
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 21 March 2024
Sec. Nutrition and Microbes
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1362529
 Vladimir A. Veselovsky1
Vladimir A. Veselovsky1 Daria I. Boldyreva1
Daria I. Boldyreva1 Evgenii I. Olekhnovich1
Evgenii I. Olekhnovich1 Ksenia M. Klimina1
Ksenia M. Klimina1 Vladislav V. Babenko1
Vladislav V. Babenko1 Natalia V. Zakharevich1
Natalia V. Zakharevich1 Andrey K. Larin1
Andrey K. Larin1 Maxim D. Morozov1
Maxim D. Morozov1 Polina Y. Zoruk1
Polina Y. Zoruk1 Petr V. Sergiev2
Petr V. Sergiev2 Olga A. Dontsova2
Olga A. Dontsova2 Igor V. Maev3
Igor V. Maev3 Tamara S. Novik4
Tamara S. Novik4 Anatoly A. Kotlobay1
Anatoly A. Kotlobay1 Vassili N. Lazarev1
Vassili N. Lazarev1 Maria A. Lagarkova1*
Maria A. Lagarkova1*Sweet-tasting proteins (SPs) are proteins of plant origin initially isolated from tropical fruits. They are thousands of times sweeter than sucrose and most artificial sweeteners. SPs are a class of proteins capable of causing a sweet taste sensation in humans when interacting with the T1R2/T1R3 receptor. SP thaumatin has already been introduced in the food industry in some countries. Other SPs, such as monellin and brazzein, are promising products. An important stage in researching SPs, in addition to confirming the absence of toxicity, mutagenicity, oncogenicity, and allergenic effects, is studying their influence on gut microbiota. In this paper we describe changes in the composition of rat gut microbiota after six months of consuming one of two recombinant SPs—brazzein or monellin. A full length 16S gene sequencing method was used for DNA library barcoding. The MaAsLin2 analysis results showed noticeable fluctuations in the relative abundances of Anaerocella delicata in brazzein-fed rat microbiota, and of Anaerutruncus rubiinfantis in monellin-fed rat microbiota, which, however, did not exceed the standard deviation. The sucrose-fed group was associated with an increase in the relative abundance of Faecalibaculum rodentium, which may contribute to obesity. Overall, prolonged consumption of the sweet proteins brazzein and monellin did not significantly change rat microbiota and did not result in the appearance of opportunistic microbiota. This provides additional evidence for the safety of these potential sweeteners.
Sugar is naturally present in all foods containing carbohydrates, including fruits, vegetables, cereals, and dairy products. The human body processes these products slowly, and sucrose provides a stable source of energy for cells. However, sugar is currently used in huge quantities in food production, leading to modern humans consuming ten times more sugar than normal. This habit has resulted in an increase in morbidity due to diseases such as metabolic syndrome, cardiovascular problems, obesity, type 2 diabetes, and more (1, 2). Sweet-testing proteins (SPs), which occur naturally primarily in tropical plants, are considered alternatives to sugar (3). SPs do not have a high caloric content or glycemic index and do not participate in carbohydrate metabolism. Due to their ample sweetness, they can be used in small quantities (4).
The human body is inhabited by trillions of symbiotic microorganisms, most of which are found within the gastrointestinal tract, mainly in the large intestine; they are collectively called the microbiota (5, 6). The composition and activity of gut microbiota (GM) change during human life and are shaped by several factors; most notably, diet and dietary factors are major determinants of GM composition and activity (7). GM changes correlate with health status (8). GM activity in humans includes the degradation of undigested proteins and carbohydrates (sugars, oligosaccharides, peptides, and amino acids), amino acid and monosaccharide fermentation, hydrogen disposal, bile-acid transformation, and vitamin synthesis (9, 10). Any change in the sugar/sweetener profile that we consume redefines the nutrient environment in our gut. How indigenous and exogenous microbes use these environments can result in detrimental or beneficial effects on the host. The dietary and microbiota components that regulate host immunity in the case of metabolic dysfunction and the cellular and molecular mechanisms involved in this process are currently being extensively investigated (11). It was shown that GM protects against developing obesity, metabolic syndrome, and pre-diabetic phenotypes by inducing commensal-specific T-helper 17 (Th17) cells. Eliminating sugar from high-fat diets protected mice from obesity and metabolic syndrome in a manner dependent on commensal-specific Th17 cells. Sugar and group 3 innate lymphoid cells (ILC3) promoted the outgrowth of Faecalibaculum rodentium that displaced Th17-inducing microbiota (12).
An important issue that artificial sugar substitute manufacturers have already faced before is the impact of these compounds on GM. The detrimental effect of saccharin and aspartame on microbiota has been demonstrated in some experiments on mice and rats (13). There is also evidence that cyclamate and saccharin have a bacteriostatic effect, which is associated with the inhibition of anaerobic glucose fermentation by microbiota (14).
Another study demonstrates that a violation of the microbiota correlates with the occurrence of glucose tolerance when using saccharin and aspartame. This could be a risk for metabolic syndrome, while using antibiotics in experimental animals, in comparison to using saccharin, eliminated glucose tolerance (15).
As for the SP effect on GM, only thaumatin’s influence on human gut bacteria has been examined (16). Thaumatin at 2 mg/mL was shown to increase the total microbial biomass across all microbiomes and promote Butyricicoccus, which is associated with beneficial effects including anti-inflammation in inflammatory bowel disease patients (17). The antimicrobial activity of brazzein in comparison with norfloxacin was also evaluated in in vitro experiments (18). The authors concluded that the effect is insignificant because the brazzein concentration that is expected to be used in food is not enough to suppress gut microorganisms. Analysis of the literature data presents a fairly large number of studies done on the effect of artificial sweeteners on microbiota, but no published studies to date on the effect of monellin and brazzein, or any SP other than thaumatin, on GM ex vivo (16, 19, 20).
Replacing sugars in the diet with SPs can help reduce the level of obesity and related diseases. Therefore, it is necessary to study the effect of SPs not only on the state of the organism itself, but also on its microbiota. In our recent study, we have shown that long-term intragastric administration of brazzein and monellin did not cause changes in animals’ physiological, biochemical, hematological, morphological, and behavioral parameters (21). In this study, we compare the effect of brazzein and monellin on the bacterial composition of rat GM relative to the effect of sucrose.
The recombinant brazzein and monellin preparation was described in Novik et al. (21). Briefly, both recombinant proteins were produced in Pichia pastoris yeast. The recombinant brazzein sequence used in this study was identical to the natural one. Recombinant single chain monellin was constructed by fusing natural monellin chain B with chain A via a Gly-Phe linker. The proteins were purified by chromatography, followed by 2 steps of ultrafiltration and lyophilization. Protein identification was performed by expression construct sequencing followed by both PAA gel electrophoresis and mass-spectrometry.
Fecal microbiota was studied on outbred rats 3–4 months old with 180–210 g average body weight. The animals were obtained from a nursery operating according to Good Laboratory Practice (GLP) standards, were bred specifically for this study, and were not involved in any previous experiments. The animals were kept in controlled conditions: at an air temperature of 20–22°C and a relative humidity of 60–70%. The distribution of animals into groups was carried out randomly, using body weight as a criterion. Individual values of body weight did not deviate from the average value of their group by more than 10%.
Two series of experiments were performed. Animals were divided into four groups for each experimental series: two experimental ones and two control ones. Each group consisted of 10 animals. Animals in the experimental groups had aqueous solutions of brazzein or monellin administered intragastrically; animals in the control groups had aqueous solutions of sucrose or distilled water administered intragastrically. Sucrose (Dia-M, Moscow, Russia) was administered intragastrically to control group animals at an equivalent dose (ED), calculated based on the daily norm for humans, 714 mg/kg body weight. The sucrose ED for rats (EDrat) was 4.284 mg/kg of body weight. Brazzein is 2000 times sweeter than sucrose; therefore, EDrat for brazzein is 2.14 mg/kg. Monellin is 3000 times sweeter than sucrose; therefore, EDrat for monellin is 1.43 mg/kg of body weight. Animals from the first experimental series received the tested proteins at a dose equivalent to 1 EDrat (2.14 mg/kg of body weight and 1.43 mg/kg of body weight for brazzein and monellin, respectively). Animals from the second experimental series received a dose equivalent to 10 EDrat (21.4 mg/kg of body weight and 14.3 mg/kg of body weight for brazzein and monellin respectively). Proteins and control solutions were administered daily at the same time for 150 days.
Six rats were randomly selected from each of the four experimental groups (receiving tested proteins in doses of 1 EDrat and 10 EDrat), a control group which had sucrose administered at a dose of 4.284 mg/kg, and a control group which had distilled water administered. On day 0 (before the start of administration of the tested proteins) and on week 3, 6, 9, 12, and 23 after the start of administration of the tested proteins (once a week), the six selected rats were placed in individual metabolic chambers for 1 h to collect 0.5 g of feces from each rat. The collected fecal samples were frozen at -80°C for subsequent analysis. At these time points, fecal samples were taken from the same rats to monitor the individual change dynamics in the rats’ microbiota during the entire experimental period.
Nucleic acids from each of 419 fecal samples were extracted using a MAGNO-sorb (AmpliSens, Russia). 400 μL of PBS was added to each sample and transferred to MagNA Lyser Green Beads (Roche, Switzerland) bead tubes for homogenization on a MagNA Lyser (30 s. 7000 rpm) (Roche, Switzerland). After homogenization, the samples were spun for 1 min at 9000 g. The supernatant was transferred to a new tube and 40 μL proteinase K were added. The samples were then heated for 20 min at 65°C and transferred to a plate where magnetic beads with MagMAX™ Viral/Pathogen Binding Solution (Thermo Fisher Scientific, United States) were added. Further DNA extraction was carried out on a KingFisher™ Purification System (Thermo Fisher Scientific, United States) according to manufacturer’s protocol. The DNA was subsequently quantified on a Qubit 4 fluorometer by an Quant-iT dsDNA BR Assay Kit (Thermo Fisher Scientific, United States).
The extracted DNA (1–5 ng) was amplified using the 27F (AGAGTTTGATYMTGGCTCAG) and 1492R (GGTTACCTTGTTAYGACTT) primers (Eurogen, Russia) and a Tersus Plus PCR kit (Eurogen, Russia) in a total volume of 25 μL. Amplification was performed on a ProFlex™ PCR System (Thermo Fisher Scientific, United States) with the following PCR conditions: initial denaturation at 95°C for 2 min, then 27 rounds of a three-step temperature cycle (95°C for 1 min, 60°C for 1 min, and 72°C for 3 min), followed by a final extension at 72°C for 2 min and cooling at 4°C. The quality of the amplicons was checked by electrophoresis in 1.5% agarose gel. The final amplicons were purified using KAPA HyperPure Beads (Roche, Switzerland) according to manufacturer’s protocol.
Libraries were prepared according to manufacturer protocol (Ligation sequencing amplicons) with modification. The amplicons were processed with the NEBNext® Ultra™ II End Repair/dA-Tailing Module (New England BioLabs Inc., MA, United States). Barcodes [Native Barcoding Kit 96 (SQK-NBD109.96)] were ligated with Blunt/TA Ligase Master Mil (New England BioLabs Inc., MA, United States). Barcoded libraries were purified using KAPA Pure Beads (Roche, Switzerland). Library concentrations were measured using the Quant-iT dsDNA Assay Kit, High Sensitivity (Thermo Fisher Scientific, United States) and samples were mixed in equimolar amounts. The final adapter (Adapter Mix II Expansion) (Oxford Nanopore Technologies, United Kingdom) was ligated to the pooled library using the NEBNext Quick Ligation Module (New England BioLabs Inc., MA, United States). The prepared DNA library (12 μL) was mixed with 37.5 μL of Sequencing Buffer, 25.5 μL of Loading Beads, loaded onto the R9.4 flow cell (FLO-MIN106; Oxford Nanopore Technologies, United Kingdom), and sequenced on MinION™ Mk1B (Oxford Nanopore Technologies, United Kingdom). MINKNOW software ver. 22.12.7 (Oxford Nanopore Technologies, United Kingdom) was used for data acquisition.
Technical sequences and bases with a quality lower than a 9 Phred score were processed using Porechop1 and NanoFilt (22) software. The resulting data were evaluated by the Emu pipeline (23) for taxonomic classification. For batch-effect correction between different sequencing technical runs, ConQur software with default parameters was used (24). Further analyses were performed using the vegan (25) and MicrobiotaProcess packages (26) for GNU/R. Alpha-diversity was estimated using the Shannon index. Beta-diversity was estimated using the Bray–Curtis dissimilarity metric and non-metric multidimensional scaling analysis (NMDS). For the statistical evaluation of observed parameters, the Three-Way analysis of variance with a Benjamini-Hochberg correction for multiple testing, implemented in standard GNU/R statistical instruments, were used. The Permutational Multivariate Analysis of Variance Using Distance Matrices (adonis2) implemented in the vegan package was used for estimating differences in microbial composition of experimental groups. Adonis2-produced p-values were processed using the Benjamini-Hochberg correction for multiple testing.
LefSe (27, 28) was used for discovery of microbial taxa significantly linked to experimental groups with parameters first. Test. alpha = 0.01, second. Test. alpha = 0.01. Multivariable association discovery was performed using MaAsLin 2 analysis (29). The MaAsLin 2 analysis was performed with the following parameters: fixed_effects = c(“gender,” “group”), reference = c (c(“group, control”)), random_effects = c(“time_point,” “sourceid”), max_significance = 0.01.
Sequencing identified 582 microbial species belonging to 265 total genera in 419 experimental rat’s fecal samples. Sample metadata is presented in Supplementary Table S1. After quality control, the fecal samples yielded 7,322 ± 2,506 reads per sample with length 1,470 ± 10 bp. A summary of the sequencing statistics is shown in Supplementary Table S2. Relative species abundances and taxonomic tables are presented in additional materials (Supplementary Tables S3, S4). The distribution of microbial genera by experimental group and time points are presented in Figure 1A.
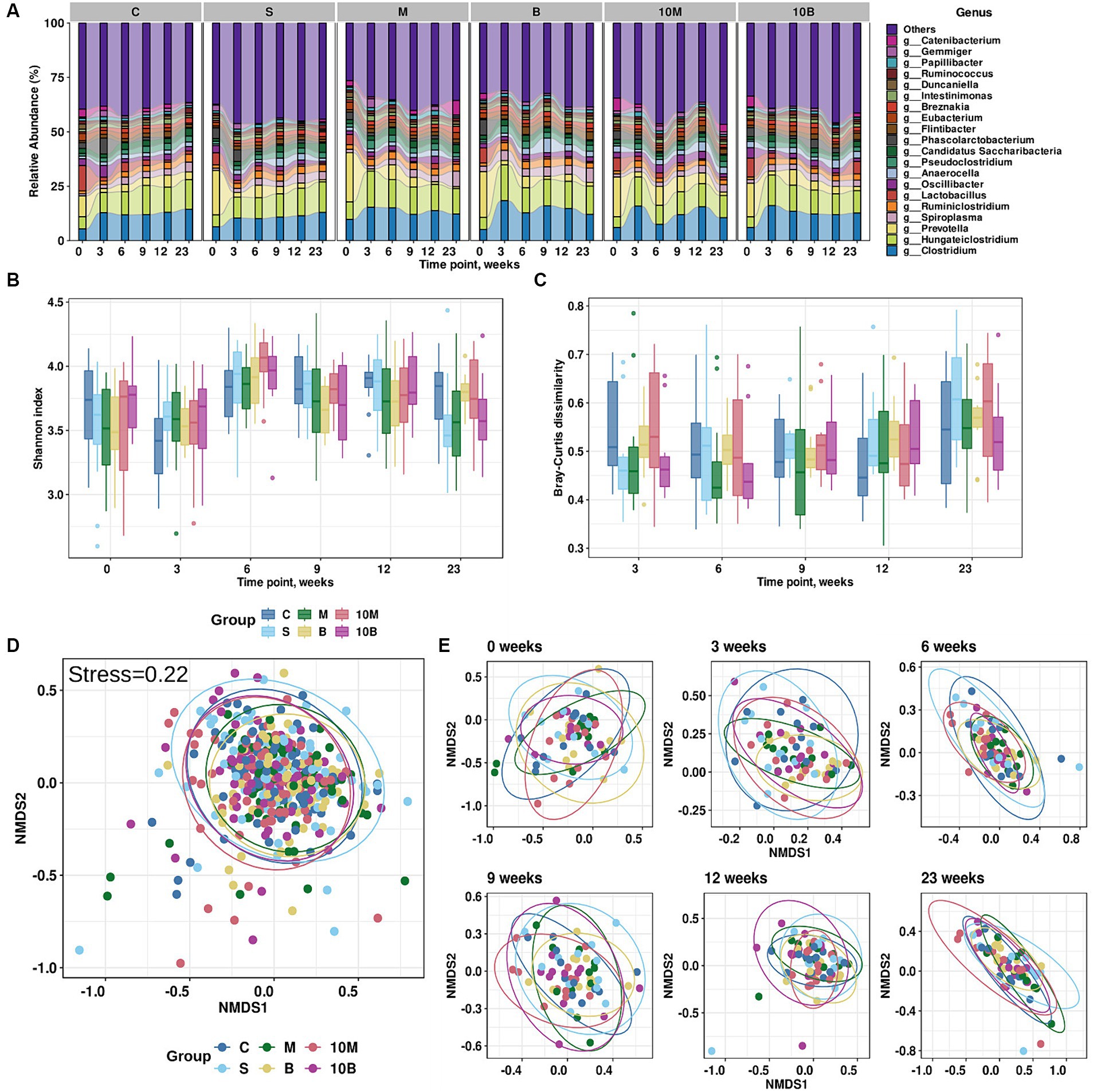
Figure 1. Results of exploratory analysis of gut microbiota changes in experimental groups. (A) Taxonomic distribution at the genus level for each experimental group across time points (weeks). X-axis indicates time points (weeks), while Y-axis shows relative abundance distributions in experimental groups. Different colors correspond to different taxonomic annotations. (B) Changes in Shannon index values for each experimental group across time points. X-axis indicates time points (weeks), Y-axis Shannon index values. Different colors correspond to different experimental groups. (C) Changes in Bray–Curtis dissimilarity calculated from baseline for each experimental group at different time points. X-axis indicates time points (weeks), Y-axis Shannon index values. Different colors correspond to different experimental groups. (D) Non-metric multidimensional scaling (NMDS) visualization of gut microbiome taxonomic profiles and Bray–Curtis dissimilarity metric (stress = 0.22). Different colors indicate different experimental groups. (E) NMDS visualization of (D) stratified by time point variable.
According to variance analysis, microbiota richness, which can be explained by alpha-diversity, depends on the time point (Three-Way ANOVA with a Benjamini-Hochberg correction for multiple testing adj. p < 0.001), but does not depend on gender or experimental group. The Shannon index distribution by experimental group and time point is presented in Figure 1B. It is worth noting that the Bray–Curtis dissimilarity calculated between the baseline time point and subsequent ones was associated not only with the time point, but also with gender (Three-Way ANOVA with a Benjamini-Hochberg correction for multiple testing, adj. p < 0.001), while the experimental group was not a statistically significant variable. The distribution of Bray–Curtis dissimilarity from the baseline time point is presented in Figure 1C. However, according to variance analysis using permutations, the overall distribution of distances between fecal samples depended on the time point (R2 = 0.080, adj. p = 0.0003) and gender (R2 = 0.007, adj. p = 0.0003), but not on the experimental group (R2 = 0.013, adj.p = 0.06; PERMANOVA using the Bray–Curtis dissimilarity with a Benjamini-Hochberg correction for multiple testing; 9,999 permutations). On the other hand, analysis of multidimensional scaling and visual inspection did not reveal clear clusters into which the samples could be divided (Figures 1D,E).
LefSe analysis was performed on the differential abundances of detected taxa linked to experimental groups. Our research data showed that in the control group where rats were treated with sucrose, the relative abundance of Faecalibaculum genera and Faecalibaculum rodentium in GM increased, while the SP-fed experimental groups were not associated with an increase in specific microorganism taxa (Supplementary Table S5). Multivariate analysis considering the influence of several factors on microbiota structure showed statistically significant associations of three bacterial species with different experimental groups. As with the LefSe analysis, rats from the sucrose-fed control group showed increases in F. rodentium according to MaAsLin2 results. In contrast to the LefSe analysis, MaAsLin 2 analysis identified additional associations such as increases in Anaerutruncus rubiinfantis in the experimental 10 EDrat monellin-fed group. Interestingly, according to relative abundance visualization, A. rubiinfantis also increased in the 1 EDrat monellin-fed group, but this result is not statistically reliable. Anaerocella delicata increased in the fetal samples of 1 EDrat brazzein-fed rats. MaAsLin 2 analysis results are presented in Figure 2. The MaAsLin 2 analysis data presented in relation to the time points showed that these associations do not tend to increase steadily in relative abundances; rather, they are fluctuant in nature and do not go beyond the standard deviation (Supplementary Figure S1). Additionally, the influence of time point on rat microbiome structure was evaluated (Supplementary Table S6).
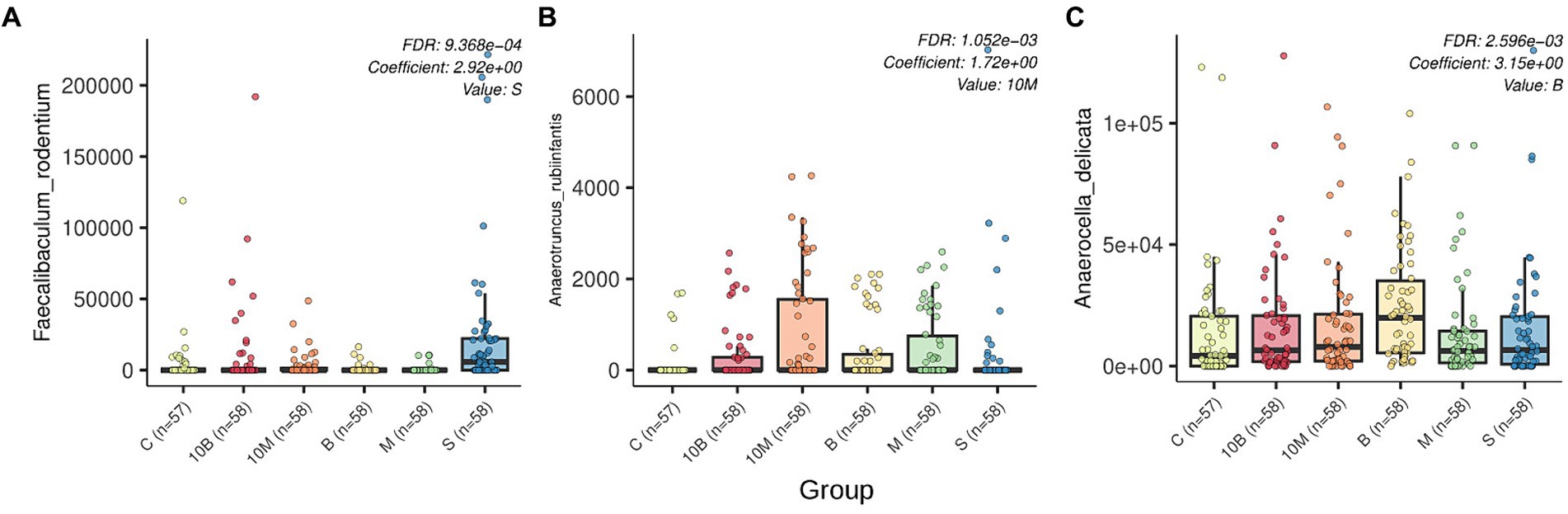
Figure 2. Box plots generated by MaAsLin2 show the differential abundance of microbial species by experimental group. FDR p-values and regression coefficients as well as experimental group variables are shown in the upper right corner of each plot. The analysis was performed with the following parameters: fixed_effects = c(“sex,” “group”), reference = c(c(“group, control”)), random_effects = c(“time_point,” “sourceid”), max_significance = 0.01. The x-axis indicates experimental group, while the y-axis indicates relative abundance. Differently abundant species are indicated by letters: (A) Faecalibaculum rodentum; (B) Anaerotruncus rubiinfantis; (C) Anaerocella delicata.
In this study we tested the effects of consuming recombinant monellin and brazzein on GM. We did not find any general structural changes in microbiota associated with sweet protein consumption. However, we detected changes in the comparative representation of individual bacterial species, such as Faecalibaculum rodentium, Anaerutruncus rubiinfantis, and Anaerocella delicata.
Our results showed an increase in Faecalibaculum rodentium when control group rats consumed sucrose. This is consistent with published reports that sucrose promotes the growth of F. rodentium, which displaces Th17-inducing microbiota (12). F. rodentium remodels retinoic acid signaling to control eosinophil-dependent homeostasis of the gut epithelium (30). Microbiome profiling identified F. rodentium as a key species involved in this regulation. In enterocytes, F. rodentium reduces the expression of retinoic acid-producing enzymes Adh1, Aldh1a1, and Rdh7, a reduction in retinoic acid signaling required to maintain specific populations of gut eosinophils. Eosinophils suppress the intraepithelial-lymphocyte-mediated production of interferon-γ, which regulates epithelial cell function (30). Microbiota-induced Th17 cells provide protection by regulating lipid absorption across the gut epithelium in an IL-17-dependent manner. Diet-induced loss of protective Th17 cells was mediated by the presence of sugar. Excluding sugar from a high-fat diet protected mice from obesity and metabolic syndrome in a manner dependent on commensal-specific Th17 cells (12).
The results showed that monellin has a minor effect on the composition of GM. It is worth noting some fluctuations in the relative abundance of Anaerotruncus rubiinfantis occurred when consuming monellin, depending on the time point of the experiment. The A. rubiinfantis strain was first discovered in 2016. This strictly anaerobic species forms gram-positive non-spore forming cocci, but there is currently no information about its functions within the host (31, 32).
Brazzein also induced some changes in GM. Bioinformatics analysis showed a shift in the relative abundance of Anaerocella delicata. A. delicata was isolated from animal farm waste in Japan (33). At this point, there is too little information to say how this strain’s enrichment might affect gut metagenome function.
The largest shift in bacterial composition is linked to the time point associated with changes in animal housing conditions, irrespective of the intake of target substances. Brazzein and monellin result in minor changes in the GM of rats. Recently, assessment of the same experimental animals’ morphological, physiological, biochemical, hematological, and behavioral characteristics allowed us to conclude that monellin and brazzein are safe and nontoxic for the mammalian organism (21). Therefore, changes in microbiota under the influence of SPs have a rather neutral character.
The problem of obesity has been quite acute in recent years. In order to avoid obesity and related diseases, more and more people are looking for substitutes to sugars in other sweeteners. In our study we examined the effect of sweet proteins brazzein and monellin on the composition of GM. The results showed that consuming such proteins affected the GM of rats to a lesser extent than changing their housing conditions from quarantine to experimental conditions.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1043060.
The animal study was approved by the study was conducted according to the guidelines of the Declaration of Helsinki and the Ethics Committee at Pharmbiomed Scientific Research Centre. The study was conducted in accordance with the local legislation and institutional requirements.
VV: Methodology, Writing – original draft, Writing – review & editing. DB: Methodology, Writing – review & editing. EO: Investigation, Writing – review & editing. KK: Conceptualization, Writing – original draft, Writing – review & editing. VB: Methodology, Writing – review & editing. NZ: Investigation, Writing – review & editing. AL: Methodology, Writing – review & editing. MM: Investigation, Writing – review & editing. PZ: Methodology, Writing – review & editing. PS: Conceptualization, Methodology, Writing – review & editing. OD: Conceptualization, Writing – review & editing. IM: Methodology, Writing – review & editing. TN: Conceptualization, Writing – original draft, Writing – review & editing. AK: Writing – original draft, Writing – review & editing. VL: Conceptualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the EFKO group of companies.
This work was performed using the core facilities of the Lopukhin FRCC PCM “Genomics, proteomics, metabolomics” (http://rcpcm.org/?p=2806). Additionally, we extend our gratitude to Anna Sokolova for her assistance in editing the English text.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1362529/full#supplementary-material
1. Rippe, JM, and Angelopoulos, TJ. Relationship between added sugars consumption and chronic disease risk factors: current understanding. Nutrients. (2016) 8:697. doi: 10.3390/NU8110697
2. Ma, X, Nan, F, Liang, H, Shu, P, Fan, X, Song, X, et al. Excessive intake of sugar: an accomplice of inflammation. Front Immunol. (2022) 13:988481. doi: 10.3389/FIMMU.2022.988481/BIBTEX
3. Ordoñez-Araque, R, and Revelo-Vizuete, B. Perspective Chapter: Sugar and Its Impact on Health. IntechOpen. (2023). doi: 10.5772/intechopen.104454
4. Kant, R. Sweet proteins – potential replacement for artificial low calorie sweeteners. Nutr J. (2005) 4:1–6. doi: 10.1186/1475-2891-4-5/FIGURES/1
5. Lobach, AR, Roberts, A, and Rowland, IR. Assessing the in vivo data on low/no-calorie sweeteners and the gut microbiota. Food Chem Toxicol. (2019) 124:385–99. doi: 10.1016/J.FCT.2018.12.005
6. Daly, K, Darby, AC, and Shirazi-Beechey, SP. Low calorie sweeteners and gut microbiota. Physiol Behav. (2016) 164:494–500. doi: 10.1016/J.PHYSBEH.2016.03.014
7. Redondo-Useros, N, Nova, E, González-Zancada, N, Díaz, LE, Gómez-Martínez, S, and Marcos, A. Microbiota and lifestyle: a special focus on diet. Nutrients. (2020) 12:1776. doi: 10.3390/NU12061776
8. Merra, G, Noce, A, Marrone, G, Cintoni, M, Tarsitano, MG, Capacci, A, et al. Influence of Mediterranean diet on human gut microbiota. Nutrients. (2020) 13:7. doi: 10.3390/NU13010007
9. Barber, TM, Valsamakis, G, Mastorakos, G, Hanson, P, Kyrou, I, Randeva, HS, et al. Dietary influences on the microbiota–gut–brain Axis. Int J Mol Sci. (2021) 22:3502. doi: 10.3390/IJMS22073502
10. Rowland, I, Gibson, G, Heinken, A, Scott, K, Swann, J, Thiele, I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. (2017) 57:1–24. doi: 10.1007/S00394-017-1445-8
11. Scheithauer, TPM, Rampanelli, E, Nieuwdorp, M, Vallance, BA, Verchere, CB, van Raalte, DH, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. (2020) 11:571731. doi: 10.3389/FIMMU.2020.571731/BIBTEX
12. Kawano, Y, Edwards, M, Huang, Y, Bilate, AM, Araujo, LP, Tanoue, T, et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell. (2022) 185:3501–3519.e20. doi: 10.1016/j.cell.2022.08.005
13. Suez, J, Korem, T, Zeevi, D, Zilberman-Schapira, G, Thaiss, CA, Maza, O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. (2014) 514:181–6. doi: 10.1038/nature13793
14. Pfeffer, M, Ziesenitz, SC, and Siebert, G. Acesulfame K, cyclamate and saccharin inhibit the anaerobic fermentation of glucose by intestinal bacteria. Z Ernahrungswiss. (1985) 24:231–5. doi: 10.1007/BF02023668/METRICS
15. Suez, J, Korem, T, Zilberman-Schapira, G, Segal, E, and Elinav, E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes. (2015) 6:149–55. doi: 10.1080/19490976.2015.1017700
16. Wang, W. Studying Changes of the Human Gut Microbiome In Response to Sweeteners Using RapidAIM. Medicine, Biology, Environmental Science. (2021). doi: 10.20381/RUOR-26509
17. Eeckhaut, V, Machiels, K, Perrier, C, Romero, C, Maes, S, Flahou, B, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. (2013) 62:1745–52. doi: 10.1136/GUTJNL-2012-303611
18. Chung, JH, Kong, JN, Choi, HE, and Kong, KH. Antioxidant, anti-inflammatory, and anti-allergic activities of the sweet-tasting protein brazzein. Food Chem. (2018) 267:163–9. doi: 10.1016/J.FOODCHEM.2017.06.084
19. Ruiz-Ojeda, FJ, Plaza-Díaz, J, Sáez-Lara, MJ, and Gil, A. Effects of sweeteners on the gut microbiota: a review of experimental studies and clinical trials. Adv Nutr. (2019) 10:S31–48. doi: 10.1093/ADVANCES/NMY037
20. Conz, A, Salmona, M, and Diomede, L. Effect of non-nutritive sweeteners on the gut microbiota. Nutrients. (2023) 15:1869. doi: 10.3390/NU15081869
21. Novik, TS, Koveshnikova, EI, Kotlobay, AA, Sycheva, LP, Kurochkina, KG, Averina, OA, et al. Sweet-tasting natural proteins Brazzein and Monellin: safe sugar substitutes for the food industry. Food Secur. (2023) 12:4065. doi: 10.3390/FOODS12224065
22. De Coster, W, D’Hert, S, Schultz, DT, Cruts, M, and Van Broeckhoven, C. Nano pack: visualizing and processing long-read sequencing data. Bioinformatics. (2018) 34:2666–9. doi: 10.1093/BIOINFORMATICS/BTY149
23. Curry, KD, Wang, Q, Nute, MG, Tyshaieva, A, Reeves, E, Soriano, S, et al. Emu: species-level microbial community profiling of full-length 16S rRNA Oxford Nanopore sequencing data. Nat Methods. (2022) 19:845–53. doi: 10.1038/s41592-022-01520-4
24. Ling, W, Lu, J, Zhao, N, Lulla, A, Plantinga, AM, Fu, W, et al. Batch effects removal for microbiome data via conditional quantile regression. Nat Commun. (2022) 13:5418. doi: 10.1038/s41467-022-33071-9
25. Oksanen, J, Blanchet, FG, Kindt, R, Legendre, P, Minchin, P, O’Hara, RB, et al. Vegan: community ecology package. R package version. 2.0–10. The R Project (2013).
26. Xu, S, Zhan, L, Tang, W, Wang, Q, Dai, Z, Zhou, L, et al. Microbiota Process: a comprehensive R package for deep mining microbiome. Innovation (Camb). (2023) 4:100388. doi: 10.1016/j.xinn.2023.100388
27. Segata, N, Izard, J, Waldron, L, Gevers, D, Miropolsky, L, Garrett, WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. (2011) 12:1–18. doi: 10.1186/GB-2011-12-6-R60/FIGURES/6
28. Beghini, F, McIver, LJ, Blanco-Míguez, A, Dubois, L, Asnicar, F, Maharjan, S, et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with biobakery 3. eLife. (2021) 10:e65088. doi: 10.7554/ELIFE.65088
29. Mallick, H, Rahnavard, A, McIver, LJ, Ma, S, Zhang, Y, Nguyen, LH, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol. (2021) 17:e1009442. doi: 10.1371/JOURNAL.PCBI.1009442
30. Cao, YG, Bae, S, Villarreal, J, Moy, M, Chun, E, Michaud, M, et al. Faecalibaculum rodentium remodels retinoic acid signaling to govern eosinophil-dependent intestinal epithelial homeostasis. Cell Host Microbe. (2022) 30:1295–1310.e8. doi: 10.1016/j.chom.2022.07.015
31. Tidjani Alou, M, Khelaifia, S, Michelle, C, Andrieu, C, Armstrong, N, Bittar, F, et al. Anaerococcus rubiinfantis sp. nov., isolated from the gut microbiota of a Senegalese infant with severe acute malnutrition. Anaerobe. (2016) 40:85–94. doi: 10.1016/J.ANAEROBE.2016.06.007
32. Pham, TPT, Cadoret, F, Alou, MT, Brah, S, Diallo, BA, Diallo, A, et al. “Urmitella timonensis” gen. Nov., sp. nov., “Blautia marasmi” sp. nov., “Lachnoclostridium pacaense” sp. nov., “Bacillus marasmi” sp. nov. and “Anaerotruncus rubiinfantis” sp. nov., isolated from stool samples of undernourished African children. New Microbes New Infect. (2017) 17:84–8. doi: 10.1016/J.NMNI.2017.02.004
Keywords: microbiome, natural sweetener, sugar substitute, intestinal bacteria, food additive, safety, symbiotic microorganism, microbiota composition
Citation: Veselovsky VA, Boldyreva DI, Olekhnovich EI, Klimina KM, Babenko VV, Zakharevich NV, Larin AK, Morozov MD, Zoruk PY, Sergiev PV, Dontsova OA, Maev IV, Novik TS, Kotlobay AA, Lazarev VN and Lagarkova MA (2024) Effect of the consumption of brazzein and monellin, two recombinant sweet-tasting proteins, on rat gut microbiota. Front. Nutr. 11:1362529. doi: 10.3389/fnut.2024.1362529
Received: 28 December 2023; Accepted: 11 March 2024;
Published: 21 March 2024.
Edited by:
Pauline M. Anton, UniLaSalle, FranceReviewed by:
Aleksandra Maria Kocot, University of Gdansk, PolandCopyright © 2024 Veselovsky, Boldyreva, Olekhnovich, Klimina, Babenko, Zakharevich, Larin, Morozov, Zoruk, Sergiev, Dontsova, Maev, Novik, Kotlobay, Lazarev and Lagarkova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria A. Lagarkova, bGFnYXJAcmNwY20ub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.