- 1College of Nursing, University of New Mexico Health Sciences Center, Albuquerque, NM, United States
- 2Pioneer Valley Life Sciences Institute, Baystate Medical Center, Springfield, MA, United States
- 3Department of Veterinary and Animal Sciences, University of Massachusetts Amherst, Amherst, MA, United States
- 4Department of Sociology, University of South Carolina, Columbia, SC, United States
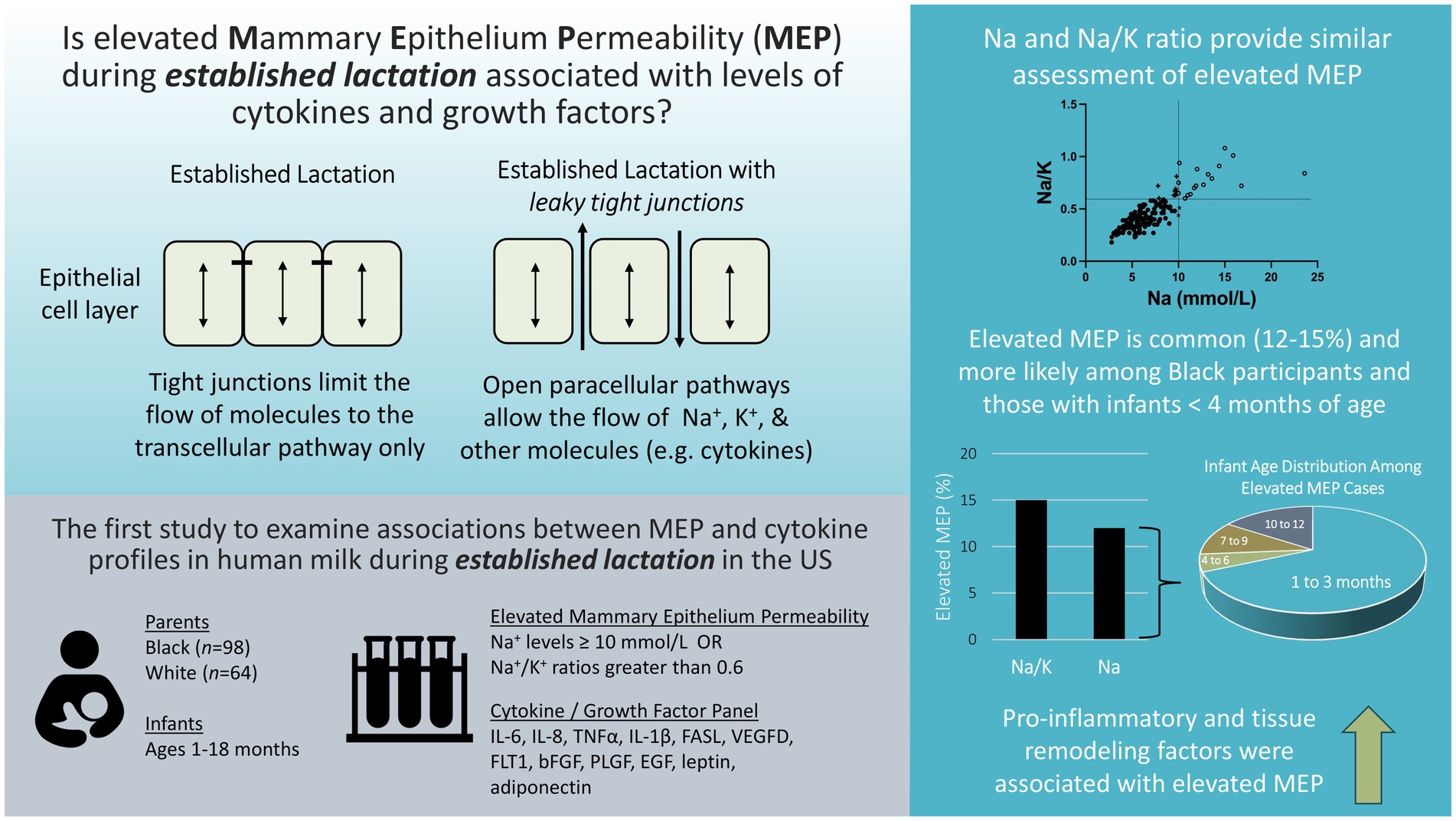
Objective: The cytokine profile of human milk may be a key indicator of mammary gland health and has been linked to infant nutrition, growth, and immune system development. The current study examines the extent to which mammary epithelium permeability (MEP) is associated with cytokine profiles during established lactation within a sample of US mothers.
Methods: Participants were drawn from a previous study of human milk cytokines. The present analysis includes 162 participants (98 Black, 64 White) with infants ranging from 1 to 18 months of age. Levels of cytokines were determined previously. Here we measure milk sodium (Na) and potassium (K) levels with ion-selective probes. Two approaches were used to define elevated MEP: Na levels ≥10 mmol/L and Na/K ratios greater than 0.6. Associations between maternal–infant characteristics, elevated MEP, and twelve analytes (IL-6, IL-8, TNFα, IL-1β, FASL, VEGFD, FLT1, bFGF, PLGF, EGF, leptin, adiponectin) were examined using bivariate associations, principal components analysis, and multivariable logistic regression models.
Results: Elevated MEP was observed in 12 and 15% of milk samples as defined by Na and Na/K cutoffs, respectively. The odds of experiencing elevated MEP (defined by Na ≥ 10 mmol/L) were higher among Black participants and declined with older infant age. All cytokines, except leptin, were positively correlated with either Na or the Na/K ratio. A pro-inflammatory factor (IL-6, IL-8, TNFα, IL-1β, EGF) and a tissue remodeling factor (FASL, VEGFD, FLT1, bFGF, PLGF, adiponectin) each contributed uniquely to raising the odds of elevated MEP as defined by either Na or the Na/K ratio.
Conclusion: This exploratory analysis of MEP and cytokine levels during established lactation indicates that elevated MEP may be more common in US populations than previously appreciated and that individuals identifying as Black may have increased odds of experiencing elevated MEP based on current definitions. Research aimed at understanding the role of MEP in mammary gland health or infant growth and development should be prioritized.
1 Introduction
Human milk is a complex biological fluid containing a multitude of cellular and molecular components integral to the health or disease state of the mother and infant. In particular, inflammatory markers in human milk appear to be associated with infant nutrition (1, 2), growth (3, 4), and immune system development (5, 6), and may be useful indicators of current (7, 8) and future mammary gland health (9, 10).
Human milk contains a variety of cytokines that act locally in the mammary gland and/or influence the growth and development of infant tissues (11). Cytokines represent a large class of secreted bioactive molecules that modulate cell-to-cell communication to impact cellular growth, viability, and differentiation, as well as immune and inflammatory responses (5). In human milk, cytokines are produced by leukocytes that have migrated into the mammary tissue from systemic circulation as well as by tissue-resident cells, such as mammary epithelium, fibroblasts, and adipocytes (5, 12). Specific cytokines are upregulated during mammary gland differentiation during pregnancy, in response to milk stasis, and during involution (13, 14). This upregulation is distinct from the rise in pro-inflammatory cytokines observed during an infection, such as mastitis. In the setting of infection, pro-inflammatory cytokines modulate the immune response of the mammary gland (7, 8).
Adipokines, such as leptin and adiponectin, are a subset of cytokines produced by adipose tissue that serve as endocrine signaling molecules with roles in regulating metabolism and body composition (11, 12). Both also play an important role in modulating mammary gland development and tissue remodeling during the lactation cycle (15). Growth factors are a class of cytokines linked to tissue growth and remodeling. They may retain bioactivity after ingestion and are important for the development of the infant intestinal barrier (11). Within the mammary gland, growth factors are important for angiogenesis, maintaining lactation, and regulating involution (16, 17).
Measurement of mammary epithelium permeability (MEP) may provide important information for the interpretation of cytokine concentrations in human milk. Prior to secretory activation (i.e., onset of mature milk), paracellular pathways between mammary epithelial cells are open allowing communication between the maternal bloodstream and the mammary gland (18). Following birth, rapid tight junction formation within the mammary epithelium facilitates paracellular pathway closure, a shift important for the establishment and maintenance of milk synthesis and secretion (19, 20). The paracellular pathway is particularly important for the movement of ions across the mammary epithelium. For example, sodium (Na) levels are high in colostrum, but decrease rapidly during the first 5 days postpartum in response to paracellular pathway closure. It has been proposed that once Na levels decrease below 10 mmol/L, milk maturity has been achieved (18, 21). In contrast, potassium (K) accumulates in milk as paracellular pathways close (18, 22). Both Na alone and as a ratio with potassium (Na/K) have been used to assess MEP. Na/K ratios of less than 0.6 have been used to indicate tight junction closure and milk maturity (18).
As long as the paracellular pathways remain closed during established lactation, milk secretion is maintained and levels of Na and the Na/K ratio remain low (19). However, both milk accumulation and inflammation have been linked to the re-opening of tight junctions and rising Na or Na/K ratios (19, 23). Our lab recently demonstrated that shifts in the Na/K ratio were closely linked to anti-SARS-CoV-2 antibody levels in human milk within a single individual over time, confirming an association between permeability and immune factors (24). Knowledge of MEP could provide important context for the interpretation of cytokine levels in human milk.
We previously examined levels of human milk cytokines in a cohort of Black and White participants during established lactation to determine associations with obesity, race, and risk factors for breast cancer (24). Of note, certain pro-inflammatory cytokines (IL-1β, FASL), growth factors (bFGF, EGF), and adipokines (leptin, adiponectin) were elevated among participants with a BMI >30. Levels of 1L-1β and leptin were found to be higher in Black participants.
The goals of the current study were (1) to determine the extent to which MEP as indicated by Na and Na/K ratios is associated with cytokine profiles in human milk during established lactation and (2) to determine if race or BMI are associated with elevated MEP. We hypothesized that higher levels of human milk pro-inflammatory cytokines would be associated with elevated permeability and that similar to observed patterns for the cytokines listed above, elevated MEP would be more common among participants with Black race or a BMI >30.
2 Methods
2.1 Study population
This is a secondary analysis of selected data from a study examining racial differences in cytokines in human milk in relation to breast cancer etiology (24). For the present study, we selected participants for whom archived whole milk samples were available. All participants had signed a consent form for a study approved by the Institutional Review Board of the University of Massachusetts Amherst (#749). Briefly, for the original study, all participants completed questionnaires on demographics and health history. Milk samples were collected between 2007 and 2013 from lactating females aged 18 years and older living in the continental United States (US). Participants had collected milk in the morning upon waking by expressing the full contents of each breast into separate glass or BPA-free plastic containers via hand expression or use of their own pump. Archived aliquots of whole milk used in this project had been stored at −20°C. Descriptive statistics regarding collection and storage and presented in Supplementary Table S1.
Of the 292 participants in the original study, frozen aliquots of whole milk were available from 167 participants (24). Of these, 5 mother-infant dyads were identified as being more than 3 SD above the mean for infant age (>798 days or 2.18 years of age). Breastfeeding practices employed by these participants were not typical of the remainder of the sample and were therefore excluded. Our final sample consisted of 162 participants (98 Black, 64 White) with infants ranging in age from 1 to 18 months.
2.2 Measurement of sodium and potassium ions
Levels of Na and K were measured using ion-selective electrode probes (Medica EasyLyte Na/K Analyzer) in 2022, providing new data for the current analysis. Briefly, 1 mL aliquots of whole milk were thawed, centrifuged at 3220 g for 3 min at 4°C and the Na and K concentrations (mmol/L) were determined in the clarified whey fraction. A total of 18 samples were run in duplicate with mean coefficients of variation (CV) of 7.8% for Na and 4.4% for K. A ratio was calculated between Na and K for each participant. Using Mann–Whitney U tests, neither Na nor the Na/K ratio were associated with prescription medication use, over-the-counter pain medication use, or shipping methods, ps < 0.10. Spearman rank correlations with years frozen at −20°C, and number of freeze–thaw cycle were also non-significant, ps < 0.10.
2.3 Measurement of cytokines and growth factors
Assays for pro-inflammatory cytokines, growth factors, and adipokines were performed in the original study (24). Briefly, multiplex and single-analyte electrochemical-luminescent sandwich assays from MesoScale Discovery (MSD, Gaithersburg, MD) were used to measure 15 analytes, of which 12 were selected for the present analysis and are shown in Table 1. IFN, TIE-2, and VEGFC were excluded due to low detectability (<35%). Thirty-eight samples, eight standards, and two of three controls were tested in duplicate on each plate according to the manufacturer’s protocols. The lower limit of detection (LLOD) for each included analyte was determined empirically, as previously reported in Murphy et al. (25), and is also reported in Table 1. Coefficients of variation (CVs) and intraclass correlation coefficients (ICCs) are also available in Murphy et al. (24).
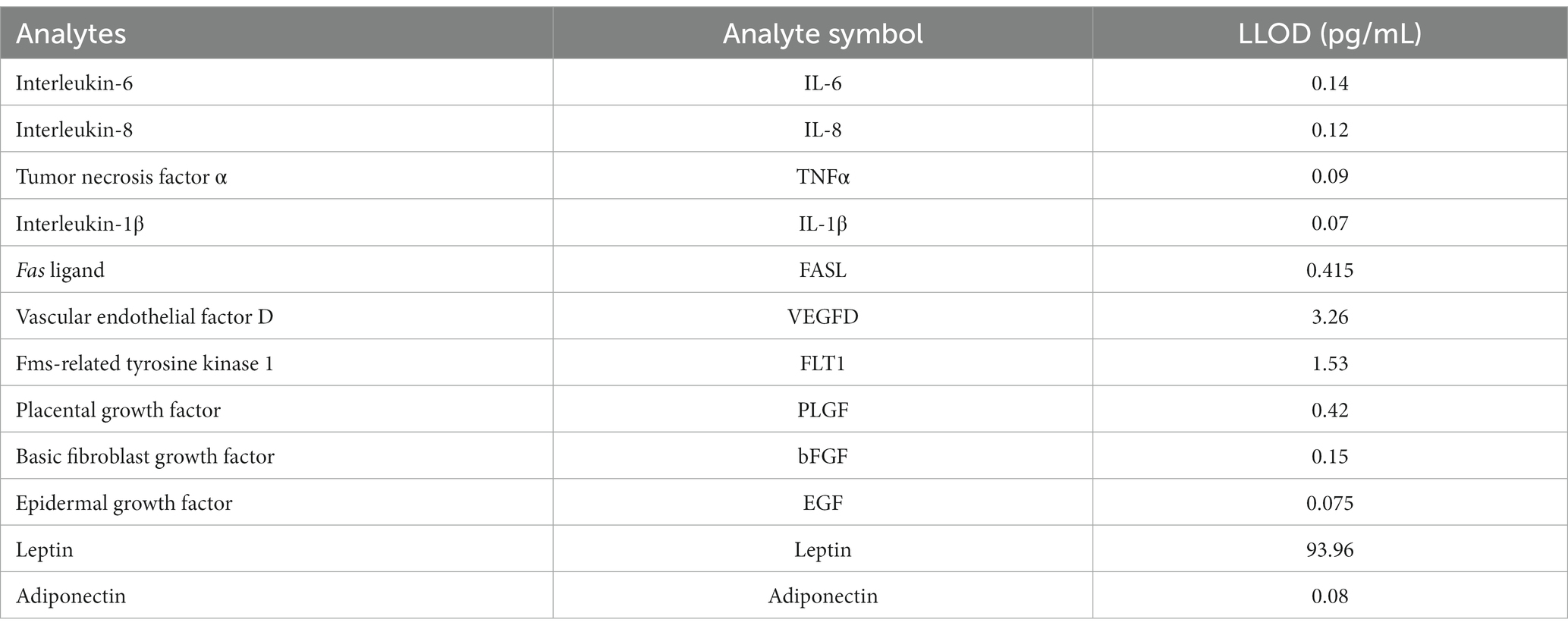
Table 1. Lower limits of detection (LLOD) for human milk analytes from Murphy et al. (24).
Mean concentrations of human milk analytes were calculated for all 162 samples with duplicate values above the LLOD. The percent of mean values below the LLOD was calculated for each analyte. Values below the LLOD were imputed with the LLOD/2. Per Keizer et al. (26), single imputation with LLOD/2 has equivalent performance to maximum likelihood imputation when less than 10% of the sample is missing. For the 162 participants included in the current study, the majority of analytes (8 of 12) had less than 10% of samples below the LLOD. However, IL-6, IL-1β, TNFα, and bFGF ranged from 13 to 20% of values below the LLOD.
2.4 Statistical analysis
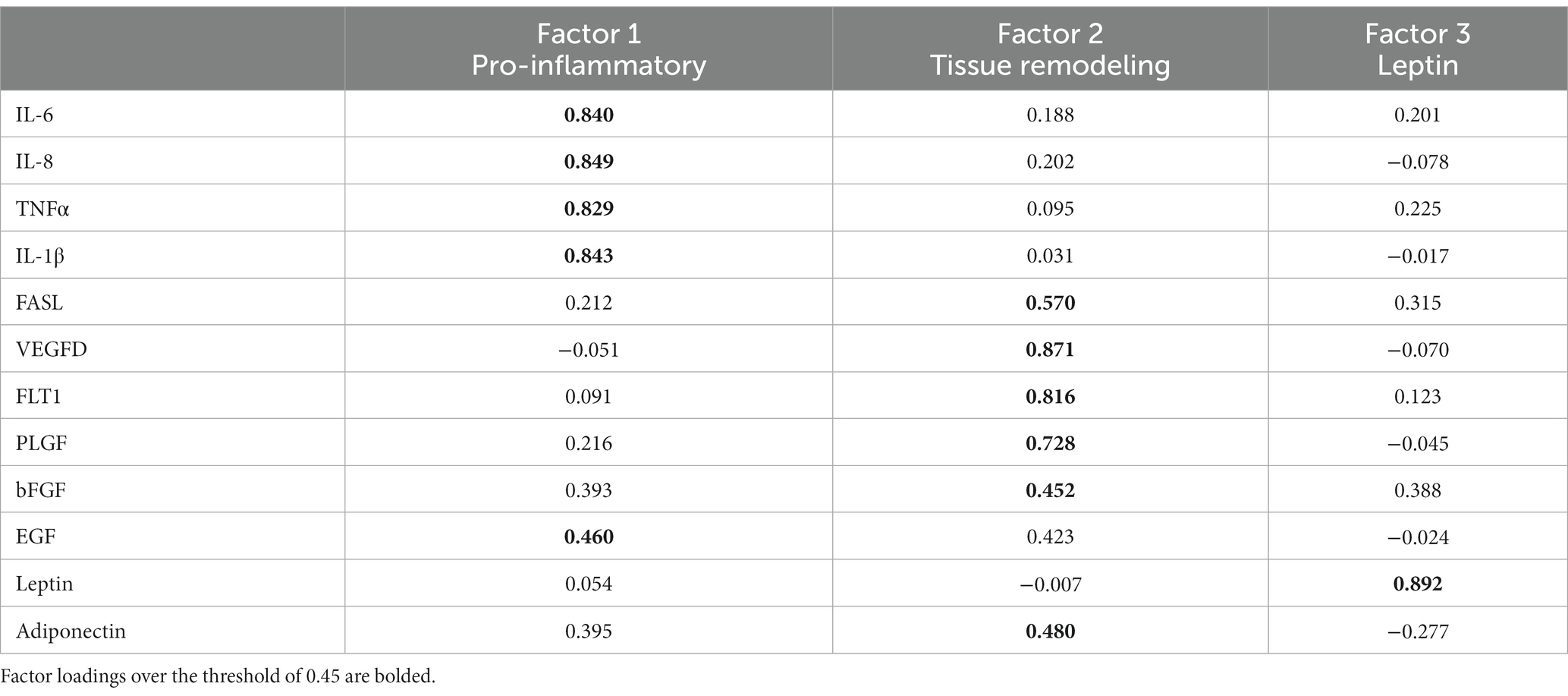
Data were cleaned and coded. All analyses were performed in SPSS 28.0. Descriptive statistics were examined. Spearman rank correlations and Mann–Whitney U tests were performed to explore bivariate associations. Two definitions of elevated MEP were examined in our dataset: Na ≥ 10 mmol/L (18, 21), and Na/K ratio ≥ 0.6 (18). Multivariable logistic regression models were fitted to predict increased MEP from maternal–infant factors. Principal component analysis with varimax rotation was used to identify affinities between pro-inflammatory cytokines, growth factors, and adipokines after normalizing (natural log) and centering analytes. Based on sample size, 0.45 was designated as the threshold for factor loadings (27). Factor scores were computed based on these results. Multivariable logistic regression models were fitted to examine factor scores as predictors of elevated MEP.
3 Results
3.1 Descriptive statistics for Na and the Na/K ratio
Descriptive statistics for Na, K, and Na/K ratios are presented in Table 2. Median concentrations of Na and K were 6.2 mmol/L (range 2.8–23.6) and 14.6 mmol/L (range 10.1–28.1) respectively. The median Na/K ratio in this sample was 0.41 with a range of 0.18–1.08. Na and the Na/K ratio were strongly correlated, r(160) = 0.79, p < 0.001. Two sets of criteria for identifying elevated MEP were examined. Using the criteria defined in the literature of 10 mmol/L for Na (18, 21), the cutoff for elevated MEP was at the 90th percentile and a total of 19 cases of elevated MEP were identified. Using the criteria of 0.6 for the Na/K ratio (18), the cutoff for elevated MEP was at the 85th percentile and 25 cases were identified. A total of 17 cases were identified by both criteria. Two cases were identified by Na ≥ 10 mmol/L alone, while 8 cases were identified solely by Na/K ≥ 0.6 (Figure 1).
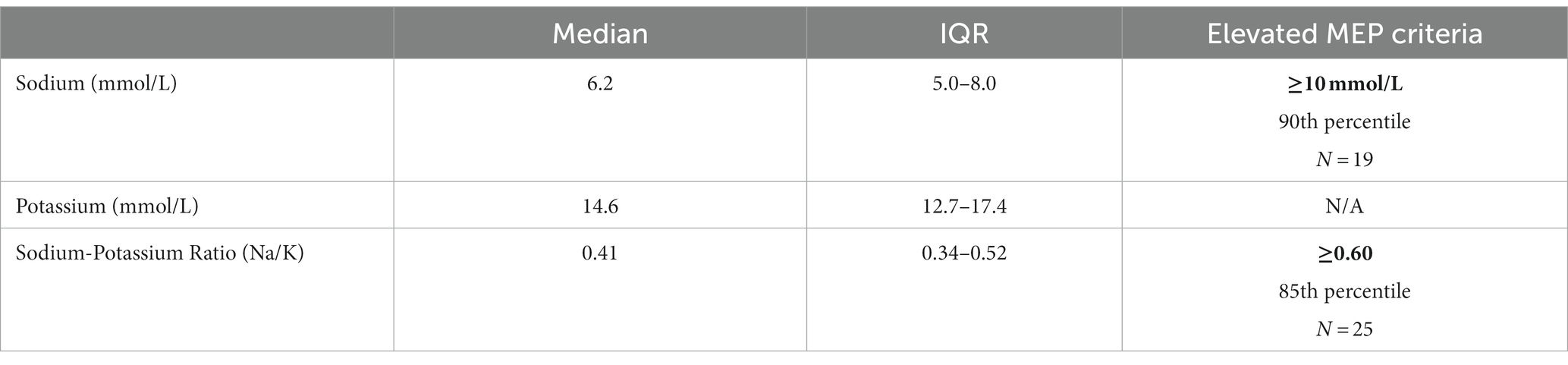
Table 2. Descriptive statistics for Na, K, and the Na/K ratio (N = 162) and criteria for defining elevated MEP (cutoffs bolded) (18).
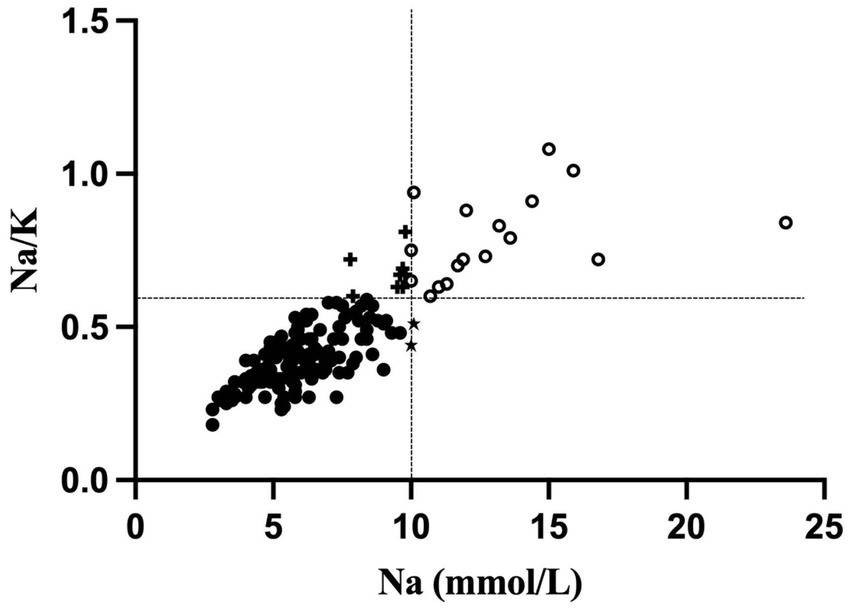
Figure 1. Scatterplot of sodium (Na; mmol/L) and the sodium-potassium (Na/K) ratio. Elevated MEP cases identified only by a Na level ≥ 10 mmol/L are indicated with a star (n = 2). Elevated MEP cases identified only by a Na/K ratio ≥ 0.6 are indicated with a plus sign (n = 8). Elevated MEP cases identified by both criteria are located in the right upper quadrant and are shown as open circles.
3.2 Associations of MEP with mother-infant characteristics
Demographic and selected health characteristics of the 162 participants for the whole sample and by participant race are presented in Table 3. There was a weak negative association between maternal age and Na, r(160) = −0.19, p < 0.05, and the Na/K ratio, r(160) = −0.18, p < 0.05. Human milk Na levels also declined with older infant age, r(160) = −0.51, p < 0.001, but this association was not observed for the Na/K ratio. The majority of cases of elevated MEP occured in infants less than 4 months of age: 68% of cases identified by Na ≥ 10 mmol/L and 52% by Na/K ≥ 0.6.
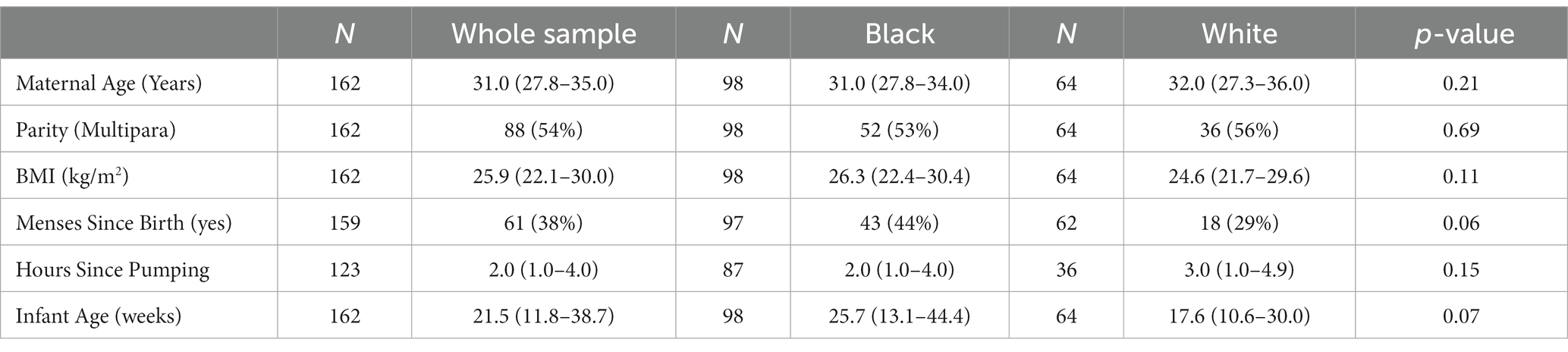
Table 3. Median (IQR) or N (%) for maternal–infant characteristics for the entire sample (N = 162) and by participant race.
Maternal and infant characteristics were evaluated in multivariable logistic regression models predicting elevated MEP. Infant age and maternal race were significant predictors in the model predicting elevated MEP as indicated by Na ≥ 10 mmol/L (Table 4). For every additional week of infant age, the odds of elevated MEP as defined by Na declined by 4%. Black participants had a higher likelihood of experiencing elevated MEP by 3.3 times. BMI over 30 was not associated with elevated MEP as indicated by Na ≥ 10 mmol/L. No maternal or infant characteristics, including race or BMI, were significant in the model predicting elevated MEP as defined by the Na/K ratio ≥ 0.6. Medication use, shipping method, nor storage conditions were significant predictors of elevated MEP as indicated by Na or Na/K.
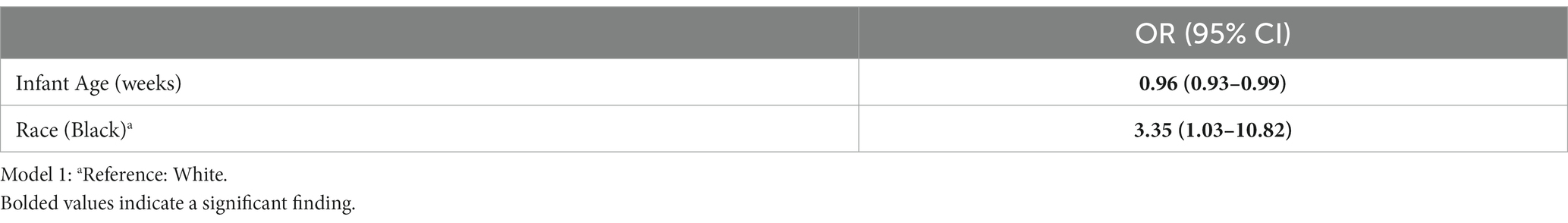
Table 4. Multivariable logistic regression model predicting elevated mammary epithelium permeability (MEP) as indicated by Na ≥ 10 mm/L (n = 162).
3.3 Human milk cytokines and associations with MEP
Descriptive statistics for the human milk cytokines are presented in Table 5. Both Na and the Na/K ratio had several significant positive associations with the selected cytokines. Nine analytes each were positively correlated with Na or the Na/K ratio, although the specific analytes varied by MEP indicator (see Table 5). Only leptin was not associated with either Na or the Na/K ratio. The overall pattern suggested that Na was more strongly associated with growth factors, while the Na/K ratio was more strongly associated with pro-inflammatory cytokines.
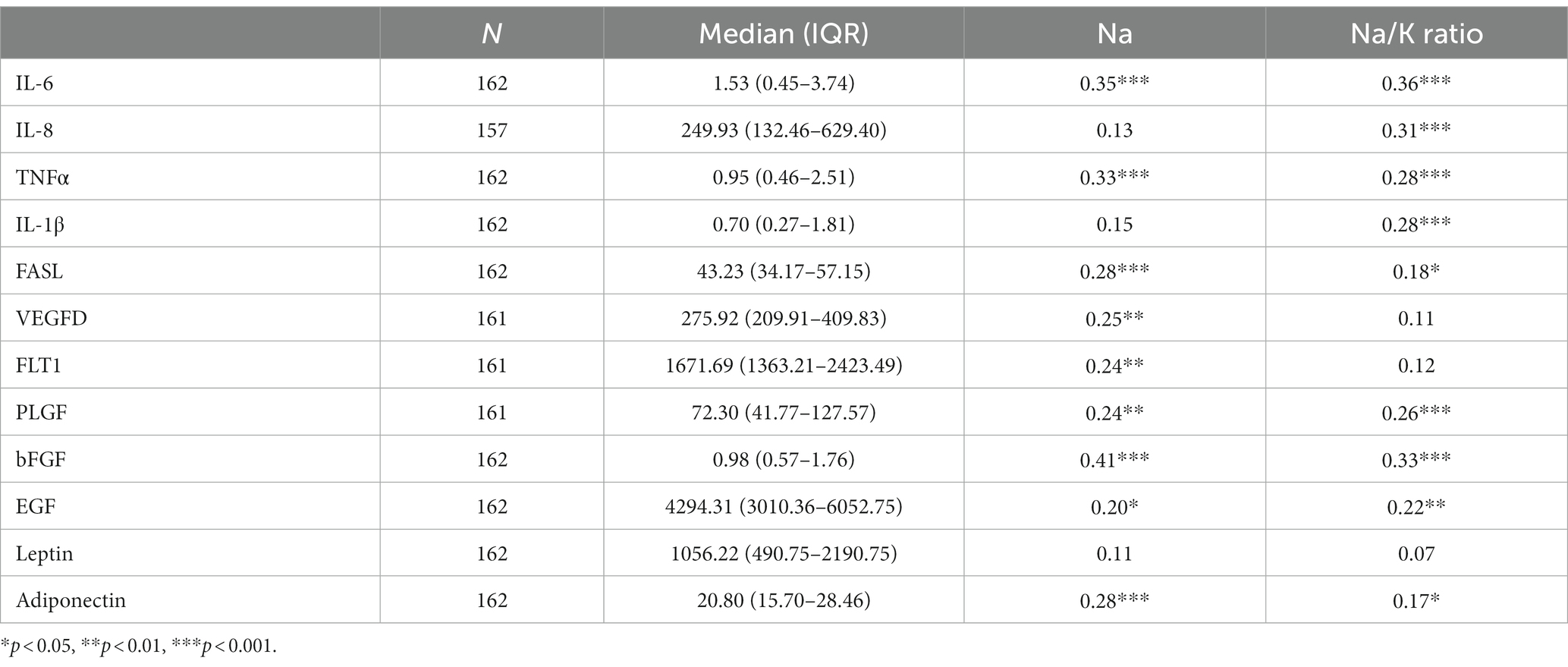
Table 5. Descriptive statistics for human milk analytes and Spearman rank correlations with Na and the Na/K ratio.
Principal components analysis, performed to consolidate the analytes, identified three unique factors (see Table 6). IL-6, IL-8, TNFα, IL-1β, and EGF loaded onto a factor representing the pro-inflammatory signature. The second factor was composed primarily of analytes involved in tissue growth and remodeling and included FASL, VEGFD, FLT1, PLGF, bFGF, and adiponectin. A single analyte, leptin, loaded onto the third factor. Pro-inflammatory and tissue remodeling factor scores were calculated based on this analysis.
Pro-inflammatory factor scores were higher in primipara as compared to multipara, Mann–Whitney U = 2,391, p < 0.05. The pro-inflammatory factor was more strongly correlated with Na/K, r(154) = 0.31, p < 0.001, than with Na alone, r(154) = 0.22, p < 0.01. In contrast, the tissue remodeling factor was more strongly correlated with Na, r(154) = 0.32, p < 0.001, than with the Na/K ratio, r(154) = 0.19, p < 0.05.
Multivariable logistic regression models were examined to evaluate pro-inflammatory and tissue remodeling factor scores as predictors of elevated MEP (Table 7). Controlling for infant age and maternal race, higher pro-inflammatory and tissue remodeling factor scores each uniquely raised the odds of elevated MEP as indicated by Na ≥ 10 mmol/L. A similar pattern was observed for the Na/K ratio where pro-inflammatory and tissue remodeling factors were uniquely associated with elevated MEP during established lactation.
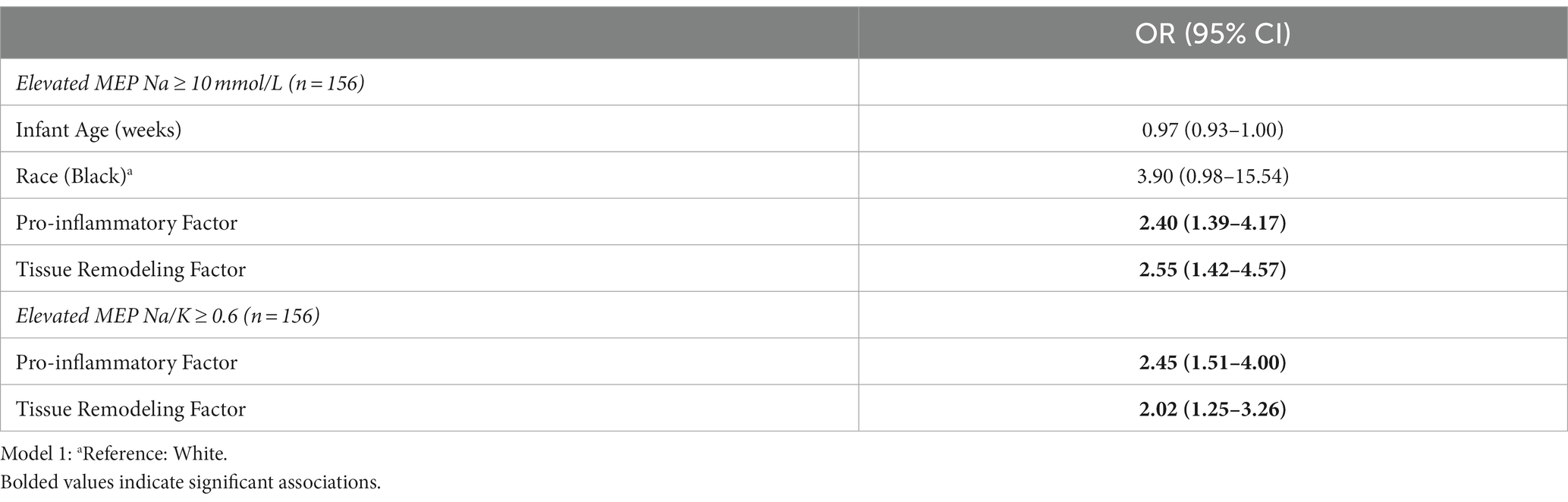
Table 7. Multivariable logistic regression models and 95% confidence intervals predicting increased mammary epithelium permeability (MEP) from pro-inflammatory and tissue remodeling factor scores.
4 Discussion
To our knowledge, this is the first study to examine associations between MEP and cytokine profiles in human milk during established lactation among parents living in the US. Of note, the percentage of participants with elevated MEP was higher in this cohort than was observed in a European cohort during established lactation using the same criteria (Na/K > 0.6) (28). Elevated MEP during established lactation occurred in 5% or less of participants in a European cohort as compared to the observed 15% in the current study. However, worldwide prevelance of elevated MEP varies significantly (28, 29). More study will be needed to identify appropriate thresholds for different sub-populations.
Historically, MEP has been studied primarily in relation to secretory activation, subclinical mastitis (SCM), mastitis, and involution. Secretory activation occurs in the first days postpartum and is associated with a precipitous decline in MEP (18, 21), while SCM and mastitis may occur at any time during lactation and are associated with significant increases in MEP (2, 28, 30–32). Therefore, MEP has been linked to developmental processes, in addition to infectious processes such as subclinical mastitis or mastitis. Our aim is to understand the physiological process of MEP which can arise as the result of multiple lactation-related states.
The milk samples assessed in the present study were all from participants nursing infants between 1 and 18 months of age, and to our knowledge, no participant was experiencing symptoms of mastitis at the time of milk collection. While we did not specifically inquire about plans for weaning, it is possible that some participants may have been in the midst of this process. MEP, as indicated by elevated Na or Na/K, is known to increase during involution (33). Of note, the odds of elevated MEP (defined as Na ≥ 10 mmol/L) declined with advancing infant age overall, possibly representing normal changes in mammary function over the course of the lactation cycle. This is in line with previous research showing a decline in subclinical mastitis with older infant age (28, 30). However, few studies have examined these trends into the second year postpartum.
As predicted, Black participants in our cohort were more likely to experience elevated MEP (defined as Na ≥ 10 mmol/L), despite a non-significant older infant age in this sub-group. Since this was the first study to assess MEP in a sizable (n = 98) sample of Black females in the US, it is unknown whether the 3.3 times greater odds of elevated MEP among Black participants was due to socioeconomic conditions impacting the frequency of breastfeeding or pumping, represents normal variation in healthy breast tissue, or was a sign of inflammation in the mammary gland. Elevated MEP among Black participants is consistent with our previous report demonstrating higher levels of some pro-inflammatory cytokines in the milk of Black women in this cohort (24).
Levels of all cytokines examined were positively associated with either Na or the Na/K ratio, with the exception of leptin. A body of research has previously identified links between cytokines and permeability. Many of these studies have suggested that inflammation may drive increased permeability (2, 4, 31, 32). Indeed, during mastitis open paracellular pathways may be adaptive, allowing cytokine-producing leukocytes access to the alveolar lumen (34). However, permeability may also increase in response to the rising alveolar pressure associated with milk accumulation. Eventually, this can lead to tissue remodeling as seen during involution. Both pro-inflammatory cytokines and tissue growth factors play a role in this process (14, 19).
In the current study, pro-inflammatory and tissue remodeling factors each uniquely raised the odds of experiencing elevated MEP as indicated by either Na ≥ 10 mmol/L or the Na/K ratio ≥ 0.6. However, when considering continuous measures of Na or the Na/K ratio, a pattern emerged where the pro-inflammatory factor was more strongly associated with the Na/K ratio, while the tissue remodeling factor was more tightly linked with Na. To our knowledge, this is the first study to examine growth factors in relation to MEP. While additional research is needed to confirm this finding, unique patterns of Na and Na/K with specific cytokines could be used to identify the physiologic processes underlying elevated MEP.
Identifying the etiology underlying MEP has important implications for both parent and infant. Elevated MEP has been linked to delayed onset of lactation (18), low milk supply (35), reduced milk nutrient content (28), and inadequate infant growth (4). Infant growth faltering has also been identified in relation to elevated cytokines in human milk (3). Growth factors in human milk may also affect the development of the infant intestinal barrier (11). Identifying persistently increased permeability could also have important implications for identifying breast cancer risk, given the established role of cytokines in tumorigenesis (9, 24, 36). Taken together, both MEP and cytokines could have important implications for both parent and infant health.
An important direction for future research is to determine how to most appropriately measure MEP. In the present study, analyses based on Na levels and Na/K ratios provide slightly different results. Using cut-off values from the literature (18), Na was the more conservative indicator of elevated MEP in this study, identifying 19 cases as compared to the 25 cases identified using the Na/K ratio. Of note, a subset of 17 cases was identified by both indicators, while 2 cases were identified by Na alone, and 8 cases by the Na/K ratio alone. As noted above, continuous measures of Na were also more closely associated with tissue growth and remodeling cytokines, while the Na/K ratio seemed to be more tightly linked with pro-inflammatory cytokines. Additional research is needed to determine how best to interpret human milk Na levels and Na/K ratios during established lactation.
There were several strengths to the current study including a cohort with a significant number of Black female participants, representation of parents with up to 18 months of established lactation, and a panel of 12 cytokines. However, there is also an important limitation. This secondary analysis used milk and questionnaire data from a study aimed at understanding factors associated with breast cancer risk. Therefore, the study design and questionnaires were not optimally structured to assess factors known to be associated with MEP. In addition, more research is needed to determine if extended freezer storage at −20°C might affect the measurement of Na by ion selective electrode.
5 Conclusion
Results presented here highlight the importance of measuring mammary epithelium permeability in studies of normal developmental and inflammatory processes in the human mammary gland, as well as in studies of the effects of human milk on infant health. Surprisingly, the human mammary gland is the only organ for which we lack routine clinical tests for normal function (37, 38). Rich information may be obtained through the measurement of MEP indicators during established lactation. Research aimed at understanding the importance of MEP for mammary gland health or infant growth and development should be prioritized.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Massachusetts Amherst Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KK: Conceptualization, Formal analysis, Writing – original draft. SS: Conceptualization, Methodology, Writing – review & editing. EB: Data curation, Investigation, Methodology, Validation, Writing – review & editing. BP: Writing – review & editing. DA: Writing – review & editing. KA: Conceptualization, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the College of Natural Sciences, University of Massachusetts-Amherst, an NIH Grant R01HD11120 to KA, SS, and KK, and a NIFA/USDA HATCH award under project number MAS00596 to KA.
Acknowledgments
We wish to thank the participants who donated milk for this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1258905/full#supplementary-material
References
1. Say, B, Dizdar, EA, Degirmencioglu, H, Uras, N, Sari, FN, Oguz, S, et al. The effect of lactational mastitis on the macronutrient content of breast milk. Early Hum Dev. (2016) 98:7–9. doi: 10.1016/j.earlhumdev.2016.03.009
2. Li, C, Solomons, NW, Scott, ME, and Koski, KG. Subclinical mastitis (SCM) and proinflammatory cytokines are associated with mineral and trace element concentrations in human breast milk. J Trace Elem Med Biol. (2018) 46:55–61. doi: 10.1016/j.jtemb.2017.11.010
3. Saso, A, Blyuss, O, Munblit, D, Faal, A, Moore, SE, and Le Doare, K. Breast Milk cytokines and early growth in Gambian infants. Front Pediatr. (2018) 6:414. doi: 10.3389/fped.2018.00414
4. Li, C, Solomons, NW, Scott, ME, and Koski, KG. Anthropometry before day 46 and growth velocity before 6 months of Guatemalan breastfed infants are associated with subclinical mastitis and milk cytokines, minerals, and trace elements. J Nutr. (2019) 149:1651–9. doi: 10.1093/jn/nxz109
5. Brenmoehl, J, Ohde, D, Wirthgen, E, and Hoeflich, A. Cytokines in milk and the role of TGF-beta. Best Pract Res Clin Endocrinol Metab. (2018) 32:47–56. doi: 10.1016/j.beem.2018.01.006
6. Palmeira, P, and Carneiro-Sampaio, M. Immunology of breast milk. Rev Assoc Medica Bras. (2016) 62:584–93. doi: 10.1590/1806-9282.62.06.584
7. Porcherie, A, Gilbert, FB, Germon, P, Cunha, P, Trotereau, A, Rossignol, C, et al. IL-17A is an important effector of the immune response of the mammary gland to Escherichia coli infection. J Immunol Baltim Md. (2016) 196:803–12. doi: 10.4049/jimmunol.1500705
8. Hunt, KM, Williams, JE, Shafii, B, Hunt, MK, Behre, R, Ting, R, et al. Mastitis is associated with increased free fatty acids, somatic cell count, and interleukin-8 concentrations in human milk. Breastfeed Med Off J Acad Breastfeed Med. (2013) 8:105–10. doi: 10.1089/bfm.2011.0141
9. Arcaro, KF, Browne, EP, Qin, W, Zhang, K, Anderton, DL, and Sauter, ER. Differential expression of cancer-related proteins in paired breast milk samples from women with breast cancer. J Hum Lact. (2012) 28:543–6. doi: 10.1177/0890334412453205
10. Qin, W, Zhang, K, Kliethermes, B, Ruhlen, RL, Browne, EP, Arcaro, KF, et al. Differential expression of cancer associated proteins in breast milk based on age at first full term pregnancy. BMC Cancer. (2012) 12:100. doi: 10.1186/1471-2407-12-100
11. Ballard, O, and Morrow, AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin N Am. (2013) 60:49–74. doi: 10.1016/j.pcl.2012.10.002
12. Kiełbasa, A, Gadzała-Kopciuch, R, and Buszewski, B. Cytokines-biogenesis and their role in human breast milk and determination. Int J Mol Sci. (2021) 22:6238. doi: 10.3390/ijms22126238
13. Watson, CJ, Oliver, CH, and Khaled, WT. Cytokine signalling in mammary gland development. J Reprod Immunol. (2011) 88:124–9. doi: 10.1016/j.jri.2010.11.006
14. Jena, MK, Jaswal, S, Kumar, S, and Mohanty, AK. Molecular mechanism of mammary gland involution: an update. Dev Biol. (2019) 445:145–55. doi: 10.1016/j.ydbio.2018.11.002
15. Colleluori, G, Perugini, J, Barbatelli, G, and Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev Endocr Metab Disord. (2021) 22:241–55. doi: 10.1007/s11154-021-09633-5
16. Lamote, I, Meyer, E, Massart-Leën, AM, and Burvenich, C. Sex steroids and growth factors in the regulation of mammary gland proliferation, differentiation, and involution. Steroids. (2004) 69:145–59. doi: 10.1016/j.steroids.2003.12.008
17. Savilahti, E, and Saarinen, KM. Colostrum TGF-beta-1 associates with the duration of breast-feeding. Eur J Nutr. (2007) 46:238–42. doi: 10.1007/s00394-007-0656-9
18. Medina Poeliniz, C, Engstrom, JL, Hoban, R, Patel, AL, and Meier, P. Measures of secretory activation for research and practice: an integrative review. Breastfeed Med. (2020) 15:191–212. doi: 10.1089/bfm.2019.0247
19. Stelwagen, K, and Singh, K. The role of tight junctions in mammary gland function. J Mammary Gland Biol Neoplasia. (2014) 19:131–8. doi: 10.1007/s10911-013-9309-1
20. Nguyen, DA, Parlow, AF, and Neville, MC. Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J Endocrinol. (2001) 170:347–56. doi: 10.1677/joe.0.1700347
21. Neville, MC, Keller, RP, Seacat, J, Casey, CE, Allen, JC, and Archer, P. Studies on human lactation. I. Within-feed and between-breast variation in selected components of human milk. Am J Clin Nutr. (1984) 40:635–46. doi: 10.1093/ajcn/40.3.635
22. Lai, CT, Gardner, H, and Geddes, D. Comparison of inductively coupled plasma optical emission spectrometry with an ion selective electrode to determine sodium and potassium levels in human Milk. Nutrients. (2018) 10:E1218. doi: 10.3390/nu10091218
23. Stelwagen, K, Farr, VC, McFadden, HA, Prosser, CG, and Davis, SR. Time course of milk accumulation-induced opening of mammary tight junctions, and blood clearance of milk components. Am J Phys. (1997) 273:R379–86. doi: 10.1152/ajpregu.1997.273.1.R379
24. Murphy, J, Pfeiffer, RM, Lynn, BCD, Caballero, AI, Browne, EP, Punska, EC, et al. Pro-inflammatory cytokines and growth factors in human milk: an exploratory analysis of racial differences to inform breast cancer etiology. Breast Cancer Res Treat. (2018) 172:209–19. doi: 10.1007/s10549-018-4907-7
25. Narayanaswamy, V, Pentecost, BT, Schoen, CN, Alfandari, D, Schneider, SS, Baker, R, et al. Neutralizing antibodies and cytokines in breast Milk after coronavirus disease 2019 (COVID-19) mRNA vaccination. Obstet Gynecol. (2022) 139:181–91. doi: 10.1097/AOG.0000000000004661
26. Keizer, RJ, Jansen, RS, Rosing, H, Thijssen, B, Beijnen, JH, Schellens, JHM, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect. (2015) 3:e00131. doi: 10.1002/prp2.131
27. Hair, J, Tatham, R, Anderson, R, and Black, W. Multivariate data analysis. 5th ed. London: Prentice Hall (1998).
28. Samuel, TM, De Castro, CA, Dubascoux, S, Affolter, M, Giuffrida, F, Billeaud, C, et al. Subclinical mastitis in a European multicenter cohort: prevalence, impact on human Milk (HM) composition, and association with infant HM intake and growth. Nutrients. (2019) 12:105. doi: 10.3390/nu12010105
29. Aryeetey, RNO, Marquis, GS, Timms, L, Lartey, A, and Brakohiapa, L. Subclinical mastitis is common among Ghanaian women lactating 3 to 4 months postpartum. J Hum Lact. (2008) 24:263–7. doi: 10.1177/0890334408316077
30. Pace, RM, Pace, CDW, Fehrenkamp, BD, Price, WJ, Lewis, M, Williams, JE, et al. Sodium and potassium concentrations and somatic cell count of human milk produced in the first six weeks postpartum and their suitability as biomarkers of clinical and subclinical mastitis. Nutrients. (2022) 14:4708. doi: 10.3390/nu14224708
31. Schaub, R, Badiou, S, Viljoen, J, Dujols, P, Bolloré, K, Van de Perre, P, et al. The immune response to sub-clinical mastitis is impaired in HIV-infected women. J Transl Med. (2018) 16:296. doi: 10.1186/s12967-018-1667-4
32. Tuaillon, E, Viljoen, J, Dujols, P, Cambonie, G, Rubbo, PA, Nagot, N, et al. Subclinical mastitis occurs frequently in association with dramatic changes in inflammatory/anti-inflammatory breast milk components. Pediatr Res. (2017) 81:556–64. doi: 10.1038/pr.2016.220
33. Kobayashi, K, Matsunaga, K, Tsugami, Y, Wakasa, H, and Nishimura, T. IL-1β is a key inflammatory cytokine that weakens lactation-specific tight junctions of mammary epithelial cells. Exp Cell Res. (2021) 409:112938. doi: 10.1016/j.yexcr.2021.112938
34. Lehmann, M, Wellnitz, O, and Bruckmaier, RM. Concomitant lipopolysaccharide-induced transfer of blood-derived components including immunoglobulins into milk. J Dairy Sci. (2013) 96:889–96. doi: 10.3168/jds.2012-5410
35. Murase, M, Wagner, EA, Chantry, JC, Dewey, KG, and Nommsen-Rivers, LA. The relation between breast milk sodium to potassium ratio and maternal report of a milk supply concern. J Pediatr. (2017) 181:294–297.e3. doi: 10.1016/j.jpeds.2016.10.044
36. King, J, Mir, H, and Singh, S. Association of cytokines and chemokines in pathogenesis of breast cancer. Prog Mol Biol Transl Sci. (2017) 151:113–36. doi: 10.1016/bs.pmbts.2017.07.003
37. Boss, M, Gardner, H, and Hartmann, P. Normal human lactation: closing the gap. F1000Res. (2018) 7:F1000 Faculty Rev-801. doi: 10.12688/f1000research.14452.1
Keywords: human milk, sodium, Na/K ratio, cytokine, growth factor, inflammation, mammary epithelium permeability, subclinical mastitis
Citation: Kivlighan KT, Schneider SS, Browne EP, Pentecost BT, Anderton DL and Arcaro KF (2024) Mammary epithelium permeability during established lactation: associations with cytokine levels in human milk. Front. Nutr. 11:1258905. doi: 10.3389/fnut.2024.1258905
Edited by:
Reinaldo B. Oria, Federal University of Ceara, BrazilReviewed by:
Marzia Giribaldi, Italian National Research Council, ItalyJing Zhu, Beijing Academy of Science and Technology, China
Bethaney Fehrenkamp, University of Idaho, United States
Soraya Mezouar, Aix-Marseille University, France
Copyright © 2024 Kivlighan, Schneider, Browne, Pentecost, Anderton and Arcaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katie T. Kivlighan, a2tpdmxpZ2hAc2FsdWQudW5tLmVkdQ==
 Katie T. Kivlighan
Katie T. Kivlighan Sallie S. Schneider
Sallie S. Schneider Eva P. Browne3
Eva P. Browne3 Kathleen F. Arcaro
Kathleen F. Arcaro