
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 01 July 2024
Sec. Nutritional Epidemiology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1362615
 Ying Chen1†
Ying Chen1† Hong Yang1†
Hong Yang1† Jie Song1
Jie Song1 Weiwei Chen1
Weiwei Chen1 Ke Liu1
Ke Liu1 Bin Liu1
Bin Liu1 Peiyang Luo2
Peiyang Luo2 Xiaohui Sun1
Xiaohui Sun1 Zhixing He3
Zhixing He3 Yingying Mao1*‡
Yingying Mao1*‡ Ding Ye1*‡
Ding Ye1*‡Background: Modifiable factors were found to be associated with the risk of irritable bowel syndrome (IBS) in observational studies, but whether these associations are causal is uncertain. We conducted a Mendelian randomization (MR) study to systematically explore the causal associations of modifiable factors with IBS.
Methods: Summary-level statistical data for IBS was obtained from a genome-wide association study (GWAS) meta-analysis of UK Biobank (40,548 cases and 293,220 controls) and the international collaborative Bellygenes initiative (12,852 cases and 139,981 controls). Genetic instruments associated with the exposures at the genome-wide significance (p < 5 × 10−8) level were selected from previous GWASs. Mendelian randomization was performed using inverse-variance weighted (IVW) method, supplemented with several sensitivity analyses to evaluate potentially causal relationships between identified contributing factors and IBS. Furthermore, we applied another database from FinnGen (8,116 IBS cases and 276,683 controls) to testify the reliability of the significant associations.
Results: Seven convincing modifiable factors were significantly associated with IBS after correction for multiple testing. Genetically predicted smoking initiation (OR = 1.12, 95% CI = 1.06–1.18, p = 1.03 × 10−4), alcohol consumption (OR = 0.47, 95% CI = 0.34–0.64, p = 3.49 × 10−6), sedentary behavior (OR = 1.17, 95% CI = 1.07–1.28, p = 4.02 × 10−4), chronotype (OR = 0.92, 95% CI = 0.88–0.96, p = 4.42 × 10−4), insomnia (OR = 1.19, 95% CI = 1.15–1.24, p = 7.59 × 10−19), education (OR = 0.80, 95% CI = 0.74–0.88, p = 5.34 × 10−7), and visceral adiposity (OR = 1.15, 95% CI = 1.06–1.24, p = 7.96 × 10−4). We additionally identified several suggestive factors, including serum magnesium, serum phosphorus, physical activity, lifetime smoking, intelligence, lean body mass, and body mass index (BMI). After pooling the effect estimates from FinnGen, the associations remained significant except for chronotype.
Conclusion: This MR analysis verified several modifiable risk factors for IBS, thus prevention strategies for IBS should be considered from multiple perspectives on these risk factors.
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that is estimated to affect around 1 in 10 people globally (1), while community prevalence appears to vary widely across different countries, partially due to the heterogeneity of diagnostic criteria and methodology (2). To be noted, the discrepancy also suggests a role for lifestyle factors in disease development (3).
Previous observational studies have summarized a large number of potential risk factors of IBS, involving micro-nutritional, behavioral, and obesity-related factors. For example, Barbalho et al. (4) have reported that vitamin D was implicated in the pathology of IBS. Additionally, there was consistent evidence to link subjectively reported poor sleep with IBS based on a systematic review (5). Besides, a well-conducted twin study from Norwegian found that low birth weight was associated with increased risk of IBS, as well as earlier onset of symptoms (6). However, whether causal associations between the potential risk factors and the development of IBS exist remains unanswered, because these findings could be prone to reverse causation and confounding factors.
The Mendelian randomization (MR) approach, using genetic variants as instrumental variables (IV) for exposure, can overcome the limitations of conventional observational studies (7). Since the genotype is determined at conception, which cannot be influenced by any stage of the disease process, therefore MR avoids the bias of reverse causality (8). On the other hand, alleles are randomly assigned when passed from parents to offspring during meiosis, so the genotype distribution in the population is unrelated to the presence of confounders, such as environmental exposure, socioeconomic status, and behavior.
In the current study, we used MR design to assess the possible causal relationship of modifiable factors (micro-nutritional, behavioral, and obesity-related factors) through a comprehensive search of relevant studies with risk of IBS.
To identify possible modifiable factors for IBS, we searched relevant system reviews, meta-analysis, and observational epidemiological studies in the PubMed database from inception up to March 20, 2022. We also conducted a review of published MR studies and excluded the traits reported until March 21, 2022, including copper, zinc, gluten-free diet, asthma (9–12). Finally, we focused on studies that have reported the association between potentially modifiable factors with available IV information and IBS risk, totaling 46 factors in our MR study (Supplementary Table S1). The summary of the study design is presented in Figure 1. The genetic instruments associated with putative risk factors were identified from the public genome-wide association studies (GWASs) of European ancestry (Supplementary Table S2).
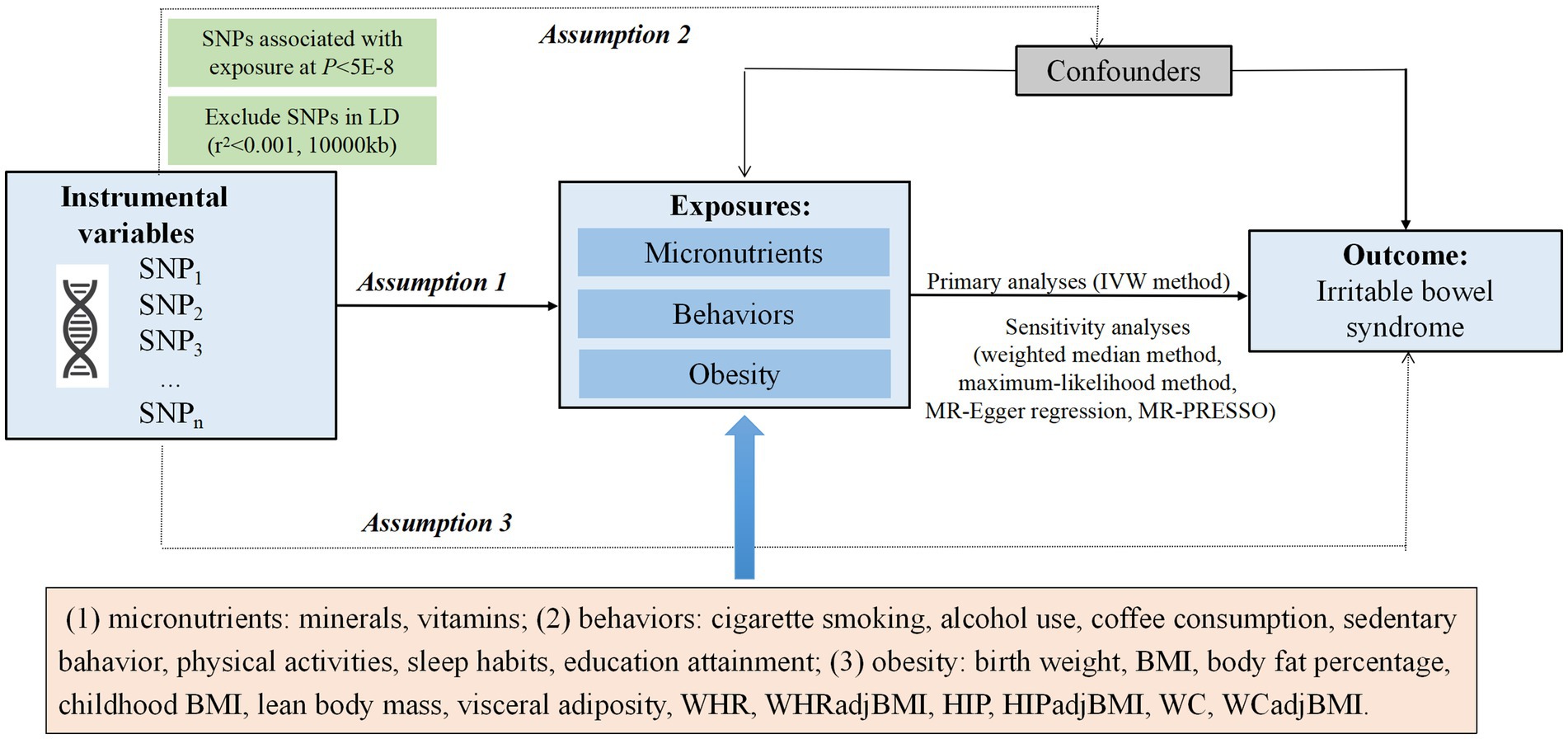
Figure 1. Overview of the Mendelian randomization (MR) design. Assumption 1 indicates that the genetic variants selected as instrumental variables (IVs) should be strongly associated with the exposure, assumption 2 indicates that the IVs should not be associated with confounders, and assumption 3 indicates that the IVs should affect the risk of the outcome only through the risk factor, not via alternative pathways. IVW, inverse-variance weighted; BMI, body mass index; WHR, waist-to-hip ratio; WHRadjBMI, waist-to-hip ratio adjusted for BMI; HIP, hip circumference; WC, waist circumference.
The first summary-level data of IBS GWAS was obtained from a GWAS meta-analysis of UK Biobank (40,548 cases and 293,220 controls) and the international collaborative Bellygenes initiative (12,852 cases and 139,981 controls) (13). The diagnostic criteria for the GWAS was based on Rome III symptom data, self-reported medical IBS diagnosis or electronic medical records. Moreover, we used another dataset from FinnGen1 involved 8,116 IBS cases and 276,683 controls, to validate the significant associations with risk of IBS and combined the results to strengthen the robustness. The diagnostic criteria for GWAS from FinnGen was based on codes of the International Classification of Diseases 9th Revision (ICD-9) and ICD-10. No ethical approval was required since we used publicly available summary data. The analytical process was in line with the STROBE-MR guidelines (14).
We selected independent single-nucleotide polymorphisms (SNPs) achieving the genome-wide significance threshold from corresponding GWASs as genetic instruments. SNPs in high linkage disequilibrium (LD) were pruned using a threshold of r2 < 0.001, distance = 10,000 kb. The retained SNPs with the lowest p-values for exposure were utilized as IVs. Detailed information of the instrumental SNPs is listed in Supplementary Table S3.
To be noted, several IVs came from UK Biobank and most cases (40,548 in 53,400, up to 76%) in the GWAS by Eijsbouts et al. (13) were also from UK biobank. Sample overlap may be biased in the direction of the observational association between the exposure and outcome (15). Therefore, we calculated the bias of these overlapped sample using a web tool.2 The estimated bias was negligible (< 0.1%), suggesting sample overlap may not substantially bias estimates (Supplementary Table S4).
F-statistics and R2 were used to evaluate the strength of the genetic instruments, with an F-statistics >10 considered adequate to avoid weak-instrument bias (16), and R2 standing for the percentage of variation in exposures explained by the instruments (17). The R2 and F-statistics were calculated using the following formular:
Where k is the number of variants, n is the sample size, MAF is the minor allele frequency. In addition, we undertook power calculation using a webtool3 (18).
In the MR analyses, we used inverse variance weighted (IVW) method for primary analysis, which provides an unbiased estimate in the absence of horizontal pleiotropy or when horizontal pleiotropy is balanced (19). Cochran’s Q test was used to evaluate the heterogeneity of the IVs. The random-effects model was applied if the Q statistic at the p < 0.05 level, otherwise, the fixed-effects model was utilized (20, 21).
Considering the IVW method may be invalid in the presence of pleiotropic IVs, we also performed sensitivity analysis using the weighted median and maximum likelihood methods. Weighted median method can provide valid causal estimates if more than 50% of the IVs are valid (22). Maximum-likelihood method produces estimates for the probability distribution parameters by maximizing the likelihood function with low standard error (23). Besides, the intercept obtained from the MR-Egger regression was used to evaluate the horizontal pleiotropy, with a p < 0.05 suggesting the presence of pleiotropy (16). Moreover, we applied MR pleiotropy residual sum and outlier (MR-PRESSO) method to detect and correct for potential outliers (24). The minimum numbers of instrumental SNPs allowed for the MR analysis were two, three, two, three and four for IVW, weighted median, maximum likelihood, MR-Egger and MR-PRESSO methods, respectively. For exposure with only one instrumental SNP, we used Wald ratio to estimate the association with risk of IBS.
All analyses were conducted using R (version 4.1.2) with “Mendelian Randomization” (25) and “MR-PRESSO” (24) packages. Results were reported as OR with corresponding 95% CIs. An observed p-value <1.09 × 10−3 (0.05 divided by 46 risk factors) was considered statistically significant based on the Bonferroni correction for multiple comparisons. p-values between 1.09 × 10−3 and 0.05 were considered as suggestive associations. Subsequently, we validated the significant findings using another independent database from FinnGen. Finally, we combined the estimates for each influencing factor from the two databases using the random-effects meta-analysis method, with p value <0.05 as the threshold for statistical significance.
The number of SNPs ranged from 1 to 425, and the explained variances varied from 0.1 to 6.6%. The F-statistics range from 10.94 to 432.49, suggesting that all SNPs had sufficient validity. Further, we performed power calculations based on the sample size of the IBS datasets. The detailed variances, F-statistics and power are displayed in Supplementary Table S2.
Overall, the MR associations of 46 modifiable factors with IBS derived from the IVW method are shown in Figure 2. Seven convincing factors were significantly associated with IBS risk after correction for multiple testing, and eight suggestive factors was potentially associated with the risk of IBS, with sensitivity analyses giving similar results (Figure 3; Supplementary Table S5). Specifically, genetically predicted smoking initiation, sedentary behavior, insomnia, visceral adiposity were statistically linked to higher IBS risk, while lifetime smoking, lean body mass, BMI were suggestively associated with increasing risk of IBS. Conversely, genetically predicted alcohol consumption, chronotype, education were statistically associated with reduced IBS risk, while serum magnesium and phosphate, intelligence, physical activity were suggestively associated with decreasing risk of IBS. Of the 15 exposures, a total of 14 modifiable factors have been confirmed in the meta-analysis (Supplementary Figure S1). MR analysis revealed non-significant association of some factors on the risk of IBS (Supplementary Table S6). Detailed information about heterogeneity between the SNPs used as instrumental variables for these associations is listed in Supplementary Tables S5, S6.
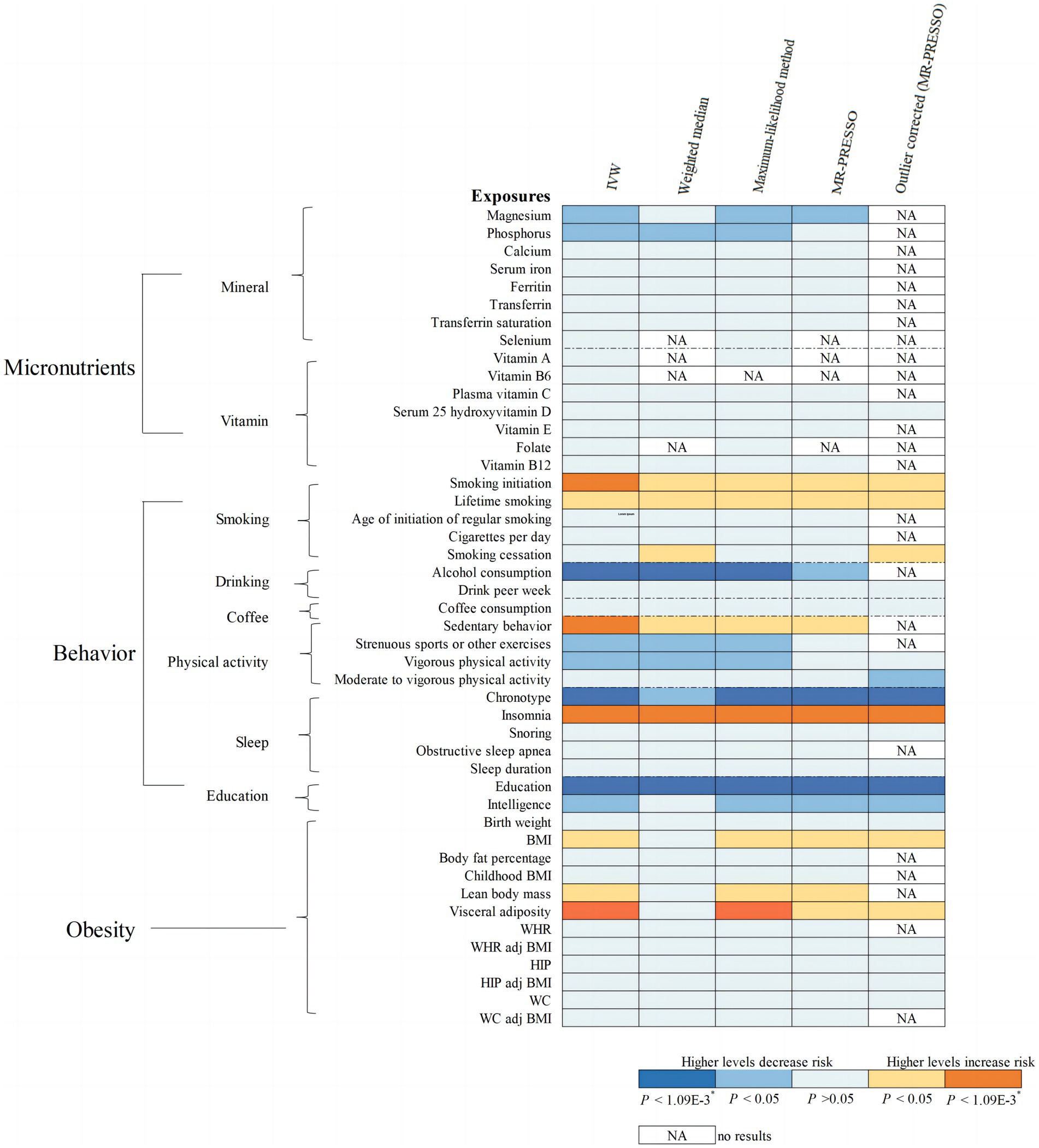
Figure 2. MR associations of 46 potential modifiable factors with irritable bowel syndrome (IBS). The results shown are derived from the primary method and sensitivity methods.
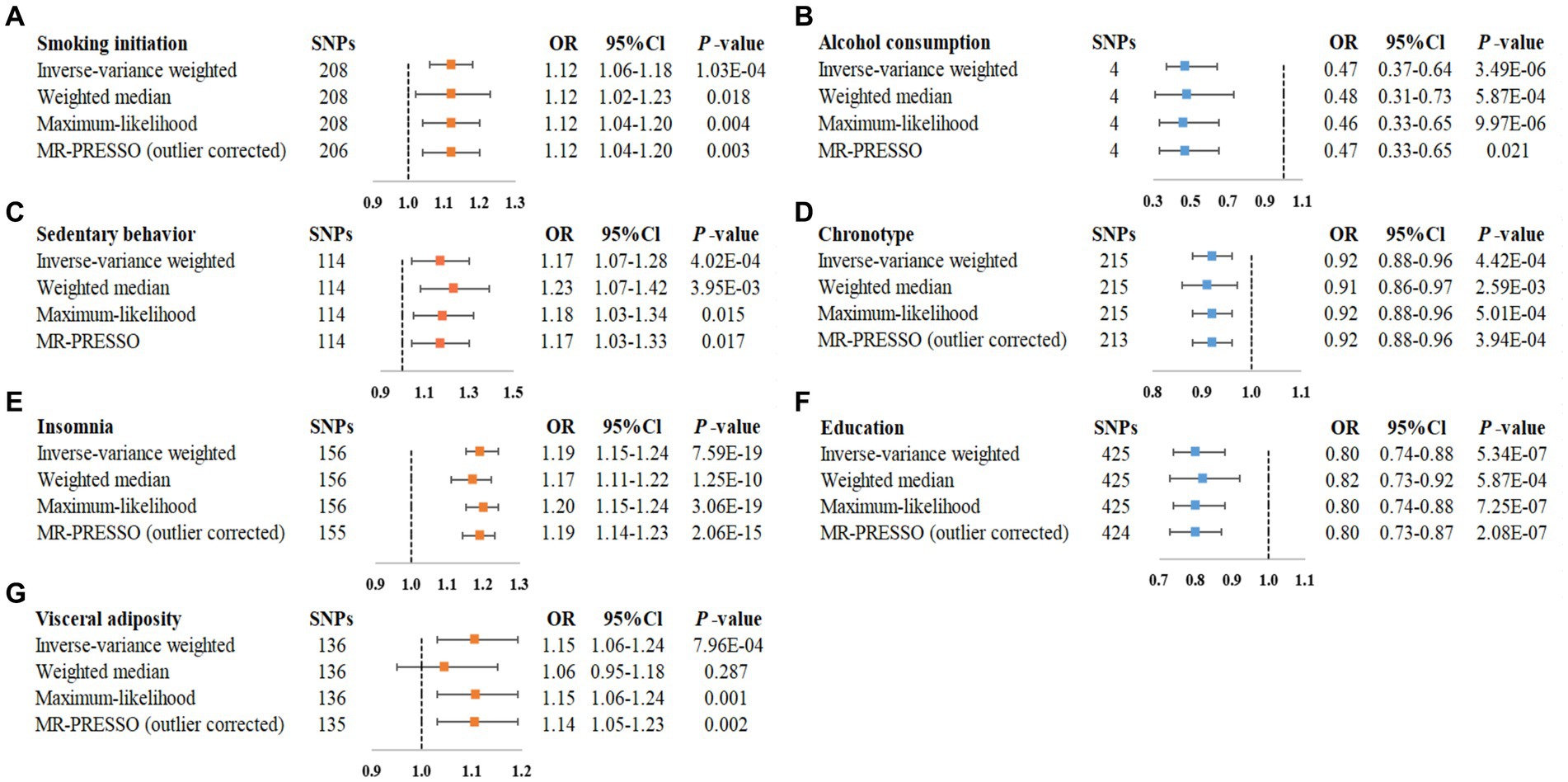
Figure 3. Statistically significant associations of genetically predicted modifiable factors and IBS in main and sensitivity analyses. (A) smoking initiation-IBS, (B) alcohol consumption-IBS, (C) sedentary behavior-IBS, (D) chronotype-IBS, (E) insomnia-IBS, (F) education-IBS, (G) visceral adiposity-IBS.
Regarding the 8 minerals and 7 vitamins investigated, a suggestive association was observed that genetically predicted serum magnesium (Mg) concentration was associated with a 67% (95% CI =19–87%) lower risk of IBS (Figure 2). We also noted a suggestive association between per 1-SD increment raised serum phosphorus concentrations and 28% decreased risk of IBS (95% CI = 10–43%; Figure 2). Sensitivity analysis provided similar results for Mg and phosphors (Supplementary Figure S2). These associations of serum Mg and phosphorus with the risk of IBS remained consistent in the combined analysis, with ORs of 0.37 (95% CI = 0.16–0.87) for serum Mg and 0.79 (95% CI = 0.64–0.98) for serum phosphorus (Supplementary Figure S1). However, our study showed no association of the other minerals or vitamins with the risk of IBS (Supplementary Table S6).
Genetically predicted smoking initiation was positively correlated with an increased risk of IBS (OR = 1.12, 95% CI = 1.06–1.18; Figure 2). Horizontal pleiotropy was not observed by MR-Egger (P for intercept = 0.621), and the other MR sensitivity approaches provided similar risk estimates (Figure 3). Two outliers were detected by the MR-PRESSO test, and the direction and magnitude of the association did not change (Figure 3). Also, genetically predicted lifetime smoking showed a suggestive association with the risk of IBS (OR = 1.17, 95% CI = 1.01–1.35; Figure 2). Results were consistent in sensitivity analyses (Supplementary Figure S2). After combining the associations from two datasets, the ORs for smoking initiation and lifetime smoking were 1.12 (95% CI = 1.06–1.18, Supplementary Figure S1) and 1.18 (95% CI = 1.04–1.33, Supplementary Figure S1), respectively.
As depicted in Figure 2, our MR analyses revealed a statistically significant association between genetically predicted alcohol consumption and lower risk of IBS (OR = 0.47, 95% CI = 0.34–0.64). Sensitivity analyses demonstrated consistent directions and similar effect estimates to IVW, with no evidence of unbalanced pleiotropy (P for intercept from MR-Egger = 0.198, Figure 3). The observed association between alcohol consumption and IBS was consistent in the meta-analysis of two databases (OR = 0.45, 95% CI = 0.34–0.61, Supplementary Figure S1).
Regarding the physical activity factors investigated, under the IVW estimation model, we noted a statistically significant association between genetically predicted sedentary behavior and a higher risk of IBS (OR = 1.17, 95% CI = 1.07–1.28; Figure 2). The association remained stable in sensitivity analyses (Figure 3), with no evidence of unbalanced pleiotropy (P for intercept from MR-Egger = 0.069). On the contrary, genetically predicted strenuous sports or other exercises (SSOE) (OR = 0.88, 95% CI = 0.78–0.99; Figure 2) as well as vigorous physical activity (VPA) (OR = 0.86, 95% CI = 0.75–0.99; Figure 2) were inversely associated with the risk of IBS, and sensitivity analysis yielded consistent results (Supplementary Figure S2). The results from the meta-analysis of two databases confirmed the findings of sedentary behavior (OR = 1.26, 95% CI = 1.05–1.50, Supplementary Figure S1), strenuous sports or other exercises (OR = 0.88, 95% CI = 0.78–0.99, Supplementary Figure S1), and vigorous physical activity (OR = 0.86, 95% CI = 0.75–0.99, Supplementary Figure S1).
As for the sleep-related traits, we observed that genetically predicted insomnia was significantly associated with increased risk of IBS by IVW method (OR = 1.19, 95% CI = 1.15–1.24, Figure 2), and sensitivity analyses yielded consistent results (Figure 3). MR-Egger regression indicated no potential pleiotropic bias for the IVs (P for intercept = 0.185). In addition, genetically predicted chronotype was inversely associated with the risk of IBS (OR = 0.92, 95% CI = 0.88–0.96; Figure 2). Results of risk estimates were consistent in sensitivity analyses (Figure 3). MR-Egger intercept test indicated no evidence of directional pleiotropy (P for intercept = 0.420). By using the MR-PRESSO test to exclude possible outliers, the effect estimates of the association between chronotype and risk of IBS did not change markedly (Figure 3). The association of insomnia with risk of IBS has been confirmed in the meta-analysis (OR = 1.19, 95% CI = 1.15–1.24, Supplementary Figure S1), but the result of chronotype did not reach significance (OR = 1.00, 95% CI = 0.85–1.17, Supplementary Figure S1).
Genetically predicted higher educational attainment was associated with significantly lower risk of IBS (OR = 0.80 per SD increase in years of education completed, 95% Cl = 0.74–0.88; Figure 2). The association was confirmed in the sensitivity analyses (Figure 3). In addition, genetically intelligence was suggestively correlated with a reduced risk of IBS (OR = 0.91, 95% CI = 0.84–0.99) (Supplementary Figure S2). MR-PRESSO identified several outliers, but a similar estimate was observed after the removal (Supplementary Figure S2). The combined effects of educational attainment and intelligence on risk of IBS were 0.76 (95% CI = 0.66–0.88, Supplementary Figure S1) and 0.91 (95% CI = 0.84–0.97, Supplementary Figure S1), respectively.
IVW analysis showed the association between genetically determined visceral adiposity and IBS was statistically significant (OR = 1.15, 95% CI = 1.06–1.24, Figure 2). Similar results were obtained in sensitivity analyses (Figure 3). Additionally, after the removal of some outliers identified by the MR-PRESSO test, the magnitude of the effect and their precision remained similar (Figure 3). Except for visceral adiposity, the MR analysis also revealed an adverse effect of genetically predicted lean body mass (OR = 1.07, 95% CI = 1.02–1.12, Figure 2) or BMI (OR = 1.08, 95% CI = 1.03–1.15, Figure 2) on IBS. Consistent results were obtained through sensitivity analyses (Supplementary Figure S2). There were consistent associations for BMI (OR = 1.08, 95% CI = 1.03–1.14), lean body mass (OR = 1.06, 95% CI = 1.01–1.10), and visceral adiposity (OR = 1.15, 95% CI = 1.07–1.23) in the pooled analysis (Supplementary Figure S1).
In summary, in this study, we applied MR approach to comprehensively investigate potential risk factors that might have causal effects on the development of IBS. Specifically, our study noted that genetically predicted smoking initiation, alcohol consumption, chronotype, insomnia, educational attainment, sedentary behavior and visceral adiposity are causally associated with risk of IBS. There was also suggestive evidence for possible association of magnesium, phosphorus, lifetime smoking, physical activity, intelligence, lean body mass, and BMI with the risk of IBS.
Previous studies have shown that smoking might be a potential risk factor for IBS, though the association was controversial. For example, a systematic review of 55 articles reported that the association between smoking and IBS cannot be confirmed (26). While a meta-analysis based on three population-based studies in Sweden reported that smokers have higher risk of developing IBS-diarrhea (OR = 2.40, 95% CI 1.12–5.16) than non-smokers, which is in line with our study (27). Cigarette smoking has been associated with detrimental effects on both visceral and peripheral hypersensitivity and gastrointestinal motility (28). On the other hand, smoking may pose an adverse effect on intestine through microbiome (29). These changes in microbiota may also be of importance for IBS. To be specific, incorporating smoking cessation programs into IBS preventive strategies is essential to mitigate the adverse effects of smoking on gut health.
It is well known that alcohol can interfere with many functions of the gut including damage to the mucosa of the intestine, increased intestinal membrane permeability and visceral sensitivity, disturbed motility of the gut, composition and activity of the microbiota (30). A population-based study of 2,648 participants reported an inverse association for drinking frequency of 2–3 times a week (OR = 0.352, 95% CI = 0.123–1.006) and drinking 3–4 standard glasses per occasion (OR = 0.716, 95% CI = 0.522–0.982) with IBS (31). Also, in a population-based study of middle-age and elder subjects showed a tendency toward less symptoms with a moderate alcohol intake (28). Similarly, a case–control study of women aged 18–48 years reported weaker symptoms with moderate and light alcohol intake, whereas aggravated symptoms after high intake of alcohol (32). Therefore, the relationship between alcohol consumption and risk of IBS may be nonlinear, while MR analysis was not able to measure potentially nonlinear relationship, so further studies are warranted. Comprehensive prevention plans for IBS should include measures to educate individuals on moderate alcohol consumption, avoidance of excessive drinking, and provision of support for alcohol cessation, ensuring personal health and welfare.
More generally, physical activity has a positive impact on gastrointestinal disorders. Consistent with our study, a cross-sectional study consisting of 4,763 Iranian adults suggested that the time spent in sedentary behavior per day is positively associated with a higher risk of IBS (OR = 1.27, 95% CI = 1.08–1.49), especially among women and individuals of normal weight (33). Previous studies have reported that spending more time in moderate physical activity is associated with improvement of symptoms (31, 34). The mechanisms behind the association have not been fully understood. One of the possible explanation is that physical activity may have protective effects on the gastrointestinal tract such as decreased gastrointestinal blood flow, increased gastrointestinal motility, increased mechanical bouncing and neuro-immuno-endocrine alterations (35). Overall, further studies are warranted to shed light on this association. Engaging in regular physical activity is linked to enhanced gut motility and lower stress levels, both of which can aid in the prevention of IBS.
Sleep disorders are symptoms frequently reported by patients with IBS, which may be associated with greater gastrointestinal symptom severity (36, 37). Recently, a meta-analysis including 11 studies from seven countries reported that shift work (OR = 2.27, 95% CI = 1.674–3.067) and poor sleep quality (OR = 4.27, 95% CI = 2.79–6.53) are significant risk factors for medical staff suffering from IBS (38). However, the observed association might be biased by reverse causation. Fortunately, a systematic review of 5 prospective studies concluded that sleep disorders are risk factors for IBS (39). Our study provided more robust evidence for the causal association of insomnia and chronotype with risk of IBS. The underlying mechanism linking sleep disturbance and IBS remain unclear. It was proposed that disruption of circadian physiology, due to shift work or sleep disturbance, may lead to increased permeability of the intestinal, alteration and dysregulation of immune and inflammatory responses (40, 41). It is recommended to address and ameliorate sleep disturbances as a strategy to prevent IBS.
Epidemiological findings for the association between educational attainment and risk of IBS were inconsistent. Several studies showed that low educated is associated with a higher prevalence of IBS (42), while Faresjö et al. (43) found no significant differences between IBS patients and healthy controls in the education level by case–control design. These conflicting results might be attributed to the study design, definitions differences, cultural differences, environmental factors, and small sample size. MR methods are more robust to provide evidence for the protective effects of educational attainment on the risk of IBS. This effect can be explained that higher levels of educational attainment are associated with health behaviors (e.g., physical activity, diet, and smoking), more cognitively-complex occupations, and better access to health care, all of which may play a role in decreasing risk of various diseases (44). Additionally, education plays a pivotal role in raising awareness about health issues, encouraging proactive measures for prevention and treatment of IBS. Investing in education is crucial in combating the prevalence of IBS and promoting overall well-being.
Obesity seems to be involved in the pathogenesis of IBS, but the previous findings were conflicting. BMI, a commonly used indicator of general obesity, has been reported to be associated with an increased risk of IBS according to a population-based study (45). However, a birth cohort study reported that increased BMI was not significantly associated with IBS (46). Given the nature of observational analysis, association observed may be due to residual or unmeasured confounding, therefore, we applied a MR approach to circumvent these limitations. The results presented here provide more convincing evidence supporting the deleterious effect of obesity on IBS, and several mechanisms may link obesity and IBS disease ranging from the disorder of intestinal motility, gut microbiota alteration, and inflammation factors. Specifically, it is possible that excess body weight increases intra-abdominal pressure and subsequently causes abnormal IBS motility mechanically (47). Second, obesity has been associated with changes in the microbiota composition, confirming the possible link with IBS (48). Moreover, visceral adipose tissue secretes a number of adipokines and cytokines leading to tissue inflammation (49). Incorporating weight control strategies into IBS preventive plans is paramount due to the significant impact of weight on gut health.
Furthermore, several suggestive risk factors were identified in the present study. Our study found that serum Mg and phosphorus concentrations decrease IBS risk by 67 and 28% per one SD increase respectively, but the association of Mg or phosphorus with the risk of IBS is inconclusive. Previous studies have shown that the diet consumed by IBS patients has low magnesium and phosphorus content (50). Roth et al. (51) reported that extraintestinal symptoms and fatigue for IBS patients were inversely associated with intakes of Mg and phosphorus. However, the exact mechanism by which Mg or phosphorus exerts its protective effect in IBS still remains unclear, and further investigation is necessary. Moreover, we found a suggestive association between genetically predicted intelligence and risk of IBS. According to a systematic review of twelve studies, there was inconclusive evidence to conclude the relationship between intelligence (including Verbal IQ, Performance IQ and experiential intelligence) and risk of IBS (52). We speculated this result may be attributed to little considerations for confounding factors. Given the tight phenotype and genetic correlation between intelligence and education level, the underlying mechanisms may be similar.
This is the first study that comprehensively assessed the causal associations between multiple exposures and risk of IBS by exploiting data from large GWASs. Many of the putative risk factors considered in this study have not previously been assessed within MR frameworks. The use of the MR design strengthened the causal inference on the exposure-IBS associations due to diminished residual confounding and reverse causality. In addition, several sensitivity analyses were performed to test the consistency of results, and reveal and correct for possible pleiotropy.
The current study has several limitations that should be considered when interpreting our findings. For MR analysis, the validity relies on the following three assumptions. The first assumption is that the genetic variants selected as IVs should be strongly associated with the modifiable risk factors. To satisfy this assumption, we selected SNPs achieving the genome-wide significant threshold, ensuring a robust association between these SNPs and the factors of interest. The second assumption is that the IVs should not be associated with any confounder which might influence both the exposures and the outcome. Given the alleles of genetic variants are randomly allocated at gamete formation, covariates are anticipated to be randomly distributed with respect to genotypes, making them generally free of confounders The third assumption is that the IVs should be associated with IBS only through the exposure, without any direct effects on the outcome. We confirmed by performing MR-Egger regression and finding no evidence of directional pleiotropy. First, we might have overlooked weak associations, especially for traits with small variance explained by SNPs. Second, we could not examine potential nonlinear relationships for risk factors using summary data. Third, our study was restricted to individuals of European ancestry, which limits the generalizability of our findings to diverse populations. Therefore, our conclusions may not be generalizable to other populations, as the participants of the included GWAS studies are Europeans. Moreover, sample overlap should be recognized between the GWAS studies of exposure and outcome, though the estimated bias was relatively low. Finally, it’s worth noting that the diagnostic criteria for IBS varied between the two GWAS studies, which could potentially impact the results.
IBS is a complex disease caused by interaction of multiple factors and the exact pathogenesis has not been fully elucidated. Our study has verified the causal association between several modifiable risk factors and IBS from insights from MR analysis, suggesting the important role of the management of micronutrient concentration, education level, weight control, sleep quality, physical activity, and smoking rates in IBS prevention.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
The study was approved by the Ethical Committee of Zhejiang Chinese Medical University on Nov 16, 2021 (No. AF-20211116-1). Because the project was mainly based on statistical analyses of publicly accessible databases and published studies, in which informed consents were obtained and ethical review were completed separately in each study, we have received ethical waiver for the research project. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YC: Formal analysis, Methodology, Writing – original draft. HY: Formal analysis, Methodology, Visualization, Writing – original draft. JS: Formal analysis, Methodology, Visualization, Writing – original draft. WC: Methodology, Software, Validation, Writing – original draft. KL: Formal analysis, Methodology, Writing – original draft. BL: Data curation, Methodology, Writing – original draft. PL: Visualization, Writing – original draft. XS: Conceptualization, Supervision, Writing – review & editing. ZH: Investigation, Supervision, Writing – review & editing. YM: Writing – review & editing. DY: Conceptualization, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (82204843, 81973663, 82174208), the Natural Science Foundation of Zhejiang Province (LQ21H260001 and LQ20H260008).
Summary statistics for IBS were obtained from the UK Biobank study and the FinnGen consortium. The authors thank all researchers for sharing these data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1362615/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Associations of genetically predicted modifiable factors with risk of IBS in two datasets and the combined effects.
SUPPLEMENTARY FIGURE S2 | Suggestive associations of genetically predicted modifiable factors and IBS in main and sensitivity analyses.
CI, confidence interval; GWAS, genome-wide association study; IV, instrumental variable; IVW, inverse-variance weighted; MR, Mendelian randomization; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier; IBS, irritable bowel syndrome; OR odds ratio; R2, variance; SD, standard deviations; SNP, single nucleotide polymorphism.
1. Lovell, RM, and Ford, AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol. (2012) 10:712–721 e4. doi: 10.1016/j.cgh.2012.02.029
2. Oka, P, Parr, H, Barberio, B, Black, CJ, Savarino, EV, and Ford, AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2020) 5:908–17. doi: 10.1016/S2468-1253(20)30217-X
3. Black, CJ, and Ford, AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. (2020) 17:473–86. doi: 10.1038/s41575-020-0286-8
4. Barbalho, SM, Goulart, RA, Araujo, AC, Guiguer, EL, and Bechara, MD. Irritable bowel syndrome: a review of the general aspects and the potential role of vitamin D. Expert Rev Gastroenterol Hepatol. (2019) 13:345–59. doi: 10.1080/17474124.2019.1570137
5. Tu, Q, Heitkemper, MM, Jarrett, ME, and Buchanan, DT. Sleep disturbances in irritable bowel syndrome: a systematic review. Neurogastroenterol Motil. (2017) 29. doi: 10.1111/nmo.12946
6. Bengtson, MB, Ronning, T, Vatn, MH, and Harris, JR. Irritable bowel syndrome in twins: genes and environment. Gut. (2006) 55:1754–9. doi: 10.1136/gut.2006.097287
7. Burgess, S, Daniel, RM, Butterworth, AS, and Thompson, SGEPIC-InterAct Consortium. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. (2015) 44:484–95. doi: 10.1093/ije/dyu176
8. Spiller, W, Slichter, D, Bowden, J, and Davey, SG. Detecting and correcting for bias in Mendelian randomization analyses using gene-by-environment interactions. Int J Epidemiol. (2019) 48:702–12. doi: 10.1093/ije/dyy204
9. Hujoel, IA, and Hujoel, MLA. The role of copper and zinc in irritable bowel syndrome: a Mendelian randomization study. Am J Epidemiol. (2022) 191:85–92. doi: 10.1093/aje/kwab180
10. Sun, Y, Chen, X, Wang, S, Deng, M, Xie, Y, Wang, X, et al. Gluten-free diet reduces the risk of irritable bowel syndrome: a Mendelian randomization analysis. Front Genet. (2021) 12:684535. doi: 10.3389/fgene.2021.684535
11. Chen, J, Chen, X, Xie, Y, Sun, Y, Wang, X, and Hesketh, T. Irritable bowel syndrome and migraine: evidence from Mendelian randomization analysis in the UK biobank. Expert Rev Gastroenterol Hepatol. (2021) 15:1233–9. doi: 10.1080/17474124.2021.1949290
12. Freuer, D, Linseisen, J, and Meisinger, C. Asthma and the risk of gastrointestinal disorders: a Mendelian randomization study. BMC Med. (2022) 20:82. doi: 10.1186/s12916-022-02283-7
13. Eijsbouts, C, Zheng, T, Kennedy, NA, Bonfiglio, F, Anderson, CA, Moutsianas, L, et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat Genet. (2021) 53:1543–52. doi: 10.1038/s41588-021-00950-8
14. Yuan, S, Chen, J, Li, X, Fan, R, Arsenault, B, Gill, D, et al. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur J Epidemiol. (2022) 37:723–33. doi: 10.1007/s10654-022-00868-3
15. Burgess, S, Davies, NM, and Thompson, SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. (2016) 40:597–608. doi: 10.1002/gepi.21998
16. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
17. Yarmolinsky, J, Bonilla, C, Haycock, PC, Langdon, RJQ, Lotta, LA, Langenberg, C, et al. Circulating selenium and prostate Cancer risk: a Mendelian randomization analysis. J Natl Cancer Inst. (2018) 110:1035–8. doi: 10.1093/jnci/djy081
18. Brion, MJ, Shakhbazov, K, and Visscher, PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
19. Burgess, S, Butterworth, A, and Thompson, SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
20. Greco, MF, Minelli, C, Sheehan, NA, and Thompson, JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
21. Greenland, S . Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. (1987) 9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298
22. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
23. Milligan, BG . Maximum-likelihood estimation of relatedness. Genetics. (2003) 163:1153–67. doi: 10.1093/genetics/163.3.1153
24. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
25. Yavorska, OO, and Burgess, S. Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. (2017) 46:1734–9. doi: 10.1093/ije/dyx034
26. Sirri, L, Grandi, S, and Tossani, E. Smoking in irritable bowel syndrome: a systematic review. J Dual Diagn. (2017) 13:184–200. doi: 10.1080/15504263.2017.1322226
27. Talley, NJ, Powell, N, Walker, MM, Jones, MP, Ronkainen, J, Forsberg, A, et al. Role of smoking in functional dyspepsia and irritable bowel syndrome: three random population-based studies. Aliment Pharmacol Ther. (2021) 54:32–42. doi: 10.1111/apt.16372
28. Lundstrom, O, Manjer, J, and Ohlsson, B. Smoking is associated with several functional gastrointestinal symptoms. Scand J Gastroenterol. (2016) 51:914–22. doi: 10.1080/00365521.2016.1174878
29. Savin, Z, Kivity, S, Yonath, H, and Yehuda, S. Smoking and the intestinal microbiome. Arch Microbiol. (2018) 200:677–84. doi: 10.1007/s00203-018-1506-2
30. Bode, C, and Bode, JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. (2003) 17:575–92. doi: 10.1016/S1521-6918(03)00034-9
31. Nilsson, D, and Ohlsson, B. Gastrointestinal symptoms and irritable bowel syndrome are associated with female sex and smoking in the general population and with unemployment in men. Front Med. (2021) 8:646658. doi: 10.3389/fmed.2021.646658
32. Reding, KW, Cain, KC, Jarrett, ME, Eugenio, MD, and Heitkemper, MM. Relationship between patterns of alcohol consumption and gastrointestinal symptoms among patients with irritable bowel syndrome. Am J Gastroenterol. (2013) 108:270–6. doi: 10.1038/ajg.2012.414
33. Sadeghian, M, Sadeghi, O, Hassanzadeh Keshteli, A, Daghaghzadeh, H, Esmaillzadeh, A, and Adibi, P. Physical activity in relation to irritable bowel syndrome among Iranian adults. PLoS One. (2018) 13:e0205806. doi: 10.1371/journal.pone.0205806
34. Johannesson, E, Simren, M, Strid, H, Bajor, A, and Sadik, R. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. (2011) 106:915–22. doi: 10.1038/ajg.2010.480
35. Peters, HP, De Vries, WR, Vanberge-Henegouwen, GP, and Akkermans, LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. (2001) 48:435–9. doi: 10.1136/gut.48.3.435
36. Fass, R, Fullerton, S, Tung, S, and Mayer, EA. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol. (2000) 95:1195–200. doi: 10.1111/j.1572-0241.2000.02009.x
37. Cremonini, F, Camilleri, M, Zinsmeister, AR, Herrick, LM, Beebe, T, and Talley, NJ. Sleep disturbances are linked to both upper and lower gastrointestinal symptoms in the general population. Neurogastroenterol Motil. (2009) 21:128–35. doi: 10.1111/j.1365-2982.2008.01181.x
38. Liu, H, Zou, Y, Kan, Y, Li, X, and Zhang, Y. Prevalence and influencing factors of irritable bowel syndrome in medical staff: a Meta-analysis. Dig Dis Sci. (2022) 67:5019–28. doi: 10.1007/s10620-022-07401-2
39. Creed, F . Review article: the incidence and risk factors for irritable bowel syndrome in population-based studies. Aliment Pharmacol Ther. (2019) 50:507–16. doi: 10.1111/apt.15396
40. Orr, WC, Fass, R, Sundaram, SS, and Scheimann, AO. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol. (2020) 5:616–24. doi: 10.1016/S2468-1253(19)30412-1
41. Summa, KC, Voigt, RM, Forsyth, CB, Shaikh, M, Cavanaugh, K, Tang, Y, et al. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS One. (2013) 8:e67102. doi: 10.1371/journal.pone.0067102
42. Farzaneh, N, Ghobaklou, M, Moghimi-Dehkordi, B, Naderi, N, and Fadai, F. Effects of demographic factors, body mass index, alcohol drinking and smoking habits on irritable bowel syndrome: a case control study. Ann Med Health Sci Res. (2013) 3:391–6. doi: 10.4103/2141-9248.117958
43. Faresjo, A, Grodzinsky, E, Johansson, S, Wallander, MA, Timpka, T, and Akerlind, I. Psychosocial factors at work and in every day life are associated with irritable bowel syndrome. Eur J Epidemiol. (2007) 22:473–80. doi: 10.1007/s10654-007-9133-2
44. Woolf, SH, and Braveman, P. Where health disparities begin: the role of social and economic determinants—and why current policies may make matters worse. Health Aff. (2011) 30:1852–9. doi: 10.1377/hlthaff.2011.0685
45. Delgado-Aros, S, Locke, GR III, Camilleri, M, Talley, NJ, Fett, S, Zinsmeister, AR, et al. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. (2004) 99:1801–6. doi: 10.1111/j.1572-0241.2004.30887.x
46. Talley, NJ, Howell, S, and Poulton, R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol. (2004) 99:1807–14. doi: 10.1111/j.1572-0241.2004.30388.x
47. Emerenziani, S, Pier Luca Guarino, M, Trillo Asensio, LM, Altomare, A, Ribolsi, M, Balestrieri, P, et al. Role of overweight and obesity in gastrointestinal disease. Nutrients. (2019) 12:111. doi: 10.3390/nu12010111
48. Ley, RE, Turnbaugh, PJ, Klein, S, and Gordon, JI. Microbial ecology: human gut microbes associated with obesity. Nature. (2006) 444:1022–3. doi: 10.1038/4441022a
49. Idrizaj, E, Garella, R, Squecco, R, and Baccari, MC. Adipocytes-released peptides involved in the control of gastrointestinal motility. Curr Protein Pept Sci. (2019) 20:614–29. doi: 10.2174/1389203720666190121115356
50. El-Salhy, M, Ostgaard, H, Gundersen, D, Hatlebakk, JG, and Hausken, T. The role of diet in the pathogenesis and management of irritable bowel syndrome (review). Int J Mol Med. (2012) 29:723–31. doi: 10.3892/ijmm.2012.926
51. Roth, B, Larsson, E, and Ohlsson, B. Poor intake of vitamins and minerals is associated with symptoms among patients with irritable bowel syndrome. J Gastroenterol Hepatol. (2022) 37:1253–62. doi: 10.1111/jgh.15830
Keywords: irritable bowel syndrome, Mendelian randomization, modifiable factors, genetics, single-nucleotide polymorphisms
Citation: Chen Y, Yang H, Song J, Chen W, Liu K, Liu B, Luo P, Sun X, He Z, Mao Y and Ye D (2024) Associations of modifiable factors with risk of irritable bowel syndrome. Front. Nutr. 11:1362615. doi: 10.3389/fnut.2024.1362615
Received: 02 February 2024; Accepted: 17 June 2024;
Published: 01 July 2024.
Edited by:
Weimin Ye, Karolinska Institutet (KI), SwedenReviewed by:
Bodil Ohlsson, Lund University, SwedenCopyright © 2024 Chen, Yang, Song, Chen, Liu, Liu, Luo, Sun, He, Mao and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingying Mao, bXl5QHpjbXUuZWR1LmNu; Ding Ye, eWVkaW5nQHpjbXUuZWR1LmNu
†These authors have contributed equally to this work
‡ORCID: Yingying Mao, https://orcid.org/0000-0003-3644-9160
Ding Ye, https://orcid.org/0000-0001-6654-7832
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.