- 1Department of Maternal and Child Health, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Institute of Systems Epidemiology, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Child and Adolescent Psychiatry, School of Academic Psychiatry, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
- 4Department of Child Health Care of Sichuan Maternal and Child Health Hospital, Chengdu, Sichuan, China
Introduction: Vitamin K deficiency may elevate the incidence of musculoskeletal disorders (MSD), whereas it lacks validation for pediatric populations and has uncertain dose recommendations. In this context, we hypothesized that serum vitamin K levels are associated with MSD in preschool children, and the widely used vitamin A and vitamin D supplements may mediate these associations based on potential mechanisms, which expects to provide guidance for future practice.
Methods: A cross-sectional study was conducted in Sichuan province in southwestern China, from January 2021 to May 2022. Serum levels of vitamin K1/K2 and 25(OH)D were determined using the high-performance liquid chromatography method, and the diagnosis of MSD was executed by clinicians. Overall and stratified logistic regression analysis based on categorized 25(OH)D levels were conducted to assess association between serum vitamin K levels and MSD prevalence after adjusting for confounders. Mediation analysis was further performed and vitamin A and D supplementation was regressed as the mediator.
Results: A total of 6,368 children aged 0–6 years old were enrolled. MSD was identified in 1179 (18.51%) of the children, while 5,189 (81.49%) of them did not present such disorder. After adjusting confounders, a significant difference was found in serum vitamin K1 level between children in MSD and Non-MSD group (OR = 0.802, 95%CI 0.745–0.864). No significant difference was found in serum vitamin K2 level between the two groups (OR = 0.975, 95%CI 0.753–1.261). The association between vitamin K1 level and MSD prevalence was partly (36.8%) mediated by vitamin A and D supplementation.
Conclusions: A low serum vitamin K1 level is connected with an increased risk of MSD among children, highlighting that vitamin A and D supplementation is a helpful intervention to prevent MSD in children with vitamin K deficiency.
Background
Musculoskeletal disorders (MSDs) are conditions that can affect the muscles, bones, and joints, which are also the largest source of global rehabilitation need, ranking fifth in disability-adjusted life years (DALYs) and first in years lived with disability (YLDs) (1, 2). Childhood, an active stage of bone mineral accumulation and critical for adult peak bone mass (3, 4), is a particularly susceptible window for prevention and treatment of MSDs (5). MSDs in childhood, such as rickets, can lead to bone deformities and difficulties with activities, which impact health substantially, growth and development of children and adolescents (6, 7). Poor treatment of musculoskeletal problems can have a negative influence on the quality of life and lead to short-term consequences like fractures, or long-term ones like osteoporosis (8–12). This implies that MSDs in children should be given special attention due to their higher potential for skeleton healing and remodeling (5).
Age is not the sole factor influencing MSDs, as MSDs have been widely reported to be closely associated with nutrient intake: studies have shown that vitamin C can foster trabecular bone formation by modulating bone matrix gene expression (13, 14), while vitamin D and its metabolites contribute to bone development by affecting intestinal calcium transport and serum calcium and phosphate homeostasis (15, 16). Furthermore, vitamin A appears to affect bone health by simulating osteoblastic activity and bone formation, as well as inhibiting osteoclastic activity and bone resorption (17). It also correlates with the availability of vitamin D receptor binding vitamin D (18). Recent studies also suggest that vitamin K deficiency may serve as a predictor of MSDs by reducing the carboxylation of vitamin K-dependent proteins, such as osteocalcin and matrix Gla protein, which regulate the calcification (19–22), thereby paving the way for etiological investigations and interventions.
Some studies to date have examined the relationship between vitamin K and adult bone health, yielding some negative correlations between serum vitamin K levels and MSDs (23, 24). However, the study on vitamin K levels and its association with MSDs in children remains limited, particularly in preschool children who are experiencing active bone mineral accumulation. Vitamin K deficiency is one of the most common malnutrition issues in Chinese children (25). Therefore, it is urgent to explore the association between serum vitamin K levels in preschool children and their MSDs. In addition, due to the potential side effects of vitamin K (26), there is insufficient evidence to recommend vitamin K supplementation for children. However, some alternative interventions can be taken to reduce the adverse effects of vitamin K deficiency. For instance, vitamin A (Vit A) and vitamin D (Vit D) are easily accessible, safe, and widely prescribed in China (27–29), acting synergistically in bone metabolism with vitamin K. Extrapolating from general population studies (30, 31), it is plausible that Vit A and Vit D supplementation in MSD patients could serve as a potential therapeutic target to enhance bone health.
This study aimed to assess 1) the association of two major subtypes of vitamin K [phylloquinone (vitamin K1) and menaquinone (vitamin K2)] in a large Chinese sample of preschool children with MSD and 2) the mediation effect of Vitamin A/D supplement in the association between Vitamin K1/K2 levels and MSDs in preschool children.
Methods
Participants and data collection sample sources
The study enrolled children aged 0–6 years old who underwent health assessments at outpatient clinics of the child health departments across 10 urban areas (32) comprising 18 maternal and child health hospitals in Sichuan Province (Figure 1) from January 2021 to May 2022. Variables considered in this study, including the participant's age, gender, parents' highest education, birth height, birth weight, gestational week and vitamin A and D supplementation reported by the participant's guardians at enrollment were extracted from medical records. Written informed consent was secured from their guardians before the study. Ethical approval for the study protocol was granted by the Ethics Committee of Sichuan Provincial Maternal and Child Health Care Hospital (2019, No. 20).
Musculoskeletal disorders
In this study, children diagnosed with diseases such as O-leg, X-leg, pectus carinatum, square skull, and bone pain were classified as having an MSD. The diagnosis of these conditions was confirmed by clinicians according to the guidelines of Practical Child Health Care (33).
Serum vitamin K1/K2 and 25(OH)D concentrations
The vitamin K and vitamin D levels in serum were assessed by high-performance liquid chromatography (HPLC) (34–36). Briefly, 2 ml of venous blood was collected in a standard biochemical tube (red cap tube) and stored at room temperature while being protected from light. Within 8 h of collection, the samples were tested. Following the internal labeling vitamin K with liquid-liquid extraction and isotope internal standard quantification, the internal standard working solution was transferred to a clear tube and subjected to centrifugation at 4,000 rpm for 10 min. The resulting supernatant was collected and blown dry before being mixed with methanol for 1 min. Lastly, vitamin K1, K2, as well as 25(OH)D in supernatant were quantified by HPLC (model L550).
Covariates
Some relative covariates were considered in the analysis. Information about socio-demographic factors and birth information was obtained through the Pediatric Health Care System, which mainly contained: age (year), gender (male or female), residential area (ten urban areas in Sichuan province), birth height (meter), birth weight (kilogram), gestational week, and parent's degree (under junior high school, senior high school or technical secondary school and college and above).
Statistical analyses
Categorical variables were reported in terms of numbers and percentages, while continuous variables were presented as means and standard deviations (SD). To compare the characteristics of MSD and NMSD groups, Pearson chi-square tests for categorical variables and t-test were employed for continuous variables. Missing values were identified and imputed by multivariate imputation using chained equations (37).
We constructed logistic regression models to examine the associations between levels of vitamin K1/K2 as well as 25(OH)D and MSDs, with the former treated as continuous variables. To further investigate the associations between vitamin K1/K2 level and MSDs conditional on 25(OH)D level, we conducted stratified analysis based on categorized 25(OH)D levels, with 25(OH)D level <12 ng/mL, ≤ 12- <20 ng/mL, and ≥20 ng/mL, representing normal, insufficient, and deficient levels of vitamin D, respectively (38). All models were adjusted for children's gender, residential area, birth height, birth weight, gestational week, and parents' highest education. Additionally, to assess the influence of serum vitamin K1/K2 concentrations on MSD via vitamin A and D supplementation, vitamin A and D supplementation were regressed on the mediator variables. In this pathway, direct, indirect, total effects and the proportion of mediation were estimated.
Furthermore, we conducted two sensitivity analyses to assess the robustness of our results. The first analysis excluded the missing data instead of using multivariate imputation, whereas the second analysis only included individuals with birth weight (2.5-4.0 kg) and gestational week (37-42 weeks) within the normal range.
All analysis were performed using R, version 4.0.3 (R Project for Statistical Computing). For all statistical tests, a significance level of two-tailed P ≤ 0.05 was employed. Estimated effects were presented with odds ratios (OR) and 95% confidence intervals (95%CI).
Result
Participant characteristics
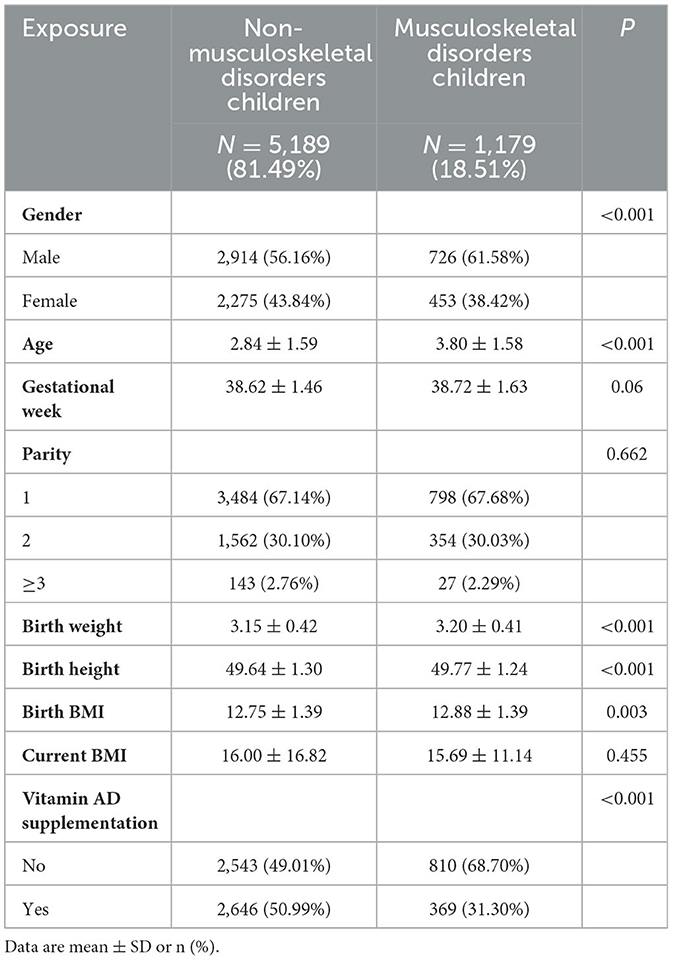
Descriptive statistics regarding the sociodemographic characteristics of the survey sample are presented in Table 1 and Supplementary Table 2. In this study, all variables were described with numbers and percentages. A total of 6,368 children aged 0–6 years old were enrolled, with 1,179 (18.51%) identified having MSDs (subtypes of MSDs are shown in Supplementary Table 2). Notably, the MSD group exhibited a greater proportion of male children in comparison to the NMSD group (P < 0.001). Regarding age, the NMSD children averaged 2.84 years, whereas MSD children averaged 3.8 years, indicating that children of the NMSD group were significantly younger than their MSD counterparts (P < 0.001). Children in the MSD group exhibited greater birth weight, height and BMI than those in the NMSD group (P < 0.05). In terms of the parity, there was no significant difference between the two groups (P = 0.67). Our study revealed no significant gestational week disparities emerged between the groups (P = 0.06). However, compared with the MSD group, the NMSD group were more likely to receive vitamin A and D supplementation (50.99 vs. 31.30%, P < 0.001).
Serum vitamin K1/K2 concentrations for children
Logistic regression models were applied to investigate the relationship between serum vitamin K1/K2 concentrations and the status of MSD in children. The models were adjusted for confounders, including residential area, kid's gender, birth height, birth weight, gestational week and parents' highest education. Our results revealed a significant difference in serum vitamin K1 levels between children in MSD and NMSD groups (OR = 0.802, 95%CI 0.745–0.864), however, there was no difference in serum vitamin K2 levels between the two groups (OR = 0.975, 95%CI 0.753–1.261). To further examine whether 25(OH)D levels modified the associations, we constructed stratified logistic regression models. The results indicated that there was no difference in the vitamin K1/K2 level among MSD and NMSD children in the vitamin D deficiency group (<12 ng/mL). Moreover, when the concentration of 25(OH)D in the serum increases as 12 ng/mL ≤ 25(OH)D <20 ng/mL or ≥20 ng/mL, vitamin K1 level was significantly associated with MSDs (95%CI 0.754–0.879,95%CI 0.740–0.871), while the vitamin K2 level remained similarly non-significant (95%CI 0.758–1.277,95%CI 0.730–1.394). More details are shown in Figure 2 and Supplementary Table 3.
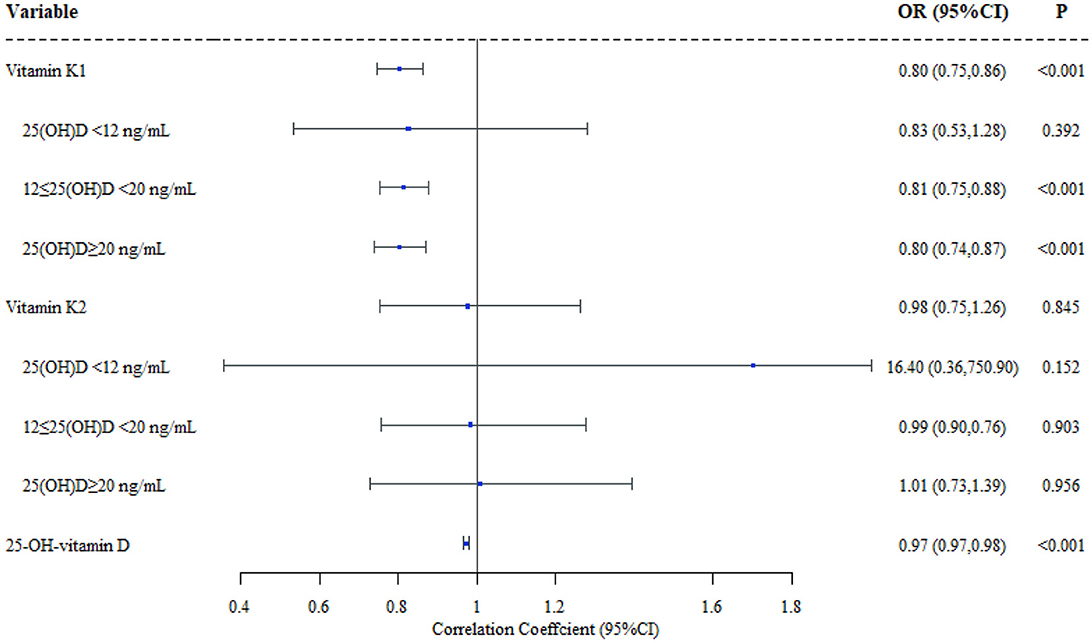
Figure 2. Effect of vitamin K1/K2/25(OH)D in children's musculoskeletal disorders (N = 6368). Adjustment of area, gender, birth height, birth weight, gestational week and parents' highest education.
Mediation of vitamin A and D supplementation
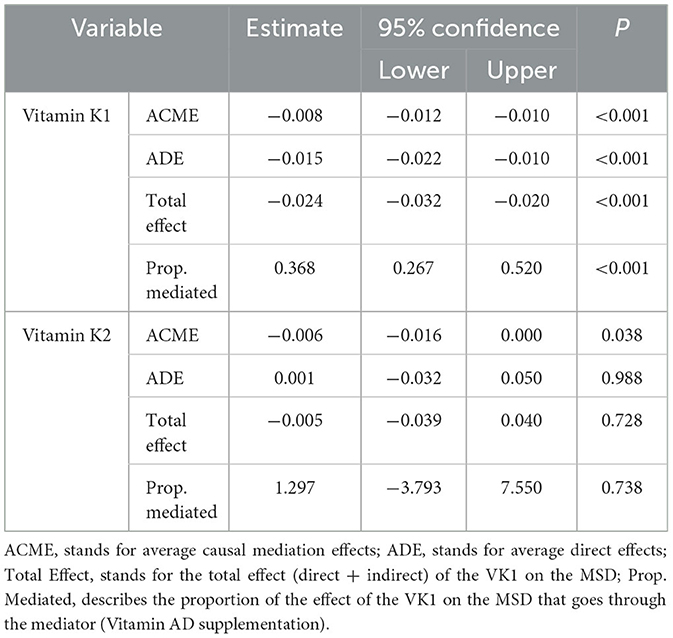
A mediation of the association between serum vitamin K1 and MSD through the vitamin A and D supplementation mediator was found. The total effect was highly significant (β = −0.024 P < 0.001). Likewise, the direct and indirect effects were β = −0.015 (P < 0.001) and β = −0.008 (P < 0.001), respectively. The proportion of the effect of vitamin K1 levels on the MSD that goes through vitamin AD supplementation is 36.8%. Conversely, the difference in the mediation of the association between serum vitamin K2 levels and MSD was not statistically significant. A comprehensive display of the total, direct, indirect effects and the proportion of mediation are displayed in Table 2.
Sensitive analysis
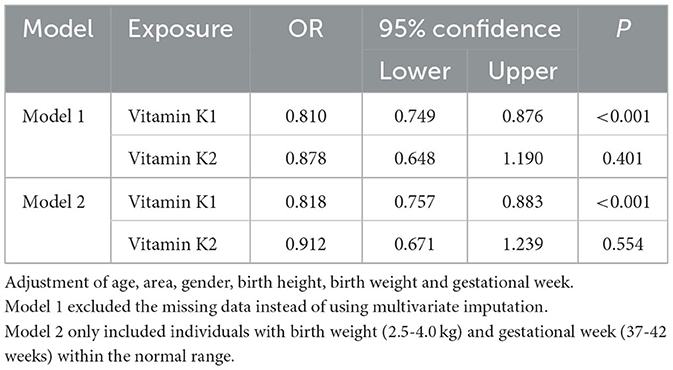
Sensitivity analyses employing logistic regression models with missing values removed (Table 3, model 1) yielded similar results, indicating a negative correlation between serum vitamin K1 concentrations and MSD prevalence (OR = 0.810, 95%CI 0.749–0.876), and no significant correlation for serum vitamin K2 concentrations (OR = 0.878, 95%CI 0.648–1.190). Furthermore, the results of logistic regression models involving participants with normal birth weight (2.5–4.0 kg) and gestational week (37-42 weeks) were consistent with the main findings (Table 3, model 2).
Discussion
The study examined the association between serum vitamin K concentration and MSDs among preschool children in Sichuan, China. Our findings demonstrate a significant association between serum vitamin K1 level and MSDs, after adjusting for children's gender, residential area, parents' highest education, birth height, birth weight and gestational week at birth. This association attenuated to null only when 25(OH)D was below <12 ng/ml. Moreover, vitamin A and D supplementation may serve as a potential influence on these associations among children. However, the serum vitamin K2 level was not associated with MSDs in any models.
To the best of our knowledge, limited studies have addressed the association between vitamin K and MSDs in preschool children (39–42). Our data indicate a significant association between serum vitamin K1 level and MSDs in children aged 0-6 years, highlighting the importance of addressing vitamin K1 deficiency and fostering proper nutrient supplementation and dietary habits in this population. A study also revealed that vitamin K1 had a greater effect on the incidence of bone fractures compared to vitamin K2 (43) which yielded results consistent with ours in general. However, findings regarding the efficacy of Vit K supplementation on bone are yet inconclusive (44, 45). While a meta-analysis has reported modest overall treatment effects (46), others have found no significant effect (47, 48). Furthermore, no randomized controlled trials have evaluated the effects of comparable doses of various forms of vitamin K on skeletal outcomes (49). Two Cochrane reviews have shown that the dosage and safety of vitamin A and D supplementation in children has been well established (50, 51). In China, vitamin A and D supplementation follows a standard, strict, and safe implementation protocol (52). Since 2013, the Chinese market for vitamin A and D drops or tablets has grown rapidly and it is widely utilized in Chinese children. Based on this study, vitamin A and D supplementation is an effective and feasible measure for children to partially block skeletal muscle disease caused by vitamin K deficiency.
Though the mechanisms linking nutrients deficiencies and bone metabolism are not fully understood, previous studies show that vitamin K may influence bone transformation through various pathways, including osteoblast differentiation, osteoclast inhibition, and activation of bone-associated vitamin K-dependent proteins, such as osteocalcin and matrix Gla protein that play essential roles in the mineralization of extracellular bone matrix (43, 53, 54). During this process, bone mineralization (55) is also closely related to the serum calcium levels, which can be regulated by vitamin D (56–58). This is consistent with our findings. It could be due to excessively low levels of vitamin D, incomplete decarboxylation of osteocalcin cannot occur which leads to inhibited bone mineralization, further manifesting as the lack of correlation between level of vitamin K and MSD occurrence. Besides, vitamin A also appears to affect bone health by simulating osteoblastic activity and inhibiting osteoclastic activity (18). Just as vitamin A and vitamin D are often used together in clinical practice, evidence shows that their pathways may overlap (59). Based on the above knowledge, we can speculate that supplementation with vitamin A or vitamin D or a combination may be a potential mediator to mitigate the adverse effects of vitamin K deficiency on MSDs, which is consistent with our study.
Our findings provide several implications for future research future research and practice. More attention should be paid to vitamin K deficiency in children, and vitamin A and D supplementation may help mitigate the adverse effects of vitamin K deficiency on MSDs. What's more, large prospective cohorts should examine the short- and long-term effects of vitamin K level on bone health in children. Ethically feasible clinical trials with safe doses can be conducted to provide further insights.
There are some strengths in this study. Firstly, we used high-performance liquid chromatography (HPLC) for vitamin K levels determination of participant from multiple regions with guaranteed consistency of measurement methods and a high detection sensitivity. Secondly, diagnosis of MSD was confirmed through physicians at the Sichuan Maternal and Child Health Hospital. The recollection bias and reporting bias has been controlled. This study is also restrained by several limitations. Firstly, the present study is a cross-sectional study that can merely show the correlation instead of causality. Secondly, despite careful adjustment for a extent range of covariates in the model, residual confounding due to the unavailability of data may exist. Thirdly, we only detected serum level of menaquinone-4, one of the subtypes of vitamin K2, which may underestimate level of exposure. Lastly, our study is conducted in a regional population with specific age group. We recommend exercising caution when extrapolating the findings of this study to children in other regions and age groups.
Conclusions
This study found that the serum vitamin K1 levels was significantly associated with MSDs, while the serum vitamin K2 levels were not. It also illustrated that vitamin AD supplementation may potentially influence these associations. These findings underscore the public health significance of vitamin A and D supplementation as a beneficial intervention for preventing MSDs attributable to vitamin K1 deficiency in children.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Sichuan Provincial Maternal and Child Health Care Hospital (2019, No. 20). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
QG and LZ are the main researchers, who has done the major work of data analysis and collection, respectively. ZS and JC are two of the main members of the supporting team, supervised by ZL, which plays an active role from the design of the study to the final drafting. The team also consists of other co-authors, including XJ, HW, XL, CY, and CX. Together, the study was guided by ZL throughout the process. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (Project No. U20A20411 to ZL).
Acknowledgments
We would like to extend their sincere thanks to the National Natural Science Foundation of China for the funding of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1239954/full#supplementary-material
References
1. GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1859–922. doi: 10.1016/S0140-6736(18)32335-3
2. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
3. NIH NIH Consensus Development Panel on Osteoporosis Prevention D, Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. (2001) 285:785–95. doi: 10.1001/jama.285.6.785
4. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. (2011) 26:1729–39. doi: 10.1002/jbmr.412
5. Ciancia S, Hogler W, Sakkers RJB, Appelman-Dijkstra NM, Boot AM, Sas TCJ, et al. Osteoporosis in children and adolescents: how to treat and monitor? Eur J Pediatr. (2023) 182:501–11. doi: 10.1007/s00431-022-04743-x
6. Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. (2010) 7:40. doi: 10.1186/1479-5868-7-40
7. Andersen LB, Riddoch C, Kriemler S, Hills AP. Physical activity and cardiovascular risk factors in children. Br J Sports Med. (2011) 45:871–6. doi: 10.1136/bjsports-2011-090333
8. Li X, Hung VWY Yu FWP, Hung ALH, Ng BKW, Cheng JCY, et al. Persistent low-normal bone mineral density in adolescent idiopathic scoliosis with different curve severity: A longitudinal study from presentation to beyond skeletal maturity and peak bone mass. Bone. (2020) 133:115217. doi: 10.1016/j.bone.2019.115217
9. Feehan AG, Zacharin MR, Lim AS, Simm PJ. A comparative study of quality of life, functional and bone outcomes in osteogenesis imperfecta with bisphosphonate therapy initiated in childhood or adulthood. Bone. (2018) 113:137–43. doi: 10.1016/j.bone.2018.05.021
10. Sakka SD. Osteoporosis in children and young adults. Best Pract Res Clin Rheumatol. (2022) 36:101776. doi: 10.1016/j.berh.2022.101776
11. Lu J, Shin Y, Yen MS, Sun SS. Peak bone mass and patterns of change in total bone mineral density and bone mineral contents from childhood into young adulthood. J Clin Densitom. (2016) 19:180–91. doi: 10.1016/j.jocd.2014.08.001
12. Kaya MH, Erbahceci F, Alkan H, Kocaman H, Buyukturan B, Canli M, et al. Factors influencing of quality of life in adolescent idiopathic scoliosis. Musculoskelet Sci Pract. (2022) 62:102628. doi: 10.1016/j.msksp.2022.102628
13. Aghajanian P, Hall S, Wongworawat MD. Mohan S. The roles and mechanisms of actions of vitamin C in bone: new developments. J Bone Miner Res. (2015) 30:1945–55. doi: 10.1002/jbmr.2709
14. Thaler R, Khani F, Sturmlechner I, Dehghani SS, Denbeigh JM, Zhou X, et al. Vitamin C epigenetically controls osteogenesis and bone mineralization. Nat Commun. (2022) 13:5883. doi: 10.1038/s41467-022-32915-8
15. Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. (2019) 40:1109–51. doi: 10.1210/er.2018-00126
16. Luo W, Liu L, Yang L, Dong Y, Liu T, Wei X, et al. The vitamin D receptor regulates miR-140-5p and targets the MAPK pathway in bone development. Metabolism. (2018) 85:139–50. doi: 10.1016/j.metabol.2018.03.018
17. Yee MMF, Chin KY, Ima-Nirwana S, Wong SK. Vitamin A and Bone Health: A Review on Current Evidence. Molecules. (2021) 26:1757. doi: 10.3390/molecules26061757
18. Riccio P, Rossano R. Diet, gut microbiota, and vitamins D + A in multiple sclerosis. Neurotherapeutics. (2018) 15:75–91. doi: 10.1007/s13311-017-0581-4
19. Azuma K, Inoue, S. Multiple modes of vitamin K actions in aging-related musculoskeletal disorders. Int J Mol Sci. (2019) 20:2844. doi: 10.3390/ijms20112844
20. Yang YJ, Kim DJ. An overview of the molecular mechanisms contributing to musculoskeletal disorders in chronic liver disease: osteoporosis, sarcopenia, and osteoporotic sarcopenia. Int J Mol Sci. (2021) 22:2604. doi: 10.3390/ijms22052604
21. Popa DS, Bigman G, Rusu ME. The role of vitamin K in humans: implication in aging and age-associated diseases. Antioxidants. (2021) 10:566. doi: 10.3390/antiox10040566
22. Iwamoto J, Sato Y, Takeda T, Matsumoto H. High-dose vitamin K supplementation reduces fracture incidence in postmenopausal women: a review of the literature. Nutr Res. (2009) 29:221–8. doi: 10.1016/j.nutres.2009.03.012
23. Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, et al. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr. (2003) 77:512–6. doi: 10.1093/ajcn/77.2.512
24. Levy-Schousboe K, Marckmann P, Frimodt-Moller M, Peters CD, Kjaergaard KD, Jensen JD, et al. Vitamin K supplementation and bone mineral density in dialysis: results of the double-blind, randomised, placebo-controlled RenaKvit trial. Nephrol Dial Transplant. (2022) 38:2131–42. doi: 10.1093/ndt/gfac315
25. Rai RK, Luo J, Tulchinsky TH. Vitamin K supplementation to prevent hemorrhagic morbidity and mortality of newborns in India and China. World J Pediatr. (2017) 13:15–9. doi: 10.1007/s12519-016-0062-6
26. Piscaer I, Wouters EFM, Vermeer C, Janssens W, Franssen FME, Janssen, et al. Vitamin K deficiency: the linking pin between COPD and cardiovascular diseases? Respir Res. (2017) 18:189. doi: 10.1186/s12931-017-0673-z
27. Subspecialty Subspecialty Group of Children Health the the Society of Pediatrics Chinese Medical Association; Editorial Board Chinese Chinese Journal of Pediatrics. [Practical guidelines for clinical issues related to vitamin D nutrition in Chinese children]. Zhonghua Er Ke Za Zhi. (2022) 60:387–94. doi: 10.3760/cma.j.cn112140-20211230-01092
28. Wagner CL, Greer FR. American Academy of Pediatrics Section on B, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. (2008) 122:1142–52. doi: 10.1542/peds.2008-1862
29. Yang Minxia XH, Zong Nian L, Tao Y, Fengzhen C. Application status and influencing factors of vitamin AD supplementation for infants in Gaoming District of Foshan City. Qingdao Med J. (2019) 51:5–9.
30. Genaro Pde S, Martini LA. Vitamin A supplementation and risk of skeletal fracture. Nutr Rev. (2004) 62:65–7. doi: 10.1111/j.1753-4887.2004.tb00026.x
31. Ali M, Uddin Z, Hossain A. Combined effect of vitamin D supplementation and physiotherapy on reducing pain among adult patients with musculoskeletal disorders: a quasi-experimental clinical trial. Front Nutr. (2021) 8:717473. doi: 10.3389/fnut.2021.717473
32. National Bureau of Statistics. Available online at: http://www.stats.gov.cn/sj/tjbz/tjyqhdmhcxhfdm/2022/51.html (accessed May 15, 2023).
33. Li H. The Principle and Practice of Pediatric Primary Care. Beijing: People's Literature Publishing House (2016).
34. Wang Chao CZ, Xuepeng X, Fan B, Yan X. Serum levels and clinical significance of vitamin K1 and vitamin K2 in young children with abnormal bone metabolism. Zhejiang Pract Med. (2022) 27:431–4. doi: 10.16794/j.cnki.cn33-1207/r.2022.05.015
35. Wakabayashi H, Onodera K, Yamato S, Shimada, K. Simultaneous determination of vitamin K analogs in human serum by sensitive and selective high-performance liquid chromatography with electrochemical detection. Nutrition. (2003) 19:661–5. doi: 10.1016/S0899-9007(03)00056-X
36. Xu B, Feng Y, Gan L, Zhang Y, Jiang W, Feng J, et al. Vitamin D status in children with short stature: accurate determination of serum vitamin D components using high-performance liquid chromatography-tandem mass spectrometry. Front Endocrinol. (2021) 12:707283. doi: 10.3389/fendo.2021.707283
37. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. (2011) 20:40–9. doi: 10.1002/mpr.329
38. Health Health Industry Standard of the People's Republic of China Method for Vitamin D Deficiency Screening. Available online at: http://www.nhc.gov.cn/wjw/yingyang/202005/91d4275bc393465191a098005a1034aa/files/8a75fd3f113c463fbf039de3f7b25bf5.pdf (accessed December 24, 2022).
39. Huang ZB, Wan SL, Lu YJ, Ning L, Liu C, Fan SW. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: a meta-analysis of randomized controlled trials. Osteoporos Int. (2015) 26:1175–86. doi: 10.1007/s00198-014-2989-6
40. Martini LA, Booth SL, Saltzman E, do Rosario Dias de Oliveira Latorre M, Wood RJ. Dietary phylloquinone depletion and repletion in postmenopausal women: effects on bone and mineral metabolism. Osteoporos Int. (2006) 17:929–35. doi: 10.1007/s00198-006-0086-1
41. Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, et al. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. (2004) 89:4904–9. doi: 10.1210/jc.2003-031673
42. Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, et al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition. (2001) 17:315–21. doi: 10.1016/S0899-9007(00)00554-2
43. Villa JKD, Diaz MAN, Pizziolo VR, Martino HSD. Effect of vitamin K in bone metabolism and vascular calcification: a review of mechanisms of action and evidences. Crit Rev Food Sci Nutr. (2017) 57:3959–70. doi: 10.1080/10408398.2016.1211616
44. Beulens JW, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K(2)) in human health. Br J Nutr. (2013) 110:1357–68. doi: 10.1017/S0007114513001013
45. Wu J, Guo B, Guan H, Mi F, Xu J, Basang; Li Y, et al. The Association Between Long-term Exposure to Ambient Air Pollution and Bone Strength in China. J Clin Endocrinol Metab. (2021) 106:e5097–108. doi: 10.1210/clinem/dgab462
46. Fang Y, Hu C, Tao X, Wan Y, Tao F. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab. (2012) 30:60–8. doi: 10.1007/s00774-011-0287-3
47. Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. (2006) 166:1256–61. doi: 10.1001/archinte.166.12.1256
48. Mott A, Bradley T, Wright K, Cockayne ES, Shearer MJ, Adamson J, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. (2019) 30:1543–59. doi: 10.1007/s00198-019-04949-0
49. Zhang Y, Shea MK, Judd SE, D'Alton ME, Kahe, K. Issues related to the research on vitamin K supplementation and bone mineral density. Eur J Clin Nutr. (2022) 76:335–9. doi: 10.1038/s41430-021-00941-2
50. Tan ML, Abrams SA, Osborn DA. Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health. Cochrane Database Syst Rev. (2020) 12:CD013046. doi: 10.1002/14651858.CD013046.pub2
51. Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst. Rev. (2017) 3:CD008524. doi: 10.1002/14651858.CD008524.pub3
52. Child Health Care Professional Committee Chinese Preventive Medicine Association. Expert consensus on clinical application of vitamin A and vitamin D in Chinese children. Chin J Child Health Care. (2021) 29:7. doi: 10.11852/zgetbjzz2020-2118
53. Akbari S, Rasouli-Ghahroudi AA. Vitamin K and bone metabolism: a review of the latest evidence in preclinical studies. Biomed Res Int. (2018) 2018:4629383. doi: 10.1155/2018/4629383
54. Palermo A, Tuccinardi D, D'Onofrio L, Watanabe M, Maggi D, Maurizi AR, et al. Vitamin K and osteoporosis: Myth or reality? Metabolism. (2017) 70:57–71. doi: 10.1016/j.metabol.2017.01.032
55. Houillier P, Nicolet-Barousse L, Maruani G, Paillard M. What keeps serum calcium levels stable? Joint Bone Spine. (2003) 70:407–13. doi: 10.1016/S1297-319X(03)00052-6
56. Avenell A, Mak JC, O'Connell, D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. (2014) 2014:CD000227. doi: 10.1002/14651858.CD000227.pub4
57. Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. (2011) 25:585–91. doi: 10.1016/j.beem.2011.05.002
58. Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. (2014) 383:146–55. doi: 10.1016/S0140-6736(13)61647-5
Keywords: preschool children, serum vitamin K levels, musculoskeletal disorders, a cross-sectional study, vitamin A and vitamin D supplement
Citation: Ge Q, Zhang L, Sun Z, Cai J, Jiang X, Wang H, Li X, Yu C, Xiao C and Liu Z (2023) The mediation effect of vitamin A and vitamin D supplement in the association between serum vitamin K levels and musculoskeletal disorders in preschool children. Front. Nutr. 10:1239954. doi: 10.3389/fnut.2023.1239954
Received: 14 June 2023; Accepted: 04 December 2023;
Published: 22 December 2023.
Edited by:
Fabio Vescini, Azienda Sanitaria Universitaria Integrata di Udine, ItalyReviewed by:
Tianlin Gao, Qingdao University, ChinaAlessandro Brunetti, Santa Maria della Misericordia Hospital in Udine, Italy
Copyright © 2023 Ge, Zhang, Sun, Cai, Jiang, Wang, Li, Yu, Xiao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenmi Liu, emhlbm1pbGl1QHNjdS5lZHUuY24=
†These authors share first authorship
 Qiaoyue Ge
Qiaoyue Ge Lu Zhang
Lu Zhang Zeyuan Sun
Zeyuan Sun Jiarui Cai1
Jiarui Cai1 Xia Jiang
Xia Jiang Zhenmi Liu
Zhenmi Liu