
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 15 May 2024
Sec. Nutritional Epidemiology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1381731
This article is part of the Research Topic Objective Dietary Assessment in Nutrition Epidemiology Studies - Volume II View all 20 articles
Introduction: The aim of this cross-sectional study was to develop an algorithm to predict athletes use of third-party tested (TPT) supplements. Therefore, a nutritional supplement questionnaire was used with a section about self-reported TPT supplement use.
Methods: Outcomes were randomly assigned to a training dataset to identify predictors using logistic regression models, or a cross-validation dataset. Training data were used to develop an algorithm with a score from 0 to 100 predicting use or non-use of TPT nutritional supplements.
Results: A total of n = 410 NCAA Division I student-athletes (age: 21.4 ± 1.6 years, 53% female, from >20 sports) were included. Then n = 320 were randomly selected, of which 34% (n = 109) of users consistently reported that all supplements they used were TPT. Analyses resulted in a 10-item algorithm associated with use or non-use of TPT. Risk quadrants provided the best fit for classifying low vs. high risk toward inconsistent TPT-use resulting in a cut-off ≥60% (χ2(4) = 61.26, P < 0.001), with reasonable AUC 0.78. There was a significant association for TPT use (yes/no) and risk behavior (low vs. high) defined from the algorithm (χ2(1)=58.6, P < 0.001). The algorithm had a high sensitivity, classifying 89% of non-TPT users correctly, while having a low specificity, classifying 49% of TPT-users correctly. This was confirmed by cross-validation (n = 34), reporting a high sensitivity (83%), despite a lower AUC (0.61).
Discussion: The algorithm classifies high-risk inconsistent TPT-users with reasonable accuracy, but lacks the specificity to classify consistent users at low risk. This approach should be useful in identifying athletes that would benefit from additional counseling.
Contaminants have been found in 19% of nutritional supplements sold in the United States (1), and recently, 38% of tested supplements contained undeclared doping substances in the Netherlands (2). In the United States, the number of doping violations with sanctions associated with supplements ranges from 0 to 17% of total investigated cases per year and has averaged around 9% of all doping cases annually since 2006 (3). One of the risk mitigation strategies to ensure athletes use safe nutritional supplements free from doping-related substances is to use products that are third-party tested (TPT) (4, 5). Despite the literature consistently mentioning the need for the use of TPT supplements (6, 7), research shows that a substantial number of athletes report inconsistently using TPT supplements (8, 9).
A reason for this low TPT compliance may be that current certification programs only cover 4–12% of the nutritional supplements currently on the market (10, 11); in addition, many of these programs do not test for banned substances. The large number of certification programs holding different standards makes it difficult for athletes to select programs that test for WADA-prohibited substances (12, 13). In addition, sports nutrition and dietary supplement knowledge are low in athletes (8, 14, 15), and access to nutrition experts, such as a sports RD, may be limited (8, 16). Overall, the endorsement of TPT nutritional supplements does not necessarily result in athlete compliance: despite many athletic departments instituting strict liability for drug testing, only 50–80% of athletes report the regular use of TPT supplements (8, 9).
Even though instituting strict liability in sport for drug testing (6, 7, 17), data suggest that 20–50% of athletes are not using TPT nutritional supplements, with high level (inter)national athletes reporting a 50% compliance with purchasing TPT supplements, vs. 67% in collegiate athletes and 80% in Olympic athletes (8, 9). These figures fall short of the 100% compliance goal of sports organizations. As to athlete attitudes, athletes normally consider it unacceptable to consume banned substances through supplements without their knowledge (9). Further, almost all student-athletes (93% based on n = 138) at a DI NCAA athletic department stated that it is important to know if supplements are tested for banned substances (8), while at the same time, 43% reported not using certified supplements (8).
Preventing the consumption of adulterated products containing substances not listed on the label is essential to limit exposing users to doping-related substances and health risks, as contaminated supplements may contain substances that negatively affect health (2). Current nutrition education curricula within athletics may improve the quality of dietary intake, but they contain little information about supplements and TPT programs (18, 19), and it is mainly the TPT programs that have their own quality assurance program testing for substances prohibited in sport that athletes should seek out (5). At the same time, the TPT programs need to be ISO 17025 accredited ensuring that the lab is deemed to be technically competent to adhere to the needed standards for lab testing, and participating brands need to adhere to their certification criteria (5). While previous research has assessed athletes’ knowledge (14, 15) and use of supplements (20–23), predictors for safe supplement use through the use of third-party tested supplements are currently unknown.
Therefore, the objective of this study was to develop an algorithm to predict whether athletes use TPT supplements while using a questionnaire assessing self-reported nutritional supplement behavior.
This cross-sectional cohort design study asked athletes from six NCAA Division I athletic departments, from October 2022 to April 2023, to fill out a questionnaire about behavior and attitudes concerning safe nutritional supplement use, including a predefined nutritional supplement list to identify supplement and third-party tested (TPT) nutritional supplement use during the last 12 months. Athletes were recruited by email, at fuel stations, or during team meetings. The results were analyzed aiming to identify predictors for TPT supplement use that could be combined in one algorithm predicting the use of uncertified nutritional supplements vs. TPT nutritional supplements, resulting in a “supplement risk behavior score.” After identifying variables predicting TPT supplement use while using the full dataset, responses from participants not delivering all answers to each of the identified predictors were removed. Then, the remaining responses were randomly assigned to two datasets, a “training dataset” and a smaller “validation dataset” to develop and confirm the validity of the algorithm predicting TPT nutritional supplement use. The algorithm was created while analyzing the outcome of the training dataset using the weight (i.e., “Estimate”) and direction of the “estimate” outcome of the logistic regression analyses. See the Supplementary material for the original questionnaire (Supplementary File 1, Original Nutritional Supplement Survey), the supplement safety screener containing the algorithm-based questions (Supplementary File 2A, S3 Nutritional Supplement Screener or Supplementary File 2B, Qualtrics file), and an Excel file with instructions for calculation of the supplement risk behavior score and interpretation of the S3 screener (Supplementary File 3, S3 Instruction, work file, and interpretation).
The participating NCAA Division I athletic departments, representing the highest-level collegiate athletes within the United States, included an estimated total of 3,580 student-athletes, ranging from 480 to 900 student-athletes per athletic department. Respondents had to be at least 18 years of age but under the age of 35, and a current member of a varsity sport at one of the participating athletic departments, while responding to at least 70% of the questionnaire. In cases of duplicate responses, the first response (identified via timestamp) was kept. Bot-generated responses were excluded before the initial response rate of 14% (n = 506) was calculated.
The study was approved by the Arizona State University Institutional Review Board (STUDY00015034). Student-athletes read and checked informed consent before accessing the questionnaire. The questionnaire, accessible through a link or QR-code, was anonymous, and upon completion, student-athletes were linked to a separate questionnaire where personal information would be provided and where they received a $17.50 virtual gift card for completion of the questionnaire.
The web-based questionnaire, was administered through Qualtrics (SAP, Seattle, WA, USA) for which each question required a response to move forward. The questionnaire, with 85 questions, was partly adapted from published literature (8, 9, 24–26), with additional newly formulated “original” questions.
The questionnaire (Supplementary File 1), consists of five main categories:
General questions: Athletic department [#1], primary sport [#1], sex [#1], age [#1], athlete status [#1]—subtotal: 5 questions.
Information sources: Nutrition information and counseling [#1], contact moments [#1], topics addressed [#1], preferred health professional [#1], preferred information source [#1], types of social media use [#1], social media frequency [#1], daily time spent on social media per day [#1], social media use related to nutritional supplements and sports foods [#1], preferred way of contacting in case of new information [#1]– subtotal: 10 questions.
Supplement knowledge: Supplement section of the nutrition for sport knowledge questionnaire (NSKQ) [#12], supplements related to doping [#1], WADA familiarity [#1], contamination [#1], implications of failed drug test [#1]– subtotal: 16 questions.
Nutritional supplement use: Age of first use [#1], purchase outside athletic department [#1], frequency of TPT supplements during last 12 months [#1], who purchases supplements [#1], location of supplement purchase [#1], estimated contamination of supplements [#1], predefined supplement checklist [#1], TPT of individual supplements [#1], TPT logo recognition [#1]– subtotal: 9 questions.
Attitudes and barriers: Find and order TPT supplements [#1], common feelings and beliefs about TPT supplements [#11], strategies for safe supplement use [#1], solutions and purchases of (safe) supplement use [#18], personality traits [#14]—subtotal: 45 questions.
For this article, only the results of the questions that directly related to self-reported personality traits concerning the use or non-use of certified TPT nutritional supplements are reported.
The NCAA reports that there are 460,000 NCAA DI-III student-athletes (27). For this purpose, a confidence level of 95% was used with a margin of error of 5%, while estimating that at least 50% of athletes use a third-party testing system while purchasing nutritional supplements (9). This resulted in a minimum number of 384 participants needed. This method of using margin of error was applied since the primary objective was to survey a sample of participants and have a large enough sample size to represent the intended population. This method of margin of effort prioritizes representativeness of the sample to the population over obtaining a sample size for a statistical analysis to detect a specific effect size. The latter is characteristic of research testing a specific hypothesis.
The demographics and descriptive data for relevant questionnaire sections are reported as percentages (%) and frequencies (n). For the development of the predictive model, cross-validation was performed using a 90:10 training/validation split. The purpose of adopting the 90:10 sample split for the cross validation was to target as large of a sample for the training dataset in order to establish a viable predictive model, while simultaneously securing a minimum of 30 complete responses in the validation sample so that the central limit theorem could be applied to a sample that was likely to be undersized in this initial stage of predictive modeling. This resulted in 320 participants in the training data without missing values. A stepwise logistic regression was run on each of the survey questions in the training dataset, while controlling for respondent sex, to determine which questions were related to the outcome of consistent vs. inconsistent TPT supplement use. For this analysis, p ≤ 0.10 was the initial threshold for retaining variables in the model. A final logistic regression model was run on the cross-validation dataset in which variables p < 0.05 were retained, with causal priority being granted to variables deemed practically relevant from a sports nutrition perspective in relation to selecting (safer) nutritional supplements. Model parameter estimates were obtained by including multiple predictor variables (as combined in the final algorithm) to evaluate the unique variance contribution of each survey question. These metrics were evaluated to ensure that each survey question contributed unique variance and predictive value above and beyond all other survey questions in the model. This process resulted in the final model, as presented in Table 1.
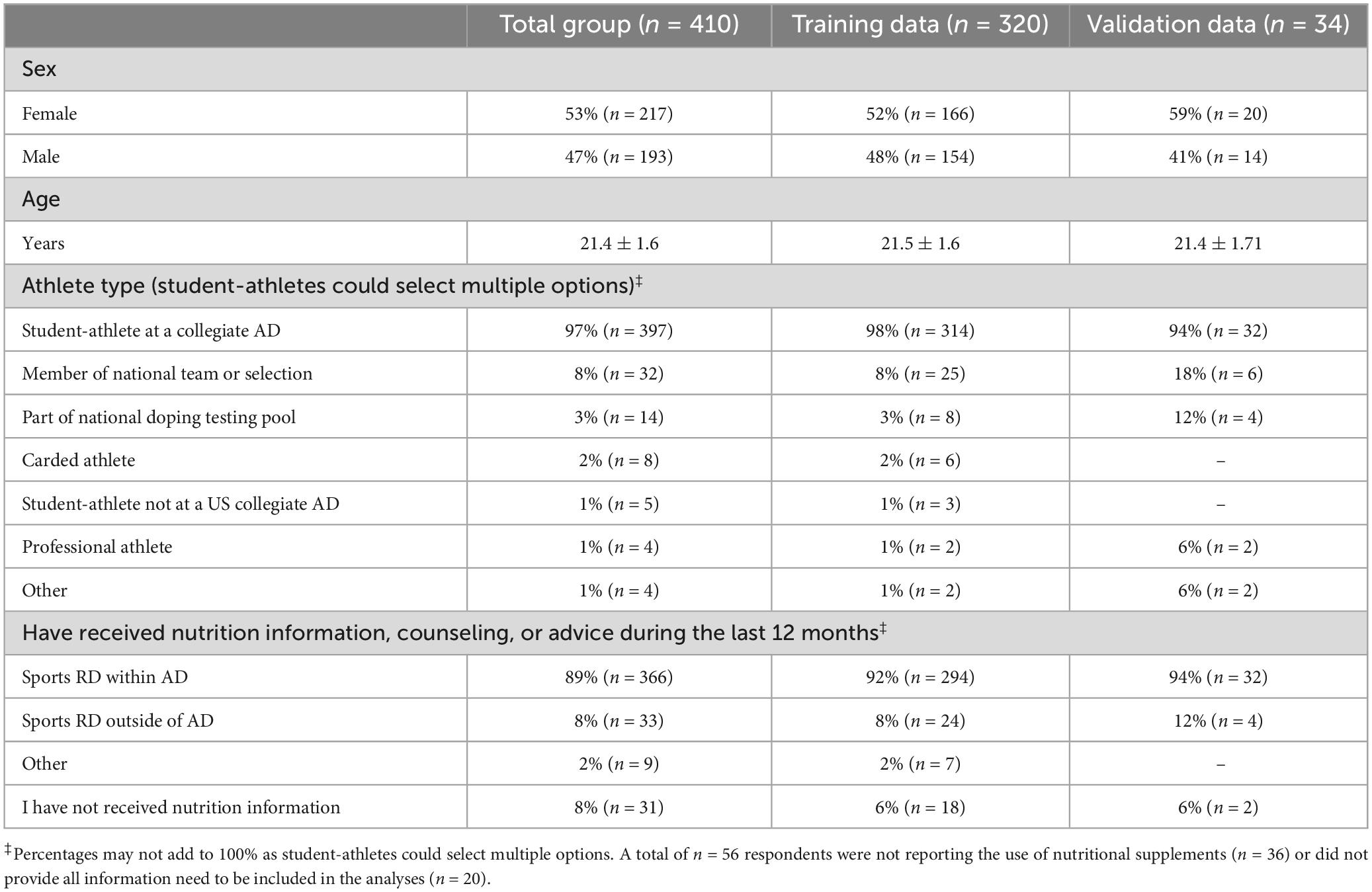
Table 1. Demographics of the NCAA Division I collegiate athletes surveyed reported as frequency (% and n) or as mean ± standard deviation, for total as well as training and cross-validation groups.
Then the risk behavior score quadrants (i.e., 0–19%, 20–39%, 40–59%, 60–79%, 80–100%) were plotted against consistent vs. inconsistent TPT use to assess potential cut-off values in determining low vs. high risk to determine the best fit for the model, and chi-square analysis was used to determine significance. Best fit in this case means that it should best predict consistent vs. inconsistent third-party tested supplement use compared with other predictor variables. Finally, after establishing the algorithm predicting low vs. high risk of TPT, a chi-square test of association was run to estimate the relationship between the use of TPT products and the predicted binary risk grouping of using TPT products. The creation of a binary risk grouping of high vs. low risk was intended to show a simplified approach of categorizing risk. Finally, the fit of the model was assessed by calculating the area under the curve (AUC), as well as true positive (TP), false positive (FP), false negative (FN), true negative (TN) scores. These values were used to calculate sensitivity (TP/TP+FN) and specificity (TN/TN+FP), as well as positive predictive value (PPV = TP/TP+FP) and negative predictive value (NPV = TN/TN+FN).
The training model was tested on the validation data to confirm its replicability and reduce the likelihood of an overfit final model. Significance was set for P < 0.05 if not described differently.
As shown in Figure 1, out of n = 506 responses a total of 91 questionnaires were excluded because athletes completed < 70% of the questions, and 5 additional questionnaires were excluded because of duplicate responses. Table 1 reports the demographics of the n = 410 NCAA Division I student-athletes (of which 53% were female, age 21.0 years or older, and IQR: 20.0 to 22.3, from > 20 sports) including those not reporting the use of nutritional supplements (9%, n = 36), as well as student-athletes reporting at least one, but often more, nutritional supplement (91%, n = 374). In addition, Table 1 shows also the demographics for the training dataset (n = 320) and cross-validation dataset (n = 34) that were created after removal of participants reporting sports foods of which the need for TPT could not be verified (< 1%, n = 2), and after removing a small number (4%, n = 18) of participants that did not provide all responses for the questions needed for the risk behavior algorithm.
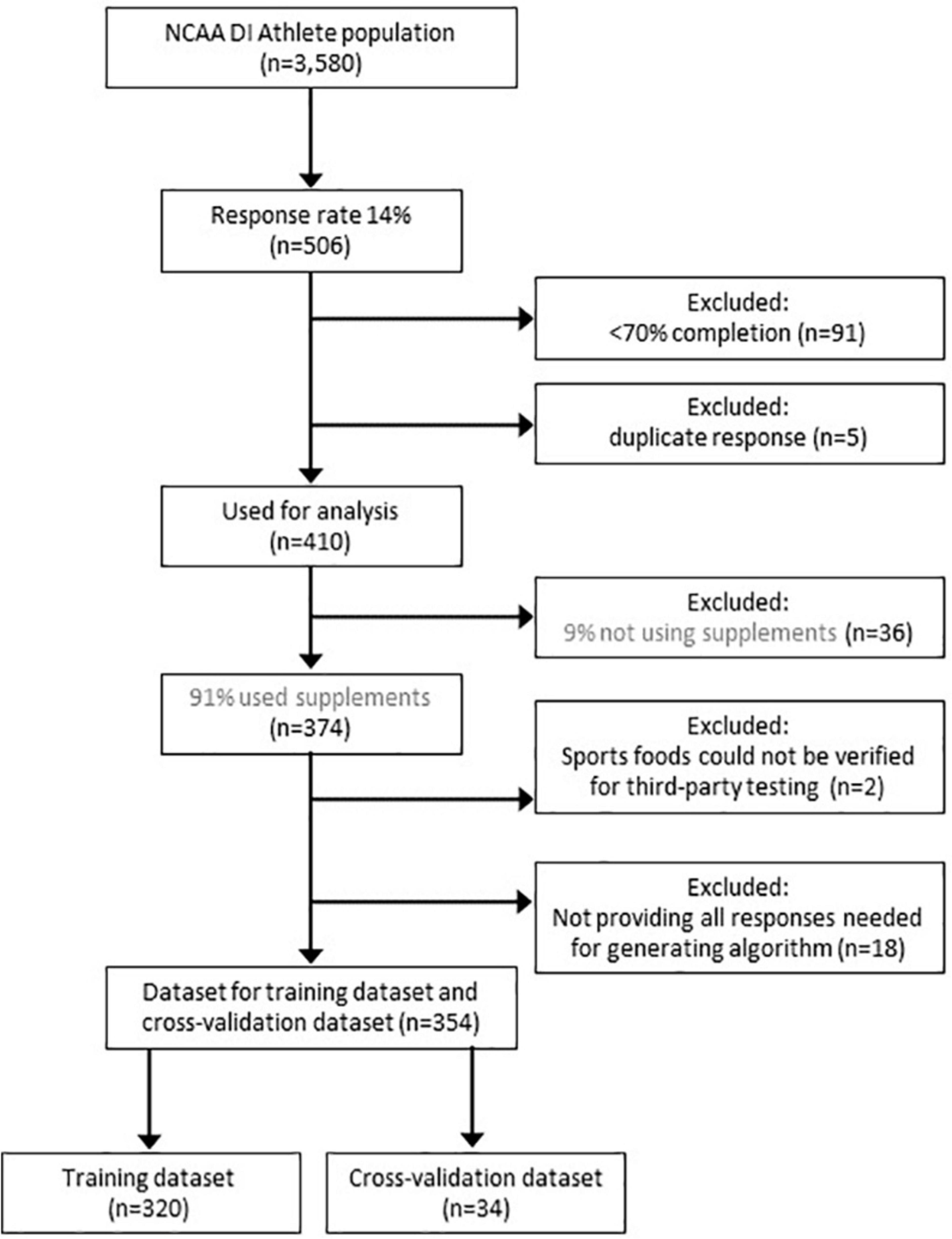
Figure 1. Consort diagram. The figure shows the response rate of NCAA Division I student-athletes, the reported use of nutritional supplements, as well as the number of responses that were excluded before constructing a training dataset and cross-validation dataset.
Based on a 90:10 training validation split, the data of a total of n = 320 participants were randomly selected for the training dataset for the development of the algorithm and to determine the best fit to classify a high vs. a low risk of not using TPT supplements. A total of 38% (n = 121) consistently used TPT vs. 62% (n = 199) inconsistently using TPT.
Table 2 contains 10 items that were significantly associated with TPT risk (i.e., consistent vs. inconsistent TPT-use) with P ≤ 0.10, it was already mentioned that these items were based on the 85 questions and the two newly created variables. Out of these 10 items, four items were part of the predefined supplement question list (i.e., multivitamin, weight gainer, caffeine, and creatine). Despite that the Wald chi-square outcome for multivitamin use was P = 0.1116, the supplement was included as it is one of the most popular dietary supplements (46%, n = 190), together with caffeine (56%, n = 229), creatine (22%, n = 91), and weight gainer (5%, n = 22).
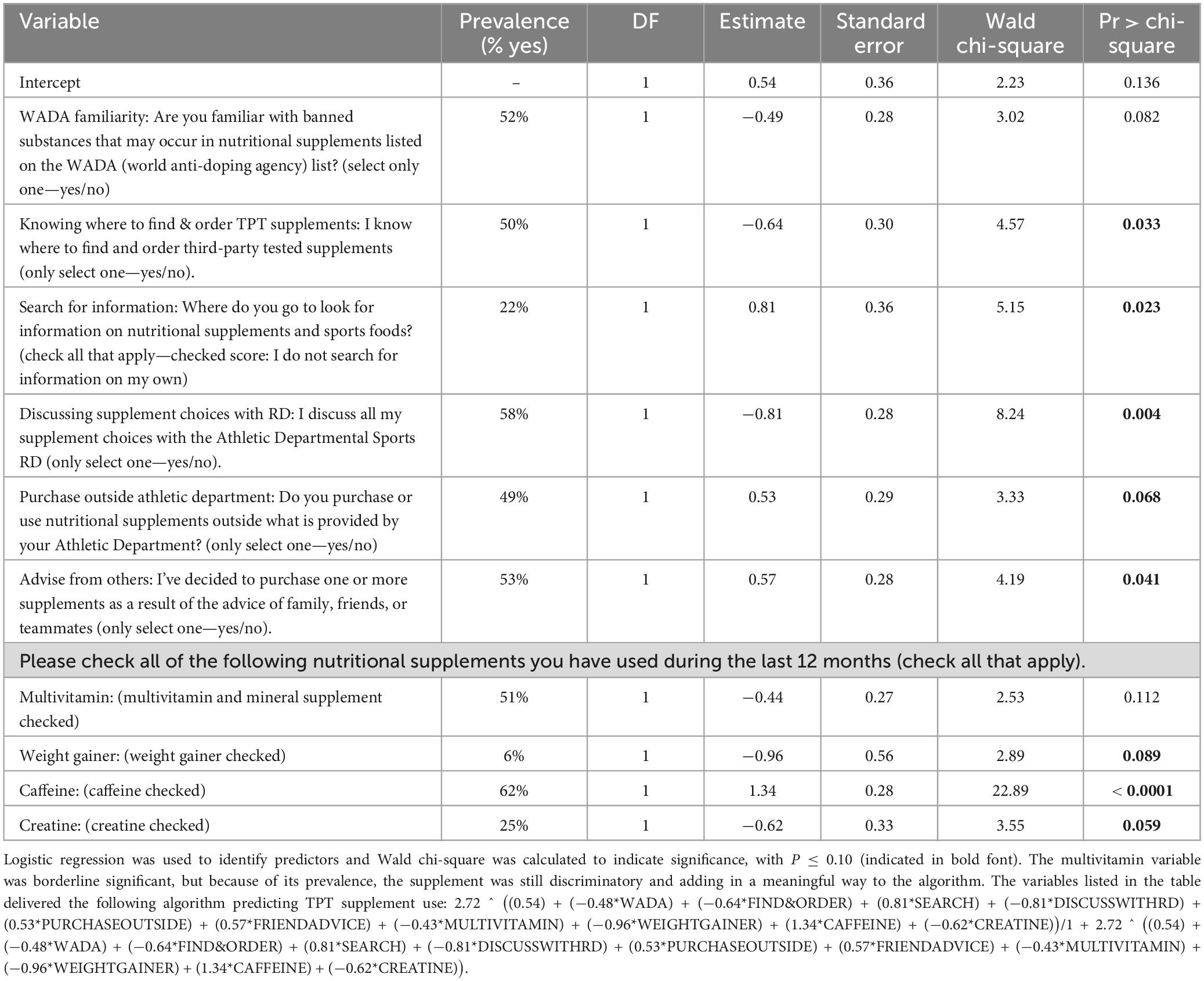
Table 2. Variables identified as predictors whereas athletes are using TPT nutritional supplements, including the prevalence per outcome for n = 320 NCAA Division I collegiate athletes.
The algorithm supporting the risk behavior score was based on the weight (i.e., “Estimate”) and direction of the “estimate” outcome as mentioned in Table 2. The variables listed in the table delivered the following algorithm predicting TPT supplement use:
In which capitalized letters reflect the following question items:
W = WADA; FO = FIND & ORDER; S = SEARCH; D = DISCUSS WITH RD; P = PURCHASE OUTSIDE; FA = FRIEND ADVICE; M = MULTIVITAMIN; W = WEIGHTGAINER; CA = CAFFEINE; CR = CREATINE.
This algorithm suggests an increased risk of not using TPT, with a higher score ranging from 0 to 1, in which a low score suggests a low risk vs. a high score suggesting a high risk.
To find the best fit indicating a low vs. high risk for inconsistent or not using TPT, risk behavior score quadrants were plotted against consistent vs. inconsistent TPT use to determine the best-fit cut-off value to determine low vs. high risk, as shown in Table 3. This table shows that the discriminatory cut-off was between quadrants three and four at the 60% mark.
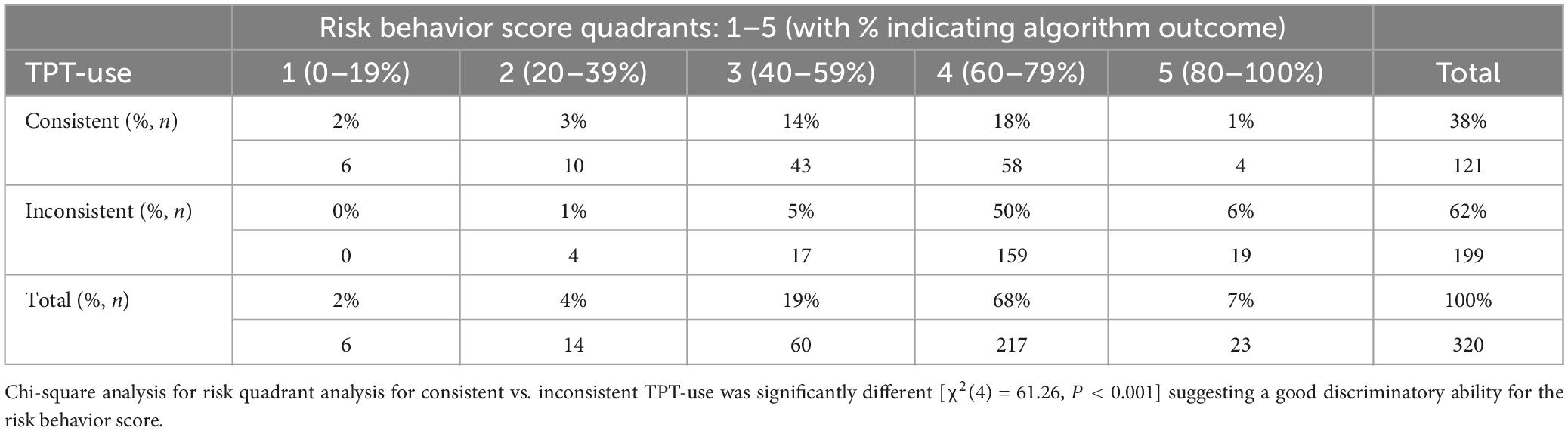
Table 3. Algorithm-based risk behavior score for using vs. not using TPT quadrant against consistent vs. inconsistent TPT-use analysis for n = 320 NCAA Division I collegiate athletes.
When classifying athletes for consistent/inconsistent TPT use according to questionnaire-based self-reporting and low/high-risk behavior based on the algorithm, only the athletes reporting all predictive variables could be included in the algorithm. When applying the results of the n = 320 student-athletes that provided answers for the variables based on the final model for the algorithm, a total of 38% (n = 121) consistently reported the use of TPT supplements, meaning that all the supplements they used were TPT. The rest of the athletes inconsistently used TPT (62%, n = 199).
As shown in Table 4 the screener was able to identify significantly different responses [χ2 (1) = 58.59, P < 0.001], indicating a strong relationship between the risk groupings and product use outcomes. The screener had a high sensitivity, the test’s ability to designate an individual with a negative outcome, as it classified 89% of the student-athletes not consistently using TPT accurately in the ≥ 60% high-risk behavior score group. Despite the test’s high sensitivity, the test had a low specificity (49%), as roughly half of the consistent TPT users were correctly classified in the < 60% low-risk behavior score group. The area under the curve (AUC) for the cut-off value ≥ 60% suggesting a high risk for inconsistent TPT was 0.78, with a positive predictive value (PVV) of 0.69, and negative predictive value (NPV) of 0.71.
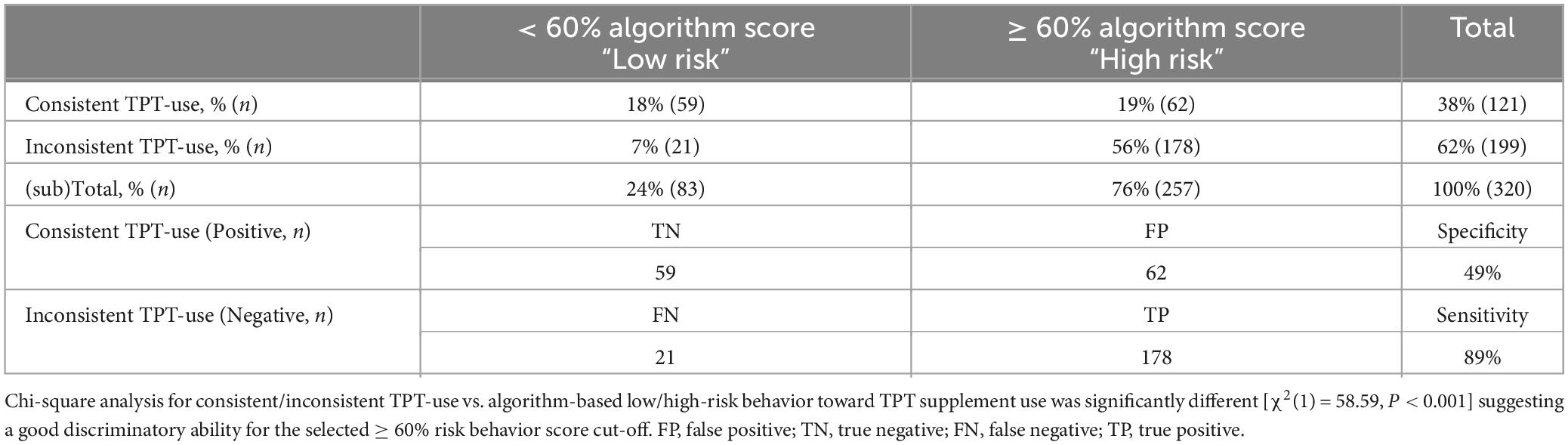
Table 4. Training data absolute and relative categorized screener-based answers for consistent/inconsistent TPT use vs. algorithm-based low/high-risk behavior toward TPT supplement use in n = 320 NCAA Division I collegiate athletes.
Based on a 90:10 training validation split, the remainder of n = 34 participants were used to validate the model from the training data in a cross-validation. A total of 29% (n = 10) consistently used TPT vs. 71% (n = 24) inconsistently using TPT.
When applying the same strategy as for the training data for the cross-validation data (Table 5), the crosstab table had very similar percentages to the training data shown in Table 4; however, the chi-square analysis was not significant [χ2(1) = 2.14, P = 0.144]. This non-significant relationship indicates that the binary risk categories did not significantly predict product use. This lack of relationship, however, could be a result of the small size of this sample. Still, the screener had a similar high sensitivity as the training dataset, as it classified 83% of the student-athletes inconsistently using TPT accurately in the ≥ 60% high-risk behavior score group. In addition, the validation data also reported a low specificity while including only 40% of the consistent TPT users in the < 60% low-risk behavior score group identifying them as “low risk.” The AUC for the cut-off value ≥ 60%, suggesting a high risk for inconsistent TPT, was lower than in the training data with 0.61, and a PVV of 0.77 and NPV of 0.50.
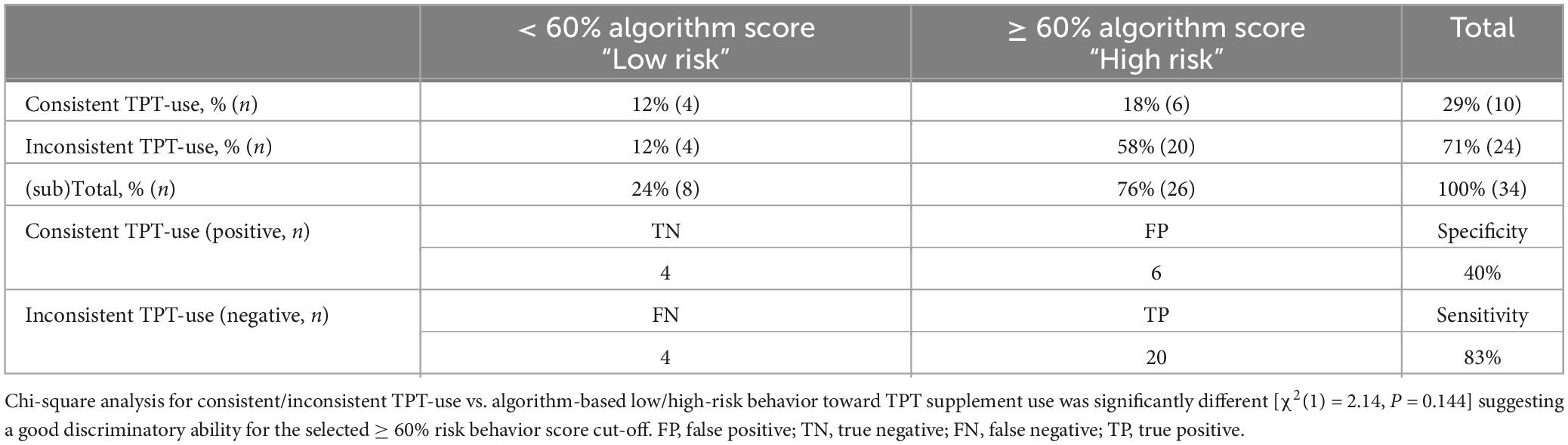
Table 5. Cross-validation absolute and relative categorized screener-based answers for consistent/inconsistent TPT use vs. algorithm-based low/high-risk behavior toward TPT supplement use in n = 34 NCAA Division I collegiate athletes.
This algorithm was determined to be very suitable for identifying inconsistent TPT users indicated by a ≥ 60% high-risk behavior score. For those consistently using TPT supplements, the algorithm provides more insight into their behavior, but due to its low specificity it cannot replace the actual checking of self-reported TPT use to identify consistent TPT users.
The developed algorithm was based on ten predictive variables including four nutritional supplements that were clearly related to TPT use. Six elements (WADA familiarity, knowing where to find and order TPT supplements, discussing supplement choices with a sports RD, and using multivitamins, weight gainer, and creatine) were associated with consistent TPT use, while the other four elements (not searching for information, purchasing products outside of the supplements provided by the athletic department, deciding to purchase supplements based on the advice of others, such as family, friends or team mates, and using caffeine) were associated with inconsistent TPT use. This section discusses, where possible, the relevance of these predictors.
To start, the majority of athletes participating in high level competitive sports need to comply with WADA regulations (28). Not complying may result in a positive doping test (4). Supplements often contain impurifications, that can be on the WADA (or in this case NCAA) prohibited substances list. This can lead to a positive doping test, hence the development of third-party testing organizations that batch test supplements (5). The analyses in the current study showed that there was indeed an association between athletes being familiar with the prohibited list from WADA and consistent TPT use. Potentially this can be related to access to a sports dietitian or sport level, or both. Despite roughly half of the athletes in the current study reporting familiarity with the WADA prohibited substance list, others may be less familiar with it. This includes for example semi-professional football players in South Africa, of which almost all (87%), never attended a workshop on safe supplement use, while reporting WADA familiarity ranging from 16% (29). On the contrary, up to 95% in Japanese Olympic and Paralympic athletes reported familiarity with the WADA prohibited substance list, still half of them used supplements without the advice of a doctor or dietitian (30).
Subsequently, being able to find and order TPT supplements was also associated with consistent TPT use, as it has been previous reported that only 22% of high school athletes knew where to find TPT supplements and only 25% of these athletes knew where to order TPT supplements, resulting in 24% reporting consistent TPT use (31). In a follow-up study on a subsample of high school athletes at the same high school, it was shown that education indeed resulted in an increased intention to select TPT supplements (32).
Almost two-thirds of the athletes discussed supplement choices with their registered dietitian, which was the fourth predictive variable, being associated with a lower risk of not using TPT supplements. This can be related with better informed choices by athletes when counseled by a dietitian (16), while in reality athletes also report lower percentages for consulting a dietitian related to supplement use, ranging in other recent publications from 25% (33) to 50% (30).
Finally, the use of three nutritional supplements: multivitamin and mineral supplements, weight gainer, and creatine were associated with consistent TPT use. Interestingly, based on previous reporting multivitamin and mineral supplements (≥ 60%), and creatine (20–30%) are among the more popular supplements, whereas weight gainer supplements have been less popular (< 10%) (8, 16, 31). Earlier no relationship was seen for multivitamin and mineral use, and creatine use and having access to a sports dietitian (16), but at the same time all three supplements can be reasonably classified as potentially effective in optimizing nutrient intake, or in providing extra protein or creatine as supported by the current sports nutrition consensus (6). More research is needed to better understand why specifically these supplements were more clearly related to TPT use than others.
The remainder of the predictors (i.e., not searching for information, purchasing outside of the athletic department, taking advice from family and friends, and the use of caffeine supplements) suggest a negative impact on TPT use. The strongest impact of a variable influencing the algorithm came from the use of caffeine. There is not a clear literature-based reason supporting the relation between using caffeine and not consistently using TPT supplements, on the other hand caffeine was one of the most frequently reported supplements (62%), and as such, because it is so frequently used it may be associated with the larger part of the athletes not being consistent TPT users. In addition, caffeine can be found for example in pre-workout supplements which seem to be more prone to adulteration (34). The next strongest impact on the algorithm was based on athletes not searching for information before using a supplement. In the current study, one-fifth of the athletes reported not to search for information on their own, which was substantially lower than earlier reported, as 50% of Japanese elite Olympic athletes reported not reviewing scientific evidence before using nutritional supplements (30). Two other predictors, both contributing with a similar weight to the algorithm, where purchasing supplements outside of what the athletic department provides, as well as taking advice from non-experts, increased the chance of inconsistent TPT use. Regarding the element of purchasing supplements outside of what is provided by the department, it is important to acknowledge that supplements provided by the athletic department is something that specifically applies to NCAA DI collegiate athletics, and potentially elite athletics (35), because these athletic programs have a larger budget than within other divisions (36). The supplements provided within the departments are likely at close to zero risk because they have registered sports dietitians on staff that organize the products available. At the same time, purchasing products outside of the athletic department by the athlete itself is therefore always subject to risk. Due to the large offering of supplements, and the relatively small number of third-party tested supplements testing for substances prohibited in sport, local stores may not the best resource for third-party tested supplements. Athletes should therefore be trained to find and order TPT supplement from third-party testing organizations. Lastly, purchasing supplements based on advice from others (family, friends, teammates) increased the risk of not using TPT supplements. Unfortunately, based on the available literature, this is a very common influence (36–38). In general, friends and family as well as teammates can normally not be considered experts, as a result the advice to a supplement is unlikely to be evidence based, and it is not unlikely that the friends and family are not familiar with third-party testing supplement procedures. Therefore, it comes back to making sure athletes consult with an expert (16, 39), which is reflected in the algorithm as discussing supplement choices with RD was associated with TPT use, whereas deciding to purchase supplements based on the advice of others, such as family, friends or team mates was associated with inconsistent or no TPT use.
The current algorithm had a high sensitivity to detect inconsistent self-reported TPT use or no TPT use, while the algorithm had a low specificity because it marked roughly half of the consistent TPT users with a high-risk profile. Sensitivity and specificity are inversely related, and therefore when sensitivity increases, specificity tends to decrease, and vice versa (40). Therefore, it is important to identify what the main objective is of a tool. In this case, as the majority of athletes reports inconsistent TPT use, it’s more important to identify correctly the non-TPT users than it is to correctly identify the users of TPT supplements. This is, because the absolute numbers of correctly classified inconsistent TPT users will be higher. It also means that when the algorithm is used on its own, without asking the complete number of supplements used, it will result in a misclassification of roughly half of the consistent TPT users. Therefore, it is recommended to have a face-to-face follow-up with a specialized sports health professional (39), such as a sports dietitian or nutrition expert (16). Further, the sensitivity and specificity of this algorithm should be confirmed in future research in an independent dataset, while also looking for optimization of the algorithm to ensure a better accuracy of risk classification of athletes.
There is currently almost no data that reports the relation between TPT supplement use and knowledge, attitude and practices of athletes. The existing data shows that there is no clear relationship between knowledge and TPT supplement use (8, 31), but this may be related to generalized low knowledge scores of these athletes. Further, most athletes find it unacceptable that supplements can contain unlabeled substances that could lead to a positive doping test (9), but despite reflecting these attitudes a large group of athletes does not report to use third-party tested supplements (8, 9, 31). Hence, the relevance for developing a screener scoring risk behavior toward the consistent use or non-use of TPT nutritional supplements lies in that many athletes currently are not selecting third-party batch tested supplements. Although nutritional supplement education may play an important role (32), this study shows that even within athletic departments that provide access to sports dietitians and nutrition education, as well as providing nutritional supplements allowed per NCAA regulations, consistent TPT use is low (38%). At the same time TPT supplement use can be even lower, but likely TPT supplement use can also increase with the right education. While using a similar approach, using a predetermined list of nutritional supplements and asking per supplement if it was TPT, only 24% of high school athletes (14–19 years, n = 225) claimed to know for sure that all their supplements were third-party tested, with TPT nutritional supplement use ranging from 0 to 100% for individual supplements (31). A subset of this high school athlete population (n = 106) was later part of a study investigating the impact of a high school athlete education program for safe nutritional supplement use, and 35–77% of supplements reported were TPT, which increased after the education module 7–36% depending on individual supplements reported (32).
It is important to emphasize that earlier studies, of which two are listed below, have asked only a general question about third-party supplement use, not specifically related to individual supplement use. Self-reported compliance numbers for TPT supplement use in these cases are slightly higher, but may substantially overestimate TPT supplement compliance. For example, the United States Anti-Doping Association (USADA) published the results of their 2022 Athlete Perceptions Survey, covering n = 994 athletes from 76 sports (80% Olympic, 20% Paralympic), while asking a generic question about the use of NSF certified for sport supplement. Finding that 56% of the athletes reported always checking for NSF certified for sport certification (41), and although this was lower than the 82% self-reported compliance to TPT in Dutch athletes using the NZVT system (i.e., the Dutch Safeguards System for Dietary Supplements) (9), both cases likely overestimated consistent TPT use.
A clear strength of this study is that the use of TPT supplements was questioned for individual supplements. Another strength was the large range of questions that was analyzed for its predictive value for TPT supplement use, while including a sample size representative for the NCAA DI student-athlete population. The response rate was with 14% higher than was anticipated, and after cleaning, the dataset reflected 11.5% of the sampled population, which was likely enough to accurately reflect the behavior of this student-athlete population, with an almost equal sex distribution, coming from six different athletic institutions throughout the United States, and develop the algorithm predicting TPT use.
At the same time, the study also holds limitations. The responses used for both the training dataset and cross-validation were minimal, and should be seen as a first step in confirming the predictive modeling process. The resultant small sample in the validation dataset may have yielded higher variability that could have a disproportionate effect, compared with actual model predictability, on reducing the model fit metrics of the validation data, such as AUC (42). As funds were a limiting factor, the cross-validation should be interpreted as a first pilot, therefore, it can be suggested that independent data collection in a much larger group of athletes should confirm these results in the future. Furthermore, it is unclear how well the algorithm can be translated to other (athlete) populations in less well funded athletic departments; for example, the ones that do not have access to supplements provided by an athletic department. At the same time, these athletes will need to select supplements on their own; consequently, they risk purchasing uncertified or not third-party tested supplements. Another limitation, as this is a first reporting of the development of this algorithm, is that no further stratifications have been performed.
Real-world limitations of this screener include the simplified perspective of questioning athletes (as the algorithm requires a binary yes/no answer), as well as the predefined list of supplements that likely needs to be updated regularly when supplement options and offerings change. Further, despite the limited number of questions and simple formatting of the screener it is not clear what the test-retest reliability is of this type of questionnaire. Finally, despite a reasonable sensitivity (allowing to identify inconsistent TPT-users), the specificity to identify consistent TPT-users correctly is low; therefore, the algorithm cannot currently used independently of questioning self-reported TPT use (which is identified as part 3 of the current screener), or without follow up from a specialist, such as a sports dietitian.
The algorithm classifies high-risk inconsistent TPT users with reasonable accuracy, but lacks the specificity to classify consistent users at low risk. Still, the algorithm can help to identify most of the inconsistent TPT users, allowing sports health professionals, such as sports dietitians and nutritionists to identify the needs of athletes not consistently using third-party tested nutritional supplements, and help athletes to select TPT supplements, while improving athlete compliance to athletic program policies and reduce doping risk. Further research is needed to have the algorithm function independently from self-reported supplement and TPT behavior in athletes.
The algorithm as described in this article has been integrated in a first version of a supplement safety screener (S3, Supplementary File 2) consisting of three parts: (1) general information; (2) Algorithm-based supplement risk behavior score; and (3) supplement use and self-reported use of TPT supplements. Additionally, materials were developed that will help to calculate the risk behavior score and to interpret the screener outcome, all included as Supplementary material (as tab in Supplementary File 2). The following sections briefly describe the content of the screener, followed by some suggestions for interpretation of screener outcome.
The general information in the current screener in part 1 is limited to the athlete’s name and team or sport. This allows the sports dietitian/nutritionist to identify the athlete for future follow-up, while also helping to organize the data per team sport, in case the screener is collected via a web-based questionnaire module. The supplement risk behavior score in part 2 is based on the variables listed in Table 1 using the algorithm as listed in the legend of this table. This algorithm can also be found in the following Excel file included as Supplementary material. There were two questions added to the screener that are not included in the algorithm, as they pose relevance for the nutritionist. This includes a question about the “Influence of teammates in trying a supplement” (to determine potential player dynamics related to supplement use) and the “Nutritional supplement purchase location” (allowing to further discuss the best outlets for supplement purchase with the athlete). Aside from the contributions of these predictors to a risk behavior score, it is important to emphasize that the individual outcomes also can provide important information about risk behavior in athletes. Finally, in part 3, supplement use and self-reported use of TPT supplements is questioned. This list is based on the pre-defined list of nutritional supplements used in this study, but it is suggested that this list should be kept up to date, to ensure that the most accurate assessment of self-reported supplement use and TPT use is performed.
The screener combines the outcome of the risk behavior scores and TPT-use, based on the outcome an athlete reporting the use of nutritional supplements will fit in one of the combinations listed in Table 6. This will help the sports dietitian/nutritionist to act, while considering the supplement and risk behavior of each individual athlete that filled out the screener. The table does not address the actions for athletes not currently reporting the use of nutritional supplements, but they should be also educated on the importance of the future use of TPT supplements in case athletes decide to start using nutritional supplements.
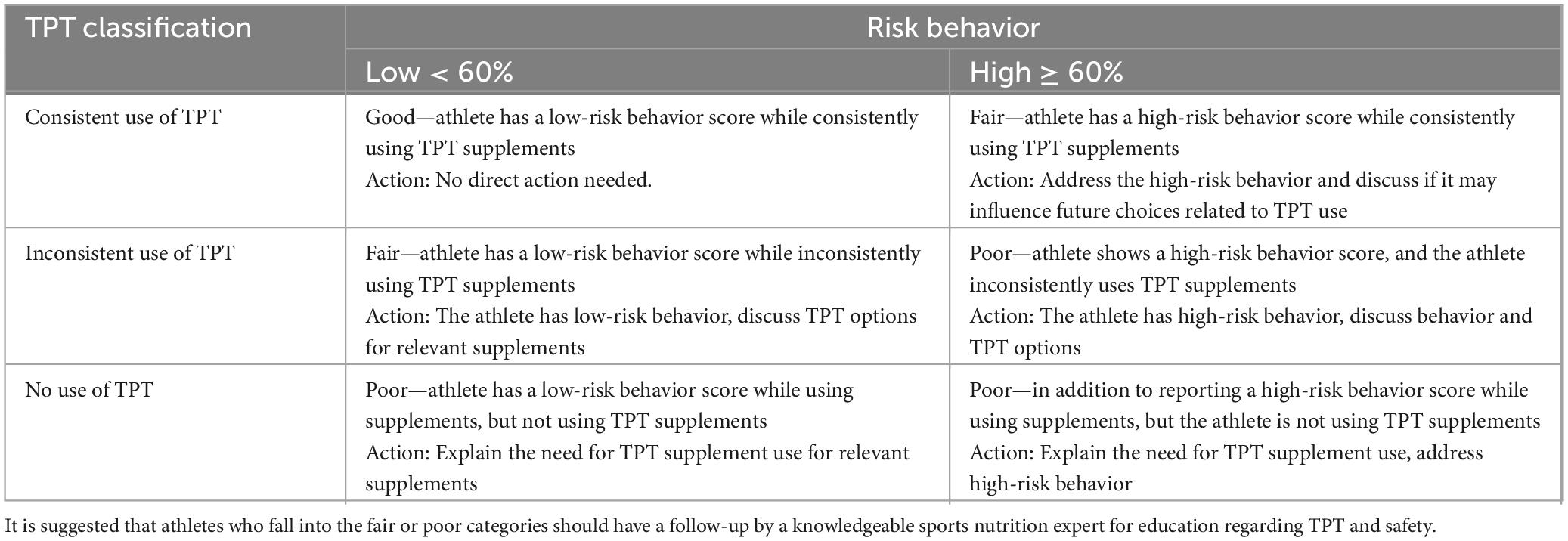
Table 6. Screener-based interpretation and suggested actions for a low- vs. high-risk behavior and TPT supplement use for those reporting the use of nutritional supplements.
It is also an option to ask only the questions of part 2. This results in a reduced screener length while being able to calculate a risk score. Specifically, when the score is ≥ 60%, this warrants follow-up for sure, but as sensitivity and specificity of the algorithm is not perfect, follow up from a specialist, such as a sports dietitian should be considered.
The datasets presented in this article may be requested upon an informal inquiry addressed to the corresponding author, who will decide on a case-by-case basis if the request can be granted. Requests to access the datasets should be directed to RmxvcmlzLndhcmRlbmFhckBhc3UuZWR1.
The studies involving humans were approved by the Arizona State University Institutional Review Board (STUDY00015034). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KS: Data curation, Formal analysis, Investigation, Project administration, Writing – review & editing. RS: Conceptualization, Formal analysis, Methodology, Validation, Writing – review & editing. CG: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Collegiate and Professional Sports Dietetic Organization (CPSDA) in the USA.
FW and CG received grants from external partners during the last 5 years that were not related to this project. FW received grants from: Arizona Parks & Trails, Pac-12 Health and Wellbeing Initiative, the Collegiate and Professional Sports Dietetic Organization, Friesland Campina N.V., Standard Process Inc., Kraft Heinz Company, FEMA, Unilever Corporation, and the Arizona Board of Regents. CG received grants from the National Institutes of Health and Beyond Meat.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1381731/full#supplementary-material
1. Geyer H, Parr MK, Mareck U, Reinhart U, Schrader Y, Schänzer W. Analysis of non-hormonal nutritional supplements for anabolic-androgenic steroids – results of an international study. Int J Sports Med. (2004) 25:124–9. doi: 10.1055/s-2004-819955
2. Duiven E, van Loon LJC, Spruijt L, Koert W, de Hon OM. Undeclared doping substances are highly prevalent in commercial sports nutrition supplements. J Sports Sci Med. (2021) 75:328–38. doi: 10.52082/jssm.2021.328
3. Outram S, Stewart B. Doping through supplement use: A review of the available empirical data. Int J Sport Nutr Exerc Metab. (2015) 25:54–9. doi: 10.1123/IJSNEM.2013-0174
4. Backhouse SH. A behaviourally informed approach to reducing the risk of inadvertent anti-doping rule violations from supplement use. Sports Med. (2023) 22:1–18. doi: 10.1007/S40279-023-01933-X
5. Eichner AK, Coyles J, Fedoruk M, Maxey TD, Lenaghan RA, Novitzky J, et al. Essential features of third-party certification programs for dietary supplements: A consensus statement. Curr Sports Med Rep. (2019) 18:178–82. doi: 10.1249/JSR.0000000000000595
6. Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br J Sports Med. (2018) 52:439–55. doi: 10.1136/bjsports-2018-099027
7. Buell JL, Franks R, Ransone J, Powers ME, Laquale KM, Carlson-Phillips A. National athletic trainers’ association position statement: Evaluation of dietary supplements for performance nutrition. J Athl Train. (2013) 48:124. doi: 10.4085/1062-6050-48.1.16
8. Vento KA, Wardenaar FC. Third-party testing nutritional supplement knowledge, attitudes, and use among an NCAA I collegiate student-athlete population. Front Sports Act Liv. (2020) 2:115. doi: 10.3389/fspor.2020.00115
9. Wardenaar FC, Hoogervorst D, Vento KA, de Hon O. Dutch Olympic and non-Olympic athletes differ in knowledge of and attitudes toward third-party supplement testing. J Diet Suppl. (2020) 18:646–54. doi: 10.1080/19390211.2020.1829248
10. Jones DR, Kasper KB, Deuster PA. Third-party evaluation: A review of dietary supplements dispensed by military treatment facilities from 2007 to 2011. Mil Med. (2015) 180:737–41. doi: 10.7205/MILMED-D-14-00500
11. Cancio A, Eliason MJ, Mercer J, Tran T, Deuster PA, Stephens MB. Third-party certification of dietary supplements: Prevalence and concerns. Mil Med. (2012) 177:1460–3. doi: 10.7205/MILMED-D-12-00069
12. Maughan RJ, Shirreffs SM, Vernec A. Making decisions about supplement use. Int J Sport Nutr Exerc Metab (2018) 28:212–9. doi: 10.1123/ijsnem.2018-0009
13. Brown AC. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol. (2017) 107:449–71. doi: 10.1016/j.fct.2016.11.001
14. Jessri M, Jessri M, RashidKhani B, Zinn C. Evaluation of Iranian college athletes’ sport nutrition knowledge. Int J Sport Nutr Exerc Metab. (2010) 20:257–63. doi: 10.1123/ijsnem.20.3.257
15. Torres-McGehee TM, Pritchett KL, Zippel D, Minton DM, Cellamare A, Sibilia M. Sports nutrition knowledge among collegiate athletes, coaches, athletic trainers, and strength and conditioning specialists. J Athl Train. (2012) 47:205–11. doi: 10.4085/1062-6050-47.2.205
16. Wardenaar FC, Ceelen IJM, Van Dijk JW, Hangelbroek RWJ, Van Roy L, Van Der Pouw B, et al. Nutritional supplement use by Dutch elite and sub-elite athletes: Does receiving dietary counselling make a difference? Int J Sport Nutr Exerc Metab. (2017) 27:32–42. doi: 10.1123/ijsnem.2016-0157
17. British Dietetics Association Sports Nutrition Group. Nutritional supplement position statement. Birmingham: British Dietetics Association Sports Nutrition Group (2022).
18. Coccia C, Fernandes SM, Altiti J. Tweeting for nutrition. J Strength Cond Res. (2018) 34:1. doi: 10.1519/jsc.0000000000002500
19. Valliant MW, Pittman H, Wenzel RK, Garner BH. Nutrition education by a registered dietitian improves dietary intake and nutrition knowledge of a NCAA female volleyball team. Nutrients. (2012) 4:506–16. doi: 10.3390/nu4060506
20. Burns RD, Schiller MR, Merrick MA, Wolf KN. Intercollegiate student athlete use of nutritional supplements and the role of athletic trainers and dietitians in nutrition counseling. J Am Diet Assoc. (2004) 104:246–9. doi: 10.1016/J.JADA.2003.11.013
21. Froiland K, Koszewski W, Hingst J, Kopecky L. Nutritional supplement use among college athletes and their sources of information. Int J Sport Nutr Exerc Metab. (2004) 14:104–20. doi: 10.1123/ijsnem.14.1.104
22. Knapik JJ, Steelman RA, Hoedebecke SS, Austin KG, Farina EK, Lieberman HR. Prevalence of dietary supplement use by athletes: Systematic review and meta-analysis. Sports Med. (2016) 46:103–23. doi: 10.1007/s40279-015-0387-7
23. Kristiansen M, Levy-Milne R, Barr S, Flint A. Dietary supplement use by varsity athletes at a Canadian university. Int J Sport Nutr Exerc Metab. (2005) 15:195–210. doi: 10.1123/ijsnem.15.2.195
24. Fardouly J, Willburger BK, Vartanian LR. Instagram use and young women’s body image concerns and self-objectification: Testing mediational pathways. New Media Soc. (2018) 20:1380–95. doi: 10.1177/1461444817694499
25. Reynolds WM. Development of reliable and valid short forms of the Marlowe-Crowne social desirability scale. J Clin Psychol. (1982) 38:119–25. doi: 10.1002/1097-4679(198201)38:13.0.CO;2-I
26. Trakman GL, Forsyth A, Hoye R, Belski R. The nutrition for sport knowledge questionnaire (NSKQ): Development and validation using classical test theory and Rasch analysis. J Int Soc Sports Nutr. (2017) 14:1–11. doi: 10.1186/s12970-017-0182-y
27. NCAA. NCAA demographics database. (2022). Available online at: https://www.ncaa.org/sports/2018/12/13/ncaa-demographics-database.aspx (accessed September 7, 2022).
28. World Anti-Doping Agency. World anti-doping code. (2024). Available online at: https://www.wada-ama.org/en/resources/world-anti-doping-code-and-international-standards/world-anti-doping-code (accessed January 17, 2024).
29. Nyawose S, Naidoo R, Naumovski N, McKune AJ. Dietary supplements and beverages: Knowledge, attitudes, and practices among semi-professional soccer players in KwaZulu-Natal, South Africa. South Afr J Sports Med. (2022) 34:1–6. doi: 10.17159/2078-516X/2022/V34I1A14018
30. Myoenzono K, Yasuda J, Takai E, Shinagawa A, Kaneko N, Yoshizaki T, et al. Investigation of supplement use and knowledge among Japanese elite athletes for the Tokyo 2020 olympic/paralympic games and the Beijing 2022 winter olympic/paralympic games. Front Sports Act Liv. (2023) 5:1258542. doi: 10.3389/FSPOR.2023.1258542
31. Wardenaar FC, Lybbert H, Morton L, Schott KD, Shumate C, Levinson S, et al. High school athletes’ use and knowledge of (safe) nutritional supplement use: An exploratory study. J Diet Suppl. (2024) 17:1–17. doi: 10.1080/19390211.2023.2301361
32. Wardenaar FC, Morton L, Nam K, Lybbert H, Schott K, Shumate C, et al. The development and efficacy of a high school athlete education program for safe nutritional supplement use. J Diet Suppl. (2023) 22:1–22. doi: 10.1080/19390211.2023.2293845
33. de Oliveira VN, Andrade MS, Sinisgalli R, Vancini RL, de Conti Teixeira Costa G, Weiss K, et al. Prevalence of dietary supplement use among male Brazilian recreational triathletes: A cross-sectional study. BMC Res Notes. (2024) 17:8. doi: 10.1186/S13104-023-06665-9
34. Lauritzen F. Dietary supplements as a major cause of anti-doping rule violations. Front Sports Act Living. (2022) 4:868228. doi: 10.3389/fspor.2022.868228
35. Shaw G, Slater G, Burke LM. Supplement use of elite Australian swimmers. Int J Sport Nutr Exerc Metab. (2016) 26:249–58. doi: 10.1123/IJSNEM.2015-0182
36. Vento KA, Delgado F, Skinner J, Wardenaar F. Funding and college-provided nutritional resources on diet quality among female athletes. J Am Coll Health. (2021) 71:1732–9. doi: 10.1080/07448481.2021.1947301
37. Hamilton KP, Ziegler J, Samavat H, Wardenaar F, Esopenko C, Womack J, et al. Nutritional supplement use and athletic characteristics among a sample of NCAA division I and division III student-athletes. J Diet Suppl. (2022) 3:1–15. doi: 10.1080/19390211.2022.2140740
38. McDaid B, Wardenaar FC, Woodside JV, Neville CE, Tobin D, Madigan S, et al. Athletes perceived level of risk associated with botanical food supplement use and their sources of information. Int J Environ Res Public Health. (2023) 20:6244. doi: 10.3390/IJERPH20136244
39. Wardenaar FC, Hoogervorst D. How sports health professionals perceive and prescribe nutritional supplements to Olympic and non-Olympic athletes. Int J Environ Res Public Health. (2022) 19:12477. doi: 10.3390/IJERPH191912477
40. Shreffler J, Huecker MR. Diagnostic testing accuracy: Sensitivity, specificity, predictive values and likelihood ratios. StatPearls. (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK557491/ (accessed January 8, 2024).
41. U.S. Anti-Doping Agency [USADA]. Athlete Perceptions Survey. (2024). Available online at: https://www.usada.org/resources/athlete-perceptions-survey/ (accessed January 5, 2024).
Keywords: batch testing, dietary supplements, sport foods, ergogenic aids, doping
Citation: Wardenaar FC, Schott KD, Seltzer RGN and Gardner CD (2024) Development of a screener to assess athlete risk behavior of not using third-party tested nutritional supplements. Front. Nutr. 11:1381731. doi: 10.3389/fnut.2024.1381731
Received: 04 February 2024; Accepted: 22 April 2024;
Published: 15 May 2024.
Edited by:
Amanda Jane Lloyd, Aberystwyth University, United KingdomReviewed by:
Douglas Kalman, Nova Southeastern University, United StatesCopyright © 2024 Wardenaar, Schott, Seltzer and Gardner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Floris C. Wardenaar, RmxvcmlzLndhcmRlbmFhckBhc3UuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.