- 1Department of Cardiology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Second Department of Internal Medicine, Affiliated Hospital of Tibet University for Nationalities, Xianyang, Shaanxi, China
Object: To explore the potential association between dietary live microbe intake and abdominal aortic calcification (AAC).
Methods: We conducted a cross-section study based on the National Health and Nutrition Examination Survey (NHANES). We categorized the participants into three groups (low, medium, and high dietary intake of live microbes) according to Sanders’s dietary live microbe classification system and participants’ 24-h dietary recall data. AAC was quantified by using dual-energy X-ray absorptiometry (DXA) and diagnosed by using the Kauppila AAC-24 score system. The analyses utilized weighted logistic regression and weighted linear regression.
Results: A total of 2,586 participants were included. After the full adjustment for covariates, compared to participants with a low dietary live microbe intake, participants with a high dietary live microbe intake had a significantly lower risk of severe AAC (OR: 0.39, 95% CI: 0.22, 0.68, p = 0.003), and the AAC score was also significantly decreased (β:−0.53, 95% CI: −0.83, −0.23, p = 0.002).
Conclusion: In this study, more dietary live microbial intake was associated with lower AAC scores and a lower risk of severe AAC. However, more research is needed to verify this.
1 Introduction
Vascular calcification (VC) is the abnormal deposition of calcium and phosphorus in the walls of blood vessels (1). Although studies on VC have primarily focused on coronary artery calcification (CAC), the multi-ethnic study of atherosclerosis (MESA) study suggests that abdominal aortic calcification (AAC) occurs earlier and is a more effective prognostic marker than CAC, independent of CAC scores (2, 3). Current studies have established a strong correlation between AAC and cardiovascular events as well as mortality (3–5), but another study still believed AAC is an underestimated cardiovascular disease risk factor (6). As the AAC severity increases, the risk of fatal cardiovascular events and mortality also increases significantly (7, 8).
Since the progression of VC is difficult to reverse, prevention and treatment are extremely important (9). Although VC is often considered the advanced stage of atherosclerosis (AS) (3), current medications targeting AS have shown limited efficacy in treating VC (10). Several drugs, such as bisphosphonates, calcium channel blockers, vitamin K, and dietary magnesium, may have therapeutic potential, but there is still a lack of robust clinical evidence (3, 11, 12). To date, there are no approved treatment strategies for preventing or managing AAC (13).
Adopting healthy diets is widely recognized as an effective approach to preventing cardiovascular diseases (CVD) (14). Healthy diets such as anti-inflammatory diets (15), Mediterranean diets (14), and DASH diets (16), decreased the risk of having AAC. These studies mainly focused on the role of food ingredients but ignored the impact of dietary live microbes. Studies have found that diets can influence gut flora composition and metabolites (17) and impact disease susceptibility (18, 19). Therefore, there is growing interest in the health effects of live microbes in diets. Recently, Sanders et al. (20) used the National Health and Nutrition Examination Survey (NHANES) database to assess the number of live mircrobes in various foods. They also discovered live microbe-rich diets improved health outcomes, including lower BMI, blood pressure, lipids, glucose, insulin, and inflammation level (21). These benefits from dietary live microbes may prevent diseases. Based on NHANES data and Sanders’ dietary live microbe classification system, we explored the association between dietary live microbes and AAC.
2 Materials and methods
2.1 Data source and participants
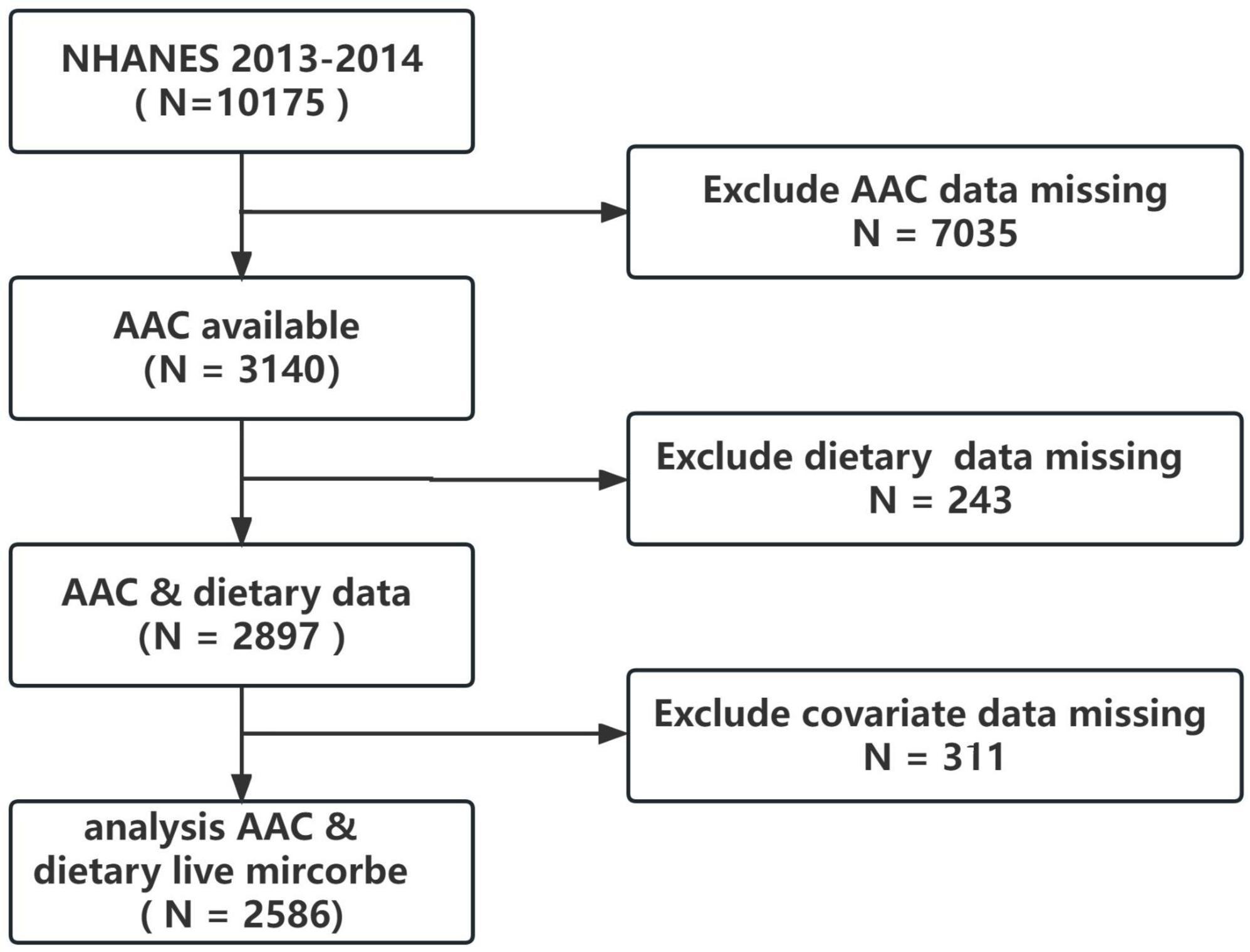
The NHANES survey design follows a complex, multistage probability sampling method to obtain nationally representative samples of the non-institutionalized US civilian population. The data collected includes demographics, physical examinations, laboratory tests, dietary information, and other questionnaires. The National Center for Health Statistics (NCHS) Institutional Review Board has approved the NHANES protocol, and participants provided informed consent before the collection of personal information, blood, and urine samples. The 2013–2014 cycle was chosen because it was the only cycle that dual X-ray absorptiometry (DXA) scans for AAC were performed. All data in this study is publicly available on the NHANES website.1 For the 2013–2014 cycle, the total population was 10,175. However, only participants over 40 years old received the DXA examination to obtain AAC score data. After excluding those with missing AAC data (n = 7035), missing dietary data (n = 243), and missing covariate data (n = 311), the final analysis consisted of 2586 participants, as depicted in Figure 1.
2.2 Dietary live microbe intake category
The dietary live microbe intake was estimated by using 24-h dietary recall data from NHANES. The food codes in the NHANES database were linked to the United States Department of Agriculture (USDA) to obtain the food composition and energy content data. A team of four experts, relying on values reported in the primary literature, estimated the levels of live microbes (CFU/g) for 9,388 food codes across 48 subgroups in the NHANES database. The experts categorized microbial levels as low (< 104 CFU/g), medium (104–107 CFU/g), or high (> 107 CFU/g) based on the quantity of live microorganisms per gram of food. Any uncertain or conflicting data was resolved through external consultation. In short, the low class is mainly pasteurized foods, the medium class is mainly fresh fruits and vegetables that have not been peeled and the high class is fermented foods and probiotic supplements that have not been pasteurized. Although this classification method is useful for estimating liver microbes in foods, it may not be suitable for estimating entire diet live microbe intake. Following the approach of Sanders et al. (20), participants were grouped into three categories according to their overall live microbe intake from all foods: (1) low diet microbe intake group (only low level foods), (2) medium diet microbe intake group (medium level but not high level foods), and (3) high diet microbe intake group (any high level foods). This previously validated method allowed for the classification of participants’ diets based on estimated live microbe content (22, 23).
2.3 Outcome
The outcome variable in this analysis was AAC. The NHANES Mobile Examination Center (MEC) conducted lateral DXA scans of the thoracic and lumbar spine in 2013–2014. Exclusion criteria included age under 40 years, pregnancy, weight over 450 pounds, or recent barium contrast use in the past 7 days. The Kauppila score system (24) was utilized to evaluate AAC from the DXA scans. This involved dividing the anterior and posterior aortic walls corresponding to vertebral levels L1–L4 into 8 segments. Each segment was scored from 0 to 3 according to the extent of calcific deposits visualized. The sum of the 8 segment scores produced the total AAC score, ranging from 0 to 24. We defined AAC presence as a score above 0 and severe AAC as a score above 6. In addition, we performed sensitivity analysis using the AAC-8 score (Schousboe score), with a total score ranging from 0 to 8 (25). A score of 3 or more is defined as severe AAC (26).
2.4 Covariates
To avoid the influence of confounding factors, covariates were included in the analysis based on known or potential relationships with AAC. Demographic factors consisted of age, gender, race, education, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking, and alcohol use. Medical conditions included hypertension, diabetes, and congestive heart failure. Laboratory measurements collected were serum calcium, phosphorus, total 25-hydroxyvitamin D, potassium, HbA1c, uric acid, creatinine, total cholesterol, white blood cells (WBC), and estimated glomerular filtration rate (eGFR). Use of antidiabetic, antihyperlipidemic, and antihypertensive medications was recorded. Dietary energy was also considered a covariate. Smoking was defined as having smoked ≥ 100 cigarettes over their lifetime. The diagnostic criteria for diabetes included self-reported diabetes, HbA1c ≥ 6.5%, fasting serum glucose ≥ 126 mg/dL, random serum glucose > 11.1 mmol, or 2-h postprandial glucose ≥ 200 mg/dL, or any self-reported insulin and antidiabetic medication use. Hypertension was defined as having a history of hypertension, an average SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg based on at least three standard consecutive seated measurements or self-reported use of any hypertension-related medications. The eGFR was calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation (27).
2.5 Statistical analyses
All analyses accounted for the complex, multistage probability sampling design of NHANES by incorporating appropriate sampling weight. The R (Core Team, Vienna, Austria, version 4.1.2) and the survey package were utilized for complex sampling analysis. Continuous variables were reported as weighted means with standard errors (SE) while categorical variables were reported as weighted proportions. Baseline clinical characteristics were compared among groups using weighted t-tests and Rao-Scott chi-square tests. The association between dietary live microbe intake and AAC score was analyzed using weighted linear regression. Weighted logistic regression examined the association between dietary live microbe intake and severe AAC. Three regression models were constructed: model 1, adjusted for non-covariates; model 2, adjusted for age, gender, race, and education; and model 3, further adjusted for BMI, smoking, alcohol use, SBP, DBP, uric acid, creatinine, eGFR, HbA1c, total cholesterol, potassium, WBC, dietary energy, medical conditions (diabetes, hypertension, and congestive heart failure), medication use (antihypertensive, antihyperlipidemic, and antidiabetic), and bone metabolism markers (serum calcium, phosphorus, and total 25-hydroxyvitamin D). To avoid bias caused by deleting samples with missing covariates, we performed multiple imputations as a sensitivity analysis. In addition, we tested the robustness of the association using the AAC-8 score system. Subgroup analyses were conducted to examine potential effect modification by stratifying weighted multivariate regression models for age, gender, education, BMI, hypertension, diabetes, eGFR, smoking, and alcohol use. Trend tests to detect potential dose-response effects. A two-sided P-value < 0.05 was considered statistically significant.
3 Results
3.1 Clinical characteristics of participants
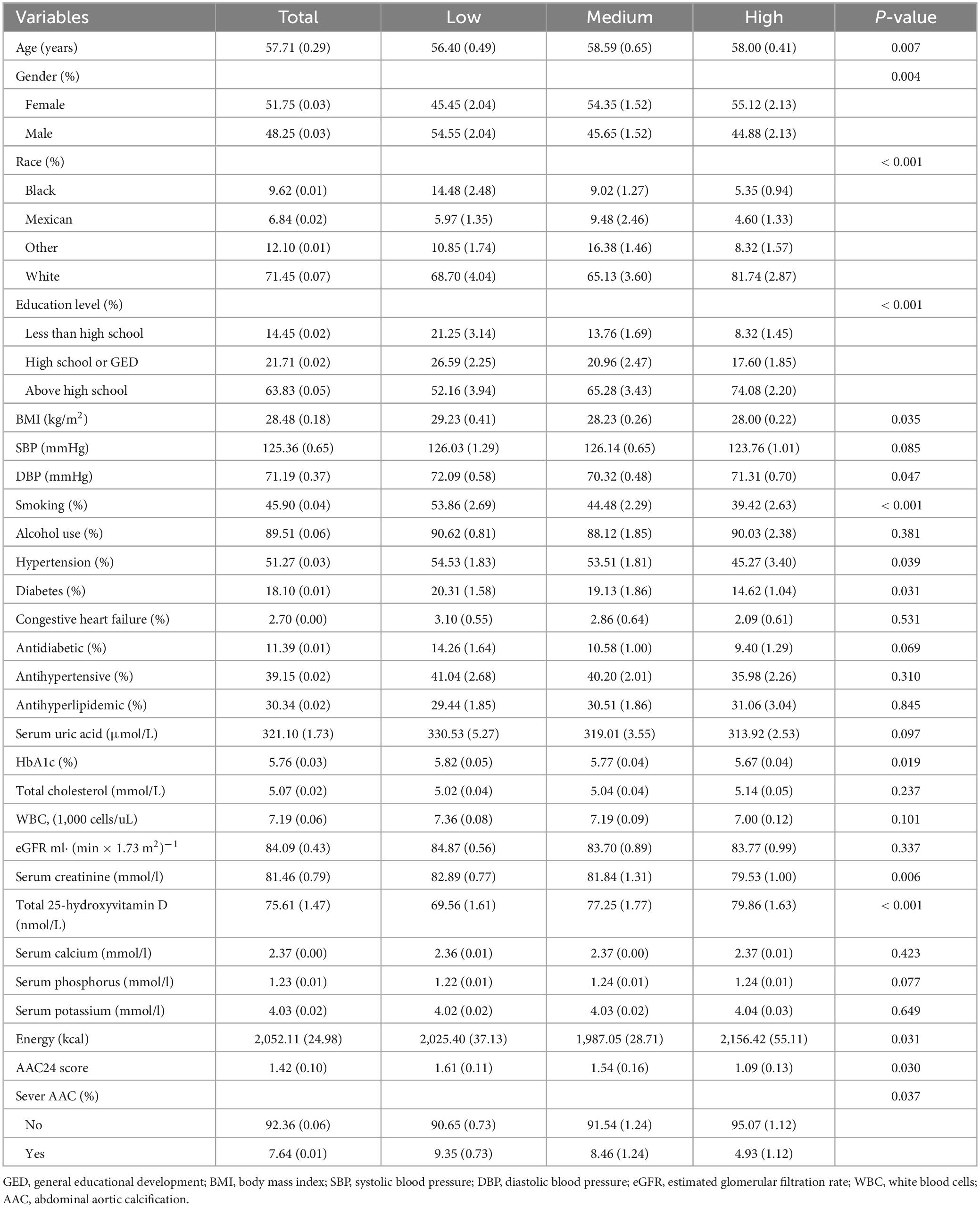
Table 1 presented the clinical characteristics of 2,586 individuals categorized by different dietary live microbe groups. The average age of the study population was 57.71 ± 0.29 years with a gender distribution of 51.75% females and 48.25% males. The average AAC scores for the overall population were 1.42 ± 0.10. Notably, there was a significant difference in the mean AAC scores among the three groups, with the high dietary live microbe intake group scoring the lowest, followed by the medium intake group. Furthermore, there were notable distinctions in several demographic and clinical parameters including age, gender, race, education, BMI, DBP, smoking, creatinine, HbA1c, total 25-hydroxyvitamin D, energy intake, and the prevalence of hypertension and diabetes among three groups (all P < 0.05). However, there were no statistically significant differences observed for SBP, alcohol use, uric acid, total cholesterol, WBC, eGFR, phosphorus, calcium, potassium, drug use, and the prevalence of congestive heart failure among the three groups.
3.2 Association between dietary live microbes and AAC
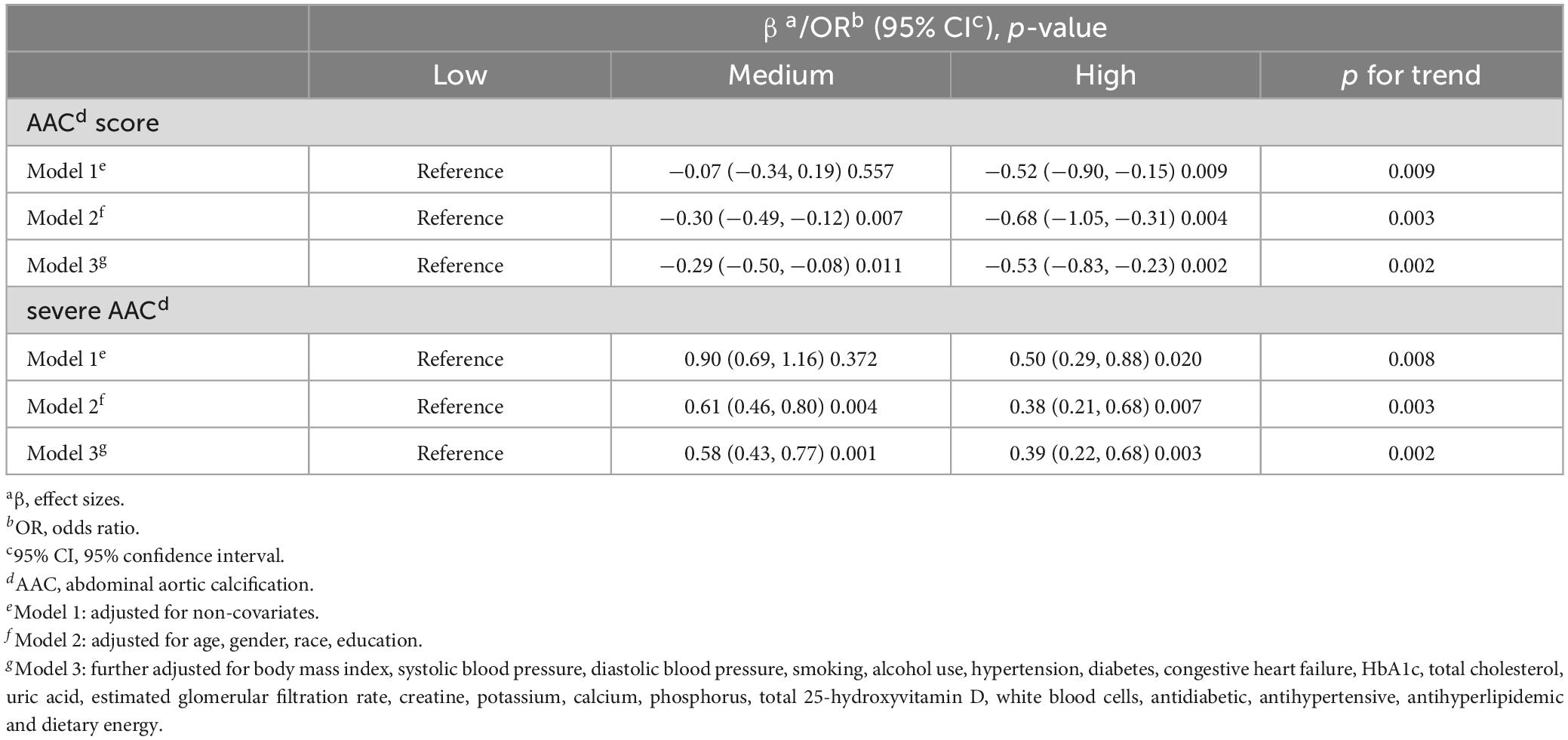
The relationship between dietary live microbes and AAC was evaluated by employing weighted multivariate linear regression and weighted multivariate logistic regression. Three models were constructed and the results are presented in Table 2. Compared to individuals with low dietary live microbe intake group, those in high dietary live microbe intake group exhibited significantly lower AAC scores (model 1: β = −0.52, 95% CI: −0.90, −0.15, p = 0.009; model 2: β = −0.68, 95% CI: −1.05, −0.31, p = 0.004; model 3: β = −0.53, 95% CI: −0.83, −0.23, p = 0.002). Furthermore, after accounting for covariates, the medium dietary live microbe intake group also demonstrated lower AAC scores than the low intake group (model 1: β = −0.07, 95% CI: −0.34, 0.19, p = 0.557; model 2: β = −0.30, 95% CI: −0.49, −0.12, p = 0.007; model 3: β = −0.29, 95% CI: −0.50, −0.08, p = 0.011).
Additionally, for severe AAC, the results were similar. Compared to individuals in low dietary live microbe intake group, those in high dietary live microbe intake group exhibited lower risk of severe AAC (model 1, OR = 0.50, 95% CI: 0.29, 0.88, p = 0.020; model 2, OR = 0.38, 95% CI: 0.21, 0.68, p = 0.007; model 3, OR = 0.39, 95% CI: 0.22, 0.68, p = 0.003). Similarly, the medium dietary live microbe intake group also showed diminished risk (model 1: OR = 0.90, 95% CI: 0.69, 1.16, p = 0.372; model 2: OR = 0.61, 95% CI: 0.46, 0.80, p = 0.004; model 3: OR = 0.58, 95% CI: 0.43, 0.77, p = 0.001). In summary, more dietary live microbe intake was associated with lower AAC scores and lower risk of severe AAC.
3.3 Subgroup analysis
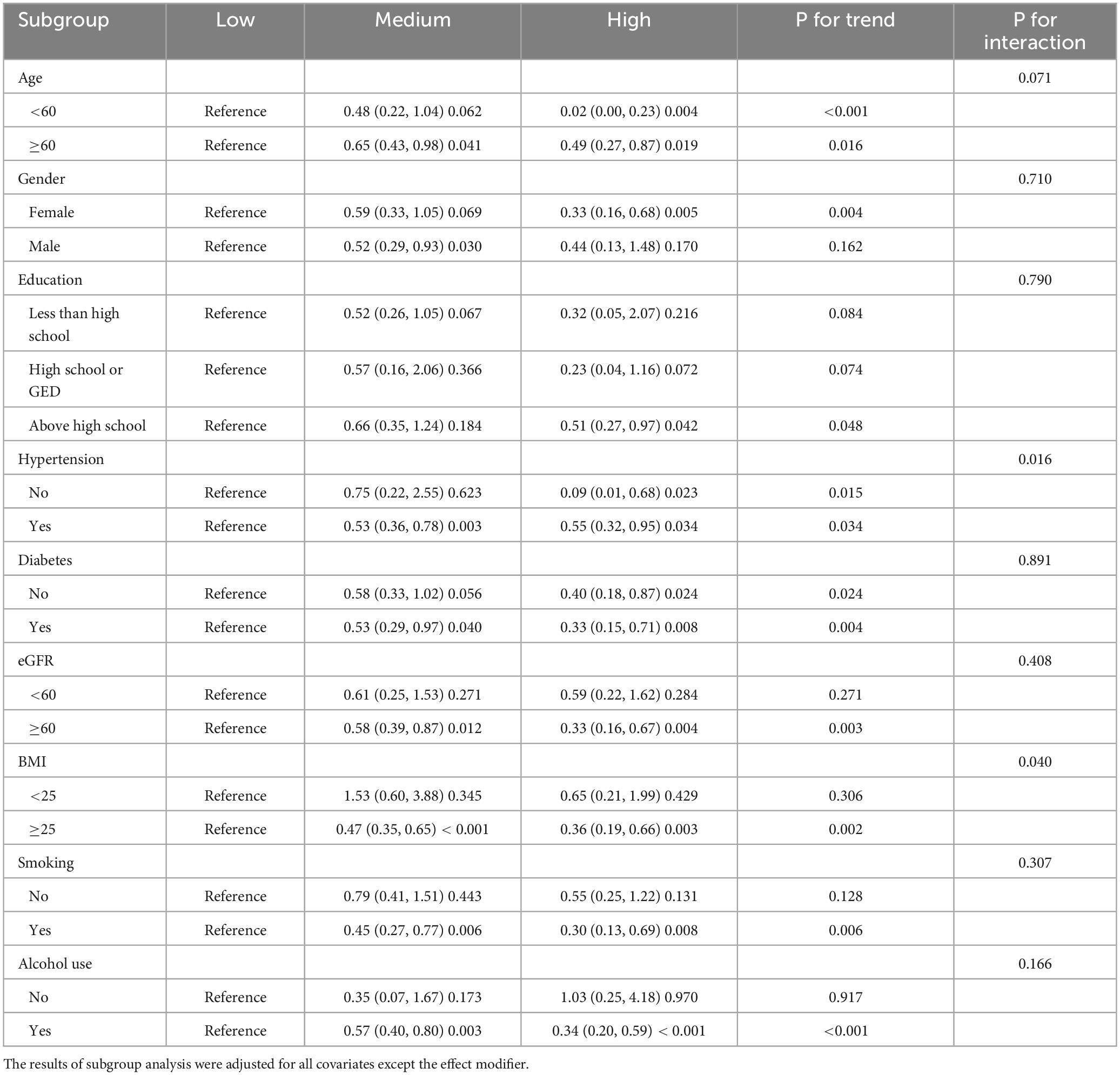
To examine the stability of the association between AAC and dietary live microbes, we conducted subgroup analyses, interaction tests, and trend tests between dietary live microbes and AAC across age, gender, education, hypertension, diabetes, eGFR, BMI, smoking, and alcohol use. Compared to those with low dietary live microbe intake, those who were older than 60 years, with hypertension, diabetes, eGFR ≥60ml ⋅ (min × 1.73 m2)−1, BMI greater than 25, smoking, or drinking were more likely to benefit from more dietary intake of live microbes. Only the interaction test for hypertension and BMI were meaningful. Interestingly, males benefited from medium live microbe diets, while females benefited from high live microbe diets. All subgroup analysis results are shown in Table 3.
3.4 Sensitivity analysis
To prevent bias caused by excluding samples with missing covariates, we also performed multiple imputations. The results showed that this correlation remained robust. In addition, we used the AAC8 scoring system to define patients with AAC8 scores greater than or equal to 3 as severe AAC. The results of the correlation analysis also remained stable. All sensitivity analysis data are in the Supplementary material.
4 Discussion
To our knowledge, this is the first study examining the association between dietary live microbe intake, assessed through NHANES 24-h recall data, and AAC within a nationally representative sample of US adults. Our findings indicated that compared to the low intake group, the high dietary live microbe intake group was associated with lower AAC scores and lower risk of severe AAC. The observed relationship persisted even after adjustment for relevant confounders, including demographics, bone metabolism markers, kidney function, other laboratory tests, comorbidities, drug use, and energy intake. In the subgroup analysis, the benefit of increased live microbe intake was particularly pronounced among age above 60 years, eGFR ≥ 60 ml⋅ (min × 1.73 m2) –1 and those with cardiovascular risk factors like hypertension, smoking, alcohol use, overweight, and diabetes. These findings underscored the potential therapeutic role of dietary live microbes, especially for individuals facing heightened cardiovascular risks, and these deserved further exploration and validation in future research.
Our findings are consistent with previous research, reinforcing the potential benefits of dietary interventions. A dietary intervention randomized controlled trial (RCT) included 90 patients with CVD risk factors who received probiotic alone, lactofermented Annurca apple puree (lfAAP), or unfermented Annurca apple puree (AAP) for 8 weeks according to a 1:1:1 allocation. At the end of the intervention, compared with other groups, the treatment effect of the lfAAP group was most obvious; HDLC increased by 61.8% compared with before intervention, while trimethylamine N-oxide (TMAO) decreased by 63% (28). Moreover, it enhanced the bioavailability of dietary polyphenols in the gut to exert antioxidant and anti-inflammatory cardiovascular benefits (28–30). A recent study found that participants who consumed medium or high levels of dietary live microbes showed better cognitive function compared to those with low intake, particularly for those with certain medical conditions like CVD, diabetes, and hypertension (23). Taking into consideration recent research, the potential health advantages associated with augmenting dietary live microbe intake, spanning improvements in blood pressure, glucose and lipid metabolism, cognitive function, intestinal nutrient absorption, and its influence on the intestinal flora, may indeed form a fundamental strategy for mitigating the risk of severe AAC (21, 23).
Previous studies have observed changes in the gut flora of VC patients. An observational study investigated differences in gut flora composition among chronic disease patients with varying degrees of aortic arch calcification (AoAC) (31). Individuals with the highest AoAC scores displayed a significant decrease in α-diversity along with a heightened prevalence of Clostridia species; those with lower AoAC scores exhibited a more favorable microbial profile, characterized by a higher abundance of beneficial bacteria such as Agathobacter (31). In another study involving 73 hemodialysis patients, notable distinctions in gut flora were discerned across various VC groups (32). Escherichia coli exhibited a positive correlation with VC and emerged as the primary contributor to VC progression; conversely, Ruminococcus, the bacterium recognized for producing short-chain fatty acids (SCFAs), displayed a negative correlation with VC and had the second most significant impact on VC (32). These SCFAs can promote tissue repair, regulate immunity, and are closely related to VC (33). In the rat vascular calcification model induced by vitamin D3 and nicotine, it was found that Akkermansia supplementation can enhance intestinal flora diversity, promote SCFA production, reduce inflammation, and ultimately alleviate VC (9). Consequently, these findings suggest a potential role for the gut flora in the development and progression of AAC.
One plausible explanatory mechanism for the observed association involves the food-gut-health axis (34). Diet can exert selective pressure on the gut flora, determining which microorganisms can colonize, persist, or become extinct in the gastrointestinal tract (35). It therefore plays a key role in shaping the composition and diversity of the gut microbiota (35, 36). Fermented Foods, fresh fruits and vegetables are an important source of probiotics, including Lactobacillus, Bifidobacterium, and Escherichia coli (37, 38). Probiotics exert anti-vascular calcification effects by acting on the intestines and throughout the body (39). First, probiotics play a pivotal role in restoring microbial equilibrium by upholding the integrity of the intestinal epithelial barrier and facilitating rebalancing (40). This maintenance protects the intestinal barrier, reduces the overpopulation of harmful bacteria, and prevents the production and leakage of harmful bacterial by-products into the circulation (41). It can regulate the expression of specific microRNAs and inhibit synthetases to reduce the production of TMAO metabolites (42, 43). Furthermore, the beneficial effects of probiotics extend to regulating the absorptive function of the intestines (44). They aid in the conversion of inorganic zinc into its organic form, thereby promoting its absorption—an action that contributes to the fight against AS and VC (44). Moreover, probiotics can promote the absorption of nutrients such as polyphenols, magnesium, vitamin D, vitamin C, and vitamin E (29, 45, 46). Not only that, it can inhibit the absorption of heavy metals and cholesterol in the intestine, and the heavy metals are thought to promote AS and calcification (47). Additionally, probiotics contribute to the production of substances associated with the prevention of VC, including vitamin K, and SCFAs (33, 48). These findings underscore the diverse and impactful role of probiotics in promoting gut-health and their potential implications for broader systemic wellbeing.
Our focus on habitual dietary microbe intake, rather than probiotic supplements, enhances the translational potential of these findings. A sustained, stable, and long-term dietary regimen significantly influences the composition and functional dynamics of the gut microbiota (49), and the impact of probiotics tends to be relatively transient (50, 51). However, we cannot directly recommend that the general population increase their intake of dietary live micorbes. Because its long-term effects are unknown, and its safety needs to be considered, especially for certain special groups including those with multiple severe infections, immune deficiencies, or gastrointestinal inflammation (52). In addition, the effects of dietary interventions enriched with dietary live microbes may vary by strain. Therefore, it may be important to emphasize tailoring the approach to individual health status and goals. Nonetheless, current evidence suggests that increasing the intake of microbe-rich foods may be a valuable strategy for improving cardiovascular health. It may have practical implications for public health and dietary guidelines. But longitudinal studies and RCTs are necessary.
There are several limitations in this study. First, in this cross-sectional study we observed the association, but causation cannot be established. Second, 24-h dietary recall data may be inaccurate due to recall bias and dietary live microbes can be affected by transportation, storage, and cooking. Third, Sanders’ dietary live microbe classification system may have lower accuracy than direct measurement. However, direct measurement requires a long time and huge expenditure, which limits its application. Fourth, the rough grouping of microbial diets may introduce estimation errors in assessing microbial intake. Fifth, our study is based on the US population, and the generalizability of the results to other populations worldwide may be limited. Sixth, although NHANES covers most of the US population through complex sampling, it does not include the hospitalized population. This results in under-assessment of critically ill patients. Seventh, despite adjusting for confounding factors, there may still be unknown confounders that have not been accounted for. Therefore, our research results should be treated with caution and can not directly guide the diet of the population. Nonetheless, our study provides new evidence for the health benefits of a diet rich in live microbes, and we call on more researchers to conduct further studies on dietary live microbes.
5 Conclusion
Our study demonstrated that more intake of dietary live microbes was associated with a reduced AAC score and the risk of severe AAC among United States adults. However, more studies are still needed to validate our findings.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Participants provided written informed consent for participation in NHANES. The data were de-identified and all participant data were obtained from publicly available NHANES. Therefore, this study did not require further approval and followed ethical guidelines.
Author contributions
XH: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review and editing. SJ: Writing – original draft, Writing – review and editing, Conceptualization. XZh: Investigation, Writing – review and editing. LS Investigation, Writing – review and editing. XL: Investigation, Writing – review and editing. LL: Investigation, Writing – review and editing. XZu: Investigation, Writing – review and editing. XC: Funding acquisition, Investigation, Supervision, Writing – review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Nos. 81900404 and 81970355).
Acknowledgments
Thanks to all NHANES staff and CDC for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1267607/full#supplementary-material
Footnotes
References
1. Passos LS, Lupieri A, Becker-Greene D, Aikawa E. Innate and adaptive immunity in cardiovascular calcification. Atherosclerosis. (2020) 306:59–67.
2. Forbang NI, Michos ED, McClelland RL, Remigio-Baker RA, Allison MA, Sandfort V, et al. Greater volume but not higher density of abdominal aortic calcium is associated with increased cardiovascular disease risk: MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. (2016) 9:e005138.
3. Bartstra JW, Mali W, Spiering W, de Jong PA. Abdominal aortic calcification: from ancient friend to modern foe. Eur J Prev Cardiol. (2021) 28:1386–91.
4. Hendriks EJ, de Jong PA, Beulens JW, van der Schouw YT, Forbang NI, Wright CM, et al. Annularity of aorto-iliac arterial calcification and risk of all-cause and cardiovascular mortality. JACC Cardiovasc Imaging. (2018) 11:1718–9. doi: 10.1016/j.jcmg.2018.01.029
5. Benjamens S, Pol RA, Glaudemans A, Wieringa I, Berger SP, Bakker SJ, et al. A high abdominal aortic calcification score by dual X-ray absorptiometry is associated with cardiovascular events after kidney transplantation. Nephrol Dial Transplant. (2018) 33:2253–9. doi: 10.1093/ndt/gfy158
6. Sethi A, Taylor DL, Ruby J, Venkataraman J, Sorokin E, Cule M, et al. Calcification of the abdominal aorta is an under-appreciated cardiovascular disease risk factor in the general population. Front Cardiovasc Med. (2022) 9:1003246. doi: 10.3389/fcvm.2022.1003246
7. Suh S, Oh T, Choi H, Kim C, Bae E, Oh K, et al. Abdominal aortic calcification and cardiovascular outcomes in chronic kidney disease: findings from KNOW-CKD Study. J Clin Med. (2022) 11:1157. doi: 10.3390/jcm11051157
8. Leow K, Szulc P, Schousboe J, Kiel D, Teixeira-Pinto A, Shaikh H, et al. Prognostic value of abdominal aortic calcification: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. (2021) 10:e017205. doi: 10.1161/JAHA.120.017205
9. Yan J, Pan Y, Shao W, Wang C, Wang R, He Y, et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling. Microbiome. (2022) 10:195. doi: 10.1186/s40168-022-01390-0
10. Vossen L, Kroon A, Schurgers L, de Leeuw P. Pharmacological and Nutritional Modulation of Vascular Calcification. Nutrients. (2019) 12:100.
11. Levy-Schousboe K, Frimodt-Møller M, Hansen D, Peters C, Kjaergaard K, Jensen J, et al. Vitamin K supplementation and arterial calcification in dialysis: results of the double-blind, randomized, placebo-controlled RenaKvit trial. Clin Kidney J. (2021) 14:2114–23. doi: 10.1093/ckj/sfab017
12. Szulc P. Abdominal aortic calcification: a reappraisal of epidemiological and pathophysiological data. Bone. (2016) 84:25–37. doi: 10.1016/j.bone.2015.12.004
13. Hutcheson J, Goettsch C. Cardiovascular calcification heterogeneity in chronic kidney disease. Circ Res. (2023) 132:993–1012.
14. Jimenez-Torres J, Alcalá-Diaz J, Torres-Peña J, Gutierrez-Mariscal F, Leon-Acuña A, Gómez-Luna P, et al. Mediterranean diet reduces atherosclerosis progression in coronary heart disease: an analysis of the CORDIOPREV randomized controlled trial. Stroke. (2021) 52:3440–9.
15. Li J, Lee D, Hu J, Tabung F, Li Y, Bhupathiraju S, et al. Dietary inflammatory potential and risk of cardiovascular disease among men and women in the U.S. J Am Coll Cardiol. (2020) 76:2181–93.
16. Chiavaroli L, Viguiliouk E, Nishi S, Blanco Mejia S, Rahelić D, Kahleová H, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. (2019) 11:338. doi: 10.3390/nu11020338
17. Valdes A, Walter J, Segal E, Spector T. Role of the gut microbiota in nutrition and health. Bmj. (2018) 361:k2179.
18. Avalos-Fernandez M, Alin T, Métayer C, Thiébaut R, Enaud R, Delhaes L. The respiratory microbiota alpha-diversity in chronic lung diseases: first systematic review and meta-analysis. Respir Res. (2022) 23:214. doi: 10.1186/s12931-022-02132-4
19. Sokol H, Leducq V, Aschard H, Pham H, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. (2017) 66:1039–48.
20. Marco M, Hutkins R, Hill C, Fulgoni V, Cifelli C, Gahche J, et al. A classification system for defining and estimating dietary intake of live microbes in US adults and children. J Nutr. (2022) 152:1729–36. doi: 10.1093/jn/nxac074
21. Hill C, Tancredi D, Cifelli C, Slavin J, Gahche J, Marco M, et al. Positive health outcomes associated with live microbe intake from foods. including fermented foods, assessed using the NHANES database. J Nutr. (2023) 153:1143–9.
22. Han L, Wang Q. Association of dietary live microbe intake with cardiovascular disease in US adults: a cross-sectional study of NHANES 2007-2018. Nutrients. (2022) 14:4908.
23. Tang H, Zhang X, Luo N, Huang J, Zhu Y. Association of dietary live microbes and non-dietary prebiotic/probiotic intake with cognitive function in older adults: evidence from NHANES. J Gerontol A Biol Sci Med Sci. (2023) [Epub ahead of print]. doi: 10.1093/gerona/glad175
24. Kauppila L, Polak J, Cupples L, Hannan M, Kiel D, Wilson P. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. (1997) 132:245–50. doi: 10.1016/s0021-9150(97)00106-8
25. Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. (2006) 17:281–9. doi: 10.1007/s00198-005-2010-5
26. Kadier K, Abulizi A, Ainiwaer A, Rehemuding R, Ma X, Ma YT. Unravelling the link between periodontitis and abdominal aortic calcification in the US adult population: a cross-sectional study based on the NHANES 2013-2014. BMJ Open. (2023) 13:e068931. doi: 10.1136/bmjopen-2022-068931
27. Levey A, Stevens L, Schmid C, Zhang Y, Castro A III, Feldman H, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009b) 150:604–12.
28. Tenore G, Caruso D, Buonomo G, D’Avino M, Ciampaglia R, Maisto M, et al. Lactofermented annurca apple puree as a functional food indicated for the control of plasma lipid and oxidative amine levels: results from a randomised clinical trial. Nutrients. (2019) 11:122. doi: 10.3390/nu11010122
29. Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. (2019) 24:370.
30. Koudoufio M, Desjardins Y, Feldman F, Spahis S, Delvin E, Levy E. Insight into polyphenol and gut microbiota crosstalk: Are their metabolites the key to understand protective effects against metabolic disorders? Antioxidants (Basel). (2020) 9:982. doi: 10.3390/antiox9100982
31. Liu Y, Peng P, Hung W, Wu P, Kao C, Wu P, et al. Comparative gut microbiome differences between high and low aortic arch calcification score in patients with chronic diseases. Int J Mol Sci. (2023) 24:5673. doi: 10.3390/ijms24065673
32. Bao W, Yang W, Su C, Lu X, He L, Zhang A. Relationship between gut microbiota and vascular calcification in hemodialysis patients. Ren Fail. (2023) 45:2148538. doi: 10.1080/0886022X.2022.2148538
33. Nogal A, Valdes A, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. (2021) 13:1–24. doi: 10.1080/19490976.2021.1897212
34. Ağagündüz D, Yılmaz B, Şahin T, Güneşliol B, Ayten Ş, Russo P. Dairy lactic acid bacteria and their potential function in dietetics: the food-gut-health axis. Foods. (2021) 10:3099.
35. Ecklu-Mensah G, Gilbert J, Devkota S. Dietary selection pressures and their impact on the gut microbiome. Cell Mol Gastroenterol Hepatol. (2022) 13:7–18.
36. Kaźmierczak-Siedlecka K, Daca A, Fic M, van de Wetering T, Folwarski M, Makarewicz W. Therapeutic methods of gut microbiota modification in colorectal cancer management - fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes. (2020) 11:1518–30. doi: 10.1080/19490976.2020.1764309
37. Vera-Santander V, Hernández-Figueroa R, Jiménez-Munguía M, Mani-López E, López-Malo A. Health benefits of consuming foods with bacterial probiotics, postbiotics, and their metabolites: a review. Molecules. (2023) 28: 1230.
38. Rezac S, Kok C, Heermann M, Hutkins R. Fermented foods as a dietary source of live organisms. Front Microbiol. (2018) 9:1785. doi: 10.3389/fmicb.2018.01785
39. Wang X, Zhang P, Zhang X. Probiotics regulate gut microbiota: an effective method to improve immunity. Molecules. (2021) 26:6076.
40. Gou H, Zhang Y, Ren L, Li Z, Zhang L. How do intestinal probiotics restore the intestinal barrier? Front Microbiol. (2022) 13:929346. doi: 10.3389/fmicb.2022.929346
41. Purdel C, Ungurianu A, Adam-Dima I, Margină D. Exploring the potential impact of probiotic use on drug metabolism and efficacy. Biomed Pharmacother. (2023) 161:114468.
42. Kim J, Choi M, Jeong J, Lim S, Kim I, Yoo H, et al. Effect of probiotics on pharmacokinetics of orally administered acetaminophen in mice. Drug Metab Dispos. (2018) 46:122–30.
43. Din A, Hassan A, Zhu Y, Yin T, Gregersen H, Wang G. Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl Microbiol Biotechnol. (2019) 103:9217–28. doi: 10.1007/s00253-019-10142-4
44. Bielik V, Kolisek M. Bioaccessibility and bioavailability of minerals in relation to a healthy gut microbiome. Int J Mol Sci. (2021) 22:6803.
45. Rizzoli R, Biver E. Are probiotics the new calcium and vitamin d for bone health? Curr Osteoporos Rep. (2020) 18:273–84.
46. Scholz-Ahrens K, Ade P, Marten B, Weber P, Timm W, Açil Y, et al. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr. (2007) 137:838s–46s. doi: 10.1093/jn/137.3.838S
47. Liang D, Liu C, Yang M. Blood cadmium and abdominal aortic calcification in population with different weight statuses: a population-based study. J Cardiovasc Transl Res. (2023) [Epub ahead of print]. doi: 10.1007/s12265-023-10414-5
48. Kang M, Baek K, Lee Y, Kim G, Seo S. Production of Vitamin K by wild-type and engineered microorganisms. Microorganisms. (2022) 10:554. doi: 10.3390/microorganisms10030554
49. Leeming E, Johnson A, Spector T, Le Roy C. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. (2019) 11:2862. doi: 10.3390/nu11122862
50. Khalesi S, Bellissimo N, Vandelanotte C, Williams S, Stanley D, Irwin C. A review of probiotic supplementation in healthy adults: helpful or hype? Eur J Clin Nutr. (2019) 73:24–37.
51. Kumar Singh A, Cabral C, Kumar R, Ganguly R, Kumar Rana H, Gupta A, et al. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients. (2019) 11:2216.
Keywords: dietary live microbes, abdominal aortic calcification, food-gut-health axis, NHANES, cross-sectional study
Citation: Huo X, Jia S, Zhang X, Sun L, Liu X, Liu L, Zuo X and Chen X (2023) Association of dietary live microbe intake with abdominal aortic calcification in US adults: a cross-sectional study of NHANES 2013–2014. Front. Nutr. 10:1267607. doi: 10.3389/fnut.2023.1267607
Received: 27 July 2023; Accepted: 10 October 2023;
Published: 24 November 2023.
Edited by:
Galya Bigman, Baltimore VA Medical Center, United StatesReviewed by:
Zanzhe Yu, Shanghai Jiao Tong University, ChinaXiaofei Hu, Army Medical University, China
Copyright © 2023 Huo, Jia, Zhang, Sun, Liu, Liu, Zuo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Chen, eGlhb3BpbmdjaGVuMTNAc2luYS5jb20=
 Xingwei Huo
Xingwei Huo Shanshan Jia1
Shanshan Jia1 Lu Liu
Lu Liu Xiaoping Chen
Xiaoping Chen