- 1Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi, China
- 2Youjiang Medical University for Nationalities, Baise, China
- 3Department of Tumor Pathology, The Key Laboratory of Molecular Pathology (Hepatobiliary Diseases) of Guangxi, Baise, Guangxi, China
- 4Guilin Medical University, Guilin, Guangxi, China
Background: Previous studies reported that variations in dietary intake patterns substantially impact human health, specifically tumorigenesis. However, confounding factors in previous cohort studies have obscured the relationship between dietary differences and the risk of oral cancer (OC).
Materials and methods: We developed an outcome dataset from genome-wide association studies (GWAS) data on three OCs within the GAME-ON project, using GWAS-META merging. We extracted 21 dietary exposures, including 10 dietary patterns, 6 vitamins, and 5 micronutrients, from the UK Biobank database, using the inverse variance weighting method as the primary statistical method. Sensitivity analysis was conducted to detect heterogeneity and pleiotropy. Serum metabolite concentrations were adjusted using multivariate Mendelian randomization.
Results: Of the 10 analyzed dietary patterns, 8 showed no significant association with the risk of developing OC. Consumption of dark chocolate (inverse variance weighted [IVW]: Odds ratio (OR) = 0.786, 95% confidence interval [CI]: 0.622–0.993, p = 0.044) and sweet pepper exhibited an inverse relationship with OC risk (IVW: OR = 0.757, 95% CI: 0.574–0.997, p = 0.048). Reverse MR analysis revealed no reverse causality. Furthermore, no significant correlation was observed between the intake of 6 vitamins and 5 micronutrients and the risk of developing OC. After using multivariable MR to adjust for serum caffeine, linoleate, theophylline, and theobromine metabolism levels, consuming dark chocolate was unrelated to a decreased risk of OC. After adjusting each serum metabolite individually, the observed p-values deviated from the original values to varying degrees, indicating that the components of dark chocolate could have different effects. Among these components, theophylline demonstrated the most significant inhibitory effect.
Conclusion: This study demonstrated a causal relationship between the intake of dark chocolate and sweet peppers and a lower risk of OC. The components of dark chocolate could have different effects.
1 Introduction
Oral cancer (OC) is a common malignancy of the head and neck region. Globally, it accounts for approximately 405,000 new cases annually (1). Tobacco use and alcohol consumption are well-documented etiological factors of OC (2, 3). In China, betel nut consumption is a common etiological factor for OC (4). An OC usually has no apparent symptoms in the early stages, making early and prompt diagnostic intervention and routine medical evaluations difficult. Patients with advanced stages of OC frequently present with symptoms, including ulcers, lumps, and lymph node metastases in the neck region. Consequently, their chances of survival at the time of diagnosis are significantly reduced (5). Numerous strategies for early OC screening have been developed to facilitate earlier detection and treatment. However, there is a critical need for more precise and effective screening approaches (6). Various dietary intakes are implicated in disease development (7, 8). Therefore, clarifying the relationship between various dietary intakes and OC is crucial to preventing disease and changing the lifestyle habits of high-risk populations.
The degree to which dietary intake affects OC remains unknown (9). Unlike single-nutrient studies that are often influenced by various confounding factors, studies of dietary intake patterns offer a more accurate reflection of the body’s condition in everyday life by summarizing and analyzing individual nutrients (10). This study employed the Mendelian randomization (MR) method to clarify these potential associations and comprehensively investigate the plausible link between various diets and OC.
The MR is a method to investigate possible associations between an exposure factor (dietary intake) and an outcome (OC). The association between exposure and outcome was analyzed at the genetic level using data sourced from public databases. One major advantage of MR is its ability to reduce potential confounders in clinical research and substantially reduce the possibility of reverse causation (11). This improves the precision, reliability, and validity of the findings.
Food often contains multiple components that can have individual or synergistic effects. Investigating the primary components of a food may be more effective in determining its mechanism of action. Although MR analysis has numerous benefits, it cannot identify the roles of individual components because of its inherent limitations. Conversely, multivariable MR (MVMR) is a method for investigating the direct or mediated effects of two or more exposures on outcomes (12). Consequently, by considering each internal component of a nutrient, MVMR enables a direct assessment of its impact on an outcome, making it easier to investigate the role of each component.
1.1 Method design
This study used a two-sample MR design to investigate the relationship between dietary intake and OC. In MR studies, single nucleotide polymorphisms (SNPs) utilized as instrumental variables (IVs) must satisfy three core criteria (11): First, SNPs must be strongly associated with the exposure factors. Second, SNPs should not be associated with confounding variables other than exposure factors. Third, SNPs must not be directly associated with the outcome, in this case, OC. Moreover, reverse MR analysis was employed to investigate the potential reverse causality effects of OC on positive outcomes. Additionally, MVMR was used to determine the direct impact of positive exposures on OC.
1.2 Data source
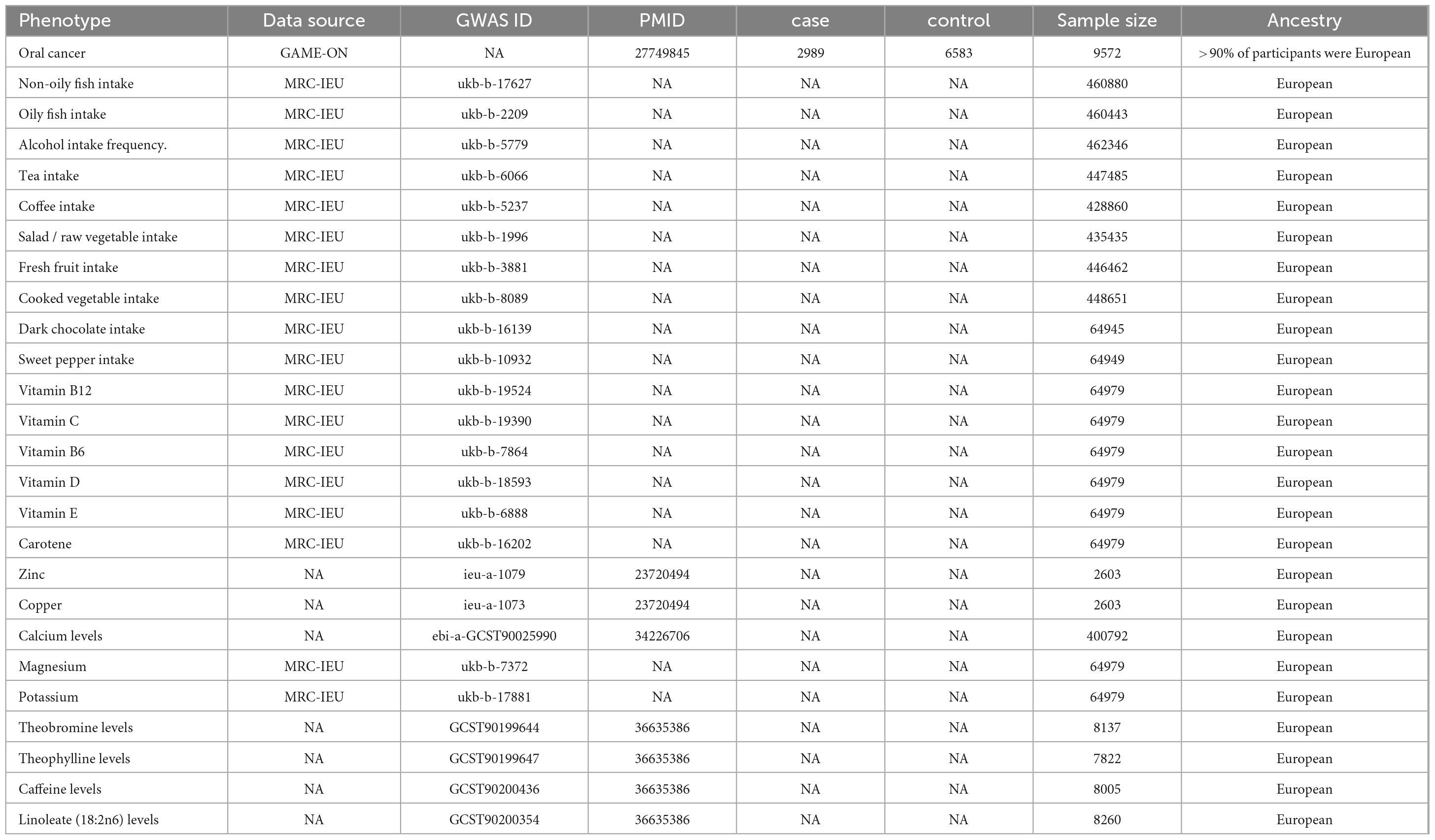
This study included 21 exposure factors, including 10 dietary intakes (oily fish, non-oily fish, alcohol, tea, coffee, cooked vegetables, salad/raw vegetables, fresh fruit, dark chocolate, and sweet pepper), 6 vitamins (vitamin B12, vitamin C, vitamin B6, vitamin D, vitamin E, and carotene), and 5 trace minerals (zinc, copper, calcium, magnesium, and potassium). Specific information about the diet in our study can be found in the publicly available information on the UK Biobank.1 We combined genome-wide association studies (GWAS) data on OC from three geographic regions using a GWAS-meta-analysis approach. The data obtained from the GAME-ON consortium included 2,989 cases and 6,583 controls, with >90% of participants predominantly of European ancestry (>70% CEU) (13). Furthermore, Chen et al. (14) and the GWAS catalog2 provide serum metabolite levels of theobromine, theophylline, caffeine, and linoleate (18:2n6). Other GWAS datasets are available through the Integrative Epidemiology Unit Open GWAS project.3 Table 1 shows the details of all the data.
1.3 Selection of instrumental variables
We identified SNPs associated with exposure factors that met a significance threshold (p < 5 × 10–6). SNPs were selected to be free of linkage disequilibrium to ensure independence, with an r2 < 0.001 within a 10,000 KB window. Surrogate SNPs were not considered. SNPs with F-statistic values <10 were regarded as weak IVs and were excluded. If the SNP was not found in the resulting GWAS dataset, it was excluded. Subsequently, we estimated that positive stranded alleles and SNPs with palindromes were eliminated. The qualifying SNPs were utilized in the subsequent analytical phase. The conditions and protocols mentioned above were used for inverse MR and MVMR analyses.
1.4 Statistical analysis
In our MR analysis, we employed five approaches: inverse variance weighted (IVW), weighted median, MR-Egger, weighted mode, and simple mode. These approaches aimed to investigate the potential association between dietary intake and the risk of developing OC. The value of p < 0.05 was deemed to be statistically significant. The directional consistency of associations, as represented by the inverse variance weighted IVW, weighted median, and MR-Egger methods, proved to be useful. The IVW was selected as the primary reference due to its excellent accuracy in identifying causality (15). The MR-Egger method was particularly responsive to horizontal pleiotropy and heterogeneity in the outcomes. The other three outcomes, weighted median, and two more served as supplementary approaches to the MR analysis. Results with inconsistent odds ratios (ORs) or evidence of horizontal pleiotropy were excluded from the analyses performed using the three MR methods: IVW, MR-Egger, and weighted median. The ORs were used to assess the impact of dietary factors on the risk of OC.
2 Results
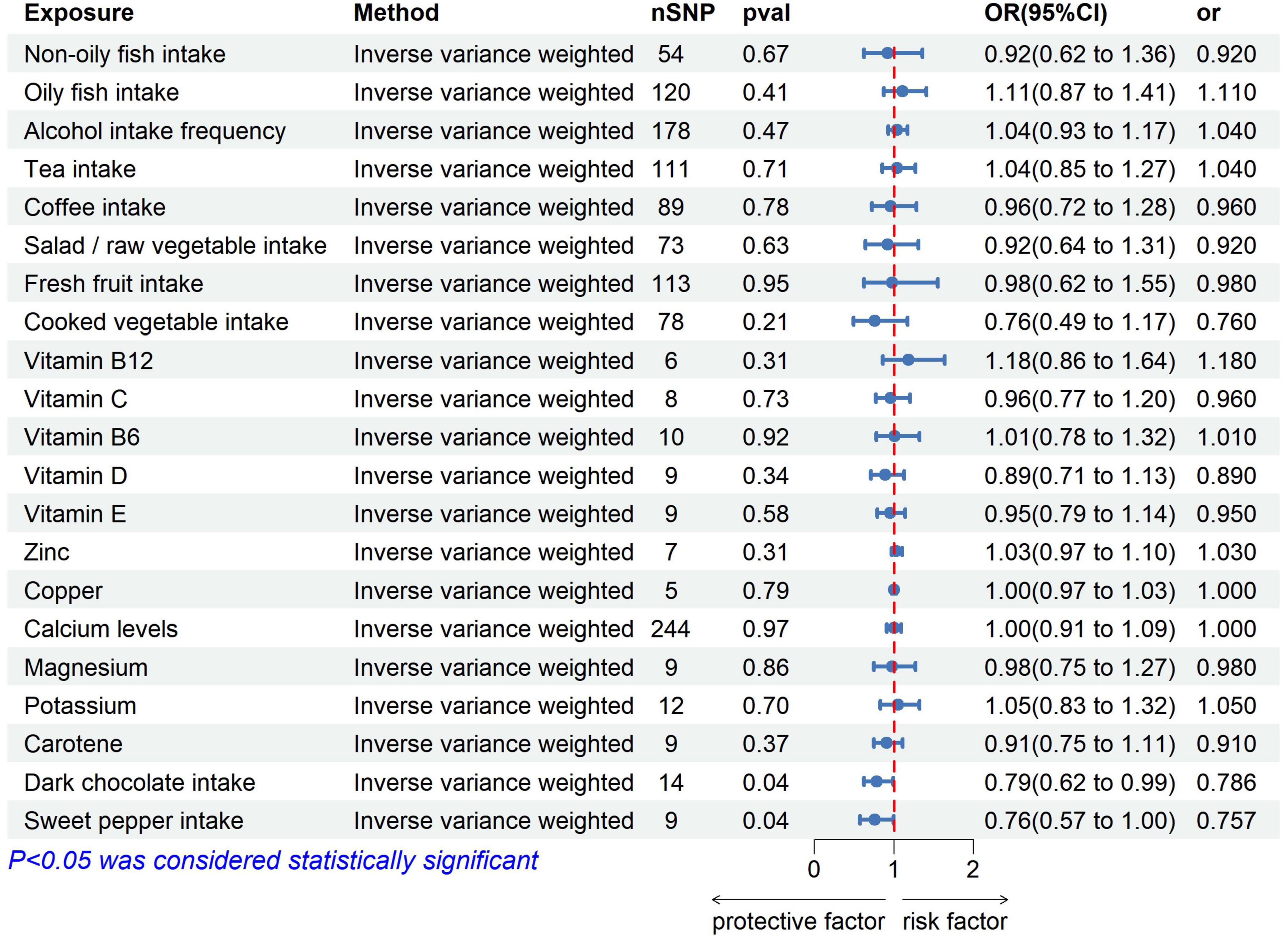
Two-sample MR results: We used five MR statistics to assess the association between 21 exposures and the risk of OC. Figure 1 depicts the IVW results for the 21 exposures.
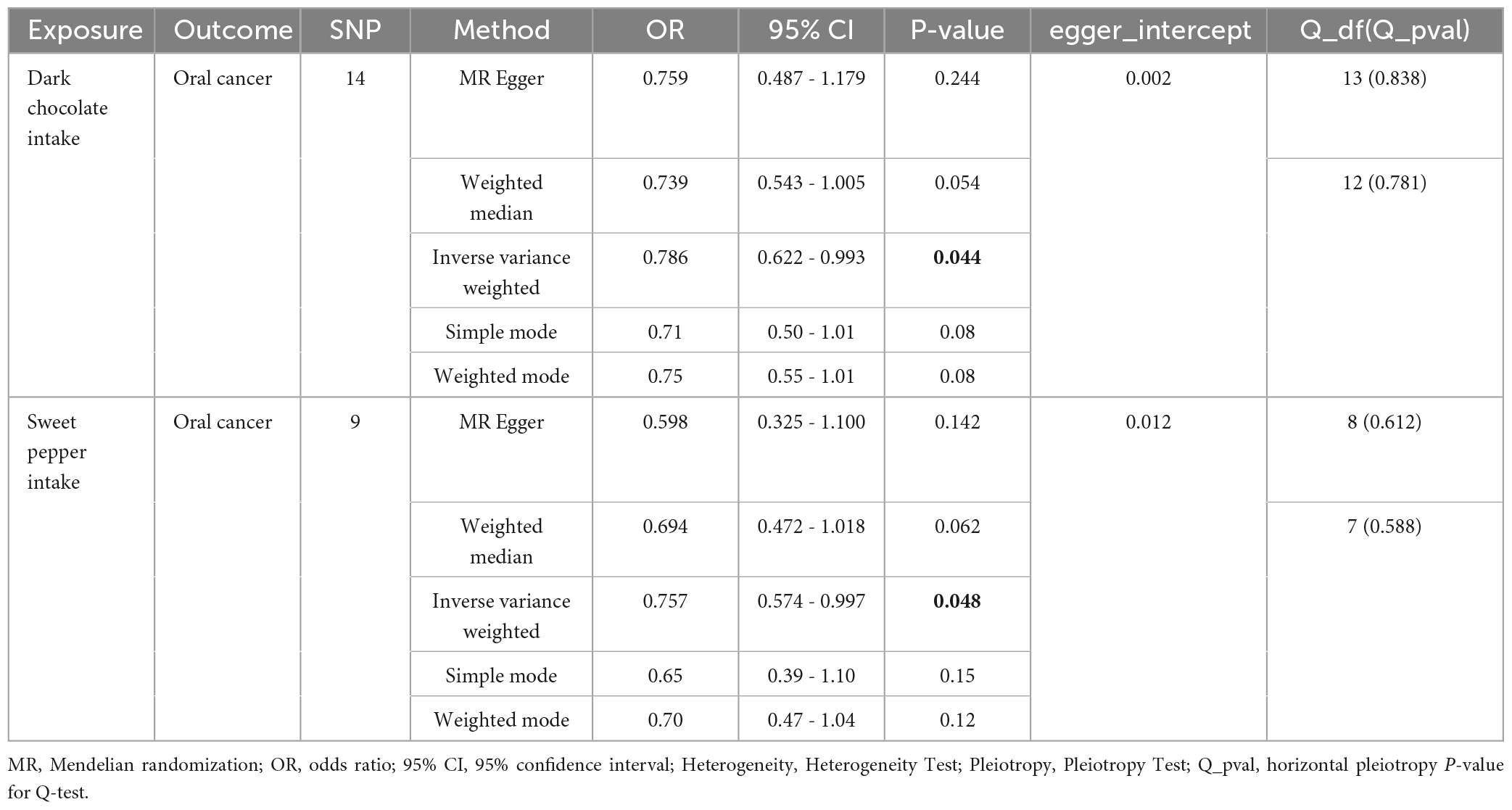
Of the 10 dietary patterns analyzed, 8 dietary intakes showed no significant association with OC risk (Supplementary Table 1). However, dark chocolate intake was inversely associated with OC risk (IVW: OR = 0.786, 95% confidence interval [CI]: 0.622–0.993, p = 0.044). Furthermore, the MR-Egger intercept test did not indicate evidence of horizontal pleiotropy (p > 0.05) (Supplementary Figure 1). The consistency of these findings was further confirmed by the leave-one-out method, suggesting the stability of the results (Supplementary Figure 2).
Similarly, sweet pepper intake was found to reduce the risk of OC (IVW: OR = 0.757, 95% CI: 0.574–0.997, p = 0.048). The MR-Egger intercept test did not indicate the presence of horizontal pleiotropy (p > 0.05) (Supplementary Figure 3). Moreover, the leave-one-out analysis revealed no outliers (Supplementary Figure 4). For both significant findings, the direction of the ORs obtained through the other three MR methods was consistent. This indicated that the results were more accurate (Table 2).
Regarding the other 6 vitamins (vitamin B12, vitamin C, vitamin B6, vitamin D, vitamin E, and carotene) and 5 trace elements (zinc, copper, calcium, magnesium, and potassium), the MR analysis indicated that the intake of these nutrients was not associated with an increased risk of OC (Supplementary Table 1).
Reverse MR results: We conducted a reverse MR analysis to determine whether the intake of dark chocolate and sweet pepper affects the risk of developing OC. The analysis revealed that dietary intake does not inversely affect OC development. Furthermore, the sensitivity analysis revealed no evidence of heterogeneity or pleiotropy. The leave-one-out analysis demonstrated consistent results, highlighting the stability of our findings (Supplementary Figures 5, 6 and Supplementary Table 2).
MVMR results: Given that numerous components in dark chocolate are known to have direct or indirect cancer-inhibitory bioactivity (16), we aimed to investigate their impact on oral carcinogenesis. Although trace elements in dark chocolate are known as active components (16), our two-sample MR analysis demonstrated that these factors do not influence the development of OC, leading to their exclusion from further analysis. Consequently, we employed MVMR to adjust serum metabolite levels for caffeine, linoleate, theophylline, and theobromine. This analysis indicated that dark chocolate intake was not associated with the risk of developing OC (Table 3).
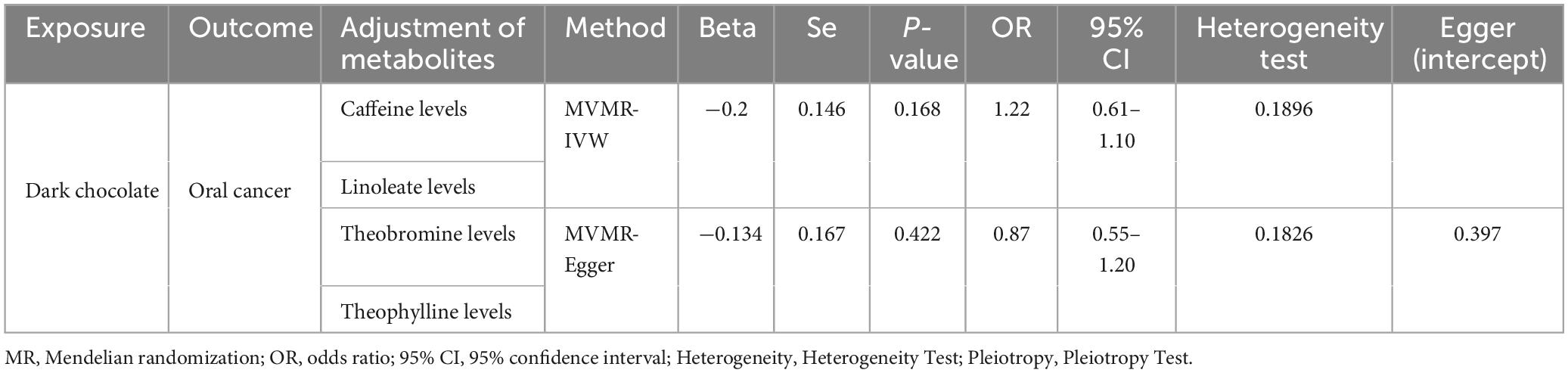
Table 3. Multivariable MR results of causal links between Dark chocolate and oral cancer after adjusting for specific serum metabolites.
We adjusted each ingredient separately in the MVMR analysis to elucidate the role of these factors in the suppression of OC by dark chocolate. After adjusting for each serum metabolite individually, we observed a decrease in p-values compared to the original values, specifically when adjusting for linoleate levels. This suggested a potential reduction in the inhibitory effect of dark chocolate on OC associated with linoleate levels. Conversely, adjusting for theobromine levels or theophylline levels resulted in higher p-values than the original values, with theophylline levels demonstrating a more pronounced increase. These findings suggest that theophylline may be the primary inhibitory factor in dark chocolate, while theobromine has a relatively limited inhibitory effect (Table 4).
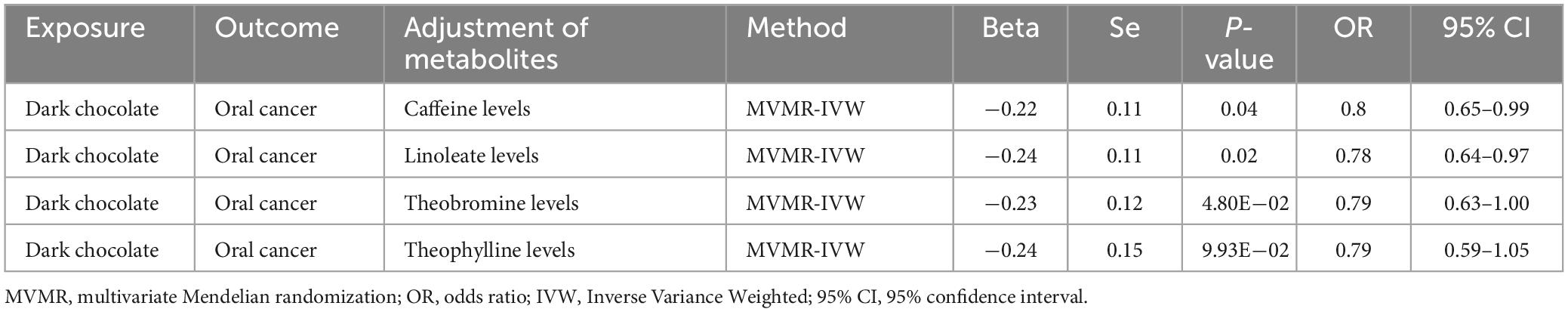
Table 4. Multivariable MR results of causal links between Dark chocolate and oral cancer after adjusting for single serum metabolites.
Despite our efforts to understand how sweet pepper may influence oral carcinogenesis, limitations in data availability restricted further investigation in this area.
3 Discussion
We found that dark chocolate and sweet pepper intake may exert a significant inhibitory effect on the development of OC. The MR analysis did not reveal any considerable association between OC risk and the remaining 8 dietary intakes (oily fish, non-oily fish, alcohol, tea, coffee, cooked vegetables, salad/raw vegetables, fresh fruit), 6 vitamins (vitamin B12, vitamin C, vitamin B6, vitamin D, vitamin E, and carotene), and 5 trace minerals (zinc, copper, calcium, magnesium, and potassium).
Considering the variety of vitamins and micronutrients in the daily diet, pooling and analyzing individual nutrients can significantly help eliminate the confounding factors often present in studies focused on specific nutrients. The findings of various studies on the association between dietary intake and OC are inconsistent (9). Concerning fish consumption, our findings are consistent with those of previous studies (17–19), which found no clear association between oily and non-oily fish intake and the development of OC. Although alcohol is considered potentially carcinogenic (20), its association with OC is unknown (21). Furthermore, the impact of tea and coffee consumption on OC is controversial (22). Our findings imply that their role may not be considerable, which is consistent with the findings of previous studies conducted by Tverdal et al. (23) and Hildebrand et al. (24). Fruits and vegetables, which are rich in vitamins, enhance antioxidant and anti-inflammatory responses, potentially inhibiting tumor growth (25). However, our findings did not demonstrate any substantial inhibitory effect of cooked vegetables, salad/raw vegetables, or fresh fruit intake on OC, inconsistent with the findings of previous studies (26, 27). Moreover, our analysis of vitamins and OC revealed no association between the intake of six common vitamins and the risk of OC, confirming our findings. Furthermore, although numerous studies (28) have highlighted the health benefits of micronutrients, our study did not find any substantial role of micronutrients in the risk of OC. We believe that the inconsistency between our study’s results and those of other experiments can be attributed to the following factors: 1. Previous observational studies may contain confounding factors, whereas our study, based on Mendelian Randomization (MR) analysis of Genome-Wide Association Studies (GWAS), minimizes the effects of confounding and reverse causation. 2. Our study is limited to European ancestries only. Variations in ancestry can influence disease susceptibility, thereby contributing to differences in results. Our findings suggest that while no direct association was found, dietary intake may still have some influence on the development of OC. These findings require further validation in future research.
Our findings indicated that dark chocolate intake may have a significant inhibitory effect on OC. Currently, studies that specifically investigate the impact of dark chocolate on OC are rare. Although some studies have proposed that various components of dark chocolate, including micronutrients, can have varying effects on overall health (16), their specific implications for OC are unknown. In this study, MVMR analysis suggested that caffeine, linoleate, theophylline, and theobromine levels may each have distinct roles in inhibiting oral carcinogenesis associated with consuming dark chocolate, particularly when controlling for several common components. Cocoa, the main ingredient in dark chocolate, contains a high percentage of methylxanthine compounds, mainly theobromine and caffeine (29). These substances are known for their potent antioxidant effects, aiding in the scavenging of free radicals, reducing DNA damage and oxidative stress, and thus potentially preventing cancer at its onset (30). Contrary to our initial expectations, our MVMR analysis revealed that caffeine in dark chocolate may not inhibit OC as anticipated. Furthermore, while theobromine has demonstrated inhibitory effects on OC, these effects are limited. Notably, theophylline in dark chocolate demonstrated a stronger inhibitory effect despite the lower percentage of theophylline in cocoa (31). Theophylline, a naturally occurring methylxanthine, is known to impact adenosine activation and downstream signaling pathways of cAMP by inhibiting cAMP phosphodiesterase activity (32). The activation of the cAMP signaling pathway plays a crucial role in the proliferation and growth of cancer cells. Notably, theophylline also inhibits the activation of NF-κB and the release of inflammatory factors such as IL-6 (33), which are implicated in creating a tumor-favorable inflammatory microenvironment that promotes cancer development and progression. Additionally, in breast cancer, theophylline has been shown to induce cell cycle arrest in the G2/M phase through the phosphorylation of cell cycle proteins B1 and Cdc2 (34). Therefore, it is evident that theophylline plays a distinct role in cancer inhibition, which aligns with the findings of our study. Dark chocolate contains a high percentage of linoleate (18:2n6) (35). These linoleic acids decrease cholesterol levels of low-density lipoproteins and fasting blood glucose concentrations by improving insulin sensitivity (36, 37). Metabolic disorders, including diabetes mellitus and obesity, are associated with an increased risk of oral carcinogenesis (38, 39). Contrary to the anticipated findings, our findings demonstrated that the inhibitory effect of dark chocolate on OC was reduced after adjusting for linoleate levels. This suggests that linoleate levels might not fulfill their anticipated role but rather may slightly reduce the inhibitory effect of dark chocolate. Further studies are required to clarify its exact role in the impact of dark chocolate.
The above findings suggest that the biological activity of dark chocolate is not attributable to a single ingredient. Furthermore, dark chocolate is specifically abundant in total polyphenols and flavonoids (40), which are known to have antioxidant properties. These may be crucial in inhibiting the development of OC (41, 42). However, the data restrictions prevented us from fully investigating and analyzing this aspect. Additionally, dark chocolate intake has been associated with improved mood (43), suggesting an additional psychological benefit. Our findings indicate that the consumption of sweet pepper may substantially inhibit the development of OC. Sweet pepper are rich in an array of phytochemicals including polyphenols, flavonoids, carotenoids, capsaicinoids, and dihydrocapsaicin. Han et al. (44) observed that capsaicinoids inhibit the activation of the NF-κB pathway and activator protein-1 (AP-1), suggesting a potential role in cancer and inflammation prevention. Further research indicates that capsaicin activates the AMPK/mTOR signaling pathway to induce autophagy in renal cancer cells, thereby reducing tumor proliferation, invasion, and epithelial-mesenchymal transition (EMT) (45). Additionally, capsaicin facilitates the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun, while inhibiting the Hedgehog/GLI pathway, exhibiting antiproliferative and anticancer properties (46). In cases of oral cancer, capsaicin has been shown to suppress tumor cell proliferation and induce apoptosis. (47). Interestingly, capsiate, another compound in sweet pepper, is effective in inhibiting tumor activity (48). Despite our efforts to further understand the underlying mechanisms, the limitations of our data prevented a more in-depth investigation.
This is a novel study on the association between various dietary patterns and OC using multiple MR analysis methods. To our knowledge, this is the first study to uncover this relationship through MR. The lack of studies that examine the relationship between dietary intake and OC highlights the importance of our findings. These findings lay the foundation for future larger-scale clinical studies and basic research to investigate this association more thoroughly. Moreover, our research bridges the gap in understanding the association between dark chocolate and OC risk. It investigates the potential mechanisms by which dark chocolate components can suppress OC, laying the foundation for future fundamental research. Our results provide insights into the role of different dietary intakes in the development of OC, offering guidance for individuals at elevated risk of OC to adjust their diets in a clinically meaningful way.
There are limitations to our study. First, to capture a wide range of associations between dietary variety and OC, we employed a significance threshold of p < 5 × 10–6 rather than the more conventional p < 5 × 10–8. Although our datasets were solely of European origin, it is crucial to conduct further studies that include diverse regions to investigate these associations globally. Second, the nature of the MR analysis prevented us from determining a dose-response relationship between various intakes and OC at this stage. Third, we did not categorize outcomes by clinical, pathological, site, or other clinical features to investigate the relationship between diet and each subtype. While we have identified possible mechanisms of action for dark chocolate, limited data prevented definitive conclusions on the role of all its ingredients.
4 Conclusion
This study demonstrated a causal association between the intake of dark chocolate and sweet peppers and a decreased risk of OC. The components of dark chocolate could have different effects.
Data availability statement
All GWAS data are available by accessing the open GWAS database (https://gwas.mrcieu.ac.uk/) or GWAS Catalog (https://www.ebi.ac.uk/gwas/), further inquiries can be directed to the corresponding author.
Ethics statement
All data for the study were obtained from publicly available databases that had been approved by the Ethics Committee, so no additional information was required. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
HW: Conceptualization, Methodology, Formal analysis, Visualization, Writing – original draft. ZZ: Conceptualization, Software, Visualization, Writing – original draft. SW: Conceptualization, Data curation, Validation, Writing – original draft. YZ: Writing – original draft. TL: Formal analysis, Validation, Writing – original draft. XH: Resources, Data curation, Writing – original draft. JY: Writing review and editing, Supervision, Project administration.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1342163/full#supplementary-material
Footnotes
- ^ https://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=100052
- ^ https://www.ebi.ac.uk/gwas/
- ^ https://gwas.mrcieu.ac.uk/
References
2. Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. (1988) 48:3282–7.
4. Nagao T, Warnakulasuriya S. Screening for oral cancer: Future prospects, research and policy development for Asia. Oral Oncol. (2020) 105:104632. doi: 10.1016/j.oraloncology.2020.104632
6. Warnakulasuriya S, Kerr AR. Oral cancer screening: Past, present, and future. J Dent Res. (2021) 100:1313–20.
7. Yin L, Yan H, Chen K, Ji Z, Zhang X, Ji G, et al. Diet-derived circulating antioxidants and risk of digestive system tumors: A Mendelian randomization study. Nutrients. (2022) 14:3724.
8. Yuan S, Chen J, Ruan X, Sun Y, Zhang K, Wang X, et al. Smoking, alcohol consumption, and 24 gastrointestinal diseases: Mendelian randomization analysis. Elife. (2023) 12:e84051.
9. Rodriguez-Molinero J, Miguelanez-Medran BDC, Puente-Gutierrez C, Delgado-Somolinos E, Martin Carreras-Presas C, Fernandez-Farhall J, et al. Association between oral cancer and diet: An update. Nutrients. (2021) 13:1299.
10. Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, et al. The dietary patterns methods project: Synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. (2015) 145:393–402. doi: 10.3945/jn.114.205336
11. Sekula P, Del Greco MF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65.
12. Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. (2019) 48:713–27.
13. Lesseur C, Diergaarde B, Olshan AF, Wunsch-Filho V, Ness AR, Liu G, et al. Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer. Nat Genet. (2016) 48:1544–50. doi: 10.1038/ng.3685
14. Chen Y, Lu T, Pettersson-Kymmer U, Stewart ID, Butler-Laporte G, Nakanishi T, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet. (2023) 55:44–53. doi: 10.1038/s41588-022-01270-1
15. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
16. Samanta S, Sarkar T, Chakraborty R, Rebezov M, Shariati MA, Thiruvengadam M, et al. Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Curr Res Food Sci. (2022) 5:1916–43. doi: 10.1016/j.crfs.2022.10.017
17. Perloy A, Maasland DHE, van den Brandt PA, Kremer B, Schouten LJ. Intake of meat and fish and risk of head-neck cancer subtypes in the Netherlands cohort study. Cancer Causes Control. (2017) 28:647–56. doi: 10.1007/s10552-017-0892-0
18. Sanchez MJ, Martinez C, Nieto A, Castellsague X, Quintana MJ, Bosch FX, et al. Oral and oropharyngeal cancer in Spain: Influence of dietary patterns. Eur J Cancer Prev. (2003) 12:49–56. doi: 10.1097/00008469-200302000-00008
19. Garavello W, Lucenteforte E, Bosetti C, La Vecchia C. The role of foods and nutrients on oral and pharyngeal cancer risk. Minerva Stomatol. (2009) 58:25–34.
21. Reidy J, McHugh E, Stassen LF. A review of the re-lationship between alcohol and oral cancer. Surgeon. (2011) 9:278–83.
22. He T, Guo X, Li X, Liao C, Yin W. Association between coffee intake and the risk of oral cavity cancer: A meta-analysis of observational studies. Eur J Cancer Prev. (2020) 29:80–8. doi: 10.1097/CEJ.0000000000000515
23. Tverdal A, Hjellvik V, Selmer R. Coffee intake and oral-oesophageal cancer: Follow-up of 389,624 Norwegian men and women 40-45 years. Br J Cancer. (2011) 105:157–61. doi: 10.1038/bjc.2011.192
24. Hildebrand JS, Patel AV, McCullough ML, Gaudet MM, Chen AY, Hayes RB, et al. Coffee, tea, and fatal oral/pharyngeal cancer in a large prospective US cohort. Am J Epidemiol. (2013) 177:50–8. doi: 10.1093/aje/kws222
25. Cirmi S, Navarra M, Woodside JV, Cantwell MM. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol Res. (2018) 133:187–94. doi: 10.1016/j.phrs.2018.05.008
27. Turati F, Rossi M, Pelucchi C, Levi F, La Vecchia C. Fruit and vegetables and cancer risk: A review of southern European studies. Br J Nutr. (2015) 113:S102–10. doi: 10.1017/S0007114515000148
28. Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med. (2005) 26:235–44.
29. Ashihara H, Kato M, Crozier A. Distribution, biosynthesis and catabolism of methylxanthines in plants. Handb Exp Pharmacol. (2011) 200:11–31.
30. Saraf S, Kaur CD. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn Rev. (2010) 4:1–11. doi: 10.4103/0973-7847.65319
31. Hurst WJ, Glinski JA, Miller KB, Apgar J, Davey MH, Stuart DA. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J Agric Food Chem. (2008) 56:8374–8. doi: 10.1021/jf801297w
32. Hashemi-Niasari F, Rabbani-Chadegani A, Razmi M, Fallah S. Synergy of theophylline reduces necrotic effect of berberine, induces cell cycle arrest and PARP, HMGB1, Bcl-2 family mediated apoptosis in MDA-MB-231 breast cancer cells. Biomed Pharmacother. (2018) 106:858–67. doi: 10.1016/j.biopha.2018.07.019
33. Ichiyama T, Hasegawa S, Matsubara T, Hayashi T, Furukawa S. Theophylline inhibits NF-kappa B activation and I kappa B alpha degradation in human pulmonary epithelial cells. Naunyn Schmiedebergs Arch Pharmacol. (2001) 364:558–61. doi: 10.1007/s00210-001-0494-x
34. Chang YL, Hsu YJ, Chen Y, Wang YW, Huang SM. Theophylline exhibits anti-cancer activity via suppressing SRSF3 in cervical and breast cancer cell lines. Oncotarget. (2017) 8:101461–74. doi: 10.18632/oncotarget.21464
35. de Oliveira LN, de Jesus Coelho Castro R, de Oliveira MA, de Oliveira LF. Lipid characterization of white, dark, and milk chocolates by FT-Raman spectroscopy and capillary zone electrophoresis. J AOAC Int. (2015) 98:1598–607. doi: 10.5740/jaoacint.15-083
36. Darand M, Hajizadeh Oghaz M, Hadi A, Atefi M, Amani R. The effect of cocoa/dark chocolate consumption on lipid profile, glycemia, and blood pressure in diabetic patients: A meta-analysis of observational studies. Phytother Res. (2021) 35:5487–501. doi: 10.1002/ptr.7183
37. Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. (2005) 46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70
38. Ramos-Garcia P, Roca-Rodriguez MDM, Aguilar-Diosdado M, Gonzalez-Moles MA. Diabetes mellitus and oral cancer/oral potentially malignant disorders: A systematic review and meta-analysis. Oral Dis. (2021) 27:404–21. doi: 10.1111/odi.13289
39. Gaudet MM, Kitahara CM, Newton CC, Bernstein L, Reynolds P, Weiderpass E, et al. Anthropometry and head and neck cancer:a pooled analysis of cohort data. Int J Epidemiol. (2015) 44:673–81.
41. Febrianto NA, Wang S, Zhu F. Chemical and biological properties of cocoa beans affected by processing: A review. Crit Rev Food Sci Nutr. (2022) 62:8403–34.
42. Jalil AM, Ismail A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health? Molecules. (2008) 13:2190–219. doi: 10.3390/molecules13092190
43. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. (2011) 15:2779–811.
44. Han SS, Keum YS, Seo HJ, Chun KS, Lee SS, Surh YJ. Capsaicin suppresses phorbol ester-induced activation of NF-kappaB/Rel and AP-1 transcription factors in mouse epidermis. Cancer Lett. (2001) 164:119–26. doi: 10.1016/s0304-3835(01)00378-0
45. Que T, Ren B, Fan Y, Liu T, Hou T, Dan W, et al. Capsaicin inhibits the migration, invasion and EMT of renal cancer cells by inducing AMPK/mTOR-mediated autophagy. Chem Biol Interact. (2022) 366:110043. doi: 10.1016/j.cbi.2022.110043
46. Bai H, Li H, Zhang W, Matkowskyj KA, Liao J, Srivastava SK, et al. Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice. Carcinogenesis. (2011) 32:1689–96. doi: 10.1093/carcin/bgr191
47. Mosqueda-Solis A, Lafuente-Ibanez de Mendoza I, Aguirre-Urizar JM, Mosqueda-Taylor A. Capsaicin intake and oral carcinogenesis: A systematic review. Med Oral Patol Oral Cir Bucal. (2021) 26:e261–8. doi: 10.4317/medoral.24570
Keywords: dark chocolate intake, sweet peppers intake, dietary intake, oral cancer, Mendelian randomization
Citation: Wang H, Zhang Z, Wu S, Zhu Y, Liang T, Huang X and Yao J (2024) Dietary patterns suggest that dark chocolate intake may have an inhibitory effect on oral cancer: a Mendelian randomization study. Front. Nutr. 11:1342163. doi: 10.3389/fnut.2024.1342163
Received: 21 November 2023; Accepted: 22 May 2024;
Published: 27 June 2024.
Edited by:
Giulia Marrone, University of Rome Tor Vergata, ItalyReviewed by:
Javier Carballo, University of Vigo, SpainMd Nur Hossain, Bangladesh Council of Scientific and Industrial Research, Bangladesh
Dimas Rahadian Aji Muhammad, Sebelas Maret University, Indonesia
Copyright © 2024 Wang, Zhang, Wu, Zhu, Liang, Huang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinguang Yao, eWFvNzc2MDY5OEAxMjYuY29t
 Hongwei Wang
Hongwei Wang Zhaoyin Zhang1,2,3
Zhaoyin Zhang1,2,3 Jinguang Yao
Jinguang Yao