- 1The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 3The Research Team on Bone and Joint Degeneration and Injury of Guangdong Provincial Academy of Chinese Medical Sciences, Guangzhou, China
- 4The Fifth Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 5Guangdong Second Chinese Medicine Hospital (Guangdong Province Enginering Technology Research Institute of Traditional Chinese Medicine), Guangzhou, China
Background: Resveratrol is a natural polyphenol compound that is widely present in herbal medicines such as Reynoutria japonica Houtt., Veratrum nigrum L., and Catsiatora Linn and is used in traditional Chinese medicine to treat metabolic bone deseases. Animal experiments have shown that resveratrol may have a strong treatment effect against osteoporosis (OP). The purpose of this study was to explore the efficacy of resveratrol in treating OP animal models based on preclinical research data.
Methods: This study was completed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched the PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI) databases from inception to May 8, 2023, to identify animal experiments on the treatment of OP with resveratrol. The effect sizes of bone mineral density (BMD), parameters of micro-CT, serum calcium, phosphorus, alkaline phosphatase (ALP) and osteocalcin were expressed as the mean differences (MDs) and 95% confidence intervals (CIs). RevMan 5.4 software was used for data analysis.
Results: This meta-analysis included a total of 15 animal experiments, including 438 OP rats. The meta-analysis results showed that compared with the control group, resveratrol (<10, 10–25, 40–50, ≥ 60 mg/kg/day) significantly increased femoral and lumbar bone mineral density (BMD) in OP rats (p < 0.05). Resveratrol (<10 mg/kg/day) significantly increased the BMD of the total body (MD = 0.01, 95% CI: 0.01 to 0.01, p < 0.001). In terms of improving the parameters related to micro-CT, resveratrol (40–50 mg/kg/day) can increase trabecular thickness and trabecular number and reduce trabecular spacing (p < 0.05). Compared with the control group, resveratrol can reduce the concentration of calcium and phosphorus in serum but has no significant effect on serum ALP and osteocalcin (p > 0.05). The results of subgroup analysis showed that resveratrol increased the whole-body BMD of SD rats (p = 0.002) but did not improve the whole-body BMD of 3-month-old rats (p = 0.17).
Conclusion: Resveratrol can increase BMD in OP rat models, and its mechanism of action may be related to improving bone microstructure and regulating calcium and phosphorus metabolism. The clinical efficacy of resveratrol in the treatment of OP deserves further research.
1. Introduction
Osteoporosis (OP) is a systemic bone disease characterized by low bone mass, damage to the microstructure of bone tissue, and increased bone fragility (1). The increased risk of bone fragility and fracture caused by OP poses a heavy economic burden to society and patients (2, 3). The aetiology of OP is complex and diverse, including the interactions between endocrine, nutritional, genetic, physiological, and immune factors (4). Among them, postmenopausal osteoporosis (PMOP) is considered strongly correlated with oestrogen deficiency (5). The increase in bone resorption and the decrease in bone formation lead to an imbalance in bone homeostasis (1, 6), which is closely related to the occurrence of OP. An epidemiological study has shown that the prevalence of OP in people over 50 years old in Europe and America is 4–6%, while in Asian populations, it is over 15% (7). According to the diagnostic criteria of the World Health Organization (WHO), the latest epidemiological research results show that the global prevalence of OP is as high as 19.7% (8, 9). The OP prevalence rates in different countries (4.1% in Netherlands to 52.0% in Türkiye) and continents (8.0% in Oceania to 26.9% in Africa) vary greatly (8, 9). As the population continues to age, OP is recognized as a major public health issue (7). At present, the treatment of OP mainly includes bisphosphonates, parathyroid drugs, or oestrogen replacement therapy (10, 11), all of which have inevitable adverse reactions. Therefore, researching and developing more alternative drugs with fewer side effects and better therapeutic effects is an important topic for OP treatment.
Botanical or traditional medicine have always been breakthrough points in new drug development, mainly due to their higher potential for drug conversion and lower incidence of adverse reactions. Traditional Chinese medicine is also commonly used for the treatment of OP, and its pharmacological mechanism usually has the characteristics of “multiple components, multiple targets, and multiple pathways.” Resveratrol is a natural polyphenol compound with a structure similar to oestrogen diethylstilbestrol, which is widely present in herbs such as Reynoutria japonica Houtt., Veratrum nigrum L., and Catsiatora Linn (12, 13). Research has shown that resveratrol competitively binds to oestrogen receptors in vitro, similar to phytoestrogens, and exerts anti-OP effects (14). Another study showed that resveratrol can affect the metabolism of bone cells and has the ability to regulate bone turnover (15). It is a natural antioxidant that can effectively prevent bone loss caused by oxidative stress in the body. Previous clinical studies have suggested that resveratrol can reduce bone loss and fracture risk in postmenopausal women or diabetes patients (16, 17). However, there is currently a lack of advanced evidence for the use of resveratrol in the treatment of OP; therefore, there is a lack of clarity regarding the application value of resveratrol.
The pre-clinical studies conclusions of animal experiments can provide key information for clinical practice and enhance the understanding of disease mechanisms among clinical and scientific researchers. At present, the clinical evidence for the treatment of OP with resveratrol is very limited, and thus, there is little information regarding the potential medicinal value of resveratrol in OP treatment. However, in the experimental field, studies have examined resveratrol treatment of OP animal models. This systematic review and meta-analysis aimed to evaluate the efficacy of resveratrol in treating OP animal models in order to provide evidence for future research on the anti-OP clinical efficacy of resveratrol.
2. Materials and methods
The implementation of this study strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18). The data source for this meta-analysis is publicly published papers, which means that ethical review was not needed.
2.1. Eligibility criteria
The inclusion criteria for this meta-analysis were as follows: (1) the study design was a controlled experiment, which means that the study protocol included both an experimental group and a control group, (2) the research object was a female rat model; the species of rats were Albino rats, SD rats, or Wistar rats; the age of rats did not exceed 6 months, and the modelling method was ovariectomy (OVX), (3) the intervention for the experimental group was resveratrol, but the dosage of resveratrol was not limited, (4) comparison: the intervention measures for the control group can be blank control (tap water or normal saline) or other drug treatments, and (5) outcome index: bone mineral density (BMD) (g/cm2) is the primary outcome measure, and secondary outcomes included trabecular thickness (Tb. Th), trabecular number (Tb. N), trabecular spacing (Tb. SP), serum calcium (mmol/l), serum phosphorus (mmol/l), serum alkaline phosphatase (ALP) (U/l), and serum osteocalcin (nmol/l); furthermore, all outcome indicators must clearly report the results of the measurement data, and the data reporting format must be mean ± standard deviation. There were no restrictions regarding publication language.
2.2. Exclusion criteria
The exclusion criteria were as follows: (1) review, meeting abstract, and case report, (2) incomplete experimental data, and (3) in vitro studies or clinical studies.
2.3. Search strategies
We searched the following four databases to obtain animal experimental studies on the treatment of OP with resveratrol: PubMed, Embase, The Cochrane Library, and China National Knowledge Infrastructure (CNKI). The search was performed from database inception to May 8, 2023. The search strategy included a combination of MeSH terms and free words, and the strategy was adjusted based on the characteristics of each database. The keywords related to resveratrol included “Resveratrol” OR “trans-Resveratrol” OR “3 5 4 trihydroxystilbene” OR “cis-Resveratrol” OR “3 4 5 stilbenetriol” OR “trans-Resveratrol-3-O-sulfate” OR “trans-Resveratrol-3-O-sulfate” OR “SRT-501” OR “trans-Resveratrol” OR “SRT501” OR “SRT-501” OR “cis-Resveratrol” OR “Resveratrol-3-sulfate” OR “3 4 5 trihydroxystilbene” OR “Resveratrol-3-sulfate.” The keywords for OP include: “Osteoporosis” OR “OP” OR “Osteoporoses” OR “bone loss” OR “bone density” OR “bone mineral density” OR “bone mass density.”
2.4. Data extraction
Two researchers independently conducted literature screening and data extraction and cross-checked the results. Disagreements were resolved by discussion or by consulting a third researcher. The following data were extracted: (1) basic information of the included study: author, title, year of publication, animal species, weight, age, and sample size, (2) specific details of intervention measures, including medication dosage and duration, (3) the various information elements of bias risk assessment, and (4) outcome indicators and outcome measurement data.
2.5. Quality evaluation of the included studies
We used the risk of bias tool for animal studies provided by the Systematic Review Center for Laboratory Animal Experience (SYRCLE) to conduct a literature quality evaluation of the included studies (19, 20). This evaluation tool has a total of 9 items, including random group allocation, groups similar at baseline, blinded group allocation, random housing, blinded interventions, random outcome assessment, blinded outcome assessment, reporting of drop-outs, and other biases. Each item can be judged as having low bias risk, high bias risk, and unclear bias risk (19, 20).
2.6. Statistical analysis
RevMan 5.4 software was used for data analysis. The outcome measures included in this study were all continuous variables, so all combined effects are expressed as the mean difference (MD) and 95% confidence interval (CI). This meta-analysis used the random-effects model for pooled data analysis. To clarify the anti-OP effect of resveratrol at different doses, we divided the drug doses of resveratrol into four groups: >10, 10–25, 40–50, and ≥ 60 mg/kg/day. In each included study, if there were 2 or more sets of satisfactory measurement data within the same dose range (the same study), the group with the lowest dose was selected for meta-analysis. Considering that differences in race and age of rats may affect the reliability of the conclusion, we conducted subgroup analyses based on those two factors. In particular, the resveratrol group used in the subgroup analysis was the lowest-dose group in each included study. We also constructed funnel plots for each outcome indicator to evaluate potential publication bias.
3. Results
3.1. Literature screening results
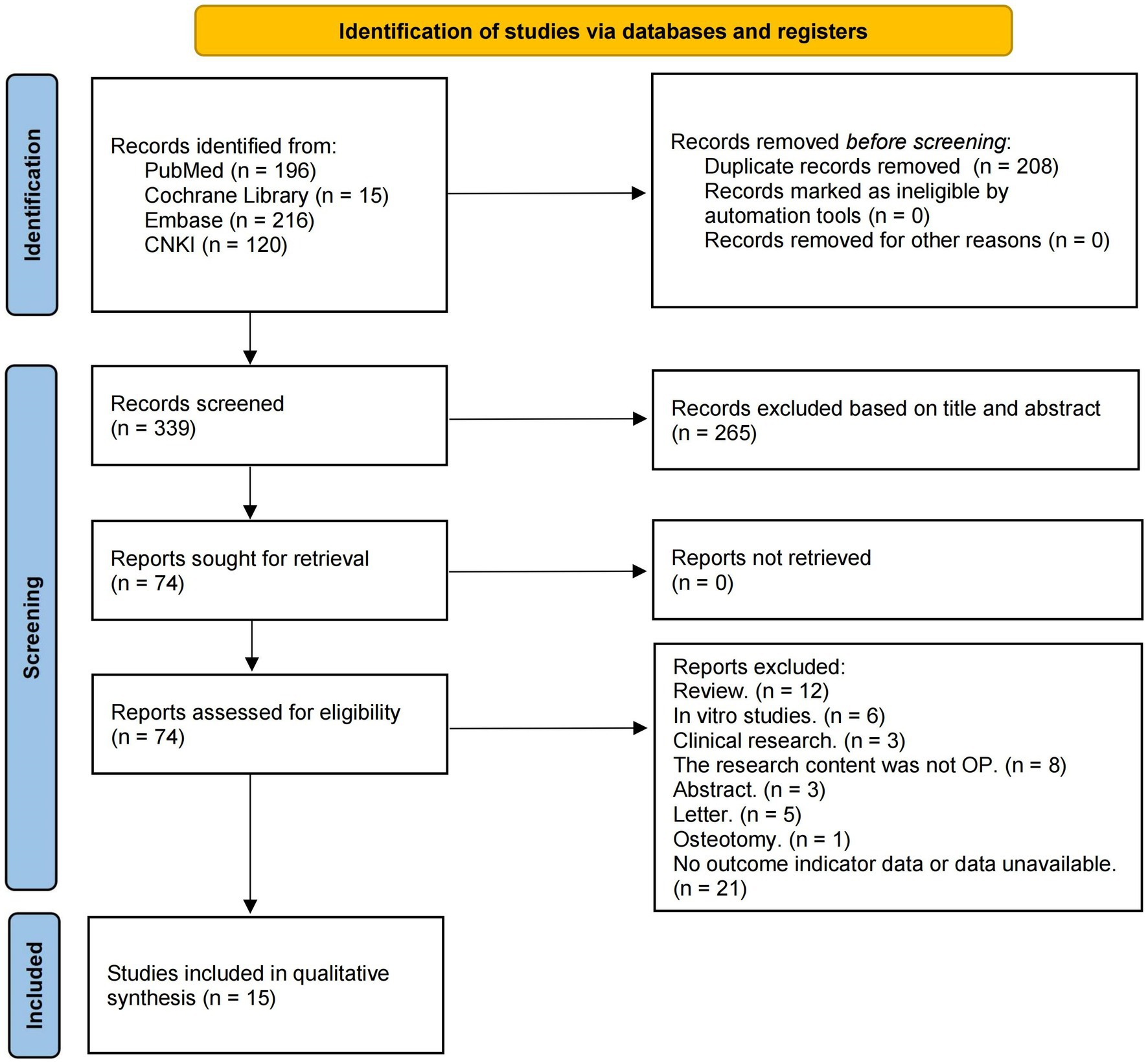
After removing duplicate literature, we initially obtained 339 articles. In the initial screening, we excluded literature that clearly did not meet the inclusion criteria based on the information provided by the title and abstract. After applying the inclusion and exclusion criteria and screening full texts, a total of 15 studies on the treatment of OP animal models with resveratrol that met the requirements of this meta-analysis were ultimately included (21–35). The search process and details are shown in Figure 1.
3.2. Characteristics of the 15 included studies
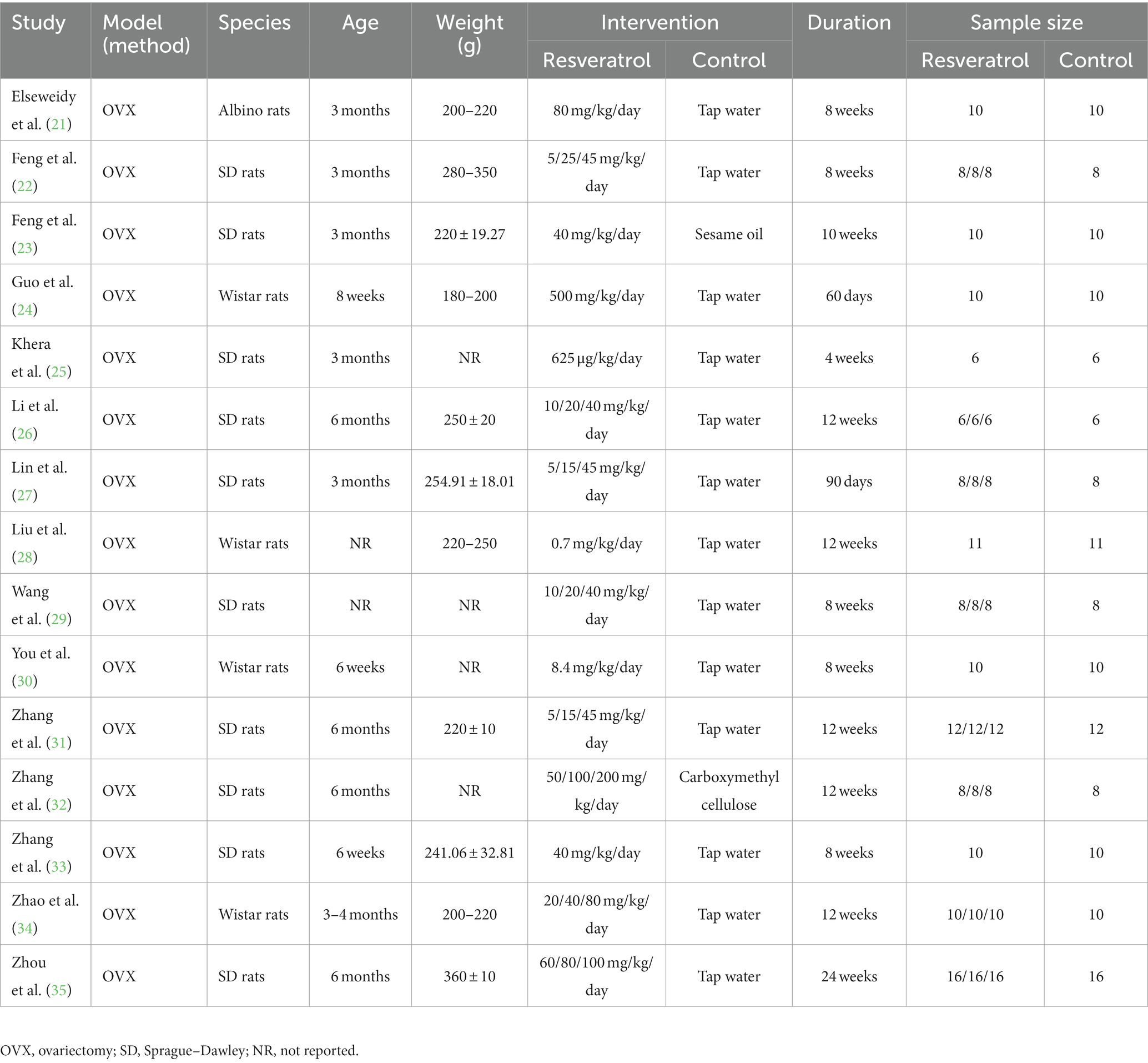
This meta-analysis included 15 experimental studies on the treatment of OP rats with resveratrol. A total of 438 rats were included in this study, including 295 in the resveratrol group and 143 in the control group. There are three types of rat strains, namely, Albino rats, SD rats, and Wistar rats. The modelling method for OP is OVX. The dosage of resveratrol varies greatly, with a minimum dosage of 625 μg/kg/day and a maximum dosage of 500 mg/kg/day. The course of medication is between 4 and 24 weeks. The specific details and characteristics of each included study are shown in Table 1.
3.3. Literature quality evaluation
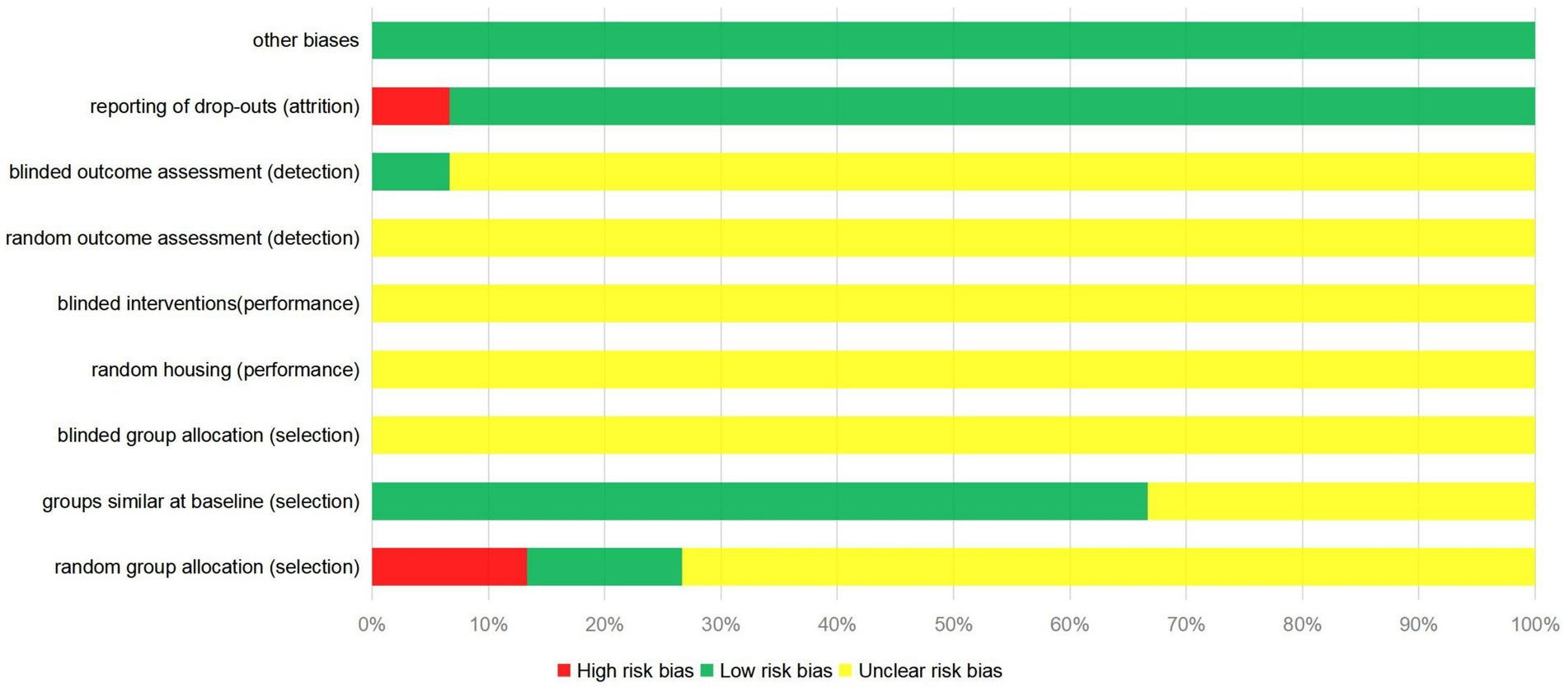
Most of the 15 studies included in this meta-analysis were evaluated for unclear risk bias. Only 2 studies used the random number table method (30, 33); 2 studies did not use random assignment (21, 27); and the remaining studies did not provide sufficient information to determine whether the experimental animals were randomly assigned. One study used a blinding method for the evaluators of results (22). One study did not provide a detailed explanation of missing data (29), which may lead to potential data reporting bias. The quality evaluation results of the literature included in the study are shown in Figure 2.
3.4. Results of meta-analysis
3.4.1. Primary outcomes
3.4.1.1. BMD of the total body
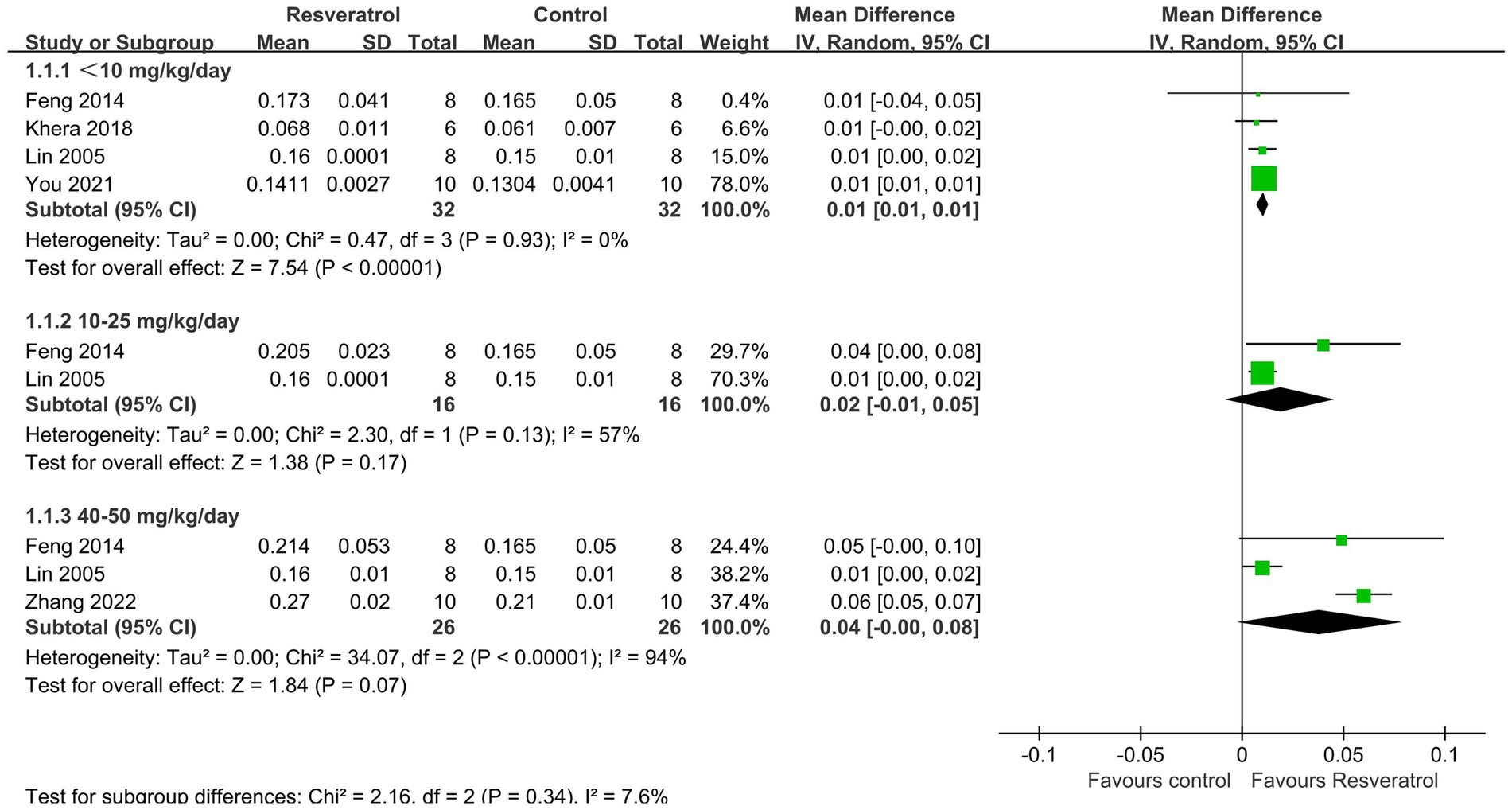
A total of 5 studies (22, 25, 27, 30, 33) reported total-body BMD (Figure 3). The meta-analysis results showed that compared with the control condition, <10 mg/kg/day resveratrol significantly increased the total-body BMD of the OP rat model (MD = 0.01, 95% CI: 0.01 to 0.01; p < 0.001), and there was no heterogeneity among the studies in this subgroup (I2 = 0%). However, resveratrol doses of 10–25 mg/kg/day and 40–50 g/kg/day showed no significant difference in total-body BMD compared with the control condition (p > 0.05).
3.4.1.2. BMD of the femur
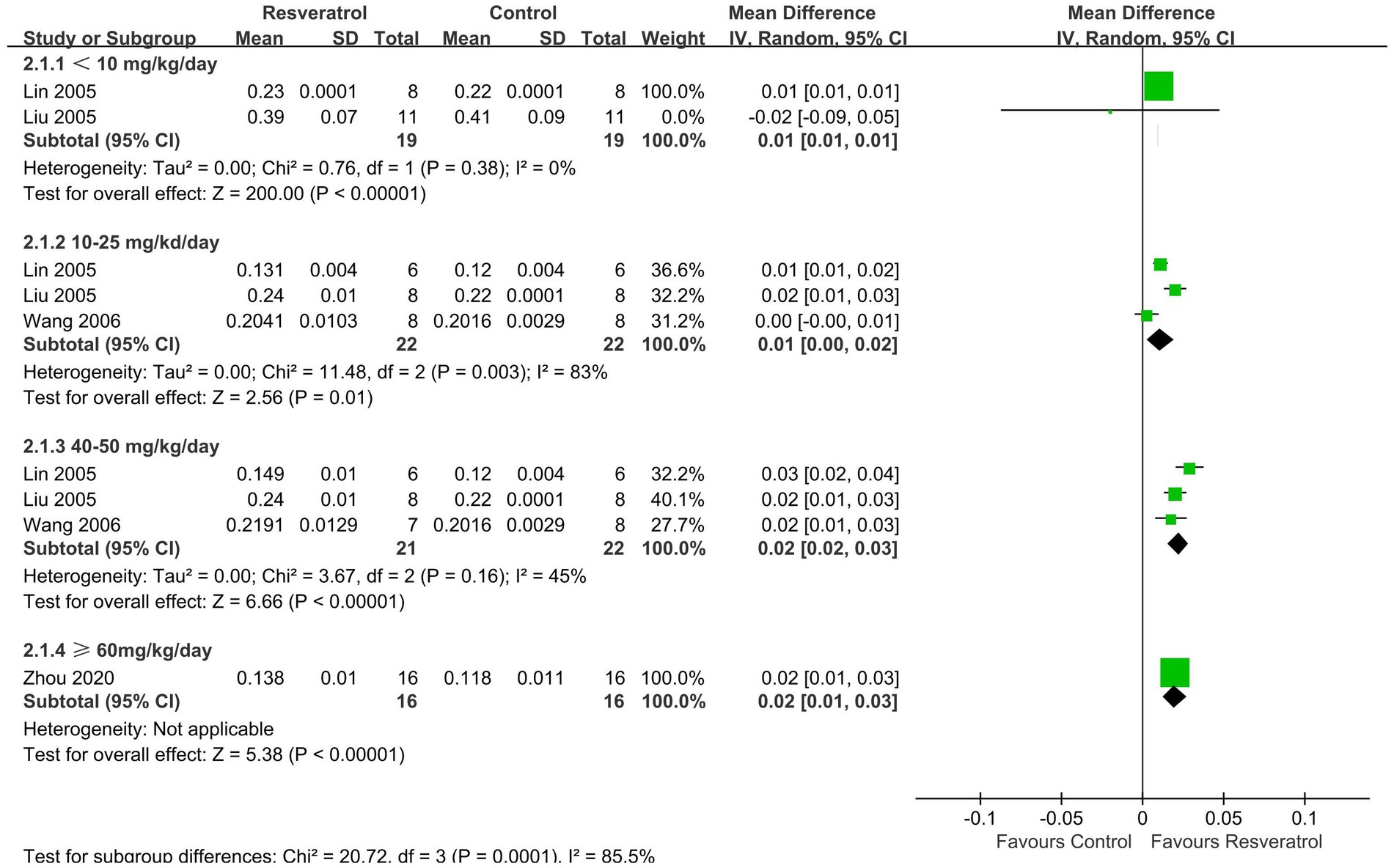
A total of 5 studies (26–29, 35) reported FBMD (Figure 4). The meta-analysis results showed that compared with the control group, four doses of resveratrol (<10, 10–25, 40–50, ≥ 60 mg/kg/day) all increased FBMD in OP rats, with MDs (95% CIs) of 0.01 (0.01, 0.01), 0.01 (0.00, 0.02), 0.02 (0.02, 0.03), and 0.02 (0.01, 0.03), respectively.
3.4.1.3. BMD of the lumbar vertebrae
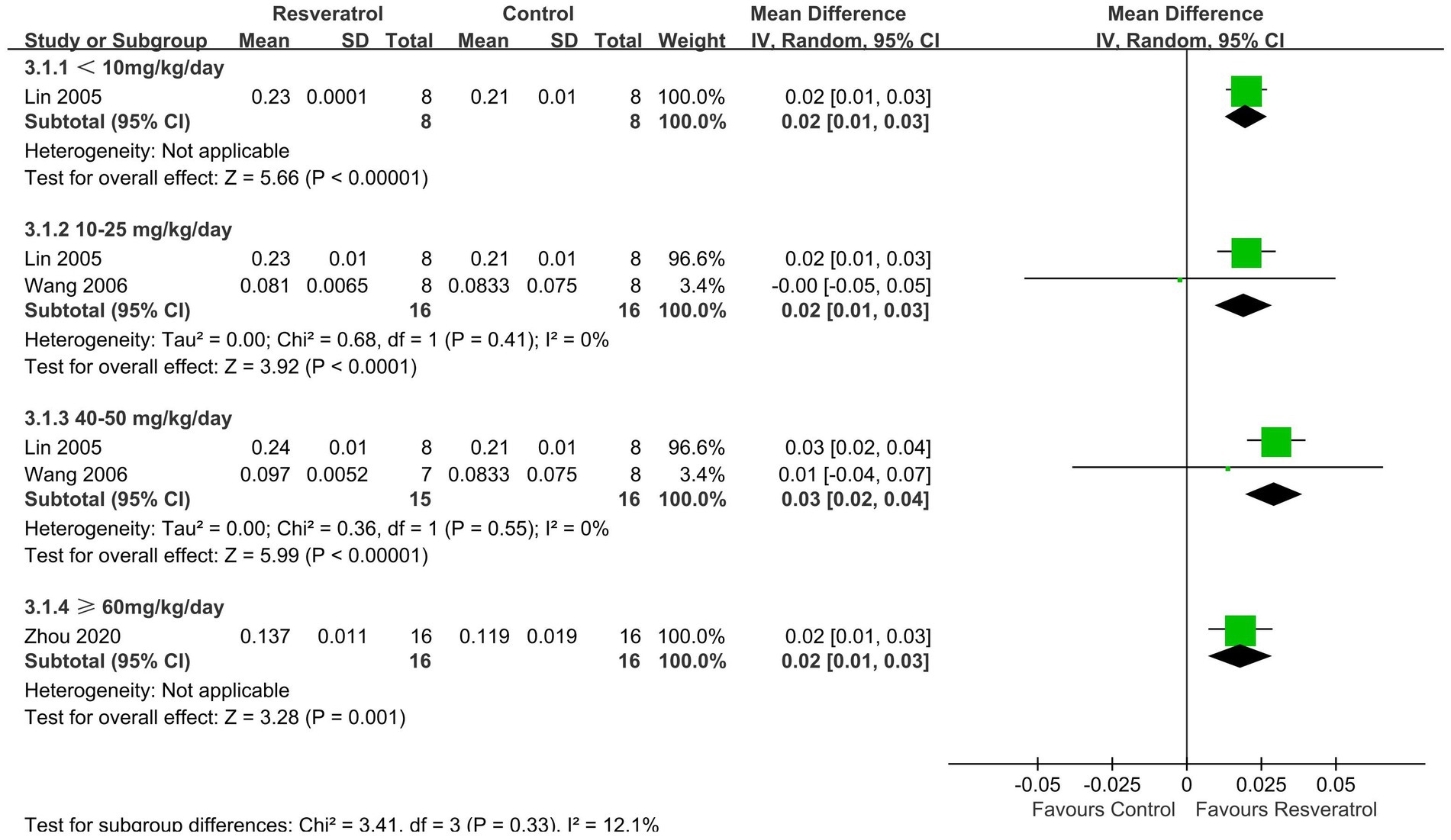
Three studies (27, 29, 35) reported LBMD (Figure 5). The meta-analysis results showed that resveratrol <10 (MD = 0.02, 95% CI: 0.01 to 0.03), 10–25 (MD = 0.02, 95% CI: 0.01 to 0.03), 40–50 (MD = 0.03, 95% CI: 0.02 to 0.04), and ≥ 60 (MD = 0.02, 95% CI: 0.01 to 0.03) mg/kg/day significantly increased LBMD compared to the control group (p < 0.05).
3.4.2. Secondary outcomes
3.4.2.1. Parameters of micro-CT
This meta-analysis analysed three parameters related to micro-CT, namely, Tb. Th (Supplementary material 1), Tb. N (Figure 6), and Tb. Sp (Supplementary material 2). The meta-analysis results showed that resveratrol (40–50 mg/kg/day) significantly increased Tb. Th (MD = 0.01, 95% CI: 0.01 to 0.01) in the OP rat model. Compared with the control group, resveratrol (<10, 40–50, ≥ 60 mg/kg/day) increased Tb. N (p < 0.05). Resveratrol (10–25, 40–50, ≥60 mg/kg/day) was more effective in reducing Tb. Sp compared to the control group, and the differences were statistically significant (p < 0.05).
3.4.2.2. Serum calcium
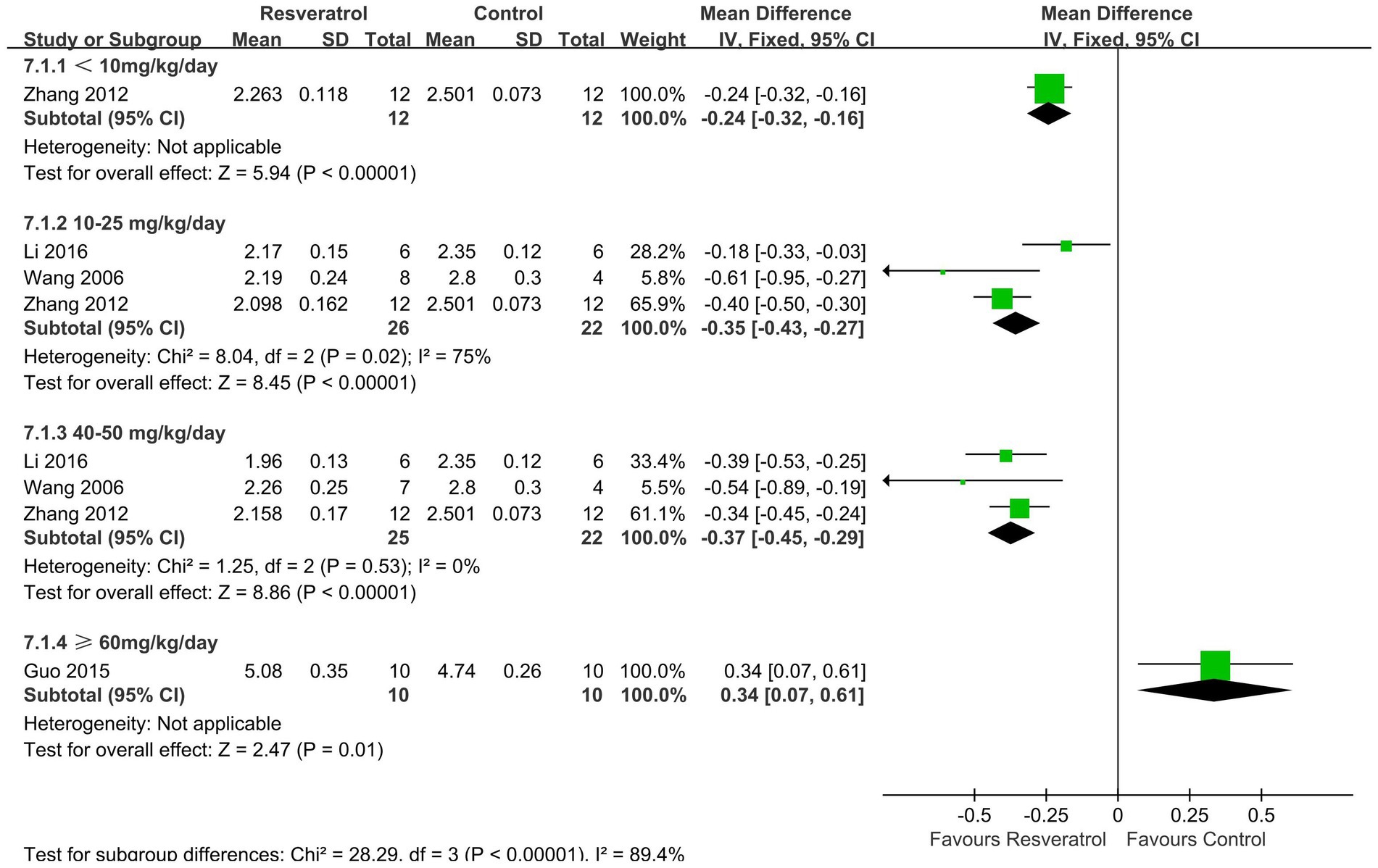
Four studies (24, 26, 29, 31) reported changes in serum calcium concentration (Figure 7). The meta-analysis results showed that resveratrol at concentrations of <10 (MD = -0.24, 95% CI: −0.32 to-0.16), 10–25 (MD = -0.35, 95% CI: −0.43 to-0.27), and 40–50 (MD = -0.37, 95% CI: −0.45 to-0.29) mg/kg/day significantly reduced serum calcium concentration compared to the control group (p < 0.001).
3.4.2.3. Serum phosphorus
Similarly, four studies (24, 26, 29, 31) reported changes in serum phosphorus concentration (Supplementary material 3). The meta-analysis results showed that resveratrol was more effective in reducing serum phosphorus concentration compared to the control group, and the differences were statistically significant (p < 0.05).
3.4.2.4. Serum ALP
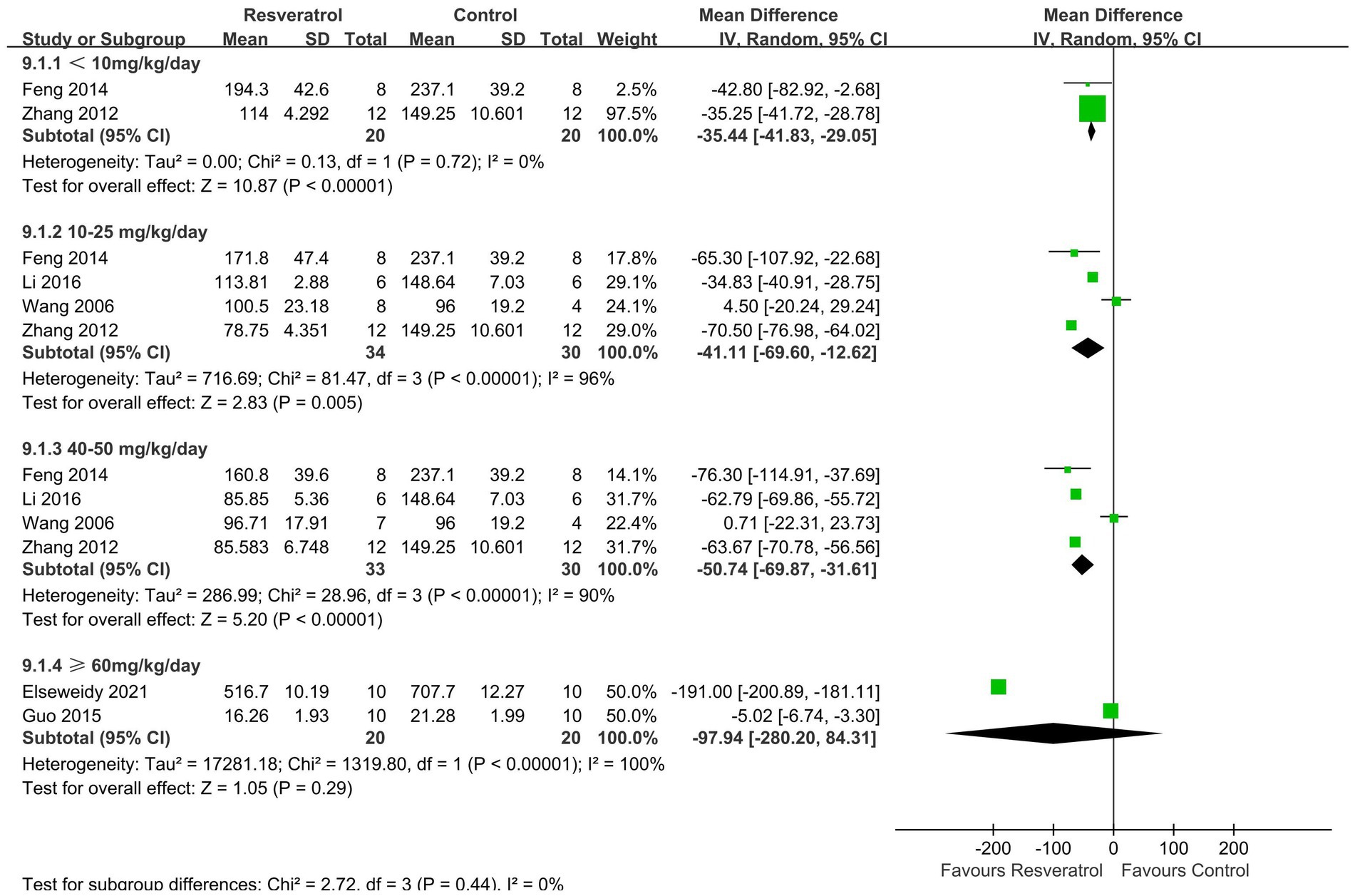
A total of 6 studies (21, 22, 24, 26, 29, 31) reported serum ALP levels (Figure 8). The meta-analysis results showed that there was no statistically significant difference in the effect of resveratrol and the control group on serum ALP (p > 0.05).
3.4.2.5. Serum osteocalcin
A total of 4 studies (22, 24, 30, 33) reported changes in serum osteocalcin levels (Supplementary material 4). The meta-analysis results showed that there was no significant difference in the effect of resveratrol on serum osteocalcin compared to the control group (p > 0.05).
3.4.3. Subgroup analysis of BMD of the total body
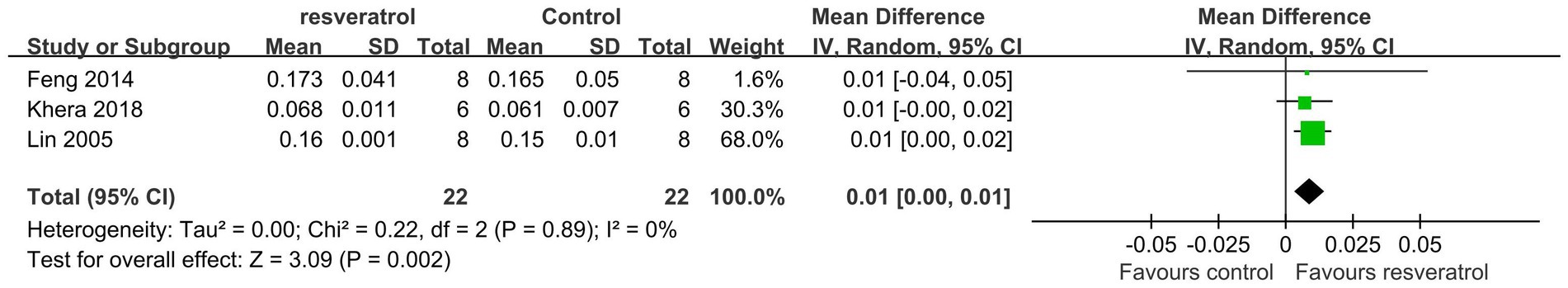
3.4.3.1. Resveratrol in SD rats
A total of 3 studies were included in the subgroup analysis (22, 25, 27). Meta-analysis results showed that compared with the control condition, resveratrol treatment resulted in a statistically significant increase in total-body BMD (MD = 0.01, 95% CI: 0.00 to 0.01; p = 0.002) (Figure 9).
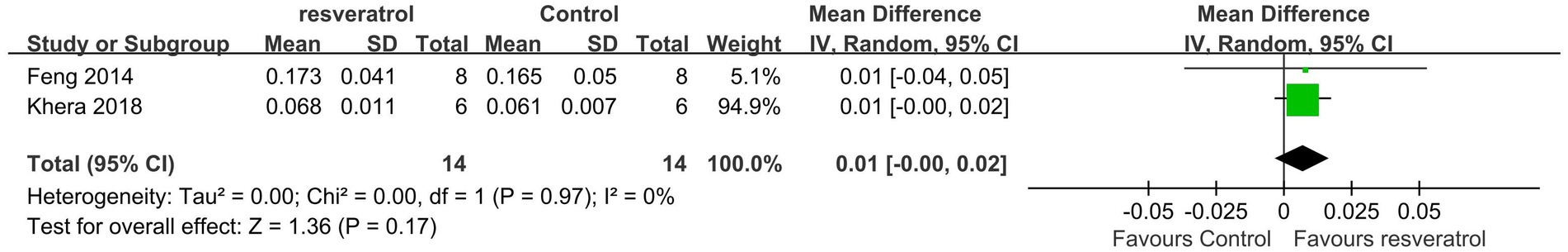
3.4.3.2. Resveratrol in 3-month-old rats
A total of 2 studies were included in the subgroup analysis (22, 25). Meta-analysis results showed that compared with the control treatment, resveratrol treatment had no statistically significant effect on improving total-body BMD (p = 0.17) (Figure 10).
3.5. Publication bias
We plotted corresponding funnel plots for all outcome indicators to evaluate publication bias. The funnel plot results show that the funnel plots of BMD of the total body, Tb. Th, serum phosphorus, and serum osteocalcin are asymmetric, indicating that there may be publication bias in these outcome indicators. The funnel plot of all outcome indicators is shown in Supplementary material 5.
4. Discussion
OP is known as the silent killer, and osteoporotic fractures are a serious complication of OP, which means that the prevention and treatment of OP are important aspects to which public health needs to pay attention. Ethnic medicine or botanical medicine has always been the focus of drug conversion. In recent years, the therapeutic effect of resveratrol on OP has received considerable attention, but research on its anti-OP efficacy or mechanism is mostly limited to animal or cell experiments, which seriously limits the progress of resveratrol in clinical application. To further clarify the anti-OP efficacy of resveratrol, this study summarizes preclinical evidence to provide support to proceed with clinical trials. This meta-analysis found that resveratrol can significantly increase FBMD and LBMD in OP rats, and this conclusion remained consistent at concentrations <10, 10–25, 40–50, and ≥ 60 mg/kg/day. In the improvement of BMD of the total body, resveratrol (<10 mg/kg/day) showed better efficacy than the control group. In terms of improving the parameters related to micro-CT, resveratrol can increase Tb. Th and Tb. N and reduce Tb.Sp. The concentration of resveratrol at 40–50 mg/kg/day can all improve these three bone microstructure indicators. In addition, resveratrol can reduce the concentration of calcium and phosphorus in serum but has no significant effect on serum ALP and osteocalcin, which was also verified in this meta-analysis. Based on preclinical animal research data, we found that resveratrol may have enormous clinical application potential in the treatment of OP, which means that resveratrol may become a candidate drug for OP treatment, but this still needs to be verified through large-scale clinical studies in the future.
Resveratrol has the characteristics of multiple targets, low cost, and low toxicity (36), and its therapeutic effect in OP is receiving increasing attention. The dynamic balance between osteoblasts and osteoclasts has always been considered the core content of OP research. An experimental study found that resveratrol can activate the osteogenic transcription factor CBFA-1 (37) and enhance the transcription of bone-specific type I collagen in a CBFA-1-dependent manner, stimulate the proliferation and differentiation of osteoblasts, and activate Sirt-1 to transform osteoblasts into osteoblasts. Research shows that resveratrol can upregulate the expression level of Sirt-1 and then upregulate the expression of FoxO1 protein to inhibit the differentiation of osteoclasts (38). The occurrence of oxidative stress can cause damage to bone cells and osteoblasts (39, 40) and lead to bone resorption activity exceeding bone formation. Resveratrol is a natural antioxidant and can effectively prevent bone loss caused by oxidative stress in the body (15), which may be the potential mechanism of its anti-OP effect. In addition, resveratrol can bind to oestrogen receptors and exert oestrogenic effects (32), thus compensating for bone loss caused by oestrogen deficiency. In addition, this meta-analysis showed that resveratrol achieved better efficacy in improving biochemical markers. Serum biochemical indicators reflect the essence of bone metabolism and the direct reflection of bone formation and bone resorption. This meta-analysis found that resveratrol has a better effect than the control treatment in reducing serum calcium concentration, which may be because resveratrol inhibits oxidative stress and reduces bone loss, thereby reducing the content of calcium entering the serum. Oxidative stress may lead to oxidative damage to bone cells and osteoblasts in the bone microenvironment, leading to imbalanced bone remodelling. The antioxidant effect of resveratrol can maintain bone homeostasis, thus stabilizing bone microstructure. Based on the undeniable regulatory role of resveratrol in bone metabolism, its clinical application in OP deserves in-depth attention.
5. Limitations
This study has limitations that should be considered when interpreting the results. First, the animal models included in the study may exhibit significant differences in factors such as species of rats, drug dosage, and sample size, which may lead to heterogeneity in the experiment and compromise the reliability of the conclusions of this study. Second, the included animal experiment reports focus on the construction of animal models and outcome evaluation, but the report on experimental design, implementation, and measurement methods is relatively brief, which may lead to poor methodological quality in literature reports, difficulty in estimating potential bias risks, and reduced data credibility. Third, there may be differences in the BMD measurement tools and serum markers used in all 15 included studies, which may lead to measurement errors between studies. Given the limitations of animal experimental design, future clinical studies targeting the treatment of OP with resveratrol should avoid these situations, which would be beneficial for improving the reliability of evidence-based data on this research topic.
6. Conclusion
This study found that resveratrol can increase BMD in OP rat models, and its mechanism of action may be closely related to improving bone microstructure and regulating calcium and phosphorus metabolism. Given that this study focuses on an OP rat model, the efficacy of resveratrol in treating OP still needs to be further validated through clinical studies in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JZ: conceptualization, validation, data curation, writing – original draft, writing – review and editing, visualization, supervision, and project administration. GZ and JY: writing – original draft, and writing – review and editing. JP: investigation, data curation, and formal analysis. BS and ML: investigation and data curation. WY: conceptualization, methodology, software, validation, formal analysis, and data curation. JL: conceptualization, supervision, validation, and funding acquisition. LZ: conceptualization, methodology, software, validation, formal analysis, data curation, writing – original draft, writing – review and editing, visualization, supervision, and funding acquisition. JZ and GZ: contributed equally to this work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82004383, 82004386), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515220131, 2022A1515010385, 2022A1515011700), the National key research and development program (2021YFC1712804), Research Fund for Bajian Talents of Guangdong Provincial Hospital of Chinese Medicine (No. BJ2022KY01), Project of Philosophy and Social Science Planning of Guangzhou (No. 2022GZQN42), Foreign Teacher Programs of Guangdong Provincial Department of Science and Technology (YKZZ [2022] No. 232) and Administration of Traditional Chinese Medicine of Guangdong Province (No. 20231109, 20225025).
Acknowledgments
The authors greatly thank the editors and reviewers for their feedback and comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1234756/full#supplementary-material
Abbreviations
OP, osteoporosis; CNKI, China National Knowledge Infrastructure; MD, mean difference; CI, confidence interval; BMD, bone mineral density; PMOP, postmenopausal osteoporosis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta analyses; NR, not reported; Tb. Th, trabecular thickness; Tb. N, trabecular number; Tb. SP, trabecular spacing; ALP, alkaline phosphatase; SYRCLE, Systematic Review Center for Laboratory Animal Experience; OVX, ovariectomy; SD, Sprague–Dawley.
References
1. Sabri, SA, Chavarria, JC, Ackert-Bicknell, C, Swanson, C, and Burger, E. Osteoporosis: an update on screening, diagnosis, evaluation, and treatment. Orthopedics. (2023) 46:e20–6. doi: 10.3928/01477447-20220719-03
2. Li, N, Cornelissen, D, Silverman, S, Pinto, D, Si, L, Kremer, I, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. PharmacoEconomics. (2020) 39:181–209. doi: 10.1007/s40273-020-00965-9
3. Yu, G, Tong, S, Liu, J, Wan, Y, Wan, M, Li, S, et al. A systematic review of cost-effectiveness analyses of sequential treatment for osteoporosis. Osteoporos Int. (2023) 34:641–58. doi: 10.1007/s00198-022-06626-1
4. Aibar-Almazán, A, Voltes-Martínez, A, Castellote-Caballero, Y, Afanador-Restrepo, DF, Carcelén-Fraile, MDC, and López-Ruiz, E. Current status of the diagnosis and management of osteoporosis. Int J Mol Sci. (2022) 23:9465. doi: 10.3390/ijms23169465
5. Arceo-Mendoza, RM, and Camacho, PM. Postmenopausal osteoporosis. Endocrinol Metab Clin N Am. (2021) 50:167–78. doi: 10.1016/j.ecl.2021.03.009
6. Patel, D, and Wairkar, S. Bone regeneration in osteoporosis: opportunities and challenges. Drug Deliv Transl Res. (2023) 13:419–32. doi: 10.1007/s13346-022-01222-6
7. Jiang, Y, Zhang, P, Zhang, X, Lv, L, and Zhou, Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif. (2021) 54:e12956. doi: 10.1111/cpr.12956
8. Xiao, PL, Cui, AY, Hsu, CJ, Peng, R, Jiang, N, Xu, XH, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. (2022) 33:2137–53. doi: 10.1007/s00198-022-06454-3
9. Salari, N, Ghasemi, H, Mohammadi, L, Behzadi, MH, Rabieenia, E, Shohaimi, S, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. (2021) 16:609. doi: 10.1186/s13018-021-02772-0
10. Gennari, L, and Bilezikian, JP. New and developing pharmacotherapy for osteoporosis in men. Expert Opin Pharmacother. (2018) 19:253–64. doi: 10.1080/14656566.2018.1428559
11. Fontalis, A, Kenanidis, E, Kotronias, RA, Papachristou, A, Anagnostis, P, Potoupnis, M, et al. Current and emerging osteoporosis pharmacotherapy for women: state of the art therapies for preventing bone loss. Expert Opin Pharmacother. (2019) 20:1123–34. doi: 10.1080/14656566.2019.1594772
12. El-Ghazaly, MA, Fadel, NA, Abdel-Naby, DH, Abd, EH, Zaki, HF, and Kenawy, SA. Amelioration of adjuvant-induced arthritis by exposure to low dose gamma radiation and resveratrol administration in rats. Int J Radiat Biol. (2020) 96:857–67. doi: 10.1080/09553002.2020.1748911
13. Liu, Y, You, Y, Lu, J, Chen, X, and Yang, Z. Recent advances in synthesis, bioactivity, and pharmacokinetics of pterostilbene, an important analog of resveratrol. Molecules. (2020) 25:5166. doi: 10.3390/molecules25215166
14. Jiang, Y, Luo, W, Wang, B, Wang, X, Gong, P, and Xiong, Y. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. (2020) 246:117422. doi: 10.1016/j.lfs.2020.117422
15. Mizutani, K, Ikeda, K, Kawai, Y, and Yamori, Y. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. (1998) 253:859–63. doi: 10.1006/bbrc.1998.9870
16. Wong, RH, Thaung Zaw, JJ, Xian, CJ, and Howe, PR. Regular supplementation with resveratrol improves bone mineral density in postmenopausal women: a randomized, placebo-controlled trial. J Bone Miner Res. (2020) 35:2121–31. doi: 10.1002/jbmr.4115
17. Bo, S, Gambino, R, Ponzo, V, Cioffi, I, Goitre, I, Evangelista, A, et al. Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutr Diabetes. (2018) 8:10–51. doi: 10.1038/s41387-018-0059-4
18. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. (2021) 18:e1003583. doi: 10.1371/journal.pmed.1003583
19. Hooijmans, CR, Rovers, MM, de Vries, RB, Leenaars, M, Ritskes-Hoitinga, M, and Langendam, MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
20. Wever, KE, Hooijmans, CR, Riksen, NP, Sterenborg, TB, Sena, ES, Ritskes-Hoitinga, M, et al. Determinants of the efficacy of cardiac ischemic preconditioning: a systematic review and meta-analysis of animal studies. PLoS One. (2015) 10:e0142021. doi: 10.1371/journal.pone.0142021
21. Elseweidy, MM, El-Swefy, SE, Shaheen, MA, Baraka, NM, and Hammad, SK. Effect of resveratrol and mesenchymal stem cell monotherapy and combined treatment in management of osteoporosis in ovariectomized rats: role of SIRT1/FOXO3a and Wnt/β-catenin pathways. Arch Biochem Biophys. (2021) 703:108856. doi: 10.1016/j.abb.2021.108856
22. Feng, J, Liu, S, Ma, S, Zhao, J, Zhang, W, Qi, W, et al. Protective effects of resveratrol on postmenopausal osteoporosis: regulation of SIRT1-NF-κB signaling pathway. Acta Biochim Biophys Sin Shanghai. (2014) 46:1024–33. doi: 10.1093/abbs/gmu103
23. Feng, YL, Jiang, XT, Ma, FF, Han, J, and Tang, XL. Resveratrol prevents osteoporosis by upregulating FoxO1 transcriptional activity. Int J Mol Med. (2018) 41:202–12. doi: 10.3892/ijmm.2017.3208
24. Guo, DW, Han, YX, Cong, L, Liang, D, and Tu, GJ. Resveratrol prevents osteoporosis in ovariectomized rats by regulating microRNA-338-3p. Mol Med Rep. (2015) 12:2098–106. doi: 10.3892/mmr.2015.3581
25. Khera, A, Kanta, P, Kalra, J, Dumir, D, and M, T. Resveratrol restores the level of key inflammatory cytokines and RANKL/OPG ratio in the femur of rat osteoporosis model. J Women Aging. (2019) 31:540–52. doi: 10.1080/08952841.2018.1522126
26. Li, C. The research of resveratrol to prevent osteoporosis in ovariectomized rats by adjusting the activity of antioxidant enzymes. Kunming, China: Kunming Medical University (2016).
27. Lin, Q, Huang, Y, Xiao, B, and Ren, G. Effects of resveratrol on bone mineral density in ovarectomized rats. Int J Biomed Sci. (2005) 1:76–81.
28. Liu, ZP, Li, WX, Yu, B, Huang, J, Sun, J, Huo, JS, et al. Effects of trans-resveratrol from polygonum cuspidatum on bone loss using the ovariectomized rat model. J Med Food. (2005) 8:14–9. doi: 10.1089/jmf.2005.8.14
29. Wang, Y. Effects of resveratrol on osteoprotegerin and osteoprotegerin ligand expression of femurs in ovariectomized rats. Lanzhou, China: Lanzhou University (2006).
30. You, L, Si, H, Gao, Y, Yang, F, and Chen, K. Effect of resveratrol on the development of osteoporosis in ovariectomized rats. Chin J Osteoporos. (2021) 27:640. doi: 10.3969/j.issn.1006-7108.2021.05.001
31. Zhang, T, Zhang, W, Bian, L, Liu, S, Yan, J, Zong, Y, et al. Protective effects and mechanisms of resveratrol on the rats suffering with osteoporosis. Acta Anat Sin. (2012) 43:679–84. doi: 10.3969/j.issn.0529-1356.2012.05.018
32. Zhang, Y, Liu, M, He, Y, Deng, N, Chen, Y, Huang, J, et al. Protective effect of resveratrol on estrogen deficiency-induced osteoporosis though attenuating NADPH oxidase 4/nuclear factor kappa B pathway by increasing miR-92b-3p expression. Int J Immunopathol Pharmacol. (2020) 34:1120272288. doi: 10.1177/2058738420941762
33. Zhang, H, Huang, Y, Li, C, and Huang, Y. Effects of estradiol combined with resveratrol on bone mineral density and biomechanics in ovariectomized osteoporosis rats. J Clin Exp Med. (2022) 21:1909–12. doi: 10.3969/j.issn.1671-4695.2022.18.002
34. Zhao, H, Li, X, Li, N, Liu, T, Liu, J, Li, Z, et al. Long-term resveratrol treatment prevents ovariectomy-induced osteopenia in rats without hyperplastic effects on the uterus. Br J Nutr. (2014) 111:836–46. doi: 10.1017/S0007114513003115
35. Zhou, Q, Li, S, Shu, L, Liu, S, and Zhang, J. Role of resveratrol in promoting cell proliferation of rats with ovariectomized osteoporosis. J Clin Med Pract. (2020) 24:86–9. doi: 10.7619/jcmp.202019025
36. Mobasheri, A, and Shakibaei, M. Osteogenic effects of resveratrolin vitro: potential for the prevention and treatment of osteoporosis. Ann N Y Acad Sci. (2013) 1290:59–66. doi: 10.1111/nyas.12145
37. Shakibaei, M, Buhrmann, C, and Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. (2011) 286:11492–505. doi: 10.1074/jbc.M110.198713
38. Yirmiya, R, Goshen, I, Bajayo, A, Kreisel, T, Feldman, S, Tam, J, et al. Depression induces bone loss through stimulation of the sympathetic nervous system. PNAS. (2006) 103:16876–81. doi: 10.1073/pnas.0604234103
39. Kimball, JS, Johnson, JP, and Carlson, DA. Oxidative stress and osteoporosis. J Bone Joint Surg. (2021) 103:1451–61. doi: 10.2106/JBJS.20.00989
Keywords: resveratrol, plant-based natural products, osteoporosis, bone mineral density, meta-analysis, evidence-based medicine
Citation: Zhao J, Zhou G, Yang J, Pan J, Sha B, Luo M, Yang W, Liu J and Zeng L (2023) Effects of resveratrol in an animal model of osteoporosis: a meta-analysis of preclinical evidence. Front. Nutr. 10:1234756. doi: 10.3389/fnut.2023.1234756
Edited by:
Ning Zhang, The Chinese University of Hong Kong, ChinaReviewed by:
Simon Chow, Stanford University, United StatesMeng Chen Michelle Li, The Chinese University of Hong Kong, China
Copyright © 2023 Zhao, Zhou, Yang, Pan, Sha, Luo, Yang, Liu and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, Z3p1Y21saXVqdW5AZm94bWFpbC5jb20=; Lingfeng Zeng, bGluZ2Zlbmd6ZW5nQGd6dWNtLmVkdS5jbg==
†ORCID: Jinlong Zhao https://orcid.org/0000-0001-7079-1336
Minghui Luo https://orcid.org/0000-0001-6831-6317
Weiyi Yang https://orcid.org/0000-0001-8657-2269
Jun Liu https://orcid.org/0000-0002-1943-3880
Lingfeng Zeng https://orcid.org/0000-0003-1311-8641
 Jinlong Zhao1,2,3†
Jinlong Zhao1,2,3† Guanghui Zhou
Guanghui Zhou Junzheng Yang
Junzheng Yang Minghui Luo
Minghui Luo Jun Liu
Jun Liu