- 1Division of Metabolic Diseases and Clinical Nutrition, Department of Specialist Medicines, University Hospital of Modena and Reggio Emilia - Policlinico, Modena, Italy
- 2Internal and Emergency Medicine, Modena - Ospedale Civile Baggiovara, Azienda Ospedaliero-Universitaria Modena, Modena, Italy
Emery–Dreifuss muscular dystrophy (EDMD) is a rare, inherited human disease. Similar to other neuromuscular dystrophies, EDMD is clinically characterized by muscle atrophy and weakness, multi-joint contractures with spine rigidity, and cardiomyopathy. Over time, muscular weakness can lead to dysphagia and a severe lowering of body mass index (BMI), worsening the prognosis. We present the case of a young male patient affected by EDMD, admitted to the hospital for pneumothorax in a severe state of undernourishment. The patient was treated with total parenteral nutrition (TPN) with Smofkabiven®, supplemented with micronutrients (vitamins and trace elements), and with minimal enteral nutrition through food. Within a year, the patient gained 8.5 kg and kept his body weight stable for the 6 years of the follow-up. In this study, we show that TPN ensures the nutritional requirements of EDMD patients in a safe and well-tolerated manner, allowing a considerable and stable improvement in nutritional status, which has a positive impact on the disease itself and the patients’ quality of life.
Introduction
EDMD is a rare slowly progressive muscular dystrophy. It is a genetically heterogeneous neuromuscular orphan spectrum disease affecting approximately 0.3–0.4 in 100,000 people (1). Several genes have been implicated in the pathogenesis of EDMD, such as EMD, LMNA, SYNE1, SYNE2, FHL1, and TMEM43 (2); however, there are still more causative genes yet to be discovered, as over 60% of patients do not have mutations in EMD or LMNA, the most common genes involved in EDMD pathogenesis (2). In EDMD, the onset of symptoms occurs within the first decade of life. Contractures of the elbows, neck extensor muscles, and Achilles’ tendons appear to be the first symptoms of the disease and occur before muscle weakness and deterioration (3). Progressive muscle degeneration begins at the end of the second decade of life in a humeroperoneal distribution (3). The cervical spine rigidity may become prominent enough to alter neck anatomy and impair swallowing, which is already present due to muscle weakness (3). Cardiac complications are found in the majority of EDMD patients, the onset is during the teenage years, and they worsen over time, frequently leading to sudden death (4). Nutritional complications often arise in EDMD dystrophies, but they are sometimes underestimated. Progressive muscle weakness leads to chewing, swallowing, and digestive problems, resulting in weight loss, which further worsens muscular performance (5). A negative energy balance due to unmet nutritional requirements, caused by an insufficient protein energy intake, may worsen the clinical outcome (6). Resting energy expenditure (REE) is mostly spent by lean body mass (LBM), composed of muscle mass and viscera, the latter contributing 10 times more to the REE (7). Only one study has explored the correlation between body composition and REE in patients with Emery–Dreifuss muscular dystrophy (EDMD). These changes include elevated body fat mass and a noteworthy decrease in LBM. Despite these alterations, EDMD patients demonstrate an increased REE, particularly when the REE is adjusted for LBM (8). These findings are unexpected because prominent progression of muscular dystrophy with no change in the viscera should cause a decrease in the daily REE. Patients with EDMD do not lose significant LBM, and muscle mass does not decrease remarkably. Therefore, causes other than muscle wasting may have been involved in these REE abnormalities, and perhaps metabolic alterations and respiratory distress may play a role (8). This evidence is in line with what has been previously observed in patients with Duchenne muscular dystrophy (DMD) (9, 10).
We present the case of an adult male patient affected by EDMD who was treated for 6 years with TPN in combination with minimal enteral feeding. We describe the positive effects of artificial nutritional therapy on nutritional parameters, body weight, and physical performance in this particular clinical setting.
Case report
A 26-year-old male patient affected by EDMD (laminopathy type A/C, LMNA gene, NM_170707, c.523_537del (p.Ala175_Ala179del)) was admitted to the hospital in July 2015 for an acute respiratory failure caused by a bilateral spontaneous pneumothorax drained with a CT-guided catheter positioning. The patient had been admitted due to worsening respiratory symptoms, including desaturation at home, requiring continuous non-invasive mechanical ventilation (NIMV) therapy in the prone position during the day and a supine position at night. In addition to EDMD with complete dysautonomia but without cognitive deterioration, the patient had a history of chronic atrial fibrillation treated with Coumadin and a previous right pneumothorax (2011), which was surgically treated. Upon admission, the patient presented with dyspnea but was alert, oriented, and cooperative. The physical examination revealed generalized loss of subcutaneous adipose tissue and concurrent compromise of muscle mass (muscle wasting in the temporal, deltoid, and quadriceps muscles) without dependent edema. He presented a flat and manageable abdomen with no ascites. Moreover, he had dry skin and mucous membranes indicative of dehydration. The ECG showed a non-sinus rhythm consistent with atrial fibrillation. Radiological investigations revealed bilateral spontaneous pneumothorax, which was treated with guided pigtail TAC drainage, resulting in the gradual improvement of hypoxemic respiratory status. A follow-up CT scan showed minimal persistence of bilateral pneumothorax (approximately 1 cm thickness). Since there were no ventilatory repercussions, their oxygen saturation values were stable, and they did not experience any fatigue or pain, it was decided not to proceed with further drainage maneuvers. During hospitalization, the patient did not develop fever and received a blood transfusion for transient anemia. Upon discharge, the patient was in good general condition but still required continuous NIV, supplemented with oxygen during nighttime hours.
During the first nutritional assessment at hospital admission, we registered the following parameters: weight 22.5 kg, height 1.64 m, and body mass index (BMI) 8.36 kg/m2. The patient weighed 33 kg until he was 10 years old, and then he had a significant weight loss. Until January 2014, his usual body mass was 23.8 kg. In June 2015, body weight was 22.8 kg and decreased to 22.5 kg a month later; therefore, in 12 months, the patient had an involuntary weight loss of 5.4%, compared to usual body weight. The patient presented dysphagia to solids, leading to a progressive decrease in oral intake, especially in the 6 months prior to hospitalization. This impacted the average daily oral energy intake, reducing it down to 500–600 kcal/day, corresponding to 50% of his nutrient requirement. The oral water intake was approximately 150–200 mL/day.
During hospitalization, the already low oral nutrition was completely suspended due to NIV. Due to severe malnutrition, the patient was treated with artificial nutrition. In particular, a midline catheter was inserted into the patient’s superior vena cava, to act as a peripherally inserted central catheter (PICC). The patient received a TPN regimen with 986 mL/day of Smofkabiven® (=900 kcal non-protein +50 g amino acids) to be administered in 24 h, daily with micronutrients (vitamins and trace elements). TPN was gradually implemented to reduce the risk of refeeding syndrome, as recommended by the American Society for Parental and Enteral Nutrition (ASPEN) guidelines (11). Furthermore, the patient was nourished with minimal enteral nutrition with natural foods (less than 200 kcal/day). A high calorie, high protein oral was also prescribed, but never actually assumed. NIV was continued during the hospitalization. After a month, the patient was discharged from the hospital with a clear improvement in his general clinical conditions, even though he needed to remain on continuous NIV. The patient was sent home with the following nutritional therapy: (1) TPN with Smofkabiven®, to be administered within approximately 18 h (overnight), along with daily micronutrients as recommended by international guidelines; (2) minimal enteral feeding using natural foods administered orally. Continuous NIV was prescribed, with nasal olive during the daytime and with a mask overnight. In addition, to start home enteral nutrition, as suggested by the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines, we scheduled the placement of a percutaneous endoscopic gastrostomy (PEG) tube, which was to be performed a month later during the nutritional follow-up (12). However, when the patient came back for control, he expressed the desire not to have the PEG tube, to avoid the constant need to adopt the prone position. Following the patient’s wish, despite the potential endoscopic feasibility of PEG placement, we decided not to insert the PEG tube but continued TPN with Smofkabiven®, along with micronutrients as optimized the month before. TPN was associated with minimal enteral feeding with natural foods as desired by the patient: 200–250 kcal/day +5–10 g protein/day and 150–200 mL/day of water.
The patient underwent clinical, metabolic, and nutritional monitoring, undertaken routinely during the 6 years of the TPN at home (Table 1). No electrolyte or fluid alterations (i.e., related to metabolic toxicity) were observed. The patient kept lipid levels and blood-chemical parameters of hepatic (cytolysis and cholestasis) and renal functions within the normal range. Any signs of pressure injury, aspiration pneumonia, or edema were detected. Patients did not develop heart failure during the follow-up period. It is also important to note that there were no infectious episodes related to venous access. The PICC has been carefully maintained; in the 6 years of treatment, it has been replaced twice, once following its suspected obstruction and the second time after its accidental removal during the dressing practice.
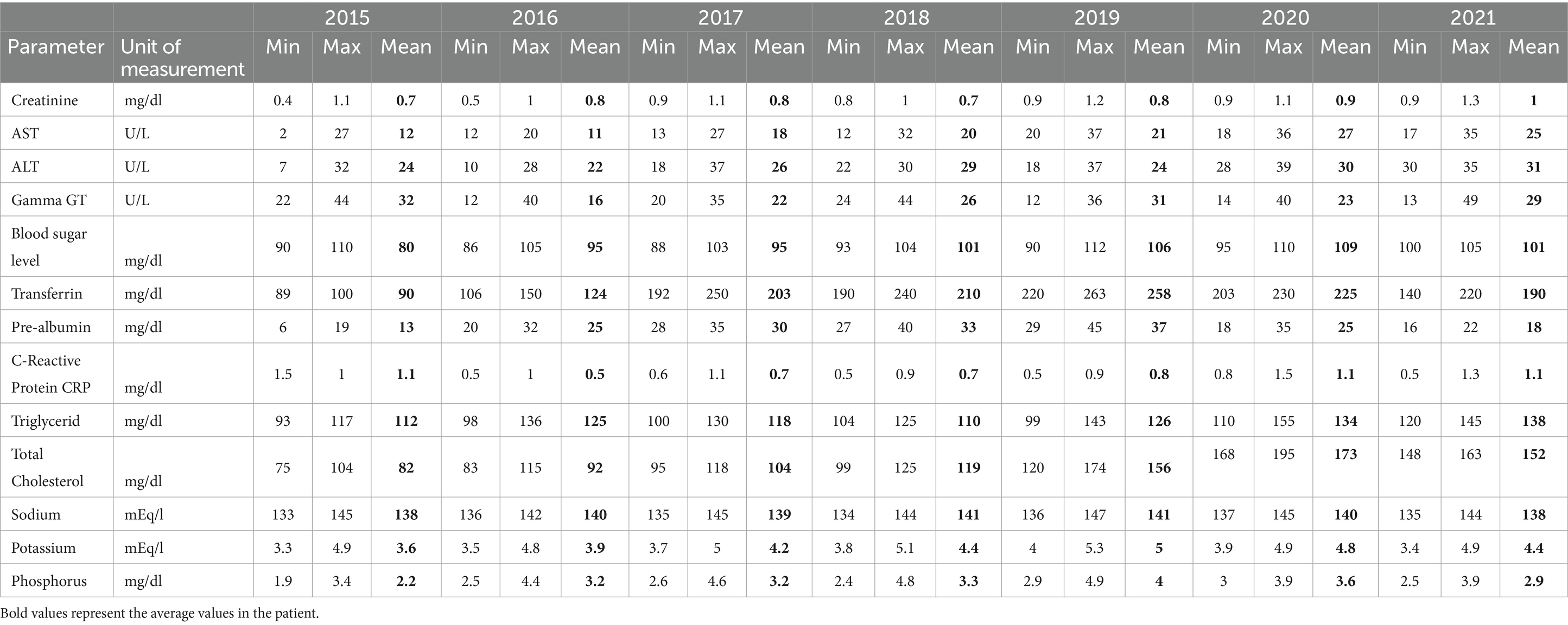
Table 1. Hematological and biochemical indices measured during the quarterly clinical-nutritional monitoring of home parenteral nutrition (HPN) are described within the entire duration of the treatment.
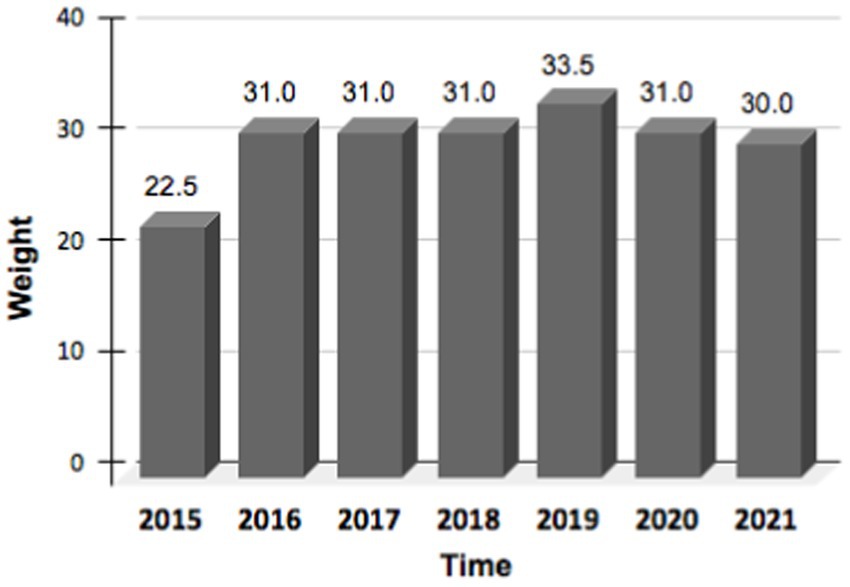
The patient’s body weight improved and was maintained during the 6 years of treatment (Figure 1). While assessments of the quality of life using validated instruments were not conducted, the patient reported a noticeable and widespread improvement in subjective wellbeing and energy perception associated with weight recovery (patient perspective). Furthermore, the nutritional solution was consistently perceived as comfortable without negatively impacting the ability to carry out the daily activities that the patient was able and willing to engage in.
Discussion
Life expectancy has increased in patients with muscular dystrophies due to medical interventions such as home ventilation, physical therapy, pacemaker or defibrillator placement, ACE inhibitors, and artificial nutrition (3). In the absence of therapeutic solutions for EDMD and other muscular dystrophies, long-term management of patients consists of appropriate clinical monitoring and symptomatic treatment (13). Nutrition could be considered a key parameter to monitor because excessive weight loss can worsen the overall physical condition of the patient (5). However, further studies are needed to better understand the effect of nutritional therapy on clinical outcomes in EDMD.
We presented the case of a patient affected by EDMD with a severe malnutrition status and a low BMI. It means that the patient was treated with Total Parenteral Nutrition (TPN), in synergy with minimal enteral feeding, for 6 years; we show that this type of nutritional therapy has contributed, over time, to ensure, in a safe and well-tolerated manner, the patient daily nutritional requirements. The patient gained weight within a year and kept it stable during the 6 years of follow-up. In this rare clinical context, TPN has made possible a significant improvement in the patient’s health and life expectancy. In addition, the patient had consistently reported a good overall tolerance to the home parenteral nutrition (HPN) treatment and an improvement in subjective well-being associated with weight recovery.
Although it was not possible to implement total enteral nutrition as recommended (12) due to the patient’s refusal, total parenteral nutrition (TPN) ensured adequate and satisfactory nutritional status. Additionally, no clinical or metabolic complications arose, as demonstrated by the trend in hematological and biochemical parameters (blood glucose, lipid profile, and hepato-renal function) throughout the entire duration of the treatment (Table 1).
EDMD typically results from a structural or functional defect in one or more proteins comprising the nuclear envelope. A deficiency or a mutation affecting any of these proteins can result in loss of structural integrity of the nucleus, which can be particularly problematic for tissues that are frequently under stress, including cardiac and skeletal muscles (2). Muscle deterioration and neck anatomy alterations lead to dysphagia over time, compromising calorie intake and resulting in severe weight loss (3). EDMD patients have an increased body fat mass and a significant decrease in LBM (8). Despite the decrease in muscle mass with no prominent change in the viscera, which accounts for the majority of REE, EDMD patients have an increased REE (7, 8). These observations suggest that an increased level of energy consumption, due to either heat consumption or metabolic changes, may require greater caloric intake than expected to maintain body weight. These alterations in REE, if not counterbalanced by increased caloric intake, may contribute to weight loss and a further deterioration in muscle performance.
Currently, there is a lack of data regarding the effects of nutritional therapy in EDMD, and no specific guidelines have been published. This report may suggest the feasibility and safety of nutritional therapy as an indication for improving the overall conditions of affected patients.
Conclusion
When enteral nutrition is not achievable, TPN can be a feasible and safe nutritional therapy for patients affected by EDMD, allowing for sufficient protein and calorie intake. This approach helps mitigate signs of malnourishment and improves overall conditions with good patient tolerance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving human samples in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FV: Conceptualization, Investigation, Writing – original draft. GP: Conceptualization, Investigation, Writing – review & editing. SG: Investigation, Writing – review & editing. RM: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by unconditionally funded by Fresenius Kabi Italia srl. The sponsor had no influence on the content.
Acknowledgments
Medical writing support for the preparation of this article was provided by Carmela Irene, PhD, on behalf of Edra S.p.A. and was unconditionally funded by Fresenius Kabi Italia Srl.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mah, J, Korngut, L, Fiest, K, Dykeman, J, Day, L, Pringsheim, T, et al. A systematic review and meta-analysis on the epidemiology of the muscular dystrophies. Can J Neurol Sci. (2016) 43:163–77. doi: 10.1017/cjn.2015.311
2. Heller, SA, Shih, R, Kalra, R, and Kang, PB. Emery-Dreifuss muscular dystrophy. Muscle Nerve. (2020) 61:436–48. doi: 10.1002/mus.26782
3. Madej-Pilarczyk, A . Clinical aspects of Emery-Dreifuss muscular dystrophy. Nucleus. (2018) 9:314–20. doi: 10.1080/19491034.2018.1462635.29633897
4. Valenti, AC, Albini, A, Imberti, JF, Vitolo, M, Bonini, N, Lattanzi, G, et al. Clinical profile, arrhythmias, and adverse cardiac outcomes in Emery-Dreifuss muscular dystrophies: a systematic review of the literature. Biology. (2022) 11:530. doi: 10.3390/biology11040530
5. Salera, S, Menni, F, Moggio, M, Guez, S, Sciacco, M, and Esposito, S. Nutritional challenges in Duchenne muscular dystrophy. Nutrients. (2017) 9:594. doi: 10.3390/nu9060594
6. Russell, DM, Leiter, LA, Whitwell, J, Marliss, EB, and Jeejeeboy, K. Skeletal muscle function during hypocaloric diets and fasting: a comparison with standard nutritional assessment parameters. Am J Clin Nutr. (1983) 37:133–8. doi: 10.1093/ajcn/37.1.133
7. McClave, SA, and Snider, HL. Dissecting the energy needs of the body. Curr Opin Clin Nutr Metab Care. (2001) 4:143–7. doi: 10.1097/00075197-200103000-00011
8. Vaisman, N, Katzenellenbogen, S, and Nevo, Y. Increased resting energy expenditure in subjects with Emery-Dreifuss muscular dystrophy. Neuromuscul Disord. (2004) 14:142–6. doi: 10.1016/j.nmd.2003.10.012
9. Hankard, R, Gottrand, F, Truck, D, Carpentier, A, Romon, M, and Farriaux, JP. Resting energy expenditure and energy substrate utilization in children with Duchenne muscular dystrophy. Pediatr Res. (1996) 40:29–33. doi: 10.1203/00006450-199607000-00006
10. Satomura, S, Yokota, I, Tatara, K, Naito, E, Ito, M, and Kuroda, Y. Paradoxical weight loss with extra energy expenditure at brown adipose tissue in adolescent patients with Duchenne muscular dystrophy. Metabolism. (2001) 50:1181–5. doi: 10.1053/meta.2001.26701
11. da Silva, JSV, Seres, DS, Sabino, K, Adams, SC, Berdahl, GJ, Citty, SW, et al. ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract. (2020) 35:178–95. doi: 10.1002/ncp.10474
12. Bischoff, SC, Austin, P, Boeykens, K, Chourdakis, M, Cuerda, C, Jonkers-Schuitema, C, et al. ESPEN practical guideline: Home enteral nutrition. Clin Nutr. (2022) 41:468–88. doi: 10.1016/j.clnu.2021.10.018
Keywords: Emery–Dreifuss muscular dystrophy, EDMD, muscular dystrophies, total parenteral nutrition, TPN, resting energy expenditure, REE
Citation: Valoriani F, Pinelli G, Gabriele S and Menozzi R (2024) Effect of nutritional therapy in Emery–Dreifuss muscular dystrophy: a case report. Front. Nutr. 11:1343548. doi: 10.3389/fnut.2024.1343548
Edited by:
Owen Kelly, Sam Houston State University, United StatesReviewed by:
Vivek Peche, Washington University in St. Louis, United StatesJorge Alonso-Pérez, Fundación Canaria Instituto de Investigación Sanitaria de Canarias (FIISC), Spain
Copyright © 2024 Valoriani, Pinelli, Gabriele and Menozzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filippo Valoriani, dmFsb3JpYW5pLmZpbGlwcG9AcG9saWNsaW5pY28ubW8uaXQ=
 Filippo Valoriani
Filippo Valoriani Giovanni Pinelli
Giovanni Pinelli Silvia Gabriele1
Silvia Gabriele1