- 1Nutrition and Health Innovation Research Institute, School of Medical and Health Sciences, Royal Perth Hospital Research Foundation, Edith Cowan University, Perth, WA, Australia
- 2The Danish Cancer Society Research Centre, Copenhagen, Denmark
- 3School of Population and Global Health, University of Western Australia, Perth, WA, Australia
- 4Centre for Healthy Ageing, Health Futures Institute, Murdoch University, Murdoch, WA, Australia
- 5Centre of Excellence for Alzheimer's Disease Research and Care, School of Medical and Health Sciences, Edith Cowan University, Joondalup, WA, Australia
- 6Australian Alzheimer's Research Foundation, Perth, WA, Australia
- 7School of Psychological Science, University of Western Australia, Perth, WA, Australia
- 8Lifestyle Approaches Towards Cognitive Health Research Group, Murdoch University, Murdoch, WA, Australia
- 9Medical School, The University of Western Australia, Royal Perth Hospital, Perth, WA, Australia
- 10Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia
- 11Clinical Diabetes and Epidemiology, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia
- 12School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
- 13Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Sciences, Deakin University, Geelong, VIC, Australia
- 14School of Psychology, The University of New South Wales, Sydney, NSW, Australia
- 15Neuroscience Research Australia (NeuRA), Randwick, NSW, Australia
- 16UNSW Ageing Futures Institute, Kensington, NSW, Australia
- 17Centre for Kidney Research, Children’s Hospital at Westmead, School of Public Health, Sydney Medical School, The University of Sydney, Sydney, NSW, Australia
Introduction: Dietary nitrate is potentially beneficial for cardiovascular, cerebrovascular, and nervous systems due to its role as a nitric oxide (NO) precursor. Increased nitrate intake improves cardiovascular health and therefore could protect against dementia, given the cardiovascular-dementia link.
Objective: To investigate the association between source-dependent nitrate intake and dementia-related mortality. As individuals with diabetes are at higher risk of dementia, a secondary aim was to investigate if the associations between nitrate and dementia varied by diabetes mellitus (DM) and pre-diabetes status.
Methods: This study involved 9,149 participants aged ≥25 years from the well-characterised Australian Diabetes, Obesity, and Lifestyle (AusDiab) Study followed over a period of 17 years. Intakes of plant-sourced, vegetable-sourced, naturally occurring animal-sourced nitrate, and processed meat (where nitrate is an allowed additive)-sourced nitrate were assessed from a 74-item food frequency questionnaire completed by participants at baseline and nitrate databases were used to estimate nitrate from these different dietary sources. Associations between source-dependent nitrate intake and dementia-related mortality were assessed using multivariable-adjusted Cox proportional hazards models adjusted for demographics, lifestyle, and dietary factors.
Results: Over 17 years of follow-up, 93 (1.0%) dementia-related deaths occurred of 1,237 (13.5%) total deaths. In multivariable-adjusted models, participants with the highest intakes of plant-sourced nitrate (median intake 98 mg/day) had a 57% lower risk of dementia-related mortality [HR (95% CI): 0.43 (0.22, 0.87)] compared to participants with lowest intakes of plant-sourced nitrate (median intake 35 mg/day). A 66% lower risk was also seen for higher intakes of vegetable-sourced nitrate [HR (95% CI): 0.34 (0.17, 0.66)]. No association was observed for animal-sourced nitrate, but the risk was two times higher amongst those who consumed the most processed meat-sourced nitrate intake [HR (95%): 2.10 (1.07, 4.12)]. The highest intake of vegetable-sourced nitrate was associated with a lower risk of dementia-related mortality for those with and without DM and pre-diabetes.
Conclusion: Encouraging the intake of nitrate-rich vegetables, such as green leafy vegetables and beetroot, may lower the risk of dementia-related mortality, particularly in individuals with (pre-) diabetes who are at a higher dementia risk.
1 Introduction
Dementia is a leading cause of mortality globally (1) and is the second leading cause of death in Australia (2). Currently, more than 55 million people have dementia with this number predicted to surge to 152 million by 2050 (3). With no cure for dementia discovered to date, identifying evidence-based preventive strategies to reduce the risk of dementia is a global research priority. Targeting modifiable risk factors is a strategy that is estimated, using population-attributable risk models, to prevent or delay up to 40% of dementia cases (4). Of the 12 modifiable risk factors identified by Lancet Commission, 5 [high blood pressure, obesity, alcohol intake, diabetes mellitus (DM), and depression] can be positively impacted by a healthy diet (4). Moreover, WHO guidelines include diet as a modifiable risk factor as do systematic reviews (5). Thus, identifying and promoting higher intakes of the protective components of a healthy dietary pattern could prevent or delay the onset of dementia.
One such potential protective dietary component is nitrate. Nitrate has been identified as an important exogenous source of the cellular signalling molecule, nitric oxide (NO) (6). NO plays a fundamental role in the regulation of the cardiovascular (7), cerebrovascular (8), and the central nervous systems (9). Increasing NO through dietary nitrate intake positively impacts the cardiovascular system (10), and could also potentially impact the cerebrovascular and central nervous systems.
We have recently shown that habitual intake of dietary nitrate from sources where nitrate is naturally present impacts cognitive performance, amongst cognitively unimpaired older adults in an apolipoprotein E (APOE) genotype contingent manner. Specifically, higher intake of dietary nitrate was associated with better language scores in non-carriers of the APOE ε4 allele and with better episodic recall and recognition memory in those at higher risk of Alzheimer’s disease (AD; the most common form of dementia) due to the presence of one or two APOE ε4 alleles (11). Given the role of NO in the cerebrovascular, and central nervous systems, the established benefit of dietary nitrate on cardiovascular health, and the recognised vascular contributions to dementia, there is a strong rationale to investigate whether habitual intake of dietary nitrate may also impact risk of dementia-related mortality.
Nitrate is found in high concentrations in green leafy vegetables and some root vegetables, contributing ~70–80% to total dietary nitrate intake (12). Nitrate is also found naturally in meat and other animal products, contributing ~10–15% to total dietary nitrate intake (12). In contrast nitrite concentration is very low in plants and higher in meat (13). Furthermore, nitrate and nitrite are both highly regulated preservatives used in processed meat, contributing to ~5% total dietary nitrate intake (14). Nitrate from plant sources has been shown to increase NO with beneficial health effects (14). In contrast, nitrate, through conversion to nitrite, also has the potential to form carcinogenic (15) and neurodegenerative N-nitrosamines (16) exogenously and endogenously. Dietary nitrate’s potential favourable (increases NO) and adverse (forms N-nitrosamines) effects are hypothesised to be source dependent (14). Plant-sourced nitrate intake is accompanied by other beneficial components such as vitamin C, flavonoids, and antioxidants that can inhibit nitrosamine production (17). The presence of nitrate and nitrite in processed meat is hypothesised to contribute to the negative health effects of processed meat intake.
The primary aim of this study was to investigate the association between habitual intakes of nitrate from different sources and dementia-related mortality in the Australian Diabetes, Obesity, and Lifestyle (AusDiab) Study. The sources of nitrate were (i) plant-sourced nitrate, (ii) vegetable-sourced nitrate, (iii) animal-sourced nitrate (excluding nitrate additives), and (iv) processed meat-sourced nitrate (including nitrate additives, but excluding fresh sausages, i.e., meat where nitrate is an allowed additive). As individuals with diabetes have a higher risk of developing dementia (18, 19), a secondary aim was to explore whether dietary nitrate was associated with fewer dementia-related mortality in participants with DM and pre-diabetes (with either impaired glucose tolerance or impaired fasting glucose).
2 Methods
2.1 Study population
The Australian Diabetes, Obesity, and Lifestyle (AusDiab) Study is a population-based longitudinal study of adults (aged ≥25 years), which recruited 11,247 men and women across Australia in 1999–2000. Further details regarding methods and response rates are described previously (20). The AusDiab study was approved by the International Diabetes Institute ethics committee and informed consent was obtained from all the participants (20).
Participants were excluded from the current study if they had implausible energy intakes [n = 546 (<2,500 kJ/day or > 14,500 kJ/day for females and < 3,300 kJ/day or 17,500 kJ/day for males)] (21, 22), whilst participants who were pregnant were not excluded from AusDiab during recruitment, they were excluded from the current study if they were pregnant at the time of recruitment (n = 60) as diet may be changed during pregnancy, had chronic renal disease (estimated glomerular filtration rate < 60; n = 899) (23, 24), or if they had missing or implausible values for covariates (n = 593). Thus, this study included 9,149 participants in the analyses.
2.2 Exposures
At baseline and 5 years, participants reported their usual intakes of 74 food and beverage items over the previous 12 months via a 74-item Cancer Council of Victoria Food Frequency Questionnaire (CCVFFQ) (25, 26). From these items, habitual intakes of different sources of dietary nitrate where nitrate is naturally present (plant-sourced nitrate, vegetable-sourced nitrate, and animal-sourced nitrate) and where nitrate is used as an additive for most products except fresh sausage (processed meat) (27), were assessed and quantified as described below. Baseline FFQ data were used for analyses as 5-year FFQ data was only available for 60% of the cohort.
2.2.1 Plant- and vegetable-sourced nitrate intake
A comprehensive plant-based food reference nitrate database with nitrate values from 304 plant-based foods from 64 countries was used to calculate nitrate values of all plant-based foods; vegetables, fruits, cereals, herbs, spices, pulses, and nuts (28). The nitrate content of plant foods differs depending on the country of cultivation; therefore, the following strategy was employed (12): the median value for each plant food was used if there were three or more references in the database for Australia; the median of values for all Oceania (Australia, New Zealand, and surrounding islands) was used if there were fewer than three references the database for Australia; the median of values for all countries in the database was used if there were fewer than three references available for Oceania. The median nitrate value (mg/g) of each plant-based food was multiplied by the estimated quantity of the plant-based food consumed (g/day). To take into account the effect of cooking, for cooked plant-based foods the assigned nitrate value was reduced by 50% (28). The nitrate values of each individual plant-based and vegetable-based food were summed to obtain total plant-sourced nitrate and total vegetable-sourced nitrate consumed per day.
2.2.2 Animal-sourced nitrate intake
Red meat, dairy, seafood, eggs, and poultry were used for the calculation of naturally occurring animal-sourced nitrate intake. Processed meat, where nitrate is as an allowed additive for most products except fresh sausage, was calculated separately due its link with detrimental health effects (29). To calculate animal-sourced nitrate intake, a recently published nitrate reference database for animal-sourced food products, with data from 51 countries, was used (13). The same strategy as described above for plant-based foods and vegetables was employed. However, the 50% reduction in value was not applied to animal products because the majority of the data sources in the animal database did not indicate cooking method clearly. To determine total animal-sourced nitrate consumed (mg/day), the amount of the specific animal-sourced food consumed (g/day) was multiplied by its median nitrate content (mg/g).
2.2.3 Total nitrate intake
The sum of nitrate intake from all food items in the FFQ including discretionary foods such as chocolate, biscuits, pizza, and crisps were used to compute total nitrate intake (mg/day). The amount of food item consumed (g/day) was multiplied by the assigned median nitrate value (mg/g) for that food item to calculate nitrate intake (mg/day). If the nitrate value for that food item was not available in any of the above listed databases, the value of zero was assigned.
2.3 Study outcomes
Dementia-related mortality, defined as death with mention of a dementia diagnosis on any part of the death certificate, was the primary outcome of this study. Information on dates and causes of death were obtained from National Death Index (NDI), using the International Classification of Diseases, Tenth Revision (ICD-10) Australian Modification format (30). Death due to dementia was defined using the ICD-10 codes; F00 (Alzheimer’s disease), G30 (Alzheimer’s disease), F01 (vascular dementia) and F03 (unspecified dementia), regardless of whether they were underlying or secondary causes of death.
2.4 Covariates
Demographic data including age, sex (male/female), education level (never to some high school, completed University, or equivalent), marital status (never married, married, de-facto, separated, divorced, and widowed), smoking status, alcohol intake, physical activity, and weekly income ($0–199, $200–399, $400–599, $600–799, $800–1,499, and $1500+) were collected at local testing centres by questionnaire at baseline. Smoking status was classified as: never smoked (<100 cigarettes in lifetime), ex-smoker (not daily for at least the previous 3 months), and current smoker (smoking daily) (31). The Active Australia Survey Questionnaire was used to record self-reported physical activity routine in the past week as described previously (32, 33). Physical activity levels were categorised as sedentary (zero physical activity), insufficient (<150 min/week), and sufficient (>150 min/week). All the participants undertook a 75-g oral glucose tolerance test except those who were pregnant or taking prescribed hypoglycaemic medication. An Olympus AU600 analyser (Olympus Optical, Tokyo, Japan) was used to measure fasting plasma glucose (FPG), 2-h plasma glucose (2-h PG), and fasting serum total cholesterol. Participants were categorised as having known diabetes mellitus (KDM) if they reported of having prescribed hypoglycaemic medication by a physician or had FPG ≥ 7.0 mmol/L or 2-h PG ≥ 11.1 mmol/L. Participants who did not report of having DM but had a FPG ≥ 7.0 mmol/L or 2-h PG ≥ 11.1 mmol/L were categorised as having newly diagnosed diabetes mellitus (NDM). Amongst those diagnosed as KDM, 92% had type 2 DM Participants were categorised as having: (1) impaired fasting glucose (IFG) if their FPG was ≥6.1 mmol/L and < 7. mmol/L and their 2-h PG was <7.8 mmol/L; (2) impaired glucose tolerance (IGT) if their 2-h PG was ≥7.8 mmol/L and < 11.1 mmol/L and their FPG < 7.0 mmol/L; and (3) normal glucose tolerance if their FPG was <6.1 mmol/L and their 2-h PG was <7.8 mmol/L. Participants with either IFG or IGT were classified as having pre-diabetes (20, 34). Height was measured without shoes using a stadiometer and was rounded to the nearest 0.5 cm. A mechanical beam balance was used to measure weight without shoes and extra clothing (35). Body mass index (BMI) was calculated by dividing weight (kg) by height (squared metres). Anthropometric details have been described previously (20, 36). Relative socio-economic situations of geographic area were used to calculate Socio-Economic Indices For Areas (SEIFA) based on 5 yearly censuses from 1999 (37). Intakes of dietary covariates were captured from the FFQ as stated previously.
2.5 Statistical analysis
Statistical analyses were performed using Stata version 15 (StataCorp, College Station, Texas 77845, United States). For all tests, statistical significance was set at p ≤ 0.05 (two-tailed). Participants were followed up for a period of 17 years from the date of study enrolment until death, or until end of follow-up date, 17 April 2017, whichever came first. Cox proportional hazards models were used to estimate the association between baseline habitual intake of plant-sourced, vegetable-sourced, naturally occurring animal-sourced, and processed meat-sourced nitrate and dementia-related mortality up to 17 years of follow-up. We investigated whether associations were non-linear using restricted cubic splines but p-values from likelihood ratio tests comparing appropriate nested models were all p > 0.05. Thus, hazard ratios (HRs) and 95% confidence intervals were attained from the models with exposure fitted as quartiles. The proportional hazards assumption was tested based on Schoenfeld residuals with no global violation of the assumption found. Covariates were selected a priori based on the current knowledge of confounding factors of nitrate intake and dementia. We used four models of adjustment: Model 1 included age and sex; Model 2 included age, sex, BMI, physical activity, smoking status (never/former/current), education level, marital status, income, SEIFA, alcohol consumption, serum cholesterol levels, presence of DM, and/or pre-diabetes; Model 3a included all the covariates adjusted for Model 2 plus energy intake; and when plant- and vegetable-sourced nitrate were the exposures of interest, Model 3b included all the covariates adjusted for in Model 2 plus potential dietary confounding variables such as intakes (g/day) of red meat, fish, saturated fatty acids, polysaturated fatty acids, monosaturated fatty acids and when naturally occurring animal and processed meat-sourced nitrate were the exposures of interest, Model 3b included all covariates in Model 2, plus intake of saturated fatty acids, polysaturated fatty acids, monosaturated fatty acids, and vegetables. We performed sensitivity analyses to check the robustness of the association of plant and vegetable-sourced nitrate with dementia-related mortality by adjusting for other factors such as intakes of flavonoids, vitamin C, and fibre. Additionally, we carried out stratified analyses by pre-diabetes and DM status at baseline to explore possible effect modification. We also investigated interactions with established risk factors for dementia, namely DM and sex. Logistic regression models were performed to obtain 17-year predicted absolute risk estimates for dementia in individuals with/without DM and pre-diabetes. An exploratory analysis was run to determine if there were any substantial changes in the intakes of dietary nitrate from different dietary sources in the participants with diet data at baseline and 5-year follow-up.
3 Results
3.1 Baseline characteristics
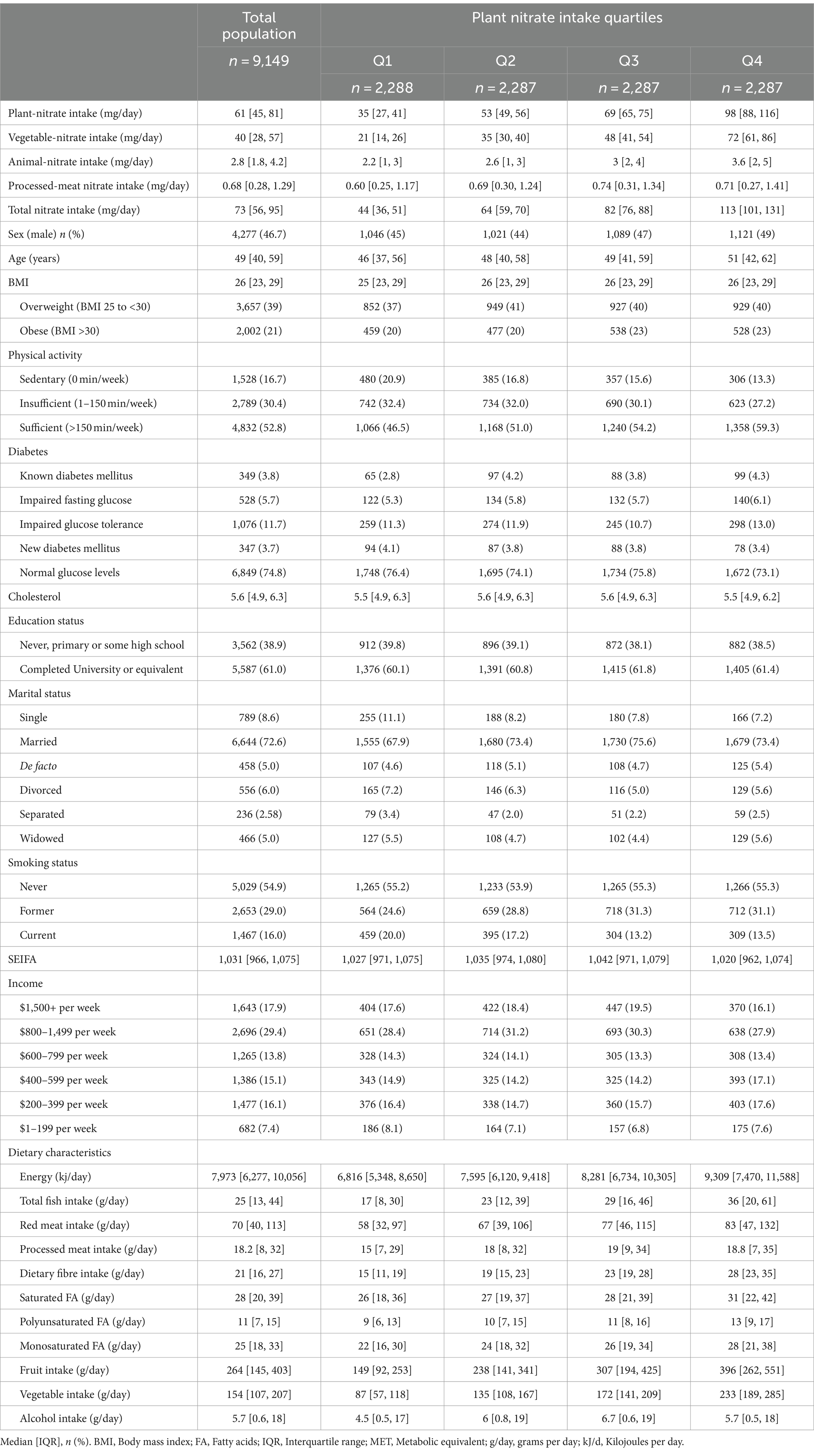
The study cohort (n = 9,149) was comprised of 46.7% males, had a median [IQR] age of 49 [40–59] years at study enrolment, and had a median [IQR] follow-up time of 16 [16–17] years. The median [IQR] intake of plant sourced nitrate was 61 [45–88] mg/day, vegetable sourced nitrate was 40 [28–57] mg/day, animal-sourced nitrate was 2.8 [1.8, 4.2] mg/day and processed meat-sourced nitrate was 0.68 [0.28, 1.29] mg/day (Table 1). Of total nitrate intake, plant-sourced nitrate contributed 86% (of this, vegetable-sourced nitrate contributed 65%, fruit nitrate 14%, whole grain nitrate 7%), animal-sourced nitrate 4%, and processed meat 1.2%. The remaining 9% was from alcohol and discretionary foods. The primary contributors to vegetable-sourced nitrate intake were lettuce (39%), zucchini (19%), cabbage (17%), pumpkin (10%), spinach (9%), and potato (8%). The main contributors to animal-sourced nitrate were yoghourt (26%), lamb (24%), beef (23%), and chicken (5%). Participants in the highest quartile of plant sourced nitrate were more likely to be older, be more physically active, have completed University or an equivalent degree, and consume higher amounts of fish, vegetables, and fruits (Table 1). Over 5-year of follow-up, there was minimal change in source dependent nitrate intake (Median change [IQR]; plant-sourced nitrate: 0.97 [−13, 16] mg/day; vegetable-sourced nitrate: 1.32 [−10, 13] mg/day; animal-sourced nitrate 0.09 [−0.85, 1.16] mg/day; and processed meat nitrate: 0 [−0.38, 0.32] mg/day).
3.2 Association between nitrate intake and dementia-related mortality in the whole cohort
Over 17 years of follow-up, 93 (1.0%) dementia mortality cases were recorded out of 1,237 (13.5%) total deaths. Participants in quartile 4 of plant sourced nitrate intake (median intake of 98 mg/day) had a 58% lower risk of dementia-related mortality [Table 2; Model 2 HR (95% CI): 0.42 (0.21, 0.82)] and a 57% lower risk after further adjusting for dietary confounders [Table 2; Model 3b: 0.43 (0.22, 0.87)], compared to the participants in quartile 1 (median intake of plant sourced nitrate 35 mg/day). Similarly, for vegetable-sourced nitrate intake, participants in quartile 4 (median intake of 72 mg/day) had a 67% lower risk of dementia-related mortality [Table 2; Model 2: 0.33, (0.17, 0.64)] and a 66% lower risk after further adjusting for dietary confounders [Table 2; Model 3b: 0.34 (0.17, 0.66)], compared to those in quartile 1 (median intake 20 mg/day). In sensitivity analyses, the associations of plant- and vegetable-sourced nitrate with dementia-related mortality remained robust with model 3b that involved further adjustment for intakes of flavonoids, vitamin C, and fibre [HRQ4vsQ1 (CI 95%): 0.36 (0.16, 0.82) for plant-sourced nitrate, and 0.28 (0.14, 0.59) for vegetable-sourced nitrate]. There was no association observed for intake of animal-sourced nitrate and dementia-related mortality (Table 2). However, for processed meat-sourced nitrate intake, participants in quartile 4 (median intake of 1.93 mg/day) had double the risk of dementia-related mortality [Table 2; Model 3b HR (95% CI): 2.10 (1.07, 4.12)], compared to the participants in quartile 1 (median intake of processed meat-sourced nitrate 0.13 mg/day) after adjustment for dietary confounders. No effect modification was observed when analyses were stratified by sex. Participants in quartile 4 of vegetable-sourced nitrate had a lower risk of dementia-related mortality in both males [Model 2; HRQ4vsQ1 (95% CI): 0.38 (0.15, 0.97)] and females [0.32 (0.12, 0.84)].
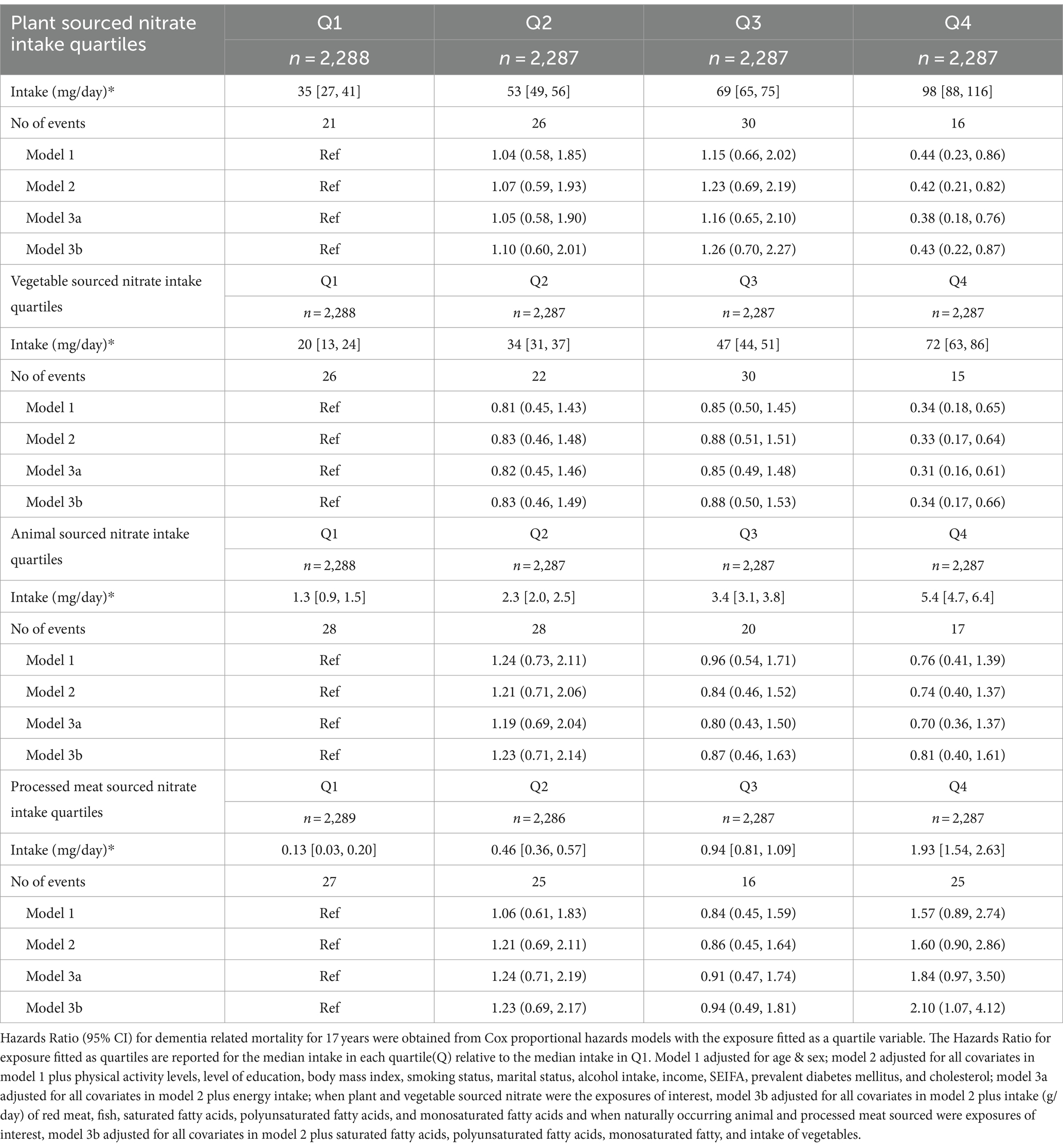
Table 2. Hazard ratios of dementia related mortality by quartiles of source-dependent dietary nitrate intake.
3.3 Association between nitrate intake and dementia-related mortality in participants with and without diabetes mellitus
Participants with DM (7.6%) and pre-diabetes (17.5%) were more likely to be older, were less likely to have completed a university or equivalent degree and were more likely to be male than participant without DM and pre-diabetes. (Supplementary Table 1). In participants with DM and pre-diabetes, a statistically significant 74 and 73% lower risk of dementia-related mortality was seen for participants with the highest, compared to the lowest, intakes of plant-sourced, and vegetable-sourced nitrate, respectively (Table 3; Model 2). For participants without DM, a statistically significant 67 and 72% lower risk of dementia-related mortality was seen for participants with the highest, compared to the lowest, intakes of vegetable-sourced, and naturally occurring animal-sourced nitrate, respectively (Table 3; Model 2 and Model 3a). On an absolute scale, participants with DM had a higher risk of dementia (Supplementary Table 2). The difference (quartile 4—quartile 1) in the 17-year predicted risk (adjusted for demographics and lifestyle risk factors) of dementia-related mortality was 1.43% for males with DM and 0.93% for males without DM, whilst for females with DM it was 2.31 and 0.47% for females without DM (Supplementary Table 2).
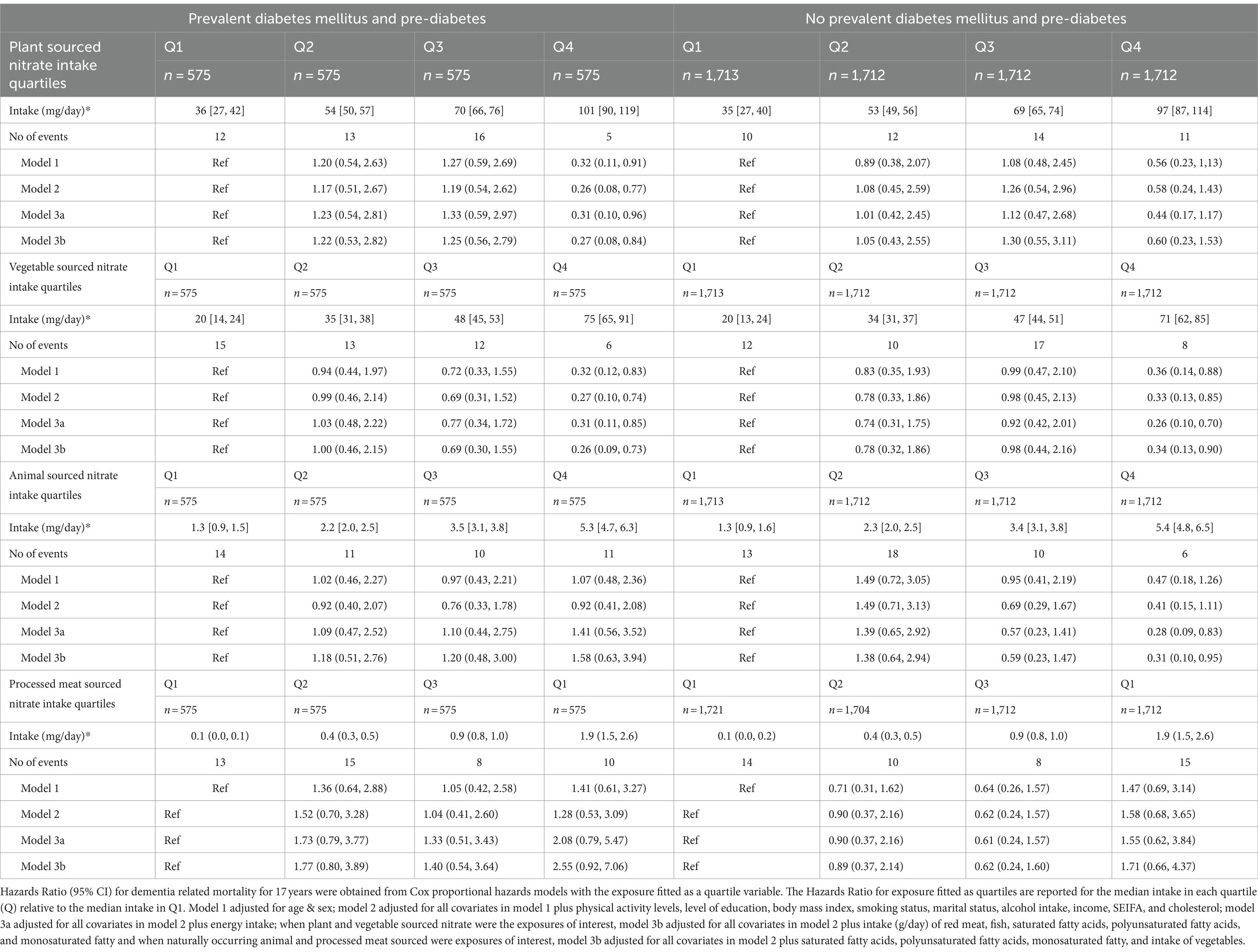
Table 3. Hazard ratios of dementia related mortality by quartiles of dietary nitrate intake stratified by prevalent diabetes mellitus and pre-diabetes.
4 Discussion
In this cohort study of 9,149 participants followed for up to 17 years, the habitual intake of plant- and vegetable-sourced nitrate was associated with a lower risk of dementia mortality, whilst processed meat-sourced nitrate intake was associated with a higher risk of dementia-related mortality. Furthermore, the inverse association between a habitual intake of vegetable-sourced nitrate and dementia-related mortality did not differ in participants with and without DM. Given that participants with DM are at a higher risk of dementia, our findings suggest that this may be an important group to target to increase their intake of nitrate-rich vegetables.
We observed that a higher plant- and vegetable-sourced nitrate intake was associated with a 58–67% lower risk of dementia mortality compared to participants with a low intake. To our knowledge, the association between different sources of nitrate intake and dementia-related mortality remained unexplored previously. However, a recent study in the population-based Rotterdam cohort consisting of 9,543 participants with a mean age of 64 years showed an association between vegetable-derived nitrate and lower risk of incident dementia [HR: 0.92 (0.86, 0.97)] over a mean follow-up period of 14.5 years (38). There is also mounting evidence that certain dietary patterns, namely the Mediterranean Diet (MedDiet), Combination of MedDiet-dietary approaches to stop hypertension (DASH) Intervention for Neurodegenerative Delay (MIND), and Japanese diets, are associated with a lower risk of dementia (39, 40). These dietary approaches have several protective components and are all high in plant-sourced dietary nitrate. A meta-analysis of studies investigating higher adherence to the Mediterranean Diet and risk of Alzheimer’s disease (AD), the most common dementia subtype, has reported an 11% lower risk of AD [Relative Risk (RR): 0.89 (0.84, 0.93)] compared to lower Mediterranean Diet adherence (41). A recent study of 60,298 participants from the United Kingdom Biobank observed a 23% lower risk of incident dementia [HR: 0.77 (0.65, 0.91)] in participants with higher MedDiet adherence after multivariable adjustment (42). The Australian Personality and Total Health (PATH) Through Life cohort with 12 years of follow-up reported a 53% lower risk of cognitive decline for participants in the highest tertile of MIND diet adherence compared to the lowest tertile [Odds Ratio (OR): 0.47 (0.24, 0.91)] (43). Furthermore, the Rush Memory and Ageing Project with an average follow-up period of 4.5 years observed that the participants in the highest tertile of MIND diet adherence had a 53% reduced risk of AD [HR: 0.47 (0.29, 0.76)], whilst participants in the middle tertile had a 35% lower risk of AD [HR: 0.65 (0.44, 0.98)]. Moreover, in the Ohsaki Cohort 2006 study with a follow-up period of 13 years, the authors reported that participants in the highest tertile of Japanese diet, a diet which comprises higher intake of seaweed, vegetables, and fish, had a 21% lower risk of incident dementia compared to the lowest tertile [HR: 0.79 (0.66, 0.95)] (40). Common components of MedDiet, MIND, and Japanese diet are green leafy vegetables and seaweed which are high in dietary nitrate.
The primary sources of dietary nitrate are plant-based foods (mainly vegetables), water, and meat. These sources differ in their nitrate content considerably, which is regulated in most countries. The Scientific Committee for Food (SCF), in 1997, and the Joint Food and Agriculture Organization/World Health Organization (WHO) Expert Committee on Food Additives (JECFA), in 2003, set the Acceptable Daily Intake (ADI) of nitrate as 0–3.7 mg/kg body weight (~260 mg/70 kg adult) on the basis of a chronic feeding study in rats with unpublished data (27). The European Food Safety Authority carried out a risk assessment of nitrate intake in 2008, which was reviewed and accepted in 2015 (44). Importantly, the ADI guidelines do not distinguish between sources of nitrate intake. The ADI of nitrate can be surpassed by intake of a single serve of nitrate-rich vegetables; for example, a single serve of rocket (80 g) comprises ~360 mg nitrate (28). Notably, clinical trials have observed that the ADI of nitrate of ~260 mg/day for a 70 kg adult was associated with beneficial effects on vascular function and blood pressure (45). Also, individuals following the DASH diet, which is rich in vegetables might consume ~1,000 mg/day of nitrate (46). Furthermore, a systematic review of 55 observational studies which assessed daily nitrate intake in adults, reported a median intake in healthy participants of 108 [87–145 mg/day] and for patient population of 110 [89–153] mg/day from studies that included individuals who developed diseases during follow-up (47). In Japan, high nitrate intake diets contain approximately 1,100 mg of nitrate/adult/day (48). However, the median nitrate intake in this Australian cohort (61 mg/day) is considerably less compared to both the ADI and the nitrate dose of ~260 mg/day demonstrated to have beneficial effects on the vascular function in clinical trials. This intake difference could explain why we only observed a lower risk of dementia related mortality in the highest quartile (98 mg/day). We observed a lower risk of dementia in the highest nitrate intake quartile, but such an association was not observed in the moderate nitrate intake quartile. Nevertheless, the median nitrate intake in this cohort was relatively low compared to other studies that investigated the association of dietary nitrate and cardiovascular disease: 61 mg/day vs. 79–128 mg/day (49–52), this is approximately one cup of raw green leafy vegetables or half a cup of cooked green leafy vegetables per day. Future studies are required to ascertain the optimal dosage of dietary nitrate to reduce the risk of dementia in an ageing population.
The mechanism via which dietary nitrate may positively impact the risk of dementia is hypothesised to be via effects on NO. Dietary nitrate improves endogenous NO levels via the nitrate-nitrite-NO-pathway, which is associated with beneficial effects on vascular health (53, 54). After ingestion of dietary nitrate, ~75% of nitrate is excreted through kidneys, whilst ~25% is taken up by salivary glands and converted to nitrite by the anaerobic bacteria present in the clefts of the tongue surface. The enteric bacterial nitrite reductase, along with low pH in the stomach, reduces nitrite to NO. The remaining nitrate and nitrite are recycled through the enterosalivary nitrate-nitrite-NO-pathway. Dietary nitrate has been found to reduce cardiovascular risk factors such as blood pressure, endothelial dysfunction, arterial stiffness, and platelet aggregation, by increasing NO through the nitrate-nitrite-NO pathway. A meta-analysis has supported the association between nitrate intake and cardiovascular health (55). Mid-life vascular risk factors have also been linked to late-life brain health, and brain vascular dysregulation has been suggested to be an early sign of AD (56, 57). Studies have also shown that endothelial-derived NO may prevent tau phosphorylation, which is a hallmark of AD (58).
We observed that a higher processed meat-sourced nitrate intake was associated with double the risk of dementia mortality compared to participants with a low intake. To our knowledge this is the first study to investigate nitrate intake from processed meat and dementia mortality. However, processed meat intake was observed to be associated with a higher risk of all-cause dementia cases in the United Kingdom Biobank cohort (59). The preservation of processed meat products with nitrate and nitrite salts is speculated to contribute to the negative health outcomes of processed meat consumption. Nitrate, through conversion to nitrite, can react with amines or amides to form genotoxic, neurodegenerative, and carcinogenic N-nitroso compounds. However, it should be noted that whether the observed association in this study is due to the presence of nitrate as an allowed additive in processed meat is unclear as processed meat contains other potential harmful compounds such as polycyclic aromatic hydrocarbons and heterocyclic aromatic amines (60), which could not be accounted for in the analyses. Notably, there was no evidence for detrimental effects of naturally occurring animal-sourced nitrate and dementia-related mortality in this study. Indeed, higher animal-sourced nitrate was associated with reduced risk amongst those without DM. However, we acknowledge that intakes of animal sourced nitrate were low compared to plant sourced nitrate in the cohort and so these findings must be interpreted with caution.
The link between DM and dementia is now well-established (61). Insulin resistance, a typical feature of type 2 DM, causes impaired glucose metabolism in the brain leading to chronic neuroinflammation (62). Furthermore, insulin resistance can contribute to formation of amyloid plaques and neurofibrillary tangles, pathological hallmarks of AD (63). The association between DM and dementia could also be due to the two-fold higher risk of a wide range of cardiovascular diseases in individuals with DM (64). As robust evidence from clinical trials demonstrates that intake of dietary nitrate is beneficial to cardiovascular health (45), data were stratified by presence of DM to explore if dietary nitrate confers protection against dementia in this high-risk population. The 17-year predicted risk of dementia-related mortality (adjusted for lifestyle-risk factors) for people with DM and pre-diabetes in the lowest vegetable-sourced nitrate intake quartile was ~3.42 (for females) or ~ 2.09 (for males) was higher than their counterparts in the highest vegetable-sourced nitrate intake quartile ~1.11 (for females) or ~ 0.66 (for males). Thus, we might expect to prevent more cases of dementia if people with DM and pre-diabetes increased their intake of nitrate-rich vegetables.
The current study has some limitations that require consideration when interpreting the findings. Primarily, we only identified dementia cases from administrative data from a single source, and are therefore likely to have missed incident dementia cases, potentially introducing selection and misclassification bias (65). Furthermore, given the observational study design, we cannot infer causality. We also cannot disregard any unmeasured confounding factors. There is also a possibility of recall bias since this is questionnaire-based dietary data and estimating nitrate intake from database may not include uncommon high nitrate foods, also, does not account for factors that determine nitrate levels of vegetables such as soil type, growing conditions, intensity of sunlight, and storage conditions. We do not attribute observed benefits entirely to the nitrate intake because the correlation between intake of vegetable-sourced nitrate and overall consumption of vegetables was strong (ρ = 0.79), which contain other beneficial compounds that can mitigate the risk of dementia-related mortality. Moreover, we only considered dietary intake data captured at baseline in our analyses. Any changes to dietary habits over time, would likely have attenuated the observed associations. Also, nitrate from water was not included as we did not know the nitrate levels of water the participants consumed. Additionally, other than being excluded at baseline for a dementia diagnosis, there was no information on cognitive impairment at baseline, which may have impacted ability to recall dietary habits accurately. Also, we could not adjust for carriage of the ε4 allele of the apolipoprotein E gene (APOE), the most common genetic risk factor for AD (66), and we could not distinguish between dementia subtypes. The current study is also limited by using dementia-related mortality as a proxy for dementia diagnosis. This meant only dementia in deceased participants was identified. Moreover, not all cases of dementia are identified on death certificates and there has been some increased identification on certificates over time (67).
Nevertheless, this cohort study has several strengths. The follow-up period of 17 years was of importance due to the prolonged nature of the dementia. The mean enrolment age (~49 years) together with the length of follow up has allowed for the examination of dietary nitrate intake from different sources in association with mid-life dementia risk factors. Consideration of mid-life risk factors along with exposure is of utmost importance in assessing late-life dementia risk as pathological changes begin to appear 10–20 years before the onset of clinical symptoms of dementia (68). Importantly, the association between higher nitrate intake and dementia-related mortality remained significant even with adjustment for dietary confounders and lifestyle factors. Finally, we used the latest comprehensive nitrate databases to calculate dietary nitrate intake from different sources (13, 28).
5 Conclusion
In this large cohort study, we observed that a higher habitual intake of plant-sourced nitrate, specifically from vegetables, was significantly associated with a lower risk of dementia-related mortality, whilst higher intakes of processed meat-sourced nitrate were associated with a higher risk of dementia. These findings suggest that encouraging the intake of nitrate-rich vegetables, may lower the risk of dementia-related mortality for those with and without (pre-) diabetes mellitus.
Data availability statement
The data analysed in this study are subject to the following licences/restrictions: the data presented in this article are not readily available since it belongs to the Baker Heart and Diabetes Institute. There are restrictions for the availability of these data, which were used under licence for the current study. Raw data are not publicly available. However, data described in the manuscript, codebook, and analytic code will be made available upon request and approval of the AusDiab Steering Committee. Requests to access these datasets should be directed to am9uYXRoYW4uc2hhd0BiYWtlci5lZHUuYXU=.
Ethics statement
The studies involving humans were approved by International Diabetes Institute Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AR: Conceptualization, Formal analysis, Methodology, Writing – original draft. NB: Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing. LZ: Writing – review & editing, Methodology. SR-B: Writing – review & editing. KM: Formal analysis, Writing – review & editing. SR-S: Supervision, Writing – review & editing. SG: Supervision, Writing – review & editing. LB: Writing – review & editing. DM: Writing – review & editing. JS: Writing – review & editing. RD: Writing – review & editing. KA: Writing – review & editing. JL: Writing – review & editing. JH: Supervision, Writing – review & editing. CB: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. For funding or logistical support, we are grateful to: National Health and Medical Research Council (NHMRC grant 233200), Australian Government Department of Health and Ageing. Abbott Australasia Pty Ltd., Alphapharm Pty Ltd., AstraZeneca, Bristol-Myers Squibb, City Health Centre-Diabetes Service-Canberra, Department of Health and Community Services—Northern Territory, Department of Health and Human Services—Tasmania, Department of Health—New South Wales, Department of Health—Western Australia, Department of Health—South Australia, Department of Human Services—Victoria, Diabetes Australia, Diabetes Australia Northern Territory, Eli Lilly Australia, Estate of the Late Edward Wilson, GlaxoSmithKline, Jack Brockhoff Foundation, Janssen-Cilag, Kidney Health Australia, Marian & FH Flack Trust, Menzies Research Institute, Merck Sharp & Dohme, Novartis Pharmaceuticals, Novo Nordisk Pharmaceuticals, Pfizer Pty Ltd., Pratt Foundation, Queensland Health, Roche Diagnostics Australia, Royal Prince Alfred Hospital, Sydney, Sanofi Aventis, Sanofi-Synthelabo, and the Victorian Government’s OIS Programme. AR is grateful for support provided by the Australian Government Research Training Programme (AGRTP) to pursue doctoral studies at Edith Cowan University, Australia. NB is funded by a National Health and Medical Research Council Early Career Fellowship (Grant number APP1159914). SR-S is supported by an NHMRC Investigator Grant (GNT1197315). LB is supported by an NHMRC of Australia Emerging Leadership Investigator Grant (ID: 1172987) and a National Heart Foundation of Australia Post-Doctoral Research Fellowship (ID: 102498). The salary of DM is supported by an NHMRC Investigator Grant (APP 2016668). The salary of JS is supported by an NHMRC Investigator Grant (APP 1173952). KA is supported by ARC Laureate Fellowship (FL190100011). The salary of JL is supported by a National Heart Foundation Future Leader Fellowship (ID: 102817). The salary of CB is supported by a Royal Perth Hospital Research Foundation ‘Lawrie Beilin’ Career Advancement Fellowship (ID: CAF 127/2020) and the Western Australian Future Health Research and Innovation Fund.
Acknowledgments
The AusDiab study, initiated and coordinated by the International Diabetes Institute, and subsequently coordinated by the Baker Heart and Diabetes Institute, gratefully acknowledges the support and assistance given by: A. Allman, B. Atkins, S. Bennett, S. Chadban, S. Colagiuri, M. de Courten, M. Dalton, M. D’Emden, D. Dunstan, T. Dwyer, D. Jolley, I. Kemp, P. Magnus, J. Mathews, D. McCarty, A. Meehan, K. O’Dea, P. Phillips, K. Polkinghorne, P. Popplewell, C. Reid, A. Stewart, R. Tapp, H. Taylor, A. Tonkin, T. Welborn, F. Wilson, P. Zimmet, and all the study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1327042/full#supplementary-material
References
1. GBD 2019 CollaboratorsNichols, E, Abd-Allah, F, Abdoli, A, Abosetugn, AE, Abrha, WA, et al. Global mortality from dementia: application of a new method and results from the global burden of disease study 2019. Alzheimers Dement. (2021) 7:e12200. doi: 10.1002/trc2.12200
2. Brown, L, Hansnata, E, and Hai Anh, LA (2017). Economic cost of dementia in Australia 2016-2056: report prepared for Alzheimer’s Australia. Alzheimer’s Australia. 84 p. Available at: https://www.dementia.org.au/files/NATIONAL/documents/The-economic-cost-of-dementia-in-Australia-2016-to-2056.pdf (Accessed November 27, 2022).
3. World Health Organization (2019). Dementia Report. Alzheimer Report. Available at: https://www.who.int/news/item/07-12-2017-dementia-number-of-people-affected-to-triple-in-next-30-years (Accessed November 27, 2022)
4. Livingston, G, Huntley, J, Sommerlad, A, Ames, D, Ballard, C, Banerjee, S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
5. Anstey, KJ, Ee, N, Eramudugolla, R, Jagger, C, and Peters, R. A systematic review of Meta-analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis. (2019) 70:S165–86. doi: 10.3233/JAD-190181
6. Lundberg, JO, and Govoni, M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. (2004) 37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027
7. Jin, RC, and Loscalzo, J. Vascular nitric oxide: formation and function. J Blood Med. (2010) 2010:147–62. doi: 10.2147/JBM.S7000
8. Toda, N, Ayajiki, K, and Okamura, T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. (2009) 61:62–97. doi: 10.1124/pr.108.000547
9. Förstermann, U, and Sessa, WC. Nitric oxide synthases: regulation and function. Eur Heart J. (2012) 33:829–37. doi: 10.1093/eurheartj/ehr304
10. Bondonno, CP, Blekkenhorst, LC, Liu, AH, Bondonno, NP, Ward, NC, Croft, KD, et al. Vegetable-derived bioactive nitrate and cardiovascular health. Mol Asp Med. (2018) 61:83–91. doi: 10.1016/j.mam.2017.08.001
11. Rajendra, A, Bondonno, NP, Murray, K, Zhong, L, Rainey-Smith, SR, Gardener, SL, et al. Habitual dietary nitrate intake and cognition in the Australian imaging, biomarkers and lifestyle study of ageing: a prospective cohort study. Clin Nutr. (2023) 42:1251–9. doi: 10.1016/j.clnu.2023.05.022
12. Blekkenhorst, LC, Prince, RL, Ward, NC, Croft, KD, Lewis, JR, Devine, A, et al. Development of a reference database for assessing dietary nitrate in vegetables. Mol Nutr Food Res. (2017) 61, 1600982. doi: 10.1002/mnfr.201600982
13. Zhong, L, Liu, AH, Blekkenhorst, LC, Bondonno, NP, Sim, M, Woodman, RJ, et al. Development of a food composition database for assessing nitrate and nitrite intake from animal-based foods. Mol Nutr Food Res. (2022) 66:e2100272. doi: 10.1002/mnfr.202100272
14. Bondonno, CP, Zhong, L, Bondonno, NP, Sim, M, Blekkenhorst, LC, Liu, A, et al. Nitrate: the Dr. Jekyll and Mr. Hyde of human health? Trends Food Sci Technol. (2023) 135:57–73. doi: 10.1016/j.tifs.2023.03.014
15. Spiegelhalder, B, Eisenbrand, G, and Preussmann, R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. (1976) 14:545–8. doi: 10.1016/s0015-6264(76)80005-3
16. de la Monte, SM, and Tong, M. Mechanisms of nitrosamine-mediated neurodegeneration: potential relevance to sporadic Alzheimer's disease. J Alzheimers Dis. (2009) 17:817–25. doi: 10.3233/JAD-2009-1098
17. Serafini, M, and Peluso, I. Functional foods for health: the interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr Pharm Des. (2016) 22:6701–15. doi: 10.2174/1381612823666161123094235
18. van Gennip, ACE, Stehouwer, CDA, van Boxtel, MPJ, Verhey, FRJ, Koster, A, Kroon, AA, et al. Association of Type 2 diabetes, according to the number of risk factors within target range, with structural brain abnormalities, cognitive performance, and risk of dementia. Diabetes Care. (2021) 44:2493–502. doi: 10.2337/dc21-0149
19. Whitmer, RA, Gilsanz, P, Quesenberry, CP, Karter, AJ, and Lacy, ME. Association of Type 1 diabetes and hypoglycemic and hyperglycemic events and risk of dementia. Neurology. (2021) 97:e275–83. doi: 10.1212/WNL.0000000000012243
20. Dunstan, DW, Zimmet, PZ, Welborn, TA, Cameron, AJ, Shaw, J, de Courten, M, et al. The Australian diabetes, obesity and lifestyle study (AusDiab)--methods and response rates. Diabetes Res Clin Pract. (2002) 57:119–29. doi: 10.1016/s0168-8227(02)00025-6
21. Rhee, JJ, Sampson, L, Cho, E, Hughes, MD, Hu, FB, and Willett, WC. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am J Epidemiol. (2015) 181:225–33. doi: 10.1093/aje/kwu308
22. Banna, JC, McCrory, MA, Fialkowski, MK, and Boushey, C. Examining plausibility of self-reported energy intake data: considerations for method selection. Front Nutr. (2015) 4:225–33. doi: 10.3389/fnut.2017.00045
23. Williams, JK, Smallwood, MJ, Benjamin, N, D'Souza, RJ, Shore, AC, Winyard, PG, et al. Renal nitrate clearance in chronic kidney disease. Nitric Oxide. (2020) 97:16–9. doi: 10.1016/j.niox.2020.01.011
24. Chen, TK, Knicely, DH, and Grams, ME. Chronic kidney disease diagnosis and management: a review. JAMA. (2019) 322:1294–304. doi: 10.1001/jama.2019.14745
25. Ireland, P, Jolley, D, Giles, G, O'Dea, K, Powles, J, Rutishauser, I, et al. Development of the Melbourne FFQ: a food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac J Clin Nutr. (1994) 3:19–31.
26. Gardener, SL, Rainey-Smith, SR, Macaulay, SL, Taddei, K, Rembach, A, Maruff, P, et al. Comparative analysis of the Cancer Council of Victoria and the online Commonwealth Scientific and Industrial Research Organisation FFQ. Br J Nutr. (2015) 114:1683–93. doi: 10.1017/S0007114515003335
27. World Health Organization (2022). Nitrate and Nitrite (WHO Food Additive series 50): 1–14 Available at: https://inchem.org/documents/jecfa/jecmono/v50je07.htm (Accessed November 2022).
28. Zhong, L, Blekkenhorst, LC, Bondonno, NP, Sim, M, Woodman, RJ, Croft, KD, et al. A food composition database for assessing nitrate intake from plant-based foods. Food Chem. (2022) 394:133411. doi: 10.1016/j.foodchem.2022.133411
29. Iqbal, R, Dehghan, M, Mente, A, Rangarajan, S, Wielgosz, A, Avezum, A, et al. Associations of unprocessed and processed meat intake with mortality and cardiovascular disease in 21 countries [prospective urban rural epidemiology (PURE) study]: a prospective cohort study. Am J Clin Nutr. (2021) 114:1049–58. doi: 10.1093/ajcn/nqaa448
30. Innes, K, Hooper, J, Bramley, M, and DahDah, P. Creation of a clinical classification: international statistical classification of diseases and related health problems—10th revision, Australian modification (ICD-10-AM). Health Inf Manag. (1997) 27:31–8. doi: 10.1177/183335839702700110
31. Dunstan, DW, Zimmet, PZ, Welborn, TA, Sicree, RA, Armstrong, TP, Atkins, RC, et al. (2000). Diabesity and Associated Disorders in Australia: The Accelerating Epidemic: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Available at: https://www.baker.edu.au/-/media/documents/impact/ausdiab/reports/ausdiab-report-2000.pdf?la=en (Accessed November 27, 2022)
32. Cameron, AJ, Welborn, TA, Zimmet, PZ, Dunstan, DW, Owen, N, Salmon, J, et al. Overweight and obesity in Australia: the 1999-2000 Australian diabetes, obesity and lifestyle study (AusDiab). Med J Aust. (2003) 178:427–32. doi: 10.5694/j.1326-5377.2003.tb05283.x
33. Armstrong, T, Bauman, A, and Davies, J. Physical Activity Patterns of Australian Adults. Results of the 1999 National Physical Activity Survey. Canberra: Australian Institute of Health and Welfare (2000).
34. Tanamas, SK, Magliano, DJ, Lynch, B, Sethi, P, Willenberg, L, Polkinghorne, KR, et al. (2012). The Australian Diabetes, Obesity and Lifestyle Study. Melbourne: Baker IDI Heart and Diabetes Institute. Available at: https://www.baker.edu.au/-/media/documents/impact/ausdiab/reports/ausdiab-report-2012.pdf?la=en (Accessed September 10, 2022).
35. Barr, ELM, Magliano, DJ, Zimmet, PZ, Polkinghorne, KR, Atkins, RC, Dunstan, DW, et al. (2005). The Australian diabetes, obesity and lifestyle study—tracking the accelerating epidemic: its causes and outcomes. International Diabetes Institute. Melbourne. Available at: https://www.baker.edu.au/Assets/Files/AUSDIAB_REPORT_2005.pdf (Accessed November 27, 2022)
36. Dalton, M, Cameron, AJ, Zimmet, PZ, Shaw, JE, Jolley, D, Dunstan, DW, et al. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. (2003) 254:555–63. doi: 10.1111/j.1365-2796.2003.01229.x
37. Australian Bureau of Statistics (2011). Socio-economic indexes for areas (SEIFA) Australia: postal areas, index of relative socio-economic advantage and disadvantage.
38. de Crom, TOE, Blekkenhorst, L, Vernooij, MW, Ikram, MK, Voortman, T, and Ikram, MA. Dietary nitrate intake in relation to the risk of dementia and imaging markers of vascular brain health: a population-based study. Am J Clin Nutr. (2023) 118:352–9. doi: 10.1016/j.ajcnut.2023.05.027
39. van den Brink, AC, Brouwer-Brolsma, EM, Berendsen, AAM, and van de Rest, O. The Mediterranean, dietary approaches to stop hypertension (DASH), and Mediterranean-DASH intervention for neurodegenerative delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer's disease-a review. Adv Nutr. (2019) 10:1040–65. doi: 10.1093/advances/nmz054
40. Matsuyama, S, Shimazu, T, Tomata, Y, Zhang, S, Abe, S, Lu, Y, et al. Japanese diet and mortality, disability, and dementia: evidence from the Ohsaki cohort study. Nutrients. (2022) 14:2034. doi: 10.3390/nu14102034
41. García-Casares, N, Gallego Fuentes, P, Barbancho, MÁ, López-Gigosos, R, García-Rodríguez, A, and Gutiérrez-Bedmar, M. Alzheimer’s disease, mild cognitive impairment and Mediterranean diet. A systematic review and dose-response meta-analysis. J Clin Med. (2021) 10:4642. doi: 10.3390/jcm10204642
42. Shannon, OM, Ranson, JM, Gregory, S, Macpherson, H, Milte, C, Lentjes, M, et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: findings from the UK biobank prospective cohort study. BMC Med. (2023) 21:81. doi: 10.1186/s12916-023-02772-3
43. Hosking, DE, Eramudugolla, R, Cherbuin, N, and Anstey, KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. (2019) 15:581–9. doi: 10.1016/j.jalz.2018.12.011
44. EFSA (2017). European Food Safety Authority. EFSA confirms safe levels for nitrites and nitrates added to food. Available at: https://www.efsa.europa.eu/en/press/news/170615 (Accessed November 22 2022).
45. Blekkenhorst, LC, Bondonno, NP, Liu, AH, Ward, NC, Prince, RL, Lewis, JR, et al. Nitrate, the oral microbiome, and cardiovascular health: a systematic literature review of human and animal studies. Am J Clin Nutr. (2018) 107:504–22. doi: 10.1093/ajcn/nqx046
46. Hord, NG, Tang, Y, and Bryan, NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. (2009) 90:1–10. doi: 10.3945/ajcn.2008.27131
47. Babateen, AM, Fornelli, G, Donini, LM, Mathers, JC, and Siervo, M. Assessment of dietary nitrate intake in humans: a systematic review. Am J Clin Nutr. (2018) 108:878–88. doi: 10.1093/ajcn/nqy108
48. Sobko, T, Marcus, C, Govoni, M, and Kamiya, S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. (2010) 22:136–40. doi: 10.1016/j.niox.2009.10.007
49. Blekkenhorst, LC, Bondonno, CP, Lewis, JR, Devine, A, Woodman, RJ, Croft, KD, et al. Association of dietary nitrate with atherosclerotic vascular disease mortality: a prospective cohort study of older adult women. Am J Clin Nutr. (2017) 106:207–16. doi: 10.3945/ajcn.116.146761
50. Bondonno, CP, Blekkenhorst, LC, Prince, RL, Ivey, KL, Lewis, JR, Devine, A, et al. Association of Vegetable Nitrate Intake with Carotid Atherosclerosis and Ischemic Cerebrovascular Disease in older women. Stroke. (2017) 48:1724–9. doi: 10.1161/STROKEAHA.117.016844
51. Jackson, JK, Patterson, AJ, MacDonald-Wicks, LK, Forder, PM, Blekkenhorst, LC, Bondonno, CP, et al. Vegetable nitrate intakes are associated with reduced self-reported cardiovascular-related complications within a representative sample of middle-aged Australian women, prospectively followed up for 15 years. Nutrients. (2019) 11:240. doi: 10.3390/nu11020240
52. Liu, AH, Bondonno, CP, Russell, J, Flood, VM, Lewis, JR, Croft, KD, et al. Relationship of dietary nitrate intake from vegetables with cardiovascular disease mortality: a prospective study in a cohort of older Australians. Eur J Nutr. (2019) 58:2741–53. doi: 10.1007/s00394-018-1823-x
53. Lundberg, JO, Weitzberg, E, and Gladwin, MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. (2008) 7:156–67. doi: 10.1038/nrd2466
54. Bondonno, CPYX, Croft, KD, Considine, MJ, Ward, NC, Rich, L, Puddey, IB, et al. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: a randomized controlled trial. Free Radic Biol Med. (2012) 52:95–102. doi: 10.1016/j.freeradbiomed.2011.09.028
55. Jackson, JK, Patterson, AJ, MacDonald-Wicks, LK, Oldmeadow, C, and McEvoy, MA. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: a systematic review and meta-analysis of human evidence. Nutr Rev. (2018) 76:348–71. doi: 10.1093/nutrit/nuy005
56. Lane, CA, Barnes, J, Nicholas, JM, Sudre, CH, Cash, DM, Malone, IB, et al. Associations between vascular risk across adulthood and brain pathology in late life: evidence from a British birth cohort. JAMA Neurol. (2020) 77:175–83. doi: 10.1001/jamaneurol.2019.3774
57. Sweeney, MD, Kisler, K, Montagne, A, Toga, AW, and Zlokovic, BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. (2018) 21:1318–31. doi: 10.1038/s41593-018-0234-x
58. Faraco, G, Hochrainer, K, Segarra, SG, Schaeffer, S, Santisteban, MM, Menon, A, et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature. (2019) 574:686–90. doi: 10.1038/s41586-019-1688-z
59. Zhang, H, Greenwood, DC, Risch, HA, Bunce, D, Hardie, LJ, and Cade, JE. Meat consumption and risk of incident dementia: cohort study of 493,888 UK biobank participants. Am J Clin Nutr. (2021) 114:175–84. doi: 10.1093/ajcn/nqab028
60. Clinton, SK, Giovannucci, EL, and Hursting, SD. The World Cancer Research Fund/American Institute for Cancer Research third expert report on diet, nutrition, physical activity, and Cancer: impact and future directions. J Nutr. (2020) 150:663–71. doi: 10.1093/jn/nxz268
61. Nguyen, TT, Ta, QTH, Nguyen, TKO, Nguyen, TTD, and Giau, VV. Type 3 diabetes and its role implications in Alzheimer's disease. Int J Mol Sci. (2020) 21:3165. doi: 10.3390/ijms21093165
62. Lee, SH, Park, SY, and Choi, CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2022) 46:15–37. doi: 10.4093/dmj.2021.0280
63. Kellar, D, and Craft, S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. (2020) 19:758–66. doi: 10.1016/S1474-4422(20)30231-3
64. Sarwar, N, Gao, P, Seshasai, SR, Gobin, R, Kaptoge, S, Di Angelantonio, E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. (2010) 375:2215–22. doi: 10.1016/S0140-6736(10)60484-9
65. Zilkens, RR, Spilsbury, K, Bruce, DG, and Semmens, JB. Linkage of hospital and death records increased identification of dementia cases and death rate estimates. Neuroepidemiology. (2009) 32:61–9. doi: 10.1159/000170908
66. Michaelson, DM . APOE ε4: the most prevalent yet understudied risk factor for Alzheimer's disease. Alzheimers Dement. (2014) 10:861–8. doi: 10.1016/j.jalz.2014.06.015
67. Adair, T, Temple, J, Anstey, KJ, and Lopez, AD. Is the rise in reported dementia mortality real? Analysis of multiple-cause-of-death data for Australia and the United States. Am J Epidemiol. (2022) 191:1270–9. doi: 10.1093/aje/kwac047
Keywords: dietary nitrate, dementia, diet, diabetes, cohort
Citation: Rajendra A, Bondonno NP, Zhong L, Radavelli-Bagatini S, Murray K, Rainey-Smith SR, Gardener SL, Blekkenhorst LC, Magliano DJ, Shaw JE, Daly RM, Anstey KJ, Lewis JR, Hodgson JM and Bondonno CP (2024) Plant but not animal sourced nitrate intake is associated with lower dementia-related mortality in the Australian Diabetes, Obesity, and Lifestyle Study. Front. Nutr. 11:1327042. doi: 10.3389/fnut.2024.1327042
Edited by:
João Laranjinha, University of Coimbra, PortugalReviewed by:
Barbara Rocha, University of Coimbra, PortugalAnna Kiss, University of Szeged, Hungary
Emmanouella Magriplis, Agricultural University of Athens, Greece
Copyright © 2024 Rajendra, Bondonno, Zhong, Radavelli-Bagatini, Murray, Rainey-Smith, Gardener, Blekkenhorst, Magliano, Shaw, Daly, Anstey, Lewis, Hodgson and Bondonno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine P. Bondonno, Yy5ib25kb25ub0BlY3UuZWR1LmF1
 Anjana Rajendra1
Anjana Rajendra1 Nicola P. Bondonno
Nicola P. Bondonno Liezhou Zhong
Liezhou Zhong Simone Radavelli-Bagatini
Simone Radavelli-Bagatini Kevin Murray
Kevin Murray Samantha L. Gardener
Samantha L. Gardener Lauren C. Blekkenhorst
Lauren C. Blekkenhorst Jonathan E. Shaw
Jonathan E. Shaw Kaarin J. Anstey
Kaarin J. Anstey Joshua R. Lewis
Joshua R. Lewis Jonathan M. Hodgson
Jonathan M. Hodgson Catherine P. Bondonno
Catherine P. Bondonno