- 1Department of Thoracic Surgery, Tangdu Hospital, Air Force Medical University, Xi'an, Shaanxi, China
- 2Department of Cardiovascular Surgery, Peking University Shenzhen Hospital, Shenzhen, China
Objective: Previous research has established a connection between Type 2 Diabetes Mellitus (T2DM), glycemic traits, dietary habits, and the risk of Pressure Ulcers (PUs). The aim of our study is to disentangle any potential causal relationship between T2DM, glycemic traits, and dietary factors, and the risk of PUs.
Methods: The exposure and outcome datasets were sourced from the IEU Open GWAS project, the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC), and the FinnGen biobank, respectively. The primary MR analysis method employed was the inverse variance-weighted method. Furthermore, we employed multivariable MR (MVMR) adjusting for BMI. Then, we investigated the possibility of a reverse association between glycemic traits and PUs through bidirectional MR. Finally, Heterogeneity and pleiotropic analysis were conducted to ensure the accuracy and robustness of the results.
Results: The findings revealed that T2DM (OR = 1.282, 95% CI: 1.138–1.445, p < 0.001) and Fasting Glucose (FG; OR = 2.111, 95% CI: 1.080–4.129, p = 0.029) were associated with an increased risk of PUs, while salad/raw vegetable intake (OR: 0.014; 95% CI: 0.001–0.278; p = 0.005) was identified as a protective element. However, no other dietary elements demonstrated a statistically significant causality with PUs. In addition, in the reverse direction, there were positive correlation between genetic susceptibility to PUs and an increase in FG (OR: 1.007, 95% CI: 1.000–1.013, p = 0.048) and Fasting Insulin (FI; OR: 1.012, 95% CI: 1.003–1.022, p = 0.011). MVMR results indicated that the causal effect of T2DM on PUs was independent of BMI (OR: 1.260, 95% CI: 1.112–1.427, p < 0.001). These results remained robust when considering weak instrument bias, pleiotropy, and heterogeneity.
Conclusion: This study establishes a causal link between genetically predicted T2DM, FG and an increased risk of PUs. Conversely, Salad/raw vegetable intake is significantly inversely associated with PUs. Simultaneously, we identified two downstream effector factor (FG and FI) that were associated with PUs. These findings may have clinical implications for both prevention and treatment.
1 Introduction
Pressure ulcers (PUs), also known as pressure sores, bed sores or decubitus ulcer, are localized injures to the skin and underlying tissue. They typically occur over bony prominences, and are classified into stages (Stage 1 to Stage 4) based on their severity (1, 2). These injuries develop when prolonged pressure is applied to the skin, often in combination with friction and shear forces. PUs are a significant concern in healthcare, particularly for individuals with limited mobility, such as those who are bedridden or use wheelchairs (3). PUs have substantial healthcare and economic implications and are present in all healthcare settings (4). Although most PUs are reasonably preventable (5), approximately 1 to 3 million individuals in the United States develop PUs each year (6), and 60,000 people die each year due to complications from PUs (7). The management costs of PU are a major problem, with a recent study estimated the annual cost in the United States to be in excess of $26.8 billion (8), and in the United Kingdom, it is estimated at £1.4–£2.1 billion per annum (9). Moreover, PUs can significantly diminish overall quality of life due to pain, the need for management procedures, extended hospital stays, and psychosocial burdens (2, 10, 11). Therefore, identifying the risk and protective factors for PUs is crucial for its prevention and management.
In developing countries and low-income households in the United States, individuals face not only a financial burden but also a higher risk of negative effects because proper nutrition or caregiver support may be deficient (12–14). Insufficient dietary habits or malnutrition may play an important role in this increase of PUs. The pathogenesis of PUs is multifactorial and may be related to genetic, environmental, and lifestyle factors. A number of studies have revealed that inadequate nutritional intake is associated with a higher risk of developing PUs (15–17). In particular, dietary patterns characterized by elevated fiber content, notably derived from whole grains, fruits, and vegetables, have demonstrated an association with enhanced glycemic control. The presence of fiber in these diets serves to decelerate the absorption of glucose, thereby mitigating spikes in blood sugar levels. Conversely, the consumption of a diet rich in added sugars and processed foods has been implicated in the elevation of blood glucose levels, potentially fostering insulin resistance. Individuals exhibiting heightened fasting blood sugar levels or impaired glucose tolerance face an augmented susceptibility to the onset of type 2 diabetes (T2DM) (18, 19). T2DM, a persistent disorder marked by compromised regulation of blood glucose, holds the potential to manifest as enduring microvascular and macrovascular complications and accumulating evidence showed that patients with type 2 diabetes mellitus (T2DM) are 1.5 to 2 times more likelihood to develop surgery-related PUs than patients with normal glucose tolerance (20, 21). The previous studies on potential risk factors for PUs are based on observational research, which may be susceptible to issues related to potential residual confounding and reverse causation (22). We now aim to assess the causal association between dietary habits, T2DM, and the likelihood of developing PUs using Mendelian randomization (MR).
Under certain assumptions, MR utilizes genetic variants as instrumental variables (IVs), offer the advantage of controlling for nonheritable confounders and reverse causation (23). To date, there has been no genetic study on the relationship between T2DM, glycemic traits, dietary habits, and PUs. Herein, we performed a two-sample MR study to explore the causal relationship between dietary habits, T2DM and PU in this study.
2 Methods
The following basic assumptions constitute the premise of MR analysis. (1) IVs must be directly associated with the exposures; (2) IVs cannot be directly correlated to the outcome only via exposure but not through other pathways; (3) IVs were independent of any potential confounding factors (Figure 1). The GWAS summary-level data used in this study were issued by the IEU open GWAS project,1 MAGIC2 and FinnGen biobank (FREEZE 9).3 This study was exempt from the approval of the Ethical Review Authority because the data used in this study was public, anonymized, and de-identified.
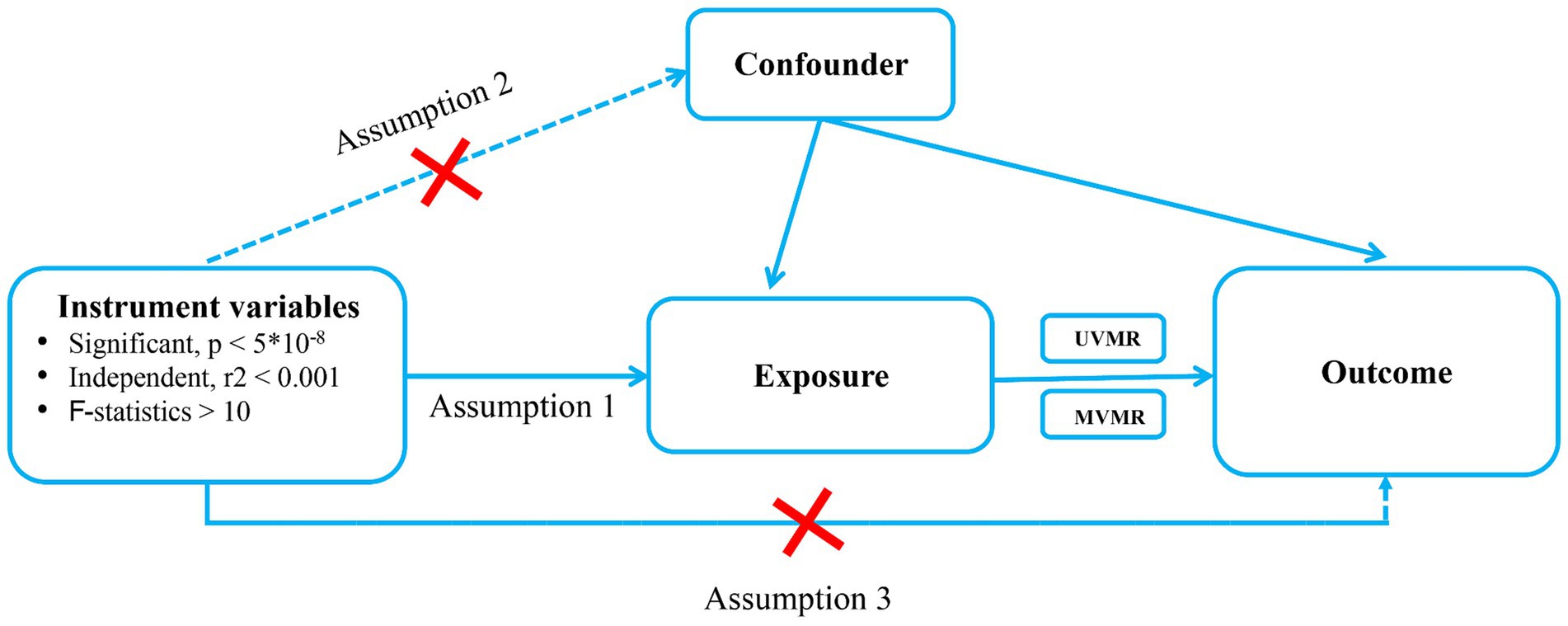
Figure 1. Schematic showing how Mendelian randomization was used to evaluate a causal association between T2DM, glycemic traits, Dietary habits and pressure ulcers in this study. T2DM, Type 2 diabetes Mellitus; UVMR, Univriable mendelian randomization; MVMR, multivariable mendelian randomization.
2.1 Data sources
We obtained genome-wide associations for 17 dietary habits, glycemic traits and a T2DM, derived from principal component (PC) analysis, by using UKBB GWAS summary statistics from Benjamin Neale’s lab,4 MAGIC and Xue et al. (24, 25), respectively. Glycemic traits and diet-associated exposure factors used in this study included fasting glucose (FG), 2 h-glucose post-challenge (2hGlu), glycated hemoglobin (HbA1c), and fasting insulin data (FI), Bread intake, Cheese intake, Drink (Alcoholic drinks per week, Alcohol intake frequency, Water intake, Coffee intake, and Tea intake), Fruit (Dried fruit intake and Fresh fruit intake), Cereal intake, Meat and Poultry (Beef intake, Pork intake, Processed meat intake, and Poultry intake), Seafood (Non-oily fish intake and Oily fish intake), and Vegetable (Salad / raw vegetable intake and Cooked vegetable intake). The GWAS summary-level data of PUs was extracted from FinnGen biobank. We did not use proxy single nucleotide polymorphisms (SNPs) when finding SNPs from the outcome, mainly because the FinnGen biobank contained enough SNPs (16,380,176 SNPs in the dataset of PUs). More information about the exposure and outcome datasets is presented in Table 1.
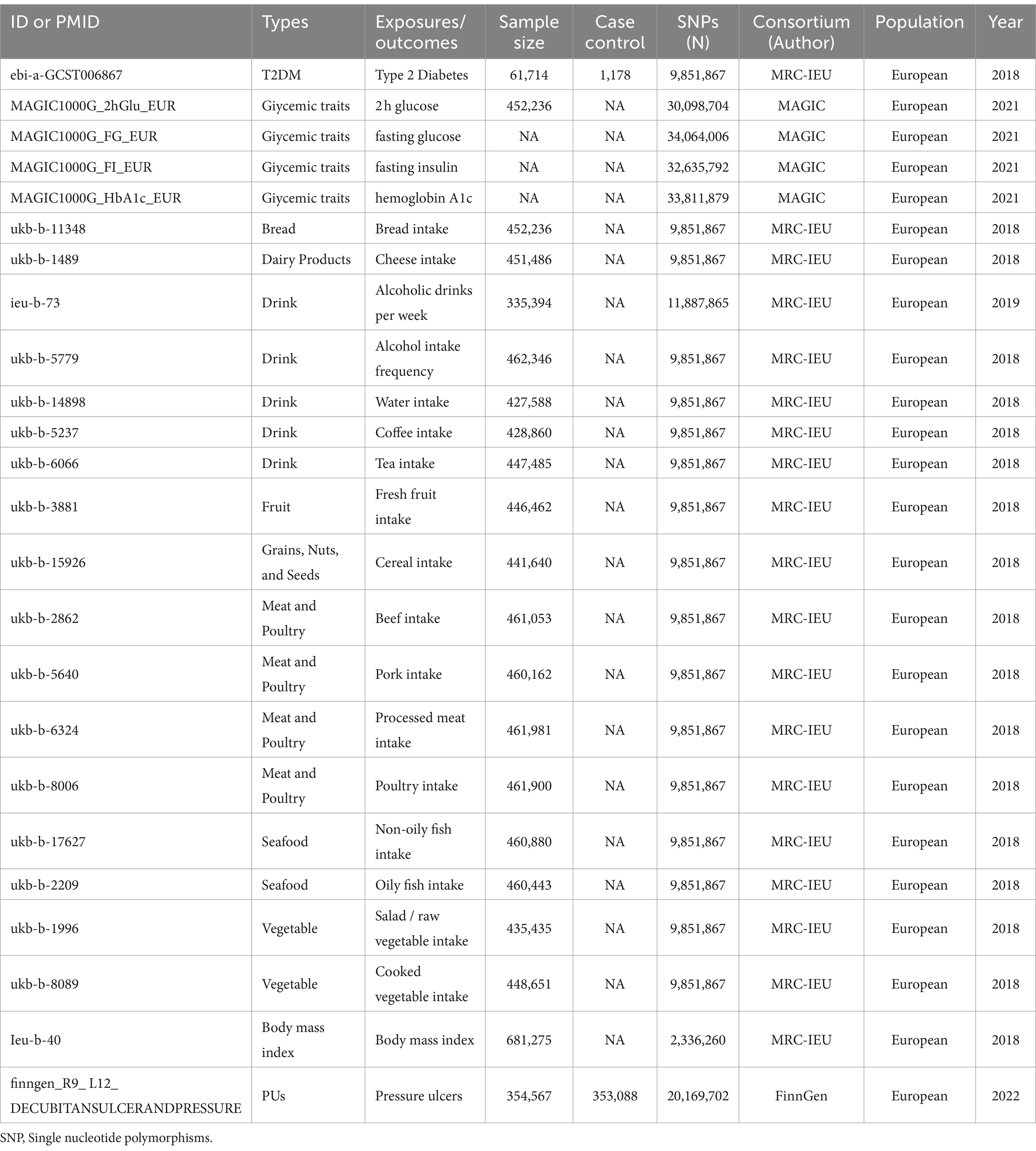
Table 1. Summary of the genome-wide association studies (GWAS) included in this two-sample MR study.
2.2 The selection of IVs
In MR analysis, IVs were utilized as mediators between exposure factors and outcomes to explore the causal relationship between exposure and outcomes. IVs are generally genetic variations, among which SNPs are the most commonly used. SNPs associated with dietary factors were extracted from the IEU open GWAS project. we screened the SNPs intensely related with exposures at the genome-wide significance level (p < 5 × 10−8), clumping window >10,000 kb, and the linkage disequilibrium level (r2 < 0.001). The F statistic greater than 10 was generally considered to meet the requirements of strong association. we searched the remaining SNPs for their associations with other phenotypes (such as body mass index and overweight (26, 27)) in PhenoScanner5 (28), a database of human genotype–phenotype associations and excluded those associated with potential confounding traits. However, in the reverse MR analysis, since very few SNPs were identified for part of PUs when they were as the exposure, a higher cutoff (p < 5e–06) was condisered as the genome-wide significant threshold.
2.3 Statistical analysis
We employed the random-effects inverse variance-weighted (IVW) method as the primary approach for calculating the causal effect. The IVW model is recognized for its strong capability in detecting causation in the two-sample MR analysis (18). To enhance the robustness of our findings, we compared the results obtained from the three different IVW methods, random-effects, fixed-effects, and multiplicative random-effects model, with those from the Weighted median, Simple mode, Weighted mode, and MR-Egger methods. The Weighted median method allows no more than 50% of invalid IVs, Simple Mode selects IVs with the most frequent occurrence in MR analysis, Weighted Mode, akin to Simple Mode, considers the weight of IVs, with higher frequency IVs receiving greater weights to enhance their contribution to causal estimation, while the MR-Egger method allows all IVs to be potentially invalidated (29). Thus, when all models yield consistent results, the evidence becomes more compelling.
We assessed heterogeneity in the IVW model using Cochran’s Q test, where a p-value of <0.05 indicates the presence of heterogeneity. It is important to note that the presence of heterogeneity does not necessarily invalidate the IVW model. Additionally, we utilized the MR-Egger method, which accommodates non-zero intercepts, to detect directional pleiotropy. To ensure the robustness of our results, we conducted a leave-one-out analysis to examine whether the removal of a SNP significantly influenced the outcomes. The MR-PRESSO method was employed to identify and address outliers. Upon detection of outliers, they were promptly removed, and the MR analysis was performed again.
Considering that BMI confound T2DM and glycemic traits in the pathogenesis of PUs, we used a multivariate MR approach to adjust for BMI, aiming to obtain an independent causal efect of T2DM and glycemic traits on the pathogenesis of PUs (30). The conditional F statistic for the BMI phenotype was calculated to evaluate the joint strength of instruments in the multivariable framework. All analyses were conducted using the TwoSampleMR package (version 0.5.7) (31), ‘MR-PRESSO’ package (version 1.0), ‘MendelianRandomization’ package (version 0.8.0) and ‘MVMR’ package (version 0.4) based on R software (version 4.2.2).
3 Results
3.1 Genetic instruments for T2DM and glycemic traits
SNPs with low allele frequencies <0.01 or no meaningful genome-wide association evidence (p < 5 × 10−8) were excluded. We identified 64, 10, 40, and 9 SNPs as LD-independent IVs (after the clumping process) in T2DM, 2hGlu, FG, FI, and HbA1c. In our study, F statistics were all significantly >10, suggesting that our results were highly trustworthy and largely unaffected by weak IVs (Supplementary Table S1).
3.2 Genetic instruments for 17 dietary habits
Supplementary Table S1 provides comprehensive information about each of the participating GWAS study. The analyses encompassed a total of 17 different dietary habits as exposures. The number of SNPs considered for each dietary habit varied between 3 and 60. We evaluated the influence of various dietary exposures on the outcome, and, in all cases, the F statistics of the identified SNPs exceeded the empirical threshold of 10, with the sum of F values ranging from 74.427 to 5216.822. This finding indicates that the results obtained are less susceptible to biases stemming from weak IVs.
3.3 Causal effects of T2DM, glycemic traits, and dietary habits on PU
In our study, a total of 3 causal associations were identified (p < 0.05 by IVW method). We found that T2DM (OR = 1.273, 95% CI: 1.040–1.557, p = 0.019) and FG (OR = 2.111, 95% CI: 1.080–4.129, p = 0.029) was related to an increased risk of PU, these discoveries were further verified by the consequences of the weighted median model (OR: 1.369; 95% CI: 1.045–1.793; p = 0.023) and (OR: 3.334; 95% CI: 1.235–9.000; p = 0.017), respectively. In contrast, salad/raw vegetable intake (OR: 0.014; 95% CI: 0.001–0.278; p = 0.005) was discovered as protective factors, and there was a statistically significant result in weighted median model (OR: 0.011; 95% CI: 0.000–0.498; p = 0.021). However, our study also indicated that Alcoholic drinks per week (OR = 1.765, 95% CI: 0.521–5.978, p = 0.361), Alcohol intake frequency (OR = 0.887, 95% CI: 0.533–1.473, p = 0.642), Bread intake (OR = 1.029, 95% CI: 0.207–5.127, p = 0.972), Cheese intake (OR = 1.202, 95% CI: 0.532–2.713, p = 0.658), Water intake (OR = 3.150, 95% CI: 0.801–12.393, p = 0.101), Cereal intake (OR = 0.750, 95% CI: 0.175–3.203, p = 0.697), Dried fruit intake (OR = 1.493, 95% CI:0.522–4.270, p = 0.455), Non-oily fish intake (OR = 10.987, 95% CI: 0.386–312.569, p = 0.161), Oily fish intake (OR = 1.427, 95% CI: 0.583–3.492, p = 0.436), Beef intake (OR = 1.092, 95% CI: 0.044–26.864, p = 0.957), Fresh fruit intake (OR = 2.750, 95% CI: 0.597–12.671, p = 0.194), Coffee intake (OR = 0.916, 95% CI:0.211–3.970, p = 0.907), Pork intake (OR = 2.745, 95% CI: 0.101–74.322, p = 0.548), Tea intake (OR = 0.666, 95% CI: 0.262–1.688, p = 0.391), Processed meat intake (OR = 0.363, 95% CI: 0.091–1.453, p = 0.152), Poultry intake (OR = 0.206, 95% CI: 0.003–14.110, p = 0.464), and Cooked vegetable intake (OR = 2.528, 95% CI: 0.166–38.386, p = 0.504) were not associated with PU in the IVW method (Figure 2; Supplementary Table S2). Albeit with there were no evidence for significant outliers, heterogeneity effect and potential pleiotropy (p > 0.05; Supplementary Tables S3, S4), we still used the fixed effect and multiplicative random effects IVW method as the major complementary approaches. The results of above two IVW methods remained the consistence with the random effect IVW. Moreover, scatter plots (Figures 3A–C) and funnel plots (Supplementary Figures S1A–C) confirmed the credibility of the results of our MR study. Based on the leave-one-out sensitively analysis (Figures 4A–C) and forest plot (Supplementary Figures S2A–C), it is suggested that the causal effect of T2DM and dietary habits on PUs was not driven by any single SNP.
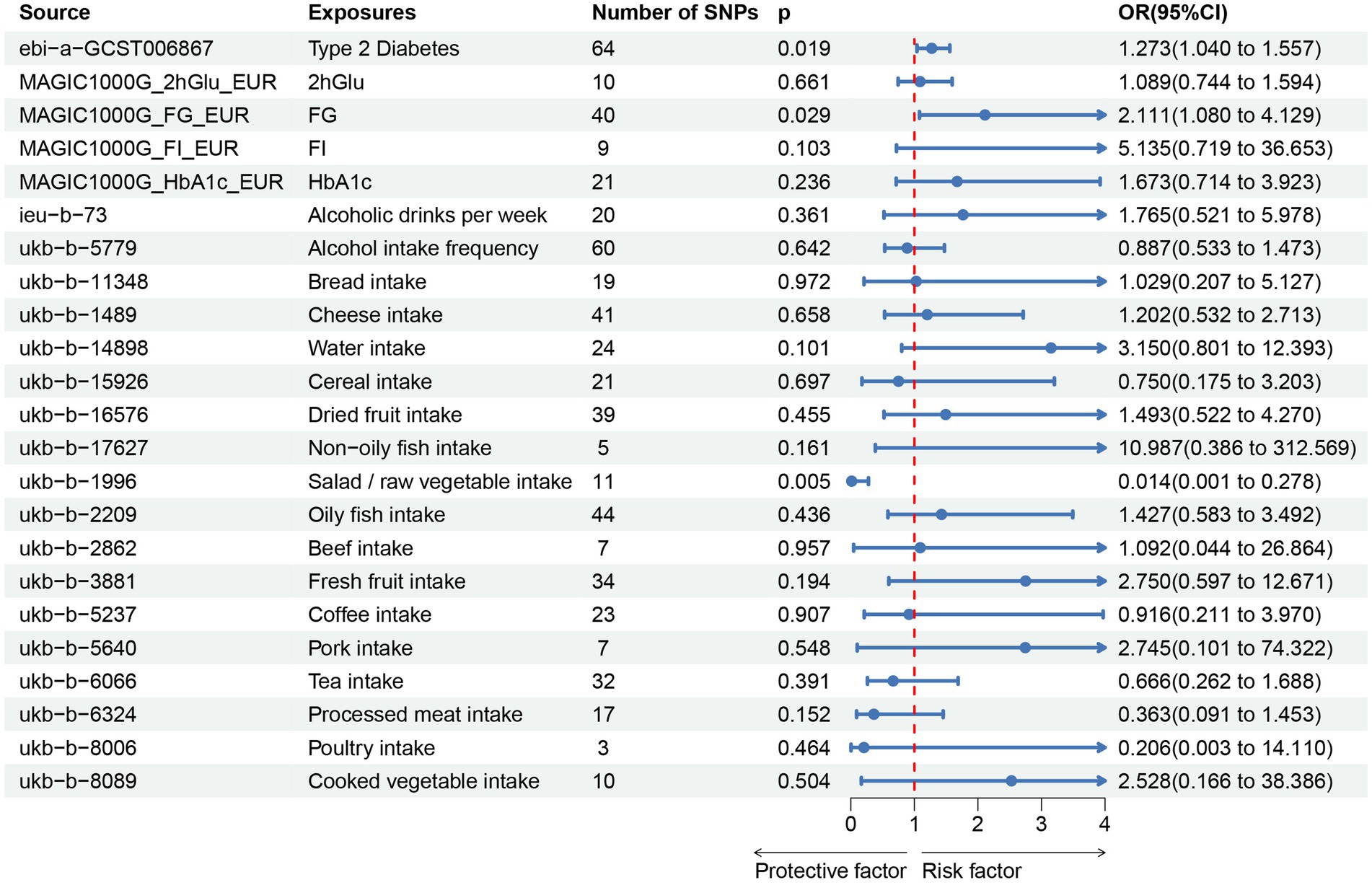
Figure 2. Associations of genetically predicted T2DM, glycemic traits, dietary habits with the risk of pressure ulcers. T2DM, Type 2 Diabetes Mellitus; FG, fasting Glucose; FI, Fasting Insulin; 2hGlu, 2 h-glucose post-challenge; HbA1c, glycated hemoglobin; and fasting insulin data (FI).
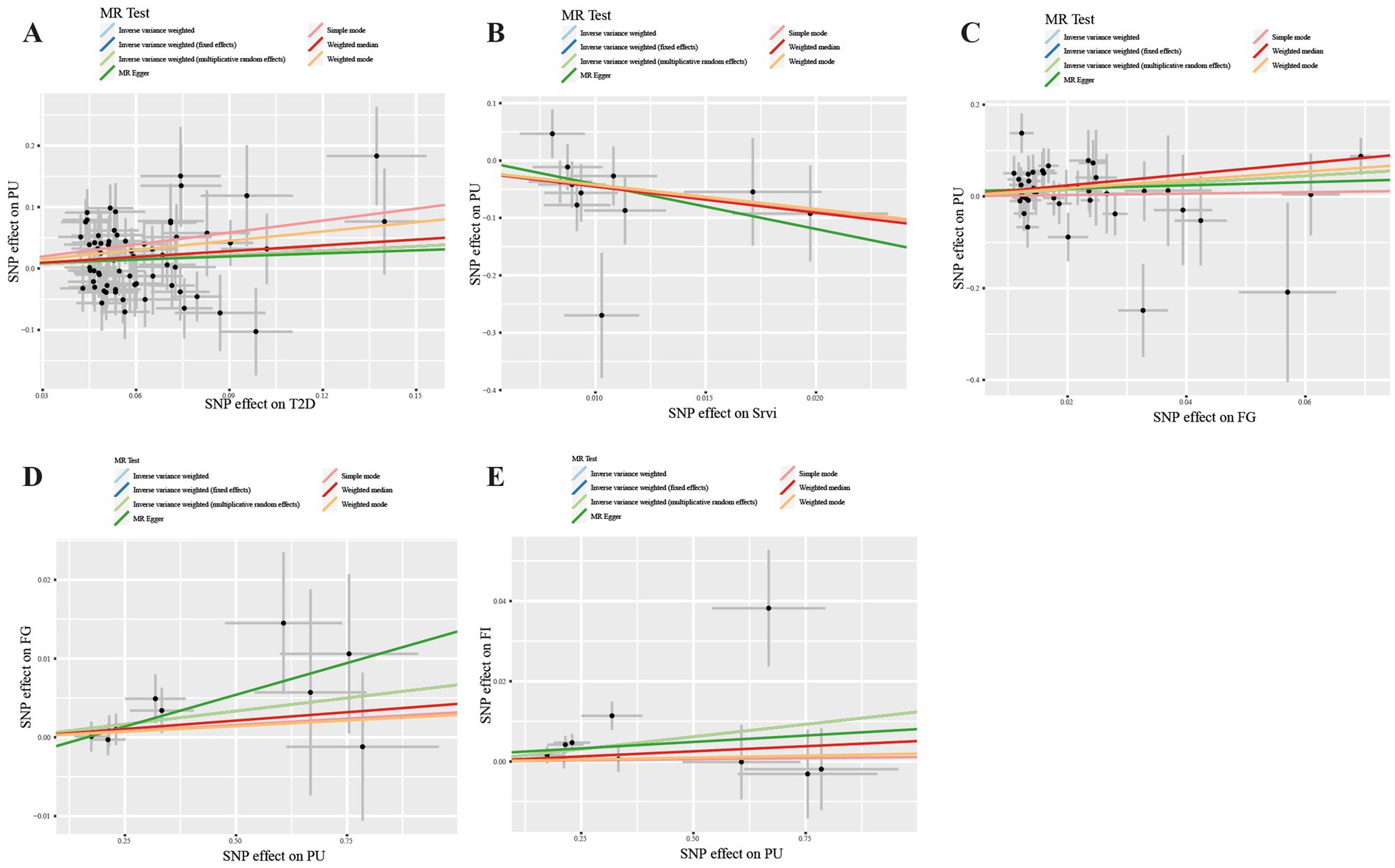
Figure 3. Scatter plot in the Mendelian randomization analysis of T2DM, salad/raw vegetable intake, glycemic traits and Pressure ulcers. Scatter plot of T2DM (A), Srvi (B), and FG (C) on PUs and PUs on FG (D) and FI (E). T2DM, Type 2 Diabetes Mellitus; Srvi, salad/raw vegetable intake; PUs, Pressure ulcers; FG, fasting Glucose; FI, Fasting Insulin.

Figure 4. Leave-one-out sensitively analysis Scatter plot in the Mendelian randomization analysis of T2DM, salad/raw vegetable intake, glycemic traits and Pressure ulcers. “Leave-one-out” plot of T2DM (A), Srvi (B), and FG (C) on PUs and PUs on FG (D) and FI (E). T2DM, Type 2 Diabetes Mellitus; Srvi, salad/raw vegetable intake; PUs, Pressure ulcers; FG, fasting Glucose; FI, Fasting Insulin.
3.4 Causal effects of PU on T2DM and glycemic traits
For the reverse MR analysis, a total of 10 SNPs implicated with PU were selected at a less stringent cut-off (p < 5 × 10−6), and all of which had F-statistics more than 10, demonstrating the positive association between genetically predicted PU patients and the levels of FG (OR: 1.007; 95% CI: 1.000–1.013; p = 0.048) and FI (OR: 1.012; 95% CI: 1.003–1.022; p = 0.011), the findings of other MR approaches were similar to the IVW method. In addition, no evidence showed the causal influence of PUs patients on T2DM (OR: 1.017; 95% CI: 0.983–1.052; p = 0.341), 2hGlu (OR: 1.024; 95% CI: 0.993–1.056; p = 0.124), and HbA1c (OR: 1.002; 95% CI: 0.996–1.007; p = 0.544; Figure 5; Supplementary Table S5). MR-PRESSO determined no horizontal pleiotropy, and no heterogeneity was observed in estimating the effect of PU patients on T2DM and Glycemic (Supplementary Tables S6, S7). Furthermore, our MR study results were supported by scatter plots (Figures 3D,E) and funnel plots (Supplementary Figures S1D,E), indicating credibility. The leave-one-out sensitivity analysis (Figures 4D,E) and forest plot (Supplementary Figures S2D,E) suggest that the causal effect of T2DM and dietary habits on pressure ulcers is not influenced by any single SNP.
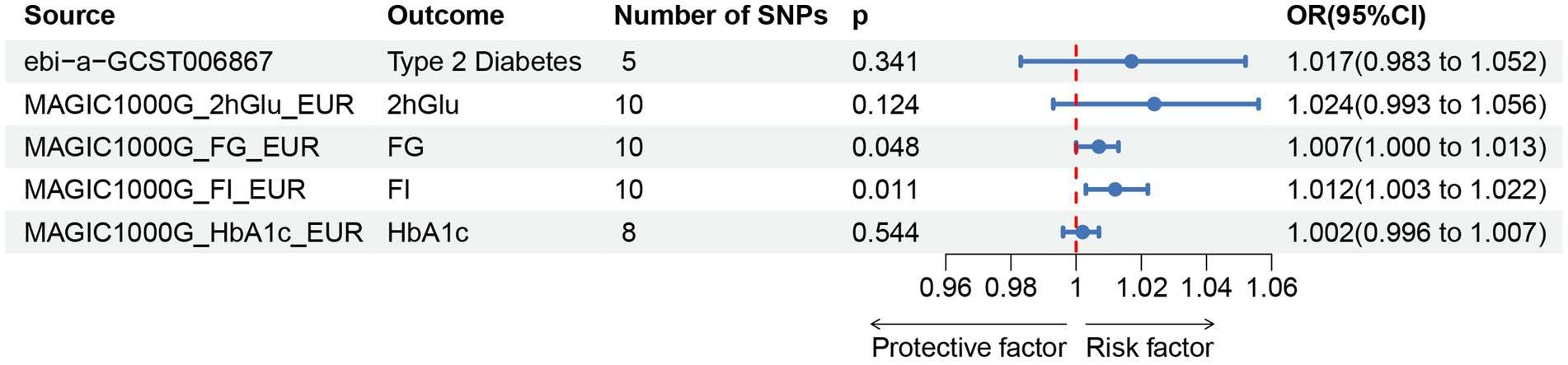
Figure 5. Associations of genetically predicted pressure ulcers on T2DM and glycemic traits.T2DM, Type 2 Diabetes Mellitus; FG, fasting Glucose; FI, Fasting Insulin; 2hGlu, 2 h-glucose post-challenge; HbA1c, glycated hemoglobin; and fasting insulin data (FI).
3.5 Multivariate MR
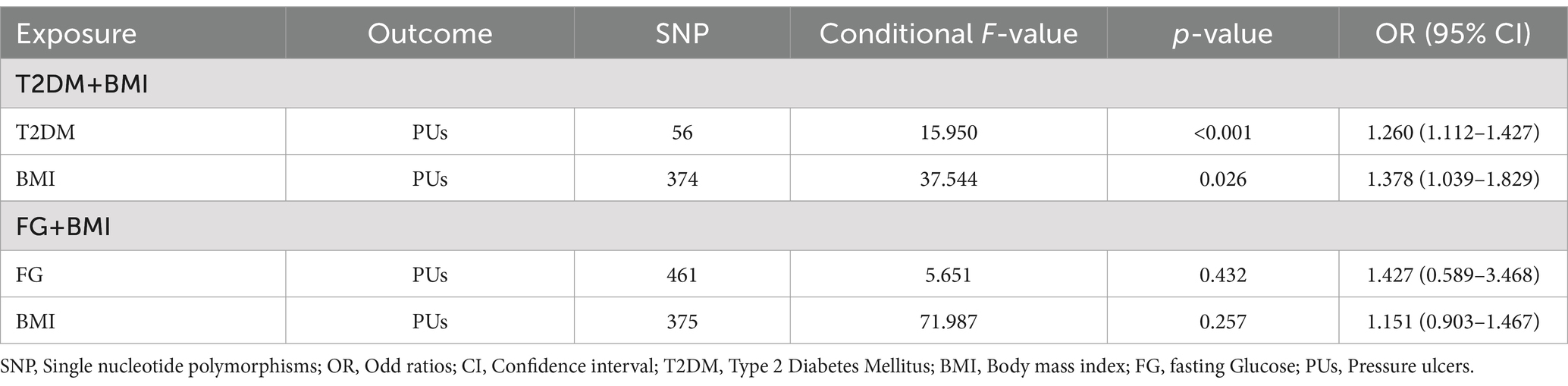
To confirm whether the correlation of T2DM or FG on PUs is independent of BMI, the multivariate MR analysis was conducted. Specifically, we identified SNPs associated with T2DM or FG and BMI separately (p < 5 × 10−8) and combined all genetic variants. Following the exclusion of duplicate IVs, we observed a significant causal association between T2DM and BMI and PUs (p < 0.001; p = 0.026. respectively) but no causal association for FG (p = 0.432; Table 2).
4 Discussion
Based on summary level data from large GWAS, we implemented a two-sample univariable MR study to investigate the causal association between dietary habits and PUs. Employing a bidirectional analytical approach for T2DM, Glycemic traits, and the risk of PUs. We further applied Multivariable MR to eliminate the potential confounding factors, specifically BMI, revealing that T2DM is the independent effect factor on PUs, allowing us to differentiate between upstream and downstream factors in the disease pathway. Our results indicated that genetically predicted T2DM and FG are positively associated with the risk of PUs, while Salad / raw vegetable intake shows a negative association with PU risk. Additionally, we observed that PUs is associated with increased levels of FG and FI. These findings were generally robust in sensitivity analysis, ensuring the reliability of our MR analyses and mitigating potential pleiotropic effects. According to our knowledge, this represents the first MR study evaluating potential causative links between T2DM, glycemic traits, dietary habits, and PUs.These findings provide valuable insights for the prevention and treatment of PUs.
Our findings reveal that T2DM and the elevated levels of FG increase the risk of PUs, this conclusion aligns with the results of numerous studies. A meta-analysis of 15 observational studies, encompassing 19,724 intraoperative PUs in patients conducted in Asia, the Americas, Europe, and Australia from 1989 to 2019, indicated that the incidence of PUs in the T2DM population increased by 50% compared to non-T2DM individuals (OR = 1.52, 95% CI 1.25–1.85) (20). Other research also confirms this finding, with a meta-analysis of six observational studies involving all 2,453 patients. Compared to patients without T2DM or normal glucose levels, individuals with T2DM were more than twice as likely to develop surgical PUs (OR = 2.15, 95% CI: 1.62–2.84) (21). One potential explanation is that T2DM induces peripheral neuropathy, impairs sensory perception, and leads to prolonged insensitivity to compression, tissue necrosis, and nonhealing of affected areas. Peripheral neuropathy, in turn, has also been associated with neuropathic ulcers and diabetic foot ulcers (32–34). Another mechanism is that T2DM and high FG levels have been shown to reduce blood flow to the skin and underlying tissues. Poor circulation can result in decreased oxygen and nutrient delivery to these tissues, making them more vulnerable to damage from pressure and friction. Impaired blood flow also slows down the healing process of PUs once they have formed (35). Additionally, T2DM can cause chronic inflammation and reduced angiogenesis, further hindering the healing processes of PUs. In mice models of T2DM, reducing inflammation by increasing levels of several pro-healing growth factors has been shown to improve wound healing (36–38). Lastly, T2DM can reduce the formation of collagen, a protein essential for skin strength and elasticity. This can affect the skin’s ability to withstand pressure and shear forces, making it more susceptible to injury and increasing the likelihood of developing PUs (39). Moreover, T2DM and a longer duration of hyperglycemia can lead to metabolic imbalances, including altered protein and nutrient metabolism (40). These potential mechanisms can affect the body’s ability to generate new tissue and repair damaged skin. Adequate nutrition is critical for the wound healing of PUs, and individuals with poorly controlled T2DM and FI levels may be at greater risk of malnutrition (41, 42). Our findings also suggest a reciprocal causation between FG and PUs, elevated FG is often associated with insulin resistance, a condition where the body’s cells become less responsive to the effects of insulin and tissue damage and inflammation caused by PUs may trigger a stress response in the body, potentially affecting insulin sensitivity and metabolism (43). Therefore, high levels of FI and FG are closely associated with the metabolic response and disease progression in PUs.
It is worth noting that our study revealed an inverse association between salad/raw vegetable intake and PUs. Consistent with previous studies, the beneficial impact could be attribeted to components found in salad/raw vegetables, which are typically rich in vitamins, minerals, sufficient water, and antioxidants. These components play crucial roles in skin health, digestive health, regular bowel movements, and tissue repair (43–46). Recent evidence emphasizes that plant polyphenolic compounds, which are prevalent in the human diet and present in substances such as curcumin (47), apigenin (48), Ocimum basilicum, and Trifolium pratense extracts (49), confer various health benefits. These compounds are well-recognized for their potent antioxidant, antimicrobial, and anti-inflammatory properties. They neutralize free radicals by inhibiting key signaling pathways, including NF-κB, transforming growth factor-beta (TGF-β), and mitogen-activated protein kinase, while also enhancing the activity of antioxidant enzymes including superoxide dismutase (SOD), peroxidases, and catalase (50). Collectively, these effects support wound healing and skin barrier repair. Additionally, these polyphenolic compounds are crucial in recruiting specific cells to sites of inflammation and accelerating the overall healing process. Adequate nutrient supply can promote healthy skin and reduce the risk of PUs development. Diets with higher vegetable intake are generally more favorable for weight management. Maintaining an appropriate weight can alleviate the pressure on the skin, reducing the likelihood of skin damage. Certain components in vegetables have anti-inflammatory and immune-supporting properties, potentially helping to lower skin inflammation levels and mitigate tissue damage caused by inflammation (51).
The relationship between alcohol intake and the risk of developing PUs is a matter of debate, with varying results from different studies. Several studies have linked alcohol consumption to an increased risk of developing PUs (52, 53). However, other research has found that the consumption of alcohol was not significantly linked to the incidence of PUs (54, 55). Despite applying MR analysis to investigate the potential association between alcohol consumption and PUs, no causal association was shown in the study. Vegetables, abundant in Vitamin C and Quercetin, are commonly recommended as dietary components for the prevention or treatment of PUs (44, 56, 57).
Our study has several strengths and limitations. Firstly, this is the first study to investigate the causal relationship between T2DM, glycemic traits, dietary habits and PUs through a Two-sample MR analysis, which is not affected by confounders and reverse causation compared with observational studies. Additionally, we conducted multivariable MR analyses to disentangle the direct causal impacts of T2DM on PUs. Finally, multiple sensitivity analyses and IV strength evaluations were conducted to ensure the robustness and reliability of the results. However, there are also some limitations. Firstly, MR analysis is based on specific assumptions that cannot be verified. Secondly, the present study primarily involved individuals of European ancestry, which may limit the generalizability of our findings to other populations. Thirdly, while we explored the association between T2DM, salad/raw vegetable intake, FI, FG and PUs from a genetic perspective, the underlying mechanisms remain unclear and warrant further investigation.
5 Conclusion
This study found that salad/raw vegetabke intake associated with a reduced risk of PUs, while T2DM and FG were associated with an increased risk of PUs. Furthermore, FI and FG may play pivotal roles as downstream factors in PUs. This study also found that various dietary habits including alcoholic drinks per week, alcohol intake frequency, bread intake, cheese intake, water intake, cereal intake, non-oily fish intake, oily fish intake, beef intake, fresh fruit intake, coffee intake, pork intake, tea intake, processed meat intake, poultry intake, and cooked vegetable intake were not associated with PUs, which were robust to different analyses and rigorous pleiotropy testing.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
PL: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. CH: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
All authors would like to thank the research groups for IEU Open GWAS project, MAGIC and the FinnGen biobank and providers of other databases mentioned in the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1375179/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Funnel plot in the Mendelian randomization analysis of T2DM, salad/raw vegetable intake, glycemic traits and Pressure ulcers. Funnel plot of T2DM (A), Srvi (B), and FG (C) on PUs and PUs on FG (D) and FI (E). T2DM, Type 2 Diabetes Mellitus; Srvi, salad/raw vegetable intake; PUs, Pressure ulcers; FG, fasting Glucose; FI, Fasting Insulin.
SUPPLEMENTARY FIGURE 2 | Forest plot in the Mendelian randomization analysis of T2DM, salad/raw vegetable intake, glycemic traits and Pressure ulcers. Forest plot of T2DM (A), Srvi (B), and FG (C) on PUs and PUs on FG (D) and FI (E). T2DM, Type 2 Diabetes Mellitus; Srvi, salad/raw vegetable intake; PUs, Pressure ulcers; FG, fasting Glucose; FI, Fasting Insulin.
Footnotes
1. ^https://gwas.mrcieu.ac.uk/
2. ^http://magicinvestigators.org/downloads/
References
1. Kottner, J, Cuddigan, J, Carville, K, Balzer, K, Berlowitz, D, Law, S, et al. Prevention and treatment of pressure ulcers/injuries: the protocol for the second update of the international clinical practice guideline 2019. J Tissue Viability. (2019) 28:51–8. doi: 10.1016/j.jtv.2019.01.001
2. Crunden, EA, Schoonhoven, L, Coleman, SB, and Worsley, PR. Reporting of pressure ulcers and medical device related pressure ulcers in policy and practice: a narrative literature review. J Tissue Viability. (2022) 31:119–29. doi: 10.1016/j.jtv.2021.10.010
3. Lindgren, M, Unosson, M, Fredrikson, M, and Ek, AC. Immobility--a major risk factor for development of pressure ulcers among adult hospitalized patients: a prospective study. Scand J Caring Sci. (2004) 18:57–64. doi: 10.1046/j.0283-9318.2003.00250.x
4. Edsberg, LE, Langemo, D, Baharestani, MM, Posthauer, ME, and Goldberg, M. Unavoidable pressure injury: state of the science and consensus outcomes. J Wound Ostomy Cont Nurs. (2014) 41:313–34. doi: 10.1097/won.0000000000000050
5. Black, JM, Cuddigan, JE, Walko, MA, Didier, LA, Lander, MJ, and Kelpe, MR. Medical device related pressure ulcers in hospitalized patients. Int Wound J. (2010) 7:358–65. doi: 10.1111/j.1742-481X.2010.00699.x
6. Mervis, JS, and Phillips, TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. (2019) 81:881–90. doi: 10.1016/j.jaad.2018.12.069
7. Lyder, CH, Wang, Y, Metersky, M, Curry, M, Kliman, R, Verzier, NR, et al. Hospital-acquired pressure ulcers: results from the National Medicare Patient Safety Monitoring System Study. J Am Geriatr Soc. (2012) 60:1603–8. doi: 10.1111/j.1532-5415.2012.04106.x
8. Bennett, G, Dealey, C, and Posnett, J. The cost of pressure ulcers in the Uk. Age Ageing. (2004) 33:230–5. doi: 10.1093/ageing/afh086
9. Padula, WV, and Delarmente, BA. The National Cost of hospital-acquired pressure injuries in the United States. Int Wound J. (2019) 16:634–40. doi: 10.1111/iwj.13071
10. Gorecki, C, Closs, SJ, Nixon, J, and Briggs, M. Patient-reported pressure ulcer pain: a mixed-methods systematic review. J Pain Symptom Manag. (2011) 42:443–59. doi: 10.1016/j.jpainsymman.2010.11.016
11. Spilsbury, K, Nelson, A, Cullum, N, Iglesias, C, Nixon, J, and Mason, S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs. (2007) 57:494–504. doi: 10.1111/j.1365-2648.2006.04140.x
12. Bereded, DT, Salih, MH, and Abebe, AE. Prevalence and risk factors of pressure ulcer in hospitalized adult patients; a single center study from Ethiopia. BMC Res Notes. (2018) 11:847. doi: 10.1186/s13104-018-3948-7
13. Saunders, LL, Krause, JS, and Acuna, J. Association of Race, socioeconomic status, and health care access with pressure ulcers after spinal cord injury. Arch Phys Med Rehabil. (2012) 93:972–7. doi: 10.1016/j.apmr.2012.02.004
14. Zakrasek, EC, Creasey, G, and Crew, JD. Pressure ulcers in people with spinal cord injury in developing nations. Spinal Cord. (2015) 53:7–13. doi: 10.1038/sc.2014.179
15. Guenter, P, Malyszek, R, Bliss, DZ, Steffe, T, O'Hara, D, LaVan, F, et al. Survey of nutritional status in newly hospitalized patients with stage iii or stage iv pressure ulcers. Adv Skin Wound Care. (2000) 13:164–8.
16. Horn, SD, Bender, SA, Ferguson, ML, Smout, RJ, Bergstrom, N, Taler, G, et al. The National Pressure Ulcer Long-Term Care Study: pressure ulcer development in Long-term care residents. J Am Geriatr Soc. (2004) 52:359–67. doi: 10.1111/j.1532-5415.2004.52106.x
17. Mathus-Vliegen, EMH. Clinical observations: nutritional status, nutrition, and pressure ulcers. Nutr Clin Pract. (2016) 16:286–91. doi: 10.1177/088453360101600505
18. Jenkins, DJA, Kendall, CWC, McKeown-Eyssen, G, Josse, RG, Silverberg, J, Booth, GL, et al. Effect of a low–glycemic index or a high–cereal Fiber diet on type 2 diabetes: a randomized trial. JAMA. (2008) 300:2742–53. doi: 10.1001/jama.2008.808
19. Venn, BJ, and Green, TJ. Glycemic index and glycemic load: measurement issues and their effect on diet–disease relationships. Eur J Clin Nutr. (2007) 61:S122–31. doi: 10.1038/sj.ejcn.1602942
20. Nasiri, E, Mollaei, A, Birami, M, Lotfi, M, and Rafiei, MH. The risk of surgery-related pressure ulcer in diabetics: a systematic review and Meta-analysis. Ann Med Surg. (2021) 65:65. doi: 10.1016/j.amsu.2021.102336
21. Liu, P, He, W, and Chen, HL. Diabetes mellitus as a risk factor for surgery-related pressure ulcers: a Meta-analysis. J Wound Ostomy Continence Nurs. (2012) 39:495–9. doi: 10.1097/WON.0b013e318265222a
22. Smith, GD, and Ebrahim, S. 'Mendelian Randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
23. Boyko, EJ. Observational research--opportunities and limitations. J Diabetes Complicat. (2013) 27:642–8. doi: 10.1016/j.jdiacomp.2013.07.007
24. Xue, A, Wu, Y, Zhu, Z, Zhang, F, Kemper, KE, Zheng, Z, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. (2018) 9:2941. doi: 10.1038/s41467-018-04951-w
25. Chen, J, Spracklen, CN, Marenne, G, Varshney, A, Corbin, LJ, Luan, J, et al. The trans-ancestral genomic architecture of glycemic traits. Nat Genet. (2021) 53:840–60. doi: 10.1038/s41588-021-00852-9
26. Chen, F, Wang, X, Pan, Y, Ni, B, and Wu, J. The paradox of obesity in pressure ulcers of critically ill patients. Int Wound J. (2023) 20:2753–63. doi: 10.1111/iwj.14152
27. Hyun, S, Li, X, Vermillion, B, Newton, C, Fall, M, Kaewprag, P, et al. Body mass index and pressure ulcers: improved predictability of pressure ulcers in intensive care patients. Am J Crit Care. (2014) 23:494–500. doi: 10.4037/ajcc2014535
28. Kamat, MA, Blackshaw, JA, Young, R, Surendran, P, Burgess, S, Danesh, J, et al. Phenoscanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
29. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
30. Davies, NM, Hill, WD, Anderson, EL, Sanderson, E, Deary, IJ, and Davey, SG. Multivariable two-sample Mendelian randomization estimates of the effects of intelligence and education on health. eLife. (2019) 8:8. doi: 10.7554/eLife.43990
31. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The Mr-Base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:7. doi: 10.7554/eLife.34408
32. Singh, N, Armstrong, DG, and Lipsky, BA. Preventing foot ulcers in patients with diabetes. JAMA. (2005) 293:217–28. doi: 10.1001/jama.293.2.217
33. Frykberg, RG, Lavery, LA, Pham, H, Harvey, C, Harkless, L, and Veves, A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. (1998) 21:1714–9. doi: 10.2337/diacare.21.10.1714
34. Pecoraro, RE, Reiber, GE, and Burgess, EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. (1990) 13:513–21. doi: 10.2337/diacare.13.5.513
35. Fromy, B, Abraham, P, Bouvet, C, Bouhanick, B, Fressinaud, P, and Saumet, JL. Early decrease of skin blood flow in response to locally applied pressure in diabetic subjects. Diabetes. (2002) 51:1214–7. doi: 10.2337/diabetes.51.4.1214
36. Dasari, N, Jiang, A, Skochdopole, A, Chung, J, Reece, EM, Vorstenbosch, J, et al. Updates in diabetic wound healing, inflammation, and scarring. Semin Plast Surg. (2021) 35:153–8. doi: 10.1055/s-0041-1731460
37. Mirza, RE, Fang, MM, Weinheimer-Haus, EM, Ennis, WJ, and Koh, TJ. Sustained Inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. (2014) 63:1103–14. doi: 10.2337/db13-0927
38. Sindrilaru, A, Peters, T, Wieschalka, S, Baican, C, Baican, A, Peter, H, et al. An unrestrained Proinflammatory M1 macrophage population induced by Iron impairs wound healing in humans and mice. J Clin Invest. (2011) 121:985–97. doi: 10.1172/JCI44490
39. Spanheimer, RG, Umpierrez, GE, and Stumpf, V. Decreased collagen production in diabetic rats. Diabetes. (1988) 37:371–6. doi: 10.2337/diab.37.4.371
40. Banday, MZ, Sameer, AS, and Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J Med. (2020) 10:174–88. doi: 10.4103/ajm.ajm_53_20
41. Alfonso-Rosa, RM, del Pozo-Cruz, B, Del Pozo-Cruz, J, Del Pozo-Cruz, JT, and Sañudo, B. The relationship between nutritional status, functional capacity, and health-related quality of life in older adults with type 2 diabetes: a pilot explanatory study. J Nutr Health Aging. (2013) 17:315–21. doi: 10.1007/s12603-013-0028-5
42. Galicia-Garcia, U, Benito-Vicente, A, Jebari, S, Larrea-Sebal, A, Siddiqi, H, Uribe, KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. (2020) 21:6275. doi: 10.3390/ijms21176275
43. Doley, J. Nutrition Management of Pressure Ulcers. Nutr Clin Pract. (2010) 25:50–60. doi: 10.1177/0884533609359294
44. Taylor, C. Importance of nutrition in preventing and treating pressure ulcers. Nurs Older People. (2017) 29:33–9. doi: 10.7748/nop.2017.e910
45. Cui, J, Lian, Y, Zhao, C, Du, H, Han, Y, Gao, W, et al. Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compr Rev Food Sci Food Saf. (2019) 18:1514–32. doi: 10.1111/1541-4337.12489
46. Evans, JA, and Johnson, EJ. The role of phytonutrients in skin health. Nutrients. (2010) 2:903–28. doi: 10.3390/nu2080903
47. Chioreanu, A, Mot, IC, Horhat, DI, Balica, NC, Sarau, CA, Morar, R, et al. Development and preliminary characterization of polyester-urethane microparticles used in curcumin drug delivery system for oropharyngeal Cancer. Medicina (Kaunas). (2022) 58:1689. doi: 10.3390/medicina58111689
48. Liu, E, Gao, H, Zhao, Y, Pang, Y, Yao, Y, Yang, Z, et al. The potential application of natural products in cutaneous wound healing: a review of preclinical evidence. Front Pharmacol. (2022) 13:900439. doi: 10.3389/fphar.2022.900439
49. Antonescu, A-IM, Antonescu, A, Miere, FG, Fritea, L, Teodorescu, AG, Vicas, L, et al. Novel Topical Formulations Based on O. Basilicum and T. pratense: antioxidant, antimicrobial, and anti-inflammatory effect. Pharmacophore. (2022) 13:80–90. doi: 10.51847/c9XdRSVT7W
50. Jakovljević, D, Momčilović, J, Bojović, B, and Stanković, M. The short-term metabolic modulation of basil (Ocimum Basilicum L. cv. 'Genovese') after exposure to cold or heat. Plants (Basel). (2021) 10:590. doi: 10.3390/plants10030590
51. Syed, RU, Moni, SS, Break, MKB, Khojali, WMA, Jafar, M, Alshammari, MD, et al. Broccoli: a multi-faceted vegetable for health: An in-depth review of its nutritional attributes, antimicrobial abilities, and anti-inflammatory properties. Antibiotics (Basel). (2023) 12:1157. doi: 10.3390/antibiotics12071157
52. Krause, JS, Vines, CL, Farley, TL, Sniezek, J, and Coker, J. An exploratory study of pressure ulcers after spinal cord injury: relationship to protective behaviors and risk factors. Arch Phys Med Rehabil. (2001) 82:107–13. doi: 10.1053/apmr.2001.18050
53. Vidal, J, and Sarrias, M. An analysis of the diverse factors concerned with the development of pressure sores in spinal cord injured patients. Spinal Cord. (1991) 29:261–7. doi: 10.1038/sc.1991.37
54. Tate, DG, Forchheimer, MB, Krause, JS, Meade, MA, and Bombardier, CH. Patterns of alcohol and substance use and abuse in persons with spinal cord injury: risk factors and Correlates11no commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(S) or upon any organization with which the author(S) is/are associated. Arch Phys Med Rehabil. (2004) 85:1837–47. doi: 10.1016/j.apmr.2004.02.022
55. Correa, GI, Fuentes, M, Gonzalez, X, Cumsille, F, Piñeros, JL, and Finkelstein, J. Predictive factors for pressure ulcers in the ambulatory stage of spinal cord injury patients. Spinal Cord. (2006) 44:734–9. doi: 10.1038/sj.sc.3101914
56. Yin, G, Wang, Z, Wang, Z, and Wang, X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp Dermatol. (2018) 27:779–86. doi: 10.1111/exd.13679
Keywords: pressure ulcers, dietary habits, glycemic traits, Mendelian randomization, type 2 diabetes, multivariate
Citation: Luo P and Huang C (2024) Causal associations between type 2 diabetes mellitus, glycemic traits, dietary habits and the risk of pressure ulcers: univariable, bidirectional and multivariable Mendelian randomization. Front. Nutr. 11:1375179. doi: 10.3389/fnut.2024.1375179
Edited by:
Jasmina D. Debeljak Martacic, University of Belgrade, SerbiaReviewed by:
Cosmin Mihai Vesa, University of Oradea, RomaniaTong Wang, Chinese Academy of Sciences (CAS), China
Copyright © 2024 Luo and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Can Huang, MTgwMTk4NjU0NDNAMTYzLmNvbQ==
 Pei Luo
Pei Luo Can Huang
Can Huang