- Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Engineering and Technology Research Center of Food Additives, College of Food and Health, Beijing Technology and Business University, Beijing, China
Some infants and young children suffer from cow's milk allergy (CMA), and have always mainly used hypoallergenic infant formula as a substitute for breast milk, but some of these formulas can still cause allergic reactions. In recent years, it has been found that probiotic nutritional interventions can regulate CMA in children. Scientific and reasonable application of probiotics to hypoallergenic infant formula is the key research direction in the future. This paper discusses the mechanism and clinical symptoms of CMA in children. This review critically ex- amines the issue of how probiotics use intestinal flora as the main vector to combine with the immune system to exert physiological functions to intervene CMA in children, with a particular focus on four mechanisms: promoting the early establishment of intestinal microecological balance, regulating the body's immunity and alleviating allergic response, enhancing the intestinal mucosal barrier function, and destroying allergen epitopes. Additionally, it overviews the development process of hypoallergenic infant formula and the research progress of probiotics in hypoallergenic infant formula. The article also offers suggestions and outlines potential future research directions and ideas in this field.
1 Introduction
Food allergy refers to the abnormal immune reaction to food proteins, which leads to the disorder of physiological function or tissue damage of the body, thus causing a series of clinical symptoms (1). According to the statistics of the World Health Organization (WHO), at present, food allergic reaction has risen to the sixth place in the global diseases, and the number of people suffering from such diseases has increased exponentially (2), affecting more than 20% of the world's population, especially children (3), and becoming the most important non-infectious disease affecting children's health. “Big-8 allergenic foods” had been identified, including gluten containing grains, crustaceans, fish, eggs, peanuts, soybeans, milk, nuts and products of the above 8 categories of substances (4). A series of investigation results show that the early life allergic reaction is mainly milk, egg allergy (5–11).
CMA is an allergic immune response to cow milk protein (CMP) that usually develops in the first few months after birth (12, 13). Cow's milk is an important source of nutrients when breastfeeding is insufficient (14). For children with CMA, different types of hydrolyzed formulas (HF) are recommended, extensively hydrolyzed formula (eHF) as the first choice for CMA treatment, and amino acid formulas (AAF) for more severe cases or those with reaction to eHF (15–18). In recent years, the infant formula adding probiotics were developed, and whether probiotics can reduce the risk of CMA, the present manuscript summarizes and discusses the mechanism and application of probiotics in early life to regulate CMA in children.
2 Mechanism and clinical symptoms of CMA in children
Cow milk contains large allergenic proteins. Most important of them are casein, β-lactoglobulin (BLG), and α-lactalbumin (19). CMA is one of the most common food allergies and ranks third among all food allergies leading to anaphylaxis (8–15% of cases) especially in childhood, affecting about 3–8% of children in different countries (20, 21). Infants are prone to CMA, which is mainly caused by the immature development of intestinal barrier and the incomplete development of immune system (22, 23). The intestinal mucosal cells of infants are sparsely arranged, the intestinal osmotic pressure is increased, and allergens are easy to enter the blood through mucosal cells to cause allergy.
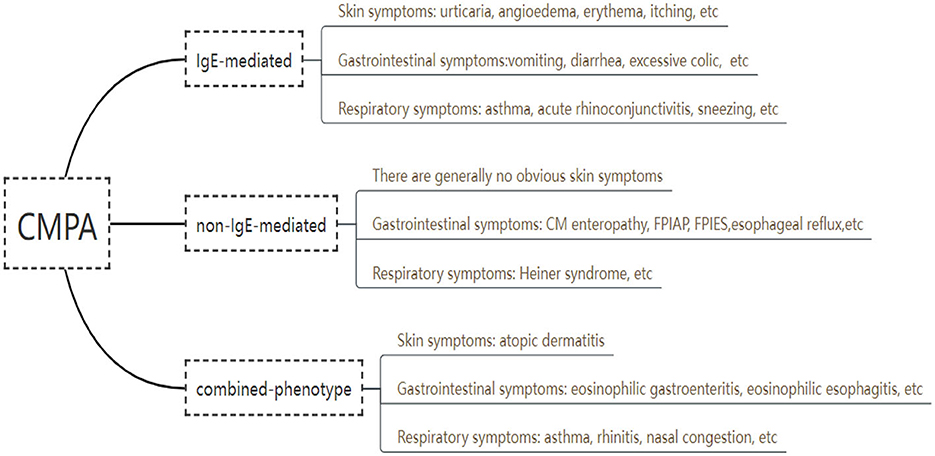
Based on immunological mechanisms, CMA can be divided into three types, including immunoglobulin E (IgE) -mediated, non IgE -mediated, and combined (Figure 1). In a Brazilian referral center of Allergy and Clinical Immunology, clinical history, laboratorial findings and test results were collected from 115 pediatric patients with CMA in 2017 through electronic medical record, the results showed that 57% of the reactions were IgE-mediated, 20% were non-IgE-mediated and 23%, mixed reactions (24). An IgE-mediated CMA is a type I hypersensitivity reaction or immediate CMA, and the clinical manifestations occur within minutes to 2 h after milk ingestions, which involves mast cell degranulation (25). Tang et al. shown that among the 234 participants who were measured by an allergen array, 9 were boiled milk sIgE-positive, 50 were yogurt sIgE-positive, 17 were buttermilk sIgE-positive, and 158 were only raw milk sIgE-positive (26). Non IgE-mediated CMA often present symptoms 2 h to even several days induced by exposure to cow milk, involving respiratory tract, gastrointestinal tract and other parts (27, 28), including type II or type III hypersensitivity reactions mediated by IgG or IgM and tissue damage caused by complement, basophils and neutrophils, and type IV hypersensitivity reactions mediated by T lymphocytes (29). The allergic mechanism of non-IgE-mediated immune response is currently under debate and still needs further research. Combined CMA may be related to the cross-inhibitory response of Th1 and Th2 in the immune system of newborn infants (30). This cross-inhibitory response may have humoral and/or cell-mediated mechanisms and may present with symptoms such as atopic dermatitis, allergic eosinophilic esophagitis, and eosinophilic gastritis.
In recent years, more and more studies have shown that the imbalance of Treg and pro-inflammatory Th17 cells (Treg/Th17) is also one of the key factors causing allergic diseases. When milk protein allergic reaction occurs, Th17 is dominant, and the number of Treg decreases (31).
3 The development of hypoallergenic infant formula
Hypoallergenic foods are those that are well tolerated in at least 90% (95% of confidence interval) individuals with allergies in double-blind, placebo-controlled trials (32, 33). Hypoallergenic infant formula is a kind of infant formula for special medical use, and it can be divided into partially hydrolyzed formulas (pHF), eHF, and AAF (see Table 1) (34). Hydrolysis may destroy the epitopes of CMA by hydrolyzing part or all of the milk proteins into small molecular peptides and amino acids, which reduces the antigenicity of CMP. pHF is mainly used for dietary management of infants with functional gastrointestinal disorders and can also be used for initial intervention feeding of non-breastfed infants at high risk of milk protein allergy (whose parents or siblings have a history of allergy). eHF and AAF are mainly used in the dietary management of infants with CMA (35–39).
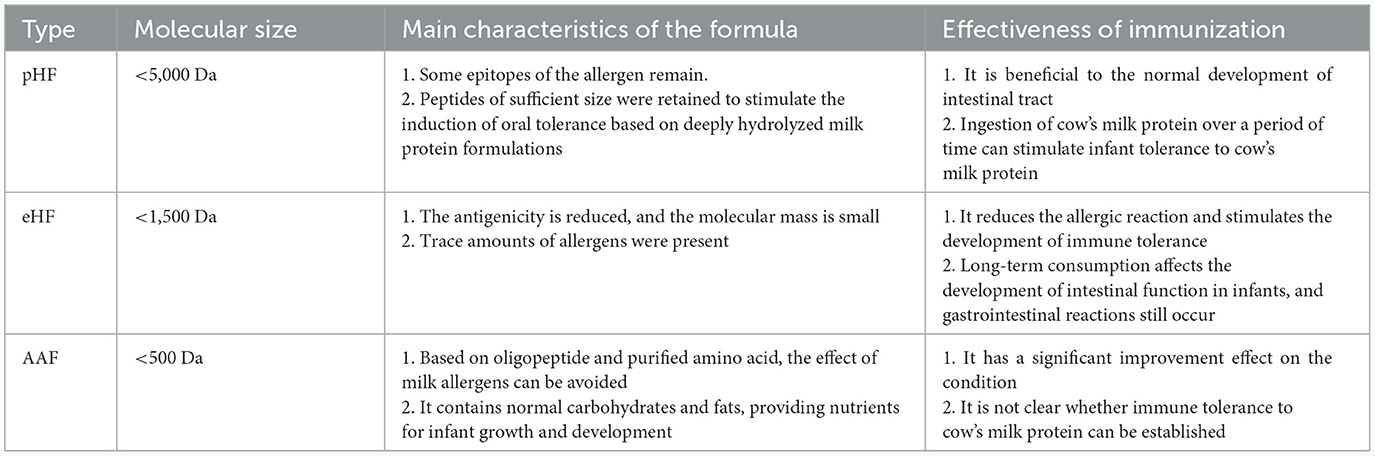
Table 1. Classification and characteristics of infant formula for special medical purposes (34).
In some pHF, B cell epitopes of cow milk allergens are still present, which can cause CMA (40). It has been shown that there is a significant difference in BLG residues between pHF and eHF. The BLG level of pHF is 40,000 times higher than that of eHF (41). pHF seems to be a better alternative to infant formula based on CMP (42). However, pHF outperforms most eHF in terms of cost and taste preference (43). Although there is no clear definition of eHF and pHF, these two infant formulas have been developed and commercially available. Hydrolyzed infant formula varies due to protein source, degree of hydrolysis, protease species, auxiliary processing techniques (such as thermal processing) and peptide profile, and allergen residues may still be present (42). The antigenicity of allergen residues in infant formula depends on the degree of hydrolysis and filtration techniques applied during the preparation; therefore, it is recommended that the safety of the hydrolyzed infant formula be first confirmed before it is introduced into the diet of CMA infants (41). The main criterion for labeling infant formula as hypoallergenic is that 90% of children or infants with CMA confirmed by double-blind, placebo-controlled trials do not exhibit allergic reactions (44).
Based on pHF and eHF research, AAF was subsequently developed. Studies have shown that the use of AAF can greatly reduce sensitization reactions (45). AAF supplementation is recommended for infants with pHF or eHF allergy, growth retardation, or multiple food allergies (45). The main problem with AAF not being widely applied is the high cost and rather unpleasant taste (46). In some studies, eHFs, and AAFs were noted to be hypoallergenic, while pHFs was not included because the allergic reaction caused by it was unpredictable (47).
Up to now, the impact of intestinal flora diversity and/or dysfunction (dysbiosis) on food allergy has attracted more people's attention, and the addition of probiotics to infant formula to develop new products has become a research hotspot. At present, there are mainly the following probiotics that are permitted to be used in infant food in China, such as Lactobacillus acidophilus NCFM, Bifidobacterium animalis subsp. Lactis Bb-12, Bifidobacterium animalis subsp. Lactis HN019, Bifidobacterium animalis subsp. Lactis Bi-07, Lacticaseibacillus rhamnosus GG (LGG), Lacticaseibacillus rhamnosus HN001, Lacticaseibacillus rhamnosus MP108, Limosilactobacillus reuteri DSM 17938, Limosilactobacillus fermentum CECT 5716, Bifidobacterium breve M-16V, Lactobacillus helveticus R0052, Bifidobacterium bifidum R0071, Bifidobacterium longum subsp. longum BB536, Bifidobacterium longum subsp. infantis R0033, etc. (48–50). The conditions under which these probiotics are used in hypoallergenic formulas need to be researched in depth.
4 Physiological functions of probiotics on children's health
As for the concept of probiotics, the most widely used is the definition of Food and Agriculture Organization (FAO) and WHO (51): Probiotics are living microorganisms that, when ingested in sufficient quantities, produce one or more demonstrated physiological functional benefits to the host.
The intestinal flora of infants is mainly dominated by Bifidobacterium, which maintains a dynamic balance with the changes of environment, diet and lifestyle in the later period (52). When the balance of intestinal flora is broken, the disordered intestinal flora will affect the occurrence and development of many diseases (53). Probiotics can play a beneficial role by regulating the abundance of intestinal flora and its metabolites. Clinical studies have found that Probio-M8 can improve asthma symptoms by regulating intestinal flora (54). In addition to directly acting on intestinal flora, probiotics can also play a role by indirectly regulating metabolites of intestinal flora, such as short-chain fatty acids (SCFA) (55), bile acids, lipids, and neurotransmitters, so as to improve the health of the body.
Different probiotic strains and doses can have different effects on health outcomes, and no one-size-fits-all strain addresses all health outcomes. However, probiotic supplementation is thought to trigger numerous immunological benefits through signaling pathways, cytokine expression. Induction of cytokine secretion by probiotic bacteria exhibited strain specificity, and the response may also vary in the presence of different species of probiotic bacteria or a mixture of probiotic bacteria (56). Ingested probiotics have been reported to interact with enterocytes and dendritic cells, Th1, Th2, and regulatory T cells (Tregs) in the gut. It has been shown that probiotics can reduce inflammation by stimulating anti-inflammatory cytokines and reducing pro-inflammatory cytokines, which in turn modulate NK cell activity and inhibit Toll-like receptor (TLR), which in turn inhibits the nuclear factor-kappa B (NF-κB) pathway (57). Probiotics have been shown to suppress intestinal inflammation by down-regulating TLR expression. Depending on the type of TLR, reduced expression of TLR can lead to multiple benefits, such as reduced NF-κB activity and other proinflammatory expressions (58).
One of the important functional indicators to monitor the immunity enhancement of probiotics is its ability to inhibit the growth of pathogenic bacteria, which can secrete peptides and organic acids to inhibit the production of harmful substances such as amines and indole, thus having the effect of inhibiting pathogenic bacteria and relieving inflammation (59). Lactobacillus rhamnosus, the most common type of lactic acid bacteria in probiotics, can produce SCFA such as acetic acid, propionic acid and butyric acid through fermentation, thereby changing the osmotic pressure inside and outside the cells, forming an acidic environment, and having a synergistic effect on inhibiting pathogenic bacteria (60). Studies have also shown that probiotics can also secrete bacteriocins for wall membrane and intracellular to inhibit the growth of pathogenic bacteria (61–63). This is undoubtedly advantageous for children whose immune defense mechanisms are not well developed.
Due to the particularity of the children population and the complexity of CMA mechanism, in the industrial production of hypoallergic infant formula, how to improve the physiological function of probiotics is a key aspect of future research (64).
5 Mechanism of probiotics in the prevention and regulation of CMA in children
Since CMA children have shown differences in their gut microbiome composition (number and diversity of species), modulation of the intestinal microbiota seems a promising strategy for the control of allergic reactions. In addition, there are some evidences on the beneficial effects of probiotics on the natural history of CMA, recovery from CMA and the appearance of other allergic manifestations in pediatric age (65–68). Hence, there is increasing interest in the use of probiotics for the prevention and treatment of food allergies.
Based on the recent studies on the effects of probiotics and their metabolites on CMA in children, we speculate that probiotics may play an important role in CMA by promoting the early establishment of intestinal microecological balance, regulating the body's immunity, and enhancing the function of intestinal mucosal barrier. In addition, lactic acid bacteria, as one of the important probiotics, also have the potential to destroy allergen epitopes and thus reduce milk sensitization.
5.1 Promote the early establishment of intestinal microecological balance
The human microbiota is a complex microbial ecosystem composed of commensal, symbiotic, and pathogenic microorganisms that can be found in the gut, skin, oral cavity, nasal passages, and urogenital tract (69, 70). Early microbiota establishment is essential for proper immune development and is beneficial for overall health status (71). The colonization of the gut is a dynamic process that is thought to begin at the fetal stage, progressing through an ecologically ordered succession of species until reaching a steady and balanced composition (which occurs approximately 1,000 days after birth) (69, 70, 72–74).
Clinical studies of eHF supplemented with probiotics showed improved symptoms in infants with CMA (75–78). The addition of Bifidobacterium and LGG is beneficial to the early establishment of intestinal microecological balance in children with CMA (79). Candy et al. (80) showed that AAF including a prebiotic blend of fructo-oligosaccharides and the probiotic strain Bifidobacterium breve M-16V improves gut microbiota in non-IgE-mediated allergic infants. Canani et al. (81) found LGG-supplemented casein formula could cause enrichment of butyric acid-producing bacteria in gut for infants with CMA, thereby promoting tolerance and reducing the risk of allergy. Yanru (82) studied the structure of intestinal flora and SCFAs in feces of children with CMA and found that the presence of Clostridium and Firmicutes was related to infant's CMA, and the composition of SCFAs in feces of children was significantly different.
Probiotics can also promote the establishment of children's intestinal microecological balance by resisting pathogen colonization, because they may temporarily occupy the vacant functional niche in the resident microbiota and secrete reactive oxygen species to inhibit pathogen growth, thereby preventing opportunistic infections and reducing the occurrence of allergies (58).
5.2 Regulating the body's immunity and alleviating the allergic response
The addition of probiotics to infant formula to assist the management of CMA has become a research hotspot, this indicates that probiotics can regulate the body's immunity. As reviewed by Servin, different Lactobacilli and Bifidobacteria strains were reported to be capable of stimulating immune cells to secrete cytokines or shifting the Th2-type response back to a Th1-type response (83, 84). Song et al. (85) found that Lactobacillus rhamnosus2016SWU.05.0601 regulated immune balance in ovalbumin-sensitized mice by modulating expression of the immune-related transcription factors and gut microbiota, decreasing the levels of Th2 and Th17 but increasing the levels of Th1 and Treg cytokines.
Zhang's study indicates that oral administration of Bifidobacteria has the capacity to suppress the skewed Th2 response in allergic mice, increasing the number of Treg and IL-10-positive cells and improve the impaired intestinal epithelial barrier function (86), and Inoue et al. (87) showed that Bifidobacterium breve M-16V modulated the systemic Th1/Th2 balance, suppressed the IgE production and reducing IL-4 levels by the in vitro and in vivo experiments. Lactobacillus casei strain Shirota (LcS) was administered intraperitoneally to ovalbumin-specific T cell receptor transgenic (OVA-TCRTg) mice, which increased IL-12 levels, decreased IgE and IgG1 levels, and restored the Th1/Th2 balance (88). The same immunological responses were also induced by Lactobacillus plantarum L-137 which could stimulate IL-12 production and reduce serum IgE and IgG levels (89).
The immunomodulatory effect of LGG on CMA has been extensively studied. Incidence of allergic symptoms decreased in children with CMA after taking extensively hydrolyzed casein formula (EHCF) containing LGG not only in mice but also in humans (90, 91). It has been reported that supplementing EHCF with LGG is more effective compared to EHCF alone in reducing CMA (44, 92). Similar results were found in another study by Thang et al., who used 3-week-old newly weaned Balb/c mice with adjuvant-free LGG sensitization to simulate CMA (93). In LGG-treated mice, Th2 responses were suppressed, resulting in remarkably lower hypersensitivity scores and CMP-specific IgG1 levels, and Th1 responses were promoted, resulting in increased levels of IFN and CMP-specific IgG2a (94). Moreover, lactic acid bacteria and its surface molecules can also affect the production of immune cells and cytokines (95). Therefore, the use of lactic acid bacteria fermentation to reduce milk sensitization has a good prospect (96–98).
Probiotics can also regulate the inflammatory signaling of intestinal epithelial cells to alleviate allergy. NF-κB and mitogen-activated protein kinase (MAPK) are two important inflammatory pathways. Studies have found that probiotics can inhibit the activation of NF-κB. The lactic acid bacteria could inhibit the phosphorylation of p38 MAPK and p65 NF-κB to mediate inflammatory responses (99). L. acidophilus L-92 could activate Th1 and Treg cells by participating in MAPK and NOD-like receptor pathways (100). In addition, DeMuri et al. (101) found that Lactobacillus acidophilus NCFM/Bifidobacterium lactis Bi-07 may alter inflammation by decreasing expression of E-selectin. Li et al. (102) indicates that Bifidobacterium breve M-16V may alter the gut microbiota to alleviate the allergy symptoms by IL-33/ST2 signaling. Wang et al. (103) showed that surface layer protein (Slp) of Lactobacillus acidophilus NCFM prevents TNF-α-stimulated cell apoptosis, as well as inhibits IL-8 secretion via inhibiting NF-κB activity, thereby exerting its anti-inflammatory activity. Chen et al. (104) found that LGG could effectively alleviate the allergic response, restore the levels of HIS, IgE, MCP, MCT, specific IgG, specific IgG1, specific IgG2a, and other inflammatory factors, and restore CD4+ T cell infiltration and the status of intestinal villi.
5.3 Enhance intestinal mucosal barrier function
The mucus layer of the intestinal mucosa is a mechanical barrier against pathogens (105). The intestinal mucosal immune system is the most complex part of the body's immune system. Intestinal commensal bacteria can stimulate the development and maturation of the intestinal mucosal immune system in the early stage, activate Th1 immune response, and inhibit IgE production to prevent allergic reactions. Moreover, research have shown that probiotics can enhance the host intestinal immune barrier and improve the immune regulation ability of Treg cells in the immune system (106). Colonized lactic acid bacteria can enhance the tight junction between epithelial cells, reduce intestinal permeability, support intestinal barrier function, and thus reduce the stimulation of allergens (107, 108). LGG could produce both a biofilm that can mechanically protect the mucosa, and different soluble factors beneficial to the gut by enhancing intestinal crypt survival, diminishing apoptosis of the intestinal epithelium, and preserving cytoskeletal integrity. Because the polysaccharides and pili present on LGG surface allow it to adhere to and temporarily colonize the intestinal mucosa (109).
5.4 Destruction of allergen epitopes thereby reducing CMA
As one of the important probiotics, lactic acid bacteria can not only regulate the composition of intestinal flora to play an immunomodulatory function or produce a variety of stimulus signals to activate immune cells, thereby triggering systemic immune response (110). On the other hand, lactic acid bacteria have a complex protease system (111), which can produce peptidase and protease to hydrolyze milk protein, destroy allergen epitopes (112), and thus reduce milk allergy (113).
Therefore, probiotic use not only emerges as a safe microbiological strategy in pediatrics for the promotion of intestinal immunity, but also becomes an important research direction for future CMA management.
6 Overviews in the application of probiotics in hypoallergenic infant formula
The application of probiotics to modulate the gut microbiome-immune axis to alleviate CMA has become a research hotspot. However, the mechanism by which the gut microbiota regulates CMA and the efficacy of probiotics are still in the preliminary exploration stage, and there are no clear and specific conclusions (114). Therefore, it is very important to locate specific strains in hypoallergenic infant formula.
Because of the different target proteins of hydrolysis, hydrolyzed infant formula is divided into hydrolyzed casein formula and hydrolyzed whey formula. However, some brands of pHF or eHF still have allergen B cell epitopes and can cause allergic reactions (115). In recent years, the combination of hydrolyzed proteases and probiotics has developed hypoallergenic milk protein hydrolysates, which have a broader application field. Probiotics have been shown to be beneficial in reducing symptoms in allergic patients, and adding probiotics to hypoallergenic infant formula is an innovative way to prevent and treat CMA (116). If a certain amount of LGG is added to eHF, it will bring many benefits to the infant, LGG more quickly induces the tolerance of infants with CMA, reduces the incidence of allergic dermatitis in infants, improves inflammation in the intestine to a certain extent, but also improves the recovery of allergic colitis (117). However, the European Society of Pediatric Nutrition and the Society of Gastroenterology believe that these new infant formulas are still not entirely satisfactory because the real safety of the probiotics added to the formulas has not been fully evaluated (118).
The research on the anti-allergic mechanism of probiotics will be carried out through in vitro and in vivo experiments and establish a safety evaluation mechanism is the key research content in the future. Moreover, for the research and development of probiotic hypoallergenic formulations, the specific probiotics used alone or in combination, the timing of the start and end of treatment, and the appropriate dose are needed to be determined deeply.
7 Suggestions and prospects
For the management of CMA in children, probiotics combined with hypoallergenic infant formula to establish immune tolerance are recommended as the main route in the future. But the data on probiotics themselves as a CMA prevention strategy are imperfect. In-depth exploration of the mechanism of probiotics regulating CMA is the focus of current research.
The application of probiotics as functional ingredients in hypoallergenic infant formula by major brands in the dairy industry has become a research hotspot. However, the health effect of probiotics has high strain tolerance, and infants with different constitutions, different genetic backgrounds and different intestinal flora should be different, and the research on probiotics can be located on the individual strains. At present, researchers have evaluated the safety and the efficacy of EHCF with LGG by randomized, double-blind trial, and the results showed that EHCF + LGG could be tolerated by the vast majority of IgE-mediated CMA children, and that the step-down approach from AFF to EHCF + LGG could promote a faster acquisition of immune tolerance (119). Although the use of probiotics is beneficial to promote immune regulation and alleviate clinical symptoms, more methodologically based and homogenized research is needed to more specifically study each type, dose, and time of probiotic supplementation for the establishment of definitive care protocols.
An in-depth comparison of the mechanism of action of probiotics added to whey protein hydrolysate vs. casein hydrolysate to reduce CMA. Based on clinical data or the real-world evidence, the mechanism of probiotics in hypoallergenic formula powders was studied. Nevertheless, there is an increased risk of functional gastrointestinal disorders among infants with CMA which could be reduced among those fed with EHCF+LGG (120), and further research is required to fully elucidate the mechanism of action of the probiotics.
Author contributions
ML: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing—original draft. CYJ: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Project administration, Visualization, Writing—review & editing.
Funding
This work was supported by the National Science and Technology Major Project of China (Beijing, 2019YFC1605002) and the National Natural Science Foundation of China (Beijing, 31872886).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Van Ree R, Poulsen LK, Wong GW, Ballmer-Weber BK, Gao Z, Jia X. Food allergy: definitions, prevalence, diagnosis and therapy. Chinese J Prevent Med. (2015) 49:87–92. doi: 10.3760/cma.j.issn.0253-9624.2015.01.020
2. Jonathan MS, Amy SP. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. (2003) 112:S118–27. doi: 10.1016/j.jaci.2003.09.033
3. Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy, Asthma Immunol Res. (2011) 3:67–73. doi: 10.4168/aair.2011.3.2.67
4. Candreva AM, Smaldini PL, Curciarello R, Cauerhff A, Fossati CA, Docena GH. Cross-reactivity between the soybean protein P34 and bovine caseins. Aller Asthma Immun. (2015) 7:60–8. doi: 10.4168/aair.2015.7.1.60
5. Jie HL. Epidemiology of food allergy in children from 31 cities in China. Int J Pediatr. (2017) 44:637–41.
6. Wang S, Jiang JX, Wang Y, Wang ZH, Wang T, Wang HS. Survey on prevalence of allergic symptoms among 0 to 24 months old children in Chinese cities. Chin J Child Health Care. (2016) 24:119–22.
7. Sha L, Shao M, Liu C, Wu Y, Chen Y. A cross-sectional study of the prevalence of food allergies among children younger than ages 14 years in a Beijing urban region. In: Allergy and Asthma Proceedings, (2019). doi: 10.2500/aap.2019.40.4193
8. Xiao YL, Pan JF, Wang LP, Duan YL. Food allergy status of infants and young children in community in Shanghai and influencing factors. J Clin Med Pract. (2018) 22:72–78. doi: 10.7619/jcmp.201811020
9. Nie J, Ran YC, Zhang YG, Chen J. The prevalence of food hypersensitivity in 0-24 months old children in Chengdu. Chin J Woman Child Health Res. (2017) 28:364–365.
10. Chen J, Niao Y, Zhang HZ, Zhao H. To investigate the prevalence of food allergy in children under 2 years of age in three cities of China. Chin J Pediatr. (2012) 50:5. doi: 10.1097/01.WOX.0000411608.35185.f5
11. Zou Y, Xu YL, Shen XM. Prevalence of food allergy in children under 3 years of age in Panzhihua city. Chin J Public Health. (2013) 29:1813–5. doi: 10.11847/zgggws2013-29-12-30
12. Johansson S, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immun. (2004) 113:832–6. doi: 10.1016/j.jaci.2003.12.591
13. Fiocchi J, Brozek H, Schünemann S, Bahna L, von Berg A, Beyer K. World Allergy organization (WAO) diagnosis and rationale for action against cow's milk allergy (DRACMA) guidelines. Pediat Allerg Immn. (2010) 21:1–125. doi: 10.1111/j.1399-3038.2010.01068.x
14. Zepeda-Ortega B, Goh A, Xepapadaki P, Sprikkelman A, Nicolaou N, Hernandez REH. Strategies and future opportunities for the prevention, diagnosis, and management of cow milk allergy. Front Immunol. (2021) 12:608372. doi: 10.3389/fimmu.2021.608372
15. Meyer R, Groetch M, Venter C. When should infants with cow's milk protein allergy use an amino acid formula? A practical guide. J Aller Clin Immunol. (2018) 6:383–99. doi: 10.1016/j.jaip.2017.09.003
16. Groetch M, Baker MG, Durban R, Meyer R, Venter C, Muraro A. The practical dietary management of food protein-induced enterocolitis syndrome. Ann Aller Asthma Immunol. (2021) 127:28–35. doi: 10.1016/j.anai.2021.03.007
17. D'Auria E, Salvatore S, Acunzo M, Peroni D, Pendezza E, Di Profio E, et al. Hydrolysed formulas in the management of cow's milk allergy: new insights, pitfalls and tips. Nutrients. (2021) 13:2762. doi: 10.3390/nu13082762
18. Salvatore S, Agosti M, Baldassarre M E, D'Auria E, Pensabene L, Nosetti L, et al. Cow's milk allergy or gastroesophageal reflux disease-can we solve the dilemma in infants? Nutrients. (2021) 13:297. doi: 10.3390/nu13020297
19. Thompson G, Zhelev Z, Peters J, Khalid S, Briscoe S, Shaw L, et al. Symptom scores in the diagnosis of pediatric cow's milk protein allergy: a systematic review. Pediatr Aller Immunol. (2021) 32:1497–507. doi: 10.1111/pai.13537
20. Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Aller Clin. (2012) 32:165–95. doi: 10.1016/j.iac.2011.10.002
21. Lajnaf R, Attia H, Ayadi MA. Technological properties and biological activities of camel a-lactalbumine: a review. Int Dairy J. (2023) 139:105563. doi: 10.1016/j.idairyj.2022.105563
22. Takiishi T, Fenero CI, Câmara NO. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barr. (2017) 5:e1373208. doi: 10.1080/21688370.2017.1373208
23. Wang L, Zhu L, Qin S. Gut microbiota modulation on intestinal mucosal adaptive immunity. J Immunol Res. (2019) 2019:4735040. doi: 10.1155/2019/4735040
24. Marino LM, Rozalem-Reali AC, Cancado BL, Gontijo JC, Pereira RA, Manhaes IB, et al. Oral tolerance versus immunological mechanism in children with cow's milk allergy. J Allergy Clin Immun. (2019) 143:AB167. doi: 10.1016/j.jaci.2018.12.509
25. Xin L, Xu ZH, Huang MJ, Wu Y, Hu LM, Chen HB. Progress on the reduction of allergenicity of bovine milk proteins by lactic acid bacteria. J Food Sci Biotechnol. (2021) 40:12–9.
26. Tang R, Lyu X, Liu Y, Zhu M, Yang X, Wu Z, et al. Four clinical phenotypes of cow's milk protein allergy based on dairy product specific IgE antibody types in North China. Front Immunol. (2022) 13:949629. doi: 10.3389/fimmu.2022.949629
27. Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Aller Clin Immunol. (2006) 117:1118–24. doi: 10.1016/j.jaci.2005.12.1352
28. Zopf Y, Hahn EG, Raithel M, Baenkler HW, Silbermann A. The differential diagnosis of food intolerance. Deutsches Ärzteblatt Int. (2009) 106:359. doi: 10.3238/arztebl.2009.0359
29. Morita H, Nomura I, Matsuda A, Saito H, Matsumoto K. Gastrointestinal food allergy in infants. Allergol Int. (2013) 62:297–307. doi: 10.2332/allergolint.13-RA-0542
30. Liu D, Cong YJ. Application of non-thermal processing technology in hypo- or non-allergenic infant formula. Sci Technol Food Ind. (2021) 42, 395–402. doi: 10.13386/j.issn1002-0306.2020080026
31. Chen J, Zhang XN, Huo QW Li MH, Shang YN, Wang JG. Progress in the mechanisms of cow's milk protein allergy in infants and its treatments. J Chin Inst Food Sci Technol. (2020) 20:289–98. doi: 10.16429/j.1009-7848.2020.07.035
32. Mahler V, Goodman RE. Definition and design of hypoallergenic foods. In:Kleine-Tebbe, J, Jakob, T., editors Molecular Allergy Diagnostics, Cham: Springer (2017). doi: 10.1007/978-3-319-42499-6_27
33. Roberts G, Grimshaw K, Beyer K, Boyle R, Lack G, Austin M, et al. Can dietary strategies in early life prevent childhood food allergy? A report from two iFAAM workshops. Clin Exper Aller. (2019) 49:1567–77. doi: 10.1111/cea.13515
34. Ministry of Health of the People's Republic of China. National Food Safety Standard - General Rules of Infant Formula for Special Medical Purposes: GB 25596-2010[S]. Beijing: Standards Press of China (2010).
35. Shi J, Zhou YD, Luo YK. Detection and analysis of binding activities of lgE antibodies from child patients allergic to cow's milk protein. J China Agric Univ. (2017) 22:40–4. doi: 10.11841/j.issn.1007-4333.2017.09.05
36. Ren WJ. Clinical efficacy of extensively hydrolyzed protein formula in the treatment of cow's milk protein allergy in infants. Maternal Child Health Care China. (2018) 33:3729–3731. doi: 10.7620/zgfybj.j.issn.1001-4411.2018.16.42
37. Wei M, Li WB, Chen PJ. Neonatal cow′s milk protein allergy: analysis of misdiagnosis of 4 neonates. Chin J Appl Clin Pediatr. (2018) 33:145–7. doi: 10.3760/cma.j.issn.2095-428X.2018.02.015
38. Zhao LF, Zhang MJ, Zhu LJ. To study the clinical characteristics of infants with cow's milk protein allergy and the efficacy and safety of amino acid free formula replacement/extensively hydrolyzed formula sequential intervention. Maternal Child Health Care China. (2020) 35:4526–9. doi: 10.19829/j.zgfybj.issn.1001-4411.2020.23.040
39. Nutten S, Maynard F, Järvi A, Rytz A, Simons PJ, Heine RG, et al. Peptide size profile and residual immunogenic milk protein or peptide content in extensively hydrolyzed infant formulas. Allergy. (2020) 75:1446. doi: 10.1111/all.14098
40. Meulenbroek LA, Oliveira S, den Hartog Jager CF, Klemans RJ, Lebens AF, Van Baalen T, et al. The degree of whey hydrolysis does not uniformly affect in vitro basophil and T cell responses of cow's milk-allergic patients. Clin Exper Aller. (2014) 44:529–39. doi: 10.1111/cea.12254
41. Kuslys M, Nutten S, Anette J, Maynard F, Affolter M, Fryer P, et al. Extensively hydrolyzed formulas for the management of cow's milk protein allergy in infants: is extensive hydrolysis sufficient to guarantee success? Clin Exper Aller. (2018) 11:48.
42. Meltretter J, Wust J, Pischetsrieder M. Modified peptides as indicators for thermal and nonthermal reactions in processed milk. J Agr Food Chem. (2014) 62:10903–15. doi: 10.1021/jf503664y
43. Verduci E, D'Elios S, Cerrato L, Comberiati P, Calvani M, Palazzo S, et al. Cow's milk substitutes for children: Nutritional aspects of milk from different mammalian species, special formula and plant-based beverages. Nutrients. (2019) 11:1739. doi: 10.3390/nu11081739
44. Parekh H, Bahna SL. Infant formulas for food allergy treatment and prevention. Pediatr Ann. (2016) 45:E150–6. doi: 10.3928/00904481-20160225-01
45. Dupont C, Kalach N, Soulaines P, Bradatan E, Lachaux A, Payot F. Safety of a new amino acid formula in infants allergic to cow's milk and intolerant to hydrolysates. J Pediatr Gastr Nutr. (2015) 61:456–63. doi: 10.1097/MPG.0000000000000803
46. Borschel MW, Baggs GE, Oliver JS. Comparison of growth of healthy term infants fed extensively hydrolyzed protein-and amino acid-based infant formulas. Nutrients. (2018) 10:289. doi: 10.3390/nu10030289
47. Sackesen C, Altintas DU, Bingol A, Bingol G, Buyuktiryaki B, Demir E, et al. Current trends in tolerance induction in cow's milk allergy: from passive to proactive strategies. Front Pediatr. (2019) 7:372. doi: 10.3389/fped.2019.00372
48. Gorbach SL. Application and safety of probiotics in infant milk powder. Chinese Institute of Food Science and Technology. In: Proceedings of the seventeenth International Symposium on Probiotics and Health. (2022) 2.
49. Fields D, Czerkies L, Sun S, Storm H, Saavedra J, Sorensen R. A randomized controlled trial assessing growth of infants fed a 100% whey extensively hydrolyzed formula compared with a casein-based extensively hydrolyzed formula. Global Pediatr Health. (2016). 3:2333794X16636613. doi: 10.1177/2333794X16636613
50. Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. (2016) 10:742–50. doi: 10.1038/ismej.2015.151
51. Littlejohns P, Cluzeau F. Guidelines for evaluation. Fam Pract. (2000) 17:S3–6. doi: 10.1093/fampra/17.suppl_1.S3
52. Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. (2018) 562:583. doi: 10.1038/s41586-018-0617-x
53. Ren CJ, Wu LM, Wangai K. Research progress about the effects of dietary polyphenols on the intestinal microbiota. Sci Technol Food Ind. (2022) 43:400–9. doi: 10.13386/j.issn1002-0306.2020090112
54. Liu A, Ma T, Xu N, Jin H, Zhao F, Kwok LY, et al. Adjunctive probiotics alleviates asthmatic symptoms via modulating the gut microbiome and serum metabolome. Microbiol Spectr. (2021) 9:e00859–21. doi: 10.1128/Spectrum.00859-21
55. Ma N, Chen X, Johnston LJ, Ma X. Gut microbiota-stem cell niche crosstalk: A new territory for maintaining intestinal homeostasis. Imeta. (2022) 1:e54. doi: 10.1002/imt2.54
56. Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci. (2014) 54:938–56. doi: 10.1080/10408398.2011.619671
57. Pourrajab B, Fatahi S. The effects of probiotic/synbiotic supplementation compared to placebo on biomarkers of oxidative stress in adults: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2022) 62:490–507. doi: 10.1080/10408398.2020.1821166
58. Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Nutrient. (2019) 10:S49–66. doi: 10.1093/advances/nmy063
59. Xiaoxu Z, Huan L, Miao X, et al. Progress in the study of antibacterial effect of probiotic metabolites on pathogens. Food Ferment Ind. (2023) 49:297–302. doi: 10.13995/j.cnki.11-1802/ts.032474
60. Kathayat D, Closs G, Helmy YA, Deblais L, Srivastava V, Rajashekara G. In vitro and in vivo evaluation of Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb12 against avian pathogenic Escherichia coli and identification of novel probiotic-derived bioactive peptides. Probiot Antimicrob Prot. (2022) 1:1–7. doi: 10.1007/s12602-021-09840-1
61. Xiang YZ Li XY, Zheng HL, Chen JY, Lin LB, Zhang QL. Purification and antibacterial properties of a novel bacteriocin against Escherichia coli from Bacillus subtilis isolated from blueberry ferments. LWT. (2021) 146:111456. doi: 10.1016/j.lwt.2021.111456
62. Mardirossian M, Pérébaskine N, Benincasa M, Gambato S, Hofmann S, Huter P, et al. The dolphin proline-rich antimicrobial peptide Tur1A inhibits protein synthesis by targeting the bacterial ribosome. Cell Chem Biol. (2018) 25:530–9. doi: 10.1016/j.chembiol.2018.02.004
63. Oakley JL, Weiser R, Powell LC, Forton J, Mahenthiralingam E, Rye PD, et al. Phenotypic and genotypic adaptations in Pseudomonas aeruginosa biofilms following long-term exposure to an alginate oligomer therapy. Msphere. (2021) 6:10–128. doi: 10.1128/mSphere.01216-20
64. Chao M, Weidong G, Yu W, Yun Q. Functional studies of probiotics and their application in functional foods. Chinese Nutrition Society. In: Proceedings of the 15th National Nutrition Science Congress of Chinese Nutrition Society. (2022) 1.
65. Canani RB, Di Costanzo M. Gut microbiota as potential therapeutic target for the treatment of cow's milk allergy. Nutrients. (2013) 5:651–62. doi: 10.3390/nu5030651
66. Scalabrin D, Harris C, Johnston WH, Berseth CL. Long-term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: a 5-year follow-up. Eur J Pediatr. (2017) 176:217–24. doi: 10.1007/s00431-016-2825-4
67. Nocerino R, Bedogni G, Carucci L, Cosenza L, Cozzolino T, Paparo L, et al. The impact of formula choice for the management of pediatric cow's milk allergy on the occurrence of other allergic manifestations: the atopic march cohort study. J Pediatr-Us. (2021) 232:183. doi: 10.1016/j.jpeds.2021.01.059
68. Tan WF, Zhou ZC, Li W, Lu H, Qiu ZM. Lactobacillus rhamnosus GG for cow's milk allergy in children: a systematic review and meta-analysis. Front Pediatr. (2021) 9:727127. doi: 10.3389/fped.2021.727127
69. Vinderola G, Burns P. Chapter 1 - The Biotics Family. In:Gomes Da Cruz A, Ranadheera CS, Nazzaro F, Mortazavian A, editors. Probiotics and Prebiotics in Foods. Academic Press (2021) 1–11. doi: 10.1016/B978-0-12-819662-5.00014-8
70. Renz H, Skevaki C. Early life microbial exposures and allergy risks: opportunities for prevention. Nat Rev Immunol. (2021) 21:177–91. doi: 10.1038/s41577-020-00420-y
71. Kong HH, Morris A. The emerging importance and challenges of the human mycobiome. Virulence. (2017) 8:310–2. doi: 10.1080/21505594.2017.1279780
72. Cukrowska B, Bierła JB, Zakrzewska M, Klukowski M, Maciorkowska E. The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients. (2020) 12:946. doi: 10.3390/nu12040946
73. Kumar H, Collado MC, Wopereis H, Salminen S, Knol J, Roeselers G. The bifidogenic effect revisited—ecology and health perspectives of bifidobacterial colonization in early life. Microorganisms. (2020) 8:1855. doi: 10.3390/microorganisms8121855
74. Vandenplas Y, Carnielli VP, Ksiazyk J, Luna MS, Migacheva N, Mosselmans JM, et al. Factors affecting early-life intestinal microbiota development. Nutrition. (2020) 78:110812. doi: 10.1016/j.nut.2020.110812
75. Canani RB, Nocerino R, Terrin G, Coruzzo A, Cosenza L, Leone L, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow's milk allergy: a randomized trial. J Aller Clin Immunol. (2012) 129:580–2. doi: 10.1016/j.jaci.2011.10.004
76. Dupont C, Hol J, Nieuwenhuis EE. An extensively hydrolysed caseinbased formula for infants with cows' milk protein allergy: tolerance/hypoallergenicity and growth catch-up. Br J Nutr. (2015) 113:1102–2. doi: 10.1017/S000711451500015X
77. Muraro A, Hoekstra MO, Meijer Y, Lifschitz C, Wampler JL, Harris C, et al. Extensively hydrolysed casein formula supplemented with Lactobacillus rhamnosus GG maintains hypoallergenic status: randomised double-blind, placebo-controlled crossover trial. BMJ Open. (2012) 2:e000637. doi: 10.1136/bmjopen-2011-000637
78. Vandenplas Y, Steenhout P, Planoudis Y, Grathwohl D. Althera Study G. Treating cow's milk protein allergy: a double-blind randomized trial comparing two extensively hydrolysed formulas with probiotics. Acta Paediatr. (2013) 102:990–8. doi: 10.1111/apa.12349
79. Chassard C, Wouters TD, Lacroix C. Probiotics tailored to the infant: A window of opportunity. Curr Opin Biotechnol. (2014) 26C:141–147. doi: 10.1016/j.copbio.2013.12.012
80. Candy DC, Van Ampting MT, Oude Nijhuis MM, Wopereis H, Butt AM, Peroni DG, et al. A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants. Pediatr Res. (2018) 83:677–86. doi: 10.1038/pr.2017.270
81. Canani RB, Di Costanzo M, Bedogni G, Amoroso A, Cosenza L, Di Scala C, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow's milk allergy: 3-year randomized controlled trial. J Aller Clin Immunol. (2017) 139:1906. doi: 10.1016/j.jaci.2016.10.050
82. Yanru. Characterization of fecal microbiota short-chain fatty acids concentrations in children with cow milk protein allergy. Northeast Agricultural University (2018).
83. Rabe H, Lundell AC, Sjöberg F, Ljung A, Strömbeck A, Gio-Batta M, et al. Neonatal gut colonization by Bifidobacterium is associated with higher childhood cytokine responses. Gut Microbes. (2020) 12:1847628. doi: 10.1080/19490976.2020.1847628
84. Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. (2004) 28:405–40. doi: 10.1016/j.femsre.2004.01.003
85. Song J, Li Y, Li J, Wang H, Zhang Y, Suo H. Lactobacillus rhamnosus 2016SWU050601 regulates immune balance in ovalbumin-sensitized mice by modulating expression of the immune-related transcription factors and gut microbiota. J Sci Food Agr. (2020) 100:4930–9. doi: 10.1002/jsfa.10554
86. Zhang LL, Chen X, Zheng PY, Luo Y, Lu GF, Liu ZQ, et al. Oral Bifidobacterium modulates intestinal immune inflammation in mice with food allergy. J Gastroenterol Hepatol. (2010) 25:928–34. doi: 10.1111/j.1440-1746.2009.06193.x
87. Inoue Y, Iwabuchi N, Xiao JZ, Yaeshima T, Iwatsuki K. Suppressive effects of bifidobacterium breve strain M-16V on T-helper type 2 immune responses in a murine model. Biol Pharmaceut Bull. (2009) 32:760–3. doi: 10.1248/bpb.32.760
88. Shida K, Takahashi R, Iwadate E, Takamizawa K, Yasui H, Sato T, et al. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin Exper Aller. (2002) 32:563–70. doi: 10.1046/j.0954-7894.2002.01354.x
89. Murosaki S, Yamamoto Y, Ito K, Inokuchi T, Kusaka H, Ikeda H, et al. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J Aller Clin Immunol. (1998) 102:57–64. doi: 10.1016/S0091-6749(98)70055-7
90. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. (2014) 69:1008–1025. doi: 10.1111/all.12429
91. Aitoro R, Simeoli R, Amoroso A, Paparo L, Nocerino R, Pirozzi C, et al. Extensively hydrolyzed casein formula alone or with L. rhamnosus GG reduces β-lactoglobulin sensitization in mice. Pediatr Aller Immunol. (2017) 28:230–7. doi: 10.1111/pai.12687
92. McGowan EC, Keet CA. Primary prevention of food allergy in children and adults: systematic review. Pediatrics. (2014) 134:S138. doi: 10.1542/peds.2014-1817J
93. Thang CL, Baurhoo B, Boye JI, Simpson BK, Zhao X. Effects of Lactobacillus rhamnosus GG supplementation on cow's milk allergy in a mouse model. Aller Asthma Clin Immunol. (2011) 7:1–9. doi: 10.1186/1710-1492-7-20
94. Rodovalho VDR, Luz SRD B, Rabah H, Carmo LRD F, Guédon E. Extracellular vesicles produced by the probiotic Propionibacterium freudenreichii CIRM-BIA 129 mitigate inflammation by modulating the NF-κB pathway. Front Microbiol. (2020) 11:1544. doi: 10.3389/fmicb.2020.01544
95. Dotterud CK, Avershina E, Sekelja M, Simpson MR, Ien T. Does maternal perinatal probiotic supplementation alter the intestinal microbiota of mother and child? J Pediatr Gastroenterol Nutr. (2015) 61:200–7. doi: 10.1097/MPG.0000000000000781
96. Nentwich I, Szepfalusi ZS, Kunz C, Spuergin P, Urbanek R. Antigenicity for humans of cow milk caseins, casein hydrolysate and casein hydrolysate fractions. Acta Veterinaria Brno. (2004) 73:291–8. doi: 10.2754/avb200473020291
97. Wang HT, Anvari S, Anagnostou K. The role of probiotics in preventing allergic disease. Children. (2019) 6:24. doi: 10.3390/children6020024
98. Strachan DP. Hay fever, hygiene, and household size. BMJ. (1989) 299:1259. doi: 10.1136/bmj.299.6710.1259
99. Nawaz M, Ma C, Basra MA, Wang J, Xu J. Amelioration of ovalbumin induced allergic symptoms in Balb/c mice by potentially probiotic strains of lactobacilli. Benef Microbes. (2015) 6:669–78. doi: 10.3920/BM2014.0141
100. Wang S, Cui J, Jiang S, Zheng C, Zhao J, Zhang H, et al. Early life gut microbiota: Consequences for health and opportunities for prevention. Crit Rev Food Sci Nutr. (2022) 13:1–25. doi: 10.1080/10408398.2022.2158451
101. DeMuri GP, Lehtoranta LM, Eickhoff JC, Lehtinen MJ, Wald ER. Ex vivo peripheral blood mononuclear cell response to R848 in children after supplementation with the probiotic Lactobacillus acidophilus NCFM/Bifidobacterium lactis Bi-07. Benef Microbes. (2021) 12:85–93. doi: 10.3920/BM2020.0068
102. Li N, Yu Y, Chen X, Gao S, Zhang Q, Xu C. Bifidobacterium breve M-16V alters the gut microbiota to alleviate OVA-induced food allergy through IL-33/ST2 signal pathway. J Cell Physiol. (2020) 235:9464–73. doi: 10.1002/jcp.29751
103. Wang HF, Zhang QX, Niu YH, Zhang X, Lu RR. Surface-layer protein from Lactobacillus acidophilus NCFM attenuates tumor necrosis factor-alpha-induced intestinal barrier dysfunction and inflammation. Int J Biol Macromol. (2019) 136:27–34. doi: 10.1016/j.ijbiomac.2019.06.041
104. Chen X, Zhao X, Hu Y, Zhang B, Zhang Y, Wang S. Lactobacillus rhamnosus GG alleviates beta-conglycinin-induced allergy by regulating the T cell receptor signaling pathway. Food Funct. (2020) 11:10554–10567. doi: 10.1039/D0FO02124E
105. Capurso L. Thirty years of Lactobacillus rhamnosus GG a review. J Clin Gastroenterol. (2019) 53:S1–41. doi: 10.1097/MCG.0000000000001170
106. Aziz N, Bonavida B. Activation of natural killer cells by probiotics. For Immunopathol Dis Therap. (2016) 7:41–55. doi: 10.1615/ForumImmunDisTher.2016017095
107. Bu G, Luo Y, Zhang Y, Chen F. Effects of fermentation by lactic acid bacteria on the antigenicity of bovine whey proteins. J Sci Food Agric. (2010) 90:2015–20. doi: 10.1002/jsfa.4046
108. Fugl A, Berhe T, Kiran A, Hussain S, Hansen EB. Characterisation of lactic acid bacteria in spontaneously fermented camel milk and selection of strains for fermentation of camel milk. Int Dairy J. (2017) 73:19–24. doi: 10.1016/j.idairyj.2017.04.007
109. Azad MA, Sarker M, Li T, Yin J. Probiotic species in the modulation of gut microbiota: an overview. Biomed Res Int. (2018) 2018:9478630. doi: 10.1155/2018/9478630
110. Finamore A, Roselli M, Britti MS, Merendino N, Mengheri E. Lactobacillus rhamnosus GG and Bifidobacterium animalis MB5 induce intestinal but not systemic antigen-specific hyporesponsiveness in ovalbumin-immunized rats. J Nutr. (2012) 142:375–81. doi: 10.3945/jn.111.148924
111. Kieliszek M, Pobiega K, Piwowarek K, Kot AM. Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules. (2021) 26:1858. doi: 10.3390/molecules26071858
112. Huang M, Li X, Wu Y, Meng X, Tong P, Yuan J. Potential allergenicity and hydrolysis assessment of bovine casein and β-casein by treatment with lactic acid bacteria. J Food Biochem. (2022) 46:1–9. doi: 10.1111/jfbc.14424
113. Donato KA, Gareau MG, Wang YJJ, Sherman PM. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling. Microbiology. (2010) 156:3288. doi: 10.1099/mic.0.040139-0
114. Yuping A, Changyu A, A. brief review of the development history of domestic infant formula from phase with an analysis on their intrisinc qualities. China Dairy Ind. (2004) 4:26–8. doi: 10.3969/j.issn.1001-2230.2004.04.008
115. Goh A, Muhardi L, Ali A, Liew WK, EstradaReyes E, Zepeda-Ortega B, et al. Differences between peptide profiles of extensive hydrolysates and their influence on functionality for the management of cow's milk allergy: a short review. Front Allergy. (2022) 3:950609. doi: 10.3389/falgy.2022.950609
116. dos Santos SC, Konstantyner T, Cocco RR. Effects of probiotics in the treatment of food hypersensitivity in children: a systematic review. Allergol Immunopathol. (2020) 48:95–104. doi: 10.1016/j.aller.2019.04.009
117. Guest JF, Fuller GW. Effectiveness of using an extensively hydrolyzed casein formula supplemented with Lactobacillus rhamnosus GG compared with an extensively hydrolysed whey formula in managing cow's milk protein allergic infants. J Comp Eff Res. (2019) 8:1317–26. doi: 10.2217/cer-2019-0088
118. Bertelsen RJ, Jensen ET, Ringel-Kulka T. Use of probiotics and prebiotics in infant feeding. Best Pract Res Clin Gastroenterol. (2016) 30:39–48. doi: 10.1016/j.bpg.2016.01.001
119. Nocerino R, Coppola S, Carucci L, de Giovanni di Santa Severina AF, Oglio F, Bedogni G, et al. The step-down approach in children with cow's milk allergy: results of a randomized controlled trial. Allergy. (2023) 78:2477–86. doi: 10.1111/all.15750
120. Nocerino R, Di Costanzo M, Bedogni G, Cosenza L, Maddalena Y, Di Scala C, et al. Dietary treatment with extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG prevents the occurrence of functional gastrointestinal disorders in children with cow's milk allergy. J Pediatr. (2019) 213:137–42. doi: 10.1016/j.jpeds.2019.06.004
Keywords: cow's milk allergy, probiotics, regulation, hypoallergenic infant formula, mechanism
Citation: Lin M and Yanjun C (2024) Research progress on the mechanism of probiotics regulating cow milk allergy in early childhood and its application in hypoallergenic infant formula. Front. Nutr. 11:1254979. doi: 10.3389/fnut.2024.1254979
Received: 08 July 2023; Accepted: 22 January 2024;
Published: 14 February 2024.
Edited by:
Rita Nocerino, University of Naples Federico II, ItalyReviewed by:
Youyou Lu, Huazhong Agricultural University, ChinaEnza D'Auria, Vittore Buzzi Children's' Hospital, Italy
Copyright © 2024 Lin and Yanjun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Yanjun, Y29uZ3lqQHRoLmJ0YnUuZWR1LmNu
 Mao Lin
Mao Lin Cong Yanjun
Cong Yanjun