- 1Department of Animal and Veterinary Sciences, The University of Vermont, Burlington, VT, United States
- 2Department of Pathology and Laboratory Medicine, The University of Vermont, Burlington, VT, United States
- 3National Dairy Council, Rosemont, IL, United States
- 4Department of Medicine, Division of Endocrinology, Metabolism, and Diabetes, The University of Vermont, Colchester, VT, United States
- 5Department of Nutrition and Food Sciences, The University of Vermont, Burlington, VT, United States
Reducing dairy fat intake is a common dietary guideline to limit energy and saturated fatty acid intake for the promotion of cardiometabolic health. However, research utilizing a holistic, food-based approach to assess the consumption of the fat found in dairy, a broad and diverse food group, may provide new insight into these guidelines. Dairy fat is comprised of a diverse assembly of fatty acids, triacylglycerols, sterols, and phospholipids, all uniquely packaged in a milk fat globule. The physical structure of this milk fat globule and its membrane is modified through different processing methods, resulting in distinctive dairy-fat matrices across each dairy product. The objectives of this narrative review were to first define and compare the dairy-fat matrix in terms of its unique composition, physical structure, and fat content across common dairy products (cow’s milk, yogurt, cheese, and butter). With this information, we examined observational studies and randomized controlled trials published within the last 10 years (2013–2023) to assess the individual effects of the dairy-fat matrix in milk, yogurt, cheese, and butter on cardiometabolic health and evaluate the implications for nutrition guidance. Searches conducted on Ovid MEDLINE and PubMed® utilizing search terms for cardiometabolic health, both broadly and regarding specific disease outcomes and risk factors, yielded 59 studies that were analyzed and included in this review. Importantly, this review stratifies by both dairy product and fat content. Though the results were heterogeneous, most studies reported no association between intake of these individual regular-fat dairy products and cardiometabolic outcome measures, thus, the current body of evidence suggests that regular-fat dairy product consumption may be incorporated within overall healthy eating patterns. Research suggests that there may be a beneficial effect of regular-fat milk and yogurt intake on outcome measures related to body weight and composition, and an effect of regular-fat cheese intake on outcome measures related to blood lipids, but more research is necessary to define the directionality of this relationship. Lastly, we identify methodological research gaps and propose future research directions to bolster the current evidence base available for ascertaining the role of dairy fat in a healthy diet.
1 Introduction
Dairy products are a prominent dietary constituent globally, as evidenced by their inclusion in food-based dietary guidelines across the world (1). Yet, these guidelines predominantly advise reducing dairy fat intake, via the consumption of primarily low-fat or fat-free dairy products, to align with other common recommendations to reduce energy and specifically the intake of saturated fatty acids (SFAs), given the high SFA content in dairy fat (1–3). However, this reductionist approach—the concept that one single nutrient or nutrient class (e.g., SFAs) leads to a certain health effect (e.g., cardiovascular diseases; CVD)—is being challenged with an emerging body of research demonstrating the influence of the food matrix, i.e., the interplay of a food’s physical structure and nutritional and bioactive composition (4–7). Dairy products exemplify the concept and importance of the food matrix as the milk, yogurt, cheese, and butter available on the market are all derived from raw milk, but have key differences such as physical structure, nutrient content and composition, as well as potentially different physiologic effects (4, 8, 9), raising the question as to whether the current body of science on cardiometabolic health effects of dairy fat is reflected in current nutrition guidance.
This narrative review considered the differences between commonly consumed dairy products (cow’s milk, yogurt, cheese, and butter) in terms of fat content, composition, and physical structure with the goal to examine and distinguish their roles in cardiometabolic health. Our aims were to: (i) define the dairy-fat matrix, (ii) describe the unique fat matrices of milk, yogurt, cheese, and butter, and (iii) examine the effect of the dairy-fat matrix of these dairy products on cardiometabolic health outcomes by stratifying by both dairy product and fat content.
2 Dairy fat physical structure and composition
Dairy fat is the most complex edible fat in nature and comprises three different levels of lipid organization with increasing complexity. The following section outlines this complexity, starting with the fundamental unit of lipids, the fatty acids (FAs), then progressing to the combination of FAs with other moieties (e.g., glycerol) to produce the macromolecules triacyglycerols (TAGs), phospholipids, and sterols, then finally the formation of the milk fat globule (MFG), a vesicle specific to milk and milk-derived dairy products, using these macromolecules. Understanding the complexity of the dairy-fat matrix is central to understanding the differences between dairy products and may be a key mechanistic consideration for their effects on cardiometabolic health.
2.1 Fatty acids
At least 400 different FAs have been identified in dairy fat including even-chain FAs and odd-chain FAs (OCFAs), cis-FAs and trans-FAs, short-chain FAs (SCFAs), medium-chain FAs (MCFAs), and long-chain FAs (LCFAs), straight-chain FAs and branched-chain FAs (BCFAs), as well as SFAs, monounsaturated FAs (MUFAs), and polyunsaturated FAs (PUFAs) (see Supplementary Table S1 for proportions of select abundant FAs). SFAs constitute approximately 68% of total FAs as the predominant FA class in dairy fat, followed by MUFAs at approximately 27% and relatively small amounts of PUFAs at approximately 4% (10); these percentages can vary widely based on a variety of factors including, but not limited to, diet, genetics, or stage of lactation (11). Palmitic acid (16:0), oleic acid (18:1 c9), and linoleic acid (18:2 c9, c12) are the individual FAs that make up the major proportion of the SFA, MUFA, and PUFA classes, respectively. The complexity of the dairy FA composition is the result of the extensive lipid metabolism occurring throughout the dairy cow (12, 13). Dairy FAs originate from both de novo synthesis and preformed FAs (i.e., FAs from feed, endogenous and microbial metabolic pathways, and microbial membranes; Figure 1). Starting with de novo synthesized FAs, ruminal bacteria ferment cellulose and hemicellulose to acetate (2:0), propionate (3:0), and butyrate (4:0), which can be taken up by various tissues for additional metabolic processing (12, 14–16). Acetate and butyrate give rise to even-chain SFAs with four to 14 carbons and half of palmitic acid in milk through de novo synthesis in the mammary gland (13, 14). In adipose tissue, propionate can be used to synthesize OCFAs, which can, in turn, be transported to the mammary gland for incorporation into milk fat, although this pathway may also occur directly in the mammary gland (16). Preformed FAs are derived from components of the cow’s diet; some appear in the milk in their original form, whereas others are transformed by endogenous or microbial metabolism. Even-chain SFAs with 18 or more carbons and half of the palmitic acid in milk are directly derived from the lipids in the diet or mobilized from adipose tissue during certain energy states (11, 13, 14, 17–19). Several MCFAs and LCFAs can be desaturated in the mammary gland or in the adipose tissue or liver prior to transport to the mammary gland (11, 13, 14). Dietary unsaturated FAs, namely oleic acid, linoleic acid, and α-linolenic acid (18:3 c9, c12, c15), are readily biohydrogenated by bacteria in the rumen, producing various trans-18:3, trans-18:2, trans-18:1 isomer intermediates, including vaccenic acid (18:1 t11), and rumenic acid (18:2 c9, t11), as well as stearic acid (18,0) as the major biohydrogenation end product (13, 19, 20). Rumen biohydrogenation has been described in greater depth by Shingfield and Wallace (19). Bacterial and protozoal species in the rumen also convert dietary branched-chain amino acids (valine, leucine, and isoleucine) or endogenously produced keto-acids (isovaleric acid, 2-methylbutyric acid, isocaproic acid, and isobutyric acid) to BCFAs for incorporation into their membranes (21). Rumen bacteria and protozoa may also produce membrane OCFAs through α-oxidation of LCFAs (16, 22). During digestion, a portion of rumen microbial cells passes out of the rumen to the small intestine where membrane FAs are taken up and transported for incorporation into the milk (23–25).
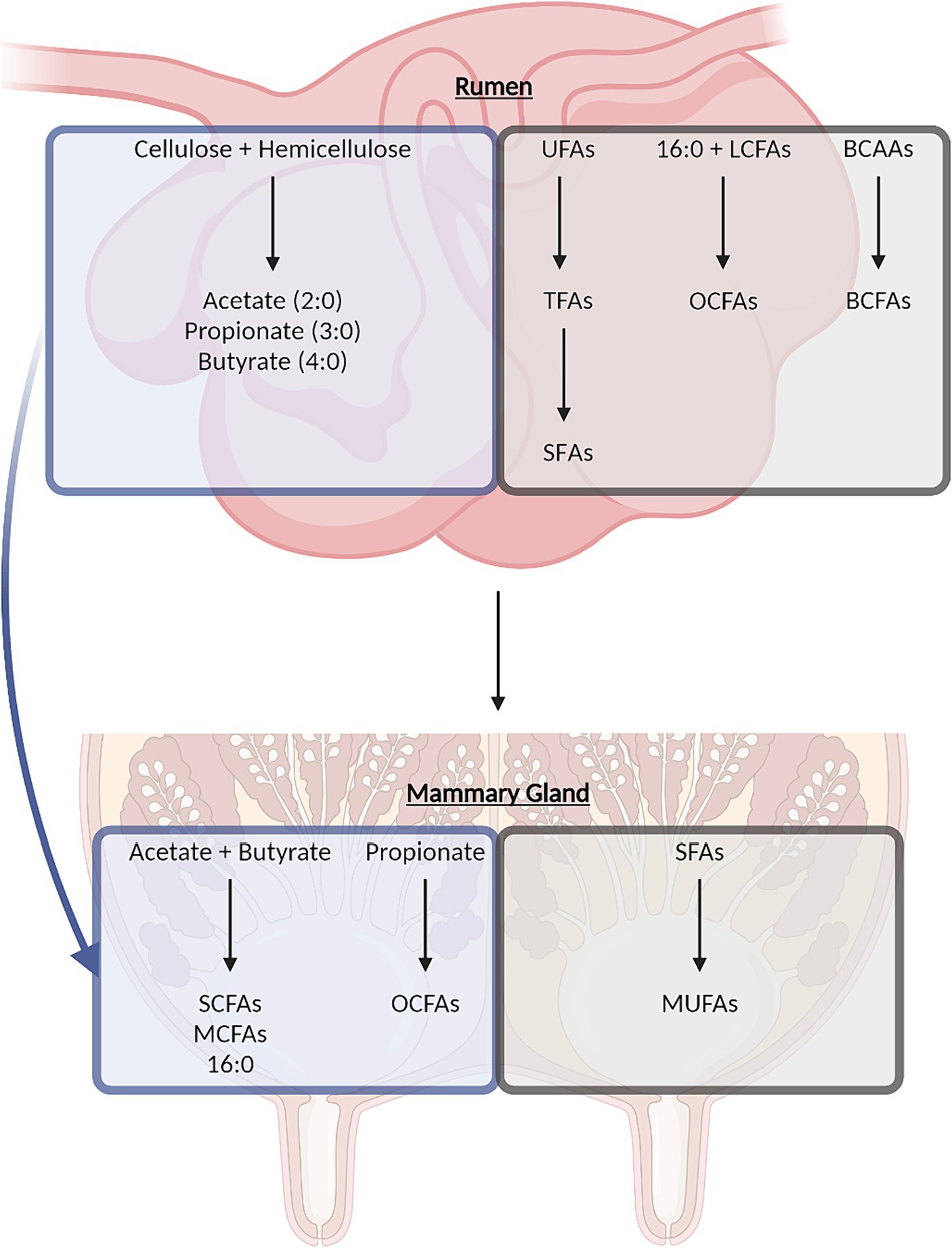
Figure 1. Overview of dairy-derived fatty acid synthesis in the rumen and mammary gland through de novo synthesis (blue boxes) or synthesis from dietary components (gray boxes). BCAAs, branched-chain amino acids; BCFAs, branched-chain fatty acids; LCFAs, long-chain fatty acids; MCFAs, medium-chain fatty acids; MUFA, monounsaturated fatty acids; OCFAs, odd-chain fatty acids; SCFAs, short-chain fatty acids; SFA, saturated fatty acids; TFAs, trans-fatty acids; UFAs, unsaturated fatty acids.
2.2 Triacyglycerols, phospholipids, and sterols
Beyond the complex array of FAs in dairy fat, this complexity multiplies as they are combined to form the secondary structures of TAGs, phospholipids, and sterols. In milk, 97–98% of FAs are found in the form of TAGs, with approximately 1% as phospholipids, and less than 1% each as sterols and free FAs (14). TAGs are categorized by their number of carbons, with 26–56 carbon TAGs as the most prevalent, while phospholipids are categorized by their polar head, with phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin (SM) as the most prevalent (26). Cholesterol is the primary sterol, comprising over 95% of the sterol class (26). Each of these lipid classes can be synthesized de novo in the mammary gland, though sterols are also produced in other tissues and are taken up by the mammary gland from plasma lipoproteins (Figure 2A) (12, 13, 27–32).
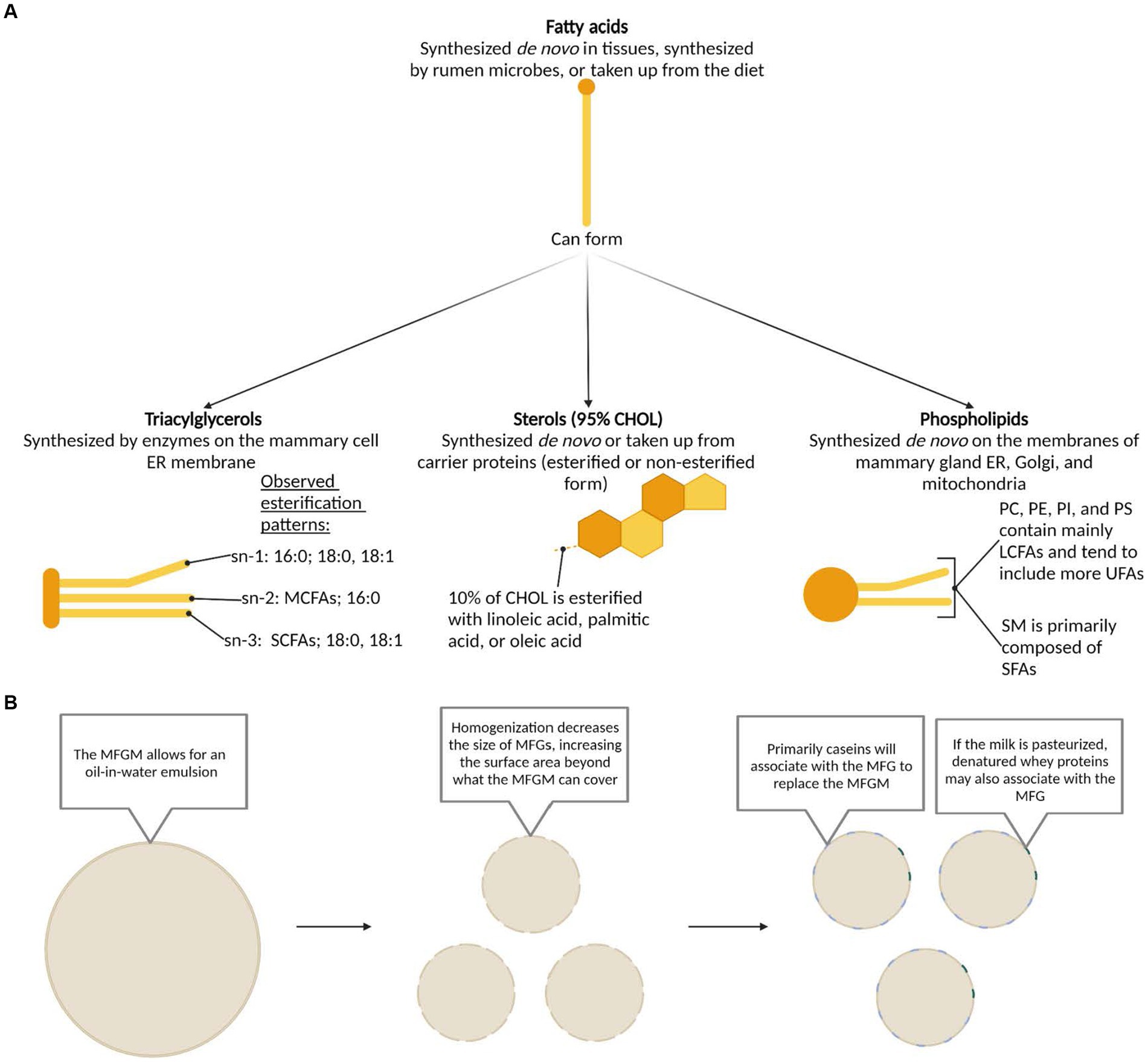
Figure 2. (A) Synthesis pathways, structures, and esterification patterns of triacylglycerols, sterols, and phospholipids formed from dairy fatty acids. Based on previously published work: fatty acids (12, 27); triacyclglycerols (12, 27, 28); sterols (12, 29, 30); phospholipids (13, 31, 32). (B) Structure of the milk fat globule membrane in milk before and after common processing methods. CHOL, cholesterol; ER, endoplasmic reticulum; LCFA, long-chain fatty acid; MCFA, medium-chain fatty acid; MFG, milk fat globule; MFGM, milk fat globule membrane; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; SCFA, short-chain fatty acid; SM, sphingomyelin; UFA, unsaturated fatty acid.
2.3 Native milk fat globule
Dairy fat is physically arranged in the form of a MFG surrounded with a distinct MFG membrane (MFGM), making it unique from other commonly consumed lipid sources. These MFGs range from 0.1 to 20 μm in diameter and the MFGM itself ranges from 8 to 10 nm in width (33, 34). An inner, central, and outer layer constitute the MFGM. The inner layer is comprised of polar lipids, derived from the endoplasmic reticulum of the mammary cell, that surrounds the central core of TAGs (35). The central layer is particularly dense, as it predominantly comprises of proteins from the cytoplasm (36, 37). The outer polar lipid bilayer is a concentrated layer of phospholipids predominantly comprised of glycerophospholipids and sphingolipids, which are derived from the cell membrane of the mammary cell (36). PE, phosphatidylinositol (PI), and phosphatidylserine (PS) tend to be located on the inner surface while PC and SM largely occur on the outer bilayer (38, 39). Transmembrane proteins, cholesterol (CHOL), glycoproteins, and enzymes, along with lipid rafts comprised of concentrated sphingomyelin and CHOL also appear on the outer layer, highlighting the influence of the unique amphipathic properties of these polar lipids (33, 34, 40, 41). The MFGM prevents the aggregation of MFGs, creating an emulsion within the milk (12) and protecting the inner TAG core from enzymatic degradation and oxidation (27). For detailed visualizations of the MFG and MFGM, we recommend the following publications (42–44).
3 Characteristics of fat in processed milk, yogurt, cheese, and butter
With an understanding of the FA composition and lipid classes present in unprocessed (i.e., raw) milk, we investigated the similarities and differences between the dairy products milk, yogurt, cheese, and butter based on their fat content, composition, and physical structure.
3.1 Dairy fat composition and content
The FA composition of milk is well documented, with comparatively less data available for yogurt, cheese, and butter (Supplementary Table S1). Overall, the percentages of FAs are similar among these dairy products while the fat content varies greatly. As listed on FoodData Central, a U.S. Department of Agriculture (i.e., USDA) food composition database of comprehensive food nutrient profiles, regular-fat (whole) milk and yogurt contain relatively low amounts of total fat [3.2 g per 100 g (45, 46)], compared to regular-fat cheese and butter [15.5–34 g per 100 g (47–68) and 82.2 g per 100 g (69), respectively], with butter having the highest total fat content. The large range of total fat content in regular-fat cheeses is due to the vast variety of cheeses available, each with key differences resulting from the unique milk base (i.e., pasteurized and/or homogenized milk), heating, acidification, salting, pressing, and/or ripening methods used (70). Among the cheeses surveyed on FoodData Central, paneer [15.5 g fat per 100 g (60)], mozzarella [20.4 g fat per 100 g (68)], and queso fresco [23.8 g fat per 100 g (58)] contained the lowest fat contents and Colby [32.1 g fat per 100 g (47)], gruyere [32.3 g fat per 100 g (53)], and cheddar [34 g fat per 100 g (62)] contained the highest fat contents, comparatively.
3.2 Dairy-fat matrix
The processing methods utilized in the dairy industry create different dairy-fat matrices between milk, yogurt, cheese, and butter. Within the food matrices of dairy products that encompass all the critical components in dairy is the dairy-fat matrix, which refers to the unique packaging and compartmentalization of the variety of lipids, as well as carbohydrates and proteins, found within and surrounding the MFG, and the specific physical and chemical interactions between these and other nutrients present. The physical structure of the MFG in these dairy products is modified by processing methods, resulting in different dairy-fat matrices and, likely, different physiologic and metabolic characteristics. Below, we provide an overview of the dairy-fat matrix in milk, yogurt, cheese, and butter for context, however, the specific metabolic implications of the alterations to the dairy-fat matrix are reviewed elsewhere (30, 71, 72).
3.2.1 Dairy-fat matrix alterations from milk processing
Unprocessed milk is comprised of an oil-in-water emulsion where the MFGM and its protected fat droplets are suspended in the aqueous phase of milk (Figure 2B) (28, 72, 73). During homogenization, the MFG size is substantially reduced, leading to an overall increase in surface area of the MFGs (74–77). Subsequently, casein primarily aggregates and covers the new surface area (30, 36, 72, 78), and thereby increases the width of the MFG (73, 79). During pasteurization, denatured whey proteins, primarily β-lactoglobulin, aggregate with the proteins of the MFGM and the caseins on the MFG (30, 74). In the case pasteurization is performed prior to homogenization, there may be more whey proteins on the surface of the MFG, along with whey-casein complexes (30). Most authors report no change in MFG size in response to pasteurization (77, 80–82), though Ren et al. (83) described an increase in MFG size as a result of heat treatment.
3.2.2 Dairy-fat matrix alterations from yogurt and cheese processing
During the production of yogurt or cheese, the coagulation of milk creates a semi-solid milk gel with MFGs interspersed in a casein protein network (76, 84, 85). The size of the MFGs and their associations with the protein network greatly impact the final physical structure, and therefore texture, of these products. Homogenized milk is frequently used as a base product for yogurt and softer cheeses as the smaller MFGs allow for better moisture retention, whereas unhomogenized milk is more commonly used for hard and semi-hard cheeses (86–88). MFGs that are larger than the pores of the protein network disrupt the network structure, while MFGs that are smaller than the pores act as inert fillers of this network (89). However, smaller MFGs may support the network structure if there are caseins present on the MFGM, such as when homogenized milk is the base product, as the caseins can interact with other proteins in the network (70, 84). In addition, it is common to observe the coalescing or aggregation of MFGs as a result of mechanical, thermal, or enzymatic processing techniques (11, 84). Notably, the high calcium content of cheese affects the dairy-fat matrix, as the interplay between milk calcium and caseins impacts the formation of the protein network in which MFGs interact (90). Research also shows that the food matrix can be impacted if texture enhancers (91, 92) or other additives (93) are included during processing.
3.2.3 Dairy-fat matrix alterations from butter processing
In contrast to milk, butter is a water-in-oil emulsion in which water droplets are suspended in the fat matrix (72, 94). During agitation, or churning, of butter, the MFG is physically disrupted, releasing the TAG core from within the MFGM to aggregate together and form butter. The MFGM fragments coalesce in a separate aqueous buttermilk phase, leaving only a residual MFGM content in butter (33, 36, 94). Though distinctly different from milk, yogurt, and cheese, butter is an important dairy product to consider as it is a concentrated source of dairy fat, presented in a microstructure inverse to that of milk.
As demonstrated, the base product milk can undergo various transformations (i.e., homogenization, heating, coagulation, fermentation, ripening, and churning, respectively) resulting in a variety of dairy products, such as homogenized milk, yogurt, cheese, or butter. These processing methods disrupt or modify the original physico-chemical assembly of dairy lipids in raw milk, resulting in a unique dairy-fat matrix. Here, we evaluate whether, and to what degree, the dairy-fat matrix’s physical (specific packaging and compartmentalization) and chemical assembly (lipid species and other bioactive nutrients) and their interactions influence the bioavailability, digestion, absorption, and metabolism of dairy fat, its moieties, and associated nutrients thereby affecting nutritional and health properties of these dairy products.
4 Examining the dairy-fat matrix of different dairy products in cardiometabolic health
Previous reviews and meta-analyses of the literature published prior to 2013 (95–103) represent the early phase of examining the differential effects of individual dairy products on cardiometabolic health. The most consistent finding across these studies was the unique effect of cheese on blood lipids, distinguishing cheese from many other dairy products (95–99). Notably, few of these summarizing works separated dairy intake by both dairy product and by fat content. With these findings, in conjunction with the differences in the dairy-fat matrix across dairy products, we sought to examine if individual dairy-fat matrices have differential effects on cardiometabolic health. Our objective was to utilize a recent snapshot of research (i.e., studies published over the last 10 years) to gain an understanding of the availability of evidence comparing individual dairy-fat matrices (i.e., comparisons stratifying by both dairy product and fat content) and the conclusions we may begin to draw on their unique effects on cardiometabolic health and implications for nutrition guidance.
4.1 Search methods
Eligible studies were identified through the Ovid MEDLINE database, using defined search parameters combining the three following categories of search terms with AND: (i) “exp Cholesterol/” OR “exp Dyslipidemias/” OR “Obesity/” OR “Obesity, Abdominal/” OR “exp Hyperglycemia/” OR “exp Insulin Resistance/” OR “Hypertension/” OR “exp Diabetes Mellitus/” OR “Cardiometabolic.mp.” OR “Inflammation/” OR “exp Cardiovascular Diseases/” OR “Stroke/” OR “Non-alcoholic Fatty Liver Disease/” OR “exp Mortality/,” (ii) “exp Dairy Products/,” and (iii) “exp Dietary Fats/.” Two additional searches of Ovid MEDLINE and PubMed® were performed with the keywords “dairy fat.mp.” and [“dairy fat” AND “cardiometabolic health”], respectively. Search results were refined to include studies published from 2013–2023 (searches conducted November 2023) that were written in the English language and performed in humans. Publications were manually excluded from this list if the study: (i) was not a primary research article, (ii) did not have outcome measures related to cardiometabolic health, (iii) used food survey methodology that did not stratify by dairy product or fat content of the dairy product, (iv) compared a component of the dairy product that was not fat, (v) compared dairy products to non-dairy products, (vi) analyzed specific fatty acids as opposed to intake of the whole dairy product, or (vii) focused on non-bovine dairy products (e.g., dairy products made from goat’s milk). Other relevant papers identified throughout the search process were also included. In total, 59 primary research articles were assessed and reported in this review. The terminology for milk-fat content varied greatly among studies, thus, the term “regular-fat,” followed by the respective milk-fat content (if defined), is used to describe what is termed high-fat, full-fat, or whole-fat in the reviewed studies.
4.2 Results
We identified 46 observational studies (Supplementary Table S2) and 13 randomized controlled trials (RCTs; Supplementary Table S3) that examined the role of dairy fat consumption on a given cardiometabolic health outcome. In the observational studies, cardiometabolic health outcomes related to obesity, type 2 diabetes (T2D), CVD, inflammation, and the metabolic syndrome (MetS) are presented. Similarly, cardiometabolic health outcomes related to obesity, hyperglycemia and insulin resistance, inflammation, hypertension, and dyslipidemia from the RCTs are presented. The observational studies mainly utilized prospective cohort designs, as opposed to a cross-section design, and the RCTs utilized either a parallel or crossover design.
4.2.1 Observational studies
4.2.1.1 Milk
Thirty-one of the observational studies focused on regular-fat milk intake (Table 1). Their study designs included analyses such as: (i) comparing the effect of one versus three daily servings of regular-fat milk, (ii) comparing the effect of non-fat milk versus regular-fat milk, and (iii) modeling the effect of substituting non-fat milk with regular-fat milk, on a given cardiometabolic health outcome.
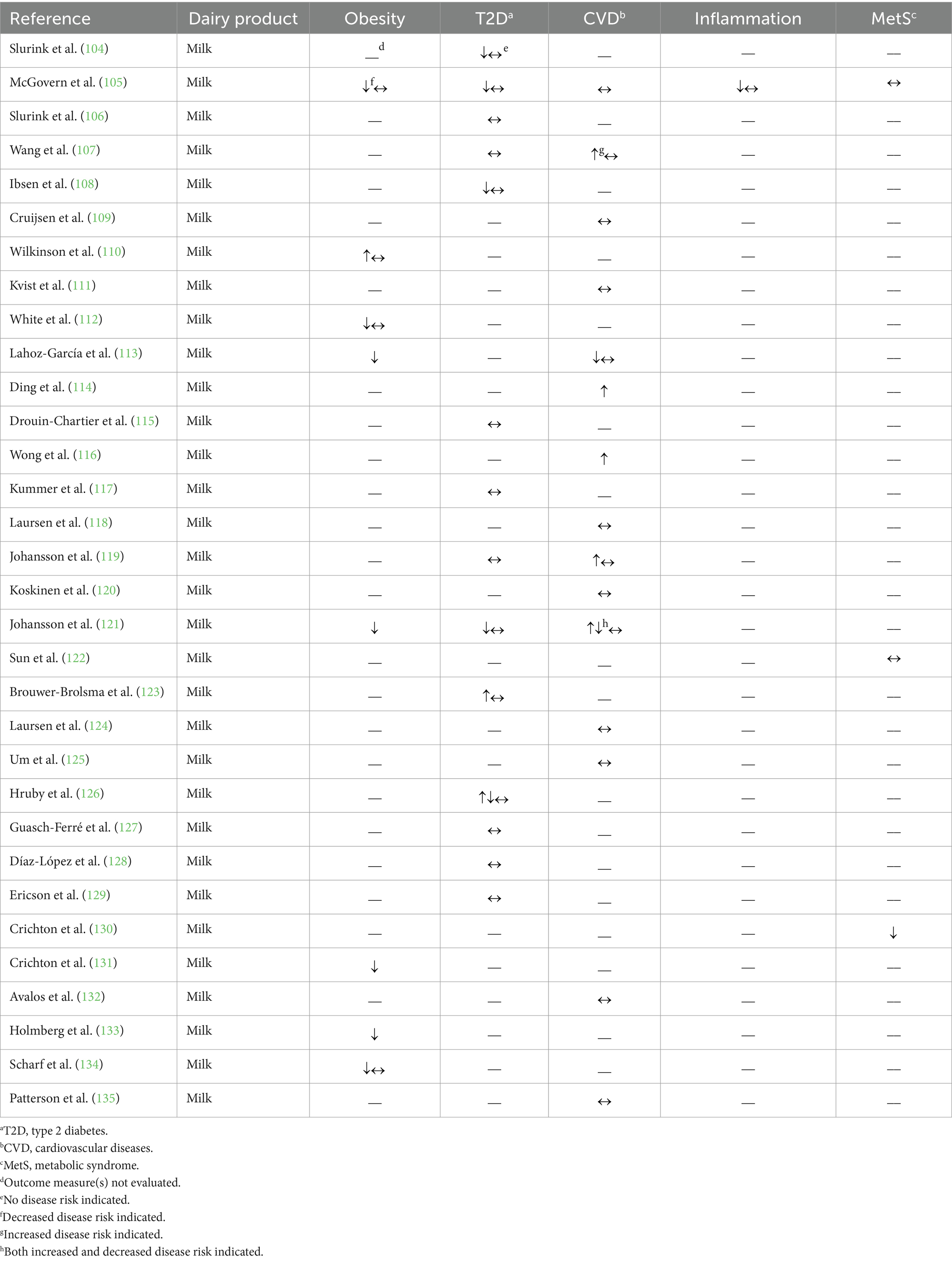
Table 1. Summary of findings from observational studies (n = 31) evaluating the effects of regular-fat milk intake on cardiometabolic disease risk factors and outcomes.
Four of the 7 observational studies that investigated the relationship of regular-fat (2–4%) milk intake and obesity were conducted in individuals across childhood stages, utilizing a variety of outcome measures including odds of overweight or obesity as well as associations with specific measurements of adiposity including body mass index (BMI) or waist circumference. Three of these studies reported either no association or an inverse association with the outcome measures evaluated (105, 112, 134) and one reported only inverse associations with the measures of adiposity reported (113). The remaining three studies were conducted in adults; Wilkinson et al. (110) observed no difference in BMI between non-fat and regular-fat (not defined) milk consumers but a 0.6 cm higher sagittal abdominal diameter associated with habitual consumption (i.e., consuming milk ≥5 times per week) of regular-fat milk. Two other studies reported a beneficial relationship between regular-fat (3.0%) milk consumption and outcome measures related to body weight and composition, specifically rates of central obesity and BMI (36 and 48% lower odds of development, respectively), compared to low-fat (≤1.5%) milk consumption (121, 133).
For outcome measures related to prediabetes, one prospective cohort study showed a non-linear association (hazard ratio crosses one at approximately 10 servings of regular-fat milk per week) between regular-fat (not defined) milk intake and prediabetes incidence in a continuous model; however, there was no association found when servings were analyzed categorically (126). Similarly, a different prospective cohort study showed no association between regular-fat (>2%) milk intake and incident prediabetes (106). Brouwer-Brolsma et al. (123) reported a positive association between regular-fat (3.5%) milk consumption and prediabetes risk (7% greater risk) when comparing between the highest and lowest tertiles of regular-fat milk intake, but no association was observed when analyzing per serving increase. Moreover, Slurink et al. (104) observed an inverse association with a relative risk of 0.89 between intake of regular-fat (>2%) milk and prediabetes risk when analyzed in a continuous model, but no association was observed when intake was analyzed in tertiles. Across multiple studies, regular-fat (2.5–4%) milk intake did not affect T2D risk (117, 119, 126–129) or T2D-related mortality (107). Similarly, substituting reduced-fat milk in place of regular-fat (not defined) milk was not associated with risk of T2D (115). Replacing regular-fat (3.5%) milk with skim milk was associated with a 0.4% higher risk of T2D in individuals aged 65–72 years but not in individuals 56–64 years old (108). Two studies focused on specific biomarkers related to T2D risk, including Homeostatic Model Assessment of Insulin Resistance and fasting blood glucose (105, 121). In children, regular-fat (2–3.25%) milk intake compared to low-fat (skim and 1%) milk intake was associated with a lower Homeostatic Model Assessment of Insulin Resistance (β coefficient approximately −0.25) in adolescence for participants with a BMI over the 85th age-appropriate percentile, but not participants with a BMI between the 5th and 85th percentiles (105). For adults, an increased regular-fat (3.0%) milk intake was not associated with fasting blood glucose, but when the authors considered adjusted means, regular-fat milk intake was associated with a 0.03 mmol/L lower fasting blood glucose concentration (121).
The majority of the identified observational studies (13 out of 15) that assessed regular-fat (≥3%) milk intake and outcomes related to CVD reported no association with at least one outcome measure (107, 109, 111, 118, 120, 124, 125, 132, 135). These outcomes included CVD-specific mortality (109, 125), myocardial infarction (MI) risk (111, 119, 135), coronary heart disease incidence (120, 132), ischemic heart disease mortality (109), stroke rate (118, 124), or stroke mortality (107, 109). Further, substitution of low-fat (≤1.5%) milk in place of regular-fat (≥3%) milk was not associated with MI risk (135). Three other studies indicated a detrimental effect of regular-fat milk intake and outcomes related to CVD, specifically citing a greater risk of cardiovascular mortality [hazard ratio 1.09 per half a serving increase per day (114)] or stroke incidence [hazard ratio 1.19 when the highest intake quartile was compared to the lowest (119)] associated with intake of regular-fat (3.0%) milk. The third study reported lower risk for heart disease mortality in individuals consuming lower-fat (2% and ≤1%) milk (hazard ratios 0.73 and 0.67, respectively) compared to individuals consuming regular-fat (3–4%) milk (107). Four studies assessed the relationship between regular-fat milk intake and concentrations of blood lipoproteins, such as total CHOL and subclasses of CHOL [e.g., low-density lipoprotein (LDL)-CHOL], but found conflicting results in terms of whether regular-fat milk consumption resulted in a beneficial or detrimental effect on the blood lipid profile (105, 113, 116, 121). Additional studies reported no association between regular-fat (2–3.25%) milk intake and blood pressure (105, 121), and a positive association (correlation coefficient 0.124) between regular-fat (not defined) milk intake and a cardiorespiratory fitness score, indicating improved cardiorespiratory fitness (113).
Only one observational study evaluated outcome measures related to inflammation, and reported a positive association (β coefficient approximately 0.25) between adiponectin concentrations and regular-fat (2–3.25%) milk consumption in participants with a BMI over the 85th age-appropriate percentile, but not in participants with a BMI between the 5th and 85th percentile (105).
Two studies utilized calculations to determine the effect of regular-fat milk consumption on general cardiometabolic health. Sun et al. (122) used the continuous MetS risk score, which composites a score based on the International Diabetes Federation risk factors for MetS [i.e., waist circumference, TAG concentration, high-density lipoprotein (HDL)-CHOL concentration, blood pressure, and fasting blood glucose concentration (136)] and found no association between regular-fat (not defined) milk intake and continuous MetS risk score. Another study, using a similar cardiometabolic risk score, also reported no association with this risk score and the consumption of regular-fat (2–3.25%) milk compared to low-fat (1% and skim) milk (105). Crichton et al. (130), alternatively, utilized a cardiovascular health score that compiled information regarding smoking, BMI, physical activity, total CHOL, blood pressure, and fasting blood glucose based on definitions from the American Heart Association, and found that the cardiovascular health score increased by approximately 0.5 points with an increasing intake of regular-fat (not defined) milk, indicating an improvement in cardiovascular health.
Collectively, recent research does not appear to indicate that reducing intake of regular-fat milk is beneficial for cardiometabolic health. Evidence suggests that there is largely no association between regular-fat milk intake and cardiometabolic health, with a potential beneficial effect on risk factors related to obesity, though additional research on the association with obesity and inflammation would bolster the current evidence base.
4.2.1.2 Yogurt
The effect of regular-fat yogurt intake on cardiometabolic health was examined in 12 recent observational studies (Table 2). Increasing intake of regular-fat (not defined) yogurt was associated with improvements in multiple facets of body composition, including 15–42% lower odds of developing abdominal (131, 140) or generalized (131) obesity. Additionally, though a larger decrease in waist circumference (absolute yearly decrease of 0.27 cm versus 0.15 cm, respectively), was observed among individuals in the highest quintile of regular-fat (not defined) yogurt consumption compared to the lowest quintile of consumption this decrease was not large enough to constitute a decrease in abdominal obesity in these same individuals (139). Another study indicated no association between increased regular-fat (≥3.9%) yogurt consumption and body weight, BMI, waist circumference, or waist to hip ratio (138).
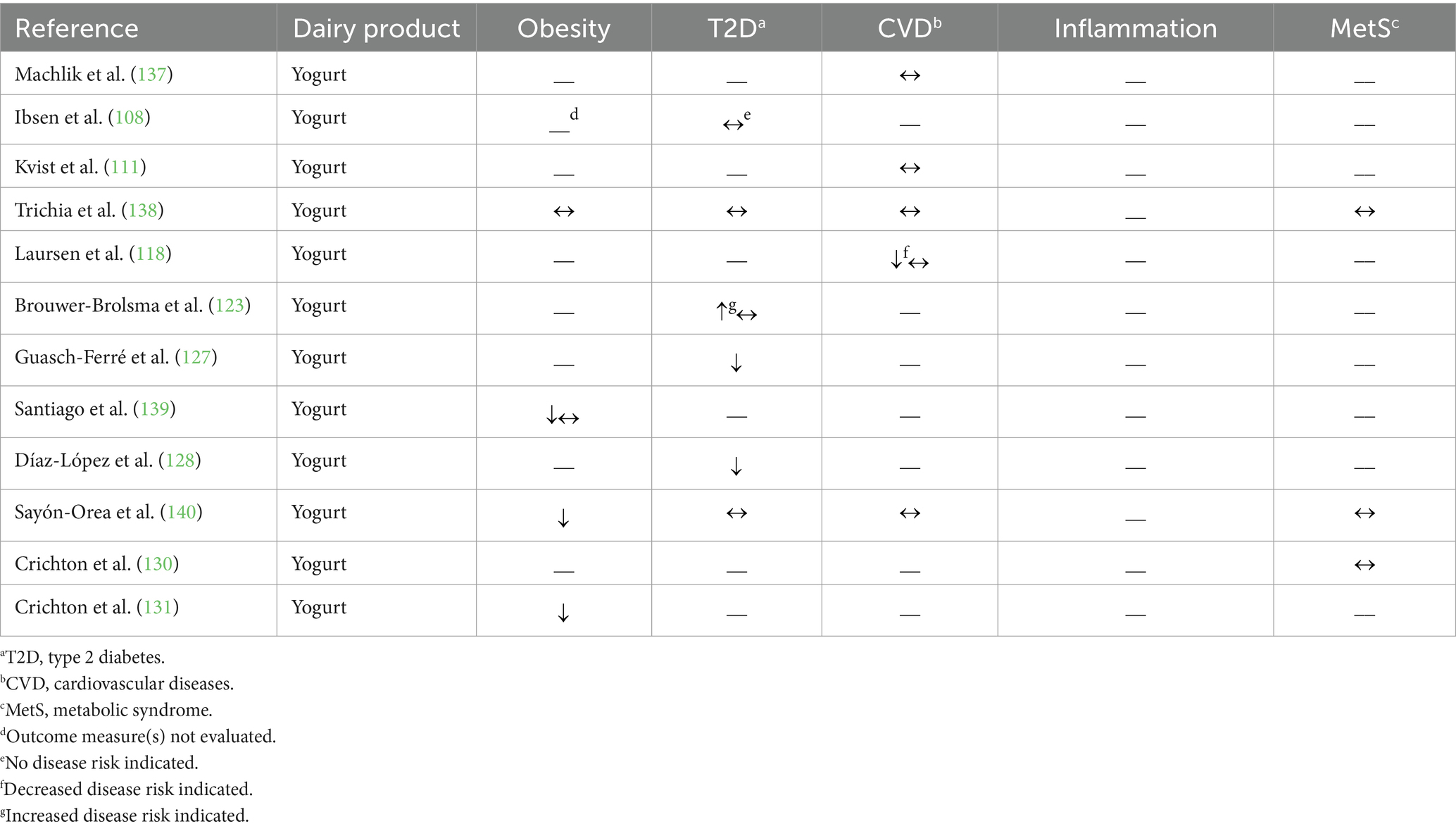
Table 2. Summary of findings from observational studies (n = 12) evaluating the effects of regular-fat yogurt intake on cardiometabolic disease risk factors and outcomes.
Brouwer-Brolsma et al. (123) observed a 7% greater risk of prediabetes associated with regular-fat (2.9%) yogurt consumption when comparing between the highest and lowest tertiles of intake (no association was observed, though, when analyzed per serving increase); however, no association was observed between regular-fat yogurt consumption and T2D risk in the same individuals. Three other prospective cohort studies reported no relationship between regular-fat (≥3.5%) yogurt consumption and T2D risk (108) or T2D risk factors of fasting blood glucose concentration (140) or hemoglobin A1c percentage (138). Moreover, two prospective cohort studies reported a 34–35% lower risk of T2D associated with regular-fat (not defined) yogurt consumption (127, 128).
Related to CVD outcomes, replacing low-fat (<2%) yogurt with regular-fat (≥3%) yogurt was not associated with risk of MI (111), total stroke (118), or hemorrhagic stroke (118); this substitution, though, was associated with a greater rate of ischemic stroke [hazard ratio 2.58 with one serving substitution per day (118)]. Three other studies reported no association between greater consumption of regular-fat (≥3.9%) yogurt and changes in blood lipid concentrations (TAG, HDL-CHOL, LDL-CHOL, or total CHOL), or blood pressure, indicators of cardiovascular health (137, 138, 140).
Sayón-Orea et al. (140) and Crichton et al. (130) evaluated the effect on regular-fat (not defined) yogurt consumption on the risk of MetS (i.e., according to the criteria set forth by the American Heart Association and the International Diabetes Federation) and cardiovascular health score, respectively; neither found regular-fat yogurt consumption to be associated with these scores. Trichia et al. (138) used a similar metabolic risk score and also reported no association between increasing regular-fat (≥3.9%) yogurt and the metabolic risk score.
In summary, the observational studies discussed above report largely no association between regular-fat yogurt consumption and cardiometabolic health. Consumption of regular-fat yogurt appears specifically effective in protecting against obesity; a weaker effect was observed on outcome measures related to T2D or CVD.
4.2.1.3 Cheese
Out of the 14 recent studies that assessed the relationship between regular-fat cheese consumption and cardiometabolic health, only three studies investigated the relationship between intake of regular-fat cheese and obesity (Table 3). One study reported no association between regular-fat (not defined) cheese consumption and both abdominal and generalized obesity (131). Trichia et al. (138) reported no association with regular-fat (≥3.9%) cheese intake and waist circumference or waist to hip ratio. These authors also found a positive association between regular-fat (≥3.9%) cheese intake and change in body weight (approximately 0.3 kg) as well as BMI (approximately 0.25 kg/m2) in participants ages 50–60 years but no association was found in participants aged 40–50 or 60–78 years (138). In the third study, participants that consumed regular-fat (≥28%) cheese, compared to lower-fat (10–17%) cheese, had 13% lower odds of overweight or obesity (121).
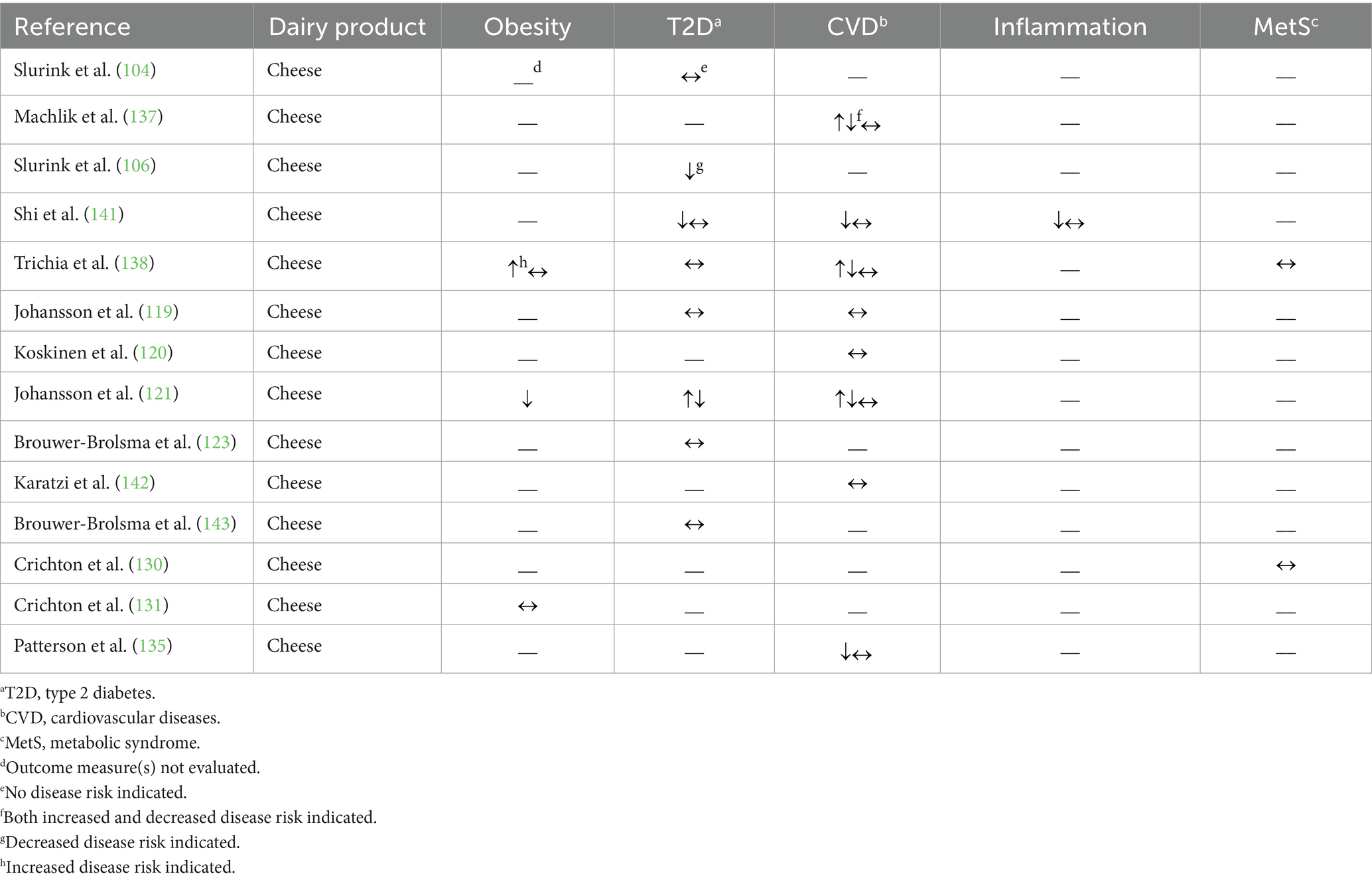
Table 3. Summary of findings from observational studies (n = 14) evaluating the effects of regular-fat cheese intake on cardiometabolic disease risk factors and outcomes.
For outcome measures related to prediabetes, Brouwer-Brolsma et al. (123) and Slurink et al. (104) observed no association between regular-fat (>20%) cheese consumption and prediabetes incidence, while Slurink et al. (106) found that a greater intake of regular-fat (>20%) cheese was associated with a 22% lower risk of prediabetes incidence. Three studies reported no association between regular-fat (≥24%) cheese consumption and T2D incidence (119, 123, 143). The results regarding the relationship between regular-fat cheese intake and the risk factors related to prediabetes and T2D are inconclusive; these include, but are not limited to (i) evidence of a beneficial relationship [increasing regular-fat (not defined) cheese intake was associated with a 0.2% lower circulating glucose concentrations (141)], (ii) evidence of an unfavorable relationship [consumers of regular-fat (≥28%) cheese had a 7% greater odds of a fasting blood glucose concentration in the prediabetic range (121)], and (iii) evidence of no relationship [increasing intake of regular-fat (≥3.9%) cheese was not associated with hemoglobin A1c concentration (138)].
In other recent work, regular-fat (≥3.5%) cheese consumption was not associated with coronary heart disease incidence (120), stroke incidence (119), or retinal vessel calibers [biomarker of CVD risk (142)]. In a cohort of Swedish women, substituting low-fat (10–17%) cheese in place of regular-fat (<17%) cheese did not affect MI risk, but increasing intake of regular-fat cheese was associated with a 17% lower MI risk (135). A study in another cohort of Swedish women and men reported no association between regular-fat (≥28%) cheese intake and MI risk (119). Four observational studies evaluated the association between regular-fat (≥3.9%) cheese intake and specific CVD risk factors, including blood pressure, lipids, and lipoproteins (121, 137, 138, 141). HDL-CHOL and LDL-CHOL concentrations were either not associated or positively associated with regular-fat (≥3.0%) cheese intake, while blood pressure and TAG concentrations were either not associated or inversely associated with regular-fat (≥3.9%) cheese intake (121, 137, 138, 141). The relationship between regular-fat (≥3.9%) cheese consumption and total CHOL concentration was less clear as there were studies that reported no association (137, 141), a positive association (138), or a combination of associations between these two variables based on different analytical stratifications and adjustments (121).
Related to inflammation, one study reported an inverse association between regular-fat (not defined) cheese intake and C-reactive protein and interleukin (IL)-6 concentrations (3.4 and 3% lower concentrations, respectively), but no association with IL-10, adiponectin, tumor necrosis factor α (TNFα), or TNFα receptor concentrations (141). Two studies reported no association between regular-fat (≥3.9%) cheese intake and cardiovascular health score (130) or metabolic risk score (138), as detailed above.
Collectively, these recent observational studies largely suggest no effect of regular-fat cheese consumption on cardiometabolic health outcomes, with some studies proposing a beneficial effect on outcome measures related to T2D and CVD.
4.2.1.4 Butter
Of the 17 identified studies that surveyed butter consumption, only two evaluated the effect of butter consumption on measures of body weight and composition (Table 4). Trichia et al. (138) found no association between butter intake and body weight, BMI, waist circumference or waist to hip ratio. Johansson et al. (121) found that increasing butter intake was associated with a 28–40% lower odds of overweight or obesity in a cross-sectional analysis, but statistical significance was attenuated in their prospective analysis.
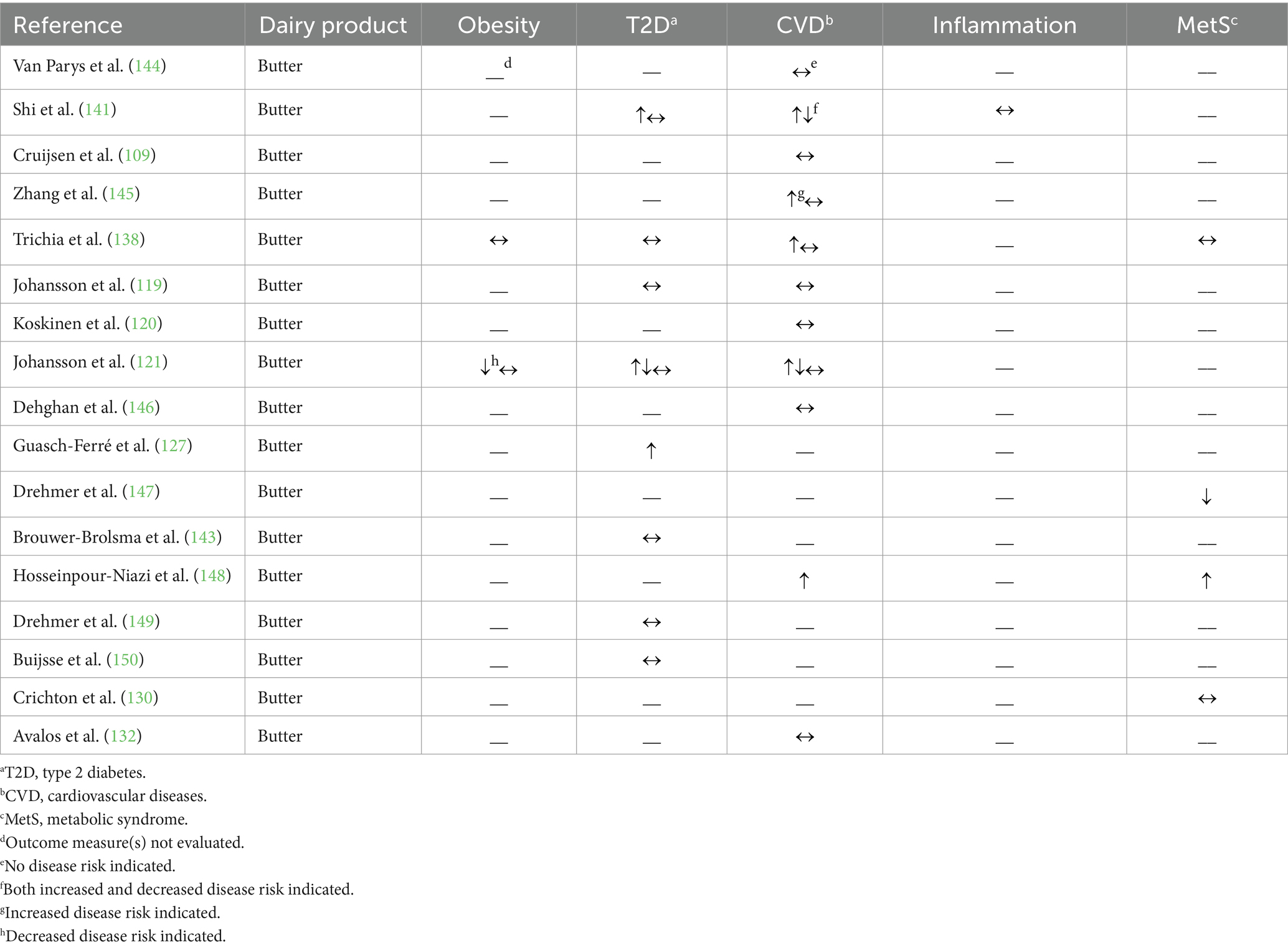
Table 4. Summary of findings from observational studies (n = 17) evaluating the effects of butter intake on cardiometabolic disease risk factors and outcomes.
Three studies published over the last 10 years reported no association between butter intake and T2D incidence (119, 143, 150); however, one study observed an increased risk of T2D when butter consumption increased [hazard ratio 2.42 with a one serving increase (127)]. In addition to T2D incidence, four studies utilized a variety of biomarkers to evaluate T2D risk, including, but not limited to, blood glucose and insulin concentrations as well as hemoglobin A1c percentage (121, 138, 141, 149). Drehmer et al. (149) found no association between butter intake and fasting blood glucose or insulin concentrations or the concentrations of blood glucose or insulin 2 hours after an oral glucose tolerance test. Shi et al. (141) identified a positive association between butter intake and circulating insulin concentrations (2.3% higher concentrations), but no association with circulating glucose concentrations, while in a cross-sectional analysis, stratified by sex, an increased butter intake was reported to be inversely associated with fasting blood glucose concentrations in male participants (12% lower odds of developing a fasting blood glucose ≥6.1 mmol/L), but was not associated with fasting blood glucose concentrations in female participants (121). Moreover, Johansson et al. (121) ascertained in their prospective analysis that an increased butter intake was positively associated with fasting blood glucose concentrations (28% higher risk of developing a fasting blood glucose ≥6.1 mmol/L). In addition, two studies reported no association between butter intake and hemoglobin A1c percentage (138, 149).
Results related to CVD are also mixed due to the variety of outcome measures utilized. Butter intake was not associated with major cardiovascular events (119, 144, 146), incident coronary heart disease (120, 132), stroke rate or stroke mortality (109, 144, 145), CVD mortality (109, 144), or ischemic heart disease mortality (109), but one study did report that greater butter intake was associated with an 8% greater risk of both CVD and heart disease mortality (145). In terms of CVD risk factors, butter intake was associated with increased LDL-CHOL and total CHOL concentrations, but the relationship with HDL-CHOL and TAG concentrations is less clear as there were studies that reported no association, a positive association, and an inverse association between butter intake and these biomarkers (121, 138, 141, 148).
Only one study evaluated the relationship between butter consumption and inflammation, and proposed no relationship, as evidenced by the lack of association with C-reactive protein, IL-6, IL-10, TNFα, or TNFα receptor concentrations (141).
Four studies analyzed the relationship between butter consumption and broader indices of cardiometabolic health, noting different relationships. Drehmer et al. (147) used a metabolic risk score (i.e., a compilation of the following metabolic risk factors: waist circumference, TAG concentration, HDL-CHOL concentration, systolic blood pressure, and fasting blood glucose concentration) and observed a 0.129-point decrease in the metabolic risk score associated with increasing butter consumption by one serving, suggesting a lower risk of MetS. Hosseinpour-Niazi et al. (148) observed 2.03 times greater odds of MetS [using the globally harmonized criteria (151)] associated with greater butter intake. Finally, Crichton et al. (130) and Trichia et al. (138) observed no association between butter consumption and cardiovascular health score, as described above, or a metabolic risk score, respectively.
Overall, the observational studies report mainly no effect of butter consumption on cardiometabolic health with fewer studies (i.e., 5 of 17 studies) reporting an adverse effect. When evaluating disease outcomes, most studies reported no association with butter consumption, but when evaluating risk factors for these outcomes, there is more evidence to suggest an adverse effect, highlighting a key area for future research. Notably, there was only one component of cardiometabolic health, blood TAG concentration, in which butter consumption appeared to be beneficial, yet this finding was not consistent across all studies that measured this marker.
4.2.1.5 Comparisons between dairy products
In addition to the aforementioned studies that evaluated the impact of dairy fat on cardiometabolic health by dairy product, three recent prospective cohort studies modeled the effects of substituting different regular-fat dairy products for one another on cardiometabolic health outcomes (Table 5; Supplementary Table S2). First, the consumption of regular-fat (3.5%) yogurt, as a substitute for regular-fat (3.5%) milk, was reported to be associated with a 0.7% reduced risk of T2D in individuals 56–59 years old per 50 g per day substitution, but not in adults 60–64 or 65–72 years old (108). Another study showed that regular-fat (3.5%) yogurt substitution in place of regular-fat (3.5%) milk was associated with a 13% lower risk of MI per 200 g per day substitution (111). In modeling how the substitution of several different dairy products affect the rate of total stroke and stroke subtypes, the substitutions of neither regular-fat (≥3%) milk nor regular-fat (≥3%) yogurt, in place of butter, were associated with a change in rate of total or hemorrhagic stroke (118). Regular-fat (≥3%) yogurt as a substitute for butter, however, was associated with a 61% lower rate of ischemic stroke per serving substitution per day (118). Moreover, regular-fat (≥3%) yogurt, as a substitute for regular-fat (≥3%) milk, was associated with a 65% lower rate of ischemic stroke per substitution per day, but not total or hemorrhagic stroke (118).

Table 5. Summary of findings from observational studies (n = 3) comparing the effect of the substitution between dairy products (milk, yogurt, cheese, or butter) on cardiometabolic disease outcomes.
In summary, these modeling-based observational studies infer that dairy fat consumed in yogurt may be beneficial for reduction of T2D and CVD risk.
4.2.2 Randomized controlled trials
4.2.2.1 Comparisons within dairy products
Out of the 13 RCTs identified, 6 RCTs evaluated the effect of consuming differing amounts of dairy fat within the same dairy product (e.g., reduced-fat versus regular-fat cheese consumption; Table 6; Supplementary Table S3). Of these, three RCTs focused specifically on milk. There was no effect of regular-fat milk intake observed for many aspects of cardiometabolic health [i.e., there was no association with outcome measures related to obesity (152, 156), hyperglycemia and insulin resistance (152, 156), inflammation (155), or blood pressure (156)]. For outcome measures related to dyslipidemia, largely no effect was found (156), but there were certain outcome measures that were affected by regular-fat milk intake (152, 155). In a 3 week trial with 17 adults, daily consumption of 500 mL (~2 cups) of regular-fat (3.5%) milk, compared to skim (0.1%) milk, did not affect blood total CHOL, LDL-CHOL, or TAG concentrations, but resulted in a 0.06 mmol/L increase in HDL-CHOL concentrations (152). Similarly, another study found that HDL-CHOL concentrations were 0.16 mmol/L higher after the daily consumption of 400 mL of regular-fat (3%) milk compared to skim (0.5%) milk in children, over a 4 month period, along with total CHOL, LDL-CHOL, and total apolipoprotein (Apo)-B concentrations (0.43 mmol/L, 0.28 mmol/L, and 0.05 g/L greater, respectively) as well as the total Apo-B:Apo-A1 ratio (0.05 g/L greater) (155). This study also reported no change in blood TAG, very low-density lipoprotein CHOL, or Apo-A1 concentrations, as well as no change in the total CHOL:HDL-CHOL ratio (155).
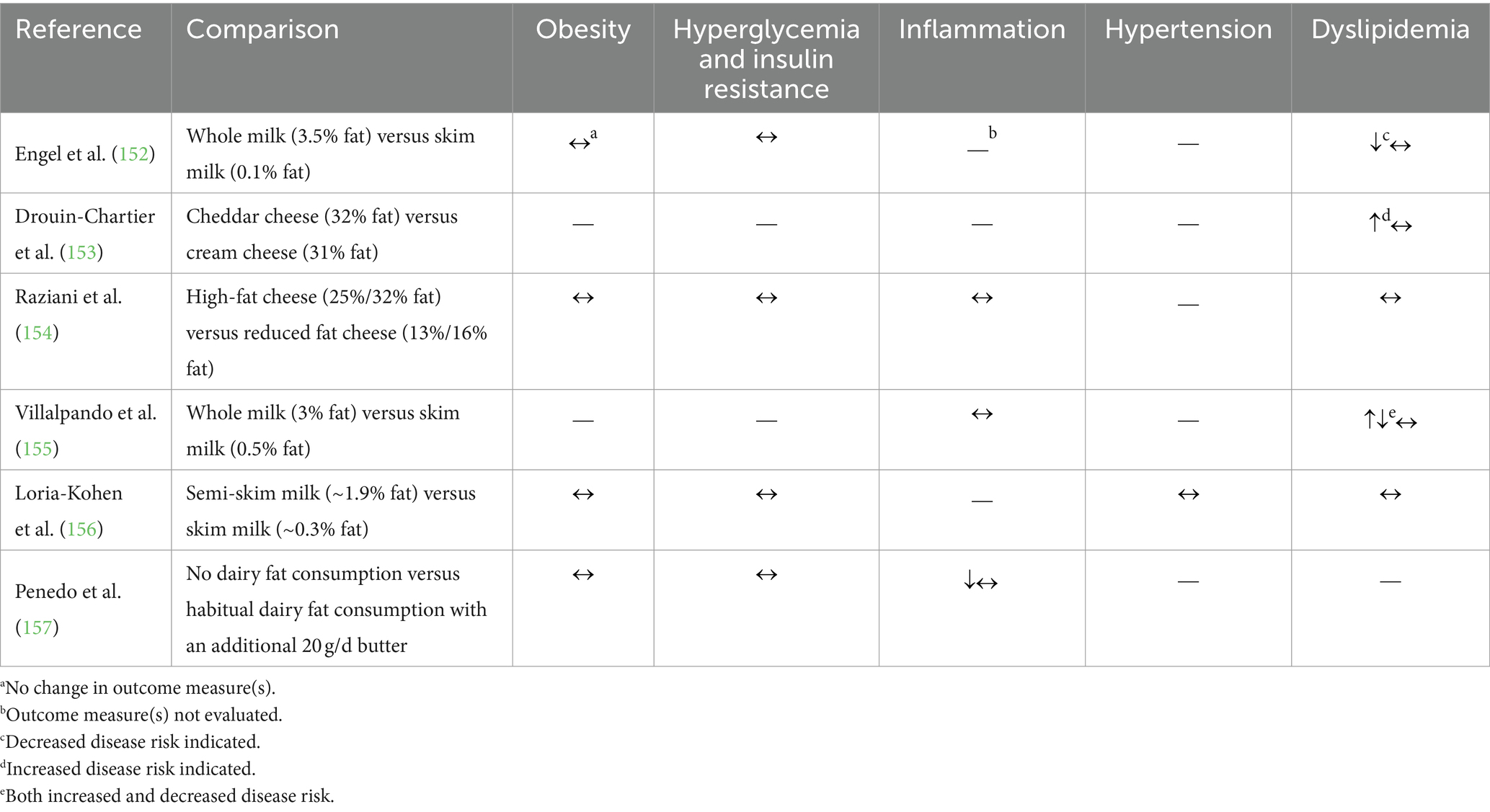
Table 6. Summary of findings from randomized controlled trials (n = 6) comparing the effects of individual dairy products (milk, cheese, or butter) on cardiometabolic risk factors.
The remaining three studies compared dairy fat intake within either cheese or butter utilizing different study designs. For example, a meal tolerance test comparing 33 g of fat from either cream cheese or cheddar cheese resulted in no differences in the area under the curve for TAG or free FA concentration change compared to baseline, but cheddar cheese consumption resulted in a larger area under the curve for Apo-B48 concentrations (60,237 ng/mL/h following cheddar cheese consumption compared to 58,528 ng/mL/h following cream cheese consumption) (153). Alternatively, no changes were observed in a 12 week RCT assessing the effect of daily consumption of regular-fat (25–32%) versus reduced-fat (13–16%) cheese on markers of body weight, blood glucose control, blood lipid profile, or inflammation (154). In a depletion-repletion study where dairy fat consumption was first eliminated for 8 weeks, then permitted with the addition of 20 g per day of butter for the subsequent 8 weeks, there were also no changes in BMI, body fat mass, or fasting blood glucose (157). Additionally, this study reported no change in C-reactive protein, adiponectin, or serum IL-4 concentrations after the repletion period, but an improvement in many other inflammatory markers [e.g., a decrease in TNFα concentration (157)].
Collectively, the limited evidence from these RCTs suggests that the consumption of regular-fat milk, cheese, or butter compared to lower-fat options may not affect cardiometabolic disease risk factors.
4.2.2.2 Comparisons between dairy products
Eight RCTs were identified that compared the effects of dairy fat consumed across different dairy products on biomarkers of cardiometabolic health (Table 7; Supplementary Table S3). Seven of the 8 RCTs compared the effects of cheese intake with either butter or milk intake; these studies mostly found that the consumption of these regular-fat dairy products did not differentially affect body weight, body composition, hyperglycemia and insulin resistance, hypertension, or inflammation (160, 163, 164). In contrast, reported effects of dairy fat consumption on markers of dyslipidemia were more variable among different types of dairy products (160–164). As an example, Rancourt-Bouchard et al. (164) identified that the consumption of regular-fat (31%) cheese enriched with γ-aminobutyric acid, compared to low-fat (1%) milk, increased total-CHOL, LDL-CHOL, and Apo-B100 concentrations (by 0.23 mmol/L, 0.21 mmol/L, and 0.05 g/L, respectively), but also increased LDL-CHOL size by 0.6 Å and Apo-A1 concentration by 0.05 g. However, there were no diet-related changes in the total CHOL to HDL-CHOL ratio, HDL-CHOL concentration, or TAG concentration in response to consuming the diet with regular-fat cheese enriched with γ-aminobutyric acid compared to the diet with low-fat milk (164). Feeney et al. (160) compared the effect of a diet containing 40 g of dairy fat consumed daily as either (i) regular-fat cheese or (ii) reduced-fat cheese and butter, for 6 weeks on cardiometabolic health markers. Greater decreases in total CHOL concentrations (0.15 mmol/L greater decrease) and LDL-CHOL concentrations (0.18 mmol/L greater concentrations), but not HDL-CHOL, TAG, or free FA concentrations, were observed in response to the regular-fat cheese diet (160). No diet-related differences were observed in fasting blood glucose or insulin concentrations (160). In a secondary analysis, no differences were seen in additional markers of hyperglycemia and insulin resistance (i.e., lipoprotein insulin resistance score) or dyslipidemia [i.e., LDL-CHOL, HDL-CHOL, very LDL-CHOL/chylomicron, and intermediate-density lipoprotein concentration or size (159)]. However, one study that did not report any differences in the blood lipid profile was a meal tolerance test that compared the consumption of 33 g of fat from either cream cheese, cheddar cheese, or butter; there were no differences in TAG, Apo-B48, or free FA concentrations between the consumption of butter and either type of cheese (153).
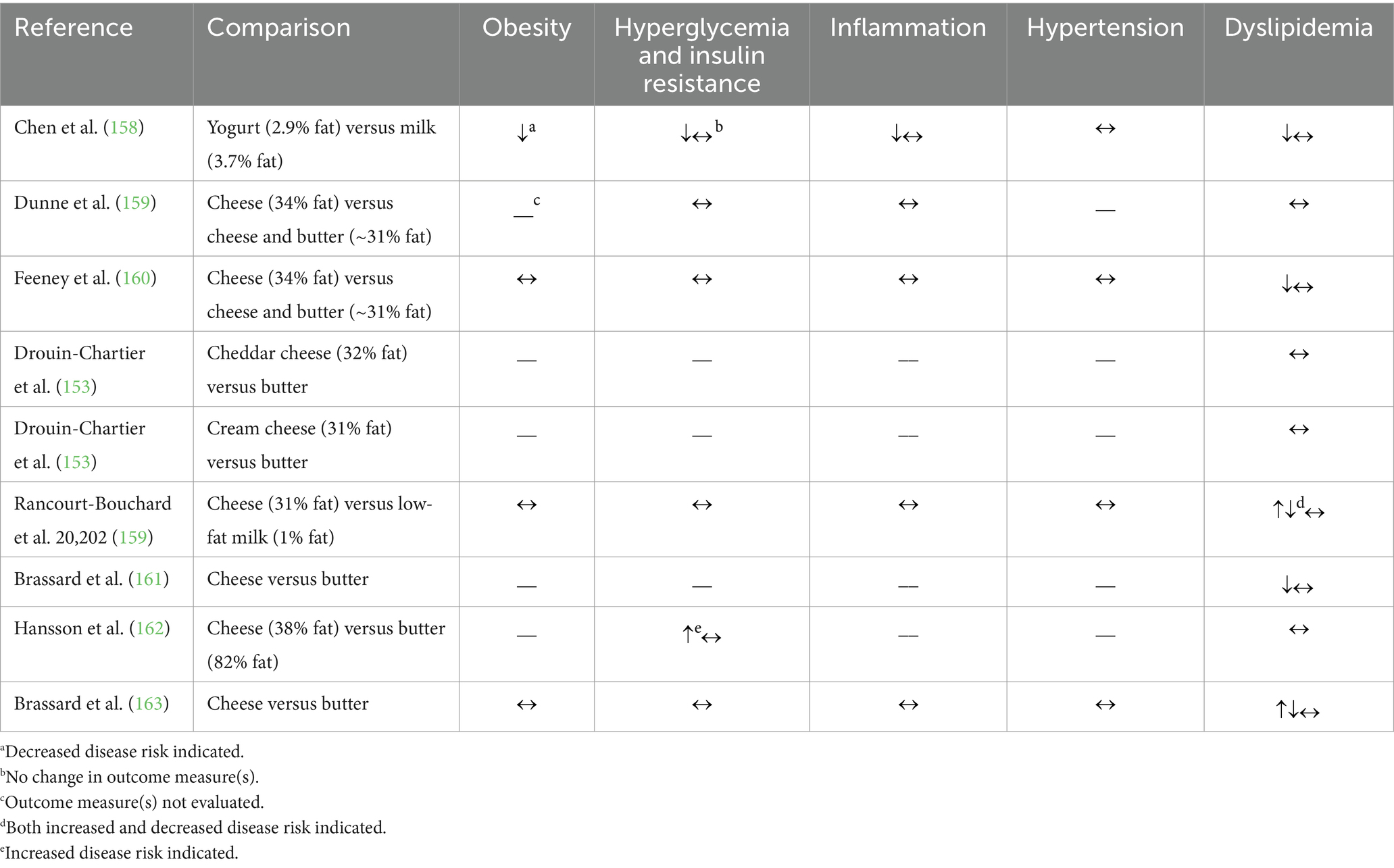
Table 7. Summary of findings from randomized controlled trials (n = 9) comparing the effects of substitution between dairy products (milk, yogurt, cheese, or butter) on cardiometabolic disease risk factors.
The eighth RCT compared daily intake of 220 g of either regular-fat (2.9%) yogurt or regular-fat (3.7%) milk in Chinese women with an elevated waist circumference and BMI (158). After 24 weeks of regular-fat yogurt consumption, the authors observed either no change or an improvement in outcome measures related to obesity and body composition, hyperglycemia and insulin resistance, inflammation, hypertension, and dyslipidemia.
In summary, these few RCTs primarily indicate no effect of dairy fat consumption on cardiometabolic risk factors when comparing intake of dairy fat in cheese to that in butter or milk. More clinical research is needed however, particularly on lipid homeostasis outcome measures as well as research including other dairy products, particularly yogurt.
4.3 Discussion
4.3.1 Findings, potential biological mechanisms, and methodological considerations
Limiting dairy fat intake is a prominent dietary recommendation to reduce energy and SFA intake to lower cardiometabolic disease risk. However, this recommendation is derived in part from a single-nutrient approach to dietary guidance, which does not fully recognize the importance of the food matrix or the dairy-fat matrix. As a result of the different dairy-fat matrices evident in milk, yogurt, cheese, and butter, our aim was to identify and evaluate observational studies and RCTs conducted within the previous 10 years that focused on the effect of the different dairy products on cardiometabolic health. There was primarily no effect observed of the consumption of these individual regular-fat dairy products on cardiometabolic health, as evidenced by both observational studies and RCTs; however, the variable nature of the observed results (Supplementary Tables S4–S6), highlight the need for additional research. Results from observational studies suggest that regular-fat milk intake may have a beneficial effect on risk factors related to obesity, RCTs however, did not report an effect. Observational studies with results on the cardiometabolic health effect of regular-fat yogurt consumption identified a more substantial beneficial effect on obesity and body weight regulation, while the results from the analyses of substitutions between dairy products demonstrate a potentially beneficial effect of regular-fat yogurt intake on T2D and CVD risk. Only one recent RCT is available to inform the limited scope of evidence on regular-fat yogurt consumption and aligns with the observational findings. Of note, the U.S. Food and Drug Administration recently announced a qualified health claim for yogurt’s potential role in reducing risk of T2D (165), and this claim was not limited to yogurts of a specific fat content (166). Results from both study types noted that consumption of regular-fat cheese may affect the blood lipid profile, in alignment with the initial work published prior to 2013 examining the differential effects of individual dairy products on cardiometabolic health, though the directionality of this effect is not definitive and requires further research. In the observational studies, results for butter were not conclusive and the reviewed studies showcased either no effect, an increased effect, or a decreased effect on cardiometabolic disease risk, while most RCTs demonstrated primarily no effect of butter consumption on cardiometabolic disease risk factors. As shown in Supplementary Table S6, 63% of the conclusions drawn from the reviewed studies indicate no effect of dairy-fat intake on cardiometabolic health outcomes, while 24 and 13% of the conclusions drawn indicate an increase or decrease, respectively, in cardiometabolic disease risk. Together, these heterogeneous results, stratified by dairy product and fat content suggest primarily no effect of consuming higher-fat varieties of dairy products on cardiometabolic health, indicating that it likely may not be necessary to limit dairy fat intake within an overall healthy, energy-balanced diet.
Many reviews have thoroughly examined the effects of the unique physico-chemical assembly of dairy products on digestion and absorption (4, 30, 71, 72, 167), with notable mechanisms including, but not limited to, the effect of MFG size on lipase action and intestinal absorption as well as the effect of calcium content on dietary fat absorption due to the interplay of calcium and FAs, and their ability to form calcium soaps, highlighting the importance of the other macro- and micronutrients present in the matrix of the dairy product. In fact, the presence of these other macro- and micronutrients in dairy products may contribute to the observed differences in health effects between dairy-derived SFAs and SFAs from other food sources (167–169). For example, the higher calcium content in cheese may be relevant to the potential effect of regular-fat cheese intake on blood lipid control (30). Moreover, specific dairy bioactives may elicit cardiometabolic health effects such as the MFGM components and their hypothesized effect on aspects of cardiometabolic health including, but not limited to, CHOL absorption and inflammation (4, 8, 41, 152, 170–173). Further, the small MFGs found in homogenized milk both on the market and homogenized milk used as a base product for yogurt (i.e., compared to hard cheeses that are typically made from unhomogenized milk) may impact satiety and lipid bioavailability (4, 30, 71), which may be important for the absorption of the MCFAs that have been thought to help with body-weight control (30, 170). The ability of specific dairy components to drive cardiometabolic health effects is dependent on their bioavailability, which is impacted by the food and fat matrix of each dairy product.
There are many nutritional, lifestyle, and methodological factors at play in the studies reviewed that ought to be considered for the existing body of literature and for future research in this area. For example, dietary patterns may have a modulatory effect of specific dairy products on cardiometabolic health, thus, an important consideration in this area of research is the diet in which these dairy products are eaten (174). A confounding mechanism may be the subsequent effect of either adding or removing dietary fat in general: e.g., an increase in caloric intake corresponding with the inclusion of dairy fat (110, 123) or a decrease in carbohydrate consumption that corresponds with an increase in dietary fat (160, 175). In these cases, it is difficult to distinguish whether the cardiometabolic health outcomes are due to an increase in overall fat consumption or rather a difference in total energy intake or distribution. A limitation across both the observational studies and RCTs identified is the lack of detail and standardization in dietary intake methodology (e.g., in the collection and reporting of dairy fat intake). In the observational studies reviewed, four dietary intake methods were described: (i) food recalls, including 24 h recalls (n = 4 studies), (ii) food records (n = 1 study), (iii) food frequency questionnaires (FFQs; n = 39 studies), or (iv) the Diet Behavior and Nutrition Survey from the National Health and Nutrition Examination Survey (n = 2 studies). Though most studies used an FFQ, the FFQs varied considerably in the number of total items or questions, ranging from a dairy-specific 15-question FFQ to a more generalized 261-question FFQ. Additionally, milk-fat content terminology is generally not standardized and is largely variable, mainly in milk with less than 2% fat content (Figure 3). Some authors use the terms “skim” or “semi-skimmed” milk, while others state the milk fat percentages. In contrast, other studies broadly define a “low-fat” milk category, including fat contents below a certain threshold, such as 2%. This terminology often creates a greater variance within this “low-fat” group than the variance between the “low-fat” and regular-fat groups. For example, there could be a difference of up to 2% between non-fat and 2% milk, compared to a difference of 1.25–1.5% between the “low-fat” and regular-fat groups. Differences in survey methods may impact the quality of the data being collected, as all methods have strengths and weaknesses (176). Similarly, differences in milk-fat content definitions greatly impact generalizability, as different authors may use different terms for the same milk-fat content or may refer to different milk-fat contents using the same term. Clear definition and standardization of dairy fat intake data collection and reporting would allow for comparison between studies and could yield more robust and cohesive research within the field.
Another limitation is the variability of study designs utilized. Across the observational studies, there were three types of analyses used: (i) comparisons between high and low intake of a regular-fat dairy product (e.g., quartiles of regular-milk intake), (ii) comparisons between intake of a low-fat dairy product with a regular-fat dairy product (e.g., skim milk compared to regular-fat milk), or (iii) substitution analyses in which a dairy product of a different type or fat content was substituted for another regular-fat dairy product (e.g., low-fat milk substituted for regular-fat milk). Similarly, the RCTs utilized different degrees of control, including: (i) providing only a controlled food item (i.e., a specific serving of the dairy product of interest), (ii) recommending a lower-fat meal prior to post-prandial experiments, (iii) limiting dairy consumption by amount or by dairy product, or (iv) restricting all dairy products beyond the dairy product of interest. In addition, many different control groups were used, with studies including comparisons of a high-fat dairy product to either a: (i) low-fat dairy product of the same type, (ii) low-fat dairy product of a different type, (iii) high-fat dairy product of a different type, and/or (iv) non-dairy control as well as a depletion/repletion design. The variety in study design provides many avenues to examine the effect of the dairy-fat matrix in cardiometabolic health outcomes and explores different dietary changes likely to be employed by consumers (e.g., increasing intake of a food or substituting it for another). Yet, the differences also impart barriers in comparing results across studies.
4.3.2 Strengths, weaknesses, and future directions
Our focus on research published within the last 10 years yields a timely review of the literature and provides an informative snapshot of emerging research to better understand the role of the dairy-fat matrix on cardiometabolic health. This narrative review evaluates the individual effects of cow’s milk, yogurt, cheese, and butter, but does not evaluate the effect of total dairy product consumption or combinations of dairy products (i.e., the consumption of different dairy products eaten within a meal or over the course of one day). Further, without evaluating the effect of total dairy fat consumption, this review does not allow for evaluation of recommendations for frequency or quantity of total dairy fat intake but is an important area of future research. Finally, with the focus on commonly consumed dairy products, this review does not consider the full range of dairy products available on the market (e.g., fermented milk or ice cream), but highlights many opportunities for future research.
The literature currently skews toward nonfermented dairy products (i.e., milk and butter) over fermented dairy products (i.e., yogurt and cheese), with only one recently conducted RCT including yogurt. Yogurt and other fermented dairy products are important topics of future research as recent observational studies suggest cardiometabolic benefits of regular-fat fermented dairy product intake (106, 129).
Diversity in study cohorts is crucial as there are disparities in cardiometabolic risk factors and outcomes by sex, age, education, race, and ethnicity that deserve recognition and future research funding and exploration (177). Most studies recruited a cohort of mostly equal numbers (less than 10% difference) of male and female participants. The average age of participants in the observational studies appeared to be split approximately evenly between the ranges of 30–50 years, 50–60 years, and 60–70 years old, while the average age of RCT participants was mostly under 50 years old. Notably, there were few studies that recruited children, and they all only evaluated the relationship between cardiometabolic health and regular-fat milk. Additionally, while a little over half of the observational studies recruited participants older than 70 years old, this was only the case for one out of the 13 RCTs. Less information is available for the educational status of the participants studied. This information was not reported in approximately a third of the observational studies and not reported in any of the RCTs. Still, it can be difficult to draw conclusions given the multiple ways this information was collected and reported, including as a binary, categorical, or continuous variable. Most observational studies had participants with a high educational status, as defined by each study. Similarly, many studies did not report information on ethnicity or race for their participants, but of the studies that did, the majority studied predominantly white individuals or individuals with European/Caucasian ethnicity. Most of the research was conducted in cohorts based in Europe and North America. Of note, Dehghan et al. (146) surveyed from 21 countries on five continents, presenting a more geographically diverse group of participants. Similarly, the racial and ethnic diversity of the participant cohorts in the RCTs is minimal. Most studies comprised of predominantly Caucasian individuals and were conducted in either primarily Canada or European countries. More diversity in participant cohorts in terms of age, education, race, and ethnicity is warranted to determine the effects of dairy fat from different products on cardiometabolic health in all people, not just a portion of the population.
Over the past decade, research and public efforts have accelerated rapidly to better understand the impact of different foods and dietary patterns beyond health outcomes, and to now further examine how diets can contribute to achieving food system transformation. A guiding framework for characterizing a sustainable food system comprises four interrelated domains: health, environment, economics, and society (178). Of note, this review sought to evaluate the role of dairy fat and its matrix on human health, and is therefore centralized within one out of the four pillars (i.e., health) of a sustainable food system; yet, all foods, including dairy foods, have different and complementary contributions to the four domains of sustainable food systems that deserve more research attention. For example, dairy farming contributes to greenhouse emissions through resources and energy to create food and ruminant livestock emission of methane gases; based on a 2008 life cycle assessment, dairy’s greenhouse gas footprint is an estimated 2% of the U.S. total (179). At the same time, dairy foods contribute essential nutrients to meet recommendations by the Food and Agriculture Organization of the United Nations, particularly for vulnerable populations including pregnant and lactating people, children and adolescence, and aging adults (180). Overall, much more research is warranted to better understand how dietary patterns that include plant and animal source foods, such as dairy foods, contribute to nutritionally adequate diets that promote human health as well as overall planetary health (181).
5 Conclusion
Our goal was to characterize the dairy-fat matrix across milk, yogurt, cheese, and butter, then examine their individual effects on cardiometabolic health to provide a critical assessment of the current body of science on the cardiometabolic health effects of dairy fat, evaluating gaps and implications in dietary guidance. Dairy fat is comprised of three increasingly complex levels of organization, starting with the FA and building to the MFG, which gives rise to unique physical and chemical properties. Further, these properties are altered by the processing methods used to create the dairy products commonly available. We aimed to evaluate if these alterations would, by way of influences on the bioavailability, digestion, absorption, and/or metabolism of dairy fat, result in unique health effects of the individual dairy-fat matrices on cardiometabolic health. Many authors have addressed this complex question using a variety of methodologies, and though multifaceted approaches are appropriate for a multifaceted question, the lack of comparable methods creates barriers in drawing clear conclusions on whether and to what degree the dairy-fat matrix of different dairy products may impact cardiometabolic health. Overall, we found that the evidence largely suggests no effect of consuming higher-fat varieties of dairy products on cardiometabolic health, with minor differences between individual dairy products, when stratified by both dairy product and fat content. More broadly, the current body of evidence suggests that regular fat dairy products may be a part of overall healthy eating patterns, but the variability in results suggest ample opportunity to bolster the current evidence base. Our recommendations for future research include replicable study designs that incorporate more variety in dairy product types and greater age, educational, socioeconomic, and racial/ethnic diversity within participant cohorts to create a cohesive literature base for dietary recommendations. Moving forward, conducting research with an understanding of the physical and chemical influences imparted by the individual dairy-fat matrices in dairy products will allow for more robust and comprehensive knowledge on the effect of the consumption of each of these unique, regular-fat dairy products on cardiometabolic health and their role in a healthy diet.
Author contributions
VT: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. AU: Conceptualization, Methodology, Writing – review & editing. JK: Funding acquisition, Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Support for this work was provided by the University of Vermont Food Systems Research Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
VMT has no conflicts of interest to declare. ALU was an employee of the National Dairy Council (NDC) at the time that this manuscript was written. JK has received honoraria and reimbursements for travel as well as research grants from the Vermont Dairy Promotion Council and the NDC/Dairy Management Inc.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1386257/full#supplementary-material
References
1. Comerford, KB, Miller, GD, Boileau, AC, Masiello Schuette, SN, Giddens, JC, and Brown, KA. Global review of dairy recommendations in food-based dietary guidelines. Front Nutr. (2021) 8:671999. doi: 10.3389/fnut.2021.671999
2. Astrup, A, Bertram, HCS, Bonjour, JP, De Groot, LCP, De Oliveira Otto, MC, Feeney, EL, et al. WHO draft guidelines on dietary saturated and trans fatty acids: time for a new approach? BMJ. (2019) 366:l4137. doi: 10.1136/bmj.l4137
3. Unger, AL, Torres-Gonzalez, M, and Kraft, J. Dairy fat consumption and the risk of metabolic syndrome: an examination of the saturated fatty acids in dairy. Nutrients. (2019) 11:2200. doi: 10.3390/nu11092200
4. Thorning, TK, Bertram, HC, Bonjour, J-P, de Groot, L, Dupont, D, Feeney, E, et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. (2017) 105:1033–45. doi: 10.3945/ajcn.116.151548
5. Fardet, A, and Rock, E. Toward a new philosophy of preventive nutrition: from a reductionist to a holistic paradigm to improve nutritional recommendations. Adv Nutr. (2014) 5:430–46. doi: 10.3945/an.114.006122
6. Mozaffarian, D . Dairy foods, obesity, and metabolic health: the role of the food matrix compared with single nutrients. Adv Nutr. (2019) 10:917S–23S. doi: 10.1093/advances/nmz053
7. U.S. Department of Agriculture . Food matrix|NAL Agricultural Thesaurus. (2023), Available at: https://agclass.nal.usda.gov/vocabularies/nalt-core/concept?uri=https%3A//lod.nal.usda.gov/nalt/17238 (Accessed June 26, 2023)
8. Weaver, CM . Dairy matrix: is the whole greater than the sum of the parts? Nutr Rev. (2021) 79:4–15. doi: 10.1093/nutrit/nuab081
9. Turgeon, SL, and Brisson, G. Symposium review: the dairy matrix—bioaccessibility and bioavailability of nutrients and physiological effects. J Dairy Sci. (2020) 103:6727–36. doi: 10.3168/jds.2019-17308
10. Gunstone, FD, and Harwood, JL, (Eds.), Occurence and characterisation of oils and fats, The lipid handbook. Boca-Raton: CRC Press (2007). 37–141
11. Palmquist, DL . Milk fat: origin of fatty acids and influence of nutritional factors thereon, P.F. Fox and P.L.H. McSweeney, (Ed.), Advanced dairy chemistry. New York: Springer (2013). 43–92
12. Jensen, RG . The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci. (2002) 85:295–350. doi: 10.3168/jds.S0022-0302(02)74079-4
13. Månsson, HL . Fatty acids in bovine milk fat. Food Nutr Res. (2008) 52:1821. doi: 10.3402/fnr.v52i0.1821
15. Brzozowska, AM, and Oprządek, J. Metabolism of fatty acids in tissues and organs of the ruminants-a review. Anim Sci Pap Reports. (2016) 34:211–20.
16. Vlaeminck, B, Fievez, V, Cabrita, ARJ, Fonseca, AJM, and Dewhurst, RJ. Factors affecting odd- and branched-chain fatty acids in milk: a review. Anim Feed Sci Technol. (2006) 131:389–417. doi: 10.1016/j.anifeedsci.2006.06.017
17. Weber, C, Hametner, C, Tuchscherer, A, Losand, B, Kanitz, E, Otten, W, et al. Variation in fat mobilization during early lactation differently affects feed intake, body condition, and lipid and glucose metabolism in high-yielding dairy cows. J Dairy Sci. (2013) 96:165–80. doi: 10.3168/jds.2012-5574
18. Hernández-Ortega, M, Martínez-Fernández, A, Soldado, A, González, A, Arriaga-Jordán, CM, Argamentería, A, et al. Effect of total mixed ration composition and daily grazing pattern on milk production, composition and fatty acids profile of dairy cows. J Dairy Res. (2014) 81:471–8. doi: 10.1017/s0022029914000399
19. Shingfield, KJ, and Wallace, RJ. Synthesis of conjugated linoleic acid in ruminants and humans, Conjugated linoleic acids and conjugated vegetable oils. The Royal Society of Chemistry (2014). 1–65
20. Bauman, DE, and Lock, AL. Conjugated linoleic acid: biosynthesis and nutritional significance, PLH McSweeney and PF Fox, (Eds.). Advanced dairy chemistry. New York: Springer (2013). 93–136
21. Taormina, VM, Unger, AL, Schiksnis, MR, Torres-Gonzalez, M, and Kraft, J. Branched-chain fatty acids—an underexplored class of dairy-derived fatty acids. Nutrients. (2020) 12:2875. doi: 10.3390/NU12092875
22. Jenkins, B, West, JA, and Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) in health and disease. Molecules. (2015) 20:2425–44. doi: 10.3390/molecules20022425
23. Vlaeminck, B, Dufour, C, Van Vuuren, AM, Cabrita, ARJ, Dewhurst, RJ, Demeyer, D, et al. Use of odd and branched-chain fatty acids in rumen contents and milk as a potential microbial marker. J Dairy Sci. (2005) 88:1031–42. doi: 10.3168/jds.s0022-0302(05)72771-5
24. Vlaeminck, B, Fievez, V, Demeyer, D, and Dewhurst, RJ. Effect of forage: concentrate ratio on fatty acid composition of rumen bacteria isolated from ruminal and duodenal digesta. J Dairy Sci. (2006) 89:2668–78. doi: 10.3168/jds.s0022-0302(06)72343-8
25. Keeney, M, Katz, I, and Allison, MJ. On the possible origin of some milk fatty acids in the rumen microbial lipids. J Am Oil Chem Soc. (1962) 39:198–201. doi: 10.1007/bf02635818
26. MacGibbon, A, and Taylor, M. Composition and structure of bovine milk lipids In: P Fox and PLH McSweeney, editors. Advanced dairy chemistry. New York: Springer (2013). 1–42.
27. Bauman, DE, Mather, IH, Wall, RJ, and Lock, AL. Major advances associated with the biosynthesis of milk. J Dairy Sci. (2006) 89:1235–43. doi: 10.3168/jds.s0022-0302(06)72192-0
28. Monks, J, Ladinsky, MS, and McManaman, JL. Organellar contacts of milk lipid droplets. Contact. (2020) 3:1–10. doi: 10.1177/2515256419897226
29. Ontsouka, EC, and Albrecht, C. Cholesterol transport and regulation in the mammary gland. J Mammary Gland Biol Neoplasia. (2014) 19:43–58. doi: 10.1007/s10911-014-9316-x
30. Michalski, MC . Specific molecular and colloidal structures of milk fat affecting lipolysis, absorption and postprandial lipemia. Eur J Lipid Sci Technol. (2009) 111:413–31. doi: 10.1002/ejlt.200800254
31. McManaman, JL . Lipid transport in the lactating mammary gland. J Mammary Gland Biol Neoplasia. (2014) 19:35–42. doi: 10.1007/s10911-014-9318-8
32. Dewettinck, K, Rombaut, R, Thienpont, N, Le, TT, Messens, K, and Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int Dairy J. (2008) 18:436–57. doi: 10.1016/j.idairyj.2007.10.014
33. Arranz, E, and Corredig, M. Invited review: milk phospholipid vesicles, their colloidal properties, and potential as delivery vehicles for bioactive molecules. J Dairy Sci. (2017) 100:4213–22. doi: 10.3168/jds.2016-12236
34. Singh, H . Symposium review: fat globules in milk and their structural modifications during gastrointestinal digestion. J Dairy Sci. (2019) 102:2749–59. doi: 10.3168/jds.2018-15507
35. Wang, C, Qiao, X, Gao, Z, Jiang, L, and Mu, Z. Advancement on milk fat globule membrane: separation, identification, and functional properties. Front Nutr. (2022) 8:807284. doi: 10.3389/fnut.2021.807284
36. Lopez, C, Cauty, C, and Guyomarc’h, F. Organization of lipids in milks, infant milk formulas and various dairy products: role of technological processes and potential impacts. Dairy Sci Technol. (2015) 95:863–93. doi: 10.1007/s13594-015-0263-0
37. Cavaletto, M, Guiffrida, M, and Conti, A. Milk fat globule membrane components—a proteomic approach In: Z Bösze , editor. Bioactive components of milk. Advances in experimental medicine and biology. New York, NY: Springer New York (2010). 129–42.
38. Contarini, G, and Povolo, M. Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. (2013) 14:2808–31. doi: 10.3390/ijms14022808
39. Verardo, V, Gómez-Caravaca, AM, Arráez-Román, D, and Hettinga, K. Recent advances in phospholipids from colostrum, milk and dairy by-products. Int J Mol Sci. (2017) 18:173. doi: 10.3390/ijms18010173
40. Wiking, L, Gregersen, SB, Hansen, SF, and Hammershøj, M. Heat-induced changes in milk fat and milk fat globules and its derived effects on acid dairy gelation—a review. Int Dairy J. (2022) 127:105213. doi: 10.1016/J.IDAIRYJ.2021.105213
41. Lopez, C . Milk fat globules enveloped by their biological membrane: unique colloidal assemblies with a specific composition and structure. Curr Opin Colloid Interface Sci. (2011) 16:391–404. doi: 10.1016/j.cocis.2011.05.007
42. Bourlieu, C, and Michalski, MC. Structure-function relationship of the milk fat globule. Curr Opin Clin Nutr Metab Care. (2015) 18:118–27. doi: 10.1097/MCO.0000000000000138
43. Torres-Gonzalez, M, and Rice Bradley, BH. Whole-Milk dairy foods: biological mechanisms underlying beneficial effects on risk markers for cardiometabolic health. Adv Nutr. (2023) 14:1523. doi: 10.1016/J.ADVNUT.2023.09.001
44. Oliveira, D, and O’Mahony, J. Composition, fractionation, techno-functional properties and applications of milk fat globule membrane-derived material, PLH McSweeney, PF Fox, and JA O’Mahony, (Eds.). Advanced dairy chemistry, Springer (2020). 169–195
45. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Milk, whole (Survey (FNDDS), 2340762). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2340762/nutrients
46. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Yogurt, whole milk, plain (Survey (FNDDS), 2340799). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2340799/nutrients
47. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Colby (Survey (FNDDS), 2341115). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341115/nutrients (Accessed January 4, 2024)
48. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Camembert (Survey (FNDDS), 2341110). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341110/nutrients (Accessed January 4, 2024)
49. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Swiss (Survey (FNDDS), 2341138), (2022). Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341138/nutrients (Accessed January 4, 2024)
50. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Provolone (Survey (FNDDS), 2341136). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341136/nutrients (Accessed January 4, 2024)
51. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Muenster (Survey (FNDDS), 2341129). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341129/nutrients (Accessed January 4, 2024)
52. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Limburger (Survey (FNDDS), 2341122). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341122/nutrients (Accessed January 4, 2024)
53. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Gruyere (Survey (FNDDS), 2341121). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341121/nutrients (Accessed January 4, 2024)
54. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Fontina (Survey (FNDDS), 2341118). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341118/nutrients (Accessed January 4, 2024)
55. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Queso Anejo, Aged Mexican Cheese (Survey (FNDDS), 2341146). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341146/nutrients (Accessed January 4, 2024)
56. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Port du Salut (Survey (FNDDS), 2341135). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341135/nutrients (Accessed January 4, 2024)
57. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Queso Cotija (Survey (FNDDS), 2341149). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341149/nutrients (Accessed January 4, 2024)
58. U.S. Department of Agriculture Agricultural Research Service. FoodData Central: Queso Fresco (Survey (FNDDS), 2341148). (2022) https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341148/nutrients (Accessed January 4, 2024)
59. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Brie (Survey (FNDDS), 2341111). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341111/nutrients (Accessed January 4, 2024)
60. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Paneer (Survey (FNDDS), 2341143). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341143/nutrients (Accessed January 4, 2024)
61. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Queso Asadero (Survey (FNDDS), 2341147). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341147/nutrients (Accessed January 4, 2024)
62. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Cheddar (Survey (FNDDS), 2341112). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341112/nutrients (Accessed January 4, 2024)
63. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Monterey (Survey (FNDDS), 2341123). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341123/nutrients (Accessed January 4, 2024)
64. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Parmesan, hard (Survey (FNDDS), 2341133). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341133/nutrients (Accessed January 4, 2024)
65. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Gouda or Edam (Survey (FNDDS), 2341120). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341120/nutrients (Accessed January 4, 2024)
66. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Colby Jack (Survey (FNDDS), 2341116). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341116/nutrients (Accessed January 4, 2024)
67. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Blue or Roquefort (Survey (FNDDS), 2341108). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341108/nutrients (Accessed January 4, 2024)
68. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Cheese, Mozzarella, NFS (Survey (FNDDS), 2341125). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2341125/nutrients (Accessed January 4, 2024)
69. U.S. Department of Agriculture Agricultural Research Service . FoodData Central: Butter, Stick (Survey (FNDDS), 2345704). (2022), Available at: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2345704/nutrients (Accessed December 4, 2023)
70. Fox, PF, Guinee, TP, Cogan, TM, and McSweeney, PL. Fundamentals of cheese science. Gaithersburg, Maryland: Aspen Publishers Inc. (2000).
71. Mulet-Cabero, AI, Mackie, AR, Brodkorb, A, and Wilde, PJ. Dairy structures and physiological responses: a matter of gastric digestion. Crit Rev Food Sci Nutr. (2020) 60:3737–52. doi: 10.1080/10408398.2019.1707159
72. Michalski, MC, Genot, C, Gayet, C, Lopez, C, Fine, F, Joffre, F, et al. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog Lipid Res. (2013) 52:354–73. doi: 10.1016/j.plipres.2013.04.004
73. Ong, L, Dagastine, RR, Kentish, SE, and Gras, SL. The effect of milk processing on the microstructure of the milk fat globule and rennet induced gel observed using confocal laser scanning microscopy. J Food Sci. (2010) 75:E135–45. doi: 10.1111/j.1750-3841.2010.01517.x
74. Lee, SJ, and Sherbon, JW. Chemical changes in bovine milk fat globule membrane caused by heat treatment and homogenization of whole milk. J Dairy Res. (2002) 69:555–67. doi: 10.1017/s002202990200571x
75. Hayes, MG, and Kelly, AL. High pressure homogenisation of raw whole bovine milk (a) effects on fat globule size and other properties. J Dairy Res. (2003) 70:297–305. doi: 10.1017/s0022029903006320
76. Lopez, C . Focus on the supramolecular structure of milk fat in dairy products. Reprod Nutr Dev. (2005) 45:497–511. doi: 10.1051/rnd:2005034
77. Zamora, A, Ferragut, V, Jaramillo, PD, Guamis, B, and Trujillo, AJ. Effects of ultra-high pressure homogenization on the cheese-making properties of milk. J Dairy Sci. (2007) 90:13–23. doi: 10.3168/jds.s0022-0302(07)72604-8
78. Lee, W, and Lucey, J. Formation and physical properties of yogurt. Asian-Australasian J Anim Sci. (2010) 23:1127–36. doi: 10.5713/ajas.2010.r.05
79. Obeid, S, Guyomarc’h, F, Francius, G, Guillemin, H, Wu, X, Pezennec, S, et al. The surface properties of milk fat globules govern their interactions with the caseins: role of homogenization and pH probed by AFM force spectroscopy. Colloids Surf B Biointerfaces. (2019) 182:110363. doi: 10.1016/j.colsurfb.2019.110363
80. Ma, Y, Zhang, L, Wu, Y, and Zhou, P. Changes in milk fat globule membrane proteome after pasteurization in human, bovine and caprine species. Food Chem. (2019) 279:209–15. doi: 10.1016/j.foodchem.2018.12.015
81. Ichimura, T, Osada, T, Yonekura, K, and Horiuchi, H. A new method for producing superior set yogurt, focusing on heat treatment and homogenization. J Dairy Sci. (2022) 105:2978–87. doi: 10.3168/jds.2021-21326
82. Zhou, X, Hadiatullah, H, Guo, T, Yao, Y, Li, C, and Wang, X. Dairy processing affects the gut digestion and microecology by changing the structure and composition of milk fat globules. J Agric Food Chem. (2021) 69:10194–205. doi: 10.1021/acs.jafc.1c04482
83. Ren, Q, Li, L, Dudu, O, and Ma, Y. Thermal and structural changes of pasteurized milk fat globules during storage. Food Biosci. (2019) 28:27–35. doi: 10.1016/j.fbio.2018.12.002
84. Lopez, C, and Briard-Bion, V. The composition, supramolecular organisation and thermal properties of milk fat: a new challenge for the quality of food products. Lait. (2007) 87:317–36. doi: 10.1051/lait:2007015
85. Gilbert, A, and Turgeon, SL. Studying stirred yogurt microstructure and its correlation to physical properties: a review. Food Hydrocoll. (2021) 121:106970. doi: 10.1016/j.foodhyd.2021.106970
86. Guinee, TP, and McSweeney, PLH. Significance of milk fat in cheese In: PLH McSweeney and PF Fox, editors. Advanced dairy chemistry. New York: Springer (2013). 377–440.
87. Huppertz, T, Uniacke-Lowe, T, and Kelly, AL. Physical chemistry of milk fat globules In: PLH McSweeney and PF Fox, editors. Advanced dairy chemistry. New York: Springer (2013). 173–212.
88. Truong, T, and Bhandari, B. Role of differentiated-size milk fat globules on the physical functionality of dairy-fat structured products In: T Truong, C Lopez, B Bhandari, and S Prakash, editors. Dairy fat products and functionality. Cham: Springer (2021). 327–54.
89. Michalski, MC, Cariou, R, Michel, F, and Garnier, C. Native vs. damaged milk fat globules: membrane properties affect the viscoelasticity of milk gels. J Dairy Sci. (2002) 85:2451–61. doi: 10.3168/jds.S0022-0302(02)74327-0
90. Hickey, CD, Auty, MAE, Wilkinson, MG, and Sheehan, JJ. The influence of cheese manufacture parameters on cheese microstructure, microbial localisation and their interactions during ripening: a review. Trends Food Sci Technol. (2015) 41:135–48. doi: 10.1016/j.tifs.2014.10.006
91. Morell, P, Hernando, I, Llorca, E, and Fiszman, S. Yogurts with an increased protein content and physically modified starch: rheological, structural, oral digestion and sensory properties related to enhanced satiating capacity. Food Res Int. (2015) 70:64–73. doi: 10.1016/j.foodres.2015.01.024
92. Ge, Z, Yin, D, Li, Z, Chen, X, and Dong, M. Effects of commercial polysaccharides stabilizers with different charges on textural, rheological, and microstructural characteristics of set yoghurts. Food Secur. (2022) 11:1764. doi: 10.3390/foods11121764
93. Bankole, AO, Irondi, EA, Awoyale, W, and Ajani, EO. Application of natural and modified additives in yogurt formulation: types, production, and rheological and nutraceutical benefits. Front Nutr. (2023) 10:1257439. doi: 10.3389/fnut.2023.1257439
94. Rønholt, S, Mortensen, K, and Knudsen, JC. The effective factors on the structure of butter and other milk fat-based products. Compr Rev Food Sci Food Saf. (2013) 12:468–82. doi: 10.1111/1541-4337.12022
95. Lecerf, J-M . Fatty acids and cardiovascular disease. Nutr Rev. (2009) 67:273–83. doi: 10.1111/j.1753-4887.2009.00194.x
96. Nestel, PJ . Effects of dairy fats within different foods on plasma lipids. J Am Coll Nutr. (2008) 27:735S–40S. doi: 10.1080/07315724.2008.10719751
97. German, JB, Gibson, RA, Krauss, RM, Nestel, P, Lamarche, B, Van Staveren, WA, et al. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur J Nutr. (2009) 48:191. doi: 10.1007/S00394-009-0002-5
98. Livingstone, KM, Lovegrove, JA, and Givens, DI. The impact of substituting SFA in dairy products with MUFA or PUFA on CVD risk: evidence from human intervention studies. Nutr Res Rev. (2012) 25:193–206. doi: 10.1017/s095442241200011x
99. Huth, PJ, and Park, KM. Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr. (2012) 3:266–85. doi: 10.3945/an.112.002030
100. Aune, D, Norat, T, Romundstad, P, and Vatten, LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. (2013) 98:1066–83. doi: 10.3945/ajcn.113.059030
101. Van Meijl, LEC, Vrolix, R, and Mensink, RP. Dairy product consumption and the metabolic syndrome. Nutr Res Rev. (2008) 21:148–57. doi: 10.1017/S0954422408116997
102. Lamarche, B . Review of the effect of dairy products on non-lipid risk factors for cardiovascular disease. J Am Coll Nutr. (2008) 27:741S. doi: 10.1080/07315724.2008.10719752
103. Soedamah-Muthu, SS, Verberne, LDM, Ding, EL, Engberink, MF, and Geleijnse, JM. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. (2012) 60:1131–7. doi: 10.1161/hypertensionaha.112.195206
104. Slurink, IAL, Chen, L, Magliano, DJ, Kupper, N, Smeets, T, and Soedamah-Muthu, SS. Dairy product consumption and incident prediabetes in the Australian diabetes, obesity, and lifestyle study with 12 years of follow-up. J Nutr. (2023) 153:1742–52. doi: 10.1016/j.tjnut.2023.03.032
105. McGovern, C, Rifas-Shiman, SL, Switkowski, KM, Woo Baidal, JA, Lightdale, JR, Hivert, MF, et al. Association of cow’s milk intake in early childhood with adiposity and cardiometabolic risk in early adolescence. Am J Clin Nutr. (2022) 116:561–71. doi: 10.1093/ajcn/nqac103
106. Slurink, IAL, den Braver, NR, Rutters, F, Kupper, N, Smeets, T, Elders, PJM, et al. Dairy product consumption and incident prediabetes in Dutch middle-aged adults: the Hoorn studies prospective cohort. Eur J Nutr. (2022) 61:183. doi: 10.1007/s00394-021-02626-9
107. Wang, S, Liu, Y, Cai, H, Li, Y, Zhang, X, Liu, J, et al. Decreased risk of all-cause and heart-specific mortality is associated with low-fat or skimmed milk consumption compared with whole milk intake: a cohort study. Clin Nutr. (2021) 40:5568–75. doi: 10.1016/j.clnu.2021.09.012
108. Ibsen, DB, Overvad, K, Laursen, ASD, Halkjær, J, Tjønneland, A, Parner, ET, et al. Changes in intake of dairy product subgroups and risk of type 2 diabetes: modelling specified food substitutions in the Danish diet, cancer and health cohort. Eur J Nutr. (2021) 60:3449–59. doi: 10.1007/s00394-021-02524-0
109. Cruijsen, E, Jacobo Cejudo, MG, Küpers, LK, Busstra, MC, and Geleijnse, JM. Dairy consumption and mortality after myocardial infarction: a prospective analysis in the alpha omega cohort. Am J Clin Nutr. (2021) 114:59–69. doi: 10.1093/ajcn/nqab026
110. Wilkinson, K, Tucker, L, Davidson, L, and Bailey, B. Milk-fat intake and differences in abdominal adiposity and BMI: evidence based on 13,544 randomly-selected adults. Nutrients. (2021) 13:1832. doi: 10.3390/nu13061832
111. Kvist, K, Laursen, ASD, Overvad, K, and Jakobsen, MU. Substitution of milk with whole-fat yogurt products or cheese is associated with a lower risk of myocardial infarction: the Danish diet, cancer and health cohort. J Nutr. (2020) 150:1252–8. doi: 10.1093/jn/nxz337
112. White, MJ, Armstrong, SC, Kay, MC, Perrin, EM, and Skinner, A. Associations between milk fat content and obesity, 1999 to 2016. Pediatr Obes. (2020) 15:e12612. doi: 10.1111/ijpo.12612
113. Lahoz-García, N, Milla-Tobarra, M, García-Hermoso, A, Hernández-Luengo, M, Pozuelo-Carrascosa, DP, and Martínez-Vizcaíno, V. Associations between dairy intake, body composition, and cardiometabolic risk factors in Spanish schoolchildren: the Cuenca study. Nutrients. (2019) 11:2940. doi: 10.3390/nu11122940
114. Ding, M, Li, J, Qi, L, Ellervik, C, Zhang, X, Manson, JE, et al. Associations of dairy intake with risk of mortality in women and men: three prospective cohort studies. BMJ. (2019) 367:6204. doi: 10.1136/bmj.l6204
115. Drouin-Chartier, JP, Li, Y, Ardisson Korat, AV, Ding, M, Lamarche, B, Manson, JE, et al. Changes in dairy product consumption and risk of type 2 diabetes: results from 3 large prospective cohorts of US men and women. Am J Clin Nutr. (2019) 110:1201–12. doi: 10.1093/ajcn/nqz180
116. Wong, VCH, Maguire, JL, Omand, JA, Dai, DWH, Lebovic, G, Parkin, PC, et al. A positive association between dietary intake of higher cow’s milk-fat percentage and non-high-density lipoprotein cholesterol in young children. J Pediatr. (2019) 211:105–111.e2. doi: 10.1016/j.jpeds.2019.03.047
117. Kummer, K, Jensen, PN, Kratz, M, Lemaitre, RN, Howard, BV, Cole, SA, et al. Full-fat dairy food intake is associated with a lower risk of incident diabetes among American Indians with low total dairy food intake. J Nutr. (2019) 149:1238–44. doi: 10.1093/jn/nxz058
118. Laursen, A, Sluijs, I, Boer, J, Verschuren, W, van der Schouw, Y, and Jakobsen, M. Substitutions between dairy products and risk of stroke: results from the European investigation into cancer and nutrition-Netherlands (EPIC-NL) cohort. Br J Nutr. (2019) 121:1398–404. doi: 10.1017/s0007114519000564
119. Johansson, I, Esberg, A, Nilsson, LM, Jansson, JH, Wennberg, P, and Winkvist, A. Dairy product intake and cardiometabolic diseases in northern Sweden: a 33-year prospective cohort study. Nutrients. (2019) 11:284. doi: 10.3390/nu11020284
120. Koskinen, TT, Virtanen, HEK, Voutilainen, S, Tuomainen, TP, Mursu, J, and Virtanen, JK. Intake of fermented and non-fermented dairy products and risk of incident CHD: the Kuopio Ischaemic heart disease risk factor study. Br J Nutr. (2018) 120:1288–97. doi: 10.1017/s0007114518002830
121. Johansson, I, Nilsson, LM, Esberg, A, Jansson, JH, and Winkvist, A. Dairy intake revisited—associations between dairy intake and lifestyle related cardio-metabolic risk factors in a high milk consuming population. Nutr J. (2018) 17:110. doi: 10.1186/s12937-018-0418-y
122. Sun, Y, Magnussen, CG, Dwyer, T, Oddy, WH, Venn, AJ, and Smith, KJ. Cross-sectional associations between dietary fat-related behaviors and continuous metabolic syndrome score among young Australian adults. Nutrients. (2018) 10:972. doi: 10.3390/nu10080972
123. Brouwer-Brolsma, E, Sluik, D, Singh-Povel, C, and Feskens, E. Dairy product consumption is associated with pre-diabetes and newly diagnosed type 2 diabetes in the lifelines cohort study. Br J Nutr. (2018) 119:442–55. doi: 10.1017/S0007114517003762
124. Laursen, ASD, Dahm, CC, Johnsen, SP, Tjønneland, A, Overvad, K, and Jakobsen, MU. Substitutions of dairy product intake and risk of stroke: a Danish cohort study. Eur J Epidemiol. (2018) 33:201–12. doi: 10.1007/s10654-017-0271-x
125. Um, CY, Judd, SE, Flanders, WD, Fedirko, V, and Bostick, RM. Associations of calcium and dairy products with all-cause and cause-specific mortality in the REasons for geographic and racial differences in stroke (REGARDS) prospective cohort study. Nutr Cancer. (2017) 69:1185–95. doi: 10.1080/01635581.2017.1367946
126. Hruby, A, Ma, J, Rogers, G, Meigs, J, and Jacques, P. Associations of dairy intake with incident prediabetes or diabetes in middle-aged adults vary by both dairy type and glycemic status. J Nutr. (2017) 147:1764–75. doi: 10.3945/jn.117.253401
127. Guasch-Ferré, M, Becerra-Tomás, N, Ruiz-Canela, M, Corella, D, Schröder, H, Estruch, R, et al. Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. (2017) 105:723–35. doi: 10.3945/ajcn.116.142034
128. Díaz-López, A, Bulló, M, Martínez-González, M, Corella, D, Estruch, R, Fitó, M, et al. Dairy product consumption and risk of type 2 diabetes in an elderly Spanish Mediterranean population at high cardiovascular risk. Eur J Nutr. (2016) 55:349–60. doi: 10.1007/s00394-015-0855-8
129. Ericson, U, Hellstrand, S, Brunkwall, L, Schulz, C, Sonestedt, E, Wallström, P, et al. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr. (2015) 101:1065–80. doi: 10.3945/ajcn.114.103010
130. Crichton, GE, and Alkerwi, A. Dairy food intake is positively associated with cardiovascular health: findings from observation of cardiovascular risk factors in Luxembourg study. Nutr Res. (2014) 34:1036–44. doi: 10.1016/j.nutres.2014.04.002
131. Crichton, GE, and Alkerwi, A. Whole-fat dairy food intake is inversely associated with obesity prevalence: findings from the observation of cardiovascular risk factors in Luxembourg study. Nutr Res. (2014) 34:936–43. doi: 10.1016/j.nutres.2014.07.014
132. Avalos, EE, Barrett-Connor, E, Kritz-Silverstein, D, Wingard, DL, Bergstrom, JN, and Al-Delaimy, WK. Is dairy product consumption associated with the incidence of CHD? Public Health Nutr. (2013) 16:2055–63. doi: 10.1017/s1368980012004168
133. Holmberg, S, and Thelin, A. High dairy fat intake related to less central obesity: a male cohort study with 12 years’ follow-up. Scand J Prim Health Care. (2013) 31:89–94. doi: 10.3109/02813432.2012.757070
134. Scharf, RJ, Demmer, RT, and DeBoer, MD. Longitudinal evaluation of milk type consumed and weight status in preschoolers. Arch Dis Child. (2013) 98:335. doi: 10.1136/archdischild-2012-302941
135. Patterson, E, Larsson, SC, Wolk, A, and Akesson, A. Association between dairy food consumption and risk of myocardial infarction in women differs by type of dairy food. J Nutr. (2013) 143:74–9. doi: 10.3945/jn.112.166330
136. Wijndaele, K, Beunen, G, Duvigneaud, N, Matton, L, Duquet, W, Thomis, M, et al. A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care. (2006) 29:2329. doi: 10.2337/dc06-1341
137. Machlik, ML, Hopstock, LA, Wilsgaard, T, and Hansson, P. Associations between intake of fermented dairy products and blood lipid concentrations are affected by fat content and dairy matrix—the Tromsø study: Tromsø7. Front Nutr. (2021) 8:773468. doi: 10.3389/fnut.2021.773468
138. Trichia, E, Luben, R, Khaw, KT, Wareham, NJ, Imamura, F, and Forouhi, NG. The associations of longitudinal changes in consumption of total and types of dairy products and markers of metabolic risk and adiposity: findings from the European investigation into Cancer and nutrition (EPIC)–Norfolk study, United Kingdom. Am J Clin Nutr. (2020) 111:1018–26. doi: 10.1093/ajcn/nqz335
139. Santiago, S-OC, Babio, N, Ruiz-Canela, M, Martí, A, Corella, D, Estruch, R, et al. Yogurt consumption and abdominal obesity reversion in the PREDIMED study. Nutr Metab Cardiovasc Dis. (2016) 26:468–75. doi: 10.1016/j.numecd.2015.11.012
140. Sayón-Orea, C, Bes-Rastrollo, M, Martí, A, Pimenta, AM, Martín-Calvo, N, and Martínez-González, MA. Association between yogurt consumption and the risk of metabolic syndrome over 6 years in the SUN study. BMC Public Health. (2015) 15:170. doi: 10.1186/s12889-015-1518-7
141. Shi, N, Olivo-Marston, S, Jin, Q, Aroke, D, Joseph, JJ, Clinton, SK, et al. Associations of dairy intake with circulating biomarkers of inflammation, insulin response and dyslipidemia among postmenopausal women. J Acad Nutr Diet. (2021) 121:1984–2002. doi: 10.1016/j.jand.2021.02.029
142. Karatzi, K, Aissopou, E, Tsirimiagou, C, Fatmeli, E, Sfikakis, P, and Protogerou, A. Association of consumption of dairy products and meat with retinal vessel calibers in subjects at increased cardiovascular risk. Nutr Metab Cardiovasc Dis. (2016) 26:752–7. doi: 10.1016/j.numecd.2016.03.006
143. Brouwer-Brolsma, EM, van Woudenbergh, GJ, Oude Elferink, SJWH, Singh-Povel, CM, Hofman, A, Dehghan, A, et al. Intake of different types of dairy and its prospective association with risk of type 2 diabetes: the Rotterdam study. Nutr Metab Cardiovasc Dis. (2016) 26:987–95. doi: 10.1016/j.numecd.2016.08.003
144. Van Parys, A, Sæle, J, Puaschitz, NG, Anfinsen, ÅM, Karlsson, T, Olsen, T, et al. The association between dairy intake and risk of cardiovascular disease and mortality in patients with stable angina pectoris. Eur J Prev Cardiol. (2023) 30:219–29. doi: 10.1093/eurjpc/zwac217
145. Zhang, Y, Zhuang, P, Wu, F, He, W, Mao, L, Jia, W, et al. Cooking oil/fat consumption and deaths from cardiometabolic diseases and other causes: prospective analysis of 521,120 individuals. BMC Med. (2021) 19:92. doi: 10.1186/s12916-021-01961-2
146. Dehghan, M, Mente, A, Rangarajan, S, Sheridan, P, Mohan, V, Iqbal, R, et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet. (2018) 392:2288–97. doi: 10.1016/s0140-6736(18)31812-9
147. Drehmer, M, Pereira, M, Schmidt, M, Alvim, S, Lotufo, P, Luft, V, et al. Total and full-fat, but not low-fat, dairy product intakes are inversely associated with metabolic syndrome in adults. J Nutr. (2016) 146:81–9. doi: 10.3945/jn.115.220699
148. Hosseinpour-Niazi, S, Mirmiran, P, Hosseini-Esfahani, F, and Azizi, F. Is the metabolic syndrome inversely associates with butter, non-hydrogenated- and hydrogenated-vegetable oils consumption: Tehran lipid and glucose study. Diabetes Res Clin Pract. (2016) 112:20–9. doi: 10.1016/j.diabres.2015.11.008
149. Drehmer, M, Pereira, MA, Schmidt, MI, Molina, MDCB, Alvim, S, Lotufo, PA, et al. Associations of dairy intake with glycemia and insulinemia, independent of obesity, in Brazilian adults: the Brazilian longitudinal study of adult health (ELSA-Brasil). Am J Clin Nutr. (2015) 101:775–82. doi: 10.3945/ajcn.114.102152
150. Buijsse, B, Boeing, H, Drogan, D, Schulze, MB, Feskens, EJ, Amiano, P, et al. Consumption of fatty foods and incident type 2 diabetes in populations from eight European countries. Eur J Clin Nutr. (2015) 69:455–61. doi: 10.1038/ejcn.2014.249
151. Alberti, KGMM, Eckel, RH, Grundy, SM, Zimmet, PZ, Cleeman, JI, Donato, KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
152. Engel, S, Elhauge, M, and Tholstrup, T. Effect of whole milk compared with skimmed milk on fasting blood lipids in healthy adults: a 3-week randomized crossover study. Eur J Clin Nutr. (2018) 72:249–54. doi: 10.1038/s41430-017-0042-5
153. Drouin-Chartier, JP, Tremblay, AJ, Maltais-Giguère, J, Charest, A, Guinot, L, Rioux, LE, et al. Differential impact of the cheese matrix on the postprandial lipid response: a randomized, crossover, controlled trial. Am J Clin Nutr. (2017) 106:1358–65. doi: 10.3945/ajcn.117.165027
154. Raziani, F, Tholstrup, T, Kristensen, M, Svanegaard, M, Ritz, C, Astrup, A, et al. High intake of regular-fat cheese compared with reduced-fat cheese does not affect LDL cholesterol or risk markers of the metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. (2016) 104:973–81. doi: 10.3945/ajcn.116.134932
155. Villalpando, S, Lara Zamudio, Y, Shamah-Levy, T, Mundo-Rosas, V, Manzano, AC, and Lamadrid-Figueroa, H. Substitution of whole cows’ milk with defatted milk for 4 months reduced serum total cholesterol, HDL-cholesterol and total apoB in a sample of Mexican school-age children (6–16 years of age). Br J Nutr. (2015) 114:788–95. doi: 10.1017/s0007114515002330
156. Loria-Kohen, V, Espinosa-Salinas, I, Ramirez de Molina, A, Casas-Agustench, P, Herranz, J, Molina, S, et al. A genetic variant of PPARA modulates cardiovascular risk biomarkers after milk consumption. Nutrition. (2014) 30:1144–50. doi: 10.1016/j.nut.2014.02.012
157. Penedo, LA, Nunes, JC, Gama, MAÔS, Leite, PEC, Quirico-Santos, TF, and Torres, AG. Intake of butter naturally enriched with cis9,trans11 conjugated linoleic acid reduces systemic inflammatory mediators in healthy young adults. J Nutr Biochem. (2013) 24:2144–51. doi: 10.1016/j.jnutbio.2013.08.006
158. Chen, Y, Feng, R, Yang, X, Dai, J, Huang, M, Ji, X, et al. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. (2019) 109:1611–9. doi: 10.1093/ajcn/nqy358
159. Dunne, S, McGillicuddy, FC, Gibney, ER, and Feeney, EL. Role of food matrix in modulating dairy fat induced changes in lipoprotein particle size distribution in a human intervention. Am J Clin Nutr. (2023) 117:111–20. doi: 10.1016/j.ajcnut.2022.10.002
160. Feeney, E, Barron, R, Dible, V, Hamilton, Z, Power, Y, Tanner, L, et al. Dairy matrix effects: response to consumption of dairy fat differs when eaten within the cheese matrix-a randomized controlled trial. Am J Clin Nutr. (2018) 108:667–74. doi: 10.1093/ajcn/nqy146
161. Brassard, D, Arsenaultm, B, Boyer, M, Bernic, D, Tessier-Grenier, M, Talbot, D, et al. Saturated fats from butter but not from cheese increase HDL-mediated cholesterol efflux capacity from J774 macrophages in men and women with abdominal obesity. J Nutr. (2018) 148:573–80. doi: 10.1093/jn/nxy014
162. Hansson, P, Holven, KB, Oyri, LKL, Brekke, HK, Biong, AS, Gjevestad, GO, et al. Meals with similar fat content from different dairy products induce different postprandial triglyceride responses in healthy adults: a randomized controlled cross-over trial. J Nutr. (2018) 149:422–31. doi: 10.1093/jn/nxy291
163. Brassard, D, Tessier-Grenier, M, Allaire, J, Rajendiran, E, She, Y, Ramprasath, V, et al. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: a randomized controlled trial. Am J Clin Nutr. (2017) 105:800–9. doi: 10.3945/ajcn.116.150300
164. Rancourt-Bouchard, M, Gigleux, I, Guay, V, Charest, A, Saint-Gelais, D, Vuillemard, J, et al. Effects of regular-fat and low-fat dairy consumption on daytime ambulatory blood pressure and other cardiometabolic risk factors: a randomized controlled feeding trial. Am J Clin Nutr. (2020) 111:42–51. doi: 10.1093/ajcn/nqz251
165. U.S. Food & Drug Administration . FDA Announces Qualified Health Claim for Yogurt and Reduced Risk of Type 2 Diabetes | FDA. (2024), Available at: https://www.fda.gov/food/cfsan-constituent-updates/fda-announces-qualified-health-claim-yogurt-and-reduced-risk-type-2-diabetes (Accessed June 20, 2024)
166. Lordan, R . A new era for food in health? The FDA announces a qualified health claim for yogurt intake and type 2 diabetes mellitus risk reduction. Diabetes Metab Syndr Clin Res Rev. (2024) 18:103006. doi: 10.1016/j.dsx.2024.103006
167. Feeney, EL, Lamichhane, P, and Sheehan, JJ. The cheese matrix: understanding the impact of cheese structure on aspects of cardiovascular health—a food science and a human nutrition perspective. Int J Dairy Technol. (2021) 74:656–70. doi: 10.1111/1471-0307.12755
168. Dunne, S, Gibney, ER, McGillicuddy, FC, and Feeney, EL. The effects of saturated fat intake from dairy on CVD markers: the role of food matrices. Proc Nutr Soc. (2024):1–9. doi: 10.1017/S0029665124000132
169. Lordan, R, Tsoupras, A, Mitra, B, and Zabetakis, I. Dairy fats and cardiovascular disease: do we really need to be concerned? Food Secur. (2018) 7:29. doi: 10.3390/foods7030029
170. Mozaffarian, D, and Wu, JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health. Circ Res. (2018) 122:369–84. doi: 10.1161/circresaha.117.309008
171. Chung, RWS, Kamili, A, Tandy, S, Weir, JM, Gaire, R, Wong, G, et al. Dietary sphingomyelin lowers hepatic lipid levels and inhibits intestinal cholesterol absorption in high-fat-fed mice. PLoS One. (2013) 8:e55949. doi: 10.1371/journal.pone.0055949
172. Lordan, R, and Zabetakis, I. Invited review: the anti-inflammatory properties of dairy lipids. J Dairy Sci. (2017) 100:4197–212. doi: 10.3168/jds.2016-12224
173. Bruno, RS, Pokala, A, Torres-Gonzalez, M, and Blesso, CN. Cardiometabolic health benefits of dairy-milk polar lipids. Nutr Rev. (2021) 79:16–35. doi: 10.1093/nutrit/nuab085
174. U.S. Department of Health and Human Services and U.S. Department of Agriculture . Dietary Guidelines for Americans, 2020–2025. (2020), Available at: https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf (Accessed April 23, 2021)
175. Astrup, A, Magkos, F, Bier, DM, Brenna, JT, de Oliveira Otto, MC, Hill, JO, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76:844–57. doi: 10.1016/j.jacc.2020.05.077
176. Bailey, RL . Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr Opin Biotechnol. (2021) 70:91–6. doi: 10.1016/j.copbio.2021.02.007
177. O’Hearn, M, Lauren, BN, Wong, JB, Kim, DD, and Mozaffarian, D. Trends and disparities in cardiometabolic health among U.S. adults, 1999–2018. J Am Coll Cardiol. (2022) 80:138–51. doi: 10.1016/j.jacc.2022.04.046
178. Drewnowski, A . The Chicago consensus on sustainable food systems science. Front Nutr. (2018) 4:300092. doi: 10.3389/FNUT.2017.00074/BIBTEX
179. Thoma, G, Popp, J, Shonnard, D, Nutter, D, Matlock, M, Ulrich, R, et al. Regional analysis of greenhouse gas emissions from USA dairy farms: a cradle to farm-gate assessment of the American dairy industry circa 2008. Int Dairy J. (2013) 31:S29–40. doi: 10.1016/J.IDAIRYJ.2012.09.010
180. Food and Agriculture Organization of the United Nations . Contribution of terrestrial animal source food to healthy diets for improved nutrition and health outcomes. (2023). Available at: https://openknowledge.fao.org/server/api/core/bitstreams/0c1bfa99-18d4-42e4-b94f-27160126f826/content
Keywords: fatty acids, milk fat globule membrane, obesity, type 2 diabetes, cardiovascular diseases, hyperglycemia, insulin resistance, inflammation
Citation: Taormina VM, Unger AL and Kraft J (2024) Full-fat dairy products and cardiometabolic health outcomes: Does the dairy-fat matrix matter? Front. Nutr. 11:1386257. doi: 10.3389/fnut.2024.1386257
Edited by:
Jasmina D. Debeljak Martacic, University of Belgrade, SerbiaReviewed by:
Diane Zimmermann, Cereal Partners Worldwide, SwitzerlandRonan Lordan, University of Pennsylvania, United States
Copyright © 2024 Taormina, Unger and Kraft. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jana Kraft, SmFuYS5LcmFmdEB1dm0uZWR1
 Victoria M. Taormina
Victoria M. Taormina Allison L. Unger2,3
Allison L. Unger2,3 Jana Kraft
Jana Kraft