
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 11 June 2024
Sec. Nutrition and Food Science Technology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1397399
 Alemu Birara Zemariam1*
Alemu Birara Zemariam1* Molalign Aligaz Adisu1
Molalign Aligaz Adisu1 Aklilu Abera Habesse1
Aklilu Abera Habesse1 Biruk Beletew Abate1
Biruk Beletew Abate1 Molla Azmeraw Bizuayehu1
Molla Azmeraw Bizuayehu1 Wubet Tazeb Wondie2
Wubet Tazeb Wondie2 Addis Wondmagegn Alamaw3
Addis Wondmagegn Alamaw3 Habtamu Setegn Ngusie4
Habtamu Setegn Ngusie4Background: Although micronutrients (MNs) are important for children’s growth and development, their intake has not received enough attention. MN deficiency is a significant public health problem, especially in developing countries like Ethiopia. However, there is a lack of empirical evidence using advanced statistical methods, such as machine learning. Therefore, this study aimed to use advanced supervised algorithms to predict the micronutrient intake status in Ethiopian children aged 6–23 months.
Methods: A total weighted of 2,499 children aged 6–23 months from the Ethiopia Demographic and Health Survey 2016 data set were utilized. The data underwent preprocessing, with 80% of the observations used for training and 20% for testing the model. Twelve machine learning algorithms were employed. To select best predictive model, their performance was assessed using different evaluation metrics in Python software. The Boruta algorithm was used to select the most relevant features. Besides, seven data balancing techniques and three hyper parameter tuning methods were employed. To determine the association between independent and targeted feature, association rule mining was conducted using the a priori algorithm in R software.
Results: According to the 2016 Ethiopia Demographic and Health Survey, out of 2,499 weighted children aged 12–23 months, 1,728 (69.15%) had MN intake. The random forest, catboost, and light gradient boosting algorithm outperformed in predicting MN intake status among all selected classifiers. Region, wealth index, place of delivery, mothers’ occupation, child age, fathers’ educational status, desire for more children, access to media exposure, religion, residence, and antenatal care (ANC) follow-up were the top attributes to predict MN intake. Association rule mining was identified the top seven best rules that most frequently associated with MN intake among children aged 6–23 months in Ethiopia.
Conclusion: The random forest, catboost, and light gradient boosting algorithm achieved a highest performance and identifying the relevant predictors of MN intake. Therefore, policymakers and healthcare providers can develop targeted interventions to enhance the uptake of micronutrient supplementation among children. Customizing strategies based on identified association rules has the potential to improve child health outcomes and decrease the impact of micronutrient deficiencies in Ethiopia.
Micronutrient intake is the provision of either a single micronutrient (MN), such as iodine, zinc, calcium, manganese, chromium, copper, fluoride, iron, folic acid, vitamin A, vitamin B-complex, or vitamin D, or a combination of MNs. These essential nutrients can be administered in the form of capsules, tablets, drops, or syrups (1). MNs are essential and required in small amounts. However, when there is a deficiency in their supply, it can have significant negative effects on the growth and development of children. These effects include stunting, wasting, delayed cognitive development, prolonged hospital stays, and weakened immunity, making children more susceptible to common childhood infections (2, 3). Despite the crucial role that MNs play in promoting healthy growth and development in children, there has been limited focus on ensuring an adequate intake of these nutrients (4). MN deficiency continues to be a widespread public health issue, particularly in developing countries like Ethiopia (5).
Due to the latency nature of clinical symptoms of MN deficiency as far as they are not detected via blood levels in an early stages with some exceptions, inadequacy of one or more of its supplementation leads to health consequence or hidden hunger (6). The deficiency of MNs, combined with stunting and wasting, contributes to approximately 45% or 3.1 million deaths in children every year (7). As per the 2019 report from the United Nations Children’s Fund, approximately 340 million children globally, with 54% of them residing in developing countries, experienced hidden hunger due to deficiencies in micronutrients (8).
To address MN deficiency and its consequences, different countries have implemented MN supplementation or improved intake strategies. Scientific evidences have shown that providing high doses of MNs like vitamin A, iron, and zinc can lead to a reduction of childhood mortality (9). Despite the efforts of programs such as the WHO 2016–2025 nutrition strategy, the inadequate intake of MNs remains a persistent issue in both developed and developing countries (10). For example, in Brazil, only 54.2% of children aged 6–59 months consumed a micronutrient supplement (11), and less than 10% of children aged 6–59 months in Ethiopia received iron supplements and deworming tablets (10, 12).
A systematic review covering Ethiopia, Nigeria, Kenya, and South Africa found varying rates of micronutrient (MN) intake, ranging from 51 to 99% for zinc, 13 to 100% for iron, and 1 to 100% for vitamin A (13). The consumption of iodized salt varied from 2% in Kenya to 96% in Ethiopia (14). In another study involving children aged 6–23 months across 20 sub-Saharan countries, it was reported that nearly 74% of the children had adequate micronutrient intake, with Ethiopian children having the lowest intake at round of to 59% (15).
The Ethiopian government has made significant efforts to address national nutrition issues, including the implementation of the first national nutrition program in 2008 (16), which prioritized ending malnutrition. It also joined the Scaling up Nutrition movement in 2012 and the Seqota Declaration in 2015 to combat child undernutrition by 2030 (17). However, despite these efforts, the issue of malnutrition and the deficiency of MN intake remain significant public health concerns in Ethiopia. According to the 2016 Ethiopian Demographic and Health Survey (EDHS), only 14% of children aged 6–23 months consumed minimum dietary diversity (12).
Various factors, including maternal socio-demographics, child characteristics, and maternal healthcare services utilization, are associated with MN intake (1, 14, 15, 18, 19). Previous studies in Ethiopia have used classical statistical methods to analyze MN intake status (14, 15), which means that the estimates are based on the previous assumptions, which may limit the potential to discover hidden information and these strategies are used to analyze features selected based on prior knowledge or logical reasoning and it is difficult to handle complex data patterns and capturing nonlinear relationships, which are often present in dietary intake data. Leveraging machine learning (ML) models can offer significant advantages and contribute to the existing empirical evidence and making the most accurate predictions enabling systems to learn from data rather than making prior assumptions (20). ML techniques excel in managing complex and nonlinear data, operate without preexisting assumptions, and capture intricate relationships among predictors (20, 21). Besides, the previous study was confined with a limited ML algorithm, data balancing techniques, and small sample size. Therefore, this study aimed to utilize 12 advanced ML techniques including association rule mining to predict MN intake status and identify its predictors using the 2016 EDHS data set. The findings will inform policymakers in planning evidence-based programs with integrated interventions to enhance MN intake. Moreover, these findings can provide valuable insights for developing context-specific strategies to address these issues, inform targeted interventions, policy-making, and resource allocation aimed at improving the nutritional status of children in Ethiopia, ultimately contributing to enhanced public health outcomes.
Data from the 2016 Ethiopian Demographic and Health Surveys (EDHS) were obtained through a formal written request to the DHS program website (22).1 The DHS Program has conducted standardized surveys in over 90 countries, gathering comprehensive and representative data on aspects such as population, health, HIV, and nutrition. The EDHS data includes information from nine regions [Tigray, Afar, Amhara, Oromia, Benishangul-Gumuz, Gambela, South Nation Nationalities and Peoples’ Region (SNNPR), Harari, and Somali] as well as two administrative cities (Addis Ababa and Dire-Dawa). The data collection procedure utilized a multi-stage stratified cluster sampling approach for each region. Stratification was performed based on urban and rural sectors, and enumeration areas were selected using probability proportional to size. Within the chosen enumeration areas, households were selected using equal probability systematic sampling (23). The study focused on children aged 6–23 months in Ethiopia within the previous 5 years. The analysis involved a weighted sample size of 2,499 children aged 6–23 months. The dataset employed in the study included 23 distinct features that were considered during the analysis.
The study variable of interest is the micronutrient intake status (MNs) among children aged 6–23 months. To determine the MN intake status, we have considered six options: consumption of food rich in vitamin A (VA) or iron within the past 24 h, consumption of micronutrient powders (MNP) or iron supplements within the past 7 days, and receipt of vitamin A supplementation (VAS) or deworming treatment within the past 6 months (24–26). To determine the intake of the minimum recommended MNs, if the respondent reported that the child had consumed at least one of the minimum recommended MNs, it was classified as a “Yes” response. Conversely, if the child had not received any of the recommended MNs, it was classified as a “No” response.
To assess the consumption of foods rich in vitamin A (VA), we analyzed the intake of seven specific food groups within the previous 24 h. These food groups included eggs, various meats (such as beef, pork, lamb, and chicken), pumpkin, carrots, and squash, dark green leafy vegetables, mangoes, papayas, and other fruits rich in VA, as well as liver, heart, and other organs, and fish or shellfish. Similarly, we assessed the consumption of iron-rich foods by examining the intake of four specific food groups within the previous 24 h. These food groups consisted of eggs, various meats, liver, heart, and other organs, as well as fish or shellfish. To determine the intake of MNP, we asked the respondents if their child had received such powders in the past 7 days. For assessing iron supplementation, we inquired whether the child had been given iron pills, sprinkles with iron, or iron syrup within the past 7 days. The researchers examined vitamin A supplementation (VAS) and deworming treatment by reviewing the integrated child health card, which contains information on immunization and growth monitoring history. They also obtained verbal responses from the mothers. These assessments were specifically conducted for children aged 6–23 months to determine if they had received VAS and deworming treatment in the last 6 months. If the respondent reported that the child had consumed at least one of these food groups, it was categorized as a “Yes” response, indicating the consumption of MN-rich foods.
The study considered several independent variables, including place of residence, region, religion, media exposure, sex of household head, age of mother, age of child, ANC visit, postnatal care (PNC) visit, family size, current marital status, working status of the mother, desire for more children, current pregnancy status, number of children, place of delivery, mode of delivery, history of diarrhea, history of cough, sex of child, working status of the father, educational status of the mother, educational status of the father, and wealth index. The selection of these independent variables was based on a comprehensive review of existing literature in the field.
The first step in ML is data pre-processing, which involves modifying or encoding the data to make it understandable by computers (27). In our ML workflow, we employed a process of continuous improvement for our models. This process included selecting and engineering features, choosing models, and tuning hyper-parameters. We refined our models continuously over an iterative approach. Figure 1 provides the details of the specific steps in our workflow.
We performed a thorough examination to identify and eliminate any duplicated data entries in our dataset. After this review, we verified that there were no redundant entries present. To address missing values, we applied the K-nearest neighbors (KNN) imputation technique (28). To detect outliers, we utilized different methods such as box plots and Grubbs’ test. Furthermore, we evaluated multicollinearity by examining the correlation matrix. We considered a correlation value above 0.8 between two variable pairs as an indication of high correlation (29, 30).
Feature engineering involves identifying, obtaining, and adjusting the most important features from the available data to develop accurate and efficient ML models (31). In our study, we employed one-hot encoding to encode nominal categorical variables and label encoding for ordinal categorical variables (32).
We did feature selection to enhance the model performance and reduce the dimensionality of the dataset (33). We utilized a feature selection method called Boruta, which assesses the importance of features by comparing their performance with randomly generated shadow features that mimic noise. Features that consistently outperformed the shadow features were considered significant and included in our predictive model (34).
Data mining and ML face difficulties due to class imbalance, which leads to reduced accuracy and biased estimate when classifying minority instances (35). To address this challenge, it is advisable to explore different data balancing techniques and select the one that performs well, as the effectiveness of these techniques can vary depending on the nature of the dataset. To mitigate this issue, we utilized seven data balancing techniques, including under-sampling, over-sampling, adaptive synthetic sampling (ADASYN), (SMOTE), synthetic minority oversampling technique with edited nearest neighbors (SMOTE ENN), SMOTE Tomik, and the near miss methods. Initially, we trained our ML algorithms using the unbalanced data. Then, we investigated the mentioned balancing techniques to train the models using balanced data. To assess the performance of each model, we compared accuracy, AUC (Area under the Curve), and other evaluation metrics. It is recommended to consider both accuracy and AUC, along with other pertinent metrics, to thoroughly evaluate model performance and make informed comparisons between various ML algorithms (36–38). Taking these factors into account, we have chosen the balancing technique that exhibited superior performance for further tuning and the final prediction of the micronutrient supplementation status.
In our study, the variable of interest, which indicated the status of micronutrient intake, necessitated a classification method as it was divided into two distinct categories: “yes” and “no.” To make accurate predictions, it was essential to choose suitable classifiers. To achieve this, we utilized the scikit-learn version 1.3.2 libraries in Python, implemented through Jupyter Notebook, to apply a range of ML algorithms.
To assess the predictive capabilities of ML algorithms in predicting the status of micronutrient supplementation, we employed 12 advanced machine learning algorithms. These algorithms encompassed support vector machines with kernel methods, Gaussian naive Bayes, logistic regression, decision tree classifier, random forest classifier, gradient boosting machines, extreme gradient boosting, AdaBoost Classifier, k-nearest neighbors, CatBoost Classifier, MLP Classifier, and ANN with tensor flow.
Creating a dependable predictive model in ML entails essential steps in model training and evaluation (39, 40). In our study, we adopted a straightforward approach by splitting the data into an 80% training set and a 20% testing set. To assess the performance of each predictive model, we employed various evaluation metrics, including accuracy, precision, recall, F1-score, and AUC. Accuracy gaged overall correctness, precision measured accurate positive predictions, recall evaluated the identification of all positive instances, and the F1-score provided a balanced measure. AUC, calculated from the area under the ROC curve, indicated the algorithm’s ability to discriminate between classes (41).
To further evaluate the model’s performance, we employed a 10-fold cross-validation techniques (28). Additionally, we conducted a comprehensive analysis of hyper parameters to refine and enhance the model’s performance. It is recommended to experiment with various tuning techniques and take the one which perform better from the others. Accordingly, we systematically explored grid search, random search, and Bayesian optimization. By comparing the results from these techniques, we identified configurations that yielded the highest performance. To improve the accuracy and reliability of the model, we also performed model calibration. Through fine-tuning the model via calibration, we enhanced its predictive capabilities to accurately forecast the desired outcome. Various kernel methods were also compared for SVM model.
Incorporating SHAP (SHapley Additive exPlanations) values and association rule mining has been highlighted by scholars for achieving diverse objectives (42, 43). Association rule mining is suitable for uncovering hidden patterns and relationships within the data, while SHAP analysis is more appropriate for understanding the impact of different features on the overall model predictions (42, 44).
Therefore, we have utilized a variety of techniques. Initially, we computed the mean SHAP values to evaluate the average impact of each feature on the model’s predictions, providing insights into the relative significance of different variables. Subsequently, we employed a waterfall plot to visually depict the cumulative effects of these variables, emphasizing their contributions to the overall prediction. Lastly, association rule mining was employed to uncover concealed patterns and relationships among the variables, enabling a more profound exploration of the dataset.
A total weighted sample of 2,499 children aged 6–23 months was included in this study. Among these children, 1,728 (69.15%) had a micronutrient intake. Approximately two-thirds (63.99%) of the participants fell within the age range of 12 to 23 months, and more than half (59.38%) of their mothers did not have any formal education. In terms of wealth status and religion, 50.5% of the respondents belonged to the poor wealth quintile, and roughly 49.74% identified as Muslim. A majority (69.07%) of the respondents had a history of antenatal care (ANC) visits, while the majority (91.04%) had not received any PNC service. Additionally, more than three-quarters (79.35%) of the respondents resided in rural areas, and nearly two-thirds (64.71%) had no access to media exposure. The detailed statistics are presented in Table 1.
Upon evaluating different methods for feature selection, we found that the Boruta algorithm produced favorable outcomes. The graphical representation of the Boruta algorithm effectively illustrated the significance of various variables, with significant variables displayed in green, insignificant variables in red, and uncertain variables in yellow (45). Our analysis of the Boruta algorithm graph (Figure 2) revealed that seven variables were considered insignificant or unimportant, four variables were uncertain, and the remaining 12 variables were deemed important for predicting the status of micronutrient intake. Consequently, we employed 16 variables to forecast micronutrient intake and explore data patterns using association rule mining.
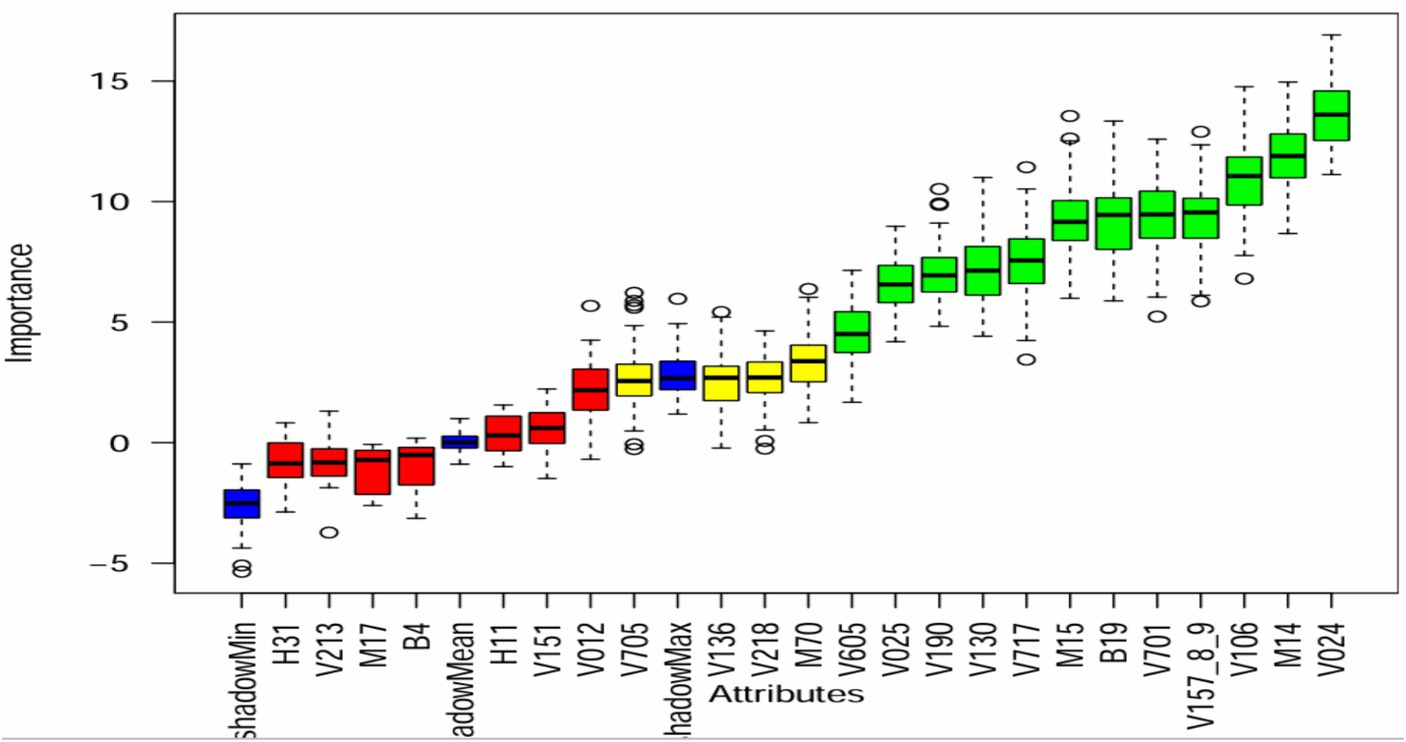
Figure 2. Feature selection using Boruta algorithm H31-had cough, V213-current pregnancy status, M17-Mode of delivery, B4-sex of child, H11-had diarrhea, V151-sex of household head, V012-mothers age, V705-husband occupation, V136-family size, V218-number of living children, M70-PNC visit, V605-desire more children, V025-residence, V190-wealth index, V130-religion, V717-mother occupation, M15-place of delivery, B19-age of child, V701-husband education status, V157_8_9-media exposure status, V106-educational status of respondent, M14-ANC visit, and V024-region.
Table 2 provides a comparison of various data balancing techniques, including under-sampling, over-sampling, Adaptive Synthetic Sampling (ADASYN), synthetic minority over-sampling technique (SMOTE), synthetic minority over-sampling technique with edited nearest neighbors (SMOTE ENN), SMOTE tomek, and the near-miss algorithm. Among these techniques, SMOTE ENN demonstrated the highest performance, achieving an AUC above 0.90 for all ML algorithms except Gaussian naive Bayes, logistic regression, and the decision tree classifier. Notably, Gaussian naive Bayes achieved an AUC of 0.85, logistic regression achieved an AUC of 0.89, and the decision tree classifier achieved an AUC of 0.84, all of which were considered acceptable. Additionally, the SMOTE ENN data balancing technique achieved an accuracy value above 85.0% across all 12 ML algorithms. These findings indicate that SMOTE ENN outperformed than the other data balancing techniques, as shown in Table 2. Consequently, we have selected SMOTE ENN as the final and most effective data balancing technique for further ML analysis and optimization. For a comprehensive visual representation comparing the performance of ML algorithms with each data balancing technique, please refer to Supplementary Figure 1.
During the analysis, we evaluated multiple ML algorithms to predict the status of micronutrient intake. To assess the algorithms’ performance, we considered various metrics such as accuracy, precision, sensitivity, specificity, recall, F1 score, and AUC. Additionally, we employed techniques like grid search, random search, and Bayesian optimization for fine-tuning the models to improve prediction accuracy. The detail of evaluation metrics is presented in Supplementary Figure 2.
The results demonstrated that all 12 algorithms performed exceptionally well, although their specific performance varied depending on the tuning technique used. When employing the grid search technique, the random forest, catboost, and light gradient boosting algorithm achieved the highest performance with an AUC of 0.98. Most algorithms achieved an AUC above 0.90, except for Gaussian naive Bayes, logistic regression, and decision trees, which had AUC values of 0.82, 0.85, and 0.87, respectively.
In the random search hyper parameter tuning, the random forest, light gradient boosting, and catboost algorithms demonstrated equally impressive performance metrics, achieving an AUC of 0.94. Similar to the results obtained from the grid search technique, most algorithms achieved an AUC above 0.90, except for Gaussian naive Bayes and decision trees, which had AUC values of 0.88. When employing the Bayesian optimization technique, the catboost algorithm followed by MLP achieved superior AUC values of 0.98 and 0.96, respectively.
In general, the comprehensive evaluation revealed excellent performance across all 12 ML algorithms, with consistent and comparable results. Different tuning techniques yielded the best outcomes for different algorithms, with random search, grid search, and Bayesian optimization demonstrating notable performance in specific cases. While some variations in performance were observed, no single technique consistently outperformed all aspects of the ML algorithms.
The random forest, light gradient boosting, and catboost algorithms with grid search and random search optimization tuning emerged as the top three performers across all metrics. In addition, the catboost algorithm followed by MLP exhibited strong performance when tuned with Bayesian optimization.
For a comprehensive comparison of the 12 ML algorithms and their performance across the three tuning techniques, please refer to Table 3. Furthermore, graphical representations illustrating the performance of each algorithm under different tuning techniques can be found in Figure 3.

Figure 3. AUC value of each ML algorithm. (A) Grid search, (B) Random search, (C) Bayesian optimization.
Based on the information presented in Figure 4, the mean SHAP value report provided valuable insights into the relative importance of different features in the classification model. Factors such as ANC visit, region, and child age emerged as the most influential variables, exerting a significant impact on the model’s predictions. This suggests that these features play a crucial role in determining the model’s predictions, while the remaining six variables have minimal influence.
The findings depicted in Figure 5, as shown by the waterfall plot, offer valuable insights into the hierarchy of feature importance for predicting the target variable. The plot highlights that ANC visit has the highest positive impact on the prediction, followed by mother occupation, child age, and mother age. On the other hand, place of delivery, wealth index, mother education, and media exposure negatively contribute to the model’s prediction. This indicates that factors such as home delivery, poor wealth status, lack of formal education, and absence of media exposure are associated with lower predicted outcomes in the model, while their absence or opposite attributes are associated with higher predicted outcomes.
By utilizing the a priori algorithm, we were able to identify significant association rules with a confidence level exceeding 95%. These rules provided valuable insights into the likelihood of micronutrient intake status among children aged 6–23 months in Ethiopia. Notably, certain variables such as region, wealth index, place of delivery, mothers’ occupation, child age, fathers’ educational status, desire for more children, access to media exposure, and ANC follow-up consistently appeared in these rules, indicating their strong association with the probability of micronutrient intake. In total, 167 rules were generated, and the following are the top seven association rules ranked by their confidence levels and corresponding lift values.
1. If children living in Benishangul Gumez region and children from medium wealth index household, the probability of micronutrient intake status is 98.7% (lift value = 1.45)
2. If children living in Gambella region, children from rich household, and whose mothers gave birth at health facility, the probability of micronutrient intake is 97.8% (lift value = 1.43)
3. If children living in Benishangul Gumuz region, children from rich household, and children who had a mother with work or occupation, the probability of micronutrient intake is 97.6% (lift value = 1.42)
4. If children living in Addis Ababa, children whose father completed higher education, and children aged 12–23 months, the probability of micronutrient intake is 96.9% (lift value = 1.4)
5. If children living in Gambella region, family wants no more children, and children born from mothers who had ANC follow-ups, the probability of micronutrient intake is 96.6% (lift value = 1.4)
6. If children living in Gambella region, children aged 12–23 months, and had a history of media exposure, the probability of being supplemented with micronutrient is 96.6% (lift value = 1.4)
7. If children living in Gambella region, children whose mother had work or occupation, and whose mothers gave birth at health facility, the probability of micronutrient intake is 95.4% (lift value = 1.37)
The aim of the study was to predict the micronutrient intake status among children aged 6–23 months in Ethiopia using advanced machine learning algorithms. Twelve different algorithms were tested, including Random Forest, Decision Tree, Naive Bayes, and others. All 12 algorithms used in the study performed well, with ROC values above the optimal threshold. The random forest classifier, catboost, and light gradient boosting classifier were particularly effective in identifying micronutrient intake status. Data balancing techniques were used to improve the accuracy of the final model. After balancing the data, the RF, Catboost, and LGB models performed the best overall. These findings are consistent with similar studies conducted in Rwanda (20) and Ethiopia (46), although the difference in data set sizes across the studies may have contributed to slight variations.
Analyzing the mean SHAP value report and waterfall plot provided valuable insights on factors influencing the prediction of micronutrient intake status among children aged 6–23 months. Factors like ANC, region, child age, and wealth index were found to be significant and influential. However, PNC utilization, father education, and desire more children had minimal impact on the classification outcome. Understanding these features can guide targeted interventions and policy decisions, improving the health and nutritional status of children in Ethiopia. These findings validate existing knowledge and evaluate the model’s effectiveness for more accurate interventions.
The other aim of the study was to identify the key predictors of micronutrient intake in children aged 6–23 months using the Boruta algorithm. Out of 23 features considered, 12 were found to be important for predicting micronutrient intake status. The Boruta algorithm revealed that machine learning models can uncover new variables and insights that traditional regression models may miss, providing valuable information for policy decisions.
The other objective of the study was to use association rule mining with the a priori algorithm to identify patterns and associations between independent predictors and the outcome variable. The top seven rules generated by the best model revealed that there was a 98.7% probability of micronutrient intake among children living in the Benishangul Gumuz region and coming from medium wealth index households is a striking observation. This result is supported with a study conducted in Ethiopia (47), Nigeria (48) and Bangladesh (49). This is the fact that household with better wealth may improve the nutritional intake for their children and Benishangul Gumuz region has agrarians’ community and they have access to serve diversified nutrient for their children. This finding suggests a strong association between the geographic region, economic status, and the likelihood of adequate micronutrient intake.
The model showed that the probability of having adequate micronutrient intake among a child living in the Gambella region, being from a rich household, and having a mother who gave birth at a health facility is claimed to be 97.8%. This result is supported by a study from recent EDHS (2016) (14). The possible justification is that, since agriculture is common in Gambella region, caregivers could get wild fruit and fish, which are good sources of micronutrients and Children from rich households, might have better access to a diverse and nutrient-rich diet, nutritional supplements, and healthcare resources. This economic advantage can contribute to a higher probability of meeting micronutrient requirements (50). Children born to mothers who deliver at health facilities may receive better post-natal care, including nutritional guidance and support. This could positively impact the child’s early development and micronutrient intake.
The reported probability of micronutrient intake is a substantial 96.9%, accompanied by a lift value of 1.4 for children living in Addis Ababa, children whose father completed higher education, and children aged 12–23 months. The result is in agreement with the studies conducted in east Africa, sub-Saharan Africa, and Nepal (15, 51, 52). This is due to the fact that higher education levels often correlate with increased awareness of nutrition and health, potentially impacting feeding practices, dietary choices, and overall child care. The higher the probability of obtaining micronutrient intake in 12–23 months could be explained by the fact that, at this age group, they could have better dietary diversity as they can eat family meals for themselves, and good complementary feeding practices are more common in urban than rural areas (14). Moreover, the late introduction of complementary foods and mothers’ and caregivers’ perceptions toward feeding diversified foods may contribute to lower consumption of micronutrients in lower age groups.
The Advanced ML result suggests that families in the Gambella region who express a desire for no more children are associated with a 95.4% probability of their children having adequate micronutrient intake. Studies support the idea that family size significantly influences the micronutrient intake of children (15, 53). This finding underscores the importance of family planning in contributing to better child nutrition outcomes. This is due to the fact that families with fewer children may have more resources available per child, enabling better access to nutritious food and healthcare. Alternatively, families with no desire for more children might be more focused on the well-being of their existing children. The result also indicates a positive association between micronutrient intake and mothers who had ANC follow-ups during pregnancy. This aligns with existing knowledge that adequate antenatal care is crucial for monitoring the health of both the mother and the developing child (14, 15). The positive correlation may be attributed to the health education and nutritional guidance provided during ANC visits. Mothers who attend ANC may receive information on proper nutrition during pregnancy and infancy, contributing to better nutritional practices. The result implies that implementing integrated healthcare approaches that combine family planning services with maternal and child health programs may further enhance the positive impact on micronutrient intake.
The advanced machine learning algorithms result shows that there is strong correlation between media exposure and micronutrient intake of children. The result is supported by studies conducted in Ethiopia and India (42, 43). This could be due to media may influence parental behavior, impacting their decision-making regarding child nutrition and encouraging them to prioritize their children’s nutritional needs.
This study had strengths in thoroughly evaluating 12 advanced machine learning algorithms and optimizing their performance through experimentation with data balancing and hyper parameter tuning. It also provided valuable insights into factors influencing micronutrient intake for targeted interventions and policies. However, limitations included reliance on existing data with potential limitations and biases, the inability to establish causal relationships or account for temporal changes, and the need for further validation in real-world settings with diverse populations to ensure reliability and generalizability.
The study shows that machine learning can accurately predict micronutrient intake status among children in Ethiopia. All 12 algorithms performed well, with the random forest, catboost and LGB classifier being the most effective. These findings have important implications for targeted interventions and public health strategies. These findings carry significant implications for public health interventions in Ethiopia, as ML algorithms can be utilized to develop targeted strategies that promote the adoption of micronutrient intake.
The study identified several important risk factors for micronutrient intake among children aged 6–23 months. Advanced ML techniques, such as SHAP value logit coefficients, were used to overcome limitations of traditional ML approaches. The developed ML model, particularly the random forest, catboost, and LGB algorithm, is valuable for informing policies and interventions to prevent and minimize the burden of MN deficiency among children aged 6–23 months.
These identified risk factors can guide policymakers and healthcare providers in designing targeted interventions for different subgroups, improving the health and nutritional status of children and mitigating the impact of MN deficiency in resource-limited areas. However, further research is necessary to translate these findings into practical applications.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: www.dhsprogram.com.
The studies involving humans were approved by central statistical agency-DHS program. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
AZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MAA: Writing – original draft, Writing – review & editing. AH: Writing – original draft, Writing – review & editing. BA: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing. WW: Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing. HN: Formal analysis, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank the measure demographic and health survey program for providing the dataset.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1397399/full#supplementary-material
ADASYN, Adaptively Generating Minority Data; AUC, Area under the Receiver Operating Characteristic Curve; EDHS, Ethiopian Demographic and Health Survey; SVM, Support Vector Machine; SMOTE, Synthetic Minority Over-Sampling Technique; SMOTE ENN: Synthetic Minority Over-Sampling Technique with Edited Nearest Neighbor; LGB, light gradient boosting; RF, random forest; XGB, eXtreme Gradient Boosting; KNN, K Nearest Neighbors; ROC, Receiver Operating Characteristic Curve; WHO, World Health Organization; MN, micronutrient; ML, machine learning.
1. Tam, E, Keats, EC, Rind, F, Das, JK, and Bhutta, ZA. Micronutrient supplementation and fortification interventions on health and development outcomes among children under-five in low-and middle-income countries: a systematic review and meta-analysis. Nutrients. (2020) 12:289. doi: 10.3390/nu12020289
2. Azadbakht, L, and Esmaillzadeh, A. Macro and micro-nutrients intake, food groups consumption and dietary habits among female students in Isfahan University of Medical Sciences. Iran Red Crescent Med J. (2012) 14:204–9.
3. Ames, BN . Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci. (2006) 103:17589–94. doi: 10.1073/pnas.0608757103
4. Velasco, I, Bath, SC, and Rayman, MP. Iodine as essential nutrient during the first 1000 days of life. Nutrients. (2018) 10:290. doi: 10.3390/nu10030290
5. Von Grebmer, K, Saltzman, A, Birol, E, Wiesman, D, Prasai, N, Yin, S, et al. Synopsis: 2014 global hunger index: the challenge of hidden hunger. Intl Food Policy Res Inst. (2014).
6. Cannell, JJ, Vieth, R, Willett, W, Zasloff, M, Hathcock, JN, White, JH, et al. Cod liver oil, vitamin a toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Ann Otol Rhinol Laryngol. (2008) 117:864–70. doi: 10.1177/000348940811701112
7. Black, RE, Victora, CG, Walker, SP, Bhutta, ZA, Christian, P, De Onis, M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. (2013) 382:427–51. doi: 10.1016/S0140-6736(13)60937-X
8. Mulat, E, Alem, G, Woyraw, W, and Temesgen, H. Uptake of minimum acceptable diet among children aged 6–23 months in orthodox religion followers during fasting season in rural area, DEMBECHA, north West Ethiopia. BMC nutrition. (2019) 5:1–10. doi: 10.1186/s40795-019-0274-y
9. Sazawal, S, Black, RE, Ramsan, M, Chwaya, HM, Dutta, A, Dhingra, U, et al. Effect of zinc supplementation on mortality in children aged 1–48 months: a community-based randomised placebo-controlled trial. Lancet. (2007) 369:927–34. doi: 10.1016/S0140-6736(07)60452-8
10. Jack, SJ, Ou, K, Chea, M, Chhin, L, Devenish, R, Dunbar, M, et al. Effect of micronutrient sprinkles on reducing anemia: a cluster-randomized effectiveness trial. Arch Pediatr Adolesc Med. (2012) 166:842–50. doi: 10.1001/archpediatrics.2012.1003
11. Freitas, MB d, Castro, IRR, Schincaglia, RM, Carneiro, LBV, Alves-Santos, NH, Normando, P, et al. Characterization of micronutrient supplements use by Brazilian children 6-59 months of age: Brazilian National Survey on child nutrition (ENANI-2019). Cad Saude Publica. (2023) 39:e00085222. doi: 10.1590/0102-311xen085222
12. CSA-Ethiopia I. Ethiopia demographic and health survey 2016: key indicators report. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF (2016).
13. Doggui, R, Al-Jawaldeh, H, El Ati, J, Barham, R, Nasreddine, L, Alqaoud, N, et al. Meta-analysis and systematic review of micro-and macro-nutrient intakes and trajectories of macro-nutrient supply in the eastern mediterranean region. Nutrients. (2021) 13:1515. doi: 10.3390/nu13051515
14. Gebremedhin, T, Aschalew, AY, Tsehay, CT, Dellie, E, and Atnafu, A. Micronutrient intake status and associated factors among children aged 6–23 months in the emerging regions of Ethiopia: a multilevel analysis of the 2016 Ethiopia demographic and health survey. PLoS One. (2021) 16:e0258954. doi: 10.1371/journal.pone.0258954
15. Engidaw, MT, Gebremariam, AD, Tiruneh, SA, Tesfa, D, Fentaw, Y, Kefale, B, et al. Micronutrient intake status and associated factors in children aged 6–23 months in sub-Saharan Africa. Sci Rep. (2023) 13:10179. doi: 10.1038/s41598-023-36497-3
16. Ayele, S, Zegeye, EA, and Nisbett, N. Multi-sectoral nutrition policy and programme design, coordination and implementation in Ethiopia. (2020). Institute of Development Studies.
17. The Federal Democratric Republic of Ethiopia. Government of Ethiopia National Nutrition Program, 2016–2020. (2016). Ethiopia FMOH.
18. Serdula, M, Lundeen, E, Nichols, E, Imanalieva, C, Minbaev, M, Mamyrbaeva, T, et al. Effects of a large-scale micronutrient powder and young child feeding education program on the micronutrient status of children 6–24 months of age in the Kyrgyz Republic. Eur J Clin Nutr. (2013) 67:703–7. doi: 10.1038/ejcn.2013.67
19. Berde, AS, Bester, P, and Kruger, IM. Coverage and factors associated with vitamin a supplementation among children aged 6–59 months in twenty-three sub-Saharan African countries. Public Health Nutr. (2019) 22:1770–6. doi: 10.1017/S1368980018004056
20. Mfateneza, E, Rutayisire, PC, Biracyaza, E, Musafiri, S, and Mpabuka, WG. Application of machine learning methods for predicting infant mortality in Rwanda: analysis of Rwanda demographic health survey 2014–15 dataset. BMC Pregnancy Childbirth. (2022) 22:388. doi: 10.1186/s12884-022-04699-8
21. Kebede Kassaw, A, Yimer, A, Abey, W, Molla, TL, and Zemariam, AB. The application of machine learning approaches to determine the predictors of anemia among under five children in Ethiopia. Sci Rep. (2023) 13:22919. doi: 10.1038/s41598-023-50128-x
22. CSA-Ethiopia International. Ethiopia Demographic and Health Survey 2016: Key indicators report. Rockville: CSA and ICF (2016).
23. Croft, T, Marshall, AM, Allen, CK, Arnold, F, Assaf, S, Balian, S, et al. Guide to DHS statistics: DHS-7 (version 2). Rockville, MD: ICF (2020).
24. Dary, O, and Hurrell, R. Guidelines on food fortification with micronutrients. World Health Organization, Food and Agricultural Organization of the United Nations: Geneva, Switzerland, (2006);2006:1–376.
26. Tabacchi, G, Wijnhoven, TM, Branca, F, Román-Vinas, B, Ribas-Barba, L, Ngo, J, et al. How is the adequacy of micronutrient intake assessed across Europe? A systematic literature review. Br J Nutr. (2009) 101:S29–36. doi: 10.1017/S0007114509990560
27. Kadhim, AI . An evaluation of preprocessing techniques for text classification. Int J Comp Sci Infor Security (IJCSIS). (2018) 16:22–32.
28. Xu, Y, and Goodacre, R. On splitting training and validation set: a comparative study of cross-validation, bootstrap and systematic sampling for estimating the generalization performance of supervised learning. J Anal Test. (2018) 2:249–62. doi: 10.1007/s41664-018-0068-2
29. Liu, X, Lei, S, Wei, Q, Wang, Y, Liang, H, and Chen, L. Machine learning-based correlation study between perioperative immunonutritional index and postoperative anastomotic leakage in patients with gastric cancer. Int J Med Sci. (2022) 19:1173–83. doi: 10.7150/ijms.72195
30. Anand, H, and Vinodchandra, S, editors. Applying correlation threshold on Apriori algorithm. 2013 IEEE International Conference ON Emerging Trends in Computing, Communication and Nanotechnology (ICECCN); (2013): IEEE.
31. Zheng, A, and Casari, A. Feature engineering for machine learning: Principles and techniques for data scientists. MDPI, Basel, Switzerland: O'Reilly Media, Inc. (2018).
32. Al-Shehari, T, and Alsowail, RA. An insider data leakage detection using one-hot encoding, synthetic minority oversampling and machine learning techniques. Entropy. (2021) 23:1258. doi: 10.3390/e23101258
33. Rawat, S, Rawat, A, Kumar, D, and Sabitha, AS. Application of machine learning and data visualization techniques for decision support in the insurance sector. Int J Info Manag Data Insights. (2021) 1:100012. doi: 10.1016/j.jjimei.2021.100012
34. Pudjihartono, N, Fadason, T, Kempa-Liehr, AW, and O'Sullivan, JM. A review of feature selection methods for machine learning-based disease risk prediction. Front Bioinfo. (2022) 2:927312. doi: 10.3389/fbinf.2022.927312
35. Arafat, MY, Hoque, S, Xu, S, and Farid, DM. Machine learning for mining imbalanced data. (2019).
36. Carrington, AM, Manuel, DG, Fieguth, PW, Ramsay, T, Osmani, V, Wernly, B, et al. Deep ROC analysis and AUC as balanced average accuracy, for improved classifier selection, audit and explanation. IEEE Trans Pattern Anal Mach Intell. (2022) 45:329–41. doi: 10.1109/TPAMI.2022.3145392
37. Bekkar, M, Djemaa, HK, and Alitouche, TA. Evaluation measures for models assessment over imbalanced data sets. J Inf Eng Appl. (2013) 3:15–33. doi: 10.5121/ijdkp.2013.3402
38. Japkowicz, N . Assessment metrics for imbalanced learning. Imbalanced learning: Foundations, algorithms, and applications. (2013) 18:187–206. doi: 10.1002/9781118646106.ch8
39. Luo, W, Phung, D, Tran, T, Gupta, S, Rana, S, Karmakar, C, et al. Guidelines for developing and reporting machine learning predictive models in biomedical research: a multidisciplinary view. J Med Internet Res. (2016) 18:e323. doi: 10.2196/jmir.5870
40. Bowles, M . Machine learning in Python: Essential techniques for predictive analysis. Elseiver, Boston: John Wiley & Sons (2015).
41. Jiang, T, Gradus, JL, and Rosellini, AJ. Supervised machine learning: a brief primer. Behav Ther. (2020) 51:675–87. doi: 10.1016/j.beth.2020.05.002
42. Gebeye, LG, Dessie, EY, and Yimam, JA. Predictors of micronutrient deficiency among children aged 6–23 months in Ethiopia: a machine learning approach. Frontiers. Nutrition. (2023) 10:10. doi: 10.3389/fnut.2023.1277048
43. Khare, S, Kavyashree, S, Gupta, D, and Jyotishi, A. Investigation of nutritional status of children based on machine learning techniques using Indian demographic and health survey data. Procedia Comp Sci. (2017) 115:338–49. doi: 10.1016/j.procs.2017.09.087
44. Vimbi, V, Shaffi, N, and Mahmud, M. Interpreting artificial intelligence models: a systematic review on the application of LIME and SHAP in Alzheimer’s disease detection. Brain Inform. (2024) 11:10.
45. Chen, R-C, Dewi, C, Huang, S-W, and Caraka, RE. Selecting critical features for data classification based on machine learning methods. J Big Data. (2020) 7:52. doi: 10.1186/s40537-020-00327-4
46. Kebede, SD, Sebastian, Y, Yeneneh, A, Chanie, AF, Melaku, MS, and Walle, AD. Prediction of contraceptive discontinuation among reproductive-age women in Ethiopia using Ethiopian demographic and health survey 2016 dataset: a machine learning approach. BMC Med Inform Decis Mak. (2023) 23:1–17. doi: 10.1186/s12911-023-02102-w
47. Lucha, TA, Engida, TA, and Mengistu, AK. Assessing the potential determinants of national vitamin a supplementation among children aged 6–35 months in Ethiopia: further analysis of the 2019 Ethiopian Mini demographic and health survey. BMC Pediatr. (2022) 22:1–7. doi: 10.1186/s12887-022-03499-5
48. Aghaji, AE, Duke, R, and Aghaji, UC. Inequitable coverage of vitamin a supplementation in Nigeria and implications for childhood blindness. BMC Public Health. (2019) 19:1–8. doi: 10.1186/s12889-019-6413-1
49. Abedin, MM, Maniruzzaman, M, Ali, M, Ahmed, N, and Ahammed, B. Assessing and determining potential factors associated with vitamin a supplementation in Bangladesh. Biostat Biometrics. (2019) 9:10–9080. doi: 10.1186/s41043-015-0008-y
50. Bakhtsiyarava, M, and Grace, K. Agricultural production diversity and child nutrition in Ethiopia. Food Secur. (2021) 13:1407–22. doi: 10.1007/s12571-021-01173-9
51. Gewa, CA, and Leslie, TF. Distribution and determinants of young child feeding practices in the east African region: demographic health survey data analysis from 2008-2011. J Health Popul Nutr. (2015) 34:1–14.
52. Baek, Y, and Chitekwe, S. Sociodemographic factors associated with inadequate food group consumption and dietary diversity among infants and young children in Nepal. PLoS One. (2019) 14:e0213610. doi: 10.1371/journal.pone.0213610
Keywords: micronutrient supplementation, children, machine learning algorithm, prediction, Ethiopia
Citation: Zemariam AB, Adisu MA, Habesse AA, Abate BB, Bizuayehu MA, Wondie WT, Alamaw AW and Ngusie HS (2024) Employing advanced supervised machine learning approaches for predicting micronutrient intake status among children aged 6–23 months in Ethiopia. Front. Nutr. 11:1397399. doi: 10.3389/fnut.2024.1397399
Received: 07 March 2024; Accepted: 22 May 2024;
Published: 11 June 2024.
Edited by:
Hettie Carina Schönfeldt, University of Pretoria, South AfricaReviewed by:
Eskezeia Y. Dessie, Cincinnati Children's Hospital Medical Center, United StatesCopyright © 2024 Zemariam, Adisu, Habesse, Abate, Bizuayehu, Wondie, Alamaw and Ngusie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alemu Birara Zemariam, YWxleGI3Mjk4QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.