
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 21 September 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1246359
This article is part of the Research Topic Vitamin D: From Pathophysiology to Clinical Impact View all 20 articles
Background: Since the association of vitamin D with atrial fibrillation (AF) risk is still unclear, we conducted this updated meta-analysis of prospective studies to identify the relationship between vitamin D or vitamin D supplementation and AF in the general population.
Methods: We conducted a comprehensive search of multiple databases up to May 2023 for studies reporting vitamin D and AF. The hazard ratios (HRs) with 95% confidence intervals (CIs) were pooled by a random-effects model.
Results: A total of seven studies were included in this meta-analysis. Vitamin D deficiency (<20 ng/ml) was associated with increased AF incidence (HR: 1.12, 95% CI: 1.005–1.25). The HR was not significant with vitamin D insufficiency (20–30 ng/ml; HR: 1.09, 95% CI: 0.98–1.21). Each 10 ng/ml increase in serum vitamin D was associated with a significantly decreased AF incidence (HR: 0.95, 95% CI: 0.93–0.97). Two studies reported the effect of vitamin D supplements on AF incidence but reached inconsistent results.
Conclusions: Vitamin D deficiency or insufficiency was associated with an increased risk of AF in the general population. The role of vitamin D supplementation in AF prevention needs further investigation.
Atrial fibrillation (AF) is the leading cardiac arrhythmia and its prevalence varies concerning geography, social economy, age, sex, and ethnicity (1–3). Once established, AF is associated with several severe complications (e.g., stroke, ischaemic heart disease) (4), mortality (4), and high healthcare costs (5). In addition, even for individuals without a history of cardiovascular diseases, AF still contributes to elevated mortality (6). Accordingly, measures to prevent and/or delay AF that can be generalized on a population level are of great value but are scarce.
Vitamin D is an important nutrient that can be ingested from in series of foods (e.g., fish, sun-dried mushrooms, milk) or dietary supplements. In addition, it can be synthesized when exposed to ultraviolet rays. In recent years, vitamin D has been hypothesized to be linked with cardiovascular diseases through its effect on the inflammation or renin-angiotensin-aldosterone (RAAS) (7). Therefore, vitamin D may be one premium option for the prevention or treatment of AF if its beneficial effects on AF risks are confirmed. In recent years, numerous studies on the association between vitamin D and AF have been performed, but they reported inconsistent findings. A dose-response meta-analysis by Liu et al. (8) demonstrated that vitamin D deficiency or inadequacy increased the risk of AF by 23 or 14%, respectively. However, significant heterogeneity existed among the included studies. In addition, a null association between vitamin D and new-onset AF was reported in a former meta-analysis by Huang et al. (9). However, they did not differentiate between the new-onset and postoperative AF. Similarly, no significant association between vitamin D and AF risk was also demonstrated by two recent Mendelian randomization studies (10).
Furthermore, the Vitamin D and OmegA-3 TriaL (VITAL) Rhythm Study, a randomized, double-blind, placebo-controlled trial, did not find a causal relationship between vitamin D supplementation and AF risk over a median follow-up of 5 years (11). Based on the evidence above, the beneficial effects of vitamin D on AF reported previously may be overestimated to some extent. Therefore, we conducted this updated meta-analysis of prospective studies to identify the relationship between vitamin D or vitamin D supplementation and AF in the general population.
This work was reported according to the PRISMA guidelines. Two authors (Xiaoli Ding and Jiying Lai) independently conducted a comprehensive search of multiple databases (PubMed, Embase, Cochrane Library) from inception to May 2023, using two sets of MeSH words and keywords: atrial fibrillation and vitamin D (Supplementary Table S1). Additionally, we searched ClinicalTrials.gov, International Standard Randomized Controlled Trial Number Registry, Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and other relevant publications to identify potential studies. Gray literatures were identified by Google Scholar database.
For the inclusion criteria concerning the association between vitamin D and AF, the following PICOS criteria were applied: (1) Population: general population, excluding studies focused on specific diseases like diabetes or established cardiovascular diseases. (2) Exposure vs. control: high serum vitamin D vs. low serum vitamin D levels. (3) Outcomes: studies reporting the association between serum vitamin D and AF incidence. (4) Study design: randomized control trials (RCTs) and observational prospective cohorts were included.
Retrospective studies (cross-sectional, case-control, and retrospective cohort), case reports, reviews, meta-analyses, and Mendelian studies were excluded. Reports on the association between vitamin D or vitamin D supplements and AF recurrence, and AF post-operation were also excluded. Regarding the studies on vitamin D supplements and AF, all the observational studies or RCTs reporting the effect of vitamin D supplements on AF incidence in adults were included, without restrictions on population, sample size, comparison, or follow-up.
The initial search results were imported into EndNote X9.1 software. Duplicate citations were eliminated using both automated and manual inspection. Subsequently, we carefully examined the titles and abstracts of the remaining citations. Based on this preliminary screening, we reviewed the full-text reports that seemed to meet the predefined inclusion criteria.
Two authors (Xiaoli Ding and Jiying Lai) independently extracted data from the included studies, and any discrepancies were resolved through discussion. The extracted information included the first author, country, publication year, gender, mean or age, study design, population, source of population, sample size, vitamin D level, measurements of vitamin D and AF, hazard ratios (HRs) with 95% confidence intervals (CIs), and adjustments.
Two authors (Xiaoli Ding and Jiying Lai) independently conducted searches for eligible studies, extracted data, assessed study quality, and synthesized the data. Disagreements were discussed by consent or advised by a third author (Zongwen Guo). To pool the HRs were pooled by random-effects models. Both vitamin D levels were analyzed as both a category and a continuous variable. In the category analysis, we examined the association between vitamin D deficiency and insufficiency compared to normal vitamin D levels. In the continuous analysis, study-specific slopes (vitamin D per 10 ng/ml increment) and 95% CIs were calculated from the natural logs of the reported HRs and CIs across different vitamin D categories (12). Considering the substantial difference in study design and quality evidence between observational studies and RCT trials, the results of vitamin D supplements and AF incidence were analyzed separately based on study design. Statistical heterogeneity between studies was assessed using the Cochran Q-test and tau2 statistics, while inconsistency across studies was measured using I2. Pre-defined subgroup analysis was conducted based on age, sex, population with vitamin D insufficiency or deficiency, region, and follow-up duration (when the number of studies exceeded five). Sensitive analyses by fixed model, excluding studies with shorter duration of follow-up (<5 years), reports without body mass index, physical activity, smoking, and hypertension were conducted.
The quality of observational studies and randomized controlled trials (RCTs) was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) and Cochrane Bias Risk Assessment Tool, respectively (13–15). The NOS evaluates bias across three domains (selection, comparability, and outcomes) using eight items, with a maximum total score of nine. A NOS score of seven or higher indicates high quality. The Cochrane Bias tool assesses bias across seven domains, including selection bias, implementation bias, measurement bias, follow-up bias, reporting bias, and other biases. The publication bias was not assessed due to the limited number of studies (N < 10).
All the statistical analyses were done by using Review Manager (RevMan) version 5.4 (The Cochrane Collaboration 2020; Nordic Cochrane Center Copenhagen, Denmark). All statistical tests were double-sided, and P < 0.05 was considered statistically significant.
The study selection is shown in Figure 1. Initially, a comprehensive search was conducted across multiple databases, including PubMed, Embase, and Cochrane Library and trial registers, resulting in a total of 775 records. After removing duplicate records (n = 228), the remaining 547 records were screened based on predetermined inclusion and exclusion criteria. Next, the remaining 31 reports were assessed for eligibility in detail in full text. Among these reports, a further review was made, resulting in the exclusion of studies (n = 24) based on the following reasons: (1) insufficiency data, n =5; (2) No targeted population or exposure, n = 4; (3) Case, case-serial, review, and meta-analysis, n = 3; (4) Retrospective studies, such as case-control studies, n = 2; (5) Inappropriate outcomes (AF recurrence, post-operative AF, n = 9; Supplementary Table S2). Finally, a total of seven studies were included in this systematic review and meta-analysis (11, 16–21). Five studies (six prospective cohorts) reported the serum vitamin D and AF incidence, whereas two studies (one RCT and one nest-case control) reported the effect of vitamin D supplements on AF incidence.
The study characteristics are shown in Table 1. Overall, these studies were published between 2015 and 2022. The sample sizes ranged from 845 to 12,577. Five studies were from the United States, one was from the Netherlands, and one was from Italy. All the study individuals were community-based general population and adjusted their results by multivariable analysis in the association between vitamin D and AF. All of the studies were scored as low bias (Supplementary Table S3 and Supplementary Figure S1).
Six prospective cohorts with 2,917 cases/28,694 participants were included (16, 18, 20, 22–24). As shown in Figure 2, vitamin D deficiency (<20 ng/ml) was associated with increased AF incidence (HR: 1.12, 95% CI: 1.005–1.25; I2 = 0%). The HR was not significant with vitamin D insufficiency (20–30 ng/ml; HR: 1.09, 95% CI: 0.98–1.21; I2 = 0%).
When vitamin D was analyzed as a continuous variable, each 10 ng/ml increase in serum vitamin D was associated with a significantly decreased AF incidence (HR: 0.95, 95% CI: 0.93–0.97, I2 = 0%; Figure 3). Excluding the study with the largest weight did not significantly change the results (HR: 0.96, 95% CI: 0.92–0.99, I2 = 0%).
An RCT showed randomization to vitamin D3 (2,000 IU/day, n = 6,272) treatments for who aged >50 years did not significantly reduce the incidence of AF compared with placebo after a median follow-up of more than 5 years (HR: 1.09; 95% CI, 0.96–1.25; P = 0.19) (11). Conversely, a nested case-control study using propensity score matching demonstrated that vitamin D treatment maintaining levels above 20 ng/ml for at least 6 months was associated with a significantly lower incidence of new-onset AF compared to untreated patients whose levels remained at or below 20 ng/ml (21) (vitamin D treated, level 21 to 29 ng/ml, HR: 0.89, 95% CI: 0.80–0.98; vitamin D treated, level ≥30 ng/ml, HR: 0.84, 95% CI: 0.73–0.0.95).
Subgroup analyses according to sample size, AF cases, mean age, and region did not find evidence of interactions (all P > 0.05). Sensitive analyses by fixed model, excluding studies with shorter duration of follow-up (<5 years), reports without body mass index, physical activity, smoking, and hypertension generated confirmed results (Table 2).
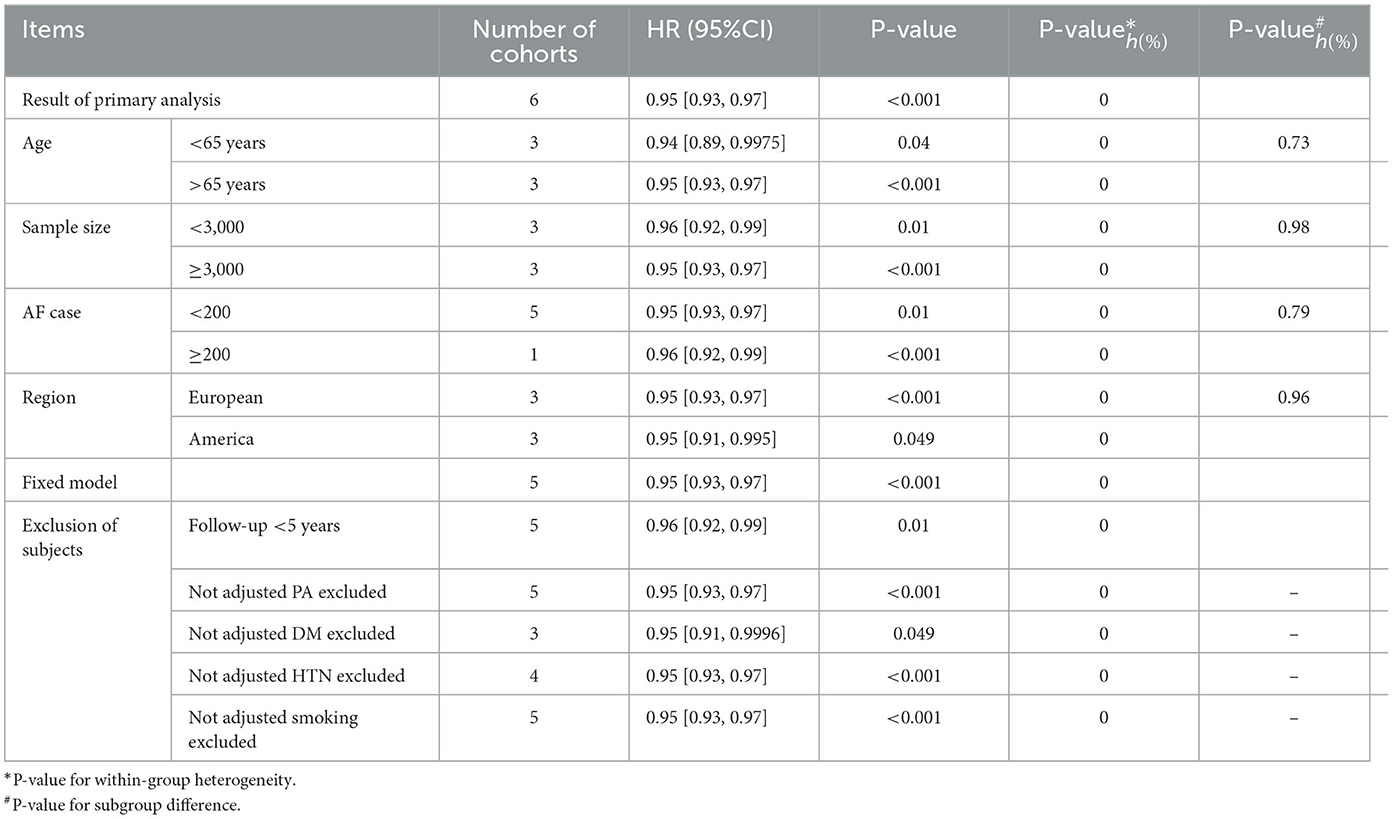
Table 2. Subgroup and sensitivity analyses-serum vitamin D and atrial fibrillation incidence, per 10 ng/ml increase.
Our current meta-analysis revealed that vitamin D deficiency but not insufficiency was associated an increased risk of AF in the general population. In addition, an increment of 10 ng/ml of vitamin D was associated with a tiny decrease in AF risk.
AF is the most common supraventricular arrhythmia with complex pathogenesis. To date, although the underlying pathogenesis of AF has not been fully understood, the disorders of the RAAS and the inflammation involvement are two key factors. Vitamin D, as an exquisite fundamental micronutrient for humans, not only plays an indispensable role in maintaining calcium-phosphate homeostasis and musculoskeletal metabolism (25) but also in the modulation of inflammatory response and RAAS activity (26). In addition, Hanafy et al. and coworkers confirmed that vitamin D can prevent or terminate the occurrence of AF through direct electromechanical effects on the left atrium of the heart failure rabbit (27). Therefore, it may be theoretically reasonable to speculate the protective effects of vitamin D on AF. However, this speculation was not fully justified in clinical practice.
To date, a number of studies investigating the association between vitamin D and AF risk have been reported but with inconclusive findings. Results from observational studies have revealed that patients with vitamin D deficiency were associated with a higher risk of AF compared with patients with normal levels (24, 28–30). Nevertheless, such an association was not identified in the prospective studies (16–18, 20). By only pooling relevant prospective studies conducted in the general population, we found that the AF risk marginally increased for individuals with vitamin D deficiency when compared with those with normal vitamin D levels. In addition, per 10 ng/ml increase in vitamin D is associated with only a 5% decrease in AF risk. In contrast, by performing a Mendelian randomization analysis using the summary statistics obtained for single-nucleotide polymorphisms (SNPs) identified from genome-wide association meta-analyses, Yang et al. (31) found no causal relationship between vitamin D levels and AF risk. Their findings were also collaborated by another relevant Mendelian randomization analysis by Zhang et al. (32). Accordingly, it is suggested that certain protective effects of vitamin D on AF occurrence may exist, but considering vitamin D as a therapeutic target to delay AF progress in clinical settings may not be recommended.
The mechanism of vitamin D deficiency on AF development is not fully understood. Murdaca et al. (33) have previously concluded that vitamin D deficiency plays a role in the pathophysiology of several autoimmune diseases such as psoriasis vulgaris, iridocyclitis, ulcerative colitis, thyrotoxicosis, and Crohn's disease. Vitamin D deficiency may alter the microbiome by changing the composition of the microbiome and the integrity of the intestinal epithelial barrier, or affect the immune system primarily through the vitamin D receptor (34). The IL31/IL33 axis plays an important role in the development of these diseases (35). It is possible that vitamin D deficiency may impair the IL31/IL33 axis favoring myocardial inflammation and AF development, which needs further investigation.
To further explore the potential benefits of vitamin D supplementation on the prevention and delay of AF, several clinical trials have been performed in clinical settings. The Women's Health Initiative (WHI) calcium and vitamin D (CaD) trial, a randomized, double-blind trial, investigated the effect of supplementation of CaD supplements in the primary prevention of AF in postmenopausal women. In this trial, postmenopausal women were randomized to either receive CaD supplements (400 IU/day of vitamin D3 and 1,000 mg/day of elemental calcium) or a placebo, and no significant difference in the incident AF rates between the two groups was observed (36). However, the study did not distinguish the independent effect of vitamin D supplementation from calcium supplementation. In addition, since previous studies reported an increased risk of cardiovascular events with calcium supplementation regardless of whether vitamin D supplementation or not (37, 38), it is possible that the increased AF risk resulting from calcium supplementation counterbalances the benefits of vitamin D supplementation to some extent. In another randomized double-blind placebo-controlled trial, Albert et al. (11) also did not find a causal relationship between vitamin D supplementation (2,000 IU/day of vitamin D) and AF risk over a median follow-up of 5 years. Nevertheless, only 12.7% of the participants in that trial had baseline vitamin D deficiency, possibly leading to the limited power to detect the potential benefits of vitamin D supplementation for this subset. In contrast, the null benefits of vitamin D supplementation and incident AF risk reported by the former two studies were not been collaborated by a recent nested case-control study (21). In the study, 39,845 participants with baseline vitamin D ≤ 20 ng/ml were included and grouped into three groups: group-A (untreated), group-B (treated, levels 21–29 ng/ml), and group-C (treated, levels ≥30 ng/ml). After being treated for ≥6 months, the AF risk was lower in group B and group C when compared with group A, and the AF risk was not significantly different between group B and C for the whole included individuals. However, for men >65 years with hypertension or diabetes mellitus, an added benefit was further acquired when the vitamin D was ≥30 ng/ml. Nevertheless, due to the observational design of the study, the findings may be confounded by the additional supplementation that was not recorded in the medical records.
Although not all the previous studies have indicated vitamin D deficiency as an independent risk factor for AF, most of the current evidence supports the potential associations of vitamin D deficiency with AF. Of note, analyzing patients with comorbidities such as heart failure and diabetes mellitus might limit the findings between vitamin D deficiency and AF because these comorbidities could increase AF occurrence regardless of vitamin D levels. Therefore, we only included prospective studies focusing on the general population, and found that vitamin D deficiency was associated with an increased risk of AF. Nevertheless, due to the observational nature of studies and several uncontrolled factors (e.g., parathyroid hormone, calcium level, and seasonal, sex, and ethnic variations in vitamin D levels), the causal relationship between vitamin D and AF risk was still inconclusive. Further prospective, randomized, double-blinded, large clinical trials in this field would be needed since they have advantages over observational studies. Although our data provided some evidence as to the importance of considering vitamin D supplementation for AF, there was still no recommendation of supplementation in clinical practice before the robust evidence.
Several limitations should be considered in this meta-analysis. First, the protocol of this systematic review and meta-analysis was not registered in the PROSPERO (International Platform of Registered Systematic Review and Meta-analysis Protocols). Second, due to the inherent limitation of observational data, the risk of bias or unexpected confounders could not be excluded. Our findings did not suggest a causal relationship between vitamin D levels and AF risks. Third, vitamin D levels could be affected by several factors (e.g., parathyroid hormone, calcium level, lifestyle, diet, and seasonal variation), which limits the findings and needs to be controlled in well-designed randomized trials. Finally, further investigations of the benefits of vitamin D supplementation on incident AF are still warranted due to the limited number of studies. In addition, the treatment dose and duration of vitamin D supplementation, baseline vitamin D levels, and targeted population should be carefully considered.
Vitamin D deficiency but not insufficiency was associated an increased risk of AF in the general population. The role of vitamin D supplementation in AF prevention needs further investigation.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by Jiangxi Provincial Health Commission Science and Technology Plan (SKJP220229732).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1246359/full#supplementary-material
1. Bosch NA, Cimini J, Walkey AJ. Atrial fibrillation. Nat Rev Dis Primers. (2022) 8:21. doi: 10.1038/s41572-022-00354-w
2. Tse HF, Wang YJ, Ahmed Ai-Abdullah M, Pizarro-Borromeo AB, Chiang CE, Krittayaphong R, et al. Stroke prevention in atrial fibrillation–an Asian stroke perspective. Heart Rhythm. (2013) 10:1082–8. doi: 10.1016/j.hrthm.2013.03.017
3. Zhang J, Johnsen SP, Guo Y, Lip GYH. Epidemiology of atrial fibrillation: geographic/ecological risk factors, age, sex, genetics. Card Electrophysiol Clin. (2021) 13:1–23. doi: 10.1016/j.ccep.2020.10.010
4. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. (2016) 354:i4482. doi: 10.1136/bmj.i4482
5. Burdett P, Lip GYH. Atrial fibrillation in the UK: predicting costs of an emerging epidemic recognizing and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes. (2022) 8:187–94. doi: 10.1093/ehjqcco/qcaa093
6. Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. (2011) 305:2080–7. doi: 10.1001/jama.2011.659
7. Cosentino N, Campodonico J, Milazzo V, De Metrio M, Brambilla M, Camera M, et al. Vitamin D and cardiovascular disease: current evidence and future perspectives. Nutrients. (2021) 13:3603. doi: 10.3390/nu13103603
8. Liu X, Wang W, Tan Z, Zhu X, Liu M, Wan R, et al. The relationship between vitamin D and risk of atrial fibrillation: a dose-response analysis of observational studies. Nutr J. (2019) 18:73. doi: 10.1186/s12937-019-0485-8
9. Huang W-L, Yang J, Yang J, Wang H-B, Yang C-J, Yang Y. Vitamin D and new-onset atrial fibrillation: a meta-analysis of randomized controlled trials. Hellenic J Cardiol. (2018) 59:72–7. doi: 10.1016/j.hjc.2017.11.006
10. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
11. Albert CM, Cook NR, Pester J, Moorthy MV, Ridge C, Danik JS, et al. Effect of marine Omega-3 fatty acid and vitamin D supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA. (2021) 325:1061–73. doi: 10.1001/jama.2021.1489
12. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
13. Liu F, Yang Y, Cheng W, Ma J, Zhu W. Reappraisal of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients: a systematic review and meta-analysis. Front Cardiovasc Med. (2021) 8:757188. doi: 10.3389/fcvm.2021.757188
14. Zhu W, Ye Z, Chen S, Wu D, He J, Dong Y, et al. Comparative effectiveness and safety of non–vitamin K antagonist oral anticoagulants in atrial fibrillation patients. Stroke. (2021) 52:1225–33. doi: 10.1161/STROKEAHA.120.031007
15. Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. (2022) 21:1–17. doi: 10.1186/s12933-022-01546-0
16. Rienstra M, Cheng S, Larson MG, McCabe EL, Booth SL, Jacques PF, et al. Vitamin D status is not related to development of atrial fibrillation in the community. Am Heart J. (2011) 162:538–41. doi: 10.1016/j.ahj.2011.06.013
17. Mathew JS, Sachs MC, Katz R, Patton KK, Heckbert SR, Hoofnagle AN, et al. Fibroblast growth factor-23 and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS). Circulation. (2014) 130:298–307. doi: 10.1161/CIRCULATIONAHA.113.005499
18. Alonso A, Misialek JR, Michos ED, Eckfeldt J, Selvin E, Soliman EZ, et al. Serum 25-hydroxyvitamin D and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Europace. (2016) 18:1143–9. doi: 10.1093/europace/euv395
19. Trevisan C, Piovesan F, Lucato P, Zanforlini BM, De Rui M, Maggi S, et al. Parathormone, vitamin D and the risk of atrial fibrillation in older adults: a prospective study. Nutr Metab Cardiovasc Dis. (2019) 29:939–45. doi: 10.1016/j.numecd.2019.05.064
20. Vitezova A, Cartolano NS, Heeringa J, Zillikens MC, Hofman A, Franco OH, et al. Vitamin D and the risk of atrial fibrillation–the Rotterdam Study. PLoS ONE. (2015) 10:e0125161. doi: 10.1371/journal.pone.0125161
21. Acharya P, Safarova MS, Dalia T, Bharati R, Ranka S, Vindhyal M, et al. Effects of Vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of atrial fibrillation. Am J Cardiol. (2022) 173:56–63. doi: 10.1016/j.amjcard.2022.02.040
22. Turin A, Bax JJ, Doukas D, Joyce C, Lopez JJ, Mathew V, et al. Interactions among vitamin D, atrial fibrillation, and the renin-angiotensin-aldosterone system. Am J Cardiol. (2018) 122:780–4. doi: 10.1016/j.amjcard.2018.05.013
23. Özsin KK, Sanri US, Toktaş F, Kahraman N, Yavuz S. Effect of plasma level of vitamin D on postoperative atrial fibrillation in patients undergoing isolated coronary artery bypass grafting. Braz J Cardiovasc Surg. (2018) 33:217–23. doi: 10.21470/1678-9741-2017-0214
24. Chen WR, Liu ZY, Shi Y, Yin DW, Wang H, Sha Y, et al. Relation of low vitamin D to nonvalvular persistent atrial fibrillation in Chinese patients. Ann Noninvasive Electrocardiol. (2014) 19:166–73. doi: 10.1111/anec.12105
25. Lips P. Vitamin D physiology. Prog Biophys Mol Biol. (2006) 92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016
26. Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. (2012) 61:450–8. doi: 10.1016/j.metabol.2011.09.007
27. Hanafy DA, Chang S-L, Lu Y-Y, Chen Y-C, Kao Y-H, Huang J-H, et al. Electromechanical effects of 1,25-dihydroxyvitamin D with antiatrial fibrillation activities. J Cardiovasc Electrophysiol. (2014) 25:317–23. doi: 10.1111/jce.12309
28. Demir M, Uyan U, Melek M. The effects of vitamin D deficiency on atrial fibrillation. Clin Appl Thromb Hemost. (2014) 20:98–103. doi: 10.1177/1076029612453762
29. Belen E, Aykan AC, Kalaycioglu E, Sungur MA, Sungur A, Cetin M. Low-level vitamin D is associated with atrial fibrillation in patients with chronic heart failure. Adv Clin Exp Med. (2016) 25:51–7. doi: 10.17219/acem/34690
30. Ozcan OU, Gurlek A, Gursoy E, Gerede DM, Erol C. Relation of vitamin D deficiency and new-onset atrial fibrillation among hypertensive patients. J Am Soc Hypertens. (2015) 9:307–12. doi: 10.1016/j.jash.2015.01.009
31. Yang S, Zhi H, Sun Y, Wang L. Circulating vitamin D levels and the risk of atrial fibrillation: a two-sample Mendelian randomization study. Front Nutr. (2022) 9:837207. doi: 10.3389/fnut.2022.837207
32. Zhang N, Wang Y, Chen Z, Liu D, Tse G, Korantzopoulos P, et al. Circulating vitamin D concentrations and risk of atrial fibrillation: a Mendelian randomization study using non-deficient range summary statistics. Front Nutr. (2022) 9:842392. doi: 10.3389/fnut.2022.842392
33. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev. (2019) 18:102350. doi: 10.1016/j.autrev.2019.102350
34. Murdaca G, Gerosa A, Paladin F, Petrocchi L, Banchero S, Gangemi S. Vitamin D and microbiota: is there a link with allergies? Int J Mol Sci. (2021) 22:4288. doi: 10.3390/ijms22084288
35. Murdaca G, Greco M, Tonacci A, Negrini S, Borro M, Puppo F, et al. IL-33/IL-31 axis in immune-mediated and allergic diseases. Int J Mol Sci. (2019) 20:5856. doi: 10.3390/ijms20235856
36. Boursiquot BC, Larson JC, Shalash OA, Vitolins MZ, Soliman EZ, Perez MV. Vitamin D with calcium supplementation and risk of atrial fibrillation in postmenopausal women. Am Heart J. (2019) 209:68–78. doi: 10.1016/j.ahj.2018.12.006
37. Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. (2010) 341:c3691. doi: 10.1136/bmj.c3691
Keywords: atrial fibrillation, vitamin D, relationship, supplementation, meta
Citation: Ding X, Lai J, Zhang H and Guo Z (2023) Vitamin D, vitamin D supplementation and atrial fibrillation risk in the general population: updated systematic review and meta-analysis of prospective studies. Front. Nutr. 10:1246359. doi: 10.3389/fnut.2023.1246359
Received: 24 June 2023; Accepted: 04 September 2023;
Published: 21 September 2023.
Edited by:
Cristina Vassalle, Gabriele Monasterio Tuscany Foundation (CNR), ItalyReviewed by:
Louise Hartley, RTI Health Solutions, United KingdomCopyright © 2023 Ding, Lai, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongwen Guo, em9uZ3dlbl9ndW9AMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.