
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 28 September 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1265662
This article is part of the Research Topic Body Composition and Cardiovascular Health View all 6 articles
Background: There are various cross-sectional studies that concluded that vitamin D is associated with blood pressure, but randomized controlled studies have not yielded consistent conclusions. Considering many limitations indeed, our study aimed to examine whether concentrations of 25(OH)D are inversely associated with blood pressure in people without a previous diagnosis of hypertension.
Method: We analyzed data from the 2005–2018 National Health and Nutrition Examination Survey. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by applying multivariable logistic regression models. The dose–response relationship was assessed by means of restricted cubic spline regression, and stratification analyses were employed to test the consistency between the subgroups.
Results: Of 17,467 participants aged ≥ 20 years without a previous diagnosis of hypertension, 4,769 had higher blood pressure. Compared with individuals whose 25(OH)D levels were in the bottom quartile (<44.3 nnol/L), adjusting for multiple confounders, the ORs for higher blood pressure were 0.90(95%CI 0.78, 1.05), 0.85(95%CI 0.72, 0.99), and 0.86(95%CI 0.72, 1.02), respectively (P for trend = 0.096). Furthermore, as a continuous variable, 25(OH)D concentrations were non-linearly associated with an increased risk of hypertension (P < 0.001). The interaction between the sleeplessness subgroup and higher blood pressure was significant (P = 0.042).
Conclusion: In adults without a previous diagnosis of hypertension in the United States, concentrations of 25(OH)D were inversely associated with higher blood pressure when it was <84 nmol/L.
Approximately 1 billion individuals worldwide suffer from hypertension, making it one of the most common chronic disorders (1). Concomitantly, hypertension is an independent risk factor for cardiovascular and cerebrovascular disorders, harms quality of life, and is the leading cause of premature mortality globally (2). Nevertheless, due to the under diagnosis and under treatment of hypertension, the rates of blood pressure (BP) control remain poor and far from satisfactory worldwide. To reduce premature mortality, prevention and control of hypertension is an essential global public health strategy (3).
As a fat-soluble steroid hormone, vitamin D is essential for calcium and phosphorus balance and bone health. In addition to dietary intake, subcutaneous endogenous synthesis is an alternative major source of vitamin D. Vitamin D first must be hydroxylated to 25-hydroxyvitamin in the liver and further to 1,25-dihydroxyvitamin in the kidney enabling bioavailability (4). Interestingly, vitamin D receptors and the vitamin D enzyme system have been discovered to exist in the majority of cells in the body, with the ability to drive the transcription of hundreds of genes (5). Therefore, many scholars reasonably hypothesize that vitamin D may affect human health more than its role in bone health. To date, extensive published studies have described that vitamin D deficiency is related to osteoporosis, cancer, cardiovascular diseases, type 2 diabetes, inflammation, and autoimmune diseases (4, 5). Regretfully, large randomized controlled trials have not found convincing evidence that supplementing with vitamin D helps prevent certain disorders, such as hypertension. Moreover, existing studies have a few limitations, such as small sample sizes, insufficient adjustment of some important covariates, and specific participants. In addition, whether complications could modify the association of interest remains unclear.
In the current study, we attempted to investigate the relationships between serum 25(OH)D and BP, detailing systolic and diastolic blood pressure, in a nationally representative sample of US adults with no previous diagnosis of hypertension.
The National Health and Nutrition Examination Survey (NHANES) is a stratified, multistage probability survey, which collected health and nutrition information among the non-institutionalized US population through interviews, physical examination, and laboratory testing. The National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC) conducted NHANES, which was approved by the Institutional Review Board of the National Center for Health Statistics. All participants or their guardians provided signed informed consent.
In the current study, we used data from seven consecutive NHANES 2-year cycles (2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018). Individuals (aged ≥ 20 years) with no previous diagnosis of hypertension were included in the analysis. We excluded participants with missing blood pressure (BP) or serum 25(OH)D measurements, with measurements of BP <3 times, and with missing serological values >20%. Outliers (through the “scatter” command in Stata) of serum 25(OH)D were further excluded. Hypertension was defined as a self-reported doctor diagnosis of hypertension and use of antihypertensive drugs. We finally had 17,467 eligible participants enrolled for analyses (Figure 1).
In the NHANES 2005–2006, serum 25(OH)D was measured by the DiaSorin assay kit [DiaSorin Corporation 25(OH)D 125I RIA kit; DiaSorin Corporation, Stillwater, Minnesota, USA]. Beginning from the 2007–2008 cycle, serum 25(OH)D concentrations were tested by a standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS). The regression approach was used to convert serum 25(OH)D data from the NHANES 2005–2006 to equivalent 25(OH)D measurements using the LC-MS/MS method. We used LC-MS/MS-equivalent data for all analyses as recommended by the CDC. Detailed information is available at https://wwwn.cdc.gov/nchs/nhanes/vitamind/analyticalnote.aspx.
Three successive blood pressure readings were taken after 5 min of calmly resting in a sitting position and establishing the maximum inflation level (MIL). A fourth try would be performed if the measurement of BP was interrupted or incomplete. All measurements of BP were taken in the mobile examination center (MEC). The absolute BP was obtained by averaging three valid measurements. Participants were categorized as having higher BP if systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥80 mmHg.
Several covariates were evaluated as potential confounding factors. Information on age (years), sex (male and female), race (non-Hispanic white, non-Hispanic black, Mexican American, and other race), education level (less than high school, high school, and college education or above), smoking status (current smoking, previous smoking, and never smoking), heavy alcohol drinking (yes and no) was acquired through household interviews utilizing standardized questionnaires. Current smoking was defined as smoking at least 100 cigarettes in one's life and still smoking, previous smoking as smoking at least 100 cigarettes in one's life but not presently smoking, and non-smoking as smoking fewer than 100 cigarettes in one's life. Alcohol consumption was estimated based on self-reported data on the average number of daily drinks. Heavy alcohol drinking was defined as having more than one drink per day for women and more than two drinks per day for men, on average, over the previous 12 months. Vitamin D supplementation refers to the intake of vitamin D2 + D3 in the previous month. During the mobile examination center visit, physical measurements such as body mass index (BMI) and waist circumference were collected. BMI was computed by dividing weight in kilos by height in meters squared.
In addition, serum samples from participants were obtained during the medical examination and frozen and stored at temperatures below −70°C until analysis. Serum concentrations of calcium, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, albumin, triglyceride, cholesterol, total bilirubin, blood urea nitrogen (BUN), uric acid (sUA), and HbA1c were included at the baseline. The Chronic Kidney Disease-Epidemiology Collaboration (CKDEPI) (6) equation was used to compute the mean estimated glomerular filtration rate (eGFR, ml/min/1.73 m2). According to the KDOQI (7) guidelines, CKD can be classified into five stages: CKD1 (eGFR ≥ 90), CKD2 (60 ≤ eGFR < 90), CKD3 (30 ≤ eGFR < 60), CKD4 (15 ≤ eGFR < 30), and CKD5 (eGFR < 15).
At the baseline, comorbidities consist of a history (yes, no) of self-reported physician-diagnosed diabetes, coronary artery disease (CAD), stroke, cancer, and sleeplessness.
Given the complex sampling design of the NHANES, all analyses were properly weighted in accordance with the analytical guidelines from the National Health and Nutrition Examination Survey for the years 2005 through 2018, which is accessible at https://wwwn.cdc.gov/Nchs/Nhanes/AnalyticGuidelines.aspx#print. As mentioned before, we combined seven consecutive 2-year cycles (2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018) to estimate with greater precision and smaller sampling error.
A complex weighting method was utilized for the data description and statistical analysis in this study by using the “Survey” package in R software. The categorical variables were described as frequencies (unweighted) and proportions (weighted), whereas the continuous variables were described as mean standard deviation. The differences were tested using the Wilcoxon rank-sum test for continuous variables and the chi-square test with Rao–Scott second-order correction for categorical variables. To investigate the association between serum 25(OH)D and higher BP, serum 25(OH)D levels were divided into quartiles, and adjusted ORs for increased blood pressure were calculated through weighted logistic regression in three distinct models. Model 1 was unadjusted. Model 2 was adjusted for age, gender, and ethnicity. Model 3 was adjusted for age, gender, ethnicity, education level, smoking status, heavy alcohol drinking, vitamin D supplementation, BMI, waist circumference, diabetes, coronary heart disease, stroke, cancer, sleeplessness, CKD, serum calcium, ALT, AST, Scr, albumin, triglyceride, cholesterol, total bilirubin, BUN, sUA, and HbA1c. The lowest quartile of serum 25(OH)D was used as the reference group. To further examine the dose–response relationship between serum 25(OH)D concentrations and BP, restricted cubic spline regression was performed with four knots, with the multivariate adjustment in Model 3. If the p-value for non-linearity was >0.05, we considered the dose–response relationship to be linear, otherwise non-linear. Stratification analyses were performed to examine whether the correlations differed for subgroups classified comorbidities including diabetes, CAD, stroke, cancer, CKD, and sleeplessness.
A two-sided p < 0.05 was used to determine statistical significance. All the data analyses were performed using the software Stata/SE V.15.1 (StataCorp LLC, College Station, TX, USA) and R software 4.2.2 (http://www.R-project.org).
Table 1 displays a broad overview of the weighted features of all 17,467 study participants according to serum 25(OH)D quartiles (Q1 < 44.3, 44.3 ≤ Q2 < 60.5, 60.5 ≤ Q3 < 77.7, and Q4 ≥ 77.7). Among the 17,467 adults (48.7% were men and 51.3% were women) without a previous diagnosis of hypertension. The average age was 42.8 years old; 65.7% were non-Hispanic white, 10.1% were Mexican American, 9.3% were non-Hispanic black, and 14.9% were “Other” races. Approximately 63.6% attained an educational level greater than a high school diploma. Across quartiles of serum 25(OH)D, significant differences (p < 0.05) were found in smoking status, heavy alcohol drinking, vitamin D supplementation, BMI, waist circumference, cancer, sleeplessness, CKD, serum calcium, ALT, AST, Scr, albumin, triglyceride, cholesterol, total bilirubin, BUN, sUA, and HbA1c. Participants with higher BP were 4,769, and the weighted percentage reached 25%. Of these, 3,382 patients suffered from higher systolic blood pressure, and 2,739 suffered from higher diastolic blood pressure.
As previously stated, we built three logistic models to explore the connection between serum 25(OH)D concentrations and BP (Table 2).
As for higher BP, in Model 1, which was unadjusted for variables, the HRs (95% CI) for the Q2 (44.3–60.5), Q3 (60.5–77.7), and Q4 (≥77.7) were 0.90 (0.80, 1.02), 0.87 (0.77, 0.98), and 0.92 (0.81, 1.04), respectively, compared with the Q1 (<44.3). After adjusting for age, gender, and ethnicity in Model 2, the HRs (95% CI) of higher BP for Q2, Q3, and Q4 were 0.86 (0.76, 0.99), 0.75 (0.65, 0.85), and 0.69 (0.59, 0.79), respectively, compared with the reference (Q1). Meanwhile, in Model 3, compared with Q1, the HRs (95% CI) for Q2, Q3, and Q4 were 0.90 (0.78, 1.05), 0.85 (0.72, 0.99), and 0.86 (0.72, 1.02) after adjusted for multiple confounders.
We further evaluated the effect of serum 25(OH)D concentrations on higher systolic and diastolic blood pressure alone. In Model 3, Q2-4 had a significantly lower HR (95% CI) of higher systolic BP than the reference (Q1). However, opposite of Q3 (60.5–77.7) and Q4 (≥77.7), Q2 (44.3–60.5) tended positive correlation with higher diastolic BP in Model 3, but it did not reach statistical significance (p > 0.05).
To address non-linearity, restricted cubic spline analyses were used to visualize the association of serum 25(OH)D concentrations and BP, indicating a U-shaped correlation (p for non-linear <0.0001, Figure 2A). Meanwhile, an approximate L-shaped relationship was found between serum 25(OH)D and elevated SBP alone (p for non-linear =0.0009, Figure 2B). However, a complex relationship, roughly Z-shaped, was found between serum 25(OH)D and DBP (p for non-linear =0.0064, Figure 2C).
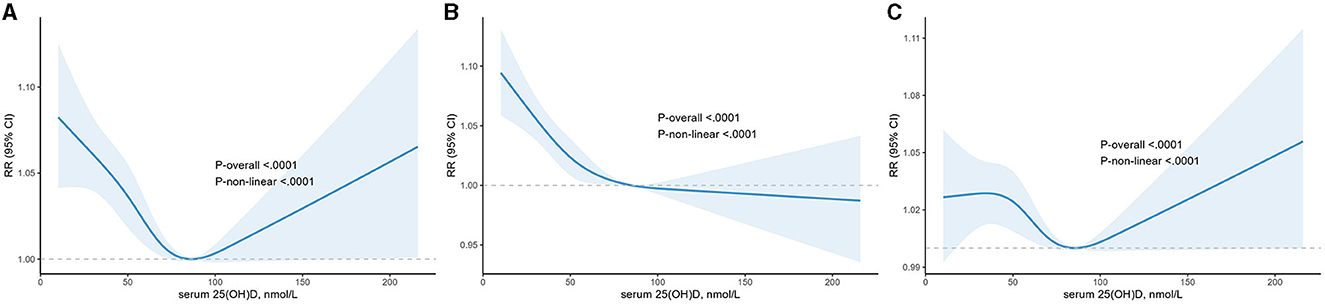
Figure 2. The dose-response analysis between serum 25(OH)D and higher blood pressure, higher systolic blood pressure and higher diastolic blood pressure with restricted cubic splines. The solid blue line and light blue area represent the estimated odds ratios and its 95% confidence interval, respectively. (A) Higher blood pressure; (B) higher systolic blood pressure; (C) higher diastolic blood pressure.
We adjusted the confounding factors in Model 3 in these subgroups. CKD1 was considered to have no chronic kidney disease (CKD), and CKD2 to CKD5 were regarded as having chronic kidney diseases. As shown in Figure 3, the effect of serum 25(OH)D on blood pressure is consistent across the subgroups except for sleeplessness (p for interaction < 0.05).
Our analyses, based on a nationally representative population from seven consecutive NHANES cycles (2005–2018), were performed to explore the relationship between serum 25(OH)D concentrations and BP in adults with no previous diagnosis of hypertension in the US. The key findings could be summarized in three points. First, after adjusting multiple variables, serum 25(OH)D was negatively correlated with BP, primarily SBP. Second, the relationships between serum 25(OH)D concentration and BP, including SBP and DBP, were non-linear. Third, the relationship was consistent across the subgroups except for sleeplessness.
Early in the 1980s, Sowers et al. (8) discovered a significant negative relationship between ingestion of vitamin D and BP. In the following decades, cross-sectional studies based on the Third National Health and Nutrition Examination Survey (NHANES III) have obtained the same conclusions (9, 10). However, prospective cohort studies elicited inconsistent results. After 4 years of follow-up, Forman et al. (11) found that compared with women without vitamin D deficiency [25(OH)D <15 ng/ml], women with vitamin D deficiency had a multivariable relative risk (RR) of 2.98 (95% CI: 1.24 to 7.20). Moreover, the risk was even more markedly elevated among men with vitamin D deficiency. Nonetheless, after an 8-year follow-up period, the RRs for hypertension incidents had reduced and were no longer statistically significant. It is noteworthy that there are only 33 participants with 25(OH)D deficiency in this study, so the representativeness of its outcomes needs to be queried. In the Tromsø study conducted by Jorde et al. (12), they proceeded with a 14-year follow-up from 1994 to 2008. They observed that, after controlling variables, serum 25(OH)D in 1994 failed to predict future hypertension or increased BP, and that there was no significant relationship between changes in serum 25(OH)D and changes in BP from 1994 to 2008. Moreover, various researchers had further investigated the relationship between vitamin D supplementation and lowering BP, obtaining negative (13–17) and positive (18–21), seemingly contradictory results, which brought into doubt the prospects of vitamin D for lowering BP. But previous studies had some limitations: small sample size (11), not taking into account some important covariates (such as chronic disease (9, 10, 12), smoking/drinking (10, 11), dietary supplement use (9, 11, 12), etc., which could significantly influence serum 25(OH)D status and prevalence of hypertension), specific participants population. However, our research was a significant cross-sectional study and employed a nationally representative sample of US adults, excluding those taking hypotensive drugs or history of physician-diagnosed hypertension participants to minimize the likelihood that they improve their BP through lifestyle changes and agents. At the same time, we adjusted for lifestyle habits, comorbidities, and serological indicators and further analyzed the effect of serum 25(OH)D concentration on systolic and diastolic blood pressure, respectively. Consistent with the previous cross-sectional studies, we found a negative relationship between serum 25(OH)D levels and higher BP. At the same time, our study further revealed that higher serum 25(OH)D levels were associated with lower SBP. As for the contribution between serum 25(OH)D levels and DBP, there was even a positive effect, but it was not statistically significant.
So far, the exact mechanism of hypotension by serum 25(OH)D is still unknown, and there are several possible core factors. An animal experiment conducted by Li et al. (22) found a several-fold increase in renin expression and plasma angiotensin II production in mice with vitamin D receptor-null (VDR-null). Thus, it gave rise to the earliest and currently widely accepted mechanism that vitamin D can lower BP by suppressing renin synthesis to downregulate the activity of the renin-angiotensin-aldosterone system (RASS) (23). Moreover, vitamin D inhibits the production of parathyroid hormone (PTH), which can increase BP by stimulating PTH2 receptors expressed on vascular smooth muscle cells, upregulating both receptors of advanced glycation end products (RAGE) expression and monocyte-macrophages cytokines and IL-6 production, and promoting the deposition of calcium in the arterial wall leading to increasing collagen deposition and vessel stiffness (24). These imply that lack of vitamin D may be a risk factor for hypertension. In our study, when serum 25(OH)D < 85 nmol/L, it was negatively correlated with SBP but not with DBP. A cross-sectional study of the biethnic population discovered that SBP was inversely associated with 25(OH)D levels in whites but not in blacks (25). Later on, Jafari et al. (26) conducted a meta-analysis of 26 randomized controlled trials for patients with diabetes and demonstrated that vitamin D improved SBP in type 2 diabetic patients. Subsequent studies have revealed similar findings (27–29), but few studies have been conducted in patients without hypertension, and our experiment fills this gap. It is well known that BP is defined as the lateral pressure exerted on the walls of blood vessels per unit area during the flow of blood (3). Part of the stroke volume is sent directly to peripheral tissues during ventricular contraction, resulting in systolic pressure, while part of it is temporarily stored in the aorta and central artery, stretching the arterial wall and rising local BP, resulting in diastolic pressure (30). BP is directly influenced by two primary factors: the volume of intravascular fluid and the capacity for vasodilation. As previously mentioned, vitamin D can downregulate the activity of the RASS, which exerts various physiological and pathophysiological effects including vasoconstriction and sodium/water retention to elevate BP via activation of angiotensin II type 1 receptor (AT1R). In addition, when vitamin D deficiency induces vascular sclerosis and stretching disturbances, the entire stroke volume flows through the arterial system and peripheral tissues, which results in increased SBP and decreased DBP (30). Further investigations of the antihypertensive mechanism of vitamin D are now warranted.
Increasing evidence supports the existence of a threshold effect for vitamin D levels (31). In our study, as mentioned previously, the relationship of vitamin D with higher blood pressure and higher SBP was U- and L-shaped, respectively. Furthermore, both of them had a cut-off value of approximately 84 nmol/L. For higher blood pressure, the risk was increased for both vitamin D levels below or above 84 nmol/L. The risk of higher SBP rose significantly below 84 nmol/L but remained marginally significant above 84 nmol/L. These outcomes indicated that subjects with vitamin D insufficiency or deficiency were more sensitive to vitamin D supplementation, and there was a risk of higher blood pressure, primarily DBP, when vitamin D is above 84 nmol/L. In support, previous studies also showed that the antihypertensive effect of vitamin D supplementation was observed only in patients with vitamin D insufficiency or deficiency (21, 32–34). However, to the best of our knowledge, a particular Z-shaped relationship between serum 25(OH)D and DBP was detected for the first time. It revealed a negative relationship between serum 25(OH)D and DBP within the range of 36 to 84 nmol/L but a positive relationship below or above the range.
Upon further subgroup analysis, we found that the inverse association between serum 25(OH)D and BP tends to be more pronounced and sustained among participants with sleeplessness. As well, recently, a cross-sectional study showed a more pronounced and stable association between vitamin D status and coronary heart disease in participants with poor sleep patterns (35). As far as we know, sleeplessness is recognized as short sleep duration and insomnia, and daytime sleepiness may be linked to reduced outdoor exercise and sunlight exposure, which may diminish serum vitamin D via endogenous synthesis (36, 37) and boost melatonin expression (38). As a result, subjects with sleeplessness had lower levels of serum vitamin D compared with those without sleeplessness, so the decline in blood pressure resulting from serum vitamin D levels was more evident according to our findings. However, further studies are needed to validate these findings.
It is important to note that there are some limitations in our study. First, there is a dearth of data on participants' salt intake and sun exposure, which are important factors in endogenous vitamin D production and hypertension, respectively. Second, we performed a single measurement of plasma 25(OH)D, which had a marked circadian variation. Third, because BP was measured merely in a single appointment, it is possible that some participants were wrongly labeled as having higher blood pressure while they were actually normotensive, and vice versa. Fourth, biases resulting from excluded and unmeasured confounders may still exist, despite our efforts to account for as many confounders as possible. Fifth, relationships between 25(OH)D and BP stratified by sleeplessness deserve to be further verified. Finally, the cross-sectional design also makes it impossible to distinguish between causes and effects.
We discovered a non-linear association between serum 25(OH)D concentration and BP in a nationally representative sample of US participants without a previous diagnosis of hypertension. Concentrations of 25(OH)D were inversely associated with blood pressure when it was <84 nmol/L. More specifically, serum 25(OH)D is adversely correlated with systolic blood pressure, and as for diastolic blood pressure, higher serum 25(OH)D concentrations were associated with higher diastolic blood pressure when serum 25(OH)D levels >84 nmol/L.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
The studies involving human participants were reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
JC: Writing—original draft, Investigation, Validation. JT: Validation, Writing—original draft. XK: Data curation, Formal analysis, Writing—review and editing. CZ: Data curation, Formal analysis, Writing—review and editing. RZ: Visualization, Writing—review and editing. JS: Visualization, Writing—review and editing. XZ: Visualization, Writing—review and editing. ZL: Conceptualization, Writing—review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lobo MD, Sobotka PA, Pathak A. Interventional procedures and future drug therapy for hypertension. Eur Heart J. (2017) 38:1101–11. doi: 10.1093/eurheartj/ehw303
2. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16:223–37. doi: 10.1038/s41581-019-0244-2
3. Ma J, Li Y, Yang X, Liu K, Zhang X, Zuo X, et al. Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. (2023) 8:168. doi: 10.1038/s41392-023-01430-7
4. Reijven PLM, Soeters PB. Vitamin D: A magic bullet or a myth? Clin Nutr. (2020) 39:2663–74. doi: 10.1016/j.clnu.2019.12.028
5. Gallagher JC, Rosen CJ. Vitamin D: 100 years of discoveries, yet controversy continues. Lancet Diab Endocrinol. (2023) 11:362–74. doi: 10.1016/S2213-8587(23)00060-8
6. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
7. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US Commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. (2014) 63:713–35. doi: 10.1053/j.ajkd.2014.01.416
8. Sowers MR, Wallace RB, Lemke JH. The association of intakes of vitamin D and calcium with blood pressure among women. Am J Clin Nutr. (1985) 42:135–42. doi: 10.1093/ajcn/42.1.135
9. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the third national health and nutrition examination survey. Am J Hypertens. (2007) 20:713–9. doi: 10.1016/j.amjhyper.2007.01.017
10. Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. (2008) 87:136–41. doi: 10.1093/ajcn/87.1.136
11. Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. (2007) 49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288
12. Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. (2010) 55:792–8. doi: 10.1161/HYPERTENSIONAHA.109.143990
13. Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. (2015) 175:745–54. doi: 10.1001/jamainternmed.2015.0237
14. Wu L, Sun D. Effects of calcium plus vitamin D supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens. (2017) 31:547–54. doi: 10.1038/jhh.2017.12
15. He S, Hao X. The effect of vitamin D3 on blood pressure in people with vitamin D deficiency: A system review and meta-analysis. Medicine (Baltimore). (2019) 98:e15284. doi: 10.1097/MD.0000000000015284
16. Abboud M. Vitamin D supplementation and blood pressure in children and adolescents: a systematic review and meta-analysis. Nutrients. (2020) 12:1163. doi: 10.3390/nu12041163
17. Gu D, Li J, Little J, Li H, Zhang X. Associations between serum sex hormone concentrations and telomere length among U.S. adults, 1999-2002. J Nutr Health Aging. (2020) 24:48–54. doi: 10.1007/s12603-019-1291-x
18. Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. (2009) 27:1948–54. doi: 10.1097/HJH.0b013e32832f075b
19. Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. (2013) 28:205–21. doi: 10.1007/s10654-013-9790-2
20. Shu L, Huang K. Effect of vitamin D supplementation on blood pressure parameters in patients with vitamin D deficiency: a systematic review and meta-analysis. J Am Soc Hypertens. (2018) 12:488–96. doi: 10.1016/j.jash.2018.04.009
21. Farapti F, Fadilla C, Yogiswara N, Adriani M. Effects of vitamin D supplementation on 25(OH)D concentrations and blood pressure in the elderly: a systematic review and meta-analysis. F1000Res. (2020) 9:633. doi: 10.12688/f1000research.24623.3
22. Li YC, Kong J, Wei M, Chen Z-F, Liu SQ, Cao L-P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. (2002) 110:229–38. doi: 10.1172/JCI0215219
23. Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. (2004) 89:387–92. doi: 10.1016/j.jsbmb.2004.03.004
24. Simeoni M, Perna AF, Fuiano G. Secondary hyperparathyroidism and hypertension: an intriguing couple. J Clin Med. (2020) 9:629. doi: 10.3390/jcm9030629
25. Sakamoto R, Jaceldo-Siegl K, Haddad E, Oda K, Fraser GE, Tonstad S. Relationship of vitamin D levels to blood pressure in a biethnic population. Nutr Metab Cardiovasc Dis. (2013) 23:776–84. doi: 10.1016/j.numecd.2012.04.014
26. Jafari T, Fallah AA, Rostampour N, Mahmoodnia L. Vitamin D ameliorates systolic but not diastolic blood pressure in patients with type 2 diabetes: Results from a meta-analysis of randomized controlled trials. Int J Vitam Nutr Res. (2018) 88:90–9. doi: 10.1024/0300-9831/a000291
27. Joukar F, Naghipour M, Hassanipour S, Salari A, Alizadeh A, Saeidi-Saedi H, et al. Association of serum levels of vitamin D with blood pressure status in northern iranian population: the PERSIAN Guilan cohort study (PGCS). Int J Gen Med. (2020) 13:99–104. doi: 10.2147/IJGM.S244472
28. Panahi Y, Namazi S, Rostami-Yalmeh J, Sahebi E, Khalili N, Jamialahmadi T, et al. Effect of vitamin D supplementation on the regulation of blood pressure in iranian patients with essential hypertension: a clinical trial. Adv Exp Med Biol. (2021) 1328:501–11. doi: 10.1007/978-3-030-73234-9_35
29. Zeng S, Bachert D, Pavkovic M, Sandner P, Hocher CF, Tsuprykov O, et al. Free vitamin D is independently associated with systolic blood pressure in diabetic patients with impaired kidney function. Clin Nephrol. (2022) 97:63–9. doi: 10.5414/CN110549
30. Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. (2012) 82:388–400. doi: 10.1038/ki.2012.131
31. Scragg R. Emerging evidence of thresholds for beneficial effects from vitamin D supplementation. Nutrients. (2018) 10:561. doi: 10.3390/nu10050561
32. Sluyter JD, Camargo CA, Stewart AW, Waayer D, Lawes CMM, Toop L, et al. Effect of monthly, high-dose, long-term vitamin d supplementation on central blood pressure parameters: a randomized controlled trial substudy. J Am Heart Assoc. (2017) 6:e006802. doi: 10.1161/JAHA.117.006802
33. Mirhosseini N, Vatanparast H, Kimball SM. The association between serum 25(OH)D status and blood pressure in participants of a community-based program taking vitamin D supplements. Nutrients. (2017) 9:1244. doi: 10.3390/nu9111244
34. Tabesh M, Azadbakht L, Faghihimani E, Tabesh M, Esmaillzadeh A. Effects of calcium plus vitamin d supplementation on anthropometric measurements and blood pressure in vitamin D insufficient people with type 2 diabetes: a randomized controlled clinical trial. J Am Coll Nutr. (2015) 34:281–9. doi: 10.1080/07315724.2014.905761
35. Wu Z, Hu H, Wang C, Rao J, Wu J, Shi Y, et al. Sleep patterns modify the association between vitamin D status and coronary heart disease: results from NHANES 2005-2008. J Nutr. (2023) 153:1398–406. doi: 10.1016/j.tjnut.2022.11.028
36. Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol. (2017) 70:89–100. doi: 10.1016/j.jacc.2017.05.031
37. Demer LL, Hsu JJ, Tintut Y. Steroid hormone vitamin D: implications for cardiovascular disease. Circ Res. (2018) 122:1576–85. doi: 10.1161/CIRCRESAHA.118.311585
Keywords: 25-hydroxyvitamin D, blood pressure, hypertension, nutrition surveys, cross-sectional study
Citation: Che J, Tong J, Kuang X, Zheng C, Zhou R, Song J, Zhan X and Liu Z (2023) Relationship between serum 25-hydroxyvitamin D concentrations and blood pressure among US adults without a previous diagnosis of hypertension: evidence from NHANES 2005–2018. Front. Nutr. 10:1265662. doi: 10.3389/fnut.2023.1265662
Received: 23 July 2023; Accepted: 04 September 2023;
Published: 28 September 2023.
Edited by:
Ian Glynn Davies, Liverpool John Moores University, United KingdomReviewed by:
Hari Krishnamurthy, Vibrant Sciences, United StatesCopyright © 2023 Che, Tong, Kuang, Zheng, Zhou, Song, Zhan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengzhang Liu, bGl1emVuZ3poYW5nNjY2QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.