
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 21 November 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1289395
Objectives: The objective of this analysis was to evaluate the effect of a diet rich in animal protein and low in glycemic index on blood pressure during pregnancy.
Design: This post hoc, secondary data analysis of a randomized controlled trial, evaluated blood pressure in pregnant participants who were randomized either to an ad libitum diet with high protein and low glycemic index, rich in dairy and seafood, or an ad libitum control diet according to national recommendations.
Setting: The study occurred in pregnant women in Copenhagen, Denmark.
Sample: A total of 279 pregnant females with overweight or obesity were enrolled.
Methods and outcome measure: Blood pressure was measured at 5 timepoints during pregnancy from gestational week 15 through week 36, and blood pressure between groups was compared.
Results: There were no differences between diet arms in systolic or diastolic blood pressure over time. There were also no differences in most blood-pressure-related pregnancy complications, including the prevalence of premature birth, preeclampsia, or hypertension, but the frequency of total cesarean sections was lower in the active than the control group (16 out of 104 vs. 30 out of 104) (p = 0.02).
Conclusion: Increased animal protein intake was not associated with changes in blood pressure in pregnant women with overweight or obesity.
Clinical trial registration: [ClinicalTrials.gov], identifier [NCT01894139].
During pregnancy, women are confronted with an onslaught of new and sometimes conflicting information about how best to maintain health through this period of metabolic adaptations (1). Differing recommendations complexify prenatal nutritional decision making; and leave questions both for mothers and healthcare providers about how best to support a healthy pregnancy through diet. Some healthcare providers like doctors and midwives lack the training to properly counsel on prenatal nutrition but still serve as a primary source of advice for pregnant women, and cultural beliefs may also conflict with national recommendations (2, 3). On top of this, current research provides differing assessments of the risk and benefit profiles of dietary components further confounding attempts for women to eat optimally during this time (4, 5).
This concern is particularly relevant for women with overweight or obesity [defined as a body mass index (BMI) ≥25 kg/m2 or ≥ 30 kg/m2, respectively], because excess body weight and body fat during pregnancy are associated with many health risks, including increased risk of cesarean section, premature delivery, excess birthweight, abnormal glucose and lipid metabolism, hypertensive disorders, postpartum hemorrhage and neonatal asphyxia (6–8). Hypertensive disorders, in particular, are associated with an increased risk of pulmonary edema, placental abruption, small-for-gestational age, and even perinatal death (9).
Not all prenatal nutrition recommendations are ambiguous. The importance of folate in early pregnancy to prevent neural tube defects and the detrimental effects of excessive alcohol consumption on offspring cognition have been well-documented, as has the importance of other micronutrients like choline and calcium, and omega-3 fatty acids (10–12). However, oftentimes, the evidence is less clear, for example when it comes to the macronutrient composition of the diet and individual food sources. Some studies support a dairy-rich diet for prevention of hypertensive disorders, while others have found no association (13–19). Relatedly, there is evidence that a diet rich in vegetable protein, but not animal protein, is inversely associated with blood pressure, though many of these effects do not persist once dietary confounders are considered and once results are adjusted for weight and BMI (20). It has also been hypothesized that the amino acid composition of different proteins, rather than the protein food source is the driving force behind the differing impacts on blood pressure, but here too, the results are mixed (21). Furthermore, some studies suggest diets high in fiber are optimal for blood pressure, but others provide mixed results (22). Ultimately though, few studies have examined the effect of the whole dietary pattern on blood pressure during pregnancy.
To address this fundamental gap in the literature, this secondary data analysis aimed to investigate the effect of consuming a diet rich in animal protein from dairy, seafood, and meat, with low glycemic index and load, versus consuming a diet following the Nordic Nutrition Recommendations, on blood pressure in pregnant females with overweight or obesity using data from the interventional study “An optimized programming of healthy children” (APPROACH) (23). We hypothesized that the additional dairy-derived protein and low glycemic index would be associated with beneficial changes in blood pressure regulation during pregnancy.
The APPROACH study was a randomized controlled trial (RCT) conducted at Copenhagen University Hospital Herlev-Gentofte and the Department of Nutrition, Exercise and Sports (NEXS) at the University of Copenhagen, Denmark, from January 2014 to December 2017. This secondary data analysis includes data from the dietary intervention period of the study, until offspring birth. The females and their partners provided signed written informed consent before commencing participation. The study was approved by the Ethics Committee of the Capital Region of Denmark (H-3-2013-119) and registered at clinicaltrials.gov (NCT01894139).
The APPROACH study included 279 pregnant females (completers gave birth to 209 infants); this secondary data analysis includes all the participants from the original RCT. Recruitment procedures have been described in detail previously (23). Briefly, females were included from early second trimester (14 weeks and 3 days to 15 weeks and 4 days) and were included if they planned to give birth at Herlev hospital, had a pre-pregnancy BMI of 28–45 kg/m2, were older than 18 years of age, and were carrying a singleton pregnancy. Females were excluded for multiple pregnancies, dairy intolerance or allergy, weight loss greater than 10 kg over the last year, alcohol or drug abuse (defined as >14 units alcohol per week pre-pregnancy), or an eating disorder or other disease that might interfere with the intervention. Additionally, females with an oral glucose tolerance test diagnostic of gestational diabetes mellitus were excluded.
The APPROACH study was a two-arm, single-blinded RCT, and has been described previously in detail (23). Briefly, the study was conducted in an outpatient setting in Denmark and the intervention lasted from early second trimester through birth. Prenatal females were randomized either to an ad libitum diet with high protein and low glycemic index (HPLGI), which was rich in dairy, seafood, and meat, or an ad libitum control diet with moderate protein and moderate glycemic index (MPMGI)—both aligned with the principles of the New Nordic Diet (23). The nutrient composition of the intervention and control diets is presented in Table 1.
Habitual diet and adherence to the interventional diets was assessed through use of a Food Frequency Questionnaire (FFQ), based on a standardized and validated FFQ by Andersen et al. (24). The FFQ was administered at 15, 28, and 36 weeks of gestation, and was validated by two 24-h recalls at gestational weeks 21 and 32 (Supplementary Figure S1). Intakes of energy and individual nutrients were then calculated using DankostPro software (Kraftvaerk FoodTech, Denmark). Since the two experimental diets differed considerably in protein intake (25–28 vs. 15–18% of energy in the HPLGI and MPMGI diets, respectively), we assessed adherence to the dietary intervention objectively, by measuring urinary urea excretion (23).
Maternal height was measured to the nearest 0.5 cm at screening by using a wall mounted stadiometer (Seca, Germany). Pre-pregnancy weight was obtained either through self-report or through the participants’ medical records. Subsequent maternal body weights were measured to the nearest gram at screening, and at each of the 9 dietary counseling sessions throughout the intervention using a medical scale (Tanita, Illinois, United States) with the female being barefoot and in light clothing or undergarments. Gestational weight gain (GWG) was calculated as [last reported weight before birth – pre-pregnancy weight].
Blood pressure was measured by sphygmomanometer by the midwives at baseline (between gestational weeks 13–15), and then again at gestational week 21 (visit 3), between weeks 24 and 26 (visit 4), week 32 (visit 6), and between weeks 35 and 36 (visit 7) (Supplementary Figure S1). A calibrated blood pressure device approved by local medical authorities was used for each measurement, although the specific brand and model varied between and within participants. Per standard clinical practice, participants sat for at least 5 min prior to measurement, were instructed not to speak before and during measurement, and the cuff was placed on a bare arm (25). Blood pressure was measured once, unless, at the midwives’ discretion, an abnormal result prompted a second measurement. Mean arterial pressure was calculated as diastolic blood pressure + (systolic blood pressure – diastolic blood pressure)/3 (26).
Sample size calculations for the APPROACH study are based on the original primary outcome of the study, which was GWG in the maternal participants (23). There was no further sample size calculation done for this secondary analysis. Baseline characteristics of the participants were analyzed by independent t-test for continuous variables and are presented as mean ± standard deviation. For categorical variables, baseline characteristics were evaluated by chi-square test and presented as absolute counts and relative frequencies (Table 2). Dietary intake during the study was compared between groups by independent t-test and presented as mean ± standard deviation using an average from the 24-h recall data or from the FFQ data, depending on the method of evaluation for each dietary component reported (Table 3). The effect of the diet on blood pressure was evaluated using a linear mixed model which included time and group (intervention/control) as fixed factors, a group-by-time interaction, and subject ID as random intercept. This type of model was selected as it allows for missing values and is most suitable when there may be correlations between repeated measures. Systolic, diastolic and mean arterial pressures were analyzed in separate linear mixed models. A model with adjustment for GWG was also constructed. Frequency of blood pressure-related complications between the two groups was evaluated by a chi-square test. The data were visually assessed for statistical assumptions, and there were no clear violations in normality, linearity, and homoscedasticity in the residual plots. A two-sided significance of p < 0.05 was used, and the analyses were carried out in SPSS Statistics for Mac version 28 (SPSS inc. Chicago, Ill, United States).
The whole sample included 279 pregnant females, with 141 of them assigned to the intervention group and 138 to the control group (Table 2). Participants were 30.4 ± 4.8 years old, with a pre-pregnancy BMI of 34.2 ± 3.8 kg/m2, and most of them were white (260 of 279). There were no significant differences in age, race, BMI, education level, blood pressure, weight, smoking status, alcohol use, or parity between the groups at baseline (Table 2). Over the course of the intervention, 70 of the 279 randomized females (25%) dropped out of the study, with no difference between groups. The females in the intervention group had an average GWG of 6.8 ± 1.3 kg, which was 1.7 kg less (p < 0.05) than those in the control group who gained an average of 8.5 ± 1.3 kg; both groups had a GWG that fell within the Institute of Medicine guidelines (23). A total of 100 (35.8%) of the participants experienced a blood pressure value above normal limits (120/80 mm Hg) at some point during pregnancy, though this did not differ by group (p = 0.594). Average vitamin D levels during pregnancy were 67 nmoL/L.
As per allocation, females in the intervention group increased their consumption of dairy protein to 10% of total energy intake, whereas those in the control group continued to consume roughly 5% of total energy as dairy protein, with the majority of dairy being low-sodium high-protein yogurt and skyr (p < 0.001) (Table 3). The HPLGI group consumed roughly 3.5% more of their daily energy intake from seafood protein than the MPMGI group, but polyunsaturated fatty acid intake was not different between groups (10.4 g/d in the MPMGI group, 10.5 g/d in the HPLGI group). The intervention and control groups consumed an average of 1,415 and 1,106 mg of calcium per day, respectively, from dairy, supplements, and other food sources (Table 3). On average, the participants daily consumed 2.7 g of potassium and 262 mg of magnesium, and approximately 2.4 g of sodium (Table 3). Sodium intakes were similar between groups. As reported previously, dietary adherence, assessed by urinary urea excretion, was high in both intervention groups and did not differ between them (22).
In the unadjusted analysis, there was a significant effect of time with systolic blood pressure increasing during pregnancy (Figure 1A), but there was no significant effect of diet on systolic blood pressure (p = 0.790). There was also no significant group-by-time interaction (Table 4). In the GWG-adjusted analysis (the only known potential confounder that differed between groups), the effect of group remained non-significant (p = 0.760) (Table 4). Similar results were obtained for diastolic blood pressure (Figure 1B and Table 5) and mean arterial pressure (Table 6).
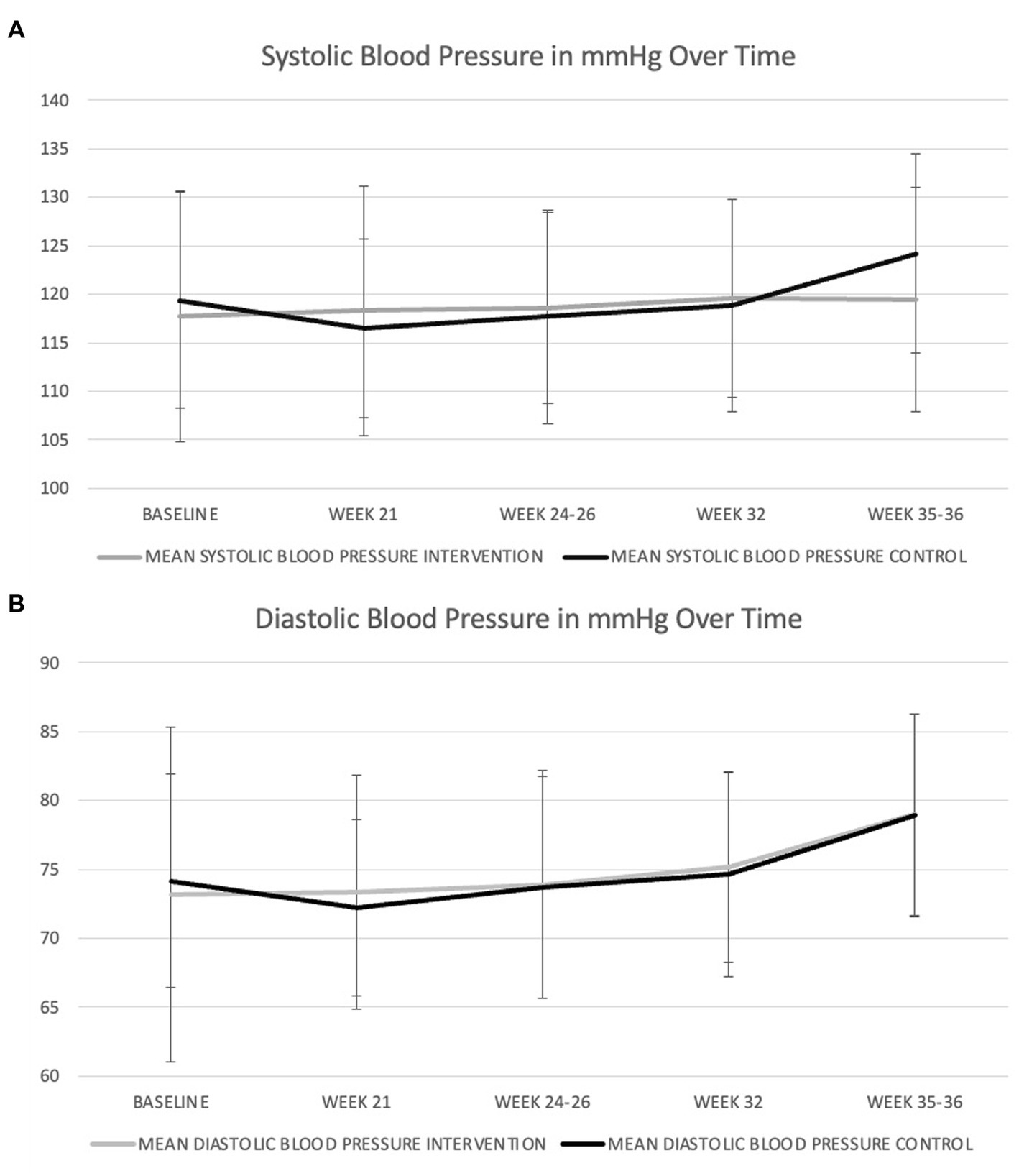
Figure 1. Changes in systolic (A) and diastolic (B) blood pressure during pregnancy in women consuming the dairy-rich diet and those consuming the average Danish diet. Data are means and standard deviations.
There were no differences between groups in the prevalence of premature birth, preeclampsia, or hypertension. There was, however, a significant difference in the prevalence of planned and total cesarean sections between groups, though there was no difference in acute cesarean sections (Table 7). There was no difference between groups in regard to previous complicated pregnancies, an important medical history which can lead to subsequent planned cesarean section. When pregnancy and birth complications were evaluated as a composite score, there were more complications in the control group than in the experimental group (p = 0.008).
Blood pressure increased during pregnancy in our participants, particularly during the last trimester, but it was largely unaffected by the experimental diet consumed during pregnancy, or the amount of GWG, and did not enter the hypertensive range for most participants. Although 40% of the participants experienced a blood pressure value above normal limits (120/80 mm Hg) at some point during pregnancy, there were few hypertension-related adverse pregnancy outcomes in either group, and diet did not seem to be significantly associated with their prevalence, except in the case of planned cesarean section. These results indicate that a diet rich in dairy, seafood, and meat, which follows the national recommendations, is not associated with unfavorable changes in blood pressure during pregnancy or adverse maternal outcomes.
Obesity is associated with higher serum parathyroid hormone (PTH) levels derived from increased vitamin D deposition in adipose tissue. This reduces systemic vitamin D bioavailability and subsequently impairs the negative feedback mechanism between vitamin D and PTH (19, 20). The obesity-driven increase in PTH could give rise to an increase in intracellular calcium and a proportional decrease in extracellular calcium—preventing the purported action of calcium on blood pressure and increasing vascular resistance (21). Furthermore, active calcium transport from the intestine depends on the binding of calcitriol [1,25(OH)2D3] to its vitamin D receptor and is diminished when vitamin D status is impaired (22). Accordingly, the physiological effects of dairy-derived micronutrients, which were consumed to greater amounts in the experimental group, may be attenuated in subjects with obesity.
The United States Endocrine Society recommends a 25-hydroxyvitamin D level of around 75 nmoL/L as normal during pregnancy, though some literature suggests a level of 100 nmoL/L for optimal maternal and offspring outcomes (27, 28). However, in Denmark, sufficiency is set much lower—at only 50 nmoL/L (29). Despite supplementation with 38 μg/d vitamin D throughout the study, well-above the recommended intake for Denmark, females in the APPROACH trial had average vitamin D levels of 67 nmoL/L—below the US Endocrine Society recommendations (29). At this level, though, it is possible that the females had suboptimal calcium absorption contributing to the lack of an effect of additional dairy-derived calcium on blood pressure and contributing to nearly half of them having an elevated blood pressure measurement at least once during the study.
Another potential mechanism involves obesity and the inhibition of magnesium and potassium’s role in nitric oxide production and endothelial relaxation and vasodilation (30). In obesity, an increased abundance of pro-inflammatory cytokines is associated with decreased nitric oxide and increased endothelial vasoconstriction—possibly countering the dairy-derived magnesium nitric oxide production, and both magnesium and potassium’s role in endothelial relaxation (31–33). Additionally, normal pregnancy is associated with increased oxidative stress, which is exacerbated in obesity (34). If persistent, the increased reactive oxygen species formation has the potential to inactivate nitric oxide (35, 36). This, too, can increase endothelial vasoconstriction and counteract the beneficial effects of magnesium and potassium on blood pressure, thereby contributing to a lack of an effect of the additional dairy consumption on blood pressure (32, 33).
Despite the high consumption of dairy foods by the participants in the experimental group, intakes of some of the blood pressure-associated micronutrients found in dairy was—in both groups—below the Nordic Nutrition Recommendations (i.e., potassium and magnesium) (29). Also, although both groups of women consumed adequate amounts of calcium; and clinical trials observing a significant reduction in blood pressure with 500–2,000 mg/d calcium supplementation did so at a higher supplemental dosage than APPROACH intakes (29, 37, 38). It is therefore conceivable that intake of these micronutrients was not sufficient to cause a beneficial effect on blood pressure in our participants, particularly given the added physiological challenges of pregnancy and obesity. Though statistically significant, the differences between groups in fish intake, a food typically rich in anti-inflammatory omega-3 fatty acids, may not have been large enough to induce clinically meaningful changes in any of the reported outcomes. This could also explain a potentially similar lack of effect from differences in glycemic index and fiber. However, given the conflicting results on the impact of glycemic index and fiber on blood pressure, it is also possible that this aspect of the prenatal diet does not play a critical role in the regulation of blood pressure during pregnancy. Most relevant studies have been observational, and none has thoroughly explored potential mechanisms (22).
The pregnancies in the present study were largely uncomplicated, particularly in regard to outcomes that might have been influenced by obesity and blood pressure. The consumption of similar amounts of fat, but with larger differences in carbohydrates, glycemic index, and protein, had no impact on the blood pressure responses during pregnancy in this at-risk group of women. Furthermore, the women consumed relatively high amounts of saturated fat, above the recommendations of <10% of daily intake, and close to the upper limit of the recommendation for sodium (2.4 g/d) with no adverse impact on their health during pregnancy, or on the health of their offspring (29). Both diets encouraged women to consume a variety of foods that fit within the guidelines of their assigned dietary intake pattern—those in the experimental group adhered to an ad libitum diet with high protein and low glycemic index, which was rich in dairy, seafood, and meat, and those in the control group to an ad libitum diet with moderate protein and moderate glycemic index.
The study had several strengths. The combination of several FFQ and 24-HR has been shown to increase precision of food intake estimates—crucial in nutrition and diet studies (39). Furthermore, following and obtaining data on the women from early second trimester until just prior to birth allowed us more accurately understand the trajectory of blood pressure and its interplay with diet throughout pregnancy. Furthermore, this study is the first of its kind in pregnant women with obesity.
However, the study also had several limitations. Blood pressure was not recorded after gestational week 37 and until birth, when a potential spike in blood pressure might have occurred. The relatively small sample size might have increased the likelihood of type 2 error, particularly regarding detecting blood-pressure related pregnancy complications. Lastly, a challenge of secondary data analysis is the inability to control the data collection process retrospectively. Though there is a standard for blood pressure measurement in Denmark, the protocol had no standard operating procedure for blood pressure, and there was no case report form confirming details which may alter blood pressure reading, including type of cuff used (manual or digital), placement of cuff over clothing, talking during the measurement, body positioning during measurement, and amount of time resting beforehand (40, 41). There is also no standard of care requirement for repeated measurements of blood pressure by the midwives involved in the treatment of the participants during pregnancy. Similarly, pre-pregnancy body weight was obtained either from the electronic medical record or self-report, increasing potential for reporting bias. Furthermore, the dietary intake might have been under-reported, as is common among people with overweight or obesity (42). However, the assessment of urea nitrogen reflected a high degree of adherence to, and a clear separation between, the experimental diets. Lastly, the APPROACH intervention was conducted in Copenhagen, Denmark, in a relatively homogeneous population, so the possibility that the results are not generalizable to other populations cannot be discounted.
Ultimately, the two diet patterns made little difference on blood pressure physiology, contradicting the 2021 American Heart Association’s guidelines for cardiovascular health which provide counsel on appropriate protein types for optimal health (43). These results provide early evidence that a varied and healthy dietary pattern contributes to healthy and successful pregnancies, regardless of its macronutrient composition and food sources, and the accompanying small variation in GWG. While further studies powered for blood pressure as the primary outcome are needed to confirm these novel findings, this secondary data analysis of the APPROACH trial addresses a critical gap in the literature by examining the effect of whole dietary patterns on blood pressure during pregnancy and puts forth some preliminary evidence which may aid in dispelling some of the myth of the “perfect” diet for pregnancy.
The data analyzed in this study is subject to the following licenses/restrictions: data may be requested from the authors of the original paper. Requests to access these datasets should be directed to bmdlQGNzbHQuZGs=.
The studies involving humans were approved by Ethics Committee of the Capital Region of Denmark (H-3-2013-119). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
EL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. FM: Data curation, Methodology, Software, Supervision, Writing – review & editing. HZ: Writing – review & editing. JS: Writing – review & editing. AA: Writing – review & editing. NG: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received research funding from the Nordea Foundation, Danish Pig Levy Foundation, Danish Dairy Research Foundation and Danish Agriculture and Food Council, and products from LEGO Charity, PharmaNord and Pharmo Vital, though there was no funding for this secondary data analysis of the original study. None of the funders had any influence on the design, execution, data handling of the investigation, or interpretation and dissemination of the results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1289395/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Timeline of relevant study events.
APPROACH, An optimized programming of healthy children; BMI, Body Mass Index; E %, percent of total energy intake; FFQ, Food Frequency Questionnaire; PIH, Pregnancy-induced hypertension; Pre-BMI, pre-pregnancy BMI; PTH, Parathyroid hormone; RCT, Randomized Control Trial.
1. Schölmerich, VLN, Ghorashi, H, Denktaş, S, and Groenewegen, P. Caught in the middle? How women deal with conflicting pregnancy-advice from health professionals and their social networks. Midwifery. (2016) 35:62–9. doi: 10.1016/j.midw.2016.02.012
2. Abayomi, JC, Charnley, MS, McCann, MT, Cassidy, L, and Newson, LM. Healthy eating advice during pregnancy lacks depth: the views of midwives and service users. Proc Nutr Soc. (2020) 79:E728. doi: 10.1017/S0029665120007144
3. Quayyum, F, and Dombrowski, SU. Barriers to nutritional pregnancy preparation and support needs in women and men: qualitative study based on the theoretical domains framework. Womens Health. (2021) 17:17455065211042182. doi: 10.1177/17455065211042182
4. Kamenju, P, Madzorera, I, Hertzmark, E, Urassa, W, and Fawzi, WW. Higher dietary intake of animal protein foods in pregnancy is associated with lower risk of adverse birth outcomes. J Nutr. (2022) 152:2546–54. doi: 10.1093/jn/nxac183
5. Yong, HY, Mohd Shariff, Z, Mohd Yusof, BN, Rejali, Z, Tee, YYS, Bindels, J, et al. Higher animal protein intake during the second trimester of pregnancy is associated with risk of GDM. Front Nutr. (2021) 8:718792. doi: 10.3389/fnut.2021.718792
6. Farshbaf-Khalili, A, Alizadeh, M, Hajebrahimi, S, Ostadrahimi, A, Malakouti, J, and Salehi-Pourmehr, H. Pre-natal and post-natal anxiety in relation to pre-pregnancy obesity: a cohort study on Iranian pregnant women. Caspian J Intern Med. (2020) 11:250–8. doi: 10.22088/cjim.11.3.250
7. Class, QA. Obesity and the increasing odds of cesarean delivery. J Psychosom Obstet Gynecol. (2021) 43:244–50. doi: 10.1080/0167482X.2021.1967926
8. Jin, Y, Dai, X, Wang, N, Hu, Y, Quan, L, and Zhu, S. Clinical observation of effects of prepregnancy body mass index and weight gain during pregnancy on neonatal weight and delivery outcome. Evid Based Complement Alternat Med. (2021) 2021:8323189. doi: 10.1155/2021/8323189
9. Beech, A, and Mangos, G. Management of hypertension in pregnancy. Aust Prescr. (2021) 44:148–52. doi: 10.18773/austprescr.2021.039
10. Blencowe, H, Cousens, S, Modell, B, and Lawn, J. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol. (2010) 39:i110–21. doi: 10.1093/ije/dyq028
11. Flak, AL, Su, S, Bertrand, J, Denny, CH, Kesmodel, US, and Cogswell, ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcohol Clin Exp Res. (2014) 38:214–26. doi: 10.1111/acer.12214
12. Danielewicz, H, Myszczyszyn, G, Dębińska, A, Myszkal, A, Boznański, A, and Hirnle, L. Diet in pregnancy-more than food. Eur J Pediatr. (2017) 176:1573–9. doi: 10.1007/s00431-017-3026-5
13. Houston, MC, and Harper, KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens. (2008) 10:3–11. doi: 10.1111/j.1751-7176.2008.08575.x
14. Groziak, SM, and Miller, GD. Natural bioactive substances in milk and colostrum: effects on the arterial blood pressure system. Br J Nutr. (2000) 84:119–25. doi: 10.1017/s0007114500002348
15. Alonso, A, Beunza, JJ, Delgado-Rodríguez, M, Martínez, JA, and Martínez-González, MA. Low-fat dairy consumption and reduced risk of hypertension: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. (2005) 82:972–9. doi: 10.1093/ajcn/82.5.972
16. Drouin-Chartier, JP, Gigleux, I, Tremblay, AJ, Poirier, L, Lamarche, B, and Couture, P. Impact of dairy consumption on essential hypertension: a clinical study. Nutr J. (2014) 13:83. doi: 10.1186/1475-2891-13-83
17. Engberink, MF, Hendriksen, MA, Schouten, EG, van Rooij, FJ, Hofman, A, Witteman, JC, et al. Inverse association between dairy intake and hypertension: the Rotterdam study. Am J Clin Nutr. (2009) 89:1877–83. doi: 10.3945/ajcn.2008.27064
18. Tsuboi, N, Sasaki, T, and Haruhara, K. Dairy intake and the risk of incidental hypertension. Hypertens Res. (2022) 45:1511–3. doi: 10.1038/s41440-022-00966-5
19. Villaverde, P, Lajous, M, MacDonald, C-J, Fagherazzi, G, Boutron-Ruault, M-C, and Bonnet, F. Dairy product consumption and hypertension risk in a prospective French cohort of women. Nutr J. (2020) 19:12. doi: 10.1186/s12937-020-0527-2
20. Elliott, P, Stamler, J, Dyer, AR, Appel, L, Dennis, B, Kesteloot, H, et al. Association between protein intake and blood pressure: the INTERMAP study. Arch Intern Med. (2006) 166:79–87. doi: 10.1001/archinte.166.1.79
21. Poggiogalle, E, Fontana, M, Giusti, AM, Pinto, A, Iannucci, G, Lenzi, A, et al. Amino acids and hypertension in adults. Nutrients. (2019) 11:1459. doi: 10.3390/nu11071459
22. Whelton, SP, Hyre, AD, Pedersen, B, Yi, Y, Whelton, PK, and He, J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. (2005) 23:475–81. doi: 10.1097/01.hjh.0000160199.51158.cf
23. Geiker, NRW, Magkos, F, Zingenberg, H, Svare, J, Chabanova, E, Thomsen, HS, et al. A high-protein low–glycemic index diet attenuates gestational weight gain in pregnant women with obesity: the “an optimized programming of healthy children” (APPROACH) randomized controlled trial. Am J Clin Nutr. (2021) 115:970–9. doi: 10.1093/ajcn/nqab405
24. Andersen, R, Brot, C, Jakobsen, J, Mejborn, H, Mølgaard, C, Skovgaard, LT, et al. Seasonal changes in vitamin D status among Danish adolescent girls and elderly women: the influence of sun exposure and vitamin D intake. Eur J Clin Nutr. (2013) 67:270–4. doi: 10.1038/ejcn.2013.3
25. Internet. Available at: https://www.rigshospitalet.dk/undersoegelse-og-behandling/find-undersoegelse-og-behandling/Sider/Blodtryksmaaling-31222.aspx (Accessed January 5, 2022).
26. DeMers, D, and Wachs, D. Physiology, mean arterial pressure In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC (2023)
27. Mithal, A, and Kalra, S. Vitamin D supplementation in pregnancy. Indian J Endocrinol Metab. (2014) 18:593–6. doi: 10.4103/2230-8210.139204
28. Wagner, CL, and Hollis, BW. The implications of vitamin D status during pregnancy on mother and her developing child. Front Endocrinol. (2018) 9:9. doi: 10.3389/fendo.2018.00500
29. Nordic Council of Ministers. Nordic nutrition recommendations 2012: integrating nutrition and physical activity. 5th ed. Copenhagen: Nordisk Ministerråd (2014).
30. Gröber, U, Schmidt, J, and Kisters, K. Magnesium in prevention and therapy. Nutrients. (2015) 7:8199–226. doi: 10.3390/nu7095388
31. Virdis, A. Endothelial dysfunction in obesity: role of inflammation. High Blood Press Cardiovasc Prevent. (2016) 23:83–5. doi: 10.1007/s40292-016-0133-8
33. Haddy, FJ, Vanhoutte, PM, and Feletou, M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. (2006) 290:R546–52. doi: 10.1152/ajpregu.00491.2005
34. Chiarello, DI, Abad, C, Rojas, D, Toledo, F, Vázquez, CM, Mate, A, et al. Oxidative stress: normal pregnancy versus preeclampsia. Biochim Biophys Acta Mol basis Dis. (2020) 1866:165354. doi: 10.1016/j.bbadis.2018.12.005
35. Mannaerts, D, Faes, E, Gielis, J, Van Craenenbroeck, E, Cos, P, Spaanderman, M, et al. Oxidative stress and endothelial function in normal pregnancy versus pre-eclampsia, a combined longitudinal and case control study. BMC Pregnancy Childbirth. (2018) 18:60. doi: 10.1186/s12884-018-1685-5
36. Ogita, H, and Liao, J. Endothelial function and oxidative stress. Endothelium. (2004) 11:123–32. doi: 10.1080/10623320490482664
37. Ritchie, LD, and King, JC. Dietary calcium and pregnancy-induced hypertension: is there a relation? Am J Clin Nutr. (2000) 71:1371S–4S. doi: 10.1093/ajcn/71.5.1371s
38. Hofmeyr, GJ, Seuc, A, Betrán, AP, Cormick, G, Singata, M, Fawcus, S, et al. The effect of calcium supplementation on blood pressure in non-pregnant women with previous pre-eclampsia: a randomized placebo-controlled study. Pregnancy Hypertens. (2021) 23:91–6. doi: 10.1016/j.preghy.2020.11.012
39. Freedman, LS, Midthune, D, Arab, L, Prentice, RL, Subar, AF, Willett, W, et al. Combining a food frequency questionnaire with 24-hour recalls to increase the precision of estimation of usual dietary intakes-evidence from the validation studies pooling Project. Am J Epidemiol. (2018) 187:2227–32. doi: 10.1093/aje/kwy126
40. Shahbabu, B, Dasgupta, A, Sarkar, K, and Sahoo, SK. Which is more accurate in measuring the blood pressure? A digital or an aneroid sphygmomanometer. J Clin Diagn Res. (2016) 10:LC11–4. doi: 10.7860/JCDR/2016/14351.7458
41. Tolonen, H, Koponen, P, Naska, A, Männistö, S, Broda, G, Palosaari, T, et al. Challenges in standardization of blood pressure measurement at the population level. BMC Med Res Methodol. (2015) 15:33. doi: 10.1186/s12874-015-0020-3
42. Heitmann, BL, and Lissner, L. Dietary underreporting by obese individuals--is it specific or non-specific? BMJ. (1995) 311:986–9. doi: 10.1136/bmj.311.7011.986
Keywords: animal protein, blood pressure, obesity, pregnancy, hypertension
Citation: Larson EA, Magkos F, Zingenberg H, Svare J, Astrup A and Geiker NRW (2023) A high protein low glycemic index diet has no adverse effect on blood pressure in pregnant women with overweight or obesity: a secondary data analysis of a randomized clinical trial. Front. Nutr. 10:1289395. doi: 10.3389/fnut.2023.1289395
Received: 05 September 2023; Accepted: 08 November 2023;
Published: 21 November 2023.
Edited by:
Kyungjoon (Joon) Lim, The University of Sydney, AustraliaReviewed by:
Ilaria Peluso, Council for Agricultural and Economics Research (CREA), ItalyCopyright © 2023 Larson, Magkos, Zingenberg, Svare, Astrup and Geiker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabeth A. Larson, ZWFsMjU0QGNvcm5lbGwuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.