- 1Digestive Endoscopy Center, Tongren Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2Digestive Endoscopy Center, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background and aim: Gallstone disease (GSD) is a major public health problem worldwide. The dietary inflammatory index (DII) and the energy-adjusted DII (E-DII) have been used to describe dietary inflammatory potential. The current study sought to investigate the pro-inflammatory role of diet on GSD among outpatients in the United States.
Methods: Cross-sectional data from 7,334 individuals older than 20 years who participated in the National Health and Nutrition Examination Survey (NHANES) from January 2017 to March 2020 were obtained. The relationship between GSD and DII was assessed using self-reported data. An association between DII and the risk of GSD was determined using sample-weighted logistic regression and restricted cubic splines (RCS). Subgroup analyzes were conducted to assess the interaction between DII and related factors. Sensitivity analysis was further used to confirm the stability of the relationship. To control for the effect of total energy intake, E-DII was calculated and analyzed.
Results: A total of 10.5% of the study participants had GSD. The DII ranged from −5.52 to 5.51, and the median DII was significantly higher for participants with GSD than those without (1.68 vs. 1.23, p < 0.001). There was a significant and stable positive relationship between DII and GSD in adjusted models (OR 1.10, 95% CI 1.00–1.20). In the fully adjusted model, subjects with DII scores in the highest tertile were more likely to have GSD than those in the lowest tertile (OR 1.52, 95% CI 1.19–1.93). An apparent dose–response association between DII and GSD was detected. The association between E-DII and GSD remained stable.
Conclusion: Higher DII/E-DII scores linked to the intake of a pro-inflammatory diet were positively associated with a higher risk of GSD. These findings suggest that pro-inflammatory dietary patterns can promote the formation of gallstones.
1 Introduction
Gallstone disease (GSD) is common in the general population and its incidence has increased in recent years. The prevalence rate of GSD among adults in Europe, the United States, and other developed countries is approximately 10–15% (1). More than 20% of patients with GSD will develop symptoms, including colic or infection, during their lifetime (2). The direct and indirect costs of GSD are a leading cause of gastrointestinal disease-related hospitalization, resulting in a significant economic burden on families and society (3, 4).
Based on their composition, gallstones can be classified into cholesterol stones, which account for >90% of all gallstones, and other stones represented by black and brown pigments (2). Gallstone formation is shown to be multifactorial (1, 5) and risk factors include age, ethnicity, genetics, female gender, and lifestyle. GSD is also linked to insulin resistance, obesity, metabolic syndrome, and diabetes, of which diet plays a vital role. Studies indicate that a high intake of cholesterol, fatty acids, carbohydrates, and legumes can increase the risk of GSD. In contrast, the consumption of unsaturated fat, coffee, fiber, ascorbic acid (vitamin C), and calcium may lower the risk of this condition (5, 6). The specific dietary pattern that contributes to the development of GSD remains poorly understood, however.
It is well established that inflammation plays an essential role in the formation of gallstones (7–9). Inflammatory processes can affect cholesterol and bile acid metabolism by changing the metabolism of protein and fat, increasing the level of bile salt, and promoting the formation of gallstones (9). While GSD is linked to inflammation, evidence on whether a pro-inflammatory diet increases the risk of GSD remains limited.
Several nutritional indices such as Diet Inflammatory Index (DII), Dietary Antioxidant Index (DAI), Dietary Phytochemical Index (DPI), Nutrition Index (NI), dietary insulin index, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean diet (MED) were reported to evaluate the effect of diet on chronic diseases (10–16). Among these evaluation indicators, the DII was originally proposed in 2009 and recalculated in 2014 to quantify the potential inflammatory level of individual dietary components by giving them a score ranging from maximum anti-inflammatory to maximum pro-inflammatory (17, 18). In addition, to adjust the influence of total energy intake, the energy-adjusted DII (E-DII) was developed (19). The DII/E-DII index has been verified by several inflammatory biomarkers, including C-reactive protein (CRP), tumor necrosis factor (TNF-a), and interleukin-I (IL). In the past decade, this index has been widely used to explore the relationship between anti- or pro-inflammatory diets and disease morbidity and mortality (13, 14, 16, 20–22). However, only a few studies have explored the specific relationship between an inflammatory diet and the development of GSD.
The current study sought to assess the cross-sectional relationship between DII/E-DII and GSD using data from the National Health and Nutrition and Examination Surveys (NHANES). We found that exposure to a pro-inflammatory diet would increase the risk of GSD.
2 Methods
2.1 Data sources
Data were obtained from individuals who participated in the National Health and Nutrition Examination Survey (NHANES) from January 2017 to March 2020. NHANES is a stratified multi-stage sampling survey conducted by the National Center for Health Statistics (NCHS) and designed to assess the health and nutrition status of Americans. The survey, which includes a family interview and a health examination, has been approved by the NCHS research ethics review board since 1999.
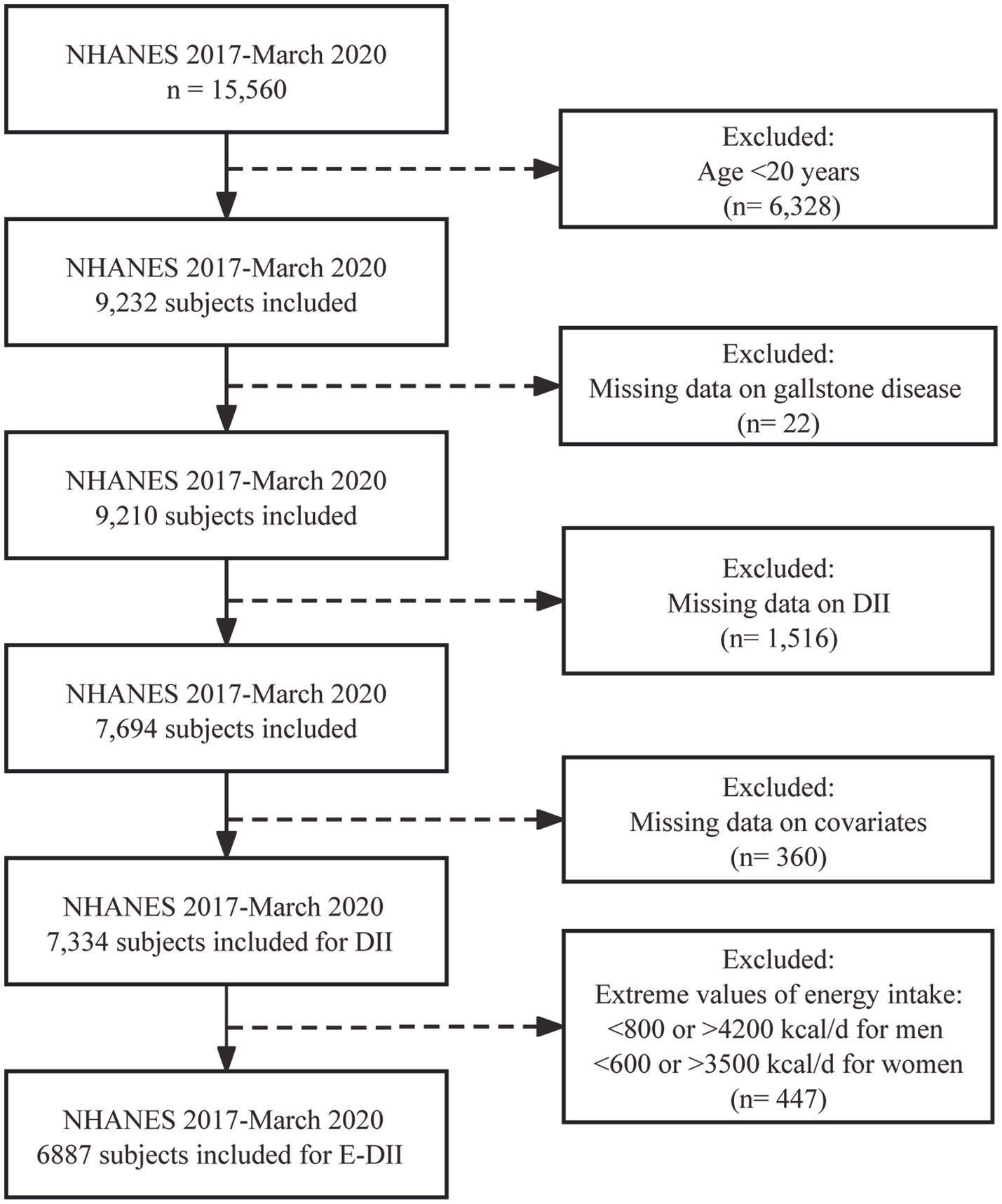
The current study included 15,560 individuals who participated in NHANES from January 2017–March 2020. After excluding individuals <20 years of age (n = 6,328), those missing data on GSD (n = 22), DII (n = 1,516), and covariates (n = 360), 7,334 participants were included in the final dataset for DII analysis. Extreme values of energy intake, including <800 kcal/d or > 4,200 kcal/d for men and < 600 kcal/d or > 3,500 kcal/d for women, were excluded when calculating the E-DII (n = 447) (23, 24). The inclusion and exclusion processes are shown in Figure 1.
2.2 Definition of GSD
The presence or absence of GSD is dependent on a patient’s self-report response to the question: “Has a doctor or other health professional ever told you that you had gallstones?”
2.3 Dietary inflammation index calculation
The DII is a potential tool to assess the anti- or pro-inflammatory quality of an individual’s diet by calculating the total potential inflammatory level of the dietary components consumed. This study calculated the exact nutritional intake of each participant using the nutritional intake information that was collected on day 1 and stored in the NHANES diet database. A total of 28 nutrients, including alcohol, vitamin A/B1/B2/B6/B12/C/D/E, β-carotene, caffeine, carbohydrate, cholesterol, energy, total fat, fiber, folic acid, iron, magnesium, zinc, selenium, monounsaturated fatty acids, niacin, n-3 fatty acids, n-6 fatty acids, protein, polyunsaturated fatty acids, and saturated fat, were used to determine the DII score in this study (Supplementary Table S2).
The specific calculation scheme of DII referred to the research of Shivappa et al. (18). Firstly, the dietary consumption information was compared to a worldwide daily intake database. The Z-score of each nutrient component was calculated based on the standard global daily mean intake and deviation (SD) values. Then it was transformed into a centered percentile, and multiplied by the respective overall inflammatory effect score to obtain the food parameter-specific DII score. Finally, all of the food parameter-specific DII scores were summated to gain an overall DII score for each individual. A higher DII score indicates that a diet is more pro-inflammatory in nature while a lower DII score indicates that a diet is more anti-inflammatory.
Accounting for the effect of total energy intake, density method was used to make energy adjustments for food and nutrient intake so that each parameter was expressed per thousand kilocalories (1,000 kcal). Then, the steps similar to the DII calculation were repeated to obtain E-DII but using an energy-adjusted global database (19).
2.4 Covariate assessment
Based on prior studies (1, 2, 25, 26), several potential confounding variables were selected as covariates for the analysis. The following demographic information was obtained: age (<40, 40–60, >60 years), gender (male or female), and race (non-Hispanic Black, non-Hispanic White, Mexican American, Other Hispanic, and Other). Body mass index (BMI) was calculated using height and weight data obtained during the NHANES mobile physical examination. Patients with a BMI >30 were categorized as obese while those ≤30 were categorized as non-obese. Participants were considered sedentary if they had ≥600 min of sedentary activity in a typical day and categorized as non-sedentary if they had <600 min/d of sedentary activity. Smoking and drinking status were classified according to the participants’ self-reported questionnaire responses. A respondent was defined as a non-smoker if they had smoked <100 cigarettes in their lifetime, and defined as a former smoker if they had smoked ≥100 cigarettes in their lifetime but did not smoke currently. Individuals who reported still smoking every day or on some days were defined as current smokers (27). Participants were further categorized as non-drinkers, light drinkers (1 to <30 drinks/month), or heavy drinkers (≥30 drinks/month). Diabetes, fatty liver, thyroid disease, and history of cancer were defined based on self-reported responses or confirmed clinical diagnoses (28–30).
2.5 Statistical analysis
Sample design and weights for the complex multi-stage cluster survey were considered using the Centers for Disease Control and Prevention (CDC) guidelines for the analysis of NHANES data. Participant characteristics were presented as means with standard deviation, SD for continuous variables, and the unweighted number of participants and weighted percentages (%) for categorical variables. Continuous variables were compared among groups using the Wilcoxon rank-sum test for complex survey samples and categorical variables were compared among groups using a weighted Chi-square test.
Sample-weighted logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) were used to measure associations between DII/E-DII scores and GSD. Four models were used after analyzing and adjusting for confounding factors. Model 1 represented the unadjusted crude model, model 2 was adjusted for sociodemographic variables (age group, sex, race, and ethnicity), and model 3 was based on model 2 and further adjusted for health-related and lifestyle factors, including sedentary activity, obesity, alcohol drinking status, smoking status, fatty liver, diabetes, and thyroid disease. To avoid over-adjustment, metabolic syndrome was defined as the presence of obesity, fatty liver, and/or diabetes (31). Model 4 was based on model 2 and further adjusted for sedentary activity, alcohol drinking status, smoking status, thyroid disease, and metabolic syndrome.
Restricted cubic splines were used to assess the dose–response relationship between GSD and DII scores, using four knots at prespecified locations according to the 5th, 35th, 65th, and 95th DII score percentiles. Subgroup analyzes were conducted using stratified multivariate regression analysis to assess the interaction between DII scores and specific covariates. p values for interactions across subgroups were calculated using the likelihood ratio test.
Given that the inclusion of the element of alcohol in the DII calculation, and data on alcohol consumption (n = 205) accounted for the largest proportion of the population participants with missing covariates (n = 360), sensitivity analyzes were performed to assess the robustness of the associations between DII and GSD after excluding alcohol intake (n = 7,539).
All statistical analyzes were performed with R software version 4.2.3 (R Core Team, Vienna, Austria. http://www.r-project.org/) using the survey package, version 4.1–1. All statistical tests were two-sided, and significance was considered at p < 0.05.
3 Results
3.1 Participant characteristics
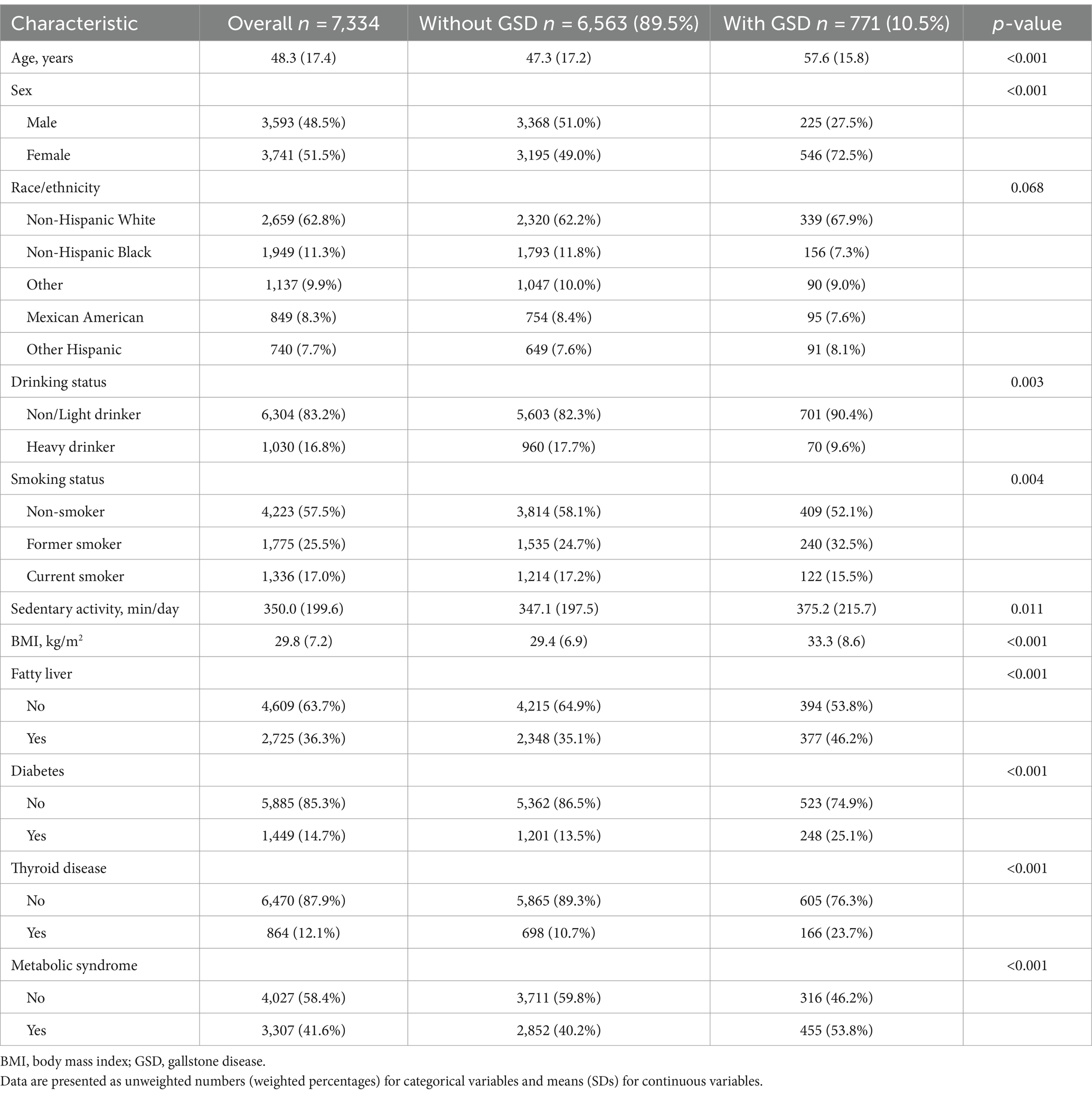
A total of 7,334 participants were included in the study analyzes for DII. The general characteristics of the participants with and without GSD are shown in Table 1. Of these, 771 and 6,563 participants did and did not have GSD, respectively, for a prevalence of 10.5%. The DII scores ranged from −5.52 (highly anti-inflammatory) to +5.51 (highly pro-inflammatory). Participants with GSD had a significantly higher DII score than those without (1.68 vs. 1.23, p < 0.001, Table 2). Participants with GSD were older, more likely to be female, and had a higher BMI value than those without (all p < 0.001). Sedentary activity, alcohol drinking status, smoking status, fatty liver, diabetes, and thyroid disease were also significantly associated with GSD (all p < 0.05).

Table 2. Dietary inflammatory index (DII) scores for January 2017–March 2020 NHANES participants with and without gallstone disease.
In further, the E-DII scores ranged from −5.25 to +5.33. There was no statistically significant difference of E-DII score between the two groups in the univariate analysis (Supplementary Table S1).
3.2 Association between DII/E-DII score and GSD risk
The association between the DII/E-DII score and the risk of GSD was determined using a sample-weighted multivariable logistic regression model (Table 3) and remained stable in each adjusted model. A higher DII score was associated with an increased risk of GSD (model 1, OR 1.22, 95% CI 1.12–1.32; model 2, OR 1.16, 95% CI 1.06–1.27; model 3, OR 1.10, 95% CI 1.00–1.20; model 4, OR 1.12, 95% CI 1.02–1.22). After full adjustment (model III), DII was associated with the presence of gallstones (OR 1.10, 95% CI 1.00–1.20). This association remained statistically significant after DII scores were grouped into tertiles. Subjects with the highest tertile DII scores had a higher risk of GSD than those with the lowest tertile DII scores (OR 1.52, 95% CI 1.19–1.93). The data also indicated that there was a linear relationship between DII scores and GSD (p for trend <0.05). Furthermore, multivariable-adjusted restricted cubic spline regression demonstrated a significant dose–response relationship between DII scores and the risk of GSD (Figure 2).

Table 3. Association between DII/E-DII and the presence of gallstone disease (GSD) among January 2017–March 2020 NHANES participants.

Figure 2. The restricted cubic spline for the association between dietary inflammatory index (DII) scores and gallstone disease (GSD).
Similar results of E-DII with GSD were obtained when grouped into tertiles. Individuals with the highest tertile E-DII scores had a higher risk of GSD than those with the lowest tertile E-DII scores (model 1, OR 1.61, 95% CI 1.07–2.42; model 2, OR 2.33, 95% CI 1.61–3.36; model 3, OR 1.90, 95% CI 1.26–2.84; model 4, OR 2.07, 95% CI 1.36–3.16) (Table 3).
3.3 Subgroup analyzes
Results of the subgroup analyzes are shown in Figure 3. No significant interactions were identified (p for interaction >0.1 for all). Effect of DII on GSD was consistent across all nine pre-specified subgroups.

Figure 3. Subgroup analyzes of the association between dietary inflammatory index (DII) and the development of gallstone disease (GSD). OR, odds ratio; CI, confidence interval. Sample-weighted logistic regression models were applied. Each stratification was adjusted for confounding factors such as age group, sex, race and ethnicity, sedentary activity, obesity, alcohol drinking status, smoking status, fatty liver, diabetes, and thyroid disease except the stratification factor itself.
3.4 Sensitivity analyzes
Excluding alcohol intake did not reduce the statistical significance of the relationship between DII score and GSD in any of the models (model 1, OR 1.22, 95% CI 1.12–1.32; model 2, OR 1.16, 95% CI 1.06–1.27; model 3, OR 1.11, 95% CI 1.01–1.23; model 4 OR 1.14, 95% CI 1.03–1.26) (Table 4).

Table 4. Association between dietary inflammatory index (DII) and the presence of gallstone disease (GSD) among January 2017–March 2020 NHANES participants, excluding alcohol intake.
4 Discussion
This study investigated the association between DII scores and GSD using data from a nationally representative study, NHANES. A robust association between DII score and GSD was observed in US adults, indicating that a pro-inflammatory diet is positively associated with an increased risk of GSD. After adjusting for all confounding factors, individuals with the highest DII/E-DII scores were shown to be at higher risk of developing GSD than those with the lowest DII/E-DII scores (OR 1.52, 1.19–1.93 95% CI, p trend <0.05 for DII, OR 1.90, 95% CI 1.26–2.84 for E-DII, Table 3). A dose–response relationship was observed between DII scores and GSD risk using restricted cubic spline regression. This association was generally consistent across subgroups. Sensitivity analysis confirmed the robustness of the primary analysis.
In recent years, the role of diet in regulating inflammation and affecting health has received widespread attention. The DII, developed by Shivappa et al. (18), is a reliable quantitative tool for evaluating the effects of diet on health by linking inflammatory cytokine levels in the blood to the outcomes of various chronic diseases (19). It was based on six of the most commonly studied inflammatory markers including IL-1β, IL-4, IL-6, IL-10, CRP, and TNF-α, and is used to quantitatively evaluate the anti- and pro-inflammatory effects of food (18, 19). A pro-inflammatory diet, that is, the higher DII score, is associated with an increased risk of several chronic noncommunicable diseases (NCD) (14, 20, 32–34), including metabolic syndrome and related diseases, cardiovascular and cerebrovascular diseases, cancers of various anatomic sites and depression and other mental health outcomes.
To the best of our knowledge, this is the first large population-based cross-sectional study to explore the association between a pro-inflammatory diet and GSD risk among a US population. One previous cross-sectional study, conducted using the Dena cohort, examined the association between DII scores and GSD (35). In contrast to findings from the current study, this report found that a pro-inflammatory diet was associated with a reduced risk of GSD. Due to the population restrictions of the geographic area, most of the participants had a similar diet and DII scores, which ranged from −0.4 to 1.43, so highly pro- or anti-inflammatory dietary data were lacking. Other studies that have synthesized and reviewed global database information have found that when the DII index covers all 45 food parameters, scores could range from −8.87 to +7.98. When it only refers to 25–30 parameters, the theoretical range of DII is usually from −5.5 to +5.5 (18, 19). Thus, the prior cross-sectional study may not be representative of people who consume a wide range of rich diets. While DII scores in the current study ranged from −5.52 (most anti-inflammatory) to +5.51 (most pro-inflammatory), which was consistent with most previous findings (18, 19). In addition, compared to the 4.3% incidence of GSD in the prior cross-sectional study (median DII −0.08), the GSD incidence in the current study was 10.5% (median DII 1.29). This reflects a likely correlation between the consumption of a pro-inflammatory diet and the development of GSD. Another case–control study was consistent with our results, in which the higher DII score, serum inflammatory and oxidative stress biomarkers were related to higher risk of GD in Iranian women (36). In our research, the E-DII was further calculated and analyzed for adjusting the effect of total energy intake, which indicated a stable and consistent correlation between E-DII and GSD.
Cholelithiasis is a critical public health issue and current researches suggest that three major pathogenic abnormalities are involved in the formation of gallstones: supersaturated gallbladder bile, precipitation and nucleation of excess cholesterol, and gallbladder hypomotility (1). Previous studies indicated that inflammation played an important role in the formation of gallstones (9, 37, 38). Higher levels of circulating inflammatory proteins and cytokines, including IL-1α, IL-6, IL-8, IL-10, IL-12 (p70), IL-13, CRP and tumor necrosis factor (TNF-α), were significantly associated with the increased risk of GSD (9, 37, 39–41). Inflammation-related histopathological changes occur in the gallbladder wall prior to the formation of cholesterol gallstones in both animal models and humans (37, 38, 42).Pro-inflammatory diet may increase the levels of circulating inflammatory proteins and cytokines in serum, which contributes to gallbladder wall fibrosis, and the impairment of gallbladder contractility (42). In addition, pro-inflammatory cytokines may lead to mucin hypersecretion, which plays an important role in the cholesterol nucleation process (41). The gallbladder hypomotility and mucin-related cholesterol nucleation predispose to the formation of gallstones (37, 43). The biological mechanisms underlying the association between pro-inflammatory diet and GSD would benefit from further researches.
This study had still several limitations. Firstly, given the cross-sectional study design of NHANES, the causal relationship between DII/E-DII scores and GSD could not be determined. Secondly, dietary data, GSD, and confounding factors were obtained from interviews or patient self-report questionnaires in the NHANES database, and are associated with an inevitable recall bias. Finally, while a sensitivity analysis was conducted, several participants were excluded due to the lack of data, which may have impacted the findings. A well-designed prospective cohort study will be necessary to explore the deeper relationship between DII/E-DII scores and GSD.
5 Conclusion
In conclusion, our findings indicate that a pro-inflammatory diet, that is, higher DII/E-DII scores, was positively associated with a higher risk of GSD. These findings indicate that pro-inflammatory dietary patterns can promote the formation of gallstones. Active dietary management and intervention should be considered to prevent the development of GSD.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics Institutional Review Board and Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JC: Writing – original draft, Conceptualization, Methodology, Funding acquisition. QZ: Software, Writing – original draft, Methodology. WW: Writing – review & editing, Conceptualization, Data curation. JL: Formal analysis, Resources, Writing – review & editing. LZ: Methodology, Visualization, Writing – review & editing. YX: Data curation, Writing – review & editing. HZ: Resources, Writing – review & editing. ZZ: Formal analysis, Visualization, Writing – review & editing. FZ: Investigation, Validation, Writing – review & editing. DY: Investigation, Validation, Writing – review & editing. YC: Project administration, Writing – review & editing, Funding acquisition. HP: Project administration, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shanghai Municipal Health Commission Health industry Clinical Research Project (No. 20234Y0016), Shanghai Municipal Natural Science Foundation (No. 21ZR1458600), Interdisciplinary Program of Shanghai Jiao Tong University (No. YG2022ZD031), and Shanghai Municipal Health Commission Key Laboratory of Gastrointestinal Tumor Innovation and Translation (No. ZDSYS-2021-01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1344699/full#supplementary-material
References
1. Di Ciaula, A, Wang, DQ, and Portincasa, P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. (2018) 34:71–80. doi: 10.1097/MOG.0000000000000423
2. Lammert, F, Gurusamy, K, Ko, CW, Miquel, JF, Mendez-Sanchez, N, Portincasa, P, et al. Gallstones. Nat Rev Dis Primers. (2016) 2:16024. doi: 10.1038/nrdp.2016.24
3. Everhart, JE, and Ruhl, CE. Burden of digestive diseases in the United States part iii: liver, biliary tract, and pancreas. Gastroenterology. (2009) 136:1134–44. doi: 10.1053/j.gastro.2009.02.038
4. Everhart, JE, and Ruhl, CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. (2009) 136:376–86. doi: 10.1053/j.gastro.2008.12.015
5. Stinton, LM, and Shaffer, EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. (2012) 6:172–87. doi: 10.5009/gnl.2012.6.2.172
6. Davidović, DB, Tomić, DV, and Jorg, JB. Dietary habits as a risk factor of gallstone disease in Serbia. Acta Chir Iugosl. (2011) 58:41–4. doi: 10.2298/ACI1104041D
7. Fremont-Rahl, JJ, Ge, Z, Umana, C, Whary, MT, Taylor, NS, Muthupalani, S, et al. An analysis of the role of the indigenous microbiota in cholesterol gallstone pathogenesis. PLoS One. (2013) 8:e70657. doi: 10.1371/journal.pone.0070657
8. Shabanzadeh, DM, Skaaby, T, Sørensen, LT, Eugen-Olsen, J, and Jørgensen, T. Metabolic biomarkers and gallstone disease - a population-based study. Scand J Gastroenterol. (2017) 52:1270–7. doi: 10.1080/00365521.2017.1365166
9. Liu, Z, Kemp, TJ, Gao, YT, Corbel, A, McGee, EE, Wang, B, et al. The Association of Circulating Inflammation Proteins and Gallstone Disease. J Gastroenterol Hepatol. (2018) 33:1920–4. doi: 10.1111/jgh.14265
10. Arabshahi, V, Amiri, R, Ghalishourani, SS, Hasaniani, N, Nozarian, S, Tavasolian, R, et al. Association between dietary insulin index and load with cardiometabolic risk factors and risk of metabolic syndrome among the patients with type 2 diabetes: a cross-sectional study. BMC Nutr. (2023) 9:141. doi: 10.1186/s40795-023-00803-z
11. Rahimlou, M, Grau, N, Banaie-Jahromi, N, Taheri, M, Khosravi, A, Mavrommatis, Y, et al. Association of Adherence to the dietary approach to stop hypertension and Mediterranean diets with blood pressure in a non-hypertensive population: results from Isfahan salt study (Iss). Nutr Metab Cardiovasc Dis. (2022) 32:109–16. doi: 10.1016/j.numecd.2021.09.029
12. Kim, M, and Park, K. Association between phytochemical index and metabolic syndrome. Nutr Res Pract. (2020) 14:252–61. doi: 10.4162/nrp.2020.14.3.252
13. Petermann-Rocha, F, Wirth, MD, Boonpor, J, Parra-Soto, S, Zhou, Z, Mathers, JC, et al. Associations between an inflammatory diet index and severe non-alcoholic fatty liver disease: a prospective study of 171,544 Uk biobank participants. BMC Med. (2023) 21:123. doi: 10.1186/s12916-023-02793-y
14. Ruiz-Canela, M, Bes-Rastrollo, M, and Martínez-González, MA. The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int J Mol Sci. (2016) 17:1265. doi: 10.3390/ijms17081265
15. Xu, Q, Qian, X, Sun, F, Liu, H, Dou, Z, and Zhang, J. Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. J Affect Disord. (2023) 322:84–90. doi: 10.1016/j.jad.2022.11.013
16. Jayanama, K, Theou, O, Godin, J, Cahill, L, Shivappa, N, Hébert, JR, et al. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age Spectrum. BMC Med. (2021) 19:64. doi: 10.1186/s12916-021-01918-5
17. Cavicchia, PP, Steck, SE, Hurley, TG, Hussey, JR, Ma, Y, Ockene, IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. (2009) 139:2365–72. doi: 10.3945/jn.109.114025
18. Shivappa, N, Steck, SE, Hurley, TG, Hussey, JR, and Hébert, JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/s1368980013002115
19. Hébert, JR, Shivappa, N, Wirth, MD, Hussey, JR, and Hurley, TG. Perspective: the dietary inflammatory index (dii)-lessons learned, improvements made, and future directions. Adv Nutr. (2019) 10:185–95. doi: 10.1093/advances/nmy071
20. Wang, X, Sun, M, Wang, L, Li, J, Xie, Z, Guo, R, et al. The role of dietary inflammatory index and physical activity in depressive symptoms: results from Nhanes 2007-2016. J Affect Disord. (2023) 335:332–9. doi: 10.1016/j.jad.2023.05.012
21. Sun, M, Fang, J, Gao, W, He, Y, Ma, Y, and Jin, L. Association of the Dietary Inflammatory Index with phenotypic age. Epidemiol Health. (2023) 45:e2023051. doi: 10.4178/epih.e2023051
22. Farazi, M, Jayedi, A, and Shab-Bidar, S. Dietary inflammatory index and the risk of non-communicable chronic disease and mortality: an umbrella review of meta-analyses of observational studies. Crit Rev Food Sci Nutr. (2023) 63:57–66. doi: 10.1080/10408398.2021.1943646
23. Chen, L, Ming, J, Chen, T, Hébert, JR, Sun, P, Zhang, L, et al. Association between dietary inflammatory index score and muscle mass and strength in older adults: a Study from National Health and nutrition examination survey (Nhanes) 1999-2002. Eur J Nutr. (2022) 61:4077–89. doi: 10.1007/s00394-022-02941-9
24. Shi, Y, Lin, F, Li, Y, Wang, Y, Chen, X, Meng, F, et al. Association of pro-Inflammatory Diet with increased risk of all-cause dementia and Alzheimer's dementia: a prospective study of 166,377 Uk biobank participants. BMC Med. (2023) 21:266. doi: 10.1186/s12916-023-02940-5
25. Cai, JS, Qiang, S, and Bao-Bing, Y. Advances of recurrent risk factors and Management of Choledocholithiasis. Scand J Gastroenterol. (2017) 52:34–43. doi: 10.1080/00365521.2016.1224382
26. Konyn, P, Alshuwaykh, O, Dennis, BB, Cholankeril, G, Ahmed, A, and Kim, D. Gallstone disease and its association with nonalcoholic fatty liver disease, all-cause and cause-specific mortality. Clin Gastroenterol Hepatol. (2023) 21:940–8.e2. doi: 10.1016/j.cgh.2022.04.043
27. Sutton, JD, Salas Martinez, ML, and Gerkovich, MM. Environmental tobacco smoke and periodontitis in United States non-smokers, 2009 to 2012. J Periodontol. (2017) 88:565–74. doi: 10.1902/jop.2017.160725
28. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2010) 33:S62–9. doi: 10.2337/dc10-S062
29. Siddiqui, MS, Vuppalanchi, R, Van Natta, ML, Hallinan, E, Kowdley, KV, Abdelmalek, M, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2019) 17:156–63.e2. doi: 10.1016/j.cgh.2018.04.043
30. Eddowes, PJ, Sasso, M, Allison, M, Tsochatzis, E, Anstee, QM, Sheridan, D, et al. Accuracy of Fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1717–30. doi: 10.1053/j.gastro.2019.01.042
31. Kassi, E, Pervanidou, P, Kaltsas, G, and Chrousos, G. Metabolic syndrome: definitions and controversies. BMC Med. (2011) 9:48. doi: 10.1186/1741-7015-9-48
32. Hariharan, R, Odjidja, EN, Scott, D, Shivappa, N, Hébert, JR, Hodge, A, et al. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes Rev. (2022) 23:e13349. doi: 10.1111/obr.13349
33. Fowler, ME, and Akinyemiju, TF. Meta-analysis of the association between dietary inflammatory index (dii) and cancer outcomes. Int J Cancer. (2017) 141:2215–27. doi: 10.1002/ijc.30922
34. Fan, L, Zhao, S, Shi, H, and Zhang, S. Role of Bmi in the relationship between dietary inflammatory index and non-alcoholic fatty liver disease: an intermediary analysis. Scand J Gastroenterol. (2023) 58:1159–65. doi: 10.1080/00365521.2023.2213791
35. Sadri, Z, Harouni, J, Vahid, F, Khosravani, Z, and Najafi, F. Association between the dietary inflammatory index with gallstone disease: finding from Dena Persian cohort. BMJ Open Gastroenterol. (2022) 9:e000944. doi: 10.1136/bmjgast-2022-000944
36. Liu, N, Feng, Y, Li, J, Ma, X, and Ma, F. Relationship between the dietary inflammatory index and kidney stone prevalence. World J Urol. (2022) 40:1545–52. doi: 10.1007/s00345-022-03998-1
37. Maurer, KJ, Carey, MC, and Fox, JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. (2009) 136:425–40. doi: 10.1053/j.gastro.2008.12.031
38. Rege, RV . Inflammatory cytokines Alter human gallbladder epithelial cell absorption/secretion. J Gastrointest Surg. (2000) 4:185–92. doi: 10.1016/s1091-255x(00)80055-4
39. Ghorbani, M, Hekmatdoost, A, Darabi, Z, Sadeghi, A, and Yari, Z. Dietary inflammatory index and risk of gallstone disease in Iranian women: a case-control study. BMC Gastroenterol. (2023) 23:311. doi: 10.1186/s12876-023-02943-9
40. Liu, T, Siyin, ST, Yao, N, Duan, N, Xu, G, Li, W, et al. Relationship between high-sensitivity C reactive protein and the risk of gallstone disease: results from the Kailuan cohort study. BMJ Open. (2020) 10:e035880. doi: 10.1136/bmjopen-2019-035880
41. Maurer, KJ, Rao, VP, Ge, Z, Rogers, AB, Oura, TJ, Carey, MC, et al. T-cell function is critical for murine cholesterol gallstone formation. Gastroenterology. (2007) 133:1304–15. doi: 10.1053/j.gastro.2007.07.005
42. van Erpecum, KJ, Wang, DQ, Moschetta, A, Ferri, D, Svelto, M, Portincasa, P, et al. Gallbladder histopathology during murine gallstone formation: relation to motility and concentrating function. J Lipid Res. (2006) 47:32–41. doi: 10.1194/jlr.M500180-JLR200
Keywords: National Health and Nutrition Examination Survey (NHANES), gallstone disease, pro-inflammatory diet, dietary inflammatory index (DII), population-based study
Citation: Cheng J, Zhuang Q, Wang W, Li J, Zhou L, Xu Y, Zhang H, Zhang Z, Zhou F, Yang D, Chu Y and Peng H (2024) Association of pro-inflammatory diet with increased risk of gallstone disease: a cross-sectional study of NHANES January 2017–March 2020. Front. Nutr. 11:1344699. doi: 10.3389/fnut.2024.1344699
Edited by:
Fei Xu, Nanjing Municipal Center for Disease Control and Prevention, ChinaReviewed by:
Binwu Sheng, First Affiliated Hospital of Xi’an Jiaotong University, ChinaMehran Rahimlou, Zanjan University of Medical Sciences, Iran
Copyright © 2024 Cheng, Zhuang, Wang, Li, Zhou, Xu, Zhang, Zhang, Zhou, Yang, Chu and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimin Chu, Y3ltMTkwNUBzaHRyaG9zcGl0YWwuY29t; Haixia Peng, cGh4MTEwMUBzaHRyaG9zcGl0YWwuY29t
†These authors have contributed equally to this work and share first authorship
 Jinnian Cheng
Jinnian Cheng Qian Zhuang
Qian Zhuang Weiyi Wang1†
Weiyi Wang1† Ying Xu
Ying Xu Fengli Zhou
Fengli Zhou Daming Yang
Daming Yang Yimin Chu
Yimin Chu Haixia Peng
Haixia Peng