
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 24 June 2024
Sec. Clinical Nutrition
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1370472
Background: Early enteral nutrition (EN) is recommended for sepsis management, but its optimal timing and clinical benefits remain uncertain. This study evaluates whether early EN improves outcomes compared to delayed EN in patients with sepsis.
Methods: We analyzed data of septic patients from the MIMIC-IV 2.2 database, focusing on those in the Medical Intensive Care Unit (MICU) and Surgical Intensive Care Unit (SICU). Patients who initiated EN within 3 days were classified into the early EN group, while those who started EN between 3 and 7 days were classified into the delayed EN group. Propensity score matching was used to compare outcomes between the groups.
Results: Among 1,111 patients, 786 (70.7%) were in the early EN group and 325 (29.3%) were in the delayed EN group. Before propensity score matching, the early EN group demonstrated lower mortality (crude OR = 0.694; 95% CI: 0.514–0.936; p = 0.018) and shorter ICU stays (8.3 [5.2, 12.3] vs. 10.0 [7.5, 14.2] days; p < 0.001). After matching, no significant difference in mortality was observed. However, the early EN group had shorter ICU stays (8.3 [5.2, 12.4] vs. 10.1 [7.5, 14.2] days; p < 0.001) and a lower incidence of AKI stage 3 (49.3% vs. 55.5%; p = 0.030). Subgroup analysis revealed that early EN significantly reduced the 28-day mortality rate in sepsis patients with lactate levels ≤4 mmol/L, with an adjusted odds ratio (aOR) of 0.579 (95% CI: 0.361, 0.930; p = 0.024).
Conclusion: Early enteral nutrition may not significantly reduce overall mortality in sepsis patients but may shorten ICU stays and decrease the incidence of AKI stage 3. Further research is needed to identify specific patient characteristics that benefit most from early EN.
Sepsis is the dysregulated immune response to infection that leads to life-threatening organ dysfunction (1, 2). Sepsis affects nearly 50 million people worldwide each year and causes approximately 11 million deaths (2). The mortality rate for patients with sepsis who were treated in the intensive care unit was as high as 41.9% (3). Sepsis mortality rates have decreased in recent years as a result of published sepsis guidelines, advances in medical technology, and antibiotic therapy, but rates remain high (4–9).
In recent years, the role of nutrition in critical illness has received increasing attention (10). In critically ill adults, early administration of nutrients has been associated with improved clinical outcomes (11). Recent research has confirmed that there is dysbiosis of the gut microbiota in patients with sepsis (12). The association between disease and disturbances in the gut microbiome has been demonstrated to result in clinical deterioration and the development of multiple organ dysfunction syndrome (MODS) (13). Therefore, timely enteral nutrition supplementation is also a crucial aspect of treating sepsis patients (14). Preclinical studies have demonstrated that earlier enteral nutrition can protect human intestinal epithelial barrier function (15, 16). For hospitalized patients, early enteral nutrition may be beneficial (17). A recent study found that early administration of enteral nutrition reduces the incidence of ventilator-associated pneumonia in patients with severe trauma who require invasive ventilation (18). The early administration of enteral nutrition in patients with sepsis and septic shock has potential physiologic advantages related to the maintenance of gut integrity and prevention of intestinal permeability, dampening of the inflammatory response, and modulation of metabolic responses that may reduce insulin resistance (19, 20). However, the benefit of early enteral nutrition for sepsis patients remains controversial. Early nutrition support in the intensive care unit has been found to be significantly associated with higher 28-day mortality rates, particularly in younger patients with less severe illness (21, 22). Recent studies have shown neither a direct benefit nor harm from early enteral nutrition in sepsis patients (23). In the most recent sepsis survivor exercise guidelines, a weak recommendation has been made that suggests early enteral nutrition therapy for sepsis patients. However, there is insufficient evidence to support this recommendation (9). The timing and benefits of enteral nutrition in patients with sepsis in the intensive care unit (ICU) have not been clearly established. In this study, we aimed to assess the potential benefits of early enteral nutrition in comparison to delayed enteral nutrition. To test this hypothesis, we conducted a retrospective cohort study utilizing the MIMIC-IV 2.2 database.
This study was a retrospective observational study utilizing the Medical Information Mart for Intensive Care IV database. (MIMIC-IV version 2.2 was most recently updated on January 5, 2023) (24). The MIMIC-IV is a large, single-center database that contains data on patients who were admitted to the intensive care unit (ICU) of a large tertiary care hospital in Boston. The database contains 73,181 hospital admissions for adult patients (18 years of age and older) who were admitted to the intensive care unit from 2008 to 2019.
All information in this database has been deidentified, making it impossible to identify individual patients. As a result, this study is not classified as human subject research and does not require consent from the patients owing to unidentified health information. The MIMIC-IV 2.2 database’s creation was approved by institutional review boards at both the Massachusetts Institute of Technology (MIT, Cambridge, MA) and BIDMC. The author, Fuchao Xu, was granted access to the MIMIC-IV 2.2 database after completing the Human Subject Research course (Certification Number: 52712098).
Patients over 18 years of age with a sepsis diagnosis who received enteral nutrition during their stay in the intensive care unit were considered for inclusion. The sepsis diagnoses complied with the definition of sepsis 3.0 (1). We analyzed only the data from the initial admission to the Medical Intensive Care Unit (MICU) and Surgical Intensive Care Unit (SICU) for each patient. Patients who spent less than 72 h in the ICU were excluded.
In our study, we collected objective patient information, including age, sex, body mass index (BMI), care unit, and race. Additionally, we recorded vital signs taken within the first 24 h of ICU admission, specifically heart rate, respiratory rate, mean arterial pressure, temperature, glucose levels, use of vasopressors, and total urine output during the first 24 h. The laboratory results included pH, arterial oxygen partial pressure (PO2), arterial carbon dioxide partial pressure (PCO2), Pao2/Fio2 ratio, lactate, white blood cell (WBC) count, hemoglobin levels, platelet count, creatinine, blood urea nitrogen, serum albumin, and blood electrolyte levels (chlorine, calcium, potassium, sodium). For variables recorded multiple times during the first 24 h, we selected the values related to the greatest disease severity. Interventions within the initial 24 h comprised invasive mechanical ventilation, continuous renal replacement therapy, invasive arterial pressure monitoring, and a peripherally inserted central catheter. The severity scores during the first day of admission into the ICU were evaluated by the Acute Physiology Score III (APS III), Oxford Acute Severity of Illness Score (OASIS), Logistic Organ Dysfunction System (LODS), Sequential Organ Failure Assessment (SOFA), and Charlson Comorbidity Index (CCI). Comorbidities, such as congestive heart failure, chronic pulmonary disease, mild liver disease, diabetes, chronic kidney disease, and cancer, were recorded. The data extraction code is available on GitHub.1 PostgreSQL tools (version 15) were used for all data extractions (25).
We included only patients with sepsis who were admitted to the MICU or SICU for the first time. The patient flow diagram is presented in Figure 1. The following exclusion criteria were applied: (1) received enteral nutrition in different ICUs; (2) no enteral nutrition admission during the ICU stay; (3) patients admitted to a care unit other than the medical ICU and surgical ICU; (4) age at ICU admission <18 years; (5) age at ICU admission >89 years (for all patients older than 89 years, the database was adjusted for their age, so we excluded them); (6) ICU stay <72 h; (7) Sepsis diagnosed >48 h after ICU admission; (8) Received parenteral nutrition; (9) Underwent gastrointestinal surgery. (We examined whether septic patients underwent gastrectomy (ICD-9-CM codes 43.5–43.9, ICD-10-PCS codes 0DB60ZZ, 0DB70ZZ), bowel resection (ICD-9-CM codes 45.7x, 45.8x, ICD-10-PCS codes 0DBB0ZZ, 0DBC0ZZ), or gastrointestinal anastomosis (ICD-9-CM codes 44.x) procedures based on their respective diagnostic codes). (10) Started enteral nutrition >7 days after ICU admission; and (11) died within 7 days after ICU admission (to avoid immortal time bias).
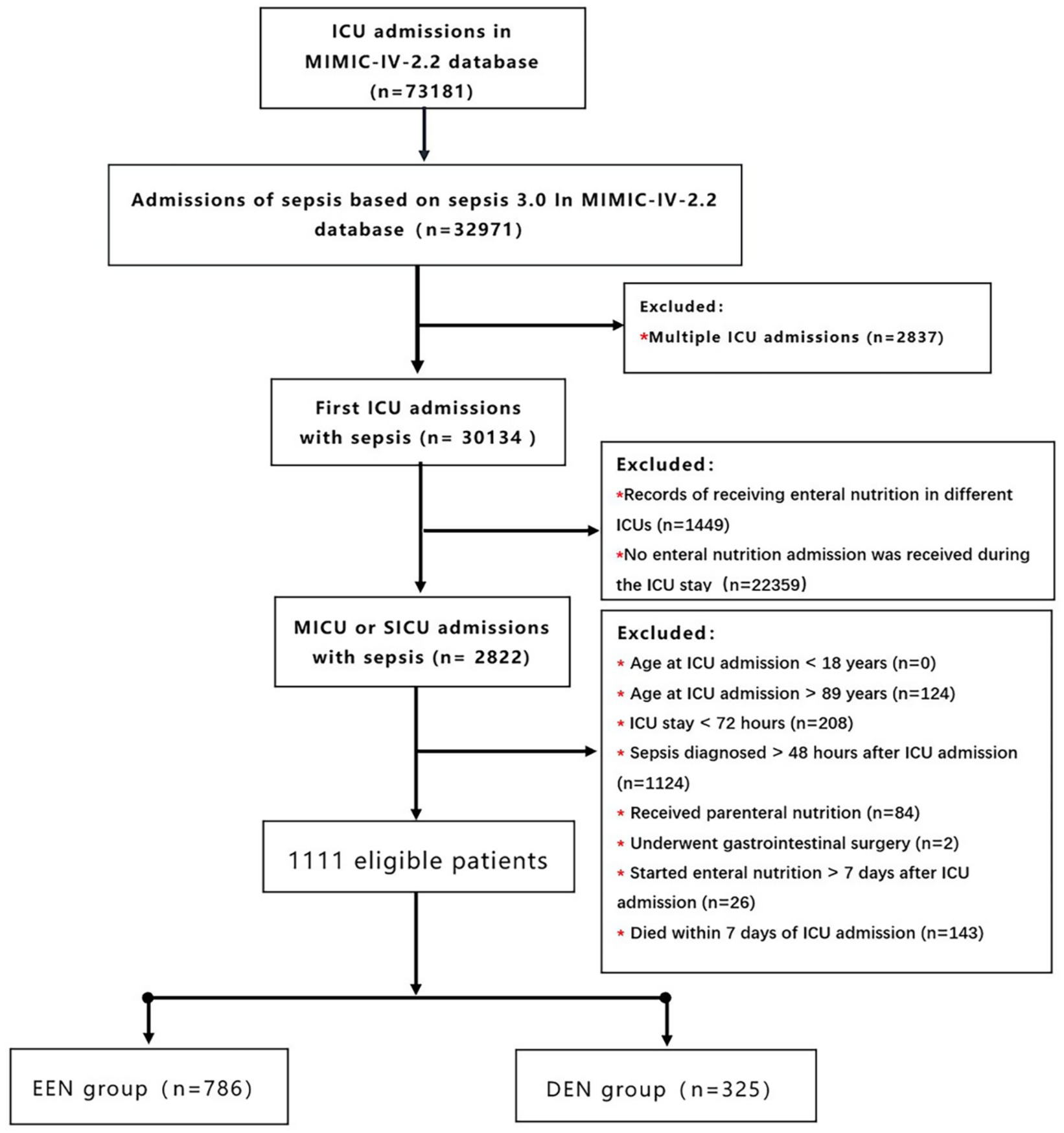
Figure 1. Flow chart of participant selection. MIMIC-IV, medical information mart for intensive care IV; ICU, intensive care unit; EEN, early enteral nutrition; DEN, delayed enteral nutrition.
By the newly published guidelines for sepsis and nutrition guidelines for critically ill patients. We divided the patients who started EN within 3 d after ICU admission into the early EN group and those who started EN 3–7 d after ICU admission into the delayed EN group (9, 26–29).
We employed the Kolmogorov–Smirnov test and the Shapiro–Wilk test to analyze continuous variables for normal distribution. Continuous variables are represented using either the mean ± standard deviation or the median (interquartile range), depending on their distribution. Categorical variables are presented as proportions. Appropriate statistical tests, such as the t test, analysis of variance, and the Mann–Whitney U test, were used for comparisons. The χ2 test was used for comparing categorical variables.
A propensity score–matching method was applied to compare the outcomes between the EEN and DEN groups (30). Propensity score matching (PSM) was performed to balance the baseline characteristics between the EEN group and the DEN group, so we used a logistic regression model to calculate the propensity score for each patient. In our study, 1:1 nearest neighbor matching was applied with a caliper width of 0.05. After PSM, standardized mean differences (SMDs) were used to evaluate the balance of characteristics between the two groups. A variable can be considered imbalanced between groups if its SMD is greater than 0.1 (31, 32). The implementation of the propensity-matching analysis is in R (version 4.3.1).
The primary outcome was 28-day mortality. Secondary outcomes included 60-day mortality, ICU mortality, length of stay in the ICU (LOS ICU), and the incidence of stage AKI. Kaplan–Meier curves were used to compare the survival of the two groups at 28 and 60 days before and after the propensity-matching procedure. Subgroup analysis was used to identify the specific population that may be more likely to benefit from EEN. We performed subgroup analyses according to age, sex, type of ICU, SOFA score, and lactate. Single-factor logistic analysis and multifactor logistic analysis were used to evaluate the association between early enteral nutrition and 28-day mortality in different subgroups. All subgroup results are presented in a forest plot. For missing data, utilize the MICE package in R to perform multiple imputation on the dataset (33) (Supplementary Figure S1). All the statistical analyses were carried out using R (version 4.3.1), and a p value of <0.05 (two-sided) was used to indicate statistical significance.
The MIMIC-IV database included 32,971 adult patients diagnosed with sepsis, from which our study cohort comprised 1,111 patients (Figure 1). Among these, 786 (70.7%) patients were classified into the early enteral nutrition (EEN) group, initiating enteral nutrition within 3 days after admission, while 325 (29.3%) patients were assigned to the delayed enteral nutrition (DEN) group, commencing enteral nutrition between 3 and 7 days after admission. Characteristics of patients in the EEN and DEN groups are summarized in Table 1. Notably, patients in the DEN group demonstrated significantly higher lactate levels (2.80 [1.70, 4.60] vs. 2.20 [1.50, 3.69]; p<0.001) and elevated disease severity scores, including LODS score (7.00 [6.00, 10.00] vs. 7.00 [5.00, 9.00]; p<0.001), and APS III score (66.00 [51.00, 82.00] vs. 57.50 [44.00, 74.00]; p<0.001) compared to the EEN group. Within the initial 24 h following ICU admission, the EEN group exhibited a higher likelihood of requiring invasive mechanical ventilation (631 (80.3%) vs. 226 (69.5%); p<0.001) and first-day urine output (1382.50 [770.50, 2233.75] vs. 1113.00 [455.00, 1955.00]; p<0.001).
Before propensity score matching (PSM), 28-day mortality was significantly lower in the early enteral nutrition group compared to the delayed enteral nutrition group (161 (20.5%) vs. 88 (27.1%); p = 0.018) (Table 2). The univariate Kaplan–Meier survival curve for 28 days also indicated that the early enteral nutrition group had a longer survival time (HR = 0.719, 95% CI: 0.546–0.947; p = 0.019) (Figure 2A). After PSM, all standardized mean differences were less than 0.1, indicating similar distributions of baseline variables in both groups (Supplementary Table S1). Following propensity matching, 28-day mortality was 4.2% lower in the early enteral nutrition group compared to the delayed enteral nutrition group, but this difference was not statistically significant (65 (22.4%) vs. 77 (26.6%); p = 0.247). The 28-day Kaplan–Meier curve post-propensity matching echoed the propensity-matched result (HR = 0.821, 95% CI: 0.591–1.141, p = 0.240) (Figure 2B). Additionally, we conducted multifactor logistic regression analysis to validate the results of propensity matching, incorporating age, gender, and covariates with p-values <0.05. The results of the multifactor logistic analysis (OR = 0.778 (0.559–1.084); p = 0.138) were consistent with those of propensity matching. Propensity matching suggested that early enteral nutrition did not significantly reduce 28-day mortality in sepsis patients compared with delayed enteral nutrition.
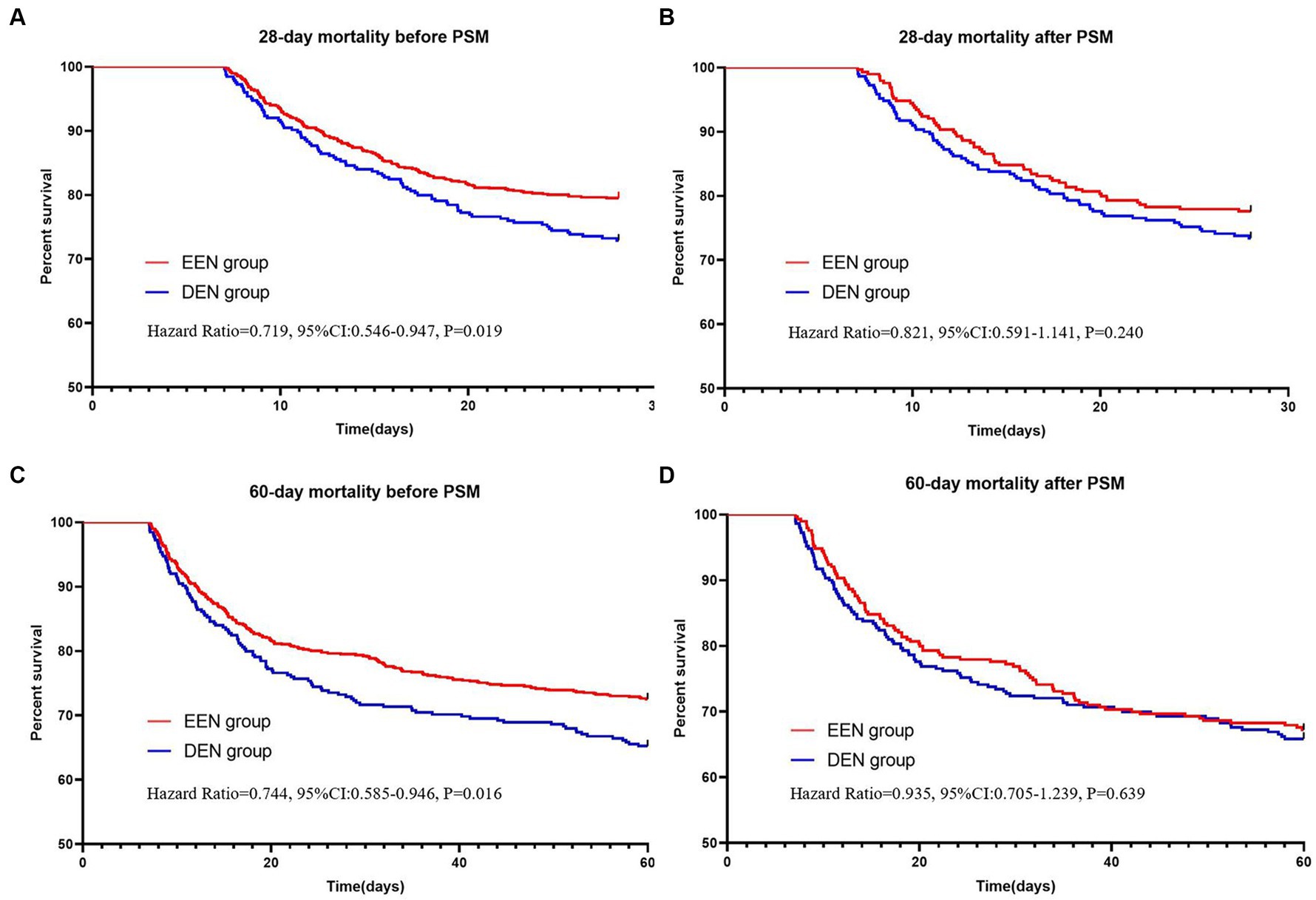
Figure 2. Kaplan–Meier survival curves of the two groups at 28 (A,B) and 60 (C,D) days before and after propensity score matching.
Before propensity matching, 60-day mortality was lower in the early enteral nutrition group than in the late enteral nutrition group (216 (27.5) vs. 113 (34.8); p = 0.017) (Table 2). The univariate Kaplan–Meier survival curve also showed longer survival in the early enteral nutrition group (HR = 0.744, 95% CI: 0.585–0.946, p = 0.017) (Figure 2C). The EEN group had shorter length of ICU stay (8.3 [5.2, 12.3] vs. 10.0 [7.5–14.2]; p < 0.001) and a lower incidence of stage 3 AKI (333 (42.4) vs. 184 (56.6); p < 0.001). After propensity matching, we found no statistically significant differences in 60-day mortality (95 (32.8) vs. 99 (34.1); p = 0.725) between the EEN and DEN groups. The 60-day Kaplan–Meier curve after propensity matching was consistent with the result after propensity matching (hazard ratio = 0.935, 95% CI: 0.705–1.239, p = 0.639) (Figure 2D). Multivariate logistic analysis was performed to verify the results of propensity matching and found that 60-day mortality (aOR = 0.859, 95% CI: 0.629–1.173, p = 0.339) in both groups were consistent with the post-PSM results. However, the EEN group still had a shorter length of ICU stay (8.3 [5.2, 12.4] vs. 110.1 [7.5, 14.2]; p < 0.001) (Figure 3) and a lower incidence of severe kidney injury (143 (49.3) vs. 161 (55.5); p = 0.030) than the DEN group after propensity matching (Figure 4).
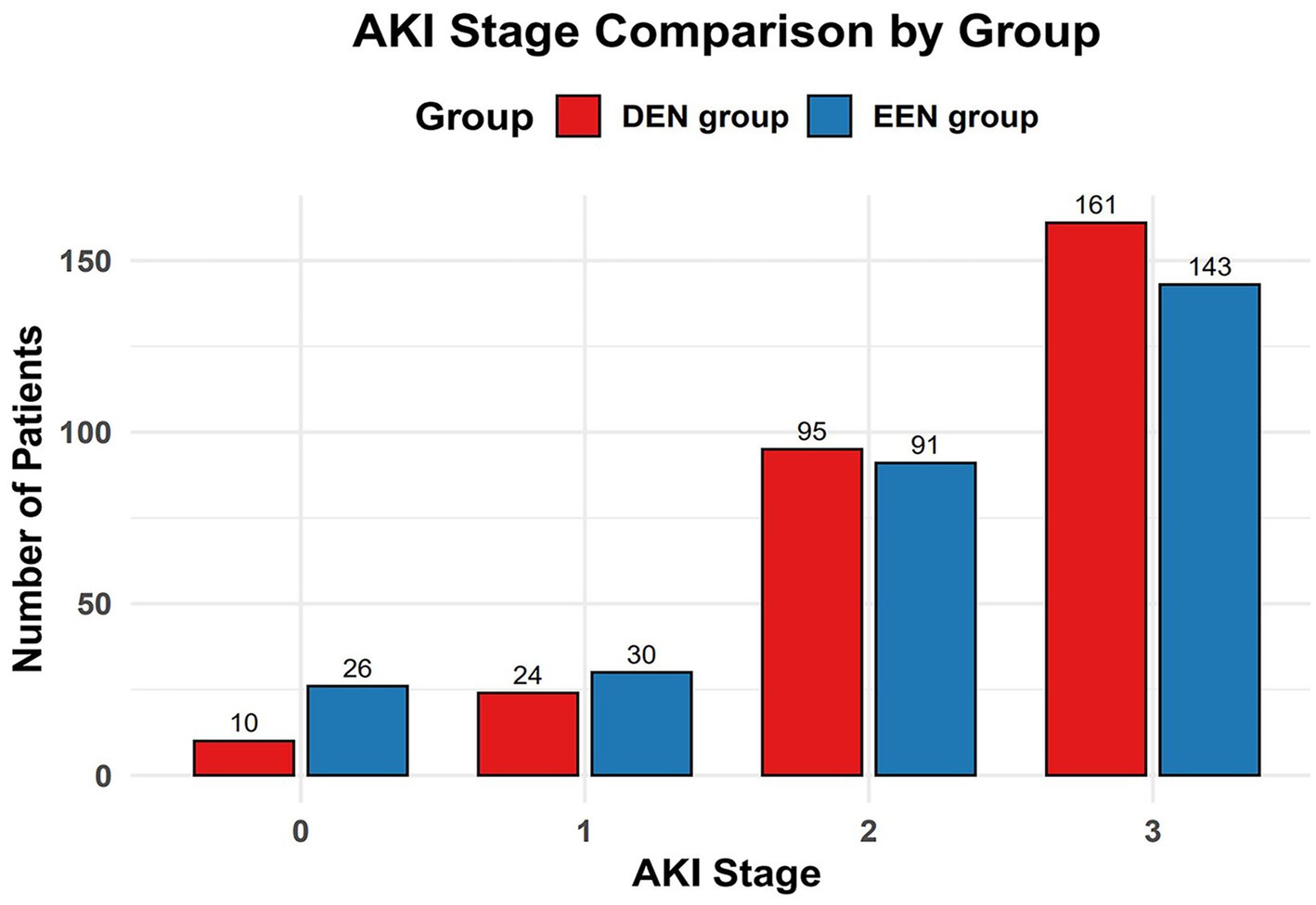
Figure 4. Comparison of AKI stage incidence between the early and delayed groups after propensity score matching.
We conducted several subgroup analyses based on propensity-matched data to explore the relationship between early enteral nutrition and 28-day mortality across different subgroups of sepsis patients. Patients were categorized based on sex, age, ICU type, SOFA score, and lactate levels. Single-factor logistic analysis and multifactor logistic analysis were utilized to assess the association between early enteral nutrition and 28-day mortality within various subgroups. The results for all subgroups are illustrated in a forest plot (Figure 5). Our findings indicate that early enteral nutrition decreased the 28-day mortality rate in patients with lactate levels ≤4 mmol/L (aOR = 0.579, 95% CI: 0.361–0.930, p = 0.024). The COX survival analysis-adjusted Kaplan–Meier survival curve is depicted in Figure 6.
The present study compared the outcomes between early and delayed EN in sepsis patients by propensity score matching based on the MIMIC IV 2.2 database. The results showed that early EN was not associated with mortality reduction but was associated with a lower incidence of severe acute kidney injury and a shorter length of ICU stay. This finding may reflect the regulatory effects of early enteral nutrition on inflammation and tissue damage. By providing nutritional support and improving intestinal mucosal barrier function, early enteral nutrition may alleviate systemic inflammatory responses and reduce the risk of renal injury. Additionally, early enteral nutrition may enhance hemodynamic stability and tissue perfusion, thereby minimizing renal damage. Overall, early enteral nutrition may reduce the incidence of stage 3 acute kidney injury through multiple pathways.
In the present study, we did not observe a reduction in mortality associated with early EN. This finding is consistent with previous studies in patients with sepsis and unselected critically ill patients (34–37). However, a recent study in critically ill patients found that early nutritional support in the ICU was significantly associated with increased mortality at 28 days, particularly in younger patients with less severe disease (21). However, many studies have shown the benefits of early enteral nutrition in the treatment of patients with many diseases (17, 21, 38). From a physiological point of view, early enteral nutrition therapy is potentially beneficial for patients with sepsis. Studies have shown that early enteral nutrition can maintain intestinal integrity and prevent intestinal permeability, thereby dampening the inflammatory response (20). Therefore, we performed subgroup analyses of the matched data to explore which sepsis patients would benefit from early enteral nutrition. We found that early enteral nutrition reduced 28-day mortality in the lactate ≤4 mmol/L groups (Figure 4). This observation is consistent with previous research, indicating the prognostic significance of lactate levels in sepsis. Multiple studies have demonstrated that lower lactate levels at the onset of sepsis are associated with better outcomes, including reduced mortality rates. Considering this established relationship, patients with initially lower lactate levels may represent a subgroup with less severe disease, and thus may benefit more from interventions such as early enteral nutrition. Our study further demonstrates that early enteral nutrition may have more pronounced benefits for patients with initially lower lactate levels, providing valuable insights for personalized treatment strategies. However, due to the complexity and severity of sepsis, the effect of enteral nutrition may not be obvious. Many internal factors influence the early enteral nutrition management of critically ill patients. For example, the performance of enteral nutrition in patients is affected by the use of large doses of catecholamines or the occurrence of vomiting, diarrhea, etc. Therefore, early enteral nutrition is not appropriate for all patients with sepsis (39).
Different sepsis patients require varying nutritional regimens. Our study indicates the necessity to optimize enteral nutrition in sepsis patients in the future, and appropriate nutritional intervention for different critically ill populations is crucial (40). In the treatment of sepsis through nutrition, it is necessary to rigorously identify individuals who are at risk of malnutrition (41). Individualized nutritional therapy represents a promising future direction for enteral nutrition therapy in sepsis patients.
There are several limitations to our study. First, to minimize possible confounding factors, we used propensity score matching. However, it may reduce the sample size of our study population. Although all patients in the EEN were matched and balance properties were satisfied, the distribution of the matched dataset was less comparable to the original dataset. Second, although we performed propensity score matching to control for confounding, some residual confounders may not be measured in this study. The study did not account for total caloric intake, progression of caloric intake, interruption of EN, baseline nutritional assessment, or intervention measures before enteral nutrition. These factors could have led to unmeasured confounding (42). Third, because of complex therapeutic interventions and subjective clinician decisions, we could not clearly explore the causal relationship between early EN and 28-day mortality. Finally, because the data we based on are from an observational database, the results reported in our study should be regarded only as a reference and must be further verified. Additional high-quality and larger sample size randomized trials are needed to investigate the optimal combination time for fluid administration and the optimal strategy to guide fluid therapy.
In conclusion, our retrospective study suggests that early enteral nutrition may not affect mortality rates when analyzed using propensity score matching. However, our findings indicate that early enteral nutrition is associated with shorter ICU stays and a lower incidence of severe acute kidney injury. Notably, subgroup analysis indicates that septic patients with lower lactate levels may derive greater benefit from early enteral nutrition. Considering the potential limitations of the propensity score method, additional randomized trials are necessary to validate the benefits of early enteral nutrition.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://physionet.org/content/mimiciv/2.2/.
MIMIC-IV database is approved by the Massachusetts Institute of Technology Institutional Review Boards. All the patients of the database are de-identified for privacy protection and informed consent is waived by the Nanjing Drum Tower Hospital Ethics Committee. The study followed the principles of the Declaration of Helsinki.
FX: Data curation, Writing – original draft. JX: Data curation, Writing – review & editing. JM: Data curation, Writing – original draft. WX: Writing – original draft. SG: Writing – review & editing. GL: Writing – review & editing. JW: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We want to sincerely thank all study group members for their collaboration and motivation during the period. We acknowledge the MIMIC IV 2.2 database for providing a valuable platform and data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1370472/full#supplementary-material
1. Singer, M, Deutschman, C, Seymour, C, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. van der Poll, T, Shankar-Hari, M, and Wiersinga, W. The immunology of sepsis. Immunity. (2021) 54:2450–64. doi: 10.1016/j.immuni.2021.10.012
3. Fleischmann-Struzek, C, Mellhammar, L, Rose, N, Cassini, A, Rudd, KE, Schlattmann, P, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. (2020) 46:1552–62. doi: 10.1007/s00134-020-06151-x
4. ARISE InvestigatorsANZICS Clinical Trials GroupPeake, S, Delaney, A, Bailey, M, Bellomo, R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. (2014) 371:1496–506. doi: 10.1056/NEJMoa1404380,
5. Kadri, S, Rhee, C, Strich, J, Morales, MK, Hohmann, S, Menchaca, J, et al. Estimating ten-year trends in septic shock incidence and mortality in United States academic medical centers using clinical data. Chest. (2017) 151:278–85. doi: 10.1016/j.chest.2016.07.010
6. Luhr, R, Cao, Y, Söderquist, B, and Cajander, S. Trends in sepsis mortality over time in randomised sepsis trials: a systematic literature review and meta-analysis of mortality in the control arm, 2002–2016. Crit Care. (2019) 23:241. doi: 10.1186/s13054-019-2528-0
7. Bauer, M, Gerlach, H, Vogelmann, T, Preissing, F, Stiefel, J, and Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care. (2020) 24:239. doi: 10.1186/s13054-020-02950-2
8. Dupuis, C, Bouadma, L, Ruckly, S, Perozziello, A, van-Gysel, D, Mageau, A, et al. Sepsis and septic shock in France: incidences, outcomes and costs of care. Ann Intensive Care. (2020) 10:145. doi: 10.1186/s13613-020-00760-x
9. Evans, L, Rhodes, A, Alhazzani, W, Antonelli, M, Coopersmith, CM, French, C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
10. Moisey, L, Merriweather, J, and Drover, J. The role of nutrition rehabilitation in the recovery of survivors of critical illness: underrecognized and underappreciated. Crit Care. (2022) 26:270. doi: 10.1186/s13054-022-04143-5
11. Matejovic, M, Huet, O, Dams, K, Elke, G, Vaquerizo Alonso, C, Csomos, A, et al. Medical nutrition therapy and clinical outcomes in critically ill adults: a European multinational, prospective observational cohort study (EuroPN). Crit Care. (2022) 26:143. doi: 10.1186/s13054-022-03997-z
12. Yang, X, Liu, D, Ren, H, Zhang, X, Zhang, J, and Yang, X. Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients. World J Gastroenterol. (2021) 27:2376–93. doi: 10.3748/wjg.v27.i19.2376
13. Adelman, M, Woodworth, M, Langelier, C, Busch, LM, Kempker, JA, Kraft, CS, et al. The gut microbiome's role in the development, maintenance, and outcomes of sepsis. Crit Care. (2020) 24:278. doi: 10.1186/s13054-020-02989-1
14. Wang, Y, Li, X, Zhang, L, Li, HN, Liu, XM, Song, W, et al. Machine learning algorithms assist early evaluation of enteral nutrition in ICU patients. Front Nutr. (2023) 10:1060398. doi: 10.3389/fnut.2023.1060398
15. Ralls, M, Demehri, F, Feng, Y, Woods Ignatoski, K, and Teitelbaum, D. Enteral nutrient deprivation in patients leads to a loss of intestinal epithelial barrier function. Surgery. (2015) 157:732–42. doi: 10.1016/j.surg.2014.12.004
16. Feng, Y, Barrett, M, Hou, Y, Yoon, H, Ochi, T, and Teitelbaum, D. Homeostasis alteration within small intestinal mucosa after acute enteral refeeding in total parenteral nutrition mouse model. Am J Physiol Gastrointest Liver Physiol. (2016) 310:G273–84. doi: 10.1152/ajpgi.00335.2015
17. Talebi, S, Zeraattalab-Motlagh, S, Vajdi, M, Nielsen, SM, Talebi, A, Ghavami, A, et al. Early vs delayed enteral nutrition or parenteral nutrition in hospitalized patients: an umbrella review of systematic reviews and meta-analyses of randomized trials. Nutr Clin Pract. (2023) 38:564–79. doi: 10.1002/ncp.10976
18. Wang, S, Zhao, X, Wang, Q, Wu, Y, Xu, J, Li, R, et al. Impact of early enteral nutrition on ventilator associated pneumonia in intubated severe trauma patients: a propensity score-matched study. Front Nutr. (2023) 10:1172526. doi: 10.3389/fnut.2023.1172526
19. Kudsk, K . Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. (2002) 183:390–8. doi: 10.1016/s0002-9610(02)00821-8
20. McClave, S, and Heyland, D. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. (2009) 24:305–15. doi: 10.1177/0884533609335176
21. Pardo, E, Lescot, T, Preiser, J, Massanet, P, Pons, A, Jaber, S, et al. Association between early nutrition support and 28-day mortality in critically ill patients: the FRANS prospective nutrition cohort study. Crit Care. (2023) 27:7. doi: 10.1186/s13054-022-04298-1
22. Reintam Blaser, A, Rooyackers, O, and Bear, D. How to avoid harm with feeding critically ill patients: a synthesis of viewpoints of a basic scientist, dietitian and intensivist. Crit Care. (2023) 27:258. doi: 10.1186/s13054-023-04543-1
23. Moon, S, Ko, R, Park, C, Suh, G, Hwang, J, and Chung, C. The effectiveness of early enteral nutrition on clinical outcomes in critically ill Sepsis patients: a systematic review. Nutrients. (2023) 15:3201. doi: 10.3390/nu15143201
24. Johnson, A, Bulgarelli, L, Shen, L, Gayles, A, Shammout, A, Horng, S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
25. Austin, T, Sun, S, Lim, Y, Nguyen, D, Lea, N, Tapuria, A, et al. An electronic healthcare record server implemented in PostgreSQL. J Healthc Eng. (2015) 6:325–44. doi: 10.1260/2040-2295.6.3.325
26. Taylor, B, McClave, S, Martindale, R, Warren, MM, Johnson, DR, Braunschweig, C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (a.S.P.E.N.). Crit Care Med. (2016) 44:390–438. doi: 10.1097/ccm.0000000000001525
27. Stewart, M, Biddle, M, and Thomas, T. Evaluation of current feeding practices in the critically ill: a retrospective chart review. Intensive Crit Care Nurs. (2017) 38:24–30. doi: 10.1016/j.iccn.2016.05.004
28. Doig, G . Parenteral versus enteral nutrition in the critically ill patient: additional sensitivity analysis supports benefit of early parenteral compared to delayed enteral nutrition. Intensive Care Med. (2013) 39:981–2. doi: 10.1007/s00134-013-2856-5
29. McClave, S, Martindale, R, Vanek, V, McCarthy, M, Roberts, P, Taylor, B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (a.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2009) 33:277–316. doi: 10.1177/0148607109335234
30. Griswold, M, Localio, A, and Mulrow, C. Propensity score adjustment with multilevel data: setting your sites on decreasing selection bias. Ann Intern Med. (2010) 152:393–5. doi: 10.7326/0003-4819-152-6-201003160-00010
31. Austin, P . An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
32. Zhang, Z, Kim, H, Lonjon, G, and Zhu, Y. Balance diagnostics after propensity score matching. Ann Transl Med. (2019) 7:16. doi: 10.21037/atm.2018.12.10
33. Pedersen, A, Mikkelsen, E, Cronin-Fenton, D, Kristensen, N, Pham, TM, Pedersen, L, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. (2017) 9:157–66. doi: 10.2147/clep.S129785
34. Wang, J, Jiang, L, Ding, S, He, SY, Liu, SB, Lu, ZJ, et al. Early enteral nutrition and Sepsis-associated acute kidney injury: a propensity score matched cohort study based on the MIMIC-III database. Yonsei Med J. (2023) 64:259–68. doi: 10.3349/ymj.2022.0276
35. Ohbe, H, Jo, T, Matsui, H, Fushimi, K, and Yasunaga, H. Early enteral nutrition in patients with severe traumatic brain injury: a propensity score-matched analysis using a nationwide inpatient database in Japan. Am J Clin Nutr. (2020) 111:378–84. doi: 10.1093/ajcn/nqz290
36. Ortiz-Reyes, L, Patel, J, Jiang, X, Coz Yataco, A, Day, AG, Shah, F, et al. Early versus delayed enteral nutrition in mechanically ventilated patients with circulatory shock: a nested cohort analysis of an international multicenter, pragmatic clinical trial. Crit Care. (2022) 26:173. doi: 10.1186/s13054-022-04047-4
37. Góes, C, Balbi, A, and Ponce, D. Evaluation of factors associated with Hypermetabolism and Hypometabolism in critically ill AKI patients. Nutrients. (2018) 10:505. doi: 10.3390/nu10040505
38. Zheng, C, Ge, Q, Luo, C, Hu, L, Shen, Y, and Xue, Q. Enteral nutrition improves the prognosis and immune nutritional status of patients in the cardiothoracic surgery recovery unit: a propensity score-matched analysis. Clin Nutr. (2022) 41:2699–705. doi: 10.1016/j.clnu.2022.10.012
39. Reintam Blaser, A, Starkopf, J, Alhazzani, W, Berger, MM, Casaer, MP, Deane, AM, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. (2017) 43:380–98. doi: 10.1007/s00134-016-4665-0
40. McKeever, L, Peterson, S, Lateef, O, and Braunschweig, C. The influence of timing in critical care nutrition. Annu Rev Nutr. (2021) 41:203–22. doi: 10.1146/annurev-nutr-111120-114108
41. Konecka, M, Kuczyńska, M, Schneider-Matyka, D, Stanisławska, M, Grochans, E, and Kamińska, M. Analysis of changes in the selected nutritional parameters of patients within a year from the admission to the enteral nutrition clinic. Nutrients. (2023) 15:1803. doi: 10.3390/nu15081803
Keywords: enteral nutrition, Sepsis, propensity score, MICU, SICU, MIMIC-IV
Citation: Xu F, Xu J, Ma J, Xu W, Gu S, Lu G and Wang J (2024) Early versus delayed enteral nutrition in ICU patients with sepsis: a propensity score-matched analysis based on the MIMIC-IV database. Front. Nutr. 11:1370472. doi: 10.3389/fnut.2024.1370472
Received: 14 January 2024; Accepted: 05 June 2024;
Published: 24 June 2024.
Edited by:
Chang Hu, Zhongnan Hospital of Wuhan University, ChinaCopyright © 2024 Xu, Xu, Ma, Xu, Gu, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Wang, d2pnYW9nb3VAYWxpeXVuLmNvbQ==; Geng Lu, bGcwODEwMjRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.