- 1Department of Nutrition and Food Health, School of Public Health, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
- 2Department of Child and Adolescent Health, School of Public Health, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
- 3Guangzhou Baiyun District Maternal and Childcare Hospital, Guangzhou, Guangdong Province, China
Background: Depression is associated with greater functional impairment and high societal costs than many other mental disorders. Research on the association between plasma polyunsaturated fatty acids (PUFAs) levels and depression have yielded inconsistent results.
Objective: To evaluate whether plasma n-3 and n-6 PUFAs levels are associated with depression in American adults.
Methods: A cross-sectional study included 2053 adults (aged ≥20 y) in the National Health and Nutrition Examination Survey (NHANES), 2011–2012. The level of plasma n-3 and n-6 PUFAs were obtained for analysis. Self-reported Patient Health Questionnaire-9 (PHQ-9) was used to identify the depression status. Binary logistic regression analysis was performed to evaluate the association between quartiles of plasma n-3 and n-6 PUFAs and depression after adjustments for confounders.
Results: The study of 2053 respondents over 20 years of age with a weighted depression prevalence of 7.29% comprised 1,043 men (weighted proportion, 49.13%) and 1,010 women (weighted, 50.87%), with a weighted mean (SE) age of 47.58 (0.67) years. Significantly increased risks of depression over non-depression were observed in the third quartiles (OR = 1.65, 95% CI = 1.05–2.62) for arachidonic acid (AA; 20:4n-6); the third quartiles (OR = 2.20, 95% CI = 1.20–4.05) for docosatetraenoic acid (DTA; 22:4n-6); the third (OR = 2.33, 95% CI = 1.34–4.07), and highest quartiles (OR = 1.83, 95% CI = 1.03–3.26) for docosapentaenoic acid (DPAn-6; 22:5n-6); and the third (OR = 2.18, 95% CI = 1.18–4.03) and highest quartiles (OR = 2.47, 95% CI = 1.31–4.68) for docosapentaenoic acid (DPAn-3; 22:5n-3); the second (OR = 2.13, 95% CI = 1.24–3.66), third (OR = 2.40, 95% CI = 1.28–4.50), and highest quartiles (OR = 2.24, 95% CI = 1.08–4.69) for AA/docosahexaenoic acid (DHA; 22:6n-3) ratio compared with the lowest quartile after adjusting for confounding factors.
Conclusion: Higher plasma levels of AA, DTA, DPAn-6, DPAn-3 PUFAs, and AA/DHA ratio may be potential risk factors for depression in US adults.
1 Introduction
Depression is a common and serious mental disorder and affects more than 280 million people globally (1). In fact, the World Health Organization ranked severe depression as the third cause of burden of disease worldwide as early as 2008 and projected that the disease will be the leading cause of disease burden worldwide by the year 2030 (2). In recent years, many studies have reported understanding the role of different influence factors, such as neurotransmitter, inflammatory markers and nutritional factors, to elucidate the underlying pathophysiology of depression in adults (3–5). Polyunsaturated fatty acids (PUFAs), as important nutrients, exhibit significant effects on the composition of the intestinal microflora as well as the function of the brain (6), and participate in numerous biological processes such as oxidation, neurotransmission, and inflammation (7, 8). Notably, PUFAs may play an important role in depression and its symptoms. Increasing evidence suggests that PUFAs could be associated with the pathophysiology of depression, as well as with the mechanisms underlying the therapeutic actions of antidepressants (9–11).
PUFAs are a class of fatty acids with two or more carbon–carbon double bonds (10). In human health, some PUFAs are considered essential nutrients, mainly including n-3 (primarily from fish, walnuts, wheat germ, and flaxseed) and n-6 PUFAs (primarily from refined vegetable oils such as corn, sunflower, and soybean), which cannot be synthesized in the body and must be obtained from dietary sources (8, 9). Various mental disorders such as Alzheimer disease (AD), dementia, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), schizophrenia, Bipolar disorders (BD) and depression have been suggested to be associated with altered levels and functions of PUFAs (6, 12–14). However, inconsistent conclusions remain, especially in the studies on depression.
The association between n-3 PUFAs and depression has been extensively investigated. Many studies showed that higher levels of n-3 PUFAs, mainly eicosapentaenoic acid (EPA; 20:5n-3) and DHA, were associated with a lower risk of depression (15–22). However, a few studies observed no apparent association with n-3 PUFAs (23–27), and, in particular, a recent longitudinal study did not support a protective effect of n-3 PUFAs on depression risk (28). In contrast to the cumulative evidence indicating the association of n-3 PUFAs with depression, the relationship between n-6 PUFAs and depression have received much less attention. Some studies found higher levels of n-6 PUFAs related to higher severity of depression, although the results of the relevant studies have been inconsistent (27, 29–32). Okubo’s study performed among Japanese breast cancer survivors indicated that a higher blood levels of n-6 PUFAs may increase the risk of depression, while the Avon Longitudinal Study (31) and Thesing’s study (33) reported no association in British and Dutch populations. Moreover, studies on the association of n-6/n-3 ratio and depression are also controversial. Some cross-sectional or longitudinal studies suggested a positive association (18, 34–36), whereas several other studies reported a negative association (37) or no association (25, 38).
Therefore, we comprehensively estimated the association of plasma n-3 and n-6 PUFAs and depression in a nationally representative sample of US adults aged 20 years and older. In order to provide a reference for elucidating the role of PUFAs on depression and a safer and more effective strategy to prevent or mitigate depressive symptoms in US adults.
2 Methods
2.1 Study design and sample
This is a cross-sectional study, done using the 2011–2012 cycle of The National Health and Nutrition Examination Survey (NHANES) data (39). NHANES is a stratified multistage probability sampling design to represent the noninstitutionalized civilian US population. Participants completed the survey through a computer-assisted personal interview and a medical examination at a mobile examination center (MEC). More detailed information regarding the survey design and data collection procedure are available elsewhere (40). The study protocol was approved by the National Center for Health Statistics research ethics review board. Written informed consent was obtained for all participants. The 2011–2012 cycle was utilized since all the main independent and dependent variables of interest were available only in this dataset (especially plasma PUFA). Since depression is more common in adult group (≥ 20 years), participants who were < 20 years were excluded for this study. Participants who did not fully respond to the depression screener questionnaire (PHQ-9) were excluded from the study (41). A total of 2053 adults from the United States were included in this study.
2.2 Determination and classification of depression status
Depression status were determined based on participant’s responses to the PHQ-9 questionnaire in the mental health-depression screener of questionnaire data of NHANES 2011–2012 cycle. PHQ-9 is the 9-item self-report depression scale that asks questions about the frequency of symptoms of depression over the past 2 weeks. Each item can be scored from 0 (not at all) to 3 (nearly every day) (42). The PHQ-9 score ranges from 0 to 27 and thus, classified in two categories. The individuals with PHQ-score < 9 were classified as “no or mild depression” and those with PHQ-score of 10 or more, were classified as “moderate to severe depression” (43, 44).
2.3 Assessment of plasma n-3 and n-6 PUFAs
Thirty fatty acids analyzed by means of gas chromatography–mass spectrometry and expressed in μmol/L were measured in serum with the goal of obtaining US reference ranges for most circulating fatty acids in a fasting subsample of participants. Briefly, total fatty acids were hexane-extracted from the matrix (100uL serum or plasma) along with an internal standard solution for fatty acid recovery. The extract was derivatized with pentafluorobenzyl bromide to form pentafluorobenzyl esters. The reaction mixture is injected onto a capillary gas chromatograph column (45). More details about plasma fatty acids profile analysis are available in the NHANES manual (40).
We chose all types of plasma n-3 and n-6 PUFAs tested in NHANES for our analysis. Moreover, we selected the most representative AA in n-6 PUFAs and the most representative DHA and EPA in n-3 PUFAs, and analyzed their ratios to reflect the different roles of n-3 and n-6 PUFAs in depression. Seventeen variables, total n-3 PUFAs, ALA, stearidonic acid (SDA; 18:4n-3), EPA, DHA, DPAn-3, total n-6 PUFAs, linoleic acid (LA; 18:2n-6), gamma-Linolenic acid (GLA; 18:3n-6), homo-gamma-Linolenic acid (DGLA; 20:3n-6), eicosadienoic acid (EDA; 20:2n-6), AA, DTA, DPAn-6, total n-6/n-3 ratio, AA/EPA ratio, and AA/DHA ratio, were evaluated in the present study.
2.4 Assessment of life’s essential 8
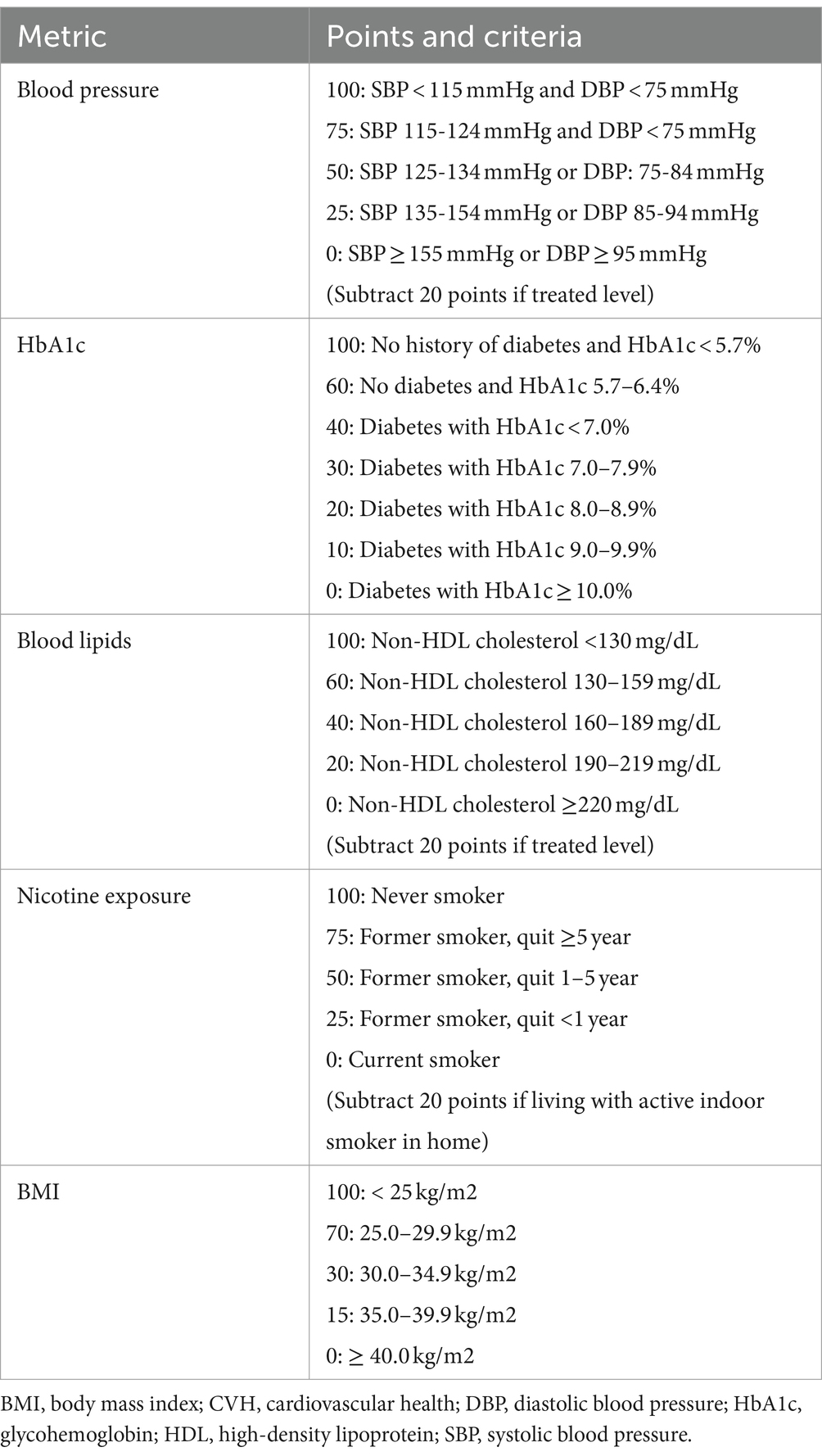
LE8 scoring algorithm consists of 4 health behaviors (diet, physical activity, nicotine exposure, and sleep health) and 4 health factors (body mass index [BMI], blood pressure, glucose, lipids) (46). This study selected 5 individual LE8 metrics (i.e., blood pressure, lipids, glucose, BMI and nicotine exposure) that were more relevant to the study variables (Table 1). LE8 has great potential to assess and promote cardiovascular health (CVH) across life course. CVH is associated with cardiovascular disease (CVD), as well as non-CVD outcomes such as cognitive impairment and depression (47). CVH-related factors routinely collected (i.e., BMI, smoking, hypertension, hypercholesterolemia, and diabetes) were more relevant to our study variables and could be used to accurately estimate individuals’ overall CVH across time even when LE8 metrics are incomplete (48).
2.5 Other covariates
Based on existing literatures on PUFAs and depression, age, sex, race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, Other Hispanic and Other race), educational level and poverty income ratio (PIR) were included in this study as other potential confounders (28, 31, 49). Highest educational level was categorized into 3 levels: (1) Less than high school/general education development (GED), (2) High school graduate/GED or equivalent and (3) higher than high school graduate/GED. PIR represent the ratio of family or unrelated individual income to their appropriate poverty threshold (50). It was categorized into three groups: (1) low: ≤ 1.3, (2) medium: 1.3–3.5, (3) high: > 3.5 (51).
2.6 Statistical analysis
Following the NHANES analytic guidelines, all analyses in this study accounted for sample weights, clustering, and stratification to generate nationally representative estimates (39).
Means and percentages of baseline characteristics were compared using t-tests for continuous variables and χ2 test for categorical variables. PUFAs were grouped into quartiles and analyzed for the inter-quartile group trend. The lowest quartile (first quartile) was defined as the reference group in each model. Binary logistic regression analysis was used to assess the relation between quartiles of plasma PUFAs level and depression. These regression analyses employed 4 sets of covariates: model 1 was crude model; model 2 adjusted for age, sex and race/ethnicity; Model 3 adjusted the ratio of family income to poverty and personal highest education level on the basis of Model 2; and Model 4 additionally adjusted cardiovascular health on the basis of Model 3.
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, United States). Statistical significance was set at 2-sided p < 0.05.
This analysis was conducted using publicly available, deidentified data and was not subject to review by the Guangdong Pharmaceutical University’s institutional review board.
3 Results
3.1 Demographic characteristics of study population
A total of 2053 US adults aged 20–80 who participated in NHANES 2011–2012 enrolled into the study (Table 2). 7.29% (weighted) subjects had a PHQ-9 score ≥ 10 and were categorized as depressed (moderate to severe depression), and 92.71% (weighted) subjects with PHQ-9 score < 10 were categorized as not depressed. The weighted mean (standard error, SE) age of the study participants was 47.58 (0.67) years, and male and female participants were almost equally distributed gender wise. Among the participants, 821 (68.10%) were non-Hispanic white, 477 (11.21%) were non-Hispanic black, 213 (7.42%) were Mexican-American, 209 (5.89%) were Other Hispanic, and 333 (7.37%) were other race/ethnicity. About 83.59% of respondent had completed at least high school/GED. Among adults, 22.40% had low income (PIR ≤ 1.3), 33.66% had medium income (1.3 < PIR ≤ 3.5) and 37.89% high income (3.5 < PIR). Average CVH score was 70.54 (0.82) and the percentages of low, moderate, and high CVH were17.62, 52.20, and 30.18%, respectively. Significantly participants with lower PIR and CVH level were more likely to have depression.
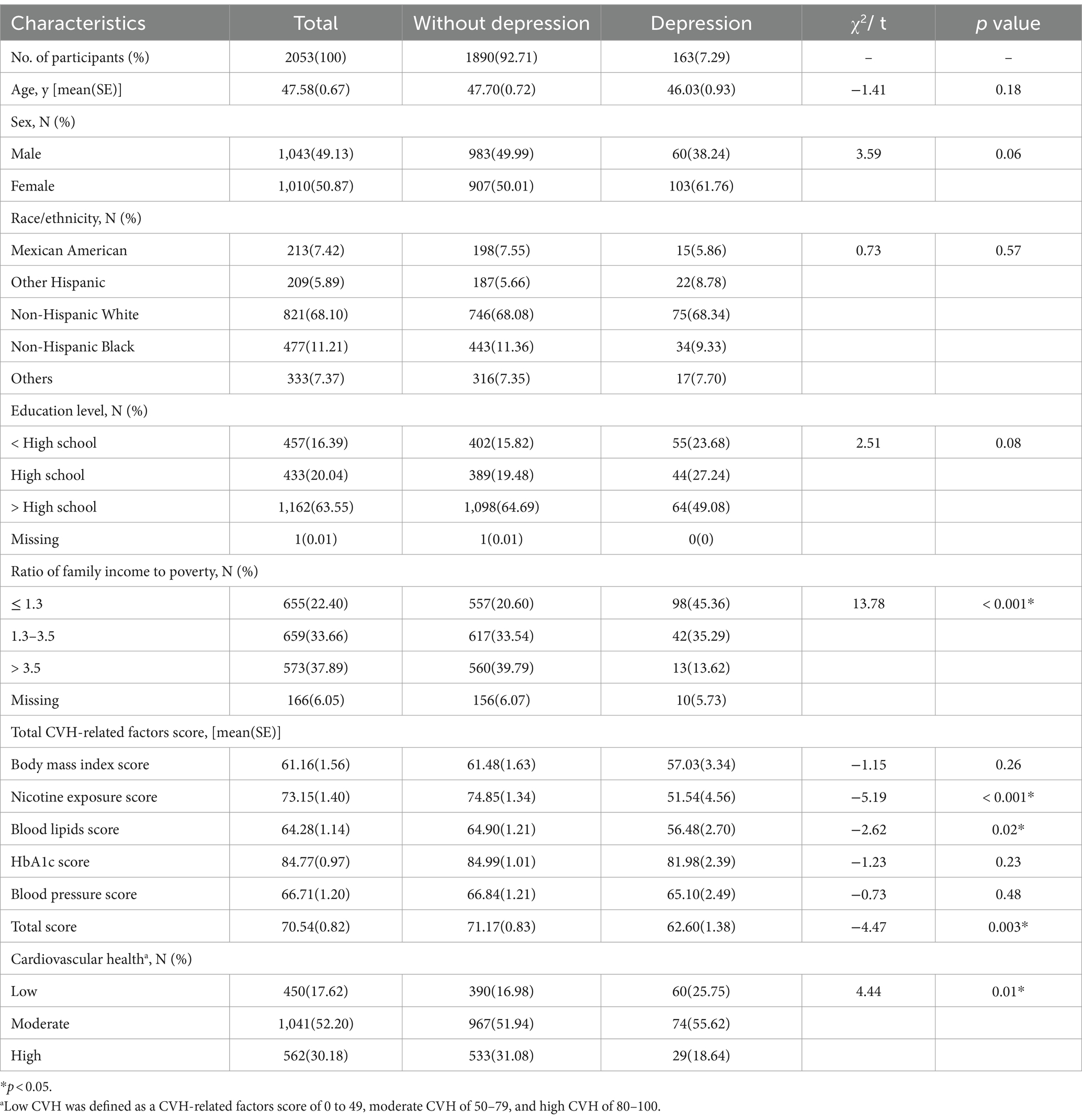
Table 2. Descriptive characteristics of all Participants for total sample and by depression level groups among US adults in NANES, 2011–2012.
3.2 The association between PUFAs and depression: univariate analyses
The univariate analyses of PUFAs as continuous variables indicated that there was an association between part of plasma PUFAs levels and depression among US adults (p < 0.05). The weighted mean (SE) of those plasma PUFAs for GLA, DGLA, EDA, EPA, DTA, DHA, DPAn-6, DPAn-3 and AA/DHA ratio were 64.67 (1.46), 167.35 (2.85), 23.92 (0.47), 70.96 (1.99), 27.36 (0.58), 162.61 (4.74), 20.78 (0.43), 53.69 (0.78), and 6.33 (0.16) respectively (Table 3); PUFAs as categorical variables indicated that there were a difference in quartiles of plasma PUFAs levels of DTA, DHA, DPAn-6, total n-6/n-3 ratio and AA/DHA ratio among US adults with depression and non-depression (p < 0.05; Table 4).
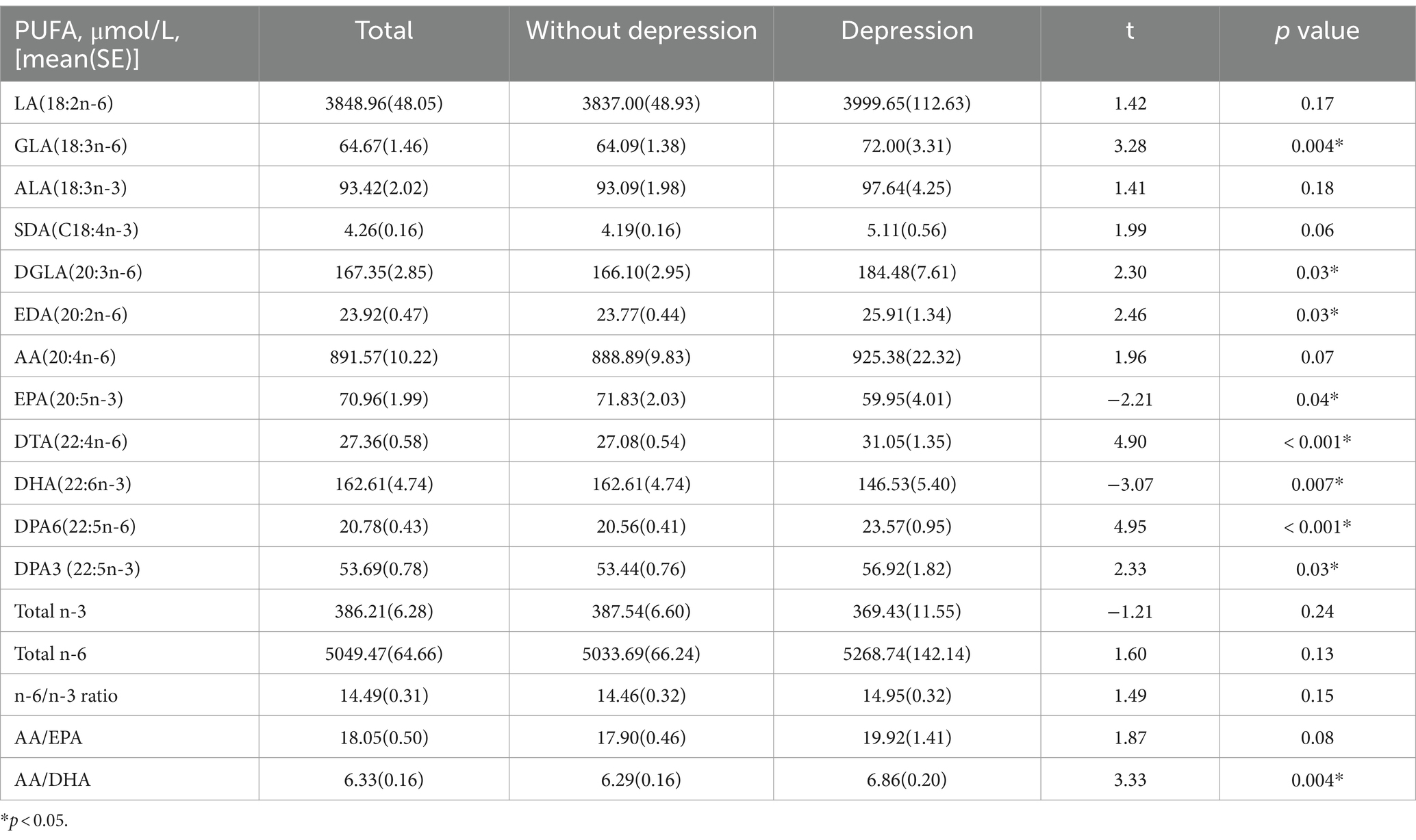
Table 3. Univariate analyses results of the relationship between continuous plasma n-3 and n-6 polyunsaturated fatty acids and depression in American adults: NHANES, 2011–2012.
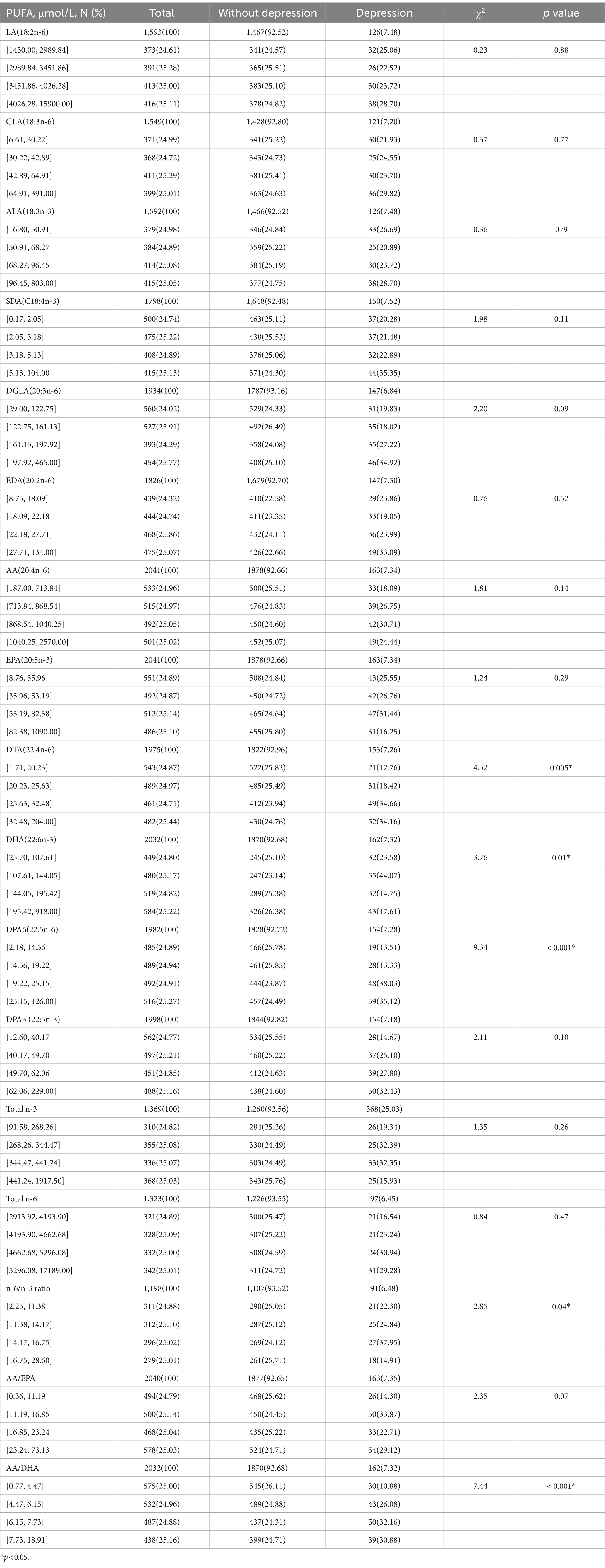
Table 4. Univariate analyses results of the relationship between quartiles of plasma n-3 and n-6 polyunsaturated fatty acids and depression in American adults: NHANES, 2011–2012.
3.3 The association between PUFAs and depression: multiple regression analysis
Table 5 present the OR and 95% CI of depression for quartiles of PUFAs concentrations, using the lowest quartile category as the reference. After controlling for age, sex, race/ethnicity, ratio of family income to poverty, personal highest education level and CVH in the fully adjusted model (model 4), there was a positive relationship between depression and PUFAs in the third quartiles (OR = 1.65, 95% CI = 1.05–2.62) for AA; the third quartiles (OR = 2.20, 95% CI = 1.20–4.05) for DTA; the third (OR = 2.33, 95% CI = 1.34–4.07), and highest quartiles (OR = 1.83, 95% CI = 1.03–3.26) for DPAn-6; and the third (OR = 2.18, 95% CI = 1.18–4.03) and highest quartiles (OR = 2.47, 95% CI = 1.31–4.68) for DPAn-3; 22:5n-3; the second (OR = 2.13, 95% CI = 1.24–3.66), third (OR = 2.40, 95% CI = 1.28–4.50), and highest quartiles (OR = 2.24, 95% CI = 1.08–4.69) for AA/DHA ratio (Table 5).
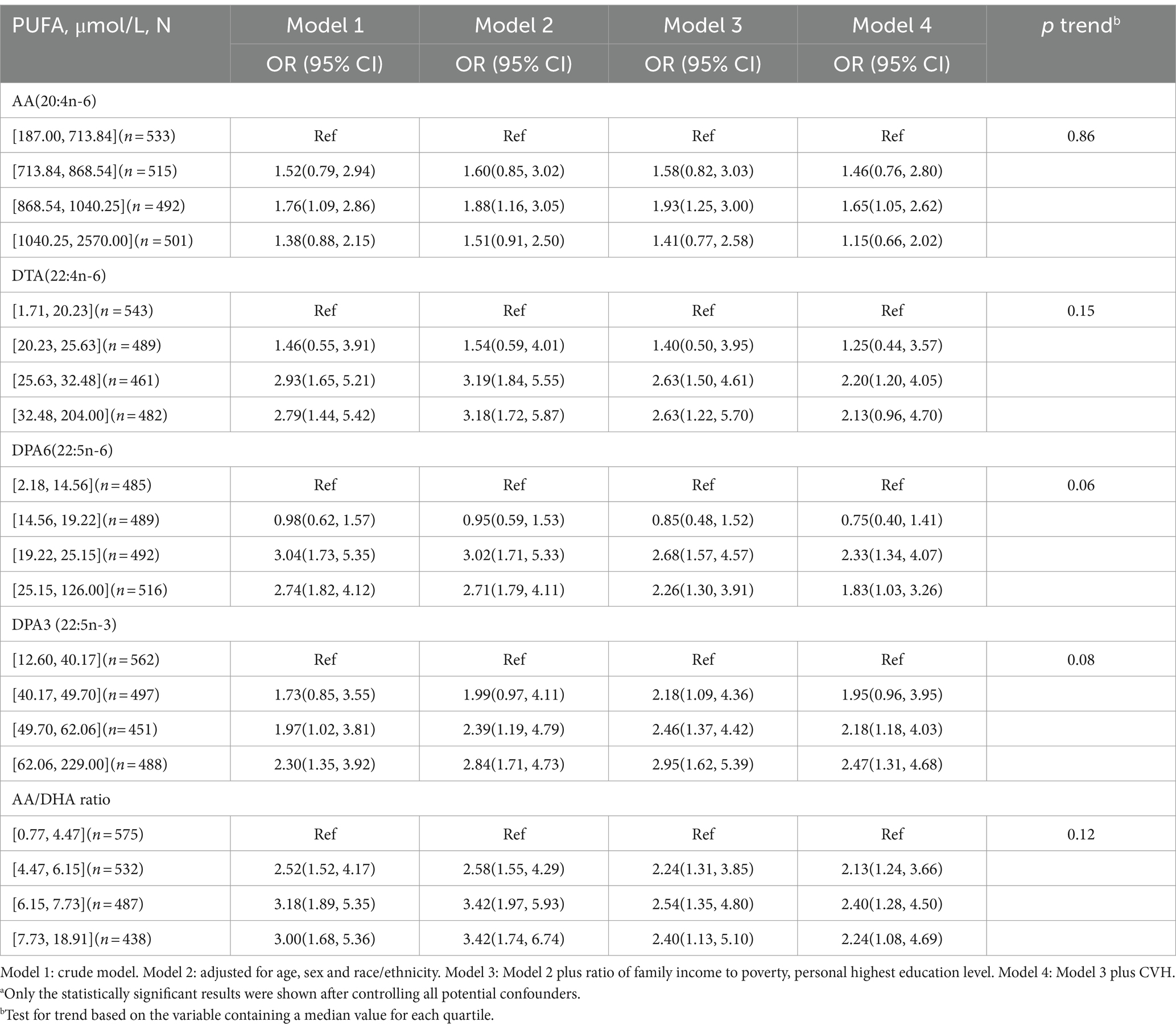
Table 5. Association of plasma n-3 and n-6 polyunsaturated fatty acids levels with depression in American adults: NHANES, 2011–2012a.
3.4 The association between PUFAs and depression: stratified analyses
Adjusted model was performed to observe the association between the PUFAs and depression by keeping all other study variables constant (Table 6). With increased plasma levels of AA, DPAn-3 and AA/DHA ratio as well as decreased plasma levels of DHA, women have an increased risk of depression. The risk of depression increases with increased plasma levels of AA and DPAn-6 among ≥60 years age group. Participants aged between 40 and 59 years old were more likely to develop depression due to elevated plasma DPAn-3 levels. Furthermore, elevated plasma AA/DHA levels in the 20–39 years age group may be a risk factor for depression.
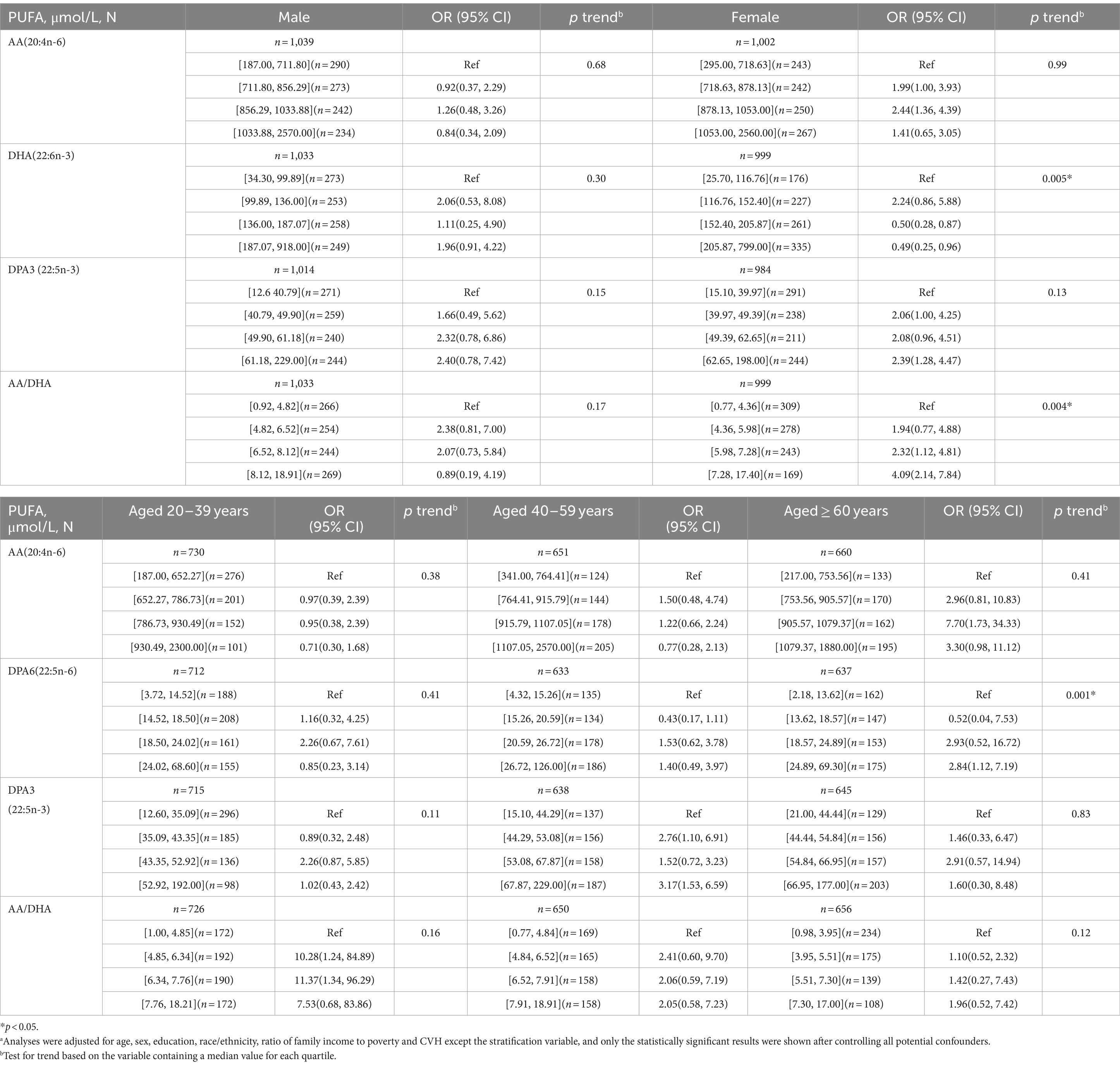
Table 6. Stratified analyses of the association of plasma n-3 and n-6 polyunsaturated fatty acids levels with depression in American adults: NHANES, 2011–2012.a
4 Discussion
Our findings suggest that there was a significant association between plasma PUFAs with moderate or severe level of depression in American adults after controlling all potential confounders. There were higher odds of developing depression among people who have higher plasma levels of n-6 PUFAs (AA, DTA, and DPAn-6), n-3 PUFAs (DPAn-3) and AA/DHA ratio. Our analysis consolidated the risk role of higher plasma levels of AA and AA/DHA ratio in depression, and was the first to report a positive association between higher plasma DTA, DPAn-6, and DPAn-3 levels and depression in America adult.
The weighted prevalence of depression among subjects was 7.29% in our study. A report from NHANES, 2009–2012 data set has indicated that 7.6% of Americans aged 12 and over had depression (52). The incidence rate is approximately the same as our study. We assessed the participants’ blood lipid levels by LE8, and the blood lipids score was 64.28 (1.14). In Lili Wang’s research on the associations between LE8 and non-alcoholic fatty liver disease among US adults, the blood lipids score was 67.0 (2.7) which is also roughly in the same way as in our study (53). Overall, our findings suggest that the prevalence of depression and blood lipid levels in our study are roughly in line with the other relevant studies.
Our data provided evidence for existence of associations of depression with higher plasma levels of AA and AA/DHA ratio in American adult sample, which is consistent with some earlier research (54–56). This may reflect the opposing effects of AA and DHA on depression, although the association between plasma DHA and depression did not persist after controlling for all the potential confounding factors. AA, is an abundant n-6 PUFA in the membrane phospholipids, where it is stored in the sn-2 position of phospholipids (57). AA-derived eicosanoids from cyclooxygenase (COX) pathways or lipoxygenase (LOX) pathways are important lipid mediators involved in a number of physiological and pathophysiological processes ranging from inflammation, allergic responses, blood lipid regulation, to cell metabolism (58). Cyclooxygenases participate in the production of pro-inflammatory eicosanoids, such as prostaglandins and thromboxanes. On the other hand, lipoxygenases generate both leukotrienes and anti-inflammatory eicosanoids like lipoxins. Consequently, an imbalance between these AA-derived eicosanoids has been suggested as a contributing factor to inflammatory effects. Inflammatory processes within the central nervous system (CNS) are essential for the development of brain pathologies, including depression (59). Increased levels of inflammatory cytokines have been reported in depressed patients (60), and found to promote abnormalities in neurotransmitter metabolism and neuroendocrine function, which are related to the pathophysiology of depression (61). Whereas DHA, an n-3 PUFA, has a certain anti-inflammatory effect through competition with AA. Furthermore, DHA also has a neuroprotective effect against decreased neurogenesis and increased neuronal apoptosis (22). Increased plasma levels of AA and/or decreased DHA may cause a disproportionate ratio of n-6 to n-3 and lead to depression through increased inflammatory processes.
This study also found elevated plasma level of other n-6 PUFAs such as DTA and DPAn-6 in patients with moderate to severe depression. DTA, a 2-carbon elongation product of AA, can be desaturated to generate DPAn-6 (62). Previous studies have shown that DTA can impair neurobehavioral development by increasing reactive oxidative species production in Caenorhabditis elegans (63), and higher DPAn-6 status also showed an association with worse mental health in older adults with mild cognitive impairment (64) and children and adolescents with bipolar disorder (65). The Western diet, which is high in processed foods with fat and sugar, is increasing the risk of depression (66). In addition, reactive oxygen species and inflammatory cytokines that could be induced by unreasonable intake of PUFAs have the importance in the progression of depression (67). Previous studies have shown that some n-6 PUFAs are able to activate inflammatory responses and lead to the accumulation of reactive oxygen species and pro-inflammatory factors (68, 69). These alterations might affect the integrity of the cell membrane, leading to alteration of intestinal flora, systemic inflammation and abnormal neurotransmitter transport in the brain (6, 70). In summary, abnormalities in plasma n-6 PUFAs metabolism may be involved in the progress of depression.
The DPAn-3 is less studied as a new player in the n-3 PUFAs family, compared to its counterparts EPA and DHA. The literature on DPAn-3 is limited, however most of the available data suggests it has beneficial health effects which is contrary to our found that higher DPAn-3 was associated with depression. Our result agrees with a minority of reports concerning DPAn-3 (71). More research remains to be done to further investigate the biological effects of this DPAn-3.
There were also some studies that did not observe an association between plasma PUFAs levels and depression (31, 33, 49, 72, 73). But most of these studies have focused only on the total n-3 PUFAs and/or the total n-6 PUFAs, or the canonical fatty acids among them. A longitudinal study in middle-aged Finnish men did not find evidence that serum polyunsaturated fatty acids would be associated with risk of depression (38). The composition ratio of sex may partially influence results. According to previous studies of dietary intervention in Western countries, the association appears to be observed more in women than in men (21, 74). We also observed a significant association for AA, DHA, DPAn-3, and AA/DHA ratio in women through a sub-analysis stratified by sex. However, this finding should be interpreted with caution in light of the source of PUFAs.
One of the strengths of this study is the use of data from a nationally representative survey with a large sample size from a multiracial/multiethnic population. Even a large number of analyses have been conducted to assess the role of PUFAs in depression from multiple perspectives, especially at the supplement and diet levels, but only a few studies with small sample sizes have examined the association between depression and plasma PUFAs. To our knowledge, our study is the first observational study to examine the association between plasma PUFAs and depression among American adult using part of the LE8 indicators as the primary covariate.
Another strength of this study is that we used plasma PUFAs, which better reflects tissue levels of PUFAs than the more subjective measures of the Food Frequency Questionnaires (FFQ) and food records. Several limitations also need to be acknowledged. A major limitation is that this study is only a cross-sectional exploratory investigation, so a causal relationship cannot be concluded. Additionally, the PHQ-9 items measure only a subset of the symptoms of depression. Apart from the nine symptoms of the PHQ, other depressive symptoms were not included in this study which may be counted as a limitation.
5 Conclusion
In conclusion, this population-based cross-sectional study examining the association between plasma PUFAs and depression risk in American adults revealed an association between higher plasma levels of AA, DTA, DPAn-6, DPAn-3 and AA/DHA ratio and increased risk of depression. The complexity of PUFAs metabolism, interactions and competition between n-3 PUFAs and n-6 PUFAs and the mechanism by which these may influence depression requires continued study.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics research ethics review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. XY: Data curation, Formal analysis, Software, Writing – review & editing. YL: Data curation, Methodology, Writing – review & editing. QL: Software, Supervision, Writing – review & editing. YX: Data curation, Project administration, Writing – review & editing. JH: Data curation, Methodology, Writing – review & editing. JG: Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – review & editing. WY: Conceptualization, Formal analysis, Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. WY, male, Doctor of Medicine, member of the Health Professional Committee of Children and Adolescent of Guangdong Provincial, member of the Children ‘s Mental Health Promotion Professional Committee of Guangdong Provincial Clinical Medical Association, has been committed to the study of children’s health. He has presided over 13 scientific research projects at all levels such as the general project of the National Natural Science Foundation of China, and participated in the compilation of 2 college planning textbooks. Published more than 30 SCI papers, including 4 Top journal articles by MW and WY, and participated in the review of Nature Communications, Epidemiology and Psychiatric Sciences, and Journal of Pediatrics, etc. National Natural Science Foundation of China (Gant no. 81973063) and Natural Science Foundation of Guangdong, China (Grant no. 2018A030313723).
Acknowledgments
The authors would like to acknowledge the support from all the team members and all staff of the National Center for Health Statistics of the US Centers for Disease Control and Prevention.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Depressive disorder (depression). Available online at: https://www.who.int/zh/news-room/fact-sheets/detail/depression (Accessed May 5, 2023).
2. World Health Organization. Global burden of mental disorders and the need for a comprehensive, coordinated response from health and social sectors at the country level: report by the secretariat World Health Organization (2012).
3. Malhi, GS, and Mann, JJ. Depression. Lancet (2018) 392:2299–312. doi: 10.1016/s0140-6736(18)31948-2
4. Beurel, E, Toups, M, and Nemeroff, CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
5. Kunugi, H. Depression and lifestyle: focusing on nutrition, exercise, and their possible relevance to molecular mechanisms. Psychiatry Clin Neurosci (2023) 77:420–33. doi: 10.1111/pcn.13551
6. Ortega, MA, Álvarez-Mon, MA, and García-Montero, C. Microbiota-gut-brain axis mechanisms in the complex network of bipolar disorders: potential clinical implications and translational opportunities. Mol Psychiatry (2023) 28:2645–73. doi: 10.1038/s41380-023-01964-w
7. Melo, HM, Santos, LE, and Ferreira, ST. Diet-derived fatty acids, brain inflammation, and mental health. Front Neurosci (2019) 13:265. doi: 10.3389/fnins.2019.00265
8. Custers, EEM, and Kiliaan, AJ. Dietary lipids from body to brain. Prog Lipid Res (2022) 85:101144. doi: 10.1016/j.plipres.2021.101144
9. Decandia, D, Landolfo, E, Sacchetti, S, Gelfo, F, Petrosini, L, and Cutuli, D. N-3 PUFA improve emotion and cognition during menopause: a systematic review. Nutrients (2022) 14:1982. doi: 10.3390/nu14091982
10. Liu, X, Hao, J, Yao, E, Cao, J, Zheng, X, Yao, D, et al. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain Behav Immun (2020) 89:357–70. doi: 10.1016/j.bbi.2020.07.022
11. Zeng, L, Lv, H, Wang, X, Xue, R, Zhou, C, Liu, X, et al. Causal effects of fatty acids on depression: Mendelian randomization study. Front Nutr (2022) 9:1010476. doi: 10.3389/fnut.2022.1010476
12. Mazahery, H, Stonehouse, W, Delshad, M, Kruger, M, Conlon, C, Beck, K, et al. Relationship between long chain n-3 polyunsaturated fatty acids and autism Spectrum disorder: systematic review and Meta-analysis of case-control and randomised controlled trials. Nutrients (2017) 9:155. doi: 10.3390/nu9020155
13. McNamara, RK, and Welge, JA. Meta-analysis of erythrocyte polyunsaturated fatty acid biostatus in bipolar disorder. Bipolar Disord (2016) 18:300–6. doi: 10.1111/bdi.12386
14. Zhang, Y, Chen, J, Qiu, J, Li, Y, Wang, J, and Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr (2016) 103:330–40. doi: 10.3945/ajcn.115.124081
15. Lin, PY, Huang, SY, and Su, KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry (2010) 68:140–7. doi: 10.1016/j.biopsych.2010.03.018
16. Ganança, L, Galfalvy, HC, Cisneros-Trujillo, S, Basseda, Z, Cooper, TB, Ren, X, et al. Relationships between inflammatory markers and suicide risk status in major depression. J Psychiatr Res (2021) 134:192–9. doi: 10.1016/j.jpsychires.2020.12.029
17. Horikawa, C, Otsuka, R, Kato, Y, Nishita, Y, Tange, C, Kakutani, S, et al. Cross-sectional association between serum concentrations of n-3 long-chain PUFA and depressive symptoms: results in Japanese community dwellers. Br J Nutr (2016) 115:672–80. doi: 10.1017/s0007114515004754
18. Davyson, E, Shen, X, Gadd, DA, Bernabeu, E, Hillary, RF, McCartney, DL, et al. Metabolomic investigation of major depressive disorder identifies a potentially causal association with polyunsaturated fatty acids. Biol Psychiatry (2023) 94:630–9. doi: 10.1016/j.biopsych.2023.01.027
19. Féart, C, Peuchant, E, Letenneur, L, Samieri, C, Montagnier, D, Fourrier-Reglat, A, et al. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City study. Am J Clin Nutr (2008) 87:1156–62. doi: 10.1093/ajcn/87.5.1156
20. Matsuoka, YJ, Sawada, N, Mimura, M, Shikimoto, R, Nozaki, S, Hamazaki, K, et al. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: a population-based prospective cohort study. Transl. Psychiatry (2017) 7:e 1242. doi: 10.1038/tp.2017.206
21. Beydoun, MA, Fanelli Kuczmarski, MT, Beydoun, HA, Hibbeln, JR, Evans, MK, and Zonderman, AB. ω-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. J Nutr (2013) 143:1743–52. doi: 10.3945/jn.113.179119
22. Borsini, A, Nicolaou, A, Camacho-Muñoz, D, Kendall, AC, di Benedetto, MG, Giacobbe, J, et al. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: relevance for major depression and for human hippocampal neurogenesis. Mol Psychiatry (2021) 26:6773–88. doi: 10.1038/s41380-021-01160-8
23. Hamazaki, K, Matsuoka, YJ, Yamaji, T, Sawada, N, Mimura, M, Nozaki, S, et al. Plasma phospholipid n-3 polyunsaturated fatty acids and major depressive disorder in Japanese elderly: the Japan public health center-based prospective study. Sci Rep (2021) 11:4003. doi: 10.1038/s41598-021-83478-5
24. Patra, BN, Khandelwal, SK, Chadda, RK, Lakshmy, R, and Abraham, RA. A controlled study of plasma fatty acids in Indian patients with depressive episode. Asian J Psychiatr (2018) 31:152–6. doi: 10.1016/j.ajp.2017.12.006
25. Rubí Vargas, M, Terrazas-Medina, EA, Leyva-López, AG, Peralta-Peña, SL, and Cupul-Uicab, LA. Depressive symptoms and serum levels of polyunsaturated fatty acids omega-3 and omega-6 among college students from northern Mexico. Nutr Hosp (2017) 35:148–52. doi: 10.20960/nh.1311
26. Tsai, AC, Lucas, M, Okereke, OI, O'Reilly, EJ, Mirzaei, F, Kawachi, I, et al. Suicide mortality in relation to dietary intake of n-3 and n-6 polyunsaturated fatty acids and fish: equivocal findings from 3 large US cohort studies. Am J Epidemiol (2014) 179:1458–66. doi: 10.1093/aje/kwu086
27. Lucas, M, Mirzaei, F, and O'Reilly, EJ. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr (2011) 93:1337–43. doi: 10.3945/ajcn.111.011817
28. Thesing, CS, Bot, M, Milaneschi, Y, Giltay, EJ, and Penninx, BWJH. Bidirectional longitudinal associations of omega-3 polyunsaturated fatty acid plasma levels with depressive disorders. J Psychiatr Res (2020) 124:1–8. doi: 10.1016/j.jpsychires.2020.02.011
29. Zhang, R, Sun, J, Li, Y, and Zhang, D. Associations of n-3, n-6 fatty acids intakes and n-6: n-3 ratio with the risk of depressive symptoms: NHANES 2009-2016. Nutrients (2020) 12:240. doi: 10.3390/nu12010240
30. Currenti, W, Godos, J, Alanazi, AM, Lanza, G, Ferri, R, Caraci, F, et al. Dietary fats and depressive symptoms in Italian adults. Nutrients (2023) 15:675. doi: 10.3390/nu15030675
31. Mongan, D, Healy, C, Jones, HJ, Zammit, S, Cannon, M, and Cotter, DR. Plasma polyunsaturated fatty acids and mental disorders in adolescence and early adulthood: cross-sectional and longitudinal associations in a general population cohort. Transl Psychiatry (2021) 11:321. doi: 10.1038/s41398-021-01425-4
32. Okubo, R, Noguchi, H, Hamazaki, K, Sekiguchi, M, Kinoshita, T, Katsumata, N, et al. Association between blood polyunsaturated fatty acid levels and depressive symptoms in breast cancer survivors. Prostaglandins Leukot Essent Fatty Acids (2018) 139:9–13. doi: 10.1016/j.plefa.2018.11.002
33. Thesing, CS, Lok, A, Milaneschi, Y, Assies, J, Bockting, CLH, Figueroa, CA, et al. Fatty acids and recurrence of major depressive disorder: combined analysis of two Dutch clinical cohorts. Acta Psychiatr Scand (2020) 141:362–73. doi: 10.1111/acps.13136
34. Shibata, M, Ohara, T, Yoshida, D, Hata, J, Mukai, N, Kawano, H, et al. Association between the ratio of serum arachidonic acid to eicosapentaenoic acid and the presence of depressive symptoms in a general Japanese population: the Hisayama study. J Affect Disord (2018) 237:73–9. doi: 10.1016/j.jad.2018.05.004
35. Berger, ME, Smesny, S, Kim, SW, Davey, CG, Rice, S, Sarnyai, Z, et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: a 7-year longitudinal study. Transl. Psychiatry (2017) 7:e 1220. doi: 10.1038/tp.2017.190
36. Hoge, A, Tabar, V, Donneau, AF, Dardenne, N, Degée, S, Timmermans, M, et al. Imbalance between Omega-6 and Omega-3 polyunsaturated fatty acids in early pregnancy is predictive of postpartum depression in a Belgian cohort. Nutrients (2019) 11:876. doi: 10.3390/nu11040876
37. Beydoun, MA, Fanelli Kuczmarski, MT, Beydoun, HA, Rostant, OS, Evans, MK, and Zonderman, AB. Associations of the ratios of n-3 to n-6 dietary fatty acids with longitudinal changes in depressive symptoms among US women. Am J Epidemiol (2015) 181:691–705. doi: 10.1093/aje/kwu334
38. Ruusunen, A, Virtanen, JK, Lehto, SM, Tolmunen, T, Kauhanen, J, and Voutilainen, S. Serum polyunsaturated fatty acids are not associated with the risk of severe depression in middle-aged Finnish men: Kuopio Ischaemic heart disease risk factor (KIHD) study. Eur J Nutr (2011) 50:89–96. doi: 10.1007/s00394-010-0118-7
39. US Centers for Disease Control and Prevention. National Health and nutrition examination survey, (2011-2012). Available at: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2011
40. US Centers for Disease Control and Prevention. National Health and nutrition examination survey. Available at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx (Accessed January 2, 2023)
41. Scinicariello, F, Przybyla, J, Carroll, Y, Eichwald, J, Decker, J, and Breysse, PN. Age and sex differences in hearing loss association with depressive symptoms: analyses of NHANES 2011-2012. Psychol Med (2019) 49:962–8. doi: 10.1017/s0033291718001617
42. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
43. Wittayanukorn, S, Qian, J, and Hansen, RA. Prevalence of depressive symptoms and predictors of treatment among U.S. adults from 2005 to 2010. Gen Hosp Psychiatry (2014) 36:330–6. doi: 10.1016/j.genhosppsych.2013.12.009
44. Rethorst, CD, Bernstein, I, and Trivedi, MH. Inflammation, obesity, and metabolic syndrome in depression: analysis of the 2009-2010 National Health and nutrition examination survey (NHANES). J Clin Psychiatry (2014) 75:e1428–32. doi: 10.4088/JCP.14m09009
45. US Centers for Disease Control and Prevention. NHANES, (2011-2012). Data documentation, codebook, and frequencies: Fatty acids- serum (FAS_G). Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/FAS_G.htm
46. Lloyd-Jones, DM, Allen, NB, Anderson, CAM, Black, T, Brewer, LC, Foraker, RE, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation (2022) 146:e18–43. doi: 10.1161/cir.0000000000001078
47. Tibuakuu, M, Okunrintemi, V, Savji, N, Stone, NJ, Virani, SS, Blankstein, R, et al. Nondietary cardiovascular health metrics with patient experience and loss of productivity among US adults without cardiovascular disease: the medical expenditure panel survey 2006 to 2015. J Am Heart Assoc (2020) 9:e016744. doi: 10.1161/jaha.120.016744
48. Zheng, Y, Huang, T, Guasch-Ferre, M, Hart, J, Laden, F, Chavarro, J, et al. Estimation of life's essential 8 score with incomplete data of individual metrics. Front Cardiovasc Med (2023) 10:1216693. doi: 10.3389/fcvm.2023.1216693
49. Thesing, CS, Bot, M, Milaneschi, Y, Giltay, EJ, and Penninx, BWJH. Omega-3 and omega-6 fatty acid levels in depressive and anxiety disorders. Psychoneuroendocrinology (2018) 87:53–62. doi: 10.1016/j.psyneuen.2017.10.005
50. Madhav, KC, Sherchand, SP, and Sherchan, S. Association between screen time and depression among US adults. Prev Med Rep (2017) 8:67–71. doi: 10.1016/j.pmedr.2017.08.005
51. Petersen, KS, Sullivan, VK, and Fulgoni, VL. Circulating concentrations of essential fatty acids, linoleic and α-linolenic acid, in US adults in 2003-2004 and 2011-2012 and the relation with risk factors for Cardiometabolic disease: an NHANES analysis. Curr Dev Nutr (2020) 4:nzaa149. doi: 10.1093/cdn/nzaa149
52. Pratt, LA, and Brody, DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief (2014) 172:1–8.
53. Wang, L, Yi, J, Guo, X, and Ren, X. Associations between life's essential 8 and non-alcoholic fatty liver disease among US adults. J Transl Med (2022) 20:616. doi: 10.1186/s12967-022-03839-0
54. Gopaldas, M, Zanderigo, F, Zhan, S, Ogden, RT, Miller, JM, Rubin-Falcone, H, et al. Brain serotonin transporter binding, plasma arachidonic acid and depression severity: a positron emission tomography study of major depression. J Affect Disord (2019) 257:495–503. doi: 10.1016/j.jad.2019.07.035
55. Conklin, SM, Manuck, SB, Yao, JK, Flory, JD, Hibbeln, JR, and Muldoon, MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med (2007) 69:932–4. doi: 10.1097/PSY.0b013e31815aaa42
56. Samieri, C, Féart, C, Letenneur, L, Dartigues, JF, Pérès, K, Auriacombe, S, et al. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr (2008) 88:714–21. doi: 10.1093/ajcn/88.3.714
57. Yui, K, Imataka, G, Nakamura, H, Ohara, N, and Naito, Y. Eicosanoids derived from arachidonic acid and their family prostaglandins and cyclooxygenase in psychiatric disorders. Curr Neuropharmacol (2015) 13:776–85. doi: 10.2174/1570159x13666151102103305
58. Yin, H, Zhou, Y, Zhu, M, Hou, S, Li, Z, Zhong, H, et al. Role of mitochondria in programmed cell death mediated by arachidonic acid-derived eicosanoids. Mitochondrion (2013) 13:209–24. doi: 10.1016/j.mito.2012.10.003
59. Regulska, M, Szuster-Głuszczak, M, Trojan, E, Leśkiewicz, M, and Basta-Kaim, A. The emerging role of the double-edged impact of arachidonic acid-derived eicosanoids in the Neuroinflammatory background of depression. Curr Neuropharmacol (2021) 19:278–93. doi: 10.2174/1570159X18666200807144530
60. Jia, Y, Liu, L, Sheng, C, Cheng, Z, Cui, L, Li, M, et al. Increased serum levels of cortisol and inflammatory cytokines in people with depression. J Nerv Ment Dis (2019) 207:271–6. doi: 10.1097/nmd.0000000000000957
61. Felger, JC, and Lotrich, FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience (2013) 246:199–229. doi: 10.1016/j.neuroscience.2013.04.060
62. Weylandt, KH. Docosapentaenoic acid derived metabolites and mediators - the new world of lipid mediator medicine in a nutshell. Eur J Pharmacol (2016) 785:108–15. doi: 10.1016/j.ejphar.2015.11.002
63. Wu, CH, Hsu, WL, Tsai, CC, Chao, HR, Wu, CY, Chen, YH, et al. 7, 10, 13,16-Docosatetraenoic acid impairs neurobehavioral development by increasing reactive oxidative species production in Caenorhabditis elegans. Life Sci (2023) 319:121500. doi: 10.1016/j.lfs.2023.121500
64. Milte, CM, Sinn, N, Street, SJ, Buckley, JD, Coates, AM, and Howe, PRC. Erythrocyte polyunsaturated fatty acid status, memory, cognition and mood in older adults with mild cognitive impairment and healthy controls. Prostaglandins Leukot Essent Fatty Acids (2011) 84:153–61. doi: 10.1016/j.plefa.2011.02.002
65. Gracious, BL, Chirieac, MC, Costescu, S, Finucane, TL, Youngstrom, EA, and Hibbeln, JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord (2010) 12:142–54. doi: 10.1111/j.1399-5618.2010.00799.x
66. Firth, J, Stubbs, B, Teasdale, SB, Ward, PB, Veronese, N, Shivappa, N, et al. Diet as a hot topic in psychiatry: a population-scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatry (2018) 17:365–7. doi: 10.1002/wps.20571
67. Reemst, K, Broos, JY, Abbink, MR, Cimetti, C, Giera, M, Kooij, G, et al. Early-life stress and dietary fatty acids impact the brain lipid/oxylipin profile into adulthood, basally and in response to LPS. Front Immunol (2022) 13:967437. doi: 10.3389/fimmu.2022.967437
68. Stachowicz, K. Application potential of modulation of cyclooxygenase-2 activity: a cognitive approach. Postępy Higieny i Medycyny Doświadczalnej (2021) 75:837–46. doi: 10.2478/ahem-2021-0022
69. Stachowicz, K. The role of polyunsaturated fatty acids in neuronal signaling in depression and cognitive processes. Arch Biochem Biophys (2023) 737:109555. doi: 10.1016/j.abb.2023.109555
70. Pascoe, MC, Crewther, SG, Carey, LM, et al. What you eat is what you are -- a role for polyunsaturated fatty acids in neuroinflammation induced depression? Clin Nutr (2011) 30:407–15. doi: 10.1016/j.clnu.2011.03.013
71. Liu, T, Wang, L, Guo, J, Zhao, T, Tang, H, Dong, F, et al. Erythrocyte membrane fatty acid composition as a potential biomarker for depression. Int J Neuropsychopharmacol (2023) 26:385–95. doi: 10.1093/ijnp/pyad021
72. Ma, J, Peng, L, and Li, S. Mendelian randomization of the causal relationship between ω-3 polyunsaturated fatty acids and major depression. Wei Sheng Yan Jiu (2023) 52:793–800. doi: 10.19813/j.cnki.weishengyanjiu.2023.05.018
73. Liu, JJ, Galfalvy, HC, Cooper, TB, Oquendo, MA, Grunebaum, MF, Mann, JJ, et al. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. J Clin Psychiatry (2013) 74:732–8. doi: 10.4088/JCP.12m07970
Keywords: n-3, n-6, polyunsaturated fatty acids, depression, NHANES, PHQ-9, American, adult
Citation: Wang M, Yan X, Li Y, Li Q, Xu Y, Huang J, Gan J and Yang W (2024) Association between plasma polyunsaturated fatty acids and depressive among US adults. Front. Nutr. 11:1342304. doi: 10.3389/fnut.2024.1342304
Edited by:
Adriana Ximenes-da-Silva, Federal University of Alagoas, BrazilReviewed by:
Xuping Gao, Peking University Sixth Hospital, ChinaMarija Takic, University of Belgrade, Serbia
Copyright © 2024 Wang, Yan, Li, Li, Xu, Huang, Gan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhan Yang, d2VuaGFuLXlhbmdAZ2RwdS5lZHUuY24=; Juan Gan, MTE5ODUzMTg5NUBxcS5jb20=
 Man Wang
Man Wang Xiaofang Yan
Xiaofang Yan Yanmei Li2
Yanmei Li2 Jitian Huang
Jitian Huang Wenhan Yang
Wenhan Yang