- Department of Neurology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: While dietary factors have shown an association with Parkinson’s disease (PD), the available data remains a subject of ongoing debate and controversy.
Aim: We sought to evaluate potential relationships between dietary consumption of nutrients and micronutrients and risk of PD in a large sample.
Methods: Cross-sectional data were retrospectively analyzed for 10,651 adults aged 40–80 years that had been collected in the US between 2007 and 2016 as a component of the nationwide National Health and Nutrition Examination Survey. Aspects of dietary intake were compared between those who reported having specific PD medication regimens or not when they completed the survey, and potential associations between diet and risk of PD were explored using binomial logistic regression. We employed Propensity Score Matching (PSM) to minimize the impact of potential confounding factors, thus enhancing the reliability of the results. Additionally, subgroup analysis based on gender and age was conducted to investigate these relationships.
Results: Higher dietary intake of iron was linked to greater PD risk [odds ratio (OR) 1.065, 95% confidence interval (CI) 1.019–1.114, p = 0.006], whereas risk decreased with higher intake of vitamin K (OR 0.999, 95% CI 0.998–1.000, p = 0.024) or vitamin C (OR 0.998, 95% CI 0.996–0.999, p = 0.039). Even after applying PSM, the connection between dietary iron intake and dietary vitamin C intake with PD risk remained substantial. Subgroup analysis results revealed a significant positive association between dietary intake of iron from food and the PD risk, which was evident among individuals under 60 years of age and among males.
Conclusion: The intake of micronutrients can influence risk of PD, which should be verified and explored further in prospective samples with other dietary habits and ethnic backgrounds.
1 Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders (1), affecting roughly 1.5% of the general population older than 65 years and 3.0% of the population older than 80 years (2). Incidence is expected to rise as the global population ages (3). In PD, selective degeneration of dopaminergic cells in the substantia nigra of the brain cause motor symptoms such as bradykinesia, postural instability, resting tremor, and rigidity, as well as non-motor symptoms such as hyposmia, sleep disorders, cognitive impairment, and autonomic dysfunction. Both types of symptoms can substantially reduce one’s quality of life and ability to live independently.
The etiology of Parkinson’s disease (PD) is widely acknowledged to be multifactorial, encompassing a combination of genetic and environmental factors (4, 5). Among these environmental factors, diet has emerged as a significant determinant. Research indicates that adopting a diet rich in antioxidants (6), or adhering to a “Mediterranean” dietary pattern characterized by an abundance of plant-based foods, fish, and olive oil (7), may potentially mitigate the risk of PD. In contrast, a diet high in cholesterol and saturated fat is associated with an increased risk of PD (8). These factors were also found to potentially influence the clinical features of PD (9). Most of this work has been conducted on relatively small or medium-sized samples, which may limit its generalizability. Moreover, in these studies, the findings regarding the connections between dietary consumption and PD were inconclusive.
To complement this evidence base, we investigated potential correlations between dietary elements and the risk of PD within a substantial sample extracted from the U.S. National Health and Nutrition Examination Survey (NHANES) (10). The present work appears to be the first use of NHANES data for exploring links between diet and PD.
2 Methods
2.1 Sample and survey
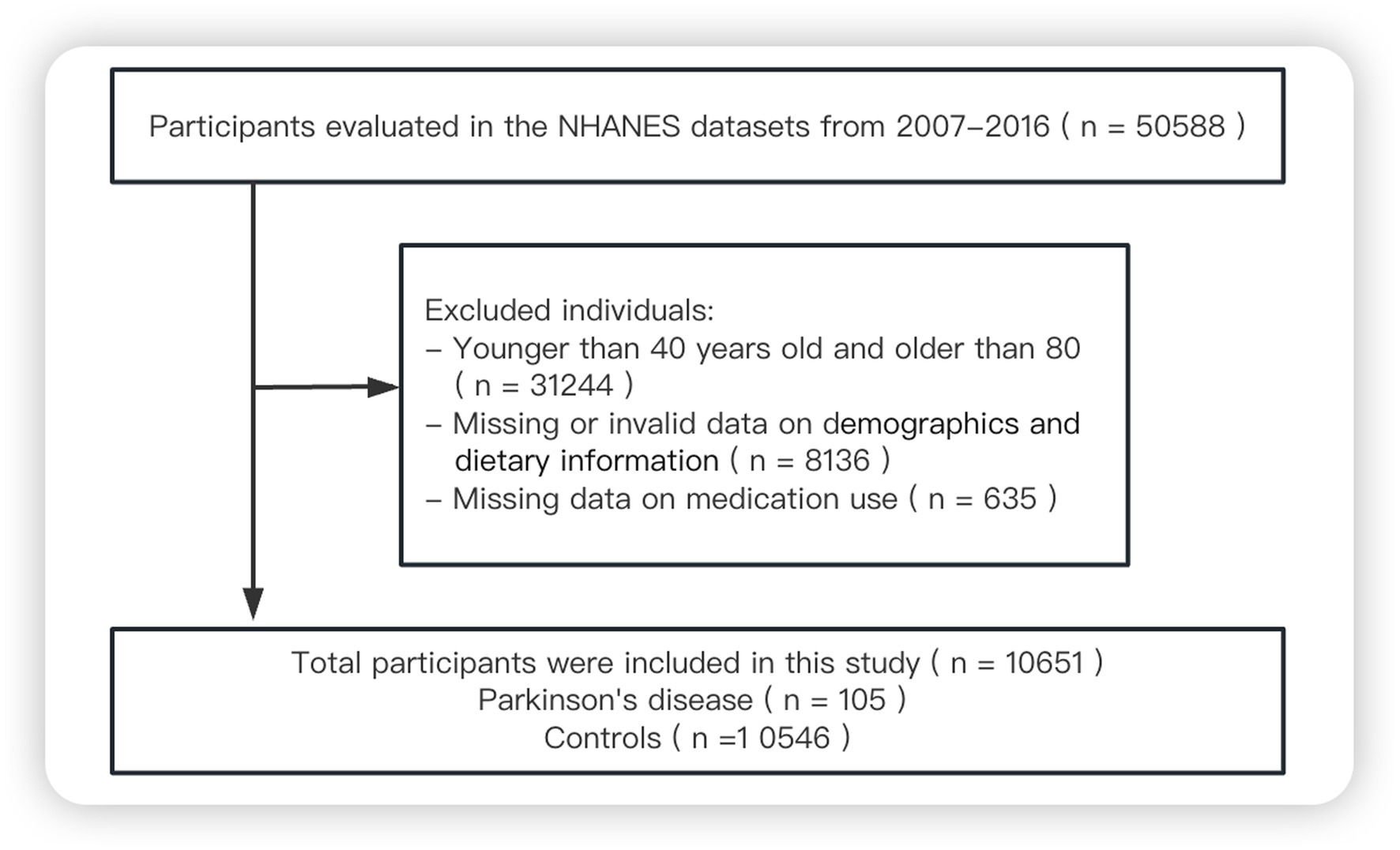
Since 1999, data on the demographics, socioeconomic status, health and diet of adults and children in the US general population have been collected through the NHANES, conducted by the National Center for Health Statistics at the Centers for Disease Control and Prevention (11). For the present study, we screened data from 50,588 respondents who participated in the NHANES in 2007–2008, 2009–2010, 2011–2012, 2013–2014 or 2015–2016. We excluded individuals younger than 40 years or older than 80 years, those with missing survey data, and those who reported taking “unsecure” PD medications (cabergoline, orphenadrine or pramipexole) (12), leaving 10,651 in the final analysis (Figure 1).
2.2 Definition of PD
Survey respondents were considered to have PD if they reported taking appropriate medications, defined as carbidopa, levodopa, methyldopa, benztropine, ropinirole, entacapone, and amantadine (12, 13).
2.3 Dietary intakes
Dietary information of respondents in each cycle was collected through 24-h diet recall interviews conducted in-person in a “mobile examination center,” followed 3–10 days later by a second diet recall interview conducted by telephone. Dietary intakes were determined by averaging data from two dietary recalls, when such information was accessible; otherwise, data from a single dietary recall were utilized. The 24-h dietary supplement usage component was administered subsequent to the 24-h dietary interview pertaining to food and beverage consumption. In the case of dietary supplements, the total intake was also computed as an average of data from two dietary recalls, when available. We further estimated total nutrient intakes by summing the nutrient intakes from food sources and dietary supplements. The survey questionnaires can be found in the supplementary materials.
2.4 Demographics, income, lifestyle, and comorbidities
NHANES gathered demographic information, including age, gender, ethnic background, educational level, poverty-income ratio, smoking habits, and alcohol consumption, through household interviews. In addition, data related to body weight and height were obtained during physical examinations conducted at the mobile examination center. Participants were categorized according to their education level, which included “below high school,” “high school,” or “above high school.” Body mass index was computed as the quotient of an individual’s weight in kilograms divided by the square of their height in meters. Respondents were categorized as “former smokers” if they reported a history of a minimum of 100 cigarettes in the past but were not currently smoking, and as “current smokers” if they reported a history of at least 100 cigarettes in the past and were presently smoking. In terms of alcohol consumption, participants were categorized as “former drinkers” if they reported having consumed a minimum of 12 alcoholic drinks per year in the past but fewer than 12 in the preceding year, and as “current drinkers” if they reported consuming at least 12 alcoholic drinks per year in the past, including the most recent year. Respondents were categorized by their coffee intake into two groups: < 100 mg/d or ≥ 100 mg/d. Respondents were considered to have comorbidities if they reported having been diagnosed with hypertension or diabetes.
2.5 Statistical analysis
Continuous data were presented as mean ± standard deviation, and categorical data were reported as n (%). Statistical significance of differences in survey variables between respondents with and without PD was assessed using appropriate statistical tests, including Student’s t-test, the Mann–Whitney U rank sum test, or the χ2 test. Factors potentially associated with PD risk were explored using binomial logistic regression. In all analyses, we applied study-specific dietary sample weights to accommodate the intricate sample design of NHANES (14). To mitigate differences between PD patients and non-PD participants, a 1:4 ratio PSM analysis was employed. This process entailed controlling for multiple confounding variables, including as age, sex, body mass index, poverty-income ratio, education, ethnicity, smoking habits, alcohol intake, hypertension, diabetes, and caffeine intake. In addition, we also performed subgroup analysis based on gender and age to investigate the relationship between dietary intakes and PD risk. Data were analyzed using R version 4.1.2 and Stata version 17.0. Results, when applicable, were reported as odds ratios (ORs) along with their corresponding 95% confidence intervals (CIs).
3 Results
3.1 Characteristics of included participants
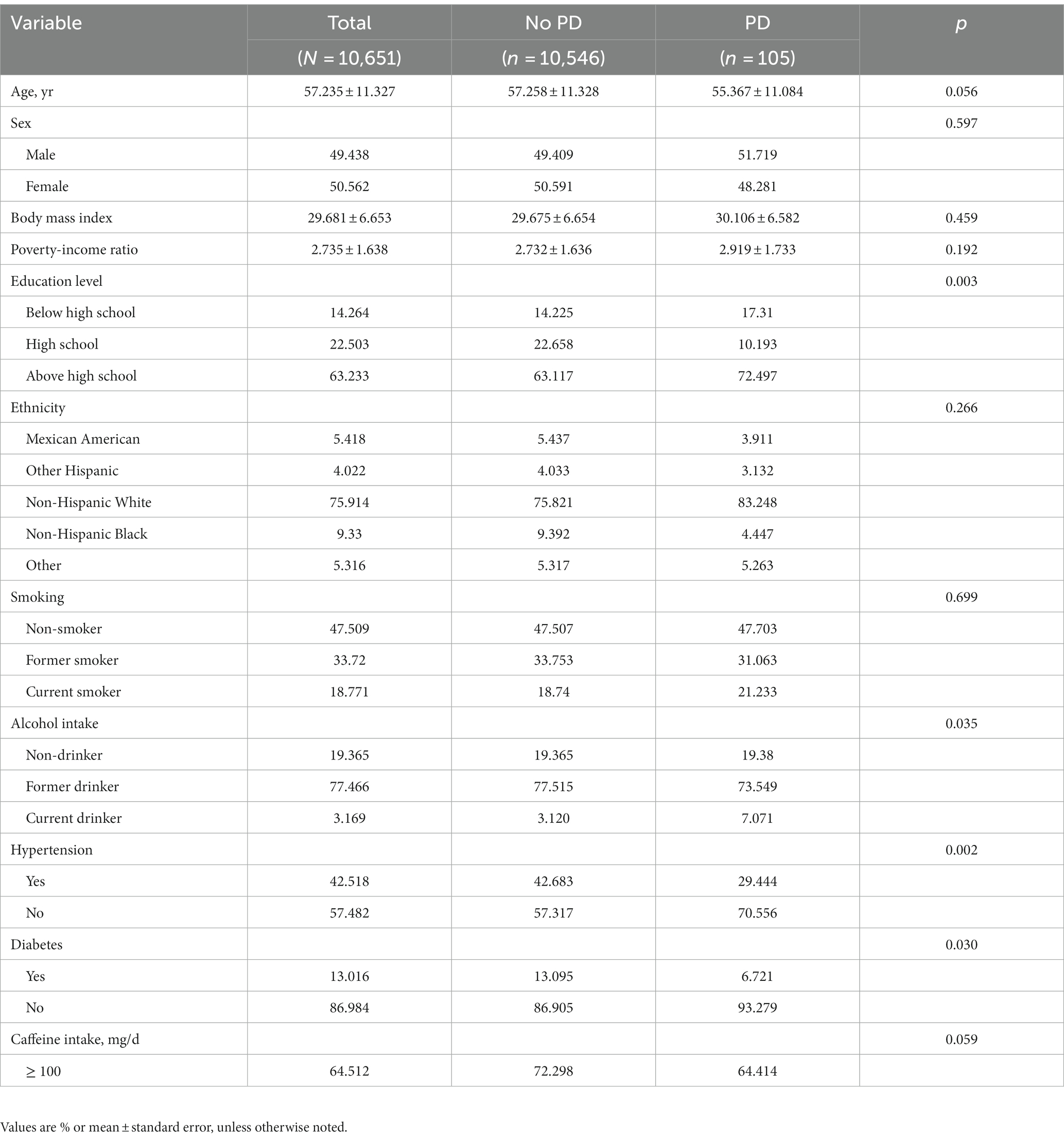
Out of the 10,651 participants, 105 (0.986%) were found to have PD. Compared to respondents without PD, those with PD had a considerably lower level of education (p = 0.003) and lower alcohol intake (p = 0.035), but significantly higher rates of hypertension (p = 0.002) and diabetes (p = 0.030) (Table 1).
3.2 Dietary intakes
Table 2 presents the dietary intakes from foods of the included participants before PSM. Patients suffering from PD took less lycopene (p = 0.034) and more vitamin B1 (p = 0.016), vitamin B2 (p = 0.014), niacin (p = 0.020), vitamin B6 (p = 0.004), folic acid (p = 0.049) and iron (p < 0.001). Participants in the no-PD group demonstrated a notably higher propensity for the utilization of dietary supplements (p = 0.001). And PD patients tend to take less vitamin C than controls (p = 0.021) (Supplementary Table S1). Additionally, the total intake of lycopene (p = 0.032) and vitamin C (p = 0.021) among PD patients is lower than controls. There was no statistically significant difference observed in the other dietary factors with PD (p > 0.05) (see Table 3).
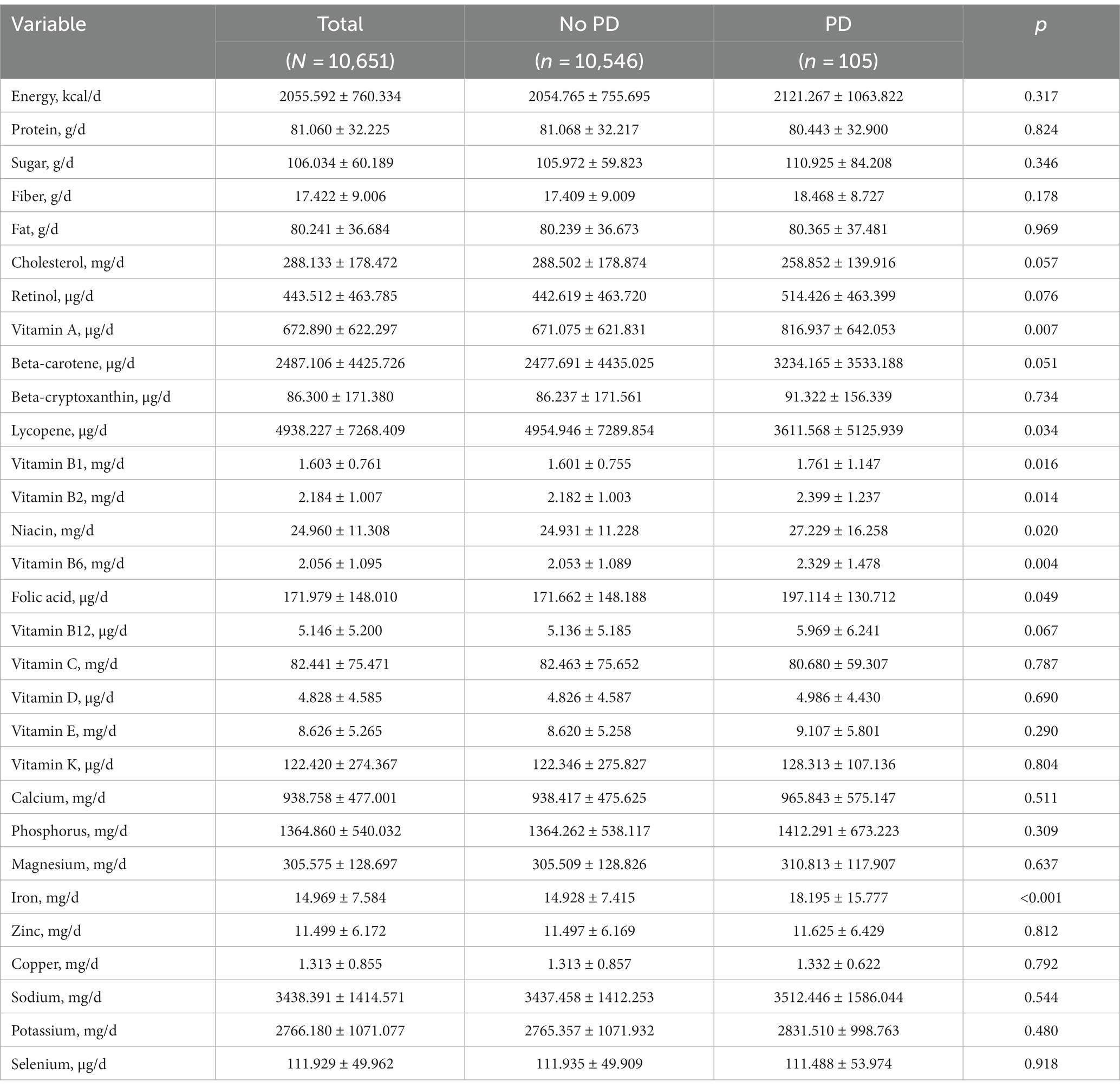
Table 2. Dietary intakes from food by study participants, stratified by Parkinson’s disease (PD) status.
3.3 Association between dietary intakes and PD risk
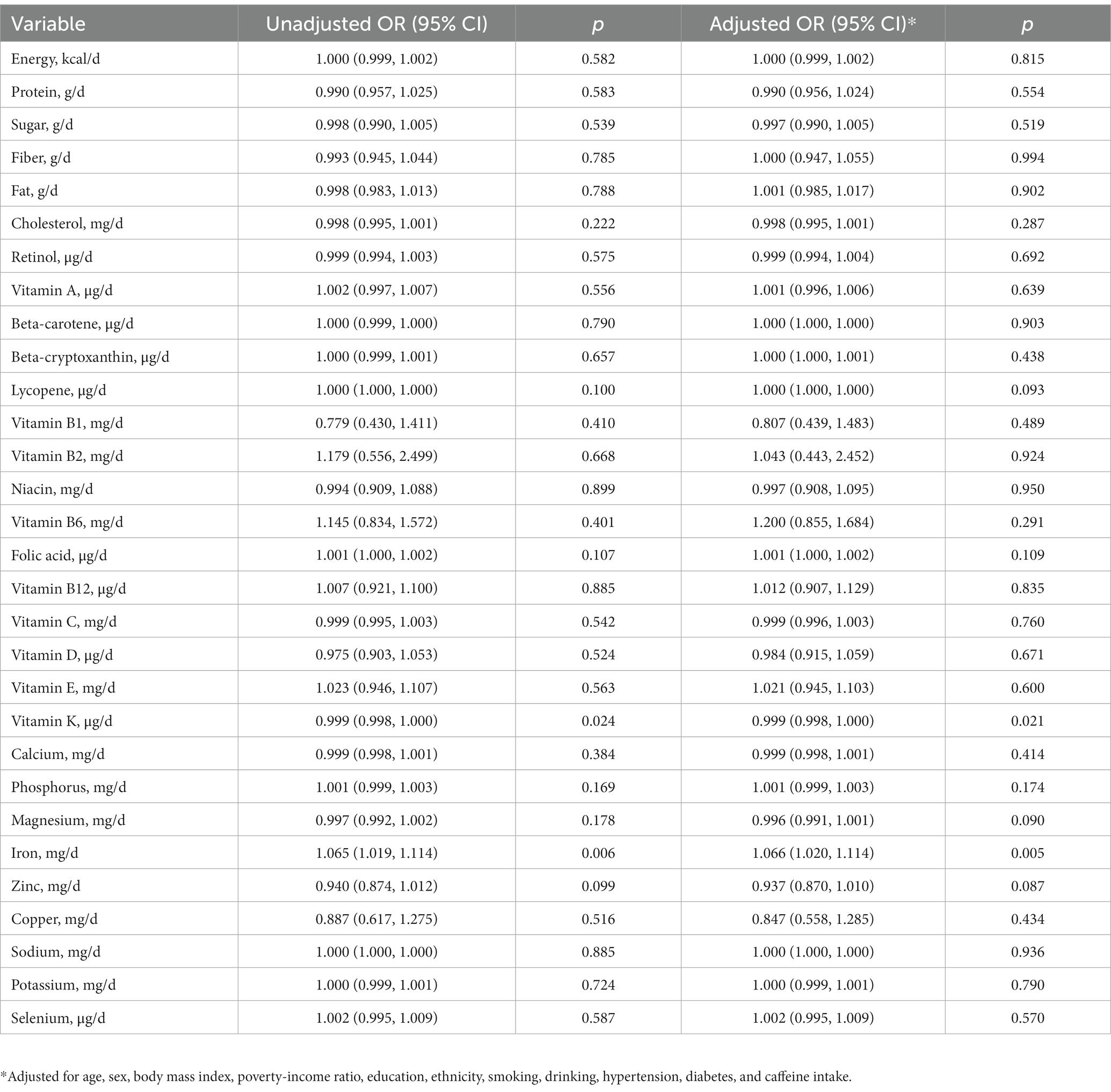
In our analysis of dietary intake solely from food sources, a negative correlation was identified between dietary intake of vitamin K and PD risk (OR 0.999, 95% CI 0.998–1.000, p = 0.024). Conversely, an increased intake of iron was related to an elevated risk of PD (OR 1.065, 95% CI 1.019–1.114, p = 0.006). These associations retained their statistical significance even following adjustments for potential confounding factors (Table 4; Supplementary Table S4). When considering the total dietary intake, including both food and dietary supplements, we identified a notable inverse correlation between vitamin C intake and the risk of PD (OR 0.998, 95% CI 0.996–0.999, p = 0.008; Table 5; Supplementary Figure S2).
3.4 Propensity score matching analysis
99 patients in the PD group were subjected to matching with 396 patients in the no-PD group. After PSM, participant characteristics were effectively balanced between the two groups (except for the type of education, Supplementary Table S2). After PSM, PD patients continued to exhibit potentially higher intake of retinol (p = 0.029), vitamin A (p = 0.008), and iron (p = 0.016) from food. Additionally, the percentage of individuals in the non-PD group using dietary supplements was significantly higher (p = 0.029). Furthermore, individuals with PD consumed significantly less vitamin C (p = 0.009) and zinc (p = 0.036).
In the logistic regression performed after PSM, it was found that higher dietary iron intake continued to be linked with an elevated risk of PD (OR 1.098, 95% CI 1.031–1.168, p = 0.003; Supplementary Table S4). Additionally, an inverse correlation was found between greater zinc intake from food and the risk of PD (OR 0.850, 95% CI 0.757–0.953, p = 0.006; Supplementary Table S4). When we examined total dietary intake from food and dietary supplements, we still found a significant negative correlation between higher intake of vitamin C and the risk of PD (OR 0.997, 95% CI 0.995–0.999, p = 0.002; Supplementary Table S3). Furthermore, an elevated intake of copper was related to an increased risk of PD (OR 1.527, 95% CI 1.016–2.295, p = 0.035). Even after accounting for potential confounding factors, these associations retained their statistical significance.
3.5 Subgroup analyses
Supplementary Table S4 presents the results of a subgroup analysis, stratified by gender and age, to examine the association between dietary intakes of vitamin C, vitamin K, iron, zinc, and copper, and the odds of developing PD. A positive correlation between dietary iron intake from food and the risk of PD clearly observed in individuals aged less than 60 years and among males. As for dietary zinc intake, the inverse association was only observed in female.
4 Discussion
In this first application of NHANES data to PD risk, we identified dietary intake of zinc, vitamins C and K as potentially protective against PD, while iron and copper intake appears to increase risk.
Our analysis of a large sample of more than 10,000 individuals may help clarify the inconsistent literature on whether vitamin C intake can decrease risk of PD (15–19). For example, a large Swedish cohort study that followed participants for 17 years linked higher dietary intake of vitamins E and C to lower risk of PD (16), but the Singapore Chinese Health Study found no correlation between dietary intake of carotenoids, as well as vitamins A, C, or E, and the risk of PD (17). Our results suggest that deficiency in vitamin C can increase risk of the disease, perhaps because the body cannot buffer oxidative stress (20).
Our work suggesting a negative correlation between dietary intake of vitamin K and PD risk should inspire further work on this topic, since the literature has neglected this potential benefit of vitamin K. We are aware of one study linking deficiency of vitamin K2 with PD progression (21). Vitamin K may reduce risk of PD by scavenging free radicals and thereby dampening injury due to reactive oxygen species that can lead to PD (22, 23).
The observed Inverse relationship between zinc Intake and the PD risk in our present study is in line with the findings from a prior Japanese case–control study including 249 cases and 368 controls (24). Zinc, an antioxidant and anti-inflammatory agent, performs distinctive and sequential functions in counteracting free radicals and safeguarding neurons from oxidative harm (25). An experimental study suggested the advantageous impact of zinc supplementation in a Drosophila PD model (26). In addition, gender may exert an influence on PD risk in the context of lower zinc intake. Nevertheless, additional research is essential to validate the sex-specific effects.
We observed that a higher intake of dietary iron from food sources was associated with an increased risk of PD. This supports the widely held belief that iron dysregulation contributes to PD and parkinsonism (27), and it aligns with the observation that individuals with PD tend to have significantly higher levels of iron in serum than healthy controls (28). Iron is an indispensable trace element within the human body. Nonetheless, its redox activity, along with the generation of free radicals during the interconversion between ferrous (Fe2+) and ferric (Fe3+) iron, also confers toxic properties (29). This redox-active metal generates an excessive amount of reactive oxygen species through the Fenton reaction, leading to pronounced oxidative stress reactions and resultant cellular damage (29). Furthermore, Fe2+ can also interact with the negatively charged C-terminus of α-synuclein, accelerating its aggregation, and the redox reactions of iron can promote the oligomerization of α-synuclein in the substantia nigra (SN) (30, 31). Excessive iron may deposit in the substantia nigra and kill dopaminergic neurons, increasing risk of PD (32). Nevertheless, the literature on the relationship between iron intake and the risk of PD has shown inconsistent findings (33–36), so our relatively large study may help to clarify this question.
Although we found iron intake from food to correlate positively with PD risk, our study did not establish a significant link between total iron intake and the risk of PD. Similarly, a large study including 124,353 participants in the US only identified a positive correlation between the risk of PD and intake of iron from food (36). On the other hand, a prospective study of nearly 390,000 people suggested that higher supplemental iron consumption was linked to greater risk of parkinsonism (37). Furthermore, our subgroup analysis indicates a positive correlation between high iron intake and PD risk in men and individuals aged under 60 years. Notably, two prior studies (38, 39) have similarly identified gender-specific associations, with an increased risk of PD associated with dietary iron intake observed exclusively in men. Our results suggest that both gender and age may be influential factors in the relationship between iron intake and the risk of PD. Menstrual blood loss is a substantial factor that influences iron levels in women (40). Additionally, a prior study (41) observed that the prevalence of anemia increased with age in individuals aged ≥65 years, for both men and women. Future research endeavors are essential to corroborate these associations and to elucidate whether the influence of iron on the risk of PD depends on its form and route of intake.
In our study, we discovered that a higher total intake of copper may be associated with an elevated risk of PD. While a previous meta-analysis (39) did not identify a connection between dietary intake of copper and PD risk, other studies (42–44) have suggested that disruptions in copper homeostasis, which can result in either increased or reduced levels, may contribute to the onset of PD.
It’s important to interpret our findings with caution since the dietary data was obtained from two cross-sectional diet assessments, which are subject to recall bias and do not capture potentially substantial day-to-day variations in dietary consumption. In addition, the study participants were not prospectively diagnosed with PD but simply asked whether they were taking anti-PD medications, which was similar to a previous study (11). This approach may have missed some cases of PD. A third limitation is that we considered nutrients and non-nutrients individually, without accounting for their potential interactions (45). Such interactions may help explain some of the inconsistencies in the literature on dietary risk factors of PD. A fourth limitation is that we collected dietary data on individuals with PD after they had been diagnosed with the disease and were taking medication, which may have confounded our analyses of dietary risk factors. Ultimately, suitably large prospective studies are needed to explore whether and how diet affects risk of PD.
5 Conclusion
Our analysis, conducted on a relatively large sample from the United States, indicates that higher iron intake from food and total copper intake are associated with an increased risk of PD. Conversely, increased total dietary intake of vitamins C and vitamins K from food, along with zinc intake, are linked to a reduced risk of PD. These findings should be considered cautiously, and they need to be replicated in other settings, possibly with biomarkers. Further research is necessary to provide a more comprehensive assessment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LL: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Software, Validation, Writing – original draft. QS: Project administration, Validation, Writing – review & editing. YB: Data curation, Methodology, Project administration, Software, Writing – review & editing. FX: Investigation, Software, Writing – review & editing. DZ: Project administration, Writing – review & editing. HH: Methodology, Supervision, Writing – review & editing. LT: Supervision, Writing – review & editing. YX: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is financed by the Key Research and Development Project of the Sichuan Science and Technology Department (2023YFS0268).
Acknowledgments
We thank all the effort made by the staff and the participants of the NHANES study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1278128/full#supplementary-material
References
1. Dorsey, ER, and Bloem, BR. The Parkinson pandemic-a call to action. JAMA Neurol. (2018) 75:9–10. doi: 10.1001/jamaneurol.2017.3299
2. Pringsheim, T, Jette, N, Frolkis, A, and Steeves, TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. (2014) 29:1583–90. doi: 10.1002/mds.25945
3. Bach, JP, Ziegler, U, Deuschl, G, Dodel, R, and Doblhammer-Reiter, G. Projected numbers of people with movement disorders in the years 2030 and 2050. Mov Disord. (2011) 26:2286–90. doi: 10.1002/mds.23878
4. Kalia, LV, and Lang, AE. Parkinson's disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
5. Cannon, JR, and Greenamyre, JT. Gene-environment interactions in Parkinson's disease: specific evidence in humans and mammalian models. Neurobiol Dis. (2013) 57:38–46. doi: 10.1016/j.nbd.2012.06.025
6. Park, HA, and Ellis, AC. Dietary antioxidants and Parkinson's disease. Antioxidants (Basel). (2020) 9:570. doi: 10.3390/antiox9070570
7. Chen, H, Zhang, SM, Hernán, MA, Willett, WC, and Ascherio, A. Diet and Parkinson's disease: a potential role of dairy products in men. Ann Neurol. (2002) 52:793–801. doi: 10.1002/ana.10381
8. Koch, S, Laabs, BH, Kasten, M, Vollstedt, EJ, Becktepe, J, Brüggemann, N, et al. Validity and prognostic value of a polygenic risk score for Parkinson's disease. Genes (Basel). (2021) 12:1859. doi: 10.3390/genes12121859
9. Belvisi, D, Pellicciari, R, Fabbrini, A, Costanzo, M, Ressa, G, Pietracupa, S, et al. Relationship between risk and protective factors and clinical features of Parkinson's disease. Parkinsonism Relat Disord. (2022) 98:80–5. doi: 10.1016/j.parkreldis.2022.04.017
10. Centers for Disease Control and Prevention. National Center for Health Statistics National Health and nutrition examination survey [internet] Centers for Disease Control and Prevention. GA, USA: Atlanta (2001).
11. Zipf, G, Chiappa, M, Porter, KS, Ostchega, Y, Lewis, BG, and Dostal, J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. (2013) 56:1–37.
12. Botelho, J, Lyra, P, Proença, L, Godinho, C, and Mendes, JJ. Machado V. Relationship between blood and standard biochemistry levels with periodontitis in Parkinson's disease patients: data from the NHANES 2011-2012. J Pers Med. (2020) 10:69. doi: 10.3390/jpm10030069
13. Fox, SH, Katzenschlager, R, Lim, SY, Ravina, B, Seppi, K, Coelho, M, et al. The Movement Disorder Society evidence-based medicine review update: treatments for the motor symptoms of Parkinson's disease. Mov Disord. (2011) 26 Suppl 3:S2–S41. doi: 10.1002/mds.23829
14. Centers for Disease Control and Prevention, National Center for Health Statistics. The National Health and nutrition examination survey (NHANES) analytic and reporting guidelines. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf
15. Yang, F, Wolk, A, Håkansson, N, Pedersen, NL, and Wirdefeldt, K. Dietary antioxidants and risk of Parkinson's disease in two population-based cohorts. Mov Disord. (2017) 32:1631–6. doi: 10.1002/mds.27120
16. Hantikainen, E, Trolle Lagerros, Y, Ye, W, Serafini, M, Adami, HO, Bellocco, R, et al. Dietary antioxidants and the risk of Parkinson disease: the Swedish National March Cohort. Neurology. (2021) 96:e895–903. doi: 10.1212/WNL.0000000000011373
17. Ferland, G. Vitamin K, an emerging nutrient in brain function. Biofactors. (2012) 38:151–7. doi: 10.1002/biof.1004
18. Ying, AF, Khan, S, Wu, Y, Jin, A, Wong, ASY, Tan, EK, et al. Dietary antioxidants and risk of Parkinson's disease in the Singapore Chinese health study. Mov Disord. (2020) 35:1765–73. doi: 10.1002/mds.28173
19. Hughes, KC, Gao, X, Kim, IY, Rimm, EB, Wang, M, Weisskopf, MG, et al. Intake of antioxidant vitamins and risk of Parkinson's disease. Mov Disord. (2016) 31:1909–14. doi: 10.1002/mds.26819
20. Medeiros, MS, Schumacher-Schuh, A, Cardoso, AM, Bochi, GV, Baldissarelli, J, Kegler, A, et al. Iron and oxidative stress in Parkinson's disease: an observational study of injury biomarkers. PLoS One. (2016) 11:e0146129. doi: 10.1371/journal.pone.0146129
21. Yu, YX, Yu, XD, Cheng, QZ, Tang, L, and Shen, MQ. The association of serum vitamin K2 levels with Parkinson's disease: from basic case-control study to big data mining analysis. Aging (Albany NY). (2020) 12:16410–9. doi: 10.18632/aging.103691
22. Simes, DC, Viegas, CSB, Araújo, N, and Marreiros, C. Vitamin K as a diet supplement with impact in human health: current evidence in age-related diseases. Nutrients. (2020) 12:138. doi: 10.3390/nu12010138
23. Li, J, Lin, JC, Wang, H, Peterson, JW, Furie, BC, Furie, B, et al. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. (2003) 23:5816–26. doi: 10.1523/JNEUROSCI.23-13-05816.2003
24. Miyake, Y, Tanaka, K, Fukushima, W, Sasaki, S, Kiyohara, C, Tsuboi, Y, et al. Dietary intake of metals and risk of Parkinson's disease: a case-control study in Japan. J Neurol Sci. (2011) 306:98–102. doi: 10.1016/j.jns.2011.03.035
25. Prasad, AS. Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol. (2014) 28:364–71. doi: 10.1016/j.jtemb.2014.07.019
26. Saini, N, and Schaffner, W. Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biol Chem. (2010) 391:513–8. doi: 10.1515/BC.2010.052
27. Zucca, FA, Segura-Aguilar, J, Ferrari, E, Muñoz, P, Paris, I, Sulzer, D, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease. Prog Neurobiol. (2017) 155:96–119. doi: 10.1016/j.pneurobio.2015.09.012
28. Fukushima, T, Tan, X, Luo, Y, and Kanda, H. Relationship between blood levels of heavy metals and Parkinson's disease in China. Neuroepidemiology. (2010) 34:18–24. doi: 10.1159/000255462
29. Belaidi, AA, and Bush, AI. Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J Neurochem. (2016) 139:179–97. doi: 10.1111/jnc.13425
30. Weinreb, O, Mandel, S, Youdim, MBH, and Amit, T. Targeting dysregulation of brain iron homeostasis in Parkinson's disease by iron chelators. Free Radic Biol Med. (2013) 62:52–64. doi: 10.1016/j.freeradbiomed.2013.01.017
31. Zecca, L, Youdim, MB, Riederer, P, Connor, JR, and Crichton, RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. (2004) 5:863–73. doi: 10.1038/nrn1537
32. Reimão, S, Ferreira, S, Nunes, RG, Pita Lobo, P, Neutel, D, Abreu, D, et al. Magnetic resonance correlation of iron content with neuromelanin in the substantia nigra of early-stage Parkinson's disease. Eur J Neurol. (2016) 23:368–74. doi: 10.1111/ene.12838
33. Logroscino, G, Marder, K, Graziano, J, Freyer, G, Slavkovich, V, Lojacono, N, et al. Dietary iron, animal fats, and risk of Parkinson's disease. Mov Disord. (1998) 13:13–6.
34. Johnson, CC, Gorell, JM, Rybicki, BA, Sanders, K, and Peterson, EL. Adult nutrient intake as a risk factor for Parkinson's disease. Int J Epidemiol. (1999) 28:1102–9. doi: 10.1093/ije/28.6.1102
35. Powers, KM, Smith-Weller, T, Franklin, GM, Longstreth, WT Jr, Swanson, PD, and Checkoway, H. Parkinson's disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology. (2003) 60:1761–6. doi: 10.1212/01.wnl.0000068021.13945.7f
36. Logroscino, G, Gao, X, Chen, H, Wing, A, and Ascherio, A. Dietary iron intake and risk of Parkinson's disease. Am J Epidemiol. (2008) 168:1381–8. doi: 10.1093/aje/kwn273
37. Takeuchi, H, and Kawashima, R. A prospective study on the relationship between Iron supplement intake, hemoglobin concentration, and risk of parkinsonism. Nutrients. (2022) 14:4671. doi: 10.3390/nu14214671
38. Powers, KM, Smith-Weller, T, Franklin, GM, Longstreth, WT Jr, Swanson, PD, and Checkoway, H. Dietary fats, cholesterol and iron as risk factors for Parkinson's disease. Parkinsonism Relat Disord. (2009) 15:47–52. doi: 10.1016/j.parkreldis.2008.03.002
39. Cheng, P, Yu, J, Huang, W, Bai, S, Zhu, X, Qi, Z, et al. Dietary intake of iron, zinc, copper, and risk of Parkinson's disease: a meta-analysis. Neurol Sci. (2015) 36:2269–75. doi: 10.1007/s10072-015-2349-0
40. Blanco-Rojo, R, Toxqui, L, López-Parra, AM, Baeza-Richer, C, Pérez-Granados, A, Arroyo-Pardo, E, et al. Influence of diet, menstruation and genetic factors on iron status: a cross-sectional study in Spanish women of childbearing age. Int J Mol Sci. (2014) 15:4077–87. doi: 10.3390/ijms15034077
41. QuickStats. Prevalence* of Anemia† among adults aged ≥65 years, by sex and age group - National Health and nutrition examination survey, 2013-2016. MMWR Morb Mortal Wkly Rep. (2018) 67:1198. doi: 10.15585/mmwr.mm6742a8
42. Bisaglia, M, and Bubacco, L. Copper ions and Parkinson's disease: why is homeostasis so relevant? Biomol Ther. (2020) 10:195. doi: 10.3390/biom10020195
43. Montes, S, Rivera-Mancia, S, Diaz-Ruiz, A, Tristan-Lopez, L, and Rios, C. Copper and copper proteins in Parkinson's disease. Oxidative Med Cell Longev. (2014) 2014:147251. doi: 10.1155/2014/147251
44. Gromadzka, G, Tarnacka, B, Flaga, A, and Adamczyk, A. Copper Dyshomeostasis in neurodegenerative diseases-therapeutic implications. Int J Mol Sci. (2020) 21:9259. doi: 10.3390/ijms21239259
Keywords: diet, Parkinson’s disease, NHANES, micronutrients, dietary intake
Citation: Liu L, Shen Q, Bao Y, Xu F, Zhang D, Huang H, Tu L and Xu Y (2023) Association between dietary intake and risk of Parkinson’s disease: cross-sectional analysis of survey data from NHANES 2007–2016. Front. Nutr. 10:1278128. doi: 10.3389/fnut.2023.1278128
Edited by:
Elżbieta Wieczorek, Wrocław University of Science and Technology, PolandReviewed by:
Tommaso Ercoli, Azienda Ospedaliero Universitaria Sassari, ItalyAnna Puścion-Jakubik, Medical University of Bialystok, Poland
Copyright © 2023 Liu, Shen, Bao, Xu, Zhang, Huang, Tu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanming Xu, bmV1cm94eW05OTlAMTYzLmNvbQ==
 Ling Liu
Ling Liu Yanming Xu
Yanming Xu