease. Eur J Vasc Endovasc Surg. (2012) 44:301–6. doi: 10.1016/j.ejvs.2012.06.017
9. Chen, YL, Wang, JL, and Li, WQ. Prediction of the risk of coronary arterial lesions in Kawasaki disease by serum 25-hydroxyvitamin D3. Eur J Pediatr. (2014) 173:1467–71. doi: 10.1007/s00431-014-2346-y
10. Sharma, C, Ganigara, M, Galeotti, C, Burns, J, Berganza, FM, Hayes, DA, et al. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol. (2021) 17:731–48. doi: 10.1038/s41584-021-00709-9
11. Zhiqun, W, Yan, Z, Ning, S, Xia, W, Gang, S, et al. Changes of serum homocysteine, N-terminal B-type natriuretic peptide precursors and ischaemic modified albumin in children with Kawasaki disease complicated with coronary artery injury. Chin J Clin. (2021) 49:859–62. doi: 10.3969/j.issn.2095-8552.2021.07.03
12. Yang, R, Li, Y, Cui, T, Zhao, M, Li, L, Jing, Y, et al. Relationship between homocysteine C-reactive protein fibrinogen and coronary artery injury in children with Kawasaki disease. Chin Med Clinic. (2020) 20:2567–8. doi: 10.11655/zgywylc2020.15.038
13. Li, YC, Chen, Y, Liu, W, and Thadhani, R. MicroRNA-mediated mechanism of VD3 regulation of innate immune response. J Steroid Biochem Mol Biol. (2014) 144:81–6. doi: 10.1016/j.jsbmb.2013.09.014
14. Patel, JD, and Chakrabarti, C. Homocysteine, folate and vitamin b12 status in patients with coronary artery disease in young age. Inte J Clin Biochem Res. (2017) 4:129–35. doi: 10.18231/2394-6377.2017.0031
15. Misra, A, Vikram, NK, Pandey, RM, Dwivedi, M, Ahmad, FU, Luthra, K, et al. Hyperhomocysteinaemia, and low intakes of folic acid and vitamin B12 in urban North India. Eur J Nutr. (2002) 41:68–77. doi: 10.1007/s003940200010
16. Hermann, W, Schorr, H, Purschwitz, K, Rassoul, F, and Richter, V. Total homocysteine, vitamin B12 and total anti oxidants status in vegetarians. Clin Chem. (2001) 47:1094–101. doi: 10.1093/clinchem/47.6.1094
17. Ortega, RM, Jemenez, A, Andres, P, Faci, M, Lollo, JM, Lozano, MC, et al. Homocysteine levels in elderly Spanish people: influence of pyridoxine, vitamin B12 and folic acid intakes. J Nutr Health Ageing. (2002) 6:69–71.
18. Viswanathan, M, Treiman, KA, Kish-Doto, J, Middleton, JC, Coker-Schwimmer, EJL, and Nicholson, WK. Folic acid supplementation for the prevention of neural tube defects: An updated evidence report and systematic review for the US preventive services task force. JAMA. (2017) 317:190–203. doi: 10.1001/jama.2016.19193
19. Barua, S, Kuizon, S, and Junaid, MA. Folic acid supplementation in pregnancy and implications in health and disease. J Biomed Sci. (2014) 21:77. doi: 10.1186/s12929-014-0077-z
20. Amarasekera, M, Martino, D, Ashley, S, Harb, H, Kesper, D, Strickland, D, et al. Genome-wide DNA methylation profiling identifies a folate-sensitive region of differential methylation upstream of ZFP57-imprinting regulator in humans. FASEB J. (2014) 28:4068–76. doi: 10.1096/fj.13-249029
21. Samblas, M, Martínez, JA, and Milagro, F. Folic acid improves the inflammatory response in LPS-activated THP-1 macrophages. Mediat Inflamm. (2018) 2018:1–8. doi: 10.1155/2018/1312626
22. Yi, X, Zhou, Y, Jiang, D, Li, X, Guo, Y, and Jiang, X. Efficacy of folic acid supplementation on endothelial function and plasma homocysteine concentration in coronary artery disease: a meta-analysis of randomized controlled trials. Exp Ther Med. (2014) 7:1100–10. doi: 10.3892/etm.2014.1553
23. Guo, H, Chi, J, Xing, Y, and Wang, P. Influence of folic acid on plasma homocysteine levels & arterial endothelial function in patients with unstable angina. Indian J Med Res. (2009) 129:279–84.
24. Zhao, L, Qi, J, Luo, F, and Zhao, N. The diagnostic value of combined detection of serum Lp-PLA2 and Hcy and color Doppler in elderly patients with acute coronary syndrome and effect on endothelial function. Evid Based Complement Alternat Med. (2022) 2022:1–7. doi: 10.1155/2022/3150670
25. Huang, J, and Zhang, S. Overexpressed neuropilin-1 in endothelial cells promotes endothelial permeability through interaction with ANGPTL4 and VEGF in Kawasaki disease. Mediat Inflamm. (2021) 2021:1–8. doi: 10.1155/2021/9914071
26. Li, S, Sun, L, Qi, L, Jia, Y, Cui, Z, Wang, Z, et al. Effect of high homocysteine level on the severity of coronary heart disease and prognosis after stent implantation. J Cardiovasc Pharmacol. (2020) 76:101–5. doi: 10.1097/FJC.0000000000000829
27. Ni, J, Zhang, L, Zhou, T, Xu, WJ, Xue, JL, Cao, N, et al. Association between the MTHFR C677T polymorphism, blood folate and vitamin B12 deficiency, and elevated serum total homocysteine in healthy individuals in Yunnan Province, China. J Chin Med Assoc. (2017) 80:147–53. doi: 10.1016/j.jcma.2016.07.005
28. Ye, M, Chen, X, Mao, S, Zhou, J, Liu, M, and Wu, Y. Effect of folic acid, vitamin B12, and B6 supplementation on the risk of cardiovascular and cerebrovascular diseases: An updated meta-analysis of randomized controlled trials. Pteridines. (2022) 33:39–48. doi: 10.1515/pteridines-2022-0041
29. Muzaffar, R, Khan, MA, Mushtaq, MH, Nasir, M, Chaudhry, M, Khan, MF, et al. Association of dietary factors with plasma homocysteine and coronary heart disease outcome. Nutr Clin Métab. (2022) 36:106–11. doi: 10.1016/j.nupar.2021.12.180
30. An, X, Fu, M, Tian, J, Xue, Y, and Xu, H. Significance of serum 25-hydroxyVitamin D3 and interleukin-6 levels in immunoglobulin treatment of Kawasaki disease in children. Exp Ther Med. (2016) 12:1476–80. doi: 10.3892/etm.2016.3492
31. Stagi, S, Rigante, D, Lepri, G, Matucci Cerinic, M, and Falcini, F. Severe vitamin D3 deficiency in patients with Kawasaki disease: a potential role in the risk to develop heart vascular abnormalities? Clin Rheumatol. (2016) 35:1865–72. doi: 10.1007/s10067-015-2970-6
32. Schoenmakers, I, Gousias, P, Jones, KS, and Prentice, A. Prediction of winter vitamin D3 status and requirements in the UK population based on 25(OH) vitamin D3 half-life and dietary intake data. J Steroid Biochem Mol Biol. (2016) 164:218–22. doi: 10.1016/j.jsbmb.2016.03.015
33. Camargo, CA Jr, Ganmaa, D, Sidbury, R, Erdenedelger, K, Radnaakhand, N, and Khandsuren, B. Randomized trial of vitamin D3 supplementation for winter-related atopic dermatitis in children. J Allergy Clin Immunol. (2014) 134:831–835.e1. doi: 10.1016/j.jaci.2014.08.002
34. Lau, AC, Duong, T, Ito, S, Wilson, GJ, and Yeung, RSM. Inhibition of matrix metalloproteinase-9 activity improves coronary outcome in an animal model of Kawasaki disease. Clin Exp Immunol. (2009) 157:300–9. doi: 10.1111/j.1365-2249.2009.03949.x
35. Wang, Y, Wang, W, Gong, F, Fu, S, Zhang, Q, Hu, J, et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum. (2013) 65:805–14. doi: 10.1002/art.37815
36. Lv, YW, Wang, J, Sun, L, Zhang, J, Cao, L, Ding, Y, et al. Understanding the pathogenesis of Kawasaki disease by network and pathway analysis. Comput Math Methods Med. (2013) 2013:989307. doi: 10.1155/2013/989307
37. Al Mheid, I, Patel, R, Murrow, J, Morris, A, Rahman, A, Fike, L, et al. Vitamin D3 status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. (2011) 58:186–92. doi: 10.1016/j.jacc.2011.02.051
38. Mccrindle, BW, Rowley, AH, Newburger, JW, Burns, JC, Bolger, AF, Gewitz, M, et al. Diagnosis, treatment, and long-term Management of Kawasaki Disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:927–99. doi: 10.1161/CIR.0000000000000484
Keywords: Kawasaki disease, coronary artery lesions, folic acid, vitamin D3, vitamin B12
Citation: Chen Y, Liu X, Li B, Li J, Meng L, Ye C, Han L, Li H, Deng LL, Su Z and Zhang X (2024) Explorative case control study on the associations of serum vitamin D3, folic acid and vitamin B12 levels on Kawasaki disease and coronary artery lesions. Front. Nutr. 11:1402316. doi: 10.3389/fnut.2024.1402316
Received: 17 March 2024; Accepted: 22 May 2024;
Published: 11 June 2024.
Edited by:
Francesca Gorini, National Research Council (CNR), ItalyReviewed by:
Dina Keumala Sari, Universitas Sumatera Utara, IndonesiaCopyright © 2024 Chen, Liu, Li, Li, Meng, Ye, Han, Li, Deng, Su and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongjian Su, c3V6aG9uZ2ppYW5Ac2luYS5jbg==; Xing Zhang, emhpY2hpODI4QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.