
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 10 January 2024
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1342100
This article is part of the Research Topic Nutritional Approaches in Chronic Liver Diseases View all 17 articles
Background: The relationship between sarcopenia and cirrhosis is unclear. In this research, our aim is to evaluate the prevalence of sarcopenia among individuals with liver cirrhosis and its correlation with survival and mortality risks.
Methods: We conducted searches on PubMed, Web of Science, EMBASE, and Cochrane for English articles published up to July 10, 2023, and additionally manually searched the bibliography of relevant articles. We incorporated research on sarcopenia in patients with cirrhosis to examine the connection between sarcopenia and the likelihood of survival and mortality. Statistical analyses were carried out utilizing the Stata version 15.1 software. Depending on the heterogeneity of the results, we employed either fixed-effects models or random-effects models for data synthesis. To assess publication bias, we employed funnel plots and conducted Egger’s test.
Results: We included 40 studies involving 8,945 patients with cirrhosis. The overall prevalence of cirrhosis was 41% (95% CI 34%–48%). Male patients and those with liver cirrhosis and hepatic encephalopathy had a higher prevalence of sarcopenia (44% for male patients and 48% for hepatic encephalopathy patients). Sarcopenia emerged as a risk factor for both survival (HR = 2.57, 95% CI 2.02–3.27, p < 0.001) and mortality (HR = 2.13, 95% CI 1.86–2.44, p < 0.001) in patients with cirrhosis. Subgroup analyses consistently yielded the same results for study sites, whether HCC patients were excluded from the cohort, whether patients were from the liver transplant cohort or had undergone tips surgery, the definition of sarcopenia (L3-SMI or other methods), and the diagnostic criteria used by patients. The presence of sarcopenia was also a significant risk factor for hepatic encephalopathy [HR = 2.27, 95% CI (1.76–2.94), p < 0.001].
Conclusion: This systematic review and meta-analysis reveal that patients with cirrhosis have a prevalence of sarcopenia of 41% and is associated with survival rate and mortality rate. Therefore, we should attach importance to the screening of sarcopenia in patients with cirrhosis, early detection of susceptible populations, and appropriate measures to reduce the occurrence and adverse outcomes.
Systematic review registration:https://www.crd.york.ac.uk/PROSPERO/#recordDetails.
Cirrhosis is the advanced stage of chronic liver diseases and is mainly attributed to hepatitis B or C virus infection, alcohol consumption, non-alcoholic fatty liver disease, and autoimmune diseases (1). This disease has resulted in more than 1.3 million deaths, making it one of the leading global causes of mortality (2). With disease progression, many complications follow, including ascites, variceal bleeding, and hepatic encephalopathy (HE), which considerably affect the prognosis of patients with cirrhosis (3). HE is defined as a spectrum of nonspecific neurological or psychiatric abnormalities ranging from subclinical alterations to coma. It is typically induced by liver failure and/or portal vein-systemic shunting. As one of the primary complications of late-stage cirrhosis, HE has an incidence rate of about 20% to 80% (4). In patients with cirrhosis, elevated ammonia concentrations, brain edema, and increased intracranial pressure can contribute to varying degrees of HE (5, 6). HE is associated with a poor prognosis, often necessitating frequent hospitalization, imposing socio-economic and psychological burdens on patients and their families, and ultimately reducing the overall survival rate (7). Therefore, it is pressingly urgent to find more risk factors for cirrhosis.
Sarcopenia is a prevalent concern in individuals diagnosed with liver cirrhosis (8). Characterized by the decline in muscle mass, strength, and physical performance (9), sarcopenia has been detected in 14% to 55% of cirrhosis patients, prompting growing interest among researchers (10). The liver holds a central position in nutrient metabolism, and the diminishment of liver functional reserves can result in a range of complications, including malnutrition and the development of sarcopenia (11). Previous studies have revealed that sarcopenia in cirrhosis patients may be attributed to liver metabolic dysfunction, reduced appetite, increased muscle autophagy, elevated serum myostatin levels, catabolic effects of systemic inflammation induced by intestinal bacterial translocation, and low testosterone levels (10). Sarcopenia in cirrhosis patients is associated with a grim prognosis (12), such as reduced quality of life, elevated hepatic venous pressure gradients, complications related to portal hypertension (such as ascites and upper gastrointestinal varices), infections (including urinary tract infections and spontaneous peritonitis) and HE (10). Reduced extrahepatic ammonia clearance in patients with sarcopenia may contribute to HE to some extent (13).
It has been demonstrated that sarcopenia affects both hepatic encephalopathy and mortality in cirrhosis patients (13), and the decline in muscle mass in cirrhosis patients for more than a year also carries unfavorable prognostic implications (14). A meta-analysis of 22 studies has confirmed that sarcopenia is an independent predictor of increased mortality in cirrhosis patients (15). The progressive and systemic loss of skeletal muscle mass and strength in patients with sarcopenia also indicates, to some extent, the poor prognosis of LC patients (16). Therefore, we have embarked on an up-to-date and more exhaustive meta-analysis to comprehensively investigate the consequences of sarcopenia in individuals with cirrhosis. We aim to appraise the survival rates and mortality rates in cirrhotic patients with sarcopenia. The secondary objectives include appraising the prevalence of sarcopenia in cirrhotic patients and examining the impact of sarcopenia on LC and HE.
This meta-analysis followed the updated PRISMA (2020) and MOOSE guidelines (17–19), and the study protocol was registered with PROSPERO (CRD42023458935).
We conducted a comprehensive search on PubMed, Embase, Cochrane, and Web of Science, spanning from the inception of these databases to July 10, 2023, with the language limited English. The combination of medical subject heading (MeSH) terms and their free-form words was used as the search strategy, such as sarcopenia [MeSH Terms] AND liver cirrhosis [MeSH Terms] OR hepatic encephalopathy [MeSH Terms]. Supplementary Table S1 provides detailed search strategies for all the included databases. We restricted our search to studies involving human subjects to maintain relevance. In our pursuit of thoroughness, we expanded our search efforts to include conference abstracts from major events such as the 2019–2020 American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL), Asia-Pacific Association for the Study of the Liver (APASL), Digestive Disease Week (DDW), and Asian Pacific Digestive Week (APDW) in the hopes of identifying additional research. Lastly, we conducted a manual examination of the reference lists of the studies included in our analysis and relevant systematic reviews and meta-analyses to identify any potential studies that may have been missed through our initial searches.
The research explored the association between sarcopenia and liver cirrhosis or liver cirrhosis accompanied by hepatic encephalopathy.
Study eligibility criteria were based on the following PICO format: P (population): people with sarcopenia and cirrhosis (patients with or without hepatic encephalopathy were recorded separately); I (intervention/predictors): NA; C (comparator): people without sarcopenia; O (outcome): correlation between sarcopenia and cirrhosis (i.e., prevalence of sarcopenia, risk of survival and death, impact of myasthenia gravis and hepatic encephalopathy); S (study design): Observational studies (i.e., longitudinal, cross-sectional, and case control studies).
The studies were excluded for the following reasons: (1) comments, editorials, letters, posters, case reports, reviews, meta-analyses, conference abstracts, guidelines, and animal experiments; (2) no clear diagnostic criteria for cirrhosis and/or sarcopenia; (3) without enough data or unavailability of full-texts; (4) not published in English.
The titles and abstracts were screened independently using a pre-planned list of inclusion and exclusion criteria. Information such as varying criteria for defining sarcopenia, the general health status of patients, country of origin, recruitment background (hospital or nursing home), or research environment (cohort or cross-section) was not excluded. Following the inclusion criteria, we included studies that involved the population diagnosed with liver cirrhosis (LC) and provided some original data related to sarcopenia (prevalence rate, survival rate, mortality rate, etc.). Further meta-analyses were performed with patients stratified by the presence or absence of hepatocellular carcinoma as a covariate.
The quality of the included studies was assessed using the modified Newcastle–Ottawa scale (20). The scale consists of three sections with eight entries, which are as follows: (1) representativeness of the exposed cohort; (2) selection of the non-exposed cohort; (3) identification of exposure; (4) demonstration that no results of interest were found at the start of the study; (5) comparability of the cohorts based on design or analysis results controlled for confounders; (6) evaluation of results; (7) follow-up of sufficient duration to obtain results; and (8) appropriateness of follow-up to the population. The total score is 9 stars. Each study was independently assessed by two authors, and studies with NOS scores ≥6 were considered of high quality. Any disputed articles were referred to a third researcher, XW, for discussion and resolution of any disagreements.
We compiled data from each of the included studies utilizing a standardized table. The subsequent details were independently extracted by two assessors, YC and MZ: the primary author’s name, publication year, study design, research location, the origin of the cirrhosis cohort (transplant waiting list or general population), the definition of sarcopenia, the methodology employed for muscle mass measurement, the number of participants, patient demographics, and clinical characteristics, including age, gender, cirrhosis etiology, hepatocellular carcinoma (HCC) presence, and pertinent outcome measures such as sarcopenia incidence, survival rate, and mortality in cirrhosis patients. When the required data were not readily accessible, we made contact with the authors to secure the essential study information.
We conducted the statistical analysis using Stata version 15.1. The forest plot illustrates the overall effect of the analysis. Heterogeneity among the studies was assessed using I2. In general, I2 ≥ 50% indicated significant heterogeneity among the studies, leading us to adopt a random-effects model. We analyzed the source of heterogeneity through sensitivity analysis (one-by-one exclusion method). Additionally, pre-planned subgroup analyses were performed based on gender, age, etiology of cirrhosis, and study site. We employed meta-regression to determine the effects of sample size, mean age of participants, proportion of males, proportion of patients with alcohol-related liver disease, viral hepatitis, and the presence or absence of hepatocellular carcinoma (HCC) on the adjusted pooled hazard ratios (HR) for survival rate and mortality. Conversely, I2 < 50% was considered indicative of small heterogeneity across studies. We used funnel plots and Egger’s tests (21) to examine the possibility of publication bias. A p-value <0.05 (two-tailed test) was considered statistically significant.
Out of the 15,895 articles initially identified, we excluded 3,257 duplications and 4,339 articles classified as reviews, guidelines, letters, and animal experiments. An additional 8,199 papers were excluded based on a preliminary review of titles and abstracts. After carefully reviewing the full texts of the remaining 138 articles, we included 40 cohort studies with data from 8,945 patients (Figure 1).
Table 1 summarizes the characteristics of all the studies encompassed in this meta-analysis. Out of the 40 studies, 18 were conducted in Asian regions (14, 22–24, 26, 29, 33, 35, 37, 38, 40, 44, 45, 48, 50, 53, 55, 59), and the remaining 22 studies were from non-Asian regions (13, 25, 27, 28, 30–32, 34, 36, 39, 41–43, 46, 47, 49, 51, 52, 54, 56–58). Among these 40 studies, 37 were cohort studies, 2 were cross-sectional studies (43, 57), and 1 was a case-control study (35). Specifically, 19 were retrospective cohort studies, and 18 were prospective cohort studies (13, 14, 27, 29, 30, 32, 33, 38–41, 49–57). The sample sizes in the included studies varied, ranging from 52 to 675 participants. In these studies, the mean age of patients ranged from 51 to 73 years. Notably, 24 studies (13, 14, 22–24, 27–29, 31, 33–35, 37, 38, 42, 44, 45, 48, 55, 57, 59) excluded all patients with hepatocellular carcinomas (HCC), while in 10 studies (28, 31, 37, 39, 46, 47, 49, 54, 55, 57), a proportion of patients ranging from 19 to 100% had HCC. Additionally, 8 studies (26, 39, 43, 46, 52, 54) neither explicitly excluded HCC patients nor reported the prevalence of HCC.
All studies were rated high quality with NOS scores ≥6. Among them, there were 5 studies (26, 28, 35, 55, 56) with 6 scores, 20 studies (13, 23, 24, 27, 32, 34, 36, 37, 39, 40, 43, 45, 47, 48, 51, 53, 54, 57–59) with 7 scores, and 15 studies (14, 22, 23, 29–31, 33, 38, 41, 42, 44, 46, 49, 50, 52) with 8 scores.
Out of the 40 studies included, 34 studies (22–38, 40–45, 47–56, 58) with a total of 7,024 participants provided data on prevalence of sarcopenia, resulting in a pooled prevalence of 41% (RR = 41, 95% CI: 34%–48%, p < 0.001). Due to significant heterogeneity between studies (I2 = 97.8%), as depicted in Figure 2A, subgroup analyses were conducted based on gender, the definition of sarcopenia, diagnostic criteria, and etiology of cirrhosis. The results revealed variations in prevalence between these subgroups. In particular, male patients exhibited a greater overall prevalence of sarcopenia in contrast to female patients. Patients diagnosed with sarcopenia according to the EWSOP2 criteria had a higher prevalence compared to those using other criteria (RR = 50, 95% CI: 44%–56%, p < 0.001). Furthermore, there was a higher prevalence of sarcopenia among patients in the liver transplant cohort compared to other patients (RR = 46, 95% CI: 17%–76%, p < 0.001). Notably, the prevalence of sarcopenia, as defined by the skeletal muscle area at the third lumbar spine (L3-SMA), was higher in the European population (RR = 67, 95% CI: 32%–100%, p < 0.001) compared to the Asian population (Europe, RR = 59, 95% CI: 38%–80%, p < 0.001; Asia, RR = 34, 95% CI: 25%–43%, p < 0.001). Surprisingly, the prevalence of patients with or without hepatocellular carcinoma was nearly the same (HCC, RR = 38, 95% CI: 26%–51%, p < 0.001; non-HCC, RR = 39, 95% CI: 31%–47%, p < 0.001). Furthermore, subgroup analyses indicated that factors such as gender, criteria for defining sarcopenia, methods of measuring muscle mass, patient origin, patient’s country, and the inclusion of HCC patients in the cohort were not sources of heterogeneity (Table 2). The result of Egger’s test indicated the presence of publication bias (p = 0.001).
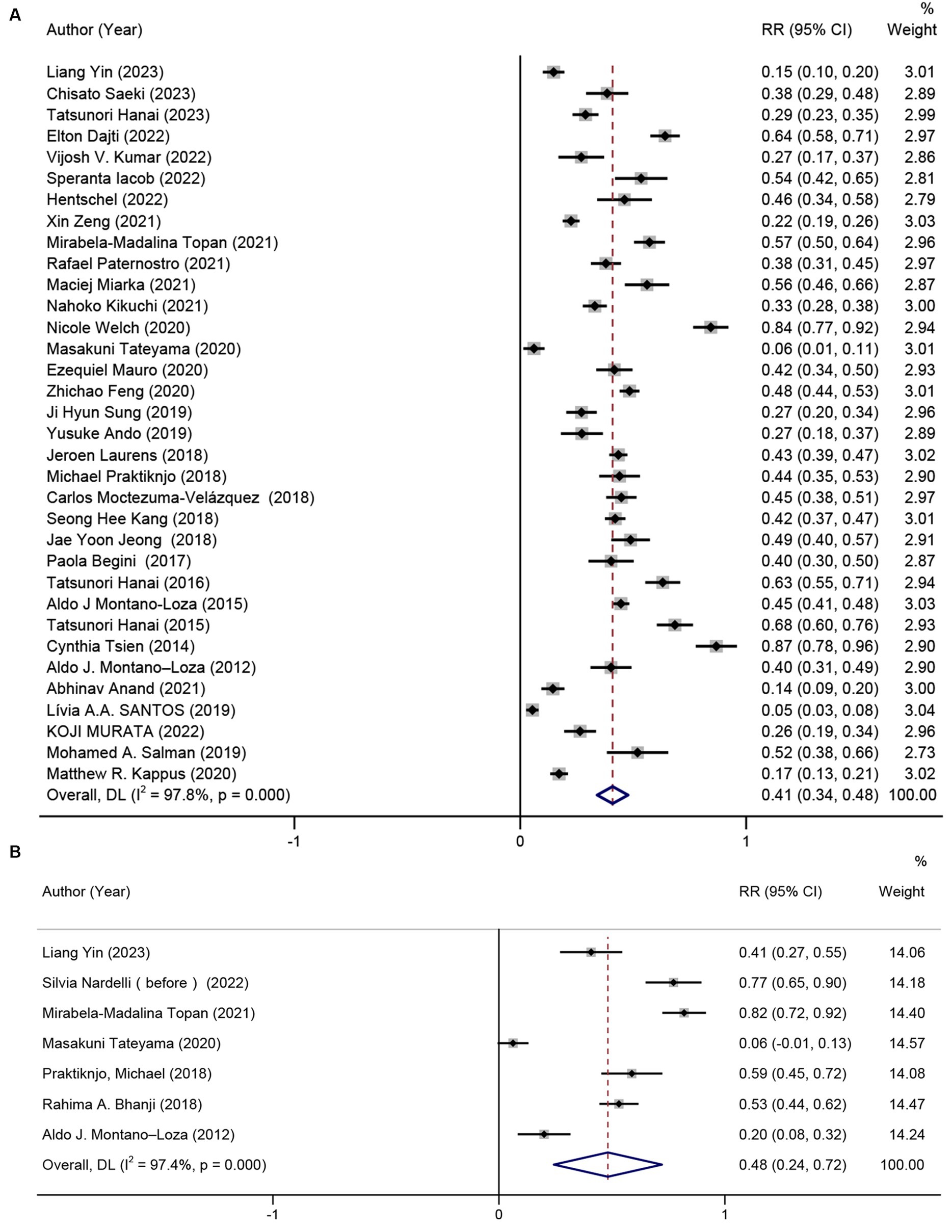
Figure 2. Prevalence of sarcopenia in patients with (A) cirrhosis, and (B) hepatic encephalopathy due to cirrhosis.
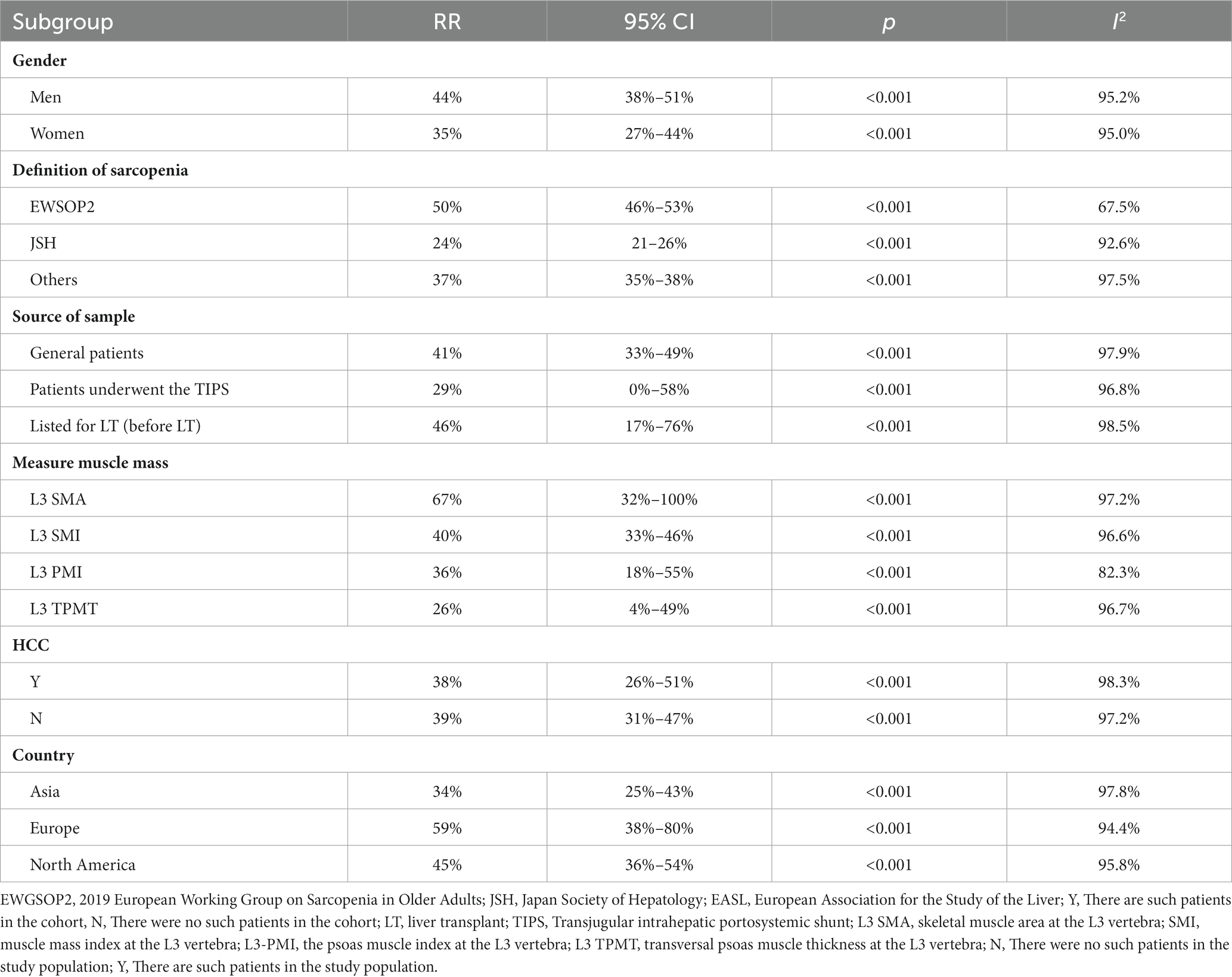
Table 2. Subgroup analysis of the correlation between depression and Internet addiction based on gender, definition of sarcopenia, measure muscle mass, HCC and country.
The prevalence of sarcopenia was reported in seven studies (13, 22, 30, 35, 42, 46, 52) (n = 426). The results unraveled that the pooled prevalence of sarcopenia was 48% (95% CI: 27%–72%, p < 0.001) in patients with cirrhosis and hepatic encephalopathy (Figure 2B). The presence of hepatic encephalopathy increased the prevalence of sarcopenia. However, there was significant heterogeneity among the studies (I2 = 97.6%). Egger’s test showed no significant publication bias (p = 0.217).
The HR results from the other three studies (13, 22, 46) were combined, revealing that sarcopenia was a risk factor for hepatic encephalopathy (HR = 2.27, 95% CI: 1.76–2.94, p < 0.001). Slightly greater heterogeneity was detected between the studies (I2 = 53%) (Figure 3A). The result of Egger’s test yielded a p-value of 0.323.
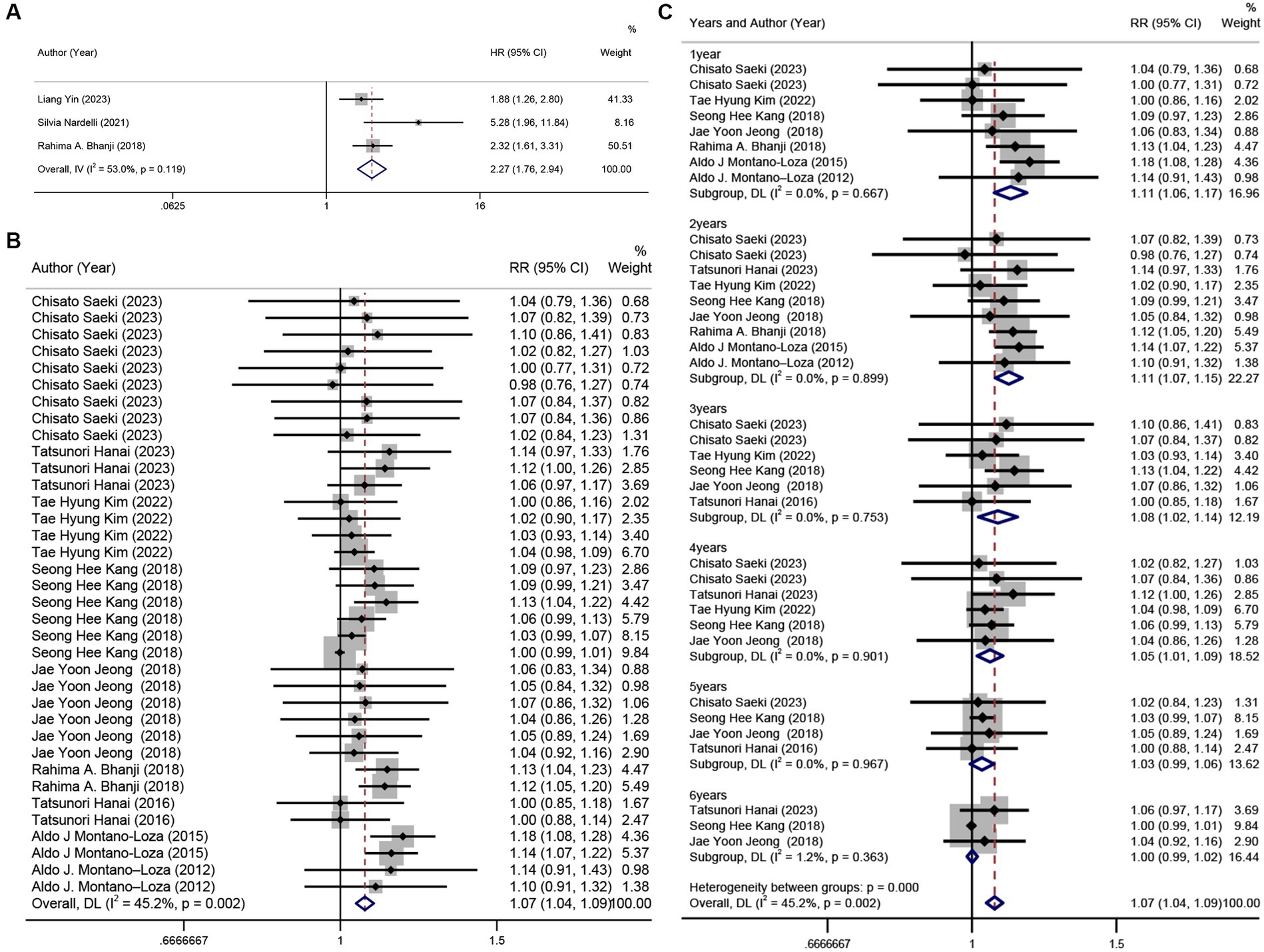
Figure 3. Forest plot. (A) univariate analysis of the incidence of hepatic encephalopathy in patients with sarcopenia and cirrhosis, (B) 6 years cumulative survival rate in patients with or without sarcopenia, (C) annual survival rate subgroups in patients with and without sarcopenia.
Ten studies (14, 23, 24, 44–46, 48, 49, 52, 59) (n = 1,218) were included in this analysis. The results revealed that sarcopenia in patients with cirrhosis was a risk factor for a 6-year survival rate (RR = 1.07, 95% CI: 1.04–1.09, p < 0.001), with low heterogeneity between studies (I2 = 45.2%) (Figure 3B). However, based on the result of Egger’s test (p < 0.001), there was evidence of publication bias among the studies. Subgroup analysis by time (years) showed that sarcopenia had a more pronounced impact on survival rates in the first 4 years, which decreased with time (Figure 3C).
By analyzing the HR for cirrhosis survival and sarcopenia in seven of these studies (14, 22, 29, 42, 44, 47, 50) (n = 1,973), we found that sarcopenia was a barrier to survival (HR = 2.57, 95% CI: 2.02–3.27), with relatively low heterogeneity between studies (I2 = 48.7%) (Figure 4A).
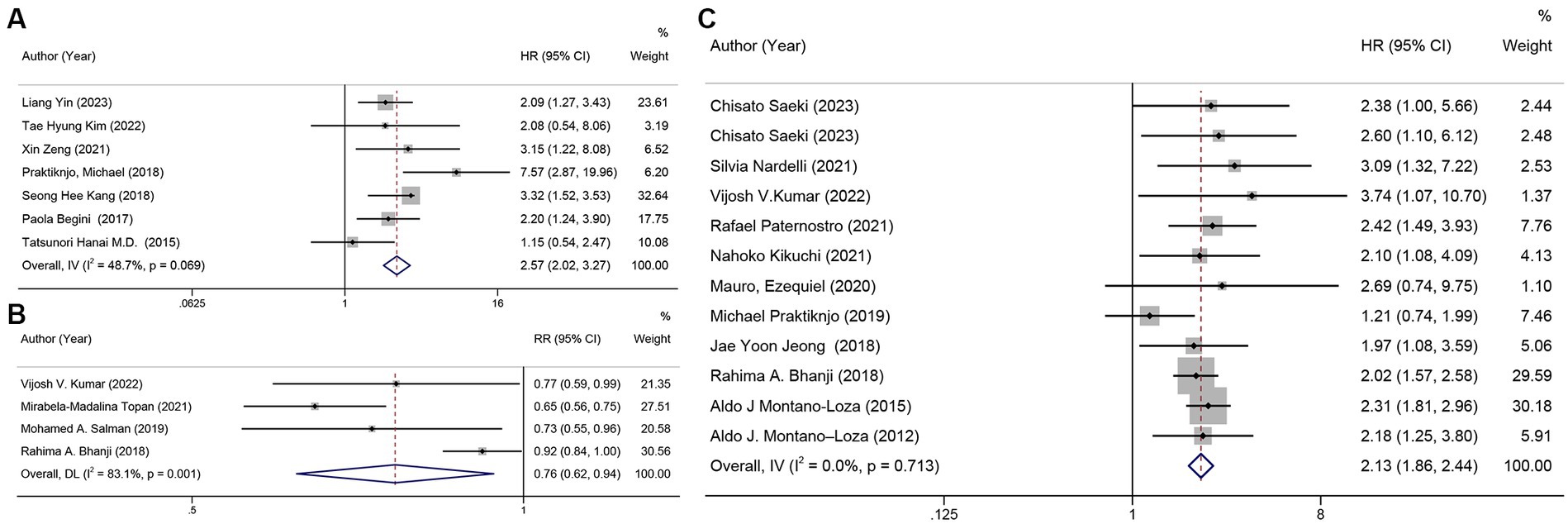
Figure 4. Forest plot. (A) univariate analysis of sarcopenia and survival rate, (B) mortality in patients with sarcopenia and cirrhosis, (C) univariate analysis of mortality in patients with sarcopenia and cirrhosis.
When excluding the cohort that included HCC patients, a separate analysis of patients without HCC revealed a pooled HR of 2.44 (95% CI: 1.85–3.22). Furthermore, sarcopenia was associated with an over 2-fold reduction in survival rates in patients with liver cirrhosis, regardless of the study site, whether HCC patients were excluded from the cohort, whether patients underwent liver transplant or TIPS surgery, whether sarcopenia was defined by L3-SMI or other methods, and diagnostic criteria (Supplementary Figures S1–S3). The funnel plot is shown in Supplementary Figure S4.
The mortality in patients with cirrhosis across four studies (26, 30, 46, 56) was meta-analyzed, and the pooled mortality rate was 76% (95% CI: 62%–94%, p = 0.011). There was a high likelihood of heterogeneity between the studies (I2 = 80.7%) (Figure 4B). In the sensitivity analysis, we excluded one study (46) after careful examination, and the pooled corrected mortality risk ratio (RR) was similar to that of the main analysis, at 68% (95% CI: 61%–77%, p < 0.001). Heterogeneity was very low (I2 = 0%). The result of Egger’s test was p = 0.464.
In a univariate analysis of data from 12 studies (13, 23, 26, 31, 33, 36, 39, 45, 46, 49, 52, 59) (n = 1,781), it was found that sarcopenia was a risk factor for mortality with a combined unadjusted hazard ratio (HR) of 2.13 (95% CI: 1.86–2.44, p < 0.001). The heterogeneity between studies was very low (I2 = 0%) (Figure 4C), and Egger’s test revealed no publication bias in the studies and that the combined HR was reliable (p = 0.959). The funnel plot is shown in Supplementary Figure S5.
In all subgroups of the mortality analysis, including country, definition of sarcopenia, presence of HCC, and source of the sample, sarcopenia consistently emerged as a risk factor for mortality. Regardless of the patient’s country and region, the definition standard of sarcopenia, and the method of measuring muscle mass, whether the cohort included ALD patients or excluded HCC patients, the HR for mortality in sarcopenia patients was consistently around 2 times higher (Supplementary Figures S6–S9).
In this analysis of 40 studies involving 8,945 patients with cirrhosis, the prevalence of sarcopenia in the cirrhotic population was found to be higher (41%) than that (33%) reported in a previous study (60). A higher prevalence was observed in male patients (44%) and those with hepatic encephalopathy (48%). The findings also illustrated a robust correlation between sarcopenia and both the survival rate and mortality in cirrhosis patients. This correlation was substantiated by subgroup analyses based on study location, sample origin, diagnostic criteria, and methodologies. Furthermore, the analysis outcomes indicated a substantial connection between sarcopenia and hepatic encephalopathy in individuals with liver cirrhosis.
A previous systematic reviews and meta-analyses included four articles that assessed the relationship between sarcopenia and cirrhosis. However, one study only pooled retrospective cohorts of patients with sarcopenia and hepatic encephalopathy (61), another study did not include hepatic encephalopathy in the analysis (15), one study solely focused on the prevalence of sarcopenia in patients with cirrhosis (60). A different meta-analysis included a large number of patients after liver transplantation, but all included studies were retrospective observational cohort studies (62). In contrast, our study conducted a more comprehensive and extensive search, additionally analyzed hepatic encephalopathy in patients with liver cirrhosis, and incorporated approximately 50% prospective studies. To ensure the quality of the study, we rigorously controlled the inclusion and exclusion criteria for articles and performed subgroup and sensitivity analyses for all outcomes.
This research revealed that the general prevalence of sarcopenia in individuals with liver cirrhosis stood at 41%, with a notably elevated prevalence among male patients. This finding was in line with previous analyses (15, 60), which could be attributed to the predominance of males in the study samples (67.08%). Furthermore, we established a strong link between sarcopenia and an increased risk of death in cirrhotic patients. Patients with sarcopenia exhibited a higher mortality rate and a 2.13-fold elevated risk of death compared to those without sarcopenia. Sarcopenia posed a significant obstacle to survival. A previous study has demonstrated a higher incidence of complications and a reduced quality of life in patients with sarcopenia (27). The survival rate of patients with sarcopenia was 2.57 times lower than that of patients without sarcopenia. In a subgroup analysis of survival, the pooled HR was higher in patients receiving transjugular intrahepatic portocaval shunts (TIPS) than in the general population (3.71 vs. 2.35). This suggests that TIPS had a more significant impact on survival, and we hypothesized that sarcopenia played a role in worsening survival among cirrhotic patients receiving TIPS. The combined HR was also higher in the cohort containing hepatocellular carcinoma (HCC) patients compared to the cohort without HCC patients (3.38 vs. 2.31), indicating that HCC had a more pronounced effect on survival rates. This highlights the connection between sarcopenia and 5 years cumulative survival in cirrhotic patients.
From a pathophysiologic point of view, sarcopenia results from an imbalance between protein synthesis and degradation caused by multiple pathways. Chronic sarcopenia is characterized by loss of muscle mass, metabolic and biochemical abnormalities, and disruption of protein homeostasis throughout the body (63). Increased hepatic gluconeogenesis in cirrhotic patients is most likely due to limited hepatic glycogen content and insulin resistance, resulting in insufficient supply of branched-chain amino acids and glucose to muscle cells. In addition, recent clinical trials have found testosterone deficiency in cirrhotic patients, indicating increased muscle cell apoptosis and myogenic protein activity (64). Sarcopenia may be also induced by chronic catabolic conditions, such as cachexia due to cirrhosis, increased energy consumption, and decreased food intake due to loss of appetite. Maintaining muscle mass and avoiding rapid loss of muscle mass and transition to sarcopenia appear to be critical for the prognosis of patients with cirrhosis. Therefore, improving survival and quality of life by monitoring body composition and screening for sarcopenia should be a priority in the clinical management of cirrhosis.
Our analysis suggests that sarcopenia constitutes a risk factor for hepatic encephalopathy. In the context of hepatic encephalopathy, ammonia plays a pivotal role in the development of HE in individuals with liver cirrhosis. Previous studies have also demonstrated that the toxicity of ammonia impacts muscles and other organs. In chronic liver disease, muscles play a critical compensatory role in ammonia clearance. However, as the ammonia clearance rate decreases in cirrhotic patients, the compensatory function of patients with sarcopenia weakens. This results in the influx of ammonia into the blood in substantial quantities, greatly increasing the likelihood of hepatic encephalopathy. During hyperammonemia, the loss of muscle mass and subsequent impairment, induced by mitochondrial dysfunction and reduced adenosine triphosphate, or altered protein modification due to various factors, contribute to the onset of sarcopenia, creating a vicious cycle (65). Diagnosing hepatic encephalopathy is vital for the prognosis of patients with liver cirrhosis. However, due to equipment and personnel constraints, hepatic encephalopathy is not routinely examined in clinical practice (66). Therefore, medical staff should be attentive to such patients and implement more targeted screening and interventions for their benefit. The earlier sarcopenia is detected, the sooner the prevention and treatment programs can be initiated to prevent significant impact on HE.
The limitations of this article can be summarized as follows. First, the patient characteristics of the included studies were inconsistent, such as their demographics (i.e., the severity of and causes of cirrhosis) and methods used to determine sarcopenia. This inconsistency may have contributed to the observed heterogeneity. To address this issue, future studies should standardize diagnostic criteria as much as possible to include more patients with cirrhosis and/or sarcopenia with the same characteristics. Second, the majority of the studies included in our analysis were observational cohort studies, potentially introducing bias due to variations in statistical methods, diagnostic criteria, cut-off values for defining sarcopenia, and the distribution of viral liver disease, alcoholic liver disease, and the percentage of cases involving hepatocellular carcinoma (HCC). We attempted to mitigate these limitations through subgroup and sensitivity analyses. Nevertheless, it is imperative to adopt standardized definitions and criteria when conducting meta-analyses of individual sarcopenia data to better elucidate the prevalence of sarcopenia and provide a more comprehensive description of cirrhosis patients with sarcopenia. Finally, we only included studies published in English and may have excluded relevant studies published in other languages, so there may be biases.
In conclusion, this systematic review and meta-analysis establishes that patients with cirrhosis have a prevalence of sarcopenia of 41%, with up to half of these individuals developing cirrhosis due to either alcoholic liver disease or viral hepatitis. Moreover, sarcopenia is closely associated with the survival and mortality rates in patients with cirrhosis, the study reveals that sarcopenia is linked to a greater than twofold rise in the risk of mortality and a decline in survival rates across most subgroups. Hepatic encephalopathy may interact with sarcopenia in patients with cirrhosis. Based on our comprehensive analysis, sarcopenia should be included as an integral component of the initial assessment for all cirrhosis patients.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
YC: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MZ: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. JG: Conceptualization, Formal analysis, Investigation, Writing – review & editing. JJ: Conceptualization, Formal analysis, Investigation, Writing – review & editing. HW: Conceptualization, Supervision, Writing – review & editing. XW: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1342100/full#supplementary-material
1. Tsochatzis, EA, Bosch, J, and Burroughs, AK. Liver cirrhosis. Lancet. (2014) 383:1749–61. doi: 10.1016/s0140-6736(14)60121-5
2. World Health Organization. Global health estimates 2019: deaths by cause, age, sex, by country and by region, 2000–2019 World Health Organization (2020). Available at: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death
3. Engelmann, C, Clària, J, Szabo, G, Bosch, J, and Bernardi, M. Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. (2021) 75:S49–s66. doi: 10.1016/j.jhep.2021.01.002
4. Marrone, G, Serra, A, Miele, L, Biolato, M, Liguori, A, Grieco, A, et al. Branched chain amino acids in hepatic encephalopathy and sarcopenia in liver cirrhosis: evidence and uncertainties. World J Gastroenterol. (2023) 29:2905–15. doi: 10.3748/wjg.v29.i19.2905
5. Garcia-Tsao, G, Abraldes, JG, Berzigotti, A, and Bosch, J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. (2017) 65:310–35. doi: 10.1002/hep.28906
6. Tranah, TH, Ballester, MP, Carbonell-Asins, JA, Ampuero, J, Alexandrino, G, Caracostea, A, et al. Plasma ammonia levels predict hospitalisation with liver-related complications and mortality in clinically stable outpatients with cirrhosis. J Hepatol. (2022) 77:1554–63. doi: 10.1016/j.jhep.2022.07.014
7. Kok, B, Whitlock, R, Ferguson, T, James Bailey, R, Warren Burak, K, Kowalczewski, J, et al. Health-related quality of life: a rapid predictor of hospitalization in patients with cirrhosis. Am J Gastroenterol. (2020) 115:575–83. doi: 10.14309/ajg.0000000000000545
8. Nishikawa, H, Fukunishi, S, Asai, A, Nishiguchi, S, and Higuchi, K. Sarcopenia and frailty in liver cirrhosis. Life. (2021) 11:399. doi: 10.3390/life11050399
9. Hari, A. Muscular abnormalities in liver cirrhosis. World J Gastroenterol. (2021) 27:4862–78. doi: 10.3748/wjg.v27.i29.4862
10. Maslennikov, R, Alieva, A, Poluektova, E, Zharikov, Y, Suslov, A, Letyagina, Y, et al. Sarcopenia in cirrhosis: prospects for therapy targeted to gut microbiota. World J Gastroenterol. (2023) 29:4236–51. doi: 10.3748/wjg.v29.i27.4236
11. Saeki, C, Kinoshita, A, Kanai, T, Ueda, K, Nakano, M, Oikawa, T, et al. The geriatric nutritional risk index predicts sarcopenia in patients with cirrhosis. Sci Rep. (2023) 13:3888. doi: 10.1038/s41598-023-31065-1
12. Lee, PC, Lee, KC, Yang, TC, Lu, HS, Cheng, TY, Chen, YJ, et al. Sarcopenia-related gut microbial changes are associated with the risk of complications in people with cirrhosis. JHEP Rep. (2023) 5:100619. doi: 10.1016/j.jhepr.2022.100619
13. Nardelli, S, Riggio, O, Gioia, S, Merli, M, Spagnoli, A, di Martino, M, et al. Risk factors for hepatic encephalopathy and mortality in cirrhosis: the role of cognitive impairment, muscle alterations and shunts. Dig Liver Dis. (2022) 54:1060–5. doi: 10.1016/j.dld.2021.12.015
14. Kim, TH, Jung, YK, Yim, HJ, Baik, JW, Yim, SY, Lee, YS, et al. Impacts of muscle mass dynamics on prognosis of outpatients with cirrhosis. Clin Mol Hepatol. (2022) 28:876–89. doi: 10.3350/cmh.2022.0231
15. Tantai, X, Liu, Y, Yeo, YH, Praktiknjo, M, Mauro, E, Hamaguchi, Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. (2022) 76:588–99. doi: 10.1016/j.jhep.2021.11.006
16. Shibamoto, A, Namisaki, T, Suzuki, J, Kubo, T, Iwai, S, Tomooka, F, et al. Hemoglobin and endotoxin levels predict sarcopenia occurrence in patients with alcoholic cirrhosis. Diagnostics. (2023) 13:2218. doi: 10.3390/diagnostics13132218
17. Stroup, DF, Berlin, JA, Morton, SC, Olkin, I, Williamson, GD, Rennie, D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
18. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:71. doi: 10.1136/bmj.n71
19. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
20. Norris, JM, Simpson, BS, Ball, R, Freeman, A, Kirkham, A, Parry, MA, et al. A modified Newcastle–Ottawa scale for assessment of study quality in genetic urological research. Eur Urol. (2021) 79:325–6. doi: 10.1016/j.eururo.2020.12.017
21. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
22. Yin, L, Chu, SL, Lv, WF, Zhou, CZ, Liu, KC, Zhu, YJ, et al. Contributory roles of sarcopenia and myosteatosis in development of overt hepatic encephalopathy and mortality after transjugular intrahepatic portosystemic shunt. World J Gastroenterol. (2023) 29:2875–87. doi: 10.3748/wjg.v29.i18.2875
23. Saeki, C, Kanai, T, Ueda, K, Nakano, M, Oikawa, T, Torisu, Y, et al. Prognostic significance of sarcopenia and severe vitamin D deficiency in patients with cirrhosis. JGH Open. (2023) 7:351–7. doi: 10.1002/jgh3.12900
24. Hanai, T, Nishimura, K, Miwa, T, Maeda, T, Imai, K, Suetsugu, A, et al. Prevalence, association, and prognostic significance of polypharmacy and sarcopenia in patients with liver cirrhosis. JGH Open. (2023) 7:208–14. doi: 10.1002/jgh3.12877
25. Dajti, E, Renzulli, M, Ravaioli, F, Marasco, G, Vara, G, Brandi, N, et al. The interplay between sarcopenia and portal hypertension predicts ascites and mortality in cirrhosis. Dig Liver Dis. (2023) 55:637–43. doi: 10.1016/j.dld.2022.11.011
26. Kumar, VV, Kothakota, SR, Nair, AK, Sasidharan, M, Kareem, H, Kanala, J, et al. Impact of sarcopenia on post-liver transplant morbidity and mortality in cirrhotic patients. Indian J Gastroenterol. (2022) 41:440–5. doi: 10.1007/s12664-022-01262-3
27. Iacob, S, Mina, V, Mandea, M, Iacob, R, Vadan, R, Boar, V, et al. Assessment of sarcopenia related quality of life using SarQoL® questionnaire in patients with liver cirrhosis. Front Nutr. (2022) 9:774044. doi: 10.3389/fnut.2022.774044
28. Hentschel, F, Schwarz, T, Lüth, S, and Schreyer, AG. Psoas muscle index predicts time to rehospitalization in liver cirrhosis: an observational study. Medicine. (2022) 101:e30259. doi: 10.1097/md.0000000000030259
29. Zeng, X, Shi, ZW, Yu, JJ, Wang, LF, Luo, YY, Jin, SM, et al. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. (2021) 12:1948–58. doi: 10.1002/jcsm.12797
30. Topan, MM, Sporea, I, Dănilă, M, Popescu, A, Ghiuchici, AM, Lupuşoru, R, et al. Impact of sarcopenia on survival and clinical outcomes in patients with liver cirrhosis. Front Nutr. (2021) 8:766451. doi: 10.3389/fnut.2021.766451
31. Paternostro, R, Bardach, C, Hofer, BS, Scheiner, B, Schwabl, P, Asenbaum, U, et al. Prognostic impact of sarcopenia in cirrhotic patients stratified by different severity of portal hypertension. Liver Int. (2021) 41:799–809. doi: 10.1111/liv.14758
32. Miarka, M, Gibiński, K, Janik, MK, Główczyńska, R, Zając, K, Pacho, R, et al. Sarcopenia-the impact on physical capacity of liver transplant patients. Life. (2021) 11:740. doi: 10.3390/life11080740
33. Kikuchi, N, Uojima, H, Hidaka, H, Iwasaki, S, Wada, N, Kubota, K, et al. Prospective study for an independent predictor of prognosis in liver cirrhosis based on the new sarcopenia criteria produced by the Japan Society of Hepatology. Hepatol Res. (2021) 51:968–78. doi: 10.1111/hepr.13698
34. Welch, N, Dasarathy, J, Runkana, A, Penumatsa, R, Bellar, A, Reen, J, et al. Continued muscle loss increases mortality in cirrhosis: impact of aetiology of liver disease. Liver Int. (2020) 40:1178–88. doi: 10.1111/liv.14358
35. Tateyama, M, Naoe, H, Tanaka, M, Tanaka, K, Narahara, S, Tokunaga, T, et al. Loss of skeletal muscle mass affects the incidence of minimal hepatic encephalopathy: a case control study. BMC Gastroenterol. (2020) 20:371. doi: 10.1186/s12876-020-01501-x
36. Mauro, E, Crespo, G, Martinez-Garmendia, A, Gutierrez-Acevedo, MN, Diaz, JM, Saidman, J, et al. Cystatin C and sarcopenia predict acute on chronic liver failure development and mortality in patients on the liver transplant waiting list. Transplantation. (2020) 104:e188–98. doi: 10.1097/tp.0000000000003222
37. Feng, Z, Zhao, H, Jiang, Y, He, Z, Sun, X, Rong, P, et al. Sarcopenia associates with increased risk of hepatocellular carcinoma among male patients with cirrhosis. Clin Nutr. (2020) 39:3132–9. doi: 10.1016/j.clnu.2020.01.021
38. Sung, JH, Uojima, H, Hidaka, H, Tanaka, Y, Wada, N, Kubota, K, et al. Risk factors for loss of skeletal muscle mass in patients with cirrhosis. Hepatol Res. (2019) 49:550–8. doi: 10.1111/hepr.13308
39. Praktiknjo, M, Clees, C, Pigliacelli, A, Fischer, S, Jansen, C, Lehmann, J, et al. Sarcopenia is associated with development of acute-on-chronic liver failure in decompensated liver cirrhosis receiving Transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol. (2019) 10:e00025. doi: 10.14309/ctg.0000000000000025
40. Ando, Y, Ishigami, M, Ito, T, Ishizu, Y, Kuzuya, T, Honda, T, et al. Sarcopenia impairs health-related quality of life in cirrhotic patients. Eur J Gastroenterol Hepatol. (2019) 31:1550–6. doi: 10.1097/meg.0000000000001472
41. van Vugt, JLA, Alferink, LJM, Buettner, S, Gaspersz, MP, Bot, D, Darwish Murad, S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. (2018) 68:707–14. doi: 10.1016/j.jhep.2017.11.030
42. Praktiknjo, M, Book, M, Luetkens, J, Pohlmann, A, Meyer, C, Thomas, D, et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology. (2018) 67:1014–26. doi: 10.1002/hep.29602
43. Moctezuma-Velázquez, C, Low, G, Mourtzakis, M, Ma, M, Burak, KW, Tandon, P, et al. Association between low testosterone levels and sarcopenia in cirrhosis: a cross-sectional study. Ann Hepatol. (2018) 17:615–23. doi: 10.5604/01.3001.0012.0930
44. Kang, SH, Jeong, WK, Baik, SK, Cha, SH, and Kim, MY. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle. (2018) 9:860–70. doi: 10.1002/jcsm.12333
45. Jeong, JY, Lim, S, Sohn, JH, Lee, JG, Jun, DW, and Kim, Y. Presence of sarcopenia and its rate of change are independently associated with long-term mortality in patients with liver cirrhosis. J Korean Med Sci. (2018) 33:e299. doi: 10.3346/jkms.2018.33.e299
46. Bhanji, RA, Moctezuma-Velazquez, C, Duarte-Rojo, A, Ebadi, M, Ghosh, S, Rose, C, et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. (2018) 12:377–86. doi: 10.1007/s12072-018-9875-9
47. Begini, P, Gigante, E, Antonelli, G, Carbonetti, F, Iannicelli, E, Anania, G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol. (2017) 16:107–14. doi: 10.5604/16652681.1226821
48. Hanai, T, Shiraki, M, Ohnishi, S, Miyazaki, T, Ideta, T, Kochi, T, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res. (2016) 46:743–51. doi: 10.1111/hepr.12616
49. Montano-Loza, AJ, Duarte-Rojo, A, Meza-Junco, J, Baracos, VE, Sawyer, MB, Pang, JX, et al. Inclusion of sarcopenia within MELD (MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. (2015) 6:e102. doi: 10.1038/ctg.2015.31
50. Hanai, T, Shiraki, M, Nishimura, K, Ohnishi, S, Imai, K, Suetsugu, A, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. (2015) 31:193–9. doi: 10.1016/j.nut.2014.07.005
51. Tsien, C, Garber, A, Narayanan, A, Shah, SN, Barnes, D, Eghtesad, B, et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. (2014) 29:1250–7. doi: 10.1111/jgh.12524
52. Montano-Loza, AJ, Meza-Junco, J, Prado, CM, Lieffers, JR, Baracos, VE, Bain, VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. (2012) 10:166–73. doi: 10.1016/j.cgh.2011.08.028
53. Anand, A, Nambirajan, A, Kumar, V, Agarwal, S, Sharma, S, Mohta, S, et al. Alterations in autophagy and mammalian target of rapamycin (mTOR) pathways mediate sarcopenia in patients with cirrhosis. J Clin Exp Hepatol. (2022) 12:510–8. doi: 10.1016/j.jceh.2021.05.004
54. Santos, LAA, Lima, TB, Ietsugu, MDV, Nunes, HRC, Qi, X, and Romeiro, FG. Anthropometric measures associated with sarcopenia in outpatients with liver cirrhosis. Nutr Diet. (2019) 76:613–9. doi: 10.1111/1747-0080.12523
55. Murata, K, Namisaki, T, Fujimoto, Y, Takeda, S, Enomoto, M, Takaya, H, et al. Clinical significance of serum zinc levels on the development of sarcopenia in cirrhotic patients. Cancer Diagn Progn. (2022) 2:184–93. doi: 10.21873/cdp.10093
56. Salman, MA, Omar, HSE, Mikhail, HMS, Tourky, M, El-Ghobary, M, Elkassar, H, et al. Sarcopenia increases 1-year mortality after surgical resection of hepatocellular carcinoma. ANZ J Surg. (2020) 90:781–5. doi: 10.1111/ans.15647
57. Ebadi, M, Bhanji, RA, Dunichand-Hoedl, AR, Mazurak, VC, Baracos, VE, and Montano-Loza, AJ. Sarcopenia severity based on computed tomography image analysis in patients with cirrhosis. Nutrients. (2020) 12:3463. doi: 10.3390/nu12113463
58. Kappus, MR, Wegermann, K, Bozdogan, E, Patel, YA, Janas, G, Shropshire, E, et al. Use of skeletal muscle index as a predictor of wait-list mortality in patients with end-stage liver disease. Liver Transpl. (2020) 26:1090–9. doi: 10.1002/lt.25802
59. Saeki, C, Kanai, T, Ueda, K, Nakano, M, Oikawa, T, Torisu, Y, et al. Osteosarcopenia predicts poor survival in patients with cirrhosis: a retrospective study. BMC Gastroenterol. (2023) 23:196. doi: 10.1186/s12876-023-02835-y
60. Mazeaud, S, Zupo, R, Couret, A, Panza, F, Sardone, R, and Castellana, F. Prevalence of sarcopenia in liver cirrhosis: a systematic review and meta-analysis. Clin Transl Gastroenterol. (2023) 14:e00584. doi: 10.14309/ctg.0000000000000584
61. Chang, KV, Chen, JD, Wu, WT, Huang, KC, Lin, HY, and Han, DS. Is sarcopenia associated with hepatic encephalopathy in liver cirrhosis? A systematic review and meta-analysis. J Formos Med Assoc. (2019) 118:833–42. doi: 10.1016/j.jfma.2018.09.011
62. Kim, G, Kang, SH, Kim, MY, and Baik, SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. (2017) 12:e0186990. doi: 10.1371/journal.pone.0186990
63. Ebadi, M, Bhanji, RA, Mazurak, VC, and Montano-Loza, AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. (2019) 54:845–59. doi: 10.1007/s00535-019-01605-6
64. Wing, SS, Lecker, SH, and Jagoe, RT. Proteolysis in illness-associated skeletal muscle atrophy: from pathways to networks. Crit Rev Clin Lab Sci. (2011) 48:49–70. doi: 10.3109/10408363.2011.586171
65. Jindal, A, and Jagdish, RK. Sarcopenia: ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. (2019) 25:270–9. doi: 10.3350/cmh.2019.0015
66. Vilstrup, H, Amodio, P, Bajaj, J, Cordoba, J, Ferenci, P, Mullen, KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. (2014) 60:715–35. doi: 10.1002/hep.27210
Keywords: sarcopenia, cirrhosis, hepatic encephalopathy, survival rate, mortality
Citation: Cui Y, Zhang M, Guo J, Jin J, Wang H and Wang X (2024) Correlation between sarcopenia and cirrhosis: a meta-analysis. Front. Nutr. 10:1342100. doi: 10.3389/fnut.2023.1342100
Received: 21 November 2023; Accepted: 27 December 2023;
Published: 10 January 2024.
Edited by:
Norma Marroni, Federal University of Rio Grande do Sul, BrazilReviewed by:
Elizangela Schemitt, Clinical Hospital of Porto Alegre, BrazilCopyright © 2024 Cui, Zhang, Guo, Jin, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinran Wang, eHdzaWN1MjAxMUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.