
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 04 December 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1265014
Background: An increasing number of studies have demonstrated that gastrointestinal inflammation may increase prostate cancer risk and raise the prostate-specific antigen (PSA) level. However, the association between ulcerative colitis (UC) and acute gastroenteritis (AGE) with PSA remains unclear and complicated. Herein, we evaluated the relationship between UC and AGE with PSA concentration using the National Health and Nutrition Examination Survey (NHANES) database and Mendelian randomization (MR) analyses.
Materials and methods: A total of 1,234 participants fit into the study after conducting the screening based on the NHANES survey conducted from 2009 to 2010. UC and AGE were the independent variables, and PSA was the dependent variable. Weighted multiple linear regressions were utilized to estimate the association of UC and AGE with PSA concentration. To detect the causal relationship between UC and AGE with PSA, a two-sample Mendelian randomized analysis was conducted.
Results: After controlling for all covariates, PSA (log2 transform) concentrations in the UC group were increased by 0.64 (0.07, 1.21). AGE was not independently associated with PSA levels after adjusting potential confounders. In patients with coronary artery disease, AGE promotes elevated PSA (log2 transform) concentrations (β = 1.20, 95% CI: 0.21–2.20, p < 0.001). Moreover, an IVW MR analysis indicated that genetically predicted UC was associated with increased PSA, and that AGE was not associated with PSA.
Conclusion: This study indicated that a positive causal association exists between UC and the PSA level. However, there is no evidence to support the relationship between AGE and the PSA level.
Gastrointestinal inflammation is a widespread disorder that affects individuals of all ages; thus, a significantly negative clinical, economic, and humanitarian effect is exerted worldwide. Acute gastroenteritis (AGE) and ulcerative colitis (UC) are characterized by the inflammation of the digestive tract. UC, an idiopathic chronic inflammatory disorder that affects the gastrointestinal tract, entails an aberrant immune response to intestinal microflora. Worldwide, tens of millions of individuals exhibit UC, and its prevalence as well as the all-cause mortality rate are gradually increasing (1). UC is more prevalent in industrialized regions, including North America and Western Europe; this notwithstanding, Asia is exhibiting an increasing incidence. Statistics report a prevalence of 286 cases per 100,000 individuals in the United States, and that of 505 per 100,000 individuals in Norway (2). AGE is defined as sudden onset diarrhea (unrelated to chronic disease, with or without nausea, vomiting, fever, or abdominal pain) that is typically occasioned by viral or bacterial infection (3, 4). Globally, AGE led to nearly 89.5 million DALYs lost and 1.45 million deaths annually (5).
Individuals with gastrointestinal inflammation may exhibit an increased risk of developing cancer, with prostate cancer (PCa) accounting for the most commonly diagnosed cancer in males and the second leading cause of cancer-related death in men worldwide (6). The prostate-specific antigen (PSA), a serine protease produced by the prostate gland, has been widely utilized in clinical practice as a screening tool for PCa. For early-stage PCa detection, serum PSA-based screening is crucial; it can potentially increase the effectiveness of treatment and reduce mortality rates (7). Although PSA is a marker for the prostate, it is not specific to cancer. PSA levels can be elevated in non-cancerous conditions such as benign prostatic hyperplasia (BPH) and prostatitis.
Several researchers have reported an association between UC and PCa (8–12). However, observations pertaining to the association between UC and PCa risk were mixed. Relatively few studies have examined the relationship between UC and AGE with PSA. Therefore, we explored the correlation of UC and AGE with PSA using the National Health and Nutrition Examination Survey (NHANES) database. Their potentially causal relationship was subsequently analyzed using two-sample MR.
NHANES is a 2-year cross-sectional survey conducted in the United States by the National Center for Health Statistics, which partly constitutes the Centers for Disease Control and Prevention, and this institution collects data representative of the health and nutrition status of non-institutional U.S. citizens. A complex multi-stage probabilistic sampling design, which combined interviews, questionnaires, diet, examination, and laboratory data, was utilized; thus, the health and nutrition status of the U.S. population was assessed. To represent a national sample, in which each individual represents approximately 50,000 US residents, the survey examines samples of approximately 5,000 individuals yearly, and it considers numerous regions. The NHANES protocols were approved by the National Center for Health Statistics Ethics Review Board, and all participants signed consent forms. More detailed information pertaining to NHANES is available at https://www.cdc.gov/nchs/nhanes/.
This study examined the relationship between UC and AGE with PSA among participants who participated in the National Health and Nutrition Examination Survey (NHANES) between 2009 and 2010. In all the participants, we excluded the following individuals: (1) female participants (n = 5,312); (2) participants who underwent rectal examination in the last 7 days (n = 16); (3) participants who underwent prostate biopsy, prostate surgery, or cystoscopy in the last 4 weeks (n = 11); (4) participants diagnosed with prostate infection or inflammation (n = 24); (5) participants diagnosed with prostate cancer (n = 88); (6) participants with missing UC data (n = 2,669); (7) participants with missing AGE data (n = 283); and (8) participants with missing PSA data (n = 900). Because only ≥40-year-old men consented to PSA testing, this analysis was limited to this age group. At the end of the screening, 1,234 of the 10,537 participants were enrolled.
Herein, the target independent variables were UC and AGE, which were obtained from self-reported personal interview questionnaire data collected in the NHANES. AGE was determined by the following medical question: Do you have a stomach or intestinal illness with vomiting or diarrhea that started during the last 30 days? Because AGE diagnosis is determined by medical history, it is appropriate to utilize the aforementioned question to determine AGE (13). UC was defined by the participant’s answer to the following question: Has a doctor or other health professional ever told you to have ulcerative colitis? The target dependent variable was PSA, which was obtained using the Hybritech PSA method contained in Beckman Access.
With regard to covariates, continuous variables included age, BMI, creatinine, ratio of family income to poverty, CRP, glycohemoglobin, HDL, and triglyceride, whereas classification variables included race, education level, marital status, smoking status, alcohol use, high blood pressure, coronary heart disease, diabetes, and stroke. The NHANES data set provided age, race, and other covariates acquisition process.
Table 1 depicts all the variables. After all the necessary variable datasets were identified using the CDC website, they were merged into a unified dataset, and an analysis was performed. In regard to NHANES, each participant was assigned a unique identifier, referred to as a “Sequence Number (SEQN),” which was utilized to identify each sampled individual during the data collection process.
We utilize R1 and EmpowerStats2 for all statistical analysis. Using the NHANES analysis guidelines, weights were considered. Means ± standard were calculated for continuous variables, and percentages were calculated for categorical variables. Due to the high right-skewed PSA distribution, a log2 transformation was utilized for analysis. To estimate the association of UC and AGE with PSA concentration, we adopted weighted univariate and multiple linear regression models; thus, we constructed three statistical models, namely Model 1, Model 2, and Model 3. In Model 1, no covariates were adjusted; in Model 2, demographic data, including age, race, education, marital status, and PIR were adjusted; and in Model 3, all the covariates presented in Table 1 were adjusted. Moreover, subgroup analyses and interaction tests were performed. All tests were considered statistically significant at p < 0.05.
To investigate the causal relationships between AGE, UC, and PSA, we conducted a two-sample MR study. Herein, two-sample MR represents a method for identifying the causal relationship between the exposure phenotype and the outcome; this method entails utilizing the genetic variants for the exposure as the instrument variables, which could leverage the publicly available dataset from large sample genome-wide association studies (GWAS) and compensate for the shortcomings typical of observational studies.
The UC genetic association data was obtained from a genome-wide association study (GWAS) analysis of 6,968 UC cases and 20,464 controls of European ancestry (GWAS ID: ieu-a-32) conducted by the IIBDGC consortium (14). In this dataset, 27,432 European (6,968 UC cases and 20,464 controls) were analyzed, and 12,255,197 single-nucleotide polymorphisms (SNPs) were identified. We utilized the presumed infectious origin summary data pertaining to diarrhea and gastroenteritis. The data, which was obtained from the FinnGen research project, comprised 26,288 cases and 305,879 control, and represents acute gastroenteritis. Outcome data were obtained from a publicly available GWAS dataset (GWAS ID: prot-a-1661) (15), and this dataset contained 3,301 Europeans with 10,534,735 SNPs.
First, SNPs were selected at a threshold of genome-wide significance (p < 5 × 10–7). Second, suitable SNPs were retained based on linkage disequilibrium as measured by r2 > 0.01 in a clumping algorithm with a cut-off value. Using the preceding approach, we screened 64 SNPs associated with UC and 7 SNPs associated with AGE.
A two-sample MR analysis between exposures and outcomes was performed using the R package “TwoSampleMR” (version 0.5.6). The inverse-variance weighted (IVW, random effects) method was utilized for primary analysis, and the MR-Egger and weighted median methods were utilized for additional analysis. When the assumption that all included SNPs can be utilized as efficient IVs is satisfied, the IVW method provides a precise estimate. MR-Egger regression can detect and adjust for pleiotropy; however, the accuracy of the produced estimate is quite low (16). The weighted median provides an accurate estimate based on the assumption that at least 50% of the IVs are valid (17). Subsequently, we utilized Cochran’s Q tests to evaluate the heterogeneity between the SNPs included in each analysis, and we utilized the MR-Egger regression to evaluate potential directional pleiotropy by testing for the intercept term. This indicates that directional pleiotropy might not exist when the intercept term approximates zero (18). To judge the stability of the MR results, the leave-one-out sensitivity test was utilized by excluding IVs one at a time (19). In addition, to assess the horizontal pleiotropy level of IVs, MR-PRESSO was utilized (20).
A total of 1,234 cancer-free male adults aged between 40 and 69 years were enrolled. The baseline characteristics of the participants as per the PSA median were depicted in Table 1. Compared to low-PSA participants, high-PSA participants were older, exhibited significantly higher family income to poverty ratio levels, and reported higher UC and stroke incidences. By contrast, low-PSA participants exhibited higher BMI and glycohemoglobin levels.
The results of the univariate and multivariate analyses, conducted by the weighted multivariable linear model, were depicted in Table 2. Model 1, an unadjusted model, indicated that PSA (log2 transform) concentrations in the UC group increased by 0.78 (0.19, 1.36), compared with the non-UC group. Model 2 was a minimally adjusted model. After adjusting for age, race, education level, marital status, and ratio of family income to poverty, PSA (log2 transform) concentrations in the UC group increased by 0.68 (0.10, 1.25), compared with the non-UC group. The fully adjusted model adjusts for age, race, education level, marital status, ratio of family income to poverty, BMI, smoking status, alcohol use, creatinine, CRP, glycohemoglobin, HDL, triglyceride, high blood pressure, coronary heart disease, diabetes, and stroke. Compared with the non-UC group, PSA (log2 transform) concentrations in the UC group increased by 0.64 (0.07, 1.21). The positive correlation between UC and PSA existed in all three models. Results obtained from the weighted linear regression indicated that there was no significant relationship between AGE and PSA across all these three models.
As depicted in Table 3, we conducted an interaction and stratified analysis by age groups, race, BMI groups, high blood pressure, coronary heart disease, diabetes, and stroke; thus, we assessed the associations between UC and PSA. Subsequently, in Model 3, no statistical significance was indicated by the interaction terms that regulate the UC–PSA association. As illustrated in Table 4, we conducted interaction and stratified analysis by age groups, race, BMI groups, high blood pressure, coronary heart disease, diabetes, and stroke; thus, we assessed the AGE–PSA associations. Significant interaction was observed only for coronary heart disease (p < 0.001). In patients with coronary artery disease, AGE promotes elevated PSA (log2 transform) concentrations (β = 1.20, 95% CI: 0.21–2.20, p = 0.023).
As depicted in Table 5, a significant causal UC–PSA association in the IVW analysis (beta = 0.048; se = 0.020; p = 0.014) was observed. Cochran’s Q statistic was 63.997 (p = 0.441), which indicates that there was no heterogeneity between IVs. MR–PRESSO testing did not reveal any outlier SNPs (p = 0.410). The MR-Egger intercept test exhibited no directional pleiotropy (intercept = 0.008, se = 0.009, p = 0.343). A leave-one-out sensitivity analysis indicated that the association between UC genetic predisposition and PSA was not considerably affected by any of the individual SNPs.
MR analysis exhibited no correlation between AGE and PSA (beta = 0.043; se = 0.211; p = 0.837). Cochran’s Q statistic was 6.162 (p = 0.291), which indicates that there was no heterogeneity between IVs. MR–PRESSO testing did not reveal any outlier SNPs (p = 0.350), and the robustness of the results was confirmed by the leave-one-out sensitivity test. The MR-Egger intercept test exhibited no directional pleiotropy (intercept = −0.074, se = 0.041, p = 0.143). A leave-one-out sensitivity analysis indicated that the overall result may be altered when removing rs73410764.
The scatter plot of the causal relationships between UC and AGE with the risk of PSA is illustrated in Figure 1, and the sensitivity analyses details are depicted in Figure 2 and Table 5.
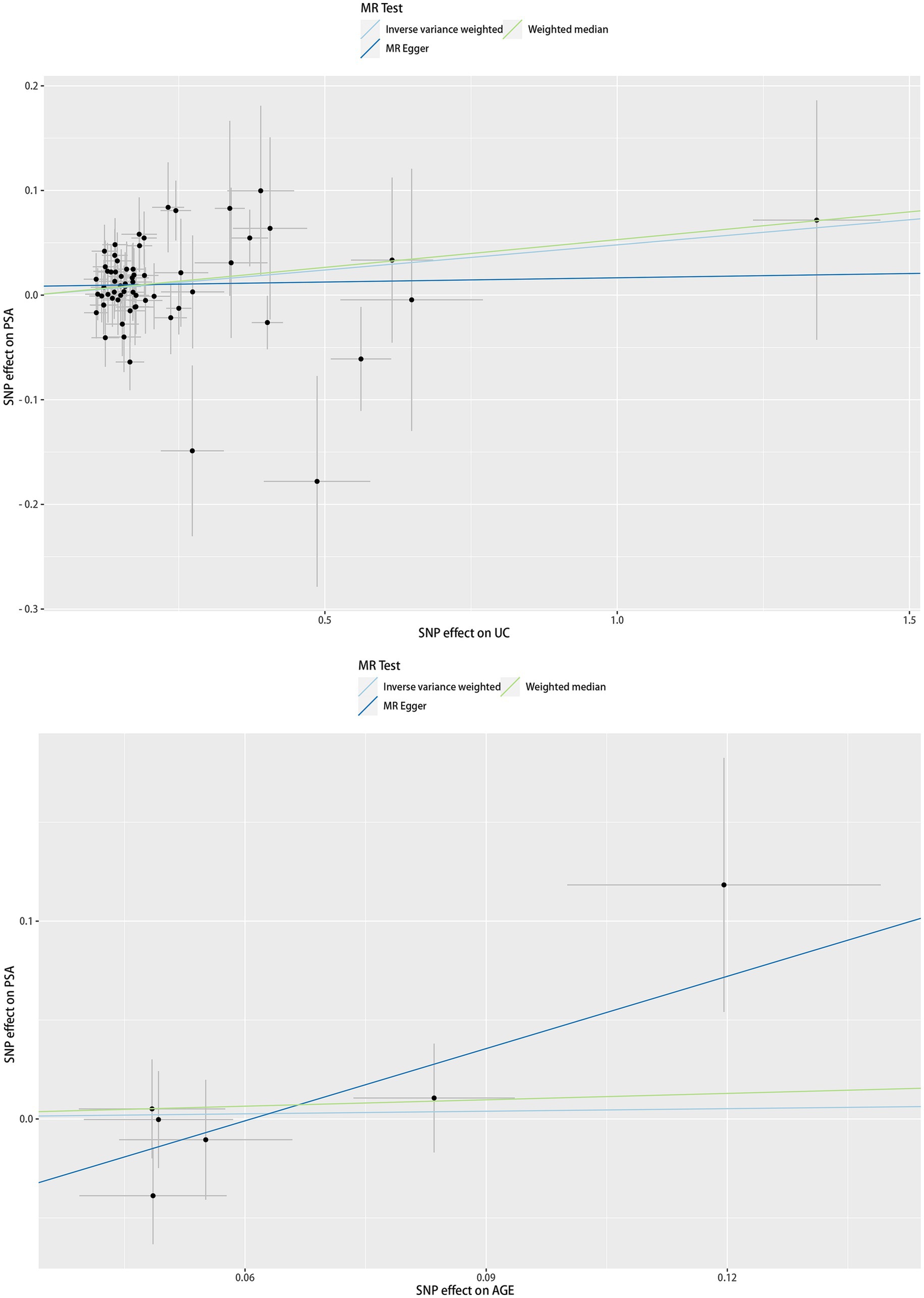
Figure 1. Scatter plots of causality. Along with the slope of each line corresponding to the effect of MR estimated under different models.
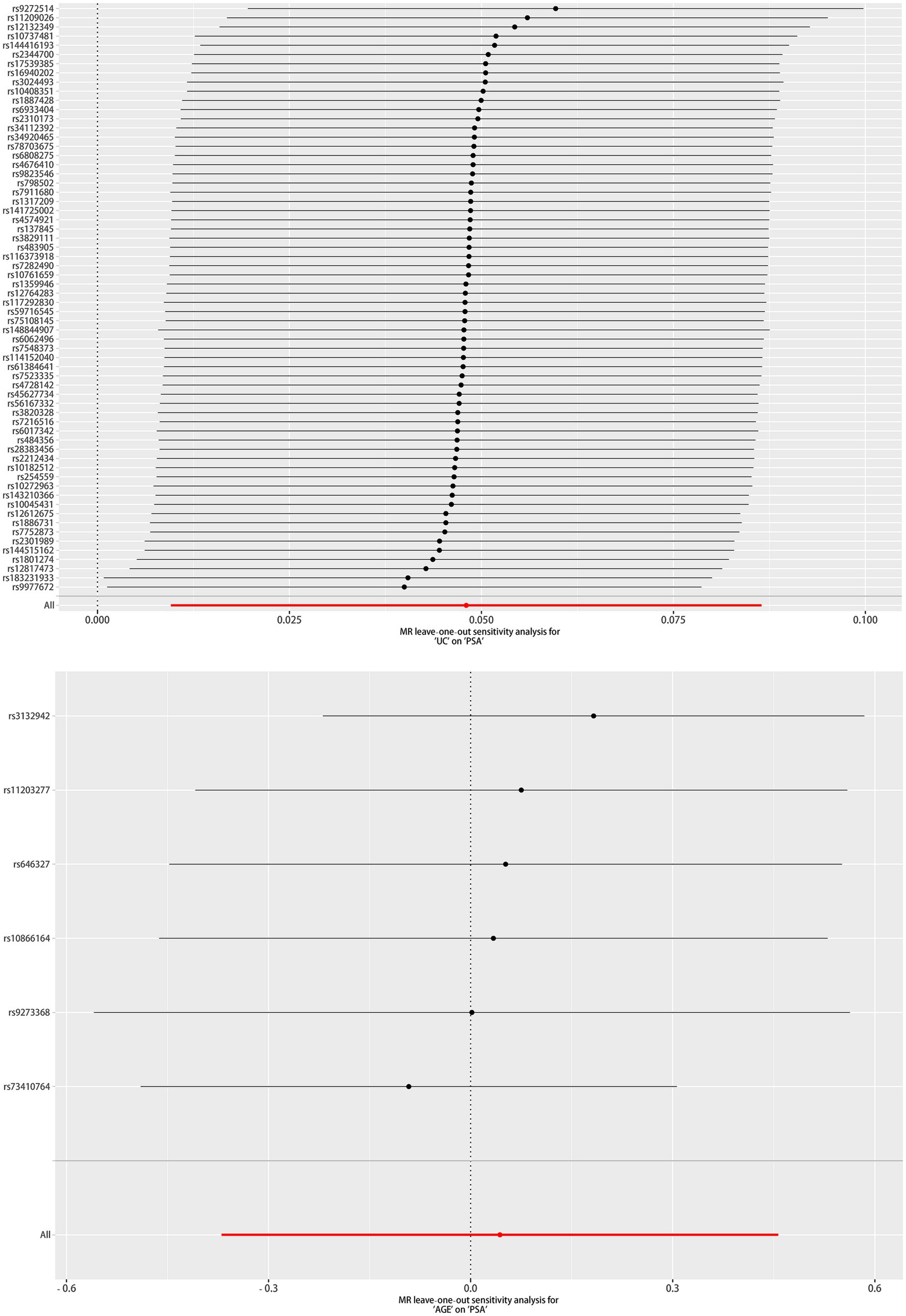
Figure 2. Leave one out of sensitivity tests. After removing the IVs one by one, compute the MR results for the remaining IVs.
The reviewed literature indicates that this pioneering study examines the relationship between UC and AGE with serum PSA concentrations based on the NHANES database and Mendelian randomization analysis. By performing an analysis of 1,234 NHANES participants, we observed that for men, UC participants exhibited higher PSA levels compared to non-UC ones. Moreover, we performed a stratified analysis, and the results have not been significantly altered; thus, the associations are robust. Furthermore, the MR analysis indicated the causal relationships between UC and PSA. Overall, no association was observed between AGE and PSA levels among men. However, using stratified analysis and interaction tests, we noted that AGE contributed to PSA elevation in the coronary heart disease population. Moreover, the MR analyses did not support causal relationships between AGE and PSA.
With respect to evaluating prostate cancer, the PSA test is currently the most widely utilized noninvasive tumor marker; however, PSA is not exclusively expressed by malignant tissues. In regard to serum, the increase in PSA levels is occasioned by the disruption of prostate gland microarchitecture, which enables PSA to cross into the extracellular space surrounding the gland. With the exception of prostate cancer, inflammation of the prostate, urinary retention, ejaculation, and ambulation influence the PSA value (21, 22).
Research into the association of UC with PCa has been limited, and, where available, such research has exhibited mixed results. Several studies have noted that UC does not promote prostate cancer (11, 23). In addition, several systematic reviews and meta-analyses have indicated that, in comparison with patients without UC, those with UC are more likely to develop cancer (24–26). Although numerous epidemiologic studies have observed relationships between UC and PCa, fewer studies have examined the UC–PSA relationships. Burns et al. documented increased PSA levels in the IBD group, and a mixed-effect regression model revealed the PSA–IBD association; however, because this study evaluated only patients presenting at an academic medical center, its external validity is limited (27).
Gut microbiota is involved in the progression of various human malignancies, including PCa. Dysbiosis of the gut microbiota crucially affects UC pathogenesis (28). Research indicates that gut dysbiosis promotes prostate cancer progression by activating the NF-κB-IL6-STAT3 axis (29). It is possible that the underlying mechanism delineating the UC–PSA association concentration is effected through the NF-κB-IL6-STAT3 pathway, and that it entails modifying the gut microbiota. There is a production dysregulation of numerous inflammatory mediators including IL-6 and IL-8 in patients with UC and in various UC experimental models (30, 31). Research has revealed that IL-6 and IL-8 correlate with increasing PSA levels (32). We propose that inflammatory mediators may partly account for the causal UC–PSA association. An existing study has indicated that chronic intestinal inflammation is associated with the increased prostatic expression of pro-inflammatory cytokines TIMP1, RANTES, and CXCL1 (33). Due to the high levels of the aforementioned factors, the activation of AKT and NF-κB pathways (34–36), which crucially affect prostate cancer development, occurs. The pro-inflammatory pathogenic microorganisms that occasion UC are introduced into the prostate through the urinary tract or circulatory system, which in turn stimulates prostate inflammation, thereby occasioning elevated PSA (37). Not only Pca and prostate inflammation but also prostate enlargement contribute to elevated PSA. The previous study that we conducted revealed that UC induced prostate enlargement (38).
The current study exhibits the following advantage. Because the study utilizes nationwide survey data, weighted data were utilized in all analyses; thus, the conclusions can be more easily generalized to the US population. In addition, a wide range of potential confounders were adequately adjusted to ensure an independent association between UC and AGE with serum PSA concentrations. In addition to the observational study based on the nationally representative NHANES, we included two-sample causal MR analyses; thus, the observations are more credible and reliable. Nevertheless, the current study exhibits some limitations, which should be considered when interpreting the results. First, due to the limited availability of UC data, the NHANES study data obtained from this study considered only the 2009–2010 period; therefore, more recent NHANES study data cannot be utilized. Second, it is probable that information bias such as information on the UC and AGE that was assessed through a questionnaire, which could affect the association, was observed.
By applying two different methods, the current study provided valid evidence to support the following notion: UC promotes elevated PSA levels, and AGE does not promote elevated PSA. On the other hand, in the population with coronary heart disease, UC promotes the increase of serum PSA levels.
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/.
The studies involving humans were approved by NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements.
HL: Methodology, Writing – original draft. JZ: Writing – original draft, Methodology. WD: Supervision, Validation, Writing – review & editing. YH: Writing – review & editing. ZS: Writing – review & editing. XJ: Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from National Natural Science Fund of China (82072808), Natural Science Fund of Guangdong Province (2019A1515010222), Guangzhou Core Medical Disciplines Project (2021–2023), Guangzhou Municipal Science and Technology Bureau Municipal Finance—Supporting Institution Jointly funded project Funds (202102010137), and Project of Medical Scientific Research in Guangdong (A2021351).
The authors extends their heartfelt gratitude to the dedicated staff members and participants involved in the NHANES study for their invaluable contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Olen, O, Askling, J, Sachs, MC, Neovius, M, Smedby, KE, Ekbom, A, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964-2014. Gut. (2020) 69:453–61. doi: 10.1136/gutjnl-2018-317572
2. Ng, SC, Shi, HY, Hamidi, N, Underwood, FE, Tang, W, Benchimol, EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
3. Florez, ID, Al-Khalifah, R, Sierra, JM, Granados, CM, Yepes-Nunez, JJ, Cuello-Garcia, C, et al. The effectiveness and safety of treatments used for acute diarrhea and acute gastroenteritis in children: protocol for a systematic review and network meta-analysis. Syst Rev. (2016) 5:14. doi: 10.1186/s13643-016-0186-8
4. Piescik-Lech, M, Shamir, R, Guarino, A, and Szajewska, H. Review article: the management of acute gastroenteritis in children. Aliment Pharmacol Ther. (2013) 37:289–303. doi: 10.1111/apt.12163
5. DALYs, GBD, and Collaborators, H. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1260–344. doi: 10.1016/S0140-6736(17)32130-X
6. Kuruma, H, and Egawa, S. Words of wisdom: re: international variation in prostate cancer incidence and mortality rates. Eur Urol. (2013) 63:583–4. doi: 10.1016/j.eururo.2012.12.012
7. Vickers, AJ. Prostate Cancer screening: time to question how to optimize the ratio of benefits and harms. Ann Intern Med. (2017) 167:509–10. doi: 10.7326/M17-2012
8. Meyers, TJ, Weiner, AB, Graff, RE, Desai, AS, Cooley, LF, Catalona, WJ, et al. Association between inflammatory bowel disease and prostate cancer: a large-scale, prospective, population-based study. Int J Cancer. (2020) 147:2735–42. doi: 10.1002/ijc.33048
9. Jung, YS, Han, M, Park, S, Kim, WH, and Cheon, JH. Cancer risk in the early stages of inflammatory bowel disease in Korean patients: a Nationwide population-based study. J Crohns Colitis. (2017) 11:954–62. doi: 10.1093/ecco-jcc/jjx040
10. Jess, T, Horvath-Puho, E, Fallingborg, J, Rasmussen, HH, and Jacobsen, BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol. (2013) 108:1869–76. doi: 10.1038/ajg.2013.249
11. Jussila, A, Virta, LJ, Pukkala, E, and Färkkilä, MA. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scand J Gastroenterol. (2013) 48:1405–13. doi: 10.3109/00365521.2013.846402
12. So, J, Tang, W, Leung, WK, Li, M, Lo, FH, Wong, MTL, et al. Cancer risk in 2621 Chinese patients with inflammatory bowel disease: a population-based cohort study. Inflamm Bowel Dis. (2017) 23:2061–8. doi: 10.1097/MIB.0000000000001240
13. Kim, HS, Rotundo, L, Nasereddin, T, Ike, A, Song, D, Babar, A, et al. Time trends and predictors of acute gastroenteritis in the United States: results from National Health and Nutrition Examination Survey 2005-2014. J Clin Gastroenterol. (2017) 51:693–700. doi: 10.1097/MCG.0000000000000907
14. Liu, JZ, van Sommeren, S, Huang, H, Ng, SC, Alberts, R, Takahashi, A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. (2015) 47:979–86. doi: 10.1038/ng.3359
15. Sun, BB, Maranville, JC, Peters, JE, Stacey, D, Staley, JR, Blackshaw, J, et al. Genomic atlas of the human plasma proteome. Nature. (2018) 558:73–9. doi: 10.1038/s41586-018-0175-2
16. Burgess, S, and Thompson, SG. Interpreting findings from mendelian randomization using the MR-egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
17. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
18. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
19. Zheng, J, Baird, D, Borges, MC, Bowden, J, Hemani, G, Haycock, P, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep. (2017) 4:330–45. doi: 10.1007/s40471-017-0128-6
20. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
21. Tchetgen, MB, and Oesterling, JE. The effect of prostatitis, urinary retention, ejaculation, and ambulation on the serum prostate-specific antigen concentration. Urol Clin North Am. (1997) 24:283–91. doi: 10.1016/S0094-0143(05)70374-8
22. Lilja, H, Ulmert, D, and Vickers, AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. (2008) 8:268–78. doi: 10.1038/nrc2351
23. Na, JE, Kim, TJ, Lee, YC, Kim, JE, Kim, ER, Hong, SN, et al. Risk of prostate cancer in patients with inflammatory bowel disease: a nationwide cohort study in South Korea. Ther Adv Gastroenterol. (2022) 15:17562848221137430. doi: 10.1177/17562848221137430
24. Haddad, A, Al-Sabbagh, MQ, Al-Ani, H, Siyam, AM, Aborajooh, E, Iwata, T, et al. Inflammatory bowel disease and prostate cancer risk: a systematic review. Arab J Urol. (2020) 18:207–12. doi: 10.1080/2090598X.2020.1761674
25. Ge, Y, Shi, Q, Yao, W, Cheng, Y, and Ma, G. The association between inflammatory bowel disease and prostate cancer risk: a meta-analysis. Prostate Cancer Prostatic Dis. (2020) 23:53–8. doi: 10.1038/s41391-019-0177-7
26. Chen, M, Yuan, C, and Xu, T. An increase in prostate cancer diagnosis during inflammatory bowel disease: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. (2020) 44:302–9. doi: 10.1016/j.clinre.2019.07.003
27. Burns, JA, Weiner, AB, Catalona, WJ, Li, EV, Schaeffer, EM, Hanauer, SB, et al. Inflammatory bowel disease and the risk of prostate cancer. Eur Urol. (2019) 75:846–52. doi: 10.1016/j.eururo.2018.11.039
28. Liu, S, Zhao, W, Lan, P, and Mou, X. The microbiome in inflammatory bowel diseases: from pathogenesis to therapy. Protein Cell. (2021) 12:331–45. doi: 10.1007/s13238-020-00745-3
29. Zhong, W, Wu, K, Long, Z, Zhou, X, Zhong, C, Wang, S, et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-kappaB-IL6-STAT3 axis. Microbiome. (2022) 10:94. doi: 10.1186/s40168-022-01289-w
30. Nakase, H, Sato, N, Mizuno, N, and Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. (2022) 21:103017. doi: 10.1016/j.autrev.2021.103017
31. Uguccioni, M, Gionchetti, P, Robbiani, DF, Rizzello, F, Peruzzo, S, Campieri, M, et al. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. (1999) 155:331–6. doi: 10.1016/S0002-9440(10)65128-0
32. Katongole, P, Sande, OJ, Nabweyambo, S, Joloba, M, Kajumbula, H, Kalungi, S, et al. IL-6 and IL-8 cytokines are associated with elevated prostate-specific antigen levels among patients with adenocarcinoma of the prostate at the Uganda Cancer Institute. Future Oncol. (2022) 18:661–7. doi: 10.2217/fon-2021-0683
33. Desai, AS, Sagar, V, Lysy, B, Weiner, AB, Ko, OS, Driscoll, C, et al. Inflammatory bowel disease induces inflammatory and pre-neoplastic changes in the prostate. Prostate Cancer Prostatic Dis. (2022) 25:463–71. doi: 10.1038/s41391-021-00392-7
34. Kuo, PL, Shen, KH, Hung, SH, and Hsu, YL. CXCL1/GROα increases cell migration and invasion of prostate cancer by decreasing fibulin-1 expression through NF-κB/HDAC1 epigenetic regulation. Carcinogenesis. (2012) 33:2477–87. doi: 10.1093/carcin/bgs299
35. Aldinucci, D, and Colombatti, A. The inflammatory chemokine CCL5 and cancer progression. Mediat Inflamm. (2014) 2014:292376:1–12. doi: 10.1155/2014/292376
36. Song, G, Xu, S, Zhang, H, Wang, Y, Xiao, C, Jiang, T, et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res. (2016) 35:148. doi: 10.1186/s13046-016-0427-7
37. Sfanos, KS, and Joshu, CE. IBD as a risk factor for prostate cancer: what is the link? Nat Rev Urol. (2019) 16:271–2. doi: 10.1038/s41585-019-0157-7
Keywords: ulcerative colitis, acute gastroenteritis, prostate specific antigen, NHANES, cross-sectional research
Citation: Li H, Zheng J, Dong W, Huang Y, Su Z and Jiang X (2023) Association of ulcerative colitis and acute gastroenteritis with prostate specific antigen: results from National Health and Nutrition Examination Survey from (2009 to 2010) and Mendelian randomization analyses. Front. Nutr. 10:1265014. doi: 10.3389/fnut.2023.1265014
Received: 21 July 2023; Accepted: 17 November 2023;
Published: 04 December 2023.
Edited by:
Fei Mao, Jiangsu University, ChinaReviewed by:
Marcos Edgar Herkenhoff, University of São Paulo, BrazilCopyright © 2023 Li, Zheng, Dong, Huang, Su and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianhan Jiang, amlhbmd4aWFuaGFuZ3pAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.