
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 20 May 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1386361
Background: Patients with nasopharyngeal carcinoma are notably susceptible to high nutritional risks. If not addressed, this susceptibility can lead to malnutrition, resulting in numerous adverse clinical outcomes. Despite the significance of this issue, there is limited comprehensive research on the topic.
Objective: The objective of our study was to identify nutritional risk factors in patients with nasopharyngeal carcinoma.
Methods: For this cross-sectional study, we recruited a total of 377 patients with nasopharyngeal carcinoma. The Nutritional Risk Screening 2002 tool was used to assess their nutritional risk. These patients were divided into a well-nourished group (n = 222) and a nutritional risk group (n = 155). Potential risk factors were screened out using univariate analysis (p < 0.1). These factors were subsequently analyzed with multivariate logistic regression analysis (p < 0.05) to identify the nutritional risk factors for these patients.
Results: Our findings indicated that increasing age (OR = 1.085, 95%CI: 1.053–1.117, p < 0.001), high number of radiation treatments (OR = 1.103, 95%CI: 1.074–1.132, p < 0.001), low BMI (OR = 0.700, 95%CI: 0.618–0.793, p < 0.001), and low albumin levels (OR = 0.852, 95%CI: 0.789–0.921, p < 0.001) are significant nutritional risk factors in patients with nasopharyngeal carcinoma.
Conclusion: Increasing age, high number of radiation treatments, low BMI, and low albumin levels are significant nutritional risk factors in patients with nasopharyngeal carcinoma.
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma, one of the most common malignancies within the nasopharynx, and a subset of head and neck cancers (1, 2). It originates from the mucosal lining of the nasopharynx and is linked with genetic factors, environmental influences, and Epstein–Barr virus infections (1, 2). The clinical signs and symptoms of NPC are categorized into four groups: (i) Nasopharyngeal tumor symptoms, such as nasal obstruction, epistaxis, and nasal discharge; (ii) Eustachian tube dysfunction symptoms, including otitis media and hearing loss; (iii) Symptoms from tumor extension towards the skull base, which may cause headaches, diplopia, facial pain, and numbness or paresthesia; and (iv) Palpable neck masses (3). Compared to other cancers, NPC is rare. About 129,000 new people were diagnosed with NPC in 2018, representing just 0.7% of all cancers diagnosed in 2018 (2). However, its geographical spread around the world is very uneven; over 70% of new patients are from East and Southeast Asia (4). NPC patients are at high nutritional risk due to the disease effects and anti-tumor treatment. A previous study showed that 85% (2,750/3232) of NPC patients were at high nutritional risk (5). Malnutrition may occur in NPC patients at nutritional risk if they do not get nutritional support on time. Malnutrition could weaken patients’ immune systems, prolong patients’ hospital stays, bring about adverse treatment effects, lead to treatment interruptions, and have negative consequences for the prognosis and quality of life (6, 7).
Early identification and intervention of nutritional risk in NPC patients can help reduce the incidence of malnutrition. Nutrition screening is defined as identifying individuals who are malnourished or at risk of malnutrition to determine whether a detailed nutrition assessment is indicated (8). Nutrition Risk Screening 2002 (NRS2002) is commonly used as an initial screening tool to identify potential nutritional risks (9, 10).
Despite the potential impact of nutritional risk on NPC patients’ prognosis and quality of life, there is limited research on identifying nutritional risk factors in this population. While several studies have investigated factors for malnutrition among NPC patients, the evaluation of these factors has not been comprehensive. For example, some studies have only assessed demographic data and failed to analyze critical blood indicators. Furthermore, although certain studies have analyzed blood biomarkers, their analysis was limited to previously validated indicators and lacked comprehensiveness. Additionally, the sample size in these studies was small, limiting their generalizability. To address the gaps in existing literature, this study utilized NRS 2002 to assess the nutritional risks in NPC patients. It performed a comprehensive analysis of demographic data, lifestyle habits, tumor stages, treatments, and blood indicators to identify nutritional risk factors in this population.
The study flow diagram is presented in Figure 1.
In this prospective cross-sectional study conducted from January 2022 to May 2023, we focused on NPC patients admitted to a general tertiary hospital. Utilizing a consecutive sampling approach, we selected all consecutively admitted NPC patients who met the inclusion criteria until our predetermined sample size was reached. The sample size was determined based on having at least 10 events for each predictor parameter. To ensure there was no duplication in data collection, meticulous checks were carried out using unique identifiers for each patient. All patients were informed, and informed consent was obtained. Based on the results of NRS 2002, we classified all NPC patients into two groups: the well-nourished group and the nutritional risk group. Demographic data, lifestyle habits, tumor stages, treatments, and blood indicators of NPC patients were analyzed to identify nutritional risk factors in this population.
Inclusion criteria: (1) all newly diagnosed cases were confirmed by pathology; (2) no history of malignant tumors in other organs; and (3) age ≥ 18 years.
Exclusion criteria: NPC patients who had severe metabolic or nutritional diseases, as well as life-threatening illnesses, psychiatric disorders, or intellectual disabilities.
NRS 2002 is widely used as a nutritional screening tool and was developed based on an analysis of 128 randomized clinical trials (10). The European Society for Parenteral and Enteral Nutrition guidelines recommend NRS 2002 for use in hospital settings as an effective and reliable nutritional screening tool (11). In addition, the Chinese Medical Association has recommended NRS 2002 to screen nutritional risk in hospitalized patients based on a report of 15,098 patients (12). Given its established reliability and effectiveness, we utilized NRS 2002 to screen the nutritional risk of NPC patients in this study.
The NRS 2002 (Figure 2) consists of two components: initial screening and final screening. Initial screening includes four judgmental questions about BMI, weight loss, food intake, and severity of illness. If the patients answered “yes” to any of the four initial screening factors, they would proceed to the final screening. Otherwise, they are not currently at nutritional risk and do not need final screening. They need to be reviewed weekly for nutritional status. The final screening includes impaired nutritional status, severity of disease, and age scores, which are combined to screen nutritional risk. A total score ≥ 3 indicates a high nutritional risk (13). NPC patients were divided into a well-nourished group and a nutritional risk group, according to the NRS 2002.
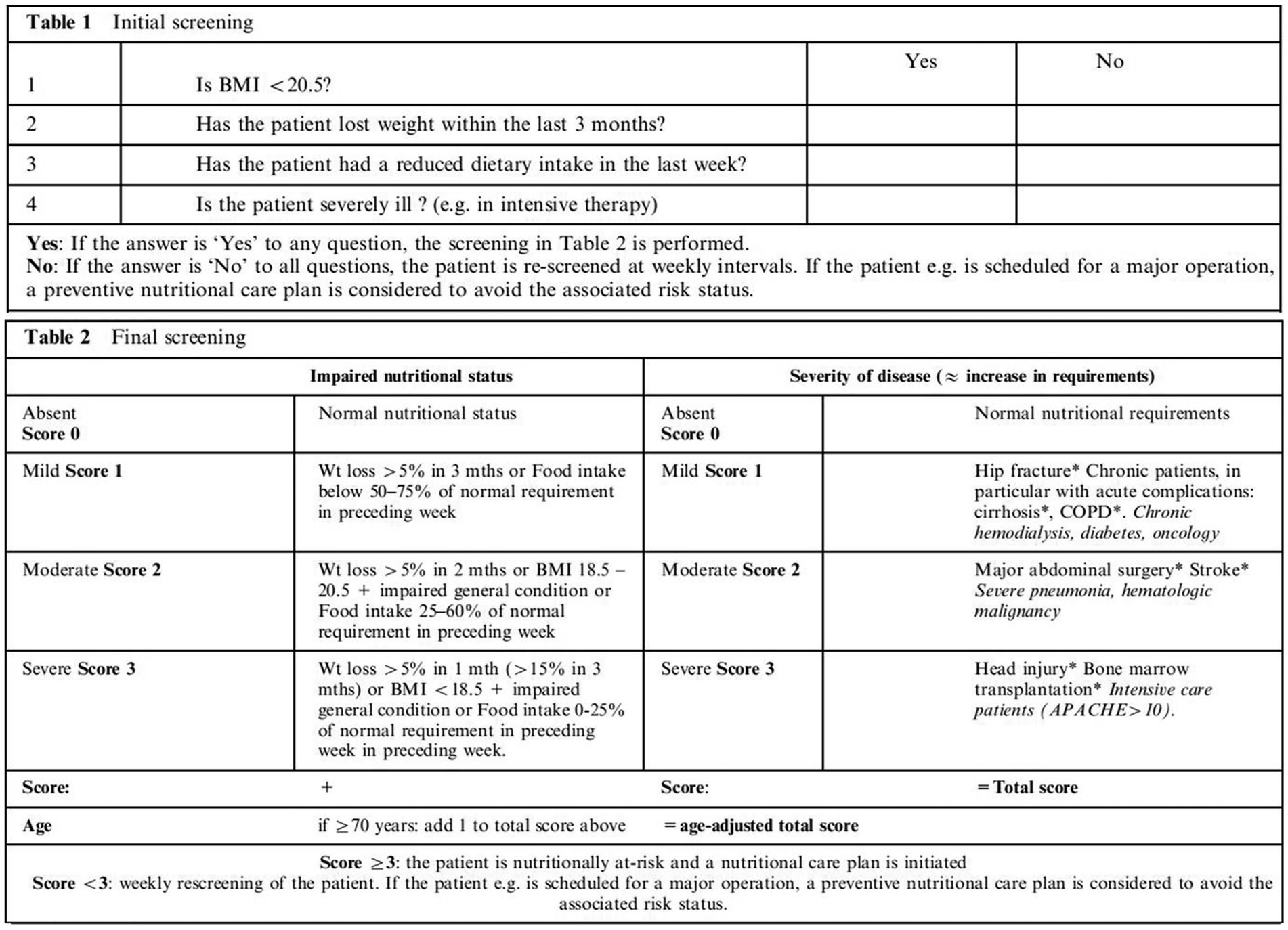
Figure 2. NRS 2002 (11). BMI, body mass index; Wt, weight; COPD, chronic obstructive pulmonary disease; APACHE, acute physiology and chronic health evaluation.
All NPC patients in this hospital undergo routine blood biochemical tests before treatment to aid physicians in formulating appropriate treatment plans. Before undergoing the blood biochemical examination, each patient’s data including gender, age, body mass index (BMI), smoking history, drinking history, and home address, were collected face to face. Tumor stage, number of radiation treatments, number of chemotherapy cycles completed, and blood biochemical indicators were collected from the hospital information system. The NRS 2002 scores were collected by NRS 2002.
The World Health Organization classification criteria were used to classify the tumor stage (14). Smoking was defined as patients with a smoking history of >2 pack-years or current smoking. Drinking was defined as consuming alcohol at least once a week for more than a year, currently drinking, or having quit drinking for less than 3 years.
In this study, patients’ data were collected on admission. Concurrently, the NRS 2002 was employed to assess nutritional risk at admission. Expertly trained nurses, proficient in the NRS 2002, were responsible for all data collection, ensuring accuracy and uniformity in the screening process.
All data were analyzed using SPSS26.0, and no data were missing. Medians with interquartile ranges [P25, P75] and means ± standard deviations (SD) were utilized to present quantitative data. First, a univariate analysis for each risk factor was performed to determine the potential risk factors. For the univariable analysis, we used the t-test, chi-squared, and Wilcoxon rank-sum tests for continuous, categorical, and graded or skewed distribution variables, respectively. Second, variables with p < 0.1 were carried forward to the logistic regression model, where we obtained ORs and 95% CIs. The significant variables with p values less than 0.05 were the nutritional risk factors in NPC patients. The investigators were trained uniformly. All data entry was double-checked.
The study was approved by the Ethical Committee of the First Affiliated Hospital of Guangxi Medical University (approval number: NO. 2022-KT-Gui Wei-005).
The study included 377 patients, with 222 in the well-nourished group and 155 in the nutritional risk group. Among them, the mean age was 50.05 ± 12.09 years (range 20–84); 294 (78.0%) patients were <60 years old, and 83 (22.0%) were ≥ 60 years old; the mean BMI was 22.30 ± 3.34 kg/m2 (range 15.24–35.80 kg/m2); 44 (11.7%) had a BMI of <18.5 kg/m2, 74 (19.6%) had a BMI of 18.5–20.5 kg/m2, and 259 (68.7%) had a BMI of >20.5 kg/m2; 285 (75.6%) were male, and 92 (24.4%) were female; 194 (51.5%) had no history of smoking, while 183 (48.5%) had a current or previous history of smoking; 228 (60.5%) had no history of drinking, while 149 (39.5%) had a current or previous history of drinking; 257 (68.2%) lived in rural locations, 37 (9.8%) in suburban locations, and 83 (22%) in urban locations; 7 (1.9%) were in stage I, 25 (6.6%) were in stage II, 96 (25.5%) were in stage III, and 249 (66.0%) were in stage IV. The median values of the NRS 2002 scores in 377 patients were (P25: 1, P75: 4), with the overall range extending from 1 to 5. The data of 377 patients are shown in Table 1. The median values of the NRS 2002 scores in the well-nourished group were (P25: 1, P75: 1), with the overall range extending from 1 to 2. The median values of the NRS 2002 scores in the nutritional risk group were (P25: 3, P75: 4), with the overall range extending from 3 to 5.
A univariate analysis for each risk factor was performed to determine the potential risk factors. Variables with p < 0.1 were the potential risk factors. In univariate analysis, there were statistically significant differences in age, BMI, the number of chemotherapy cycles completed, the number of radiation treatments, albumin, gamma-glutamyl transpeptidase, total bile acids, aspartate aminotransferase, alanine aminotransferase, prealbumin, cholinesterase, uric acid, potassium, and retinol binding protein (p < 0.1). The results are shown in Table 2.
Variables with p < 0.1 were included in the multivariable logistic regression model. The significant variables with p values less than 0.05 were the nutritional risk factors in NPC patients. The logistic regression analysis results showed statistically significant differences in age, BMI, the number of radiation treatments, and albumin (p < 0.05). Specifically, for every one-unit increase in age, the odds of nutritional risk increased by a factor of 1.085 (OR = 1.085, 95%CI: 1.053–1.117, p < 0.001). On the other hand, for every one-unit increase in BMI, the odds of nutritional risk decreased by a factor of 0.700 (OR = 0.700, 95%CI: 0.618–0.793, p < 0.001). Furthermore, for every one-unit increase in the number of radiation treatments, the odds of nutritional risk increased by a factor of 1.103 (OR = 1.103, 95%CI: 1.074–1.132, p < 0.001), and for every one-unit decrease in albumin levels, the odds of nutritional risk increased by a factor of 0.852 (OR = 0.852, 95%CI: 0.789–0.921, p < 0.001). These findings suggested that increasing age, high number of radiation treatments, low BMI, and low albumin levels were nutritional risk factors in NPC patients. The results are shown in Table 3.
NPC patients have a high nutritional risk due to various factors, such as the disease itself and treatment-related toxicities. The failure to provide timely nutritional support to NPC patients at nutritional risk can lead to malnutrition, resulting in adverse consequences. Despite the importance of this issue, limited studies have explored the nutritional risk factors in NPC patients. Our study aimed to identify the nutritional risk factors in NPC patients using the NRS 2002 to address this gap. Our findings showed that increasing age, high number of radiation treatments, low BMI, and low albumin levels were significant nutritional risk factors in NPC patients.
Nutritional risk is more common in older adults (15), as aging may be accompanied by the accumulation of diseases and impairments, such as depressive symptoms, cognitive and physical decline, emotional variations (16), and poor oral health (17). Moreover, elderly patients may have problems such as gastrointestinal hypofunction, weakened digestion and absorption capabilities, reduced liver function, and a diminished ability to metabolize nutrients, often combined with underlying diseases (18, 19). All of these factors may directly influence the balance between nutritional needs and intake (16). Even with adequate nutrition and energy intake, altered nutrient needs, compromised nutrient metabolism, and drug-nutrient interactions may affect the nutritional status of older people (20).
Our findings suggested that advanced age (OR = 1.085, 95%CI: 1.053–1.117, p < 0.001) was a nutritional risk factor in NPC patients. In the well-nourished group, 7.2% (16/222) of NPC patients were ≥ 60 years old. However, in the nutritional risk group, 43.2% (67/155) of NPC patients were ≥ 60 years old.
The loss of appetite in patients during the radiotherapy phase of cancer treatment may be caused by the side effects of the treatment. Loss of appetite in patients can lead to serious nutritional problems that adversely affect a patient’s disease prognosis, treatment outcomes, and quality of life (21). Radiotherapy can cause side effects that can lead to reduced food intake, loss of nutrients, changes in energy expenditure, and weight loss (22–24). Weight loss, mucositis, and reduced food intake occur in about 80% of patients receiving radiotherapy to the head and neck or esophagus (25).
Our findings suggested that the greater the number of radiation treatments, the greater the nutritional risk in NPC patients (OR = 1.103, 95%CI: 1.074–1.132, p < 0.001). In our study, among patients who underwent ≥10 cycles of radiotherapy, 14.0% (31/222) were in the well-nourished group, while 63.2% (98/155) were in the nutritional risk group.
BMI = weight (kg)/height2 (m2). BMI is a measure of the nutritional status of adults. A low BMI indicates a high risk of inadequate nutritional intake. BMI is often used to aid in screening for nutritional risk using many nutritional screening or assessment tools. For example, the NRS 2002 incorporates pre-screening with four questions. “Is the BMI of the patient <20.5 kg/m2” is one of the four questions in the pre-screening. Screening is performed if one of the four questions is answered positively (26). BMI is also a standard screening parameter in Mini Nutritional Assessment (MNA). In addition, the MNA score was positively correlated with BMI (27). A phenotypic criteria (non-Asian: low BMI < 20 kg/m2 if <70 years or < 22 kg/m2 if >70 years; Asia: low BMI < 18.5 kg/m2 if <70 years or < 20 kg/m2 if >70 years) was included in the Global Leadership Initiative on Malnutrition (GLIM) (28). Furthermore, BMI < 18.5 kg/m2 was defined as malnutrition based on the consensus statement of the European Society of Clinical Nutrition and Metabolism (29).
Our study showed that the low BMI (OR = 0.700, 95%CI: 0.618–0.793, p < 0.001) was a nutritional risk factor in NPC patients. In our study, the BMI was 23.59 ± 2.99 kg/m2 in the well-nourished group and 20.47 ± 2.94 kg/m2 in the nutritional risk group.
Traditionally, albumin has been used as a nutritional marker to quantify the amount of plasma circulating protein and is thus considered to reflect nutritional status. Albumin <35 g/L indicates hypoalbuminemia and persistent hypoalbuminemia is an important indicator of malnutrition (30). Dietary protein intake (DPI) directly influences albumin concentrations, and inadequate DPI could lead to a decrease in the rate of albumin, which may have little impact on albumin levels in the short term. Although the decrease in the rate of albumin was small, it was clinically significant. Eckart et al. demonstrated that nutritional risk was associated with albumin in adult patients (31). In addition, albumin is a visceral protein that is a sensitive indicator of marginal nutrient deficiency and can reflect changes in protein-caloric nutrition. Albumin has been used as a marker of nutritional status in orthopedic patients (32). Prenner et al. demonstrated that albumin was an objective parameter that could provide time-effective and cost-controlled evidence regarding malnutrition in patients after heart transplantation.
Our study showed that the lower the albumin levels, the higher the nutritional risk in NPC patients (OR = 0.852, 95%CI: 0.789–0.921, p < 0.001). The reasons might be as follows: Firstly, treatment for NPC can induce acute inflammation, such as acute oral mucositis (33), dysphagia (34), and gastrointestinal reactions (35), which can affect nutritional intake. Secondly, the acute inflammations (33, 34, 36)caused by treatment for NPC may reduce albumin concentrations. In our study, patients in the nutritional risk group received more radiotherapy than those in the well-nourished group. Finally, the albumin level is reduced with the progression of NPC (37).
This study provides several notable advantages compared to previous research studies. Firstly, our research comprehensively analyzed various factors, such as demographic data, lifestyle habits, tumor stage, treatments, and blood indicators of NPC patients, to identify nutritional risk factors in this population. Secondly, our study had a large sample size, enhancing the findings’ validity and reliability. Thirdly, we used NRS2002 as the primary nutritional screening tool, a widely accepted method for identifying nutritional risk in hospitalized patients.
Despite its strengths, our study has limitations. The cross-sectional design prevents us from inferring causality between variables and nutritional risk in NPC patients. To draw causal conclusions, randomized controlled trials (RCTs) or longitudinal studies are required. Additionally, we did not examine the link between patient psychology and nutritional risk.
Our study identifies nutritional risk factors in NPC patients, providing theoretical and practical guidance for clinical nutritional risk screening and support. Equipped with a deep understanding of factors linked to nutritional risks, nurses can educate patients more effectively, helping them grasp their risks and suggesting strategies for managing and mitigating these vulnerabilities. By collaborating closely with dieticians, nurses can develop tailored dietary and nutritional regimens that meet the daily needs of NPC patients and enhance their overall quality of life and treatment outcomes. Prompt identification of nutritional factors allows nursing professionals to intervene early, preventing further deterioration in patients’ nutritional health. This proactive approach not only prevents related complications but also can reduce the length of hospital stays, alleviating the burden on the healthcare system. In summation, our study offers valuable guidance for nursing and medical staff, ensuring enhanced care for NPC patients, improved nutritional health, and an overall better quality of life.
Increasing age, high number of radiation treatments, low BMI, and low albumin levels were nutritional risk factors in NPC patients. These results may be useful in guiding clinical nutritional risk screening and interventions for this population. Further research is needed to confirm these findings and investigate additional nutritional risk factors in NPC patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Ethical Committee of the First Affiliated Hospital of Guangxi Medical University (approval number: No. 2022-KT-Gui Wei-005). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
PW: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. XH: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. LX: Data curation, Formal analysis, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. JinL: Formal analysis, Investigation, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. JieL: Data curation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. JY: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. TL: Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study is financially supported by the Self-raised Scientific Research Fund of the Health and Family Planning Commission of the Guangxi Zhuang Autonomous Region (No. Z-A20230577).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Alsafadi, N, Alqarni, MS, Attar, M, Mgarry, R, and Bokhari, A. Nasopharyngeal Cancer: prevalence, outcome, and impact on health-related quality of life at Princess Norah oncology center, Jeddah, Saudi Arabia. Cureus. (2020) 12:e8199. doi: 10.7759/cureus.8199
2. Chen, YP, Chan, ATC, Le, QT, Blanchard, P, Sun, Y, and Ma, J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/s0140-6736(19)30956-0
3. Adham, M, Lazim, NM, and Carlos, R. Clinical presentation of nasopharyngeal carcinoma In An evidence-based approach to the management of nasopharyngeal cancer. B Abdullah, A Balasubramanian, and NM Lazim. Malaysia: Elsevier (2020). 93–109.
4. Vai, A, Molinelli, S, Rossi, E, Iacovelli, NA, Magro, G, Cavallo, A, et al. Proton radiation therapy for nasopharyngeal Cancer patients: Dosimetric and NTCP evaluation supporting clinical decision. Cancers (Basel). (2022) 14:1109. doi: 10.3390/cancers14051109
5. Peng, H, Chen, BB, Tang, LL, Chen, L, Li, WF, Zhang, Y, et al. Prognostic value of nutritional risk screening 2002 scale in nasopharyngeal carcinoma: a large-scale cohort study. Cancer Sci. (2018) 109:1909–19. doi: 10.1111/cas.13603
6. Ji, J, Jiang, DD, Xu, Z, Yang, YQ, Qian, KY, and Zhang, MX. Continuous quality improvement of nutrition management during radiotherapy in patients with nasopharyngeal carcinoma. Nurs Open. (2021) 8:3261–70. doi: 10.1002/nop2.1039
7. Tribolet, P, Kaegi-Braun, N, Gressies, C, Baumgartner, A, Wagner, KH, Stanga, Z, et al. Handgrip strength values depend on tumor entity and predict 180-day mortality in malnourished Cancer patients. Nutrients. (2022) 14:2173. doi: 10.3390/nu14102173
8. Charney, P. Nutrition screening vs nutrition assessment: how do they differ? Nutr Clin Pract. (2008) 23:366–72. doi: 10.1177/0884533608321131
9. Xu, JY, Tian, XD, Song, JH, Chen, J, Yang, YM, and Wei, JM. Preoperative nutrition support may reduce the prevalence of postoperative pancreatic fistula after open Pancreaticoduodenectomy in patients with high nutritional risk determined by NRS2002. Biomed Res Int. (2021) 2021:6691966–7. doi: 10.1155/2021/6691966
10. Kondrup, J, Rasmussen, HH, Hamberg, O, and Stanga, Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. (2003) 22:321–36. doi: 10.1016/S0261-5614(02)00214-5
11. Kondrup, J, Allison, SP, Elia, M, Vellas, B, and Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. (2003) 22:415–21. doi: 10.1016/s0261-5614(03)00098-0
12. Dou, L, Wang, X, Cao, Y, Hu, A, and Li, L. Relationship between postoperative recovery and nutrition risk screened by NRS 2002 and nutrition support status in patients with gastrointestinal Cancer. Nutr Cancer. (2020) 72:33–40. doi: 10.1080/01635581.2019.1612927
13. Ye, XJ, Ji, YB, Ma, BW, Huang, DD, Chen, WZ, Pan, ZY, et al. Comparison of three common nutritional screening tools with the new European Society for Clinical Nutrition and Metabolism (ESPEN) criteria for malnutrition among patients with geriatric gastrointestinal cancer: a prospective study in China. BMJ Open. (2018) 8:e019750. doi: 10.1136/bmjopen-2017-019750
14. Thompson, L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. (2006) 85:74. doi: 10.1177/014556130608500201
15. de Morais, C, Oliveira, B, Afonso, C, Lumbers, M, Raats, M, and de Almeida, MD. Nutritional risk of European elderly. Eur J Clin Nutr. (2013) 67:1215–9. doi: 10.1038/ejcn.2013.175
16. van Bokhorst-de van der Schueren, MAE, Lonterman-Monasch, S, de Vries, OJ, Danner, SA, Kramer, MHH, and Muller, M. Prevalence and determinants for malnutrition in geriatric outpatients. Clin Nutr. (2013) 32:1007–11. doi: 10.1016/j.clnu.2013.05.007
17. Mann, T, Heuberger, R, and Wong, H. The association between chewing and swallowing difficulties and nutritional status in older adults. Aust Dent J. (2013) 58:200–6. doi: 10.1111/adj.12064
18. Amarya, S, Singh, K, and Sabharwal, M. Changes during aging and their association with malnutrition. J Clin Gerontol Geriatr. (2015) 6:78–84. doi: 10.1016/j.jcgg.2015.05.003
19. Hickson, M. Malnutrition and ageing. Postgrad Med J. (2006) 82:2–8. doi: 10.1136/pgmj.2005.037564
20. Bernstein, M. Nutritional needs of the older adult. Phys Med Rehabil Clin N Am. (2017) 28:747–66. doi: 10.1016/j.pmr.2017.06.008
21. Poirier, VJ, Kaser-Hotz, B, Vail, DM, and Straw, RC. Efficacy and toxicity of an accelerated hypofractionated radiation therapy protocol in cats with oral squamous cell carcinoma. Vet Radiol Ultrasound. (2013) 54:81–8. doi: 10.1111/j.1740-8261.2012.01970.x
22. de Oliveira, FS, Howell, D, Lopes Carvalho, A, de Oliveira, FR, and Eluf, NJ. Adherence to intensive nutrition care in head and neck cancer patients undergoing radiotherapy. Eur Arch Otorrinolaringol. (2021) 278:3507–14. doi: 10.1007/s00405-020-06550-2
23. Jin, S, Lu, Q, Sun, Y, Xiao, S, Zheng, B, Pang, D, et al. Nutrition impact symptoms and weight loss in head and neck cancer during radiotherapy: a longitudinal study. BMJ Support Palliat Care. (2021) 11:17–24. doi: 10.1136/bmjspcare-2019-002077
24. Suzuki, H, Asakawa, A, Amitani, H, Nakamura, N, and Inui, A. Cancer cachexia--pathophysiology and management. J Gastroenterol. (2013) 48:574–94. doi: 10.1007/s00535-013-0787-0
25. Muscaritoli, M, Arends, J, Bachmann, P, Baracos, V, Barthelemy, N, Bertz, H, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr. (2021) 40:2898–913. doi: 10.1016/j.clnu.2021.02.005
26. Reber, E, Gomes, F, Vasiloglou, MF, Schuetz, P, and Stanga, Z. Nutritional risk screening and assessment. J Clin Med. (2019) 8:1065. doi: 10.3390/jcm8071065
27. Sukkriang, N, and Somrak, K. Correlation between Mini nutritional assessment and anthropometric measurements among community-dwelling elderly individuals in rural southern Thailand. J Multidiscip Healthc. (2021) 14:1509–20. doi: 10.2147/jmdh.S315652
28. Cederholm, T, Jensen, GL, Correia, M, Gonzalez, MC, Fukushima, R, Higashiguchi, T, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. (2019) 10:207–17. doi: 10.1002/jcsm.12383
29. Cederholm, T, Bosaeus, I, Barazzoni, R, Bauer, J, Van Gossum, A, Klek, S, et al. Diagnostic criteria for malnutrition – an ESPEN consensus statement. Clin Nutr. (2015) 34:335–40. doi: 10.1016/j.clnu.2015.03.001
30. Tunç, S, Cetinkaya, A, and Duman, O. Spectroscopic investigations of the interactions of tramadol hydrochloride and 5-azacytidine drugs with human serum albumin and human hemoglobin proteins. J Photochem Photobiol B. (2013) 120:59–65. doi: 10.1016/j.jphotobiol.2013.01.011
31. Eckart, A, Struja, T, Kutz, A, Baumgartner, A, Baumgartner, T, Zurfluh, S, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. (2020) 133:713–22.e7. doi: 10.1016/j.amjmed.2019.10.031
32. Cross, MB, Yi, PH, Thomas, CF, Garcia, J, and Della Valle, CJ. Evaluation of malnutrition in orthopaedic surgery. J Am Acad Orthop Surg. (2014) 22:193–9. doi: 10.5435/jaaos-22-03-193
33. Li, PJ, Li, KX, Jin, T, Lin, HM, Fang, JB, Yang, SY, et al. Predictive model and precaution for Oral mucositis during chemo-radiotherapy in nasopharyngeal carcinoma patients. Front Oncol. (2020) 10:596822. doi: 10.3389/fonc.2020.596822
34. Yang, G, Feng, D, Li, F, Luo, B, Zhu, J, Yang, Q, et al. A randomized, controlled phase II trial of maxillofacial and oral massage in attenuating severe radiotherapy-induced oral mucositis and lipid metabolite changes in nasopharyngeal carcinoma. Radiother Oncol. (2021) 163:76–82. doi: 10.1016/j.radonc.2021.07.024
35. Lu, WJ, Li, G, and Gao, L. Colonic perforation in a nasopharyngeal carcinoma patient treated with fluorouracil: a case report. World J Clin Cases. (2020) 8:1693–7. doi: 10.12998/wjcc.v8.i9.1693
36. Fang, KC, Lee, CH, Chuang, HC, Huang, TL, Chien, CY, Tsai, WL, et al. Acute radiation dermatitis among patients with nasopharyngeal carcinoma treated with proton beam therapy: prognostic factors and treatment outcomes. Int Wound J. (2023) 20:499–507. doi: 10.1111/iwj.13897
Keywords: nasopharyngeal carcinoma, nutrition risk screening 2002, nutrition, nutritional risk, factor
Citation: Wang P, Huang X, Xue L, Liao J, Liu J, Yu J and Li T (2024) Nutritional risk factors in patients with nasopharyngeal carcinoma: a cross-sectional study. Front. Nutr. 11:1386361. doi: 10.3389/fnut.2024.1386361
Received: 21 February 2024; Accepted: 07 May 2024;
Published: 20 May 2024.
Edited by:
Eswar Shankar, The Ohio State University, United StatesReviewed by:
Kate Ormiston, The Ohio State University, United StatesCopyright © 2024 Wang, Huang, Xue, Liao, Liu, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueling Huang, amllc2hvdXVwbUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.