- 1Department of Infectious Diseases, Department of Clinical Nutrition, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
- 2Department of Ultrasound, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
- 3Department of Clinical Nutrition, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
Background: Sarcopenia and sarcopenic obesity are associated with an increased possibility of adverse clinical outcomes; however, the effects of sarcopenia and sarcopenic obesity on patients with primary liver cancer remain controversial. Therefore, the present study aimed to determine the impact of sarcopenia and sarcopenic obesity on survival in patients with primary liver cancer.
Methods: We searched studies published in English in PubMed, Embase, Web of Science, and Cochrane Library databases up to 13 November 2022. Cohort studies that reported the association among sarcopenia, sarcopenic obesity, and patient survival were included.
Results: A total of 64 cohort studies with data on 11,970 patients with primary liver cancer were included in the meta-analysis. Sarcopenia was associated with poor overall survival in patients with primary liver cancer [adjusted hazard ratio (HR) 2.11, 95% confidence interval (CI): 1.89–2.36, P < 0.0001], with similar findings for sarcopenic obesity (adjusted HR: 2.87, 95% CI: 2.23–3.70, P < 0.0001). Sarcopenia was also associated with poor overall survival across the subgroups analyzed by ethnicity, type of liver cancer, treatment modalities, method used to define sarcopenia, and etiology of liver cancer. We also found a negative correlation among sarcopenia, sarcopenic obesity, and recurrence-free/disease-free survival (adjusted HR: 1.73, 95% CI: 1.50–1.99, P < 0.001; adjusted HR: 2.28, 95% CI: 1.54–3.35, P < 0.001, respectively).
Conclusion: Sarcopenia and sarcopenic obesity were significantly associated with poor overall survival and recurrence-free/disease-free survival in patients with primary liver cancer.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=378433, PROSPERO [42022378433].
1. Introduction
Primary liver cancer is the sixth most frequently occurring cancer and ranks as the third leading cause of cancer-related mortality worldwide, accounting for 8.3% of total cancer deaths (1), thus resulting in a global medical and economic burden. Hepatocellular carcinoma (HCC) is the predominant type of primary liver cancer, comprising 75%−85% of the cases, and intrahepatic cholangiocarcinoma (ICC) follows as the subsequent type (1). There are significant gender and racial differences in morbidity and mortality due to primary liver cancer; both morbidity and mortality rates are two-fold to three-fold higher in men than in women in most regions, and the disease is more common among Asians due to a high prevalence of hepatitis B and C (1). It is therefore critical to identify patients with high mortality risk based on the patient's prognosis for determining individualized treatments and improving the survival rate of patients with primary liver cancer. In recent years, researchers have made several efforts to determine the factors that influence the clinical outcomes of patients with liver cancer. Thus far, the Barcelona Clinic Liver Cancer (BCLC), Model for End-Stage Liver Disease (MELD), and the albumin–bilirubin (ALBI) scores have been used clinically to evaluate the prognosis of patients with liver cancer; however, these prognostic tools cannot adequately capture the nutritional and functional status of these patients.
Sarcopenia, as a marker of malnutrition, has been defined by the European Working Group on Sarcopenia in Older People (EWGSOP2) in 2018 as the presence of both low muscle mass and low muscle function (strength or performance) (2). It is difficult to diagnose sarcopenia because of different measuring methods and cutoff values. Sarcopenia is usually evaluated based on grip strength, dual-energy X-ray absorptiometry, computed tomography (CT), and magnetic resonance imaging (MRI) (2). Sarcopenia increases the risk of worse clinical outcomes such as reduced quality of life, development of complications, higher hospitalization cost, and death (3–6). Previous studies have shown that the hospitalization cost of older patients with sarcopenia on admission was five-fold more than those without sarcopenia (7). Sarcopenia is a common condition in patients with oncological and chronic diseases. In a systematic review and meta-analysis that included 38 studies on sarcopenia and solid cancer outcomes, sarcopenia was significantly associated with the poor overall survival of patients (8). Similarly, according to a recent umbrella review of meta-analyses, sarcopenia is associated with adverse clinical outcomes across 12 cancer types: gastric, hepatocellular, urothelial, head and neck, hematologic malignancy, pancreatic, breast, colorectal, lung, esophageal, hematologic malignancies, and ovarian (9). The existence of sarcopenia was found to be associated with a higher risk of death in patients with liver cirrhosis (10), which is likely to progress into liver cancer.
The rate of fat deposition tends to increase in sarcopenic patients, resulting in systemic inflammatory activation and insulin resistance, which subsequently leads to progressive muscle reduction and fat accumulation, especially in conditions such as aging and cachexia (11). This vicious cycle finally results in sarcopenic obesity (SO), which is defined as the co-existence of obesity and sarcopenia (12). More recently, SO has received increasing interest from oncologists because of its adverse outcomes in patients with cancer. SO is an independent prognostic factor affecting the risk of adverse outcomes in oncological patients (13–15).
Several studies, however, reported no association between sarcopenia and prognosis in patients with HCC (16–20). The influence of sarcopenia on survival in patients with liver cancer remains controversial, and a comprehensive analysis based on evidence-based medicine is required. To the best of our knowledge, previous meta-analyses have focused only on HCC patients and did not include data on patients with ICC. Recently, an increasing number of studies have examined the prognostic factors of liver cancer patients. Hence, we analyzed and summarized the relationship among sarcopenia, SO, and survival in patients with primary liver cancer. Our study aimed to determine the impact of sarcopenia and SO on the survival of patients with primary liver cancer.
2. Methods
2.1. Search strategy
We searched studies relevant to the association of sarcopenia, SO, and survival of patients with liver cancer in PubMed, Embase, Web of Science, and Cochrane Library databases up to 13 November 2022. The search keywords included sarcopenia, muscle depletion, muscle weakness, liver cancer, and liver neoplasm. The detailed search strategies are presented in Supplementary Table S1. We restricted the studies to those published in English and conducted on humans. We also retrieved potential studies by reading through the relevant systematic reviews and meta-analyses. The present meta-analysis adhered to the PRISMA guidelines (21), and its protocol was registered on PROSPERO (CRD 42022378433).
2.2. Criteria for selection
Studies that met the following criteria were included: (1) participants: patients with liver cancer confirmed by clinical/imaging or liver biopsy criteria (may include patients evaluated or already listed for liver transplantation), including HCC and ICC; (2) exposures: pretreatment for sarcopenia and/or SO; (3) outcomes: the impact of sarcopenia and/or SO on patient survival; and (4) study design: prospective or retrospective cohort study.
Studies that met the following criteria were excluded: (1) studies lacking the criteria for diagnosing sarcopenia or SO; (2) studies lacking the statistical data on the impact of sarcopenia and/or SO on survival [hazard ratio (HR) and 95% confidence interval (CI)]; and (3) reviews, case reports, editorials, letters, posters, and/or conference abstracts.
The authors XL and XH independently screened the title/abstract of all the identified citations for eligibility by using the abovementioned inclusion/exclusion criteria. Next, they retrieved and rescreened the full texts of relevant articles. For studies with overlapping cohorts, studies having the latest data and/or a larger sample size and/or more data available for subgroup analysis were used. Disagreements were resolved by consensus or discussion.
2.3. Data acquisition and quality assessment
XL and XH extracted the following data independently from each included study: first author's name, first author's country, published year, ethnicity, study type, type of liver cancer, treatment modalities, etiology of liver cancer, enrolled numbers, patient demographics (including age and sex), duration of follow-up, definitions of sarcopenia and SO, cutoff values of sarcopenia, and number of sarcopenia or SO patients. The quality of the enrolled studies was independently evaluated by the two authors according to the Newcastle–Ottawa Scale (NOS). Discrepancies between both investigators were resolved by consensus and discussion.
2.4. Statistical analysis
The outcomes for the association between sarcopenia and overall survival (OS), recurrence-free survival (RFS), or disease-free survival (DFS) were reported as crude and adjusted HR with corresponding 95% CI values. HR and 95% CI values were directly extracted from univariate and multivariate Cox regression analyses. The impact of sarcopenia and SO on the OS of primary liver cancer patients was assessed by the pooled unadjusted HR or adjusted HR and 95% CI by using a random-effects model (DerSimonian–Laird method) (22). A subgroup analysis for the adjusted HRs was conducted according to ethnicity, type of liver cancer, treatment modalities, method used to define sarcopenia, and etiology of liver cancer. Cochran's Q statistic and I2 were used to evaluate heterogeneity, with a P-value of <0.1 and I2 > 50% considered to show meaningful heterogeneity (23). We assessed publication bias through the utilization of funnel plots in meta-analysis and quantified it using Egger's regression test. We also conducted a meta-analysis of single proportions to determine the prevalence of sarcopenia. All analyses were performed with STATA software v15.0 (StataCorp, College Station, TX, USA), and a P-value of <0.05 was considered to be statistically significant.
3. Results
3.1. Study search and characteristics
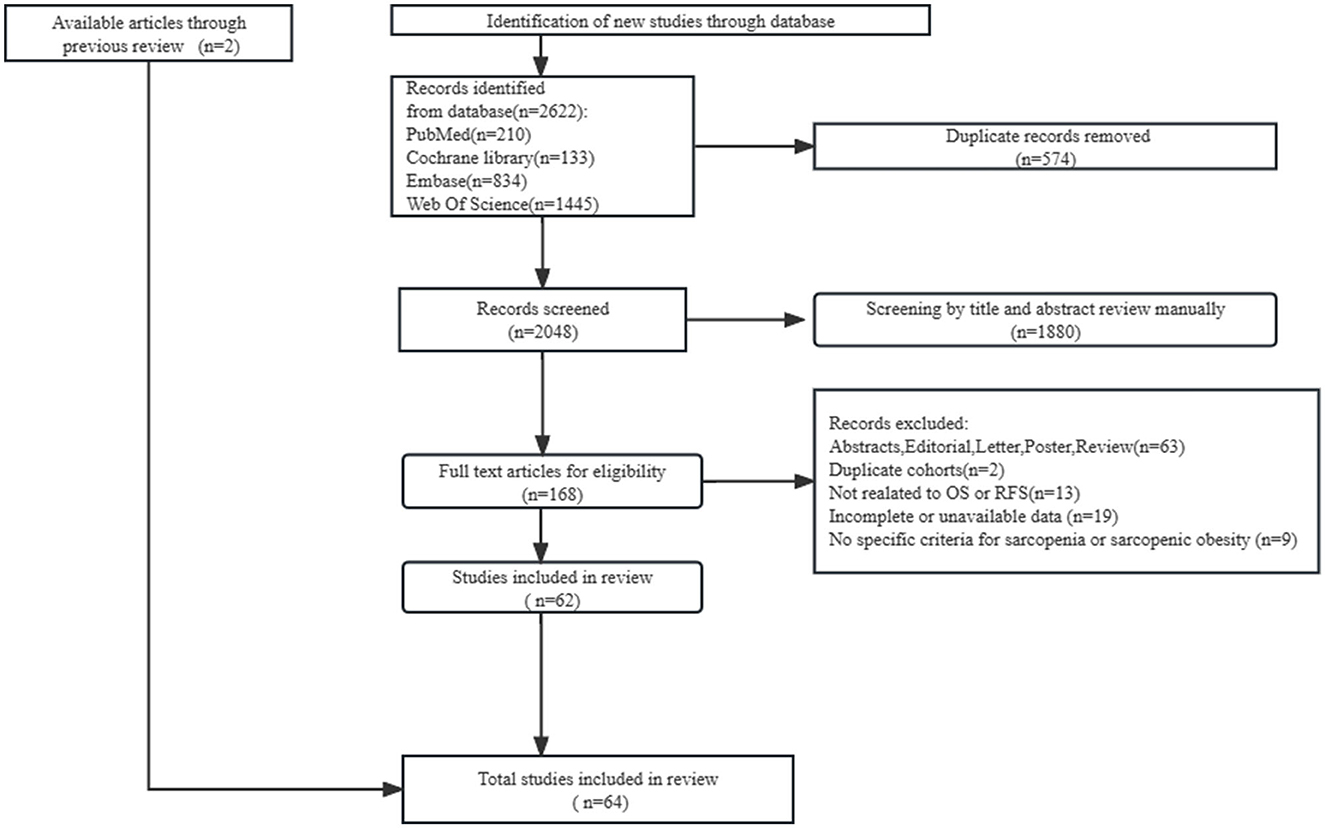
The search in PubMed, Embase, Web of Science, and Cochrane Library databases yielded 2,622 relevant citations, of which 574 duplicates and 1,880 unavailable titles/abstracts were excluded. After reviewing the full text of the remaining 168 publications and previous reviews, we included 64 eligible cohort studies with 11,970 patients (Figure 1).
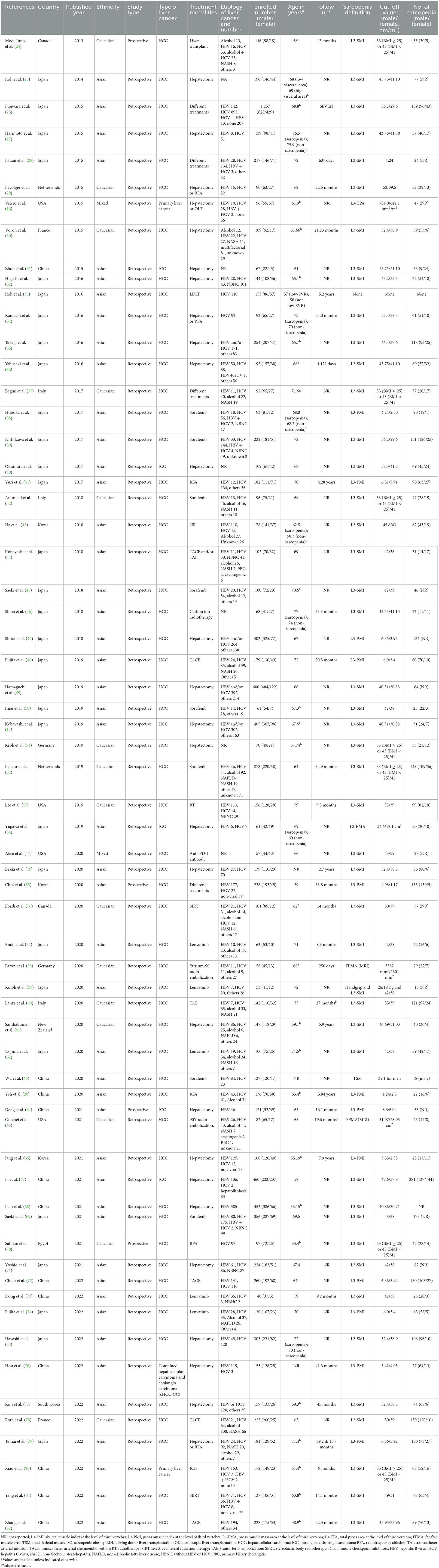
The demographics and characteristics of the included studies are shown in Table 1. In general, 47 of the 64 included studies were conducted in Asia, predominantly in Japan (n = 31), and 17 studies were from non-Asian regions. Four studies were prospective, and 60 studies were retrospective.
Of the 11,970 patients, 8,919 were men (74.51%) and 3,051 were women (25.49%), with a median (or mean) age of 51.4–77 years. The median or mean age was not reported in three studies (19, 63, 76). Fifty-six of the 64 studies involved patients with HCC. Seventeen studies enrolled patients with resectable HCC; the remaining treatment regimens included transarterial chemoembolization (TACE), liver transplantation (LT), radiofrequency ablation (RFA), and administration of kinase inhibitors. The curative treatment included liver resection, RFA, and LT, whereas the palliative treatment included intra-arterial chemoembolization, administration of kinase inhibitors, and systemic chemotherapy. Viral hepatitis was the most common etiology of liver cancer, with HCV as the primary cause of viral hepatitis in most included studies (n = 36). The other causes of liver cancer include alcohol consumption, non-alcoholic steatohepatitis (NASH), and others. Six studies did not report the etiology of liver cancer. Thirty of the 64 studies reported the duration of follow-up (median or mean), which ranged from 8.3 months to 7.9 years.
3.2. Quality assessment
The quality of the included studies was determined by NOS. Patient selection, comparability, and outcomes were used to evaluate the methodological quality of each study. Supplementary Table S2 shows the evaluation of the quality of the included studies. Based on the NOS score of ≥7, all the included studies were considered to have high quality.
3.3. Definition of sarcopenia and SO
The approaches to identifying sarcopenia and SO in patients were different. In most studies (n = 61), the areas of visceral/subcutaneous fat and skeletal muscle were determined by a transverse analysis at the level of the third lumbar vertebra (L3). In 45 studies, sarcopenia was defined by the sex-specific cutoff values of the L3-SMI (skeletal muscle index, cm2/m2), which showed a slight variation in those studies (shown in Table 1). Seven studies used different values to define sarcopenia depending on body mass index (BMI) (24, 37, 42, 51, 52, 70, 80). The majority of studies (44 studies) used cutoff values between 40 and 55 cm2/m2 in men. Nine studies evaluated sarcopenia based on the L3-PMI (psoas muscle index, cm2/m2), and one study used the total skeletal muscle mass (TSM) index. The other two studies defined sarcopenia as low fat-free muscle area (FFMA) based on MRI evaluation. According to these sex-specific cutoff values, 3,957 patients were diagnosed with sarcopenia in 62 studies, yielding a pooled prevalence of 43.2% (95% CI: 37.8%–48.5%). Among the 50 studies reporting the prevalence according to gender, a slight difference in prevalence was observed between female and male patients, with a higher pooled prevalence of 45% among men compared to 42.2% among women.
Four of the 64 studies reported the impact of SO on the survival of liver cancer patients. Regarding the definition of SO, Itoh et al. (33) used low skeletal muscle mass-to-visceral fat area ratio (SVR) to define SO, while Kobayashi et al. (14), Kroh et al. (51), and Liao et al. (68) used the co-existence of sarcopenia and obesity to define SO. However, variations were noted in the definition of obesity. Obesity was defined as the area of visceral adipose tissue at the level of the third lumbar vertebra ≥100 cm2 in both men and women by Kobayashi et al., while the patients were considered obese if their BMI was ≥25 kg/m2 in Liao's study; in Kroh's study, obesity was defined by categorizing individuals within the highest two quintiles body fat percentage for men and women.
3.4. OS in sarcopenic patients
Not all the eligible studies reported the HRs of OS. After calculating the data in a univariate analysis of 51 (n = 9,615) studies, we found that sarcopenia was related to lower OS, with a pooled unadjusted HR of 1.94 (95% CI: 1.76–2.13, P < 0.0001; shown in Supplementary Figure S1). A multivariate analysis of 47 studies (n = 8,285) revealed that the risk of death in sarcopenic patients was 2.11-fold higher than that in non-sarcopenic patients (95% CI: 1.89–2.36, P < 0.0001; shown in Figure 2). In most studies, HR was adjusted for age, gender, BMI, alpha-fetoprotein (AFP), tumor stage, and comorbidity. According to sensitivity analysis, no individual study had a significant impact on the pooled unadjusted HR and adjusted HR, thus indicating that the results were robust (Supplementary Figure S2). The test for heterogeneity showed a moderate result for both univariate and multivariate analyses (I2 = 44.5% and P < 0.1 for the unadjusted HRs; I2 = 41.4% and P < 0.1 for the adjusted HRs). The funnel plots were asymmetric in both univariate and multivariate analyses (Supplementary Figures S3, S4). Potential publication bias was significant for both unadjusted HRs (P = 0.000 < 0.05) and adjusted HRs (P = 0.000 < 0.05), according to Egger's test. Therefore, we used the trim-and-fill method by imputing the potential unpublished articles for unadjusted HRs and adjusted HRs to achieve symmetry in the funnel plot (Supplementary Figures S3, S4). The pooled unadjusted and adjusted HRs were 5.42 (95% CI: 4.60–6.50) and 5.251 (95% CI: 4.53–6.87), respectively. We further conducted a subgroup analysis on OS for the adjusted HRs as designed previously. Interestingly, the results showed that sarcopenia was consistently correlated with poor OS across all the analyzed subgroups. Sarcopenia (vs. non-sarcopenia) was associated with low OS in both Asian and non-Asian regions with summary adjusted HR of 2.10 (95% CI: 1.84–2.39) and 2.18 (95% CI: 1.78–2.66), respectively; in patients with HCC and ICC (summary adjusted HR: 2.07, 95% CI: 1.84–2.33; summary adjusted HR: 2.91, 95% CI: 2.15–3.94, respectively); in patients treated with curative and palliative therapies (pooled adjusted HR: 2.45, 95% CI: 2.01–3.00; pooled adjusted HR: 1.93, 95% CI: 171–2.18, respectively); in patients defined by L3-SMI, L3-PMI, and FFMA (MRI; pooled adjusted HR: 2.07, 95% CI: 1.82–2.35; pooled adjusted HR: 2.36, 95% CI: 1.68–3.31; pooled adjusted HR: 2.23, 95% CI: 1.36–3.65, respectively); and in patients with only HCV-related and other causes (pooled adjusted HR: 5.28, 95% CI: 2.14–13.04; pooled adjusted HR: 2.06, 95% CI: 1.85–2.29, respectively; shown in Supplementary Table S3).
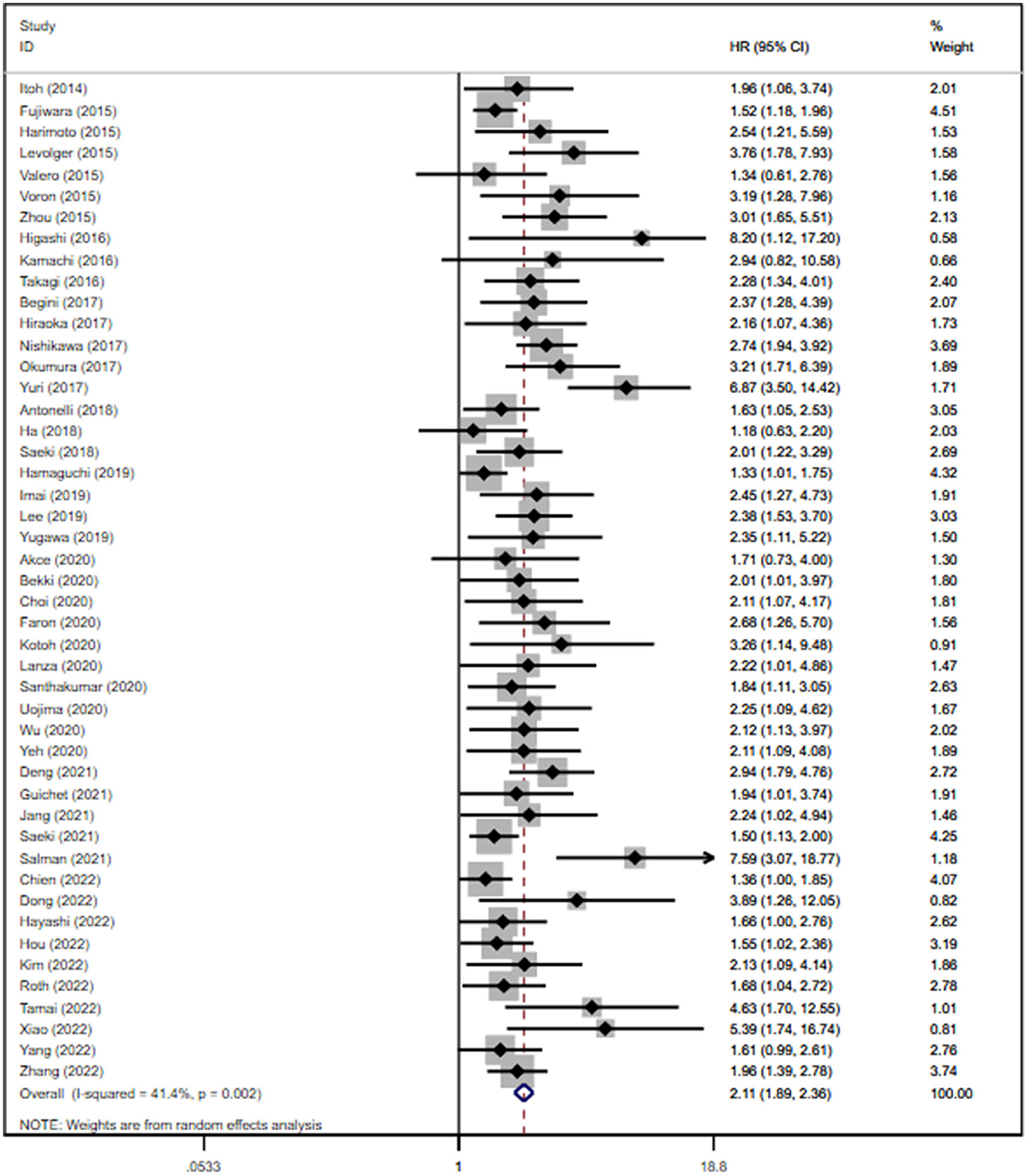
Figure 2. Forest plot of the pooled adjusted hazard ratios for the association between sarcopenia and overall survival in patients with primary liver cancer.
3.5. OS in patients with SO
Only four studies reported statistical data regarding the influence of SO on patient survival (14, 33, 51, 68). The prevalence of SO varied greatly among the studies, ranging from 6.67% to 30.00% (characteristics shown in Table 1). We conducted respective analyses of studies reporting unadjusted and adjusted HRs for OS. Patients with SO had a higher risk of death than those without SO (pooled unadjusted HR: 2.08. 95% CI 1.67–2.60, P < 0.0001; pooled adjusted HR: 2.87; 95% CI: 2.23–3.70, P < 0.0001), as shown in Supplementary Figure S5 and Figure 3. The subgroup analysis was not conducted because of the limited number of studies reporting SO data.
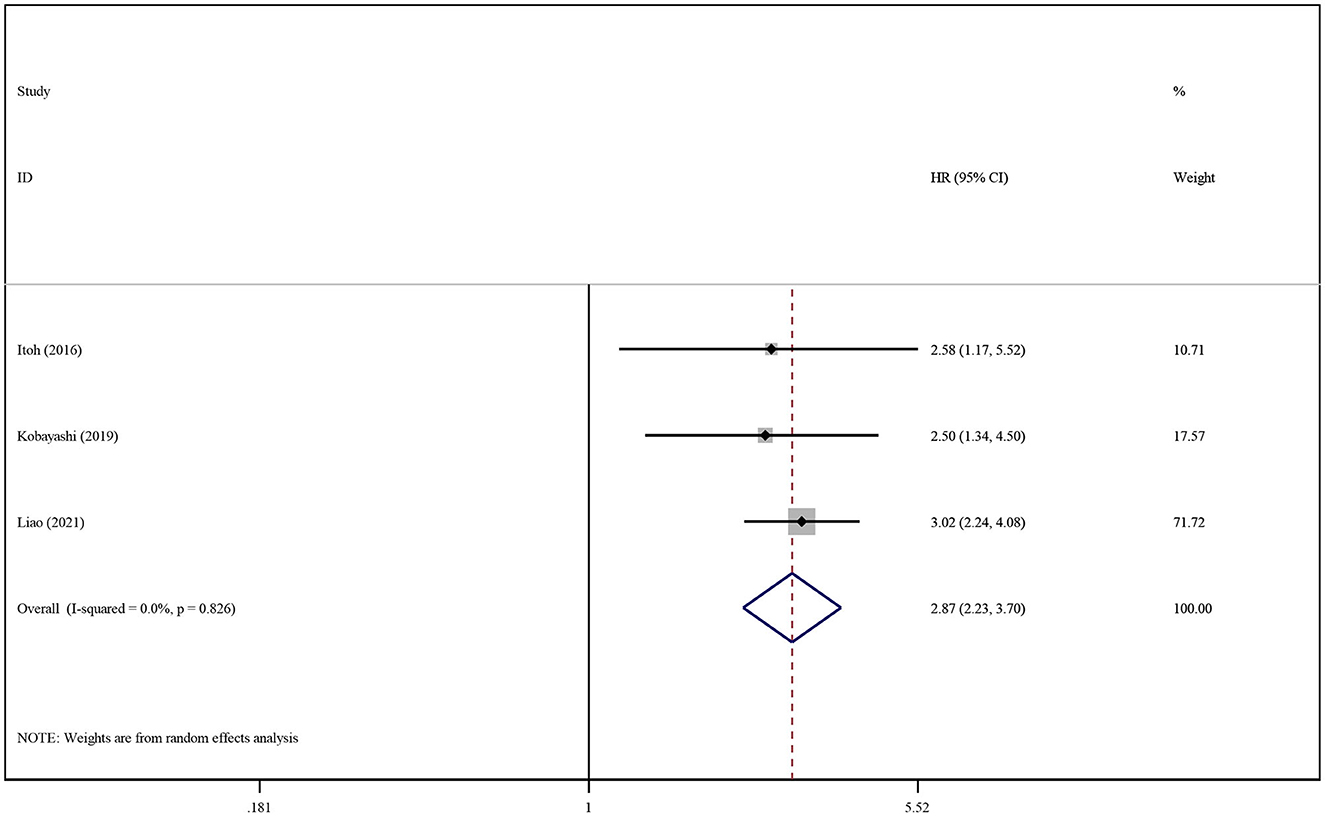
Figure 3. Forest plot of the pooled adjusted hazard ratios for the association between sarcopenic obesity and overall survival in patients with primary liver cancer.
3.6. RFS/DFS of the study patients
We conducted separate analyses of studies reporting unadjusted and adjusted HRs for RFS/DFS. A univariate analysis of 16 studies showed poor RFS/DFS in patients with sarcopenia (HR: 1.74, 95% CI: 1.50–2.02, P < 0.0001; Supplementary Figure S6), while a multivariate analysis of 11 studies showed a similar result (HR: 1.73, 95% CI: 1.50–1.99, P < 0.0001; Figure 4).
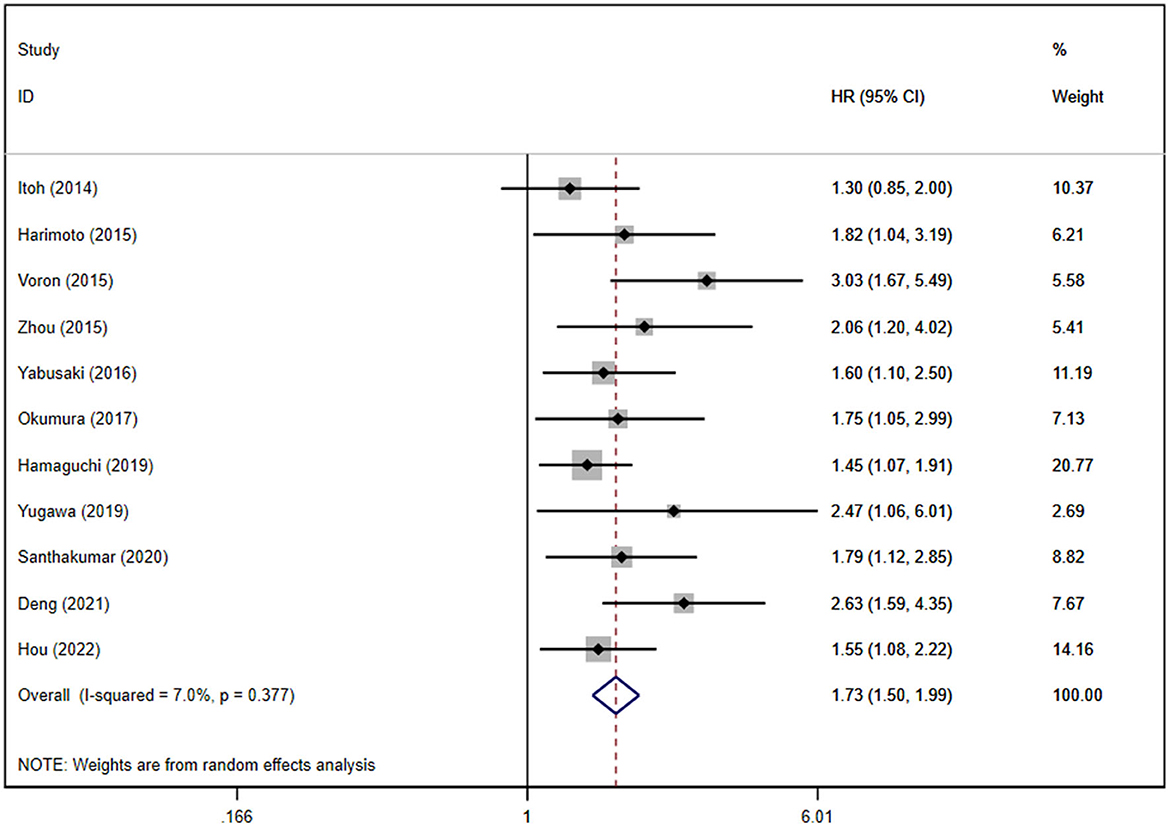
Figure 4. Forest plot of the pooled adjusted hazard ratios for the association between sarcopenia and recurrence-free survival or disease-free survival in patients with primary liver cancer.
Moderate heterogeneity was observed in univariate analysis, while mild heterogeneity was noted in multivariate analysis (P = 0.027 and I2 = 44.8% for the unadjusted HRs; P = 0.377 and I2 = 7.0% for the adjusted HRs). Publication bias was not found in the univariate analysis (Egger's test, P = 0.587), whereas the multivariate analysis showed the existence of publication bias (Supplementary Figure S7). We further conducted a subgroup analysis on RFS/DFS for the adjusted HRs according to ethnicity, type of liver cancer, and sarcopenia definitions. However, we did not perform a subgroup analysis on RFS/DFS for the adjusted HRs based on treatment modalities because all subjects were treated by hepatectomy. The results are shown in Supplementary Table S4.
The association between SO existence and its influence on RFS/DFS in HCC was analyzed in the same manner. The summary of crude and adjusted HRs were 1.80 (95% CI: 1.33–2.44, P < 0.001; Supplementary Figure S8) and 2.28 (95% CI: 1.54–3.35, P < 0.001; Figure 5), respectively; this indicated that SO was associated with a lower RFS/DFS.
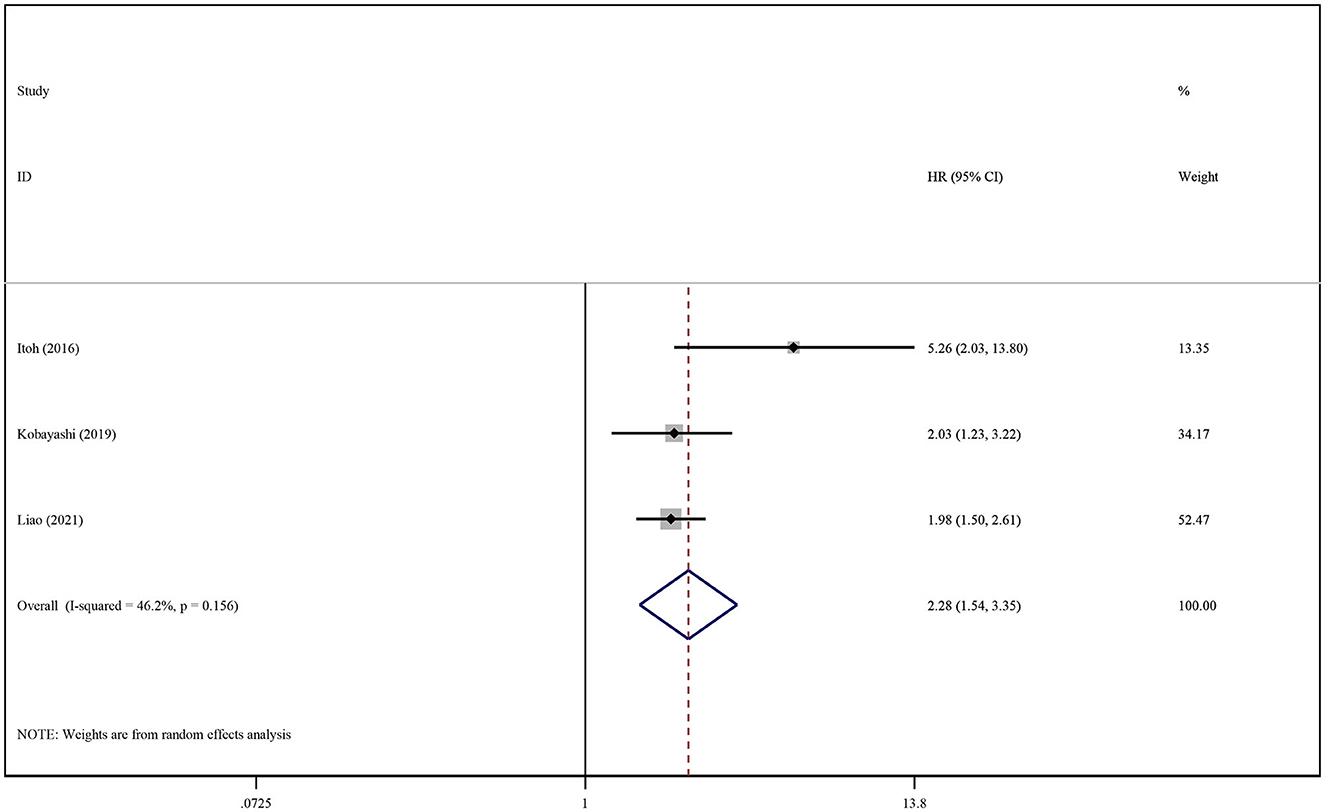
Figure 5. Forest plot of the pooled adjusted hazard ratios for the association between sarcopenic obesity and recurrence-free survival or disease-free survival in patients with primary liver cancer.
4. Discussion
In the present meta-analysis, we analyzed 64 studies comprising 11,970 participants diagnosed with primary liver cancer. Sarcopenia is a common disorder in this population, with a pooled prevalence of 43.2%, and it is more prevalent in men. The results revealed a robust association between sarcopenia and/or SO and patient survival. A strong relationship between sarcopenia and adverse clinical outcomes in cancer patients has been reported in prior studies, including depression (83), risk of fall (84), higher risk of complications (85), and cancer recurrence and mortality (9, 13, 15, 86). In 2022, a meta-analysis comprising 280 publications involving 81,814 patients with solid tumors demonstrated that sarcopenia is a prevalent condition in oncological patients with a prevalence of 35.3%; moreover, it is particularly higher in patients with specific cancer types such as esophageal cancer, urothelial cancer, cholangiocarcinoma, prostate cancer, and thyroid cancer (87). A recent study reported that SO affected 20% of cancer patients, demonstrating a significant association with various poor outcomes in cancer patients, such as low OS, RFS, and longer length of hospital stay, particularly in patients with oropharyngeal cancer and liver cancer (88). A previous systematic review of three articles involving 1,515 liver transplant recipients demonstrated a two-fold increase in mortality rates linked to pre-transplant SO (89).
The underlying mechanisms of sarcopenia and SO are poorly understood. According to prior studies, sarcopenia and SO are multifactorial conditions. The key mechanisms of sarcopenia include aging, inflammation, hormonal changes, inactivity, and low-protein intake. The available evidence indicates that the elderly population, especially individuals aged 65 years and above, is susceptible to anabolic resistance due to decreased availability of post-prandial amino acid, diminished muscle perfusion, and decreased digestive ability caused by the sequestration of amino acids in the splanchnic region (90). Body fat increases with age until 70 years. This accumulation of body fat activates macrophages, mast cells, and T lymphocytes, resulting in the secretion of inflammatory factors such as tumor necrosis factor (TNF), leptin, IL-6, and growth hormone (GH), which induces an array of inflammatory responses (91). Inflammatory factors such as TNF-α and IL-6 facilitate skeletal muscle wasting; the former directly catabolizes skeletal muscle, leading to increased gluconeogenesis, proteolysis, and upregulation of uncoupling proteins (UCPs) 2 and 3 in cachectic skeletal muscle, while the latter suppresses protein synthesis in muscle cells by the Janus kinase signaling pathway (92). Testosterone not only modulates inflammation in skeletal muscle by activating satellite cells to promote muscle regeneration but also increases the utilization of amino acids and androgen receptor expression in skeletal muscle to promote muscle protein synthesis; however, the levels of testosterone decline with age, which is likely to have a negative effect on muscle mass (93). Inactivity can affect muscle metabolism, further exacerbating the catabolic response and decreasing muscle protein synthesis (11). Prior studies have shown that exercise can improve muscle strength and mass, and both resistance training and aerobic exercise are beneficial to sarcopenia (94, 95). A previous review elaborated on the mechanisms of SO, which included lipotoxicity, adipose tissue inflammation, adipose tissue dysfunction, insulin resistance, and systemic chronic sterile low-grade inflammation. The authors proposed intricate interactions between adipose tissue and skeletal muscle, leading to the establishment of a detrimental vicious circle as individual's age, resulting in chronic low-grade local inflammation and systemic inflammation (91). Currently, there is a lack of specific medicines to treat SO. Lifestyle intervention is the most important method to treat SO, including calorie restriction; aerobic exercise; resistance exercise; and supplementation of protein, calcium, and vitamin D (93). Exercise intervention can positively change body composition and improve body weight, BMI, fat mass, body fat percentage, grip strength, and walking speed in the SO population; nutritional intervention can decrease fat mass with no improvement in grip strength (96).
To the best of our knowledge, this is the first meta-analysis to demonstrate a significant relationship between sarcopenia, SO, and survival in patients with primary liver cancer in a large sample. The study included patients who underwent either curative or palliative treatment. A strength of this meta-analysis is that the study population included patients with HCC and ICC for the first time. Although a prior meta-analysis of studies published before 2017 examined the association between sarcopenia and mortality in HCC patients, one of the studies included not only patients with HCC but also metastatic liver cancer (97). Another meta-analysis included cohorts that overlapped (98), while one study focused only on the prognosis of sarcopenia in HCC patients treated with sorafenib or lenvatinib (99).
Additionally, in the present meta-analysis, a comprehensive search was performed, and studies with overlapping cohorts were excluded. Consequently, our meta-analysis added 40 additional studies that were not analyzed in previous meta-analyses, thus contributing to 64 included studies. We found a correlation between SO and decreased OS. There is, however, a lack of extensive research on the effect of SO on survival in patients diagnosed with liver cancer.
Our study has several limitations. First, this study was constrained by insufficient data from each of the included studies, which is inherent to the nature of meta-analysis. Additionally, not all studies reported an adjusted HR, which could potentially restrict our ability to determine the precise magnitude of the mortality risk between sarcopenic and non-sarcopenic patients. Second, the selection of adjusted variables for the multivariate Cox regression models varied among the studies. Third, the significant results of Egger's tests indicated the presence of publication bias.
Sarcopenia was defined as the presence of both low muscle mass and impaired muscle function, according to EWGSOP2 (2). However, the diagnosis of sarcopenia remains controversial. On the one hand, there is a lack of standardized and feasible methods to measure muscle function or physical performance. Importantly, this limitation applies not only to the current meta-analysis but also to all existing studies that have investigated the impact of sarcopenia and/or SO on patients with malignant carcinomas. On the other hand, only prospective cohort studies are likely to document muscle function. Therefore, all the included studies can only partially define sarcopenia by measuring muscle mass, depending on CT/MRI images at the L3 level, which can be easily obtained from medical records. Hence, the retrospective nature of the included studies is recognized as a limitation of the current study. Given that the majority of the included studies were retrospective cohort studies, it is probable that the results were influenced by selection bias, as only patients who underwent CT scans were included. Furthermore, several methods were used to measure muscle mass, and the studies evaluating SMI and PMI at the L3 level based on CT imaging were included in the present meta-analysis; this may partially result in heterogeneity. We observed slight variations in the actual sex-specific cutoff values used in different studies. In some studies, the authors predefined the thresholds, whereas other studies derived these thresholds from their own study population to calculate the cutoff values. The thresholds were higher in European and American populations than in Asian populations, which might be explained by the fact that previous western studies were deemed unsuitable for Asian patients. Additionally, different cutoff values might change the magnitude of the association between sarcopenia and survival. Hence, we performed a subgroup analysis to overcome these limitations. The subgroup analysis showed a significant association between sarcopenia and poor OS and RFS/DFS across all the analyzed subgroups. Further prospective studies evaluating both muscle mass and muscle function are necessary to accurately and timely identify sarcopenic patients and to better clarify the relationship between muscle loss and survival.
Unlike previous studies examining the association between sarcopenia and survival in liver cancer patients, there is limited research on the association between SO and survival in liver cancer patients. Of the five studies searched, one study was excluded because it involved patients with other diseases, such as liver cirrhosis and cholestatic diseases (100). Furthermore, the four studies defined SO by using different approaches. Hence, further studies are required to confirm the association between SO and survival.
5. Conclusion
Sarcopenia and SO exhibited a significant association with reduced OS and RFS/DFS in patients with liver cancer. Additionally, the evaluation of sarcopenia and SO needs a consensus regarding their definitions and the utilization of appropriate cutoff values. We suggest that patients with liver cancer should undergo initial evaluation for sarcopenia and SO and receive regular monitoring because of poor prognosis. Further prospective studies are required to integrate sarcopenia and SO into an established prognostic scale specifically tailored for patients with liver cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XL and ST: study design. XL and XH: methodology and acquisition of data. XL: formal analysis and writing—original draft preparation. XL, XH, LL, and ST: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1233973/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
3. Beaudart C, Biver E, Reginster JY, Rizzoli R, Rolland Y, Bautmans I, et al. Validation of the Sarqol®, a specific health-related quality of life questionnaire for sarcopenia. J Cachexia Sarcopenia Muscle. (2017) 8:238–44. doi: 10.1002/jcsm.12149
4. Baracos VE, Arribas L. Sarcopenic Obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. (2018) 29(suppl_2):ii1–9. doi: 10.1093/annonc/mdx810
5. Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. (2012) 10:166–73, 73.e1. doi: 10.1016/j.cgh.2011.08.028
6. Zeng X, Shi ZW, Yu JJ, Wang LF, Luo YY, Jin SM, et al. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. (2021) 12:1948–58. doi: 10.1002/jcsm.12797
7. Antunes AC, Araújo DA, Veríssimo MT, Amaral TF. Sarcopenia and hospitalisation costs in older adults: a cross-sectional study. Nutr Diet. (2017) 74:46–50. doi: 10.1111/1747-0080.12287
8. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
9. Xia L, Zhao R, Wan Q, Wu Y, Zhou Y, Wang Y, et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. (2020) 9:7964–78. doi: 10.1002/cam4.3428
10. Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. (2022) 76:588–99. doi: 10.1016/j.jhep.2021.11.006
11. Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. (2014) 33:737–48. doi: 10.1016/j.clnu.2014.03.007
12. Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. (2022) 41:990–1000. doi: 10.1016/j.clnu.2021.11.014
13. Ji W, Liu X, Zheng K, Liu P, Zhao Y, Lu J, et al. Thresholds of visceral fat area and percent of body fat to define sarcopenic obesity and its clinical consequences in chinese cancer patients. Clin Nutr. (2022) 41:737–45. doi: 10.1016/j.clnu.2022.01.033
14. Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg. (2019) 269:924–31. doi: 10.1097/SLA.0000000000002555
15. Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Yao S, et al. Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann Surg Oncol. (2017) 24:3732–40. doi: 10.1245/s10434-017-6077-y
16. Valero V 3rd, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. (2015) 19:272–81. doi: 10.1007/s11605-014-2680-4
17. Akce M, Liu Y, Zakka K, Martini DJ, Draper A, Alese OB, et al. Impact of sarcopenia, BMI, and inflammatory biomarkers on survival in advanced hepatocellular carcinoma treated with anti-Pd-1 antibody. Am J Clin Oncol. (2021) 44:74–81. doi: 10.1097/COC.0000000000000787
18. Uchikawa S, Kawaoka T, Namba M, Kodama K, Ohya K, Morio K, et al. Skeletal muscle loss during tyrosine kinase inhibitor treatment for advanced hepatocellular carcinoma patients. Liver Cancer. (2020) 9:148–55. doi: 10.1159/000503829
19. Bekki T, Abe T, Amano H, Hattori M, Kobayashi T, Nakahara M, et al. Impact of low skeletal muscle mass index and perioperative blood transfusion on the prognosis for HCC following curative resection. BMC Gastroenterol. (2020) 20:328. doi: 10.1186/s12876-020-01472-z
20. Yeh WS, Chiang PL, Kee KM, Chang CD, Lu SN, Chen CH, et al. Pre-sarcopenia is the prognostic factor of overall survival in early-stage hepatoma patients undergoing radiofrequency ablation. Medicine. (2020) 99:e20455. doi: 10.1097/MD.0000000000020455
21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. (2013) 47:861–70. doi: 10.1097/MCG.0b013e318293a825
25. Itoh S, Shirabe K, Matsumoto Y, Yoshiya S, Muto J, Harimoto N, et al. Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann Surg Oncol. (2014) 21:3063–8. doi: 10.1245/s10434-014-3686-6
26. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. (2015) 63:131–40. doi: 10.1016/j.jhep.2015.02.031
27. Harimoto N, Yoshizumi T, Shimokawa M, Sakata K, Kimura K, Itoh S, et al. Sarcopenia is a poor prognostic factor following hepatic resection in patients aged 70 years and older with hepatocellular carcinoma. Hepatol Res. (2016) 46:1247–55. doi: 10.1111/hepr.12674
28. Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. (2015) 50:323–32. doi: 10.1007/s00535-014-0964-9
29. Levolger S, van Vledder MG, Muslem R, Koek M, Niessen WJ, de Man RA, et al. Sarcopenia impairs survival in patients with potentially curable hepatocellular carcinoma. J Surg Oncol. (2015) 112:208–13. doi: 10.1002/jso.23976
30. Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. (2015) 261:1173–83. doi: 10.1097/SLA.0000000000000743
31. Zhou GT, Bao HL, Zeng QQ, Hu WJ, Zhang QY. Sarcopenia as a prognostic factor in hepatolithiasis-associated intrahepatic cholangiocarcinoma patients following hepatectomy: a retrospective study. Int J Clin Exp Med. (2015) 8:18245–54.
32. Higashi T, Hayashi H, Taki K, Sakamoto K, Kuroki H, Nitta H, et al. Sarcopenia, but not visceral fat amount, is a risk factor of postoperative complications after major hepatectomy. Int J Clin Oncol. (2016) 21:310–9. doi: 10.1007/s10147-015-0898-0
33. Itoh S, Yoshizumi T, Kimura K, Okabe H, Harimoto N, Ikegami T, et al. Effect of sarcopenic obesity on outcomes of living-donor liver transplantation for hepatocellular carcinoma. Anticancer Res. (2016) 36:3029–34. doi: 10.21873/anticanres.11137
34. Kamachi S, Mizuta T, Otsuka T, Nakashita S, Ide Y, Miyoshi A, et al. sarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatment. Hepatol Res. (2016) 46:201–8. doi: 10.1111/hepr.12562
35. Takagi K, Yagi T, Yoshida R, Shinoura S, Umeda Y, Nobuoka D, et al. Sarcopenia and American Society of anesthesiologists physical status in the assessment of outcomes of hepatocellular carcinoma patients undergoing hepatectomy. Acta Med Okayama. (2016) 70:363–70. doi: 10.18926/AMO/54594
36. Yabusaki N, Fujii T, Yamada S, Suzuki K, Sugimoto H, Kanda M, et al. Adverse impact of low skeletal muscle index on the prognosis of hepatocellular carcinoma after hepatic resection. Int J Surg. (2016) 30:136–42. doi: 10.1016/j.ijsu.2016.04.049
37. Begini P, Gigante E, Antonelli G, Carbonetti F, Iannicelli E, Anania G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol. (2017) 16:107–14. doi: 10.5604/16652681.1226821
38. Hiraoka A, Hirooka M, Koizumi Y, Izumoto H, Ueki H, Kaneto M, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. (2017) 47:558–65. doi: 10.1111/hepr.12780
39. Nishikawa H, Nishijima N, Enomoto H, Sakamoto A, Nasu A, Komekado H, et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol Lett. (2017) 14:1637–47. doi: 10.3892/ol.2017.6287
40. Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Fujimoto Y, et al. Impact of skeletal muscle mass, muscle quality, and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann Surg Oncol. (2017) 24:1037–45. doi: 10.1245/s10434-016-5668-3
41. Yuri Y, Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, et al. Implication of psoas muscle index on survival for hepatocellular carcinoma undergoing radiofrequency ablation therapy. J Cancer. (2017) 8:1507–16. doi: 10.7150/jca.19175
42. Antonelli G, Gigante E, Iavarone M, Begini P, Sangiovanni A, Iannicelli E, et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United European Gastroenterol J. (2018) 6:1039–48. doi: 10.1177/2050640618781188
43. Ha Y, Kim D, Han S, Chon YE, Lee YB, Kim MN, et al. Sarcopenia predicts prognosis in patients with newly diagnosed hepatocellular carcinoma, independent of tumor stage and liver function. Cancer Res Treat. (2018) 50:843–51. doi: 10.4143/crt.2017.232
44. Kobayashi T, Kawai H, Nakano O, Abe S, Kamimura H, Sakamaki A, et al. Rapidly declining skeletal muscle mass predicts poor prognosis of hepatocellular carcinoma treated with transcatheter intra-arterial therapies. BMC Cancer. (2018) 18:756. doi: 10.1186/s12885-018-4673-2
45. Saeki I, Yamasaki T, Maeda M, Kawano R, Hisanaga T, Iwamoto T, et al. No muscle depletion with high visceral fat as a novel beneficial biomarker of sorafenib for hepatocellular carcinoma. Liver Cancer. (2018) 7:359–71. doi: 10.1159/000487858
46. Shiba S, Shibuya K, Katoh H, Koyama Y, Okamoto M, Abe T, et al. No deterioration in clinical outcomes of carbon ion radiotherapy for sarcopenia patients with hepatocellular carcinoma. Anticancer Res. (2018) 38:3579–86. doi: 10.21873/anticanres.12631
47. Shirai H, Kaido T, Hamaguchi Y, Kobayashi A, Okumura S, Yao SY, et al. Preoperative low muscle mass and low muscle quality negatively impact on pulmonary function in patients undergoing hepatectomy for hepatocellular carcinoma. Liver Cancer. (2018) 7:76–89. doi: 10.1159/000484487
48. Fujita M, Takahashi A, Hayashi M, Okai K, Abe K, Ohira H. Skeletal muscle volume loss during transarterial chemoembolization predicts poor prognosis in patients with hepatocellular carcinoma. Hepatol Res. (2019) 49:778–86. doi: 10.1111/hepr.13331
49. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yao SY, et al. Preoperative visceral adiposity and muscularity predict poor outcomes after hepatectomy for hepatocellular carcinoma. Liver Cancer. (2019) 8:92–109. doi: 10.1159/000488779
50. Imai K, Takai K, Miwa T, Taguchi D, Hanai T, Suetsugu A, et al. Rapid depletions of subcutaneous fat mass and skeletal muscle mass predict worse survival in patients with hepatocellular carcinoma treated with sorafenib. Cancers. (2019) 11:1206. doi: 10.3390/cancers11081206
51. Kroh A, Uschner D, Lodewick T, Eickhoff RM, Schöning W, Ulmer FT, et al. Impact of body composition on survival and morbidity after liver resection in hepatocellular carcinoma patients. Hepatobiliary Pancreat Dis Int. (2019) 18:28–37. doi: 10.1016/j.hbpd.2018.07.008
52. Labeur TA, van Vugt JLA, Ten Cate DWG, Takkenberg RB, Ijzermans JNM, Koerkamp BG, et al. Body composition is an independent predictor of outcome in patients with hepatocellular carcinoma treated with sorafenib. Liver Cancer. (2019) 8:255–70. doi: 10.1159/000493586
53. Lee J, Cho Y, Park S, Kim JW, Lee IJ. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with radiotherapy. Front Oncol. (2019) 9:1075. doi: 10.3389/fonc.2019.01075
54. Yugawa K, Itoh S, Kurihara T, Yoshiya S, Mano Y, Takeishi K, et al. Skeletal muscle mass predicts the prognosis of patients with intrahepatic cholangiocarcinoma. Am J Surg. (2019) 218:952–8. doi: 10.1016/j.amjsurg.2019.03.010
55. Choi K, Jang HY, Ahn JM, Hwang SH, Chung JW, Choi YS, et al. The association of the serum levels of myostatin, follistatin, and interleukin-6 with sarcopenia, and their impacts on survival in patients with hepatocellular carcinoma. Clin Mol Hepatol. (2020) 26:492–505. doi: 10.3350/cmh.2020.0005
56. Ebadi M, Moctezuma-Velazquez C, Meza-Junco J, Baracos VE, DunichandHoedl AR, Ghosh S, et al. Visceral adipose tissue radiodensity is linked to prognosis in hepatocellular carcinoma patients treated with selective internal radiation therapy. Cancers. (2020) 12:356. doi: 10.3390/cancers12020356
57. Endo K, Kuroda H, Kanazawa J, Sato T, Fujiwara Y, Abe T, et al. Impact of grip strength in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Cancers. (2020) 12:2146. doi: 10.3390/cancers12082146
58. Faron A, Sprinkart AM, Pieper CC, Kuetting DLR, Fimmers R, Block W, et al. Yttrium-90 radioembolization for hepatocellular carcinoma: outcome prediction with MRI derived fat-free muscle area. Eur J Radiol. (2020) 125:108889. doi: 10.1016/j.ejrad.2020.108889
59. Kotoh Y, Saeki I, Yamasaki T, Sasaki R, Tanabe N, Oono T, et al. Effect of handgrip strength on clinical outcomes of patients with hepatocellular carcinoma treated with lenvatinib. Appl Sci. (2020) 10:5403. doi: 10.3390/app10165403
60. Lanza E, Masetti C, Messana G, Muglia R, Pugliese N, Ceriani R, et al. Sarcopenia as a predictor of survival in patients undergoing bland transarterial embolization for unresectable hepatocellular carcinoma. PLoS ONE. (2020) 15:e0232371. doi: 10.1371/journal.pone.0232371
61. Santhakumar C, Bartlett AS, Plank LD, Wells CI, Wu LY, Gane EJ, et al. Sarcopenia negatively impacts long-term outcomes following curative resection for hepatocellular carcinoma: results of a long-term follow-up study. GastroHep. (2020) 2:215–23. doi: 10.1002/ygh2.412
62. Uojima H, Chuma M, Tanaka Y, Hidaka H, Nakazawa T, Iwabuchi S, et al. Skeletal muscle mass influences tolerability and prognosis in hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer. (2020) 9:193–206. doi: 10.1159/000504604
63. Wu CH, Liang PC, Hsu CH, Chang FT, Shao YY, Ting-Fang Shih T. Total skeletal, psoas and rectus abdominis muscle mass as prognostic factors for patients with advanced hepatocellular carcinoma. J Formos Med Assoc. (2020) 120:559–66. doi: 10.1016/j.jfma.2020.07.005
64. Deng L, Wang Y, Zhao J, Tong Y, Zhang S, Jin C, et al. The prognostic value of sarcopenia combined with hepatolithiasis in intrahepatic cholangiocarcinoma patients after surgery: a prospective cohort study. Eur J Surg Oncol. (2021) 47(3 Pt B):603–12. doi: 10.1016/j.ejso.2020.09.002
65. Guichet PL, Taslakian B, Zhan C, Aaltonen E, Farquharson S, Hickey R, et al. MRI-derived sarcopenia associated with increased mortality following yttrium-90 radioembolization of hepatocellular carcinoma. Cardiovasc Intervent Radiol. (2021) 44:1561–9. doi: 10.1007/s00270-021-02874-6
66. Jang HY, Choi GH, Hwang SH, Jang ES, Kim JW, Ahn JM, et al. Sarcopenia and visceral adiposity predict poor overall survival in hepatocellular carcinoma patients after curative hepatic resection. Transl Cancer Res. (2021) 10:854–66. doi: 10.21037/tcr-20-2974
67. Li H, Dai JL, Lan T, Liu HL, Wang JJ, Cai BL, et al. Combination of albumin-globulin score and skeletal muscle index predicts long-term outcomes of intrahepatic cholangiocarcinoma patients after curative resection. Clin Nutr. (2021) 40:3891–900. doi: 10.1016/j.clnu.2021.04.038
68. Liao CY, Li G, Bai YN, Zhou SQ, Huang L, Yan ML, et al. Prognostic value and association of sarcopenic obesity and systemic inflammatory indexes in patients with hepatocellular carcinoma following hepatectomy and the establishment of novel predictive nomograms. J Gastrointest Oncol. (2021) 12:669. doi: 10.21037/jgo-20-341
69. Saeki I, Yamasaki T, Yamauchi Y, Takami T, Kawaoka T, Uchikawa S, et al. Skeletal muscle volume is an independent predictor of survival after sorafenib treatment failure for hepatocellular carcinoma. Cancers. (2021) 13:2247. doi: 10.3390/cancers13092247
70. Salman A, Salman M, Moustafa A, Shaaban HED, El-Mikkawy A, Labib S, et al. Impact of sarcopenia on two-year mortality in patients with HCV-associated hepatocellular carcinoma after radiofrequency ablation. J Hepatocell Carcinoma. (2021) 8:313–20. doi: 10.2147/JHC.S300680
71. Yoshio S, Shimagaki T, Hashida R, Kawaguchi T, Tsutsui Y, Sakamoto Y, et al. Myostatin as a fibroblast-activating factor impacts on postoperative outcome in patients with hepatocellular carcinoma. Hepatol Res. (2021) 51:803–12. doi: 10.1111/hepr.13667
72. Chien TP, Huang SF, Chan WH, Pan KT, Yu MC, Lee WC, et al. The combination of sarcopenia and biochemical factors can predict the survival of hepatocellular carcinoma patients receiving transarterial chemoembolization. Front Oncol. (2022) 12:1005571. doi: 10.3389/fonc.2022.1005571
73. Dong D, Shi JY, Shang X, Liu B, Xu WL, Cui GZ, et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma treated with lenvatinib: a retrospective analysis. Medicine. (2022) 101:e28680. doi: 10.1097/MD.0000000000028680
74. Fujita M, Abe K, Kuroda H, Oikawa T, Ninomiya M, Masamune A, et al. Influence of skeletal muscle volume loss during lenvatinib treatment on prognosis in unresectable hepatocellular carcinoma: a multicenter study in Tohoku, Japan. Sci Rep. (2022) 12:6479. doi: 10.1038/s41598-022-10514-3
75. Hayashi H, Shimizu A, Kubota K, Notake T, Masuo H, Yoshizawa T, et al. Combination of sarcopenia and prognostic nutritional index to predict long-term outcomes in patients undergoing initial hepatectomy for hepatocellular carcinoma. Asian J Surg. (2022). doi: 10.1016/j.asjsur.2022.07.122
76. Hou GM, Jiang C, Du JP, Yuan KF. Sarcopenia predicts an adverse prognosis in patients with combined hepatocellular carcinoma and cholangiocarcinoma after surgery. Cancer Med. (2022) 11:317–31. doi: 10.1002/cam4.4448
77. Kim H, Choi HZ, Choi JM, Kang BM, Lee JW, Hwang JW. Sarcopenia with systemic inflammation can predict survival in patients with hepatocellular carcinoma undergoing curative resection. J Gastrointest Oncol. (2022) 13:744–53. doi: 10.21037/jgo-21-802
78. Roth G, Teyssier Y, Benhamou M, Abousalihac M, Caruso S, Sengel C, et al. Impact of sarcopenia on tumor response and survival outcomes in patients with hepatocellular carcinoma treated by trans-arterial (chemo)-embolization. World J Gastroenterol. (2022) 28:5324–37. doi: 10.3748/wjg.v28.i36.5324
79. Tamai Y, Iwasa M, Eguchi A, Shigefuku R, Sugimoto R, Tanaka H, et al. The prognostic role of controlling nutritional status and skeletal muscle mass in patients with hepatocellular carcinoma after curative treatment. Eur J Gastroenterol Hepatol. (2022) 34:1269–76. doi: 10.1097/MEG.0000000000002459
80. Xiao LS, Li RN, Cui H, Hong C, Huang CY, Li QM, et al. Use of computed tomography-derived body composition to determine the prognosis of patients with primary liver cancer treated with immune checkpoint inhibitors: a retrospective cohort study. BMC Cancer. (2022) 22:737. doi: 10.1186/s12885-022-09823-7
81. Yang JF, Huang WY, Lo CH, Lee MS, Lin CS, Shen PC, et al. Significant muscle loss after stereotactic body radiotherapy predicts worse survival in patients with hepatocellular carcinoma. Sci Rep. (2022) 12:19100. doi: 10.1038/s41598-022-21443-6
82. Zhang JX, Yan HT, Ding Y, Liu J, Liu S, Zu QQ, et al. Low psoas-muscle index is associated with decreased survival in hepatocellular carcinoma treated with transarterial chemoembolization. Ann Med. (2022) 54:1562–9. doi: 10.1080/07853890.2022.2081872
83. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing. (2017) 46:738–46. doi: 10.1093/ageing/afx094
84. Woo N, Kim SH. Sarcopenia influences fall-related injuries in community-dwelling older adults. Geriatr Nurs. (2014) 35:279–82. doi: 10.1016/j.gerinurse.2014.03.001
85. Thormann M, Omari J, Pech M, Damm R, Croner R, Perrakis A, et al. Low skeletal muscle mass and post-operative complications after surgery for liver malignancies: a meta-analysis. Langenbecks Arch Surg. (2022) 407:1369–79. doi: 10.1007/s00423-022-02541-5
86. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. (2018) 4:798–804. doi: 10.1001/jamaoncol.2018.0137
87. Surov A, Wienke A. Prevalence of sarcopenia in patients with solid tumors: a meta-analysis based on 81,814 patients. J Parenter Enteral Nutr. (2022) 46:1761–8. doi: 10.1002/jpen.2415
88. Gao Q, Hu K, Gao J, Shang Y, Mei F, Zhao L, et al. Prevalence and prognostic value of sarcopenic obesity in patients with cancer: a systematic review and meta-analysis. Nutrition. (2022) 101:111704. doi: 10.1016/j.nut.2022.111704
89. Hegyi PJ, Soós A, Hegyi P, Szakács Z, Hanák L, Váncsa S, et al. Pre-transplant sarcopenic obesity worsens the survival after liver transplantation: a meta-analysis and a systematic review. Front Med. (2020) 7:599434. doi: 10.3389/fmed.2020.599434
90. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the prot-age study group. J Am Med Dir Assoc. (2013) 14:542–59. doi: 10.1016/j.jamda.2013.05.021
91. Kalinkovich A, Livshits G. Sarcopenic Obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
92. Peixoto da Silva S, Santos JMO, Costa ESMP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. (2020) 11:619–35. doi: 10.1002/jcsm.12528
93. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. (2018) 14:513–37. doi: 10.1038/s41574-018-0062-9
94. Koya S, Kawaguchi T, Hashida R, Hirota K, Bekki M, Goto E, et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J Gastroenterol Hepatol. (2019) 34:580–8. doi: 10.1111/jgh.14538
95. Cao AL, Ferrucci LM, Caan BJ, Irwin ML. Effect of exercise on sarcopenia among cancer survivors: a systematic review. Cancers. (2022) 14:786. doi: 10.3390/cancers14030786
96. Hsu KJ, Liao CD, Tsai MW, Chen CN. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: a meta-analysis. Nutrients. (2019) 11:2163. doi: 10.3390/nu11092163
97. Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. (2018) 7:90–103. doi: 10.1159/000484950
98. March C, Omari J, Thormann M, Pech M, Wienke A, Surov A. Prevalence and role of low skeletal muscle mass (LSMM) in hepatocellular carcinoma. A systematic review and meta-analysis. Clin Nutr ESPEN. (2022) 49:103–13. doi: 10.1016/j.clnesp.2022.04.009
99. Guan J, Yang Q, Chen C, Wang G, Zhu HH. Prognostic value of low skeletal muscle mass in hepatocellular carcinoma patients treated with sorafenib or lenvatinib: a meta-analysis. EXCLI J. (2021) 20:1–16. doi: 10.17179/excli2020-3111
Keywords: primary liver cancer, sarcopenia, sarcopenic obesity, muscle depletion, survival
Citation: Li X, Huang X, Lei L and Tong S (2023) Impact of sarcopenia and sarcopenic obesity on survival in patients with primary liver cancer: a systematic review and meta-analysis. Front. Nutr. 10:1233973. doi: 10.3389/fnut.2023.1233973
Received: 03 June 2023; Accepted: 28 September 2023;
Published: 19 October 2023.
Edited by:
Owen Kelly, Sam Houston State University, United StatesReviewed by:
Péter Jenő Hegyi, University of Pécs, HungaryHanping Shi, Capital Medical University, China
Copyright © 2023 Li, Huang, Lei and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiwen Tong, dHN3Y3FtdUBjcW11LmVkdS5jbg==
 Xuanmei Li
Xuanmei Li Xue Huang
Xue Huang Lifu Lei
Lifu Lei Shiwen Tong
Shiwen Tong