- 1Department of Community Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Department of Clinical Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
- 3Research Development Center, Arash Women’s Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 4Food Safety Research Center (Salt), Semnan University of Medical Sciences, Semnan, Iran
- 5Department of Clinical Nutrition and Dietetics, Faculty of Nutrition and Food Technology, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Objectives: The current survey aimed to investigate the link between energy-adjusted dietary inflammatory index (E-DII) and risk factors for CVD including markers of endothelial and systemic inflammation in Iranian hemodialysis patients.
Methods: Patients on hemodialysis for at least 6 months prior to enrollment were considered eligible in this cross-sectional study. The usual dietary intakes of the hemodialysis individuals were examined through 4 non-consecutive days including 2 dialysis days and 2 non-dialysis days using a 24-h recall approach to calculate E-DII. Multiple linear regression analysis was utilized to investigate the link between E-DII and selected biomarkers of inflammation and oxidative stress including high-sensitive C reactive protein (hs-CRP), serum intercellular adhesion molecule (sICAM), serum vascular cell adhesion molecule (sVCAM), malondialdehyde, and nitric oxide (NO), sE-selectin, and endothelin-1, and beta (β) and 95% confidence interval (CI) was reported. Value of p < 0.05 was considered statistically significant.
Results: Overall, 291 hemodialysis patients make up our study population. In the crude model, the E-DII score was positively associated with a higher sVCAM-1 (β = 177.39; 95% CI: 60.51, 294.26; ptrend = 0.003). Further adjustment for potential confounders attenuated the findings in a way that an increase of 128.72 in the sVCAM-1 was observed when the E-DII score increased from −2.68 to −1.14 (95% CI: 13.50, 243.94). After controlling for potential confounders, E-DII was associated with sE-selectin in hemodialysis patients in the highest category of E-DII as compared to the lowest category (β = 4.11; 95% CI: 0.22, 8.00; ptrend = 0.039).
Conclusion: The present findings suggest that adherence to a pro-inflammatory diet among hemodialysis patients is associated with a higher inflammatory status as evidenced by sVCAM-1 and sE-selectin; however, bidirectionality may exist and the role of residual confounders should be taken into account. Therefore, more longitudinal investigations are needed to elucidate the role of diet on the inflammatory status of hemodialysis patients.
Introduction
The prevalence of cardiovascular disease (CVD) has been estimated to be 20 times higher among hemodialysis (HD) patients compared to the general population and also CVD accounts for nearly 50% of deaths in these patients (1–3). Chronic systemic inflammation is known as one of the main mediators of increased CVD risk in HD patients (4–6). Chronic inflammation increases the production of pro-inflammatory cytokines subsequently leading to the synthesis of the markers of systemic inflammation [i.e., C-reactive protein (CRP)] and vascular inflammation [i.e., soluble vascular cell adhesion molecule (sVCAM-1), soluble intercellular adhesion molecule (sICAM-1), and sE-selectin] (7).
Iran, like other developing countries, has experienced a rapid nutritional transition with a great interest in Western-style diet (8). However, undergoing HD may change the usual dietary intakes of individuals with a greater recommendation for high-quality protein and the possible necessity to limit foods and beverages that have lots of sodium, phosphorous, and potassium (9). Diet has an important role in immunonutrition through various nutrients which are suggested to influence inflammatory, immunological, and nutritional parameters (10). The dietary inflammatory index (DII) is a population-based, literature-based dietary scoring tool that provides the potential inflammatory properties of a diet (11). It represents the overall pro−/anti-inflammatory properties of the diet rather than individual foods and nutrients (11). It was shown that a pro-inflammatory diet, as indicated by a higher DII score, is associated with a higher prevalence of chronic kidney disease (CKD) and reduced kidney function (12, 13). The dietary inflammatory index was also shown to be positively associated with higher rates of mortality in HD patients (14). Also, a diet with higher pro-inflammatory potential was suggested to be linked with lower quality of life in patients undergoing HD (15). Moreover, the DII score was shown to be associated with CRP among HD patients (16).
Previous studies suggested an association between DII and CVD risk factors among different study populations (17–20); however, this issue was overlooked in HD patients. Due to the presence of reverse epidemiology phenomena or risk factor reversal, the traditional risk factors of CVD cannot explain the high prevalence of this phenomenon in HD patients (21, 22). Hence, novel risk factors have been suggested by previous studies to be measured in these patients for determining the risk of CVD (4, 5).
This study seeks to investigate the inflammatory potential of diet, calculated by DII, concerning risk factors for CVD including markers of endothelial and systemic inflammation. The findings of this study can be used to inform health agencies regarding the possible role of diet in reducing the CVD risk factors of HD patients.
Methods
Population and study design
In the current cross-sectional study, adult HD patients from 50 HD centers in Tehran, Iran, were evaluated sequentially from August 2019 to June 2020. First, the list of all the HD centers in Tehran was obtained from the Iran Dialysis Center, and then by referring to each of the 50 HD centers in Tehran, the names of all the HD patients were taken, and then the names of the patients who met the eligibility criteria to be enrolled in this study were recorded (n = 2,302). Second, the names of HD centers in Tehran were sorted alphabetically, and then the names of the patients in these centers were listed. Finally, 291 out of 2,302 subjects were selected using the systematic sampling method. Adult subjects (age ≥ 18 years) on HD for at least 6 months prior to enrollment were included. A history of HIV infection, malignancies, chronic or acute pancreatitis, liver disease, and inflammatory diseases were considered as the exclusion criteria. All patients were on HD three times a week (4 h per session) via bicarbonate dialysate and polysulfone capillary dialyzers. All of the enrolled HD patients provided written informed consent forms. Upon approval by the Ethics Committee of the National Nutrition and Food Technology Research Institute of Iran (IR.SBMU.NNFTRI.REC.1387.319), the study was initiated under the Declaration of Helsinki.
Dietary assessment
The usual dietary intakes of the HD individuals were examined through 4 non-consecutive days including 2 dialysis days and 2 non-dialysis days using a 24-h recall approach. Since dietary intakes of patients may be different on dialysis vs. non-dialysis days, both days were selected to capture day-to-day variation in diet (23). Through a face-to-face interview with a trained dietitian, participants were asked to recall all the drinks and food items consumed within 24 h. Portion sizes models were used to help people in estimating portion size and improve accuracy. Using Nutritionist IV software (First Databank® Inc., Hearst Corp., San Bruno, CA, United States) and the USDA food and nutrient database (24), dietary intakes were analyzed to determine the daily intakes of energy, macronutrients, and micronutrients of HD patients.
DII calculation
We used an approach suggested by Shivappa et al. to calculate energy-adjusted DII (E-DII). Before the E-DII calculation, the energy-adjusted amount of each food item was calculated using the residual technique (25). Of 45 dietary items suggested by Shivappa et al., 28 food items were available for E-DII calculation including vitamins A, D, E, B1, B2, B3, B6, B9, B12, C, β-carotene, n-3 fatty acids, n-6 fatty acids, cholesterol, saturated fatty acids (SFA), trans fatty acids (TFA), polyunsaturated fatty acids (PUFA), mono-unsaturated fatty acids (MUFA), magnesium, zinc, iron, selenium, caffeine, dietary fiber, carbohydrate, fat, protein, and energy (11). Initially, subjects’ dietary consumption was subtracted from the “standard global mean” and then it was divided by the “global standard deviation” to calculate the Z score of each dietary parameter. Subsequently, the Z score of each food item was transformed to the centered percentile to minimize data skewness and then multiplied by the score of inflammatory properties of each food item. Lastly, the overall E-DII for each participant was calculated by summing up the inflammatory scores of 28 food items calculated previously. Shivappa et al. suggested a DII score range of −8.87 to +7.98 with higher DII values representing a diet with pro-inflammatory properties, while lower values indicate a diet with anti-inflammatory features (11).
Biochemical parameters
A venous blood sample (10 mL) was obtained from each patient before dialysis and after 12–14 h of fasting. Then, blood samples were centrifuged at 2000 rpm for 10 min to separate serum and subsequently, the extracted serum was transferred to sterile microtubes and stored at −70°C until the time of biochemical analysis. Serum albumin, urea, and creatinine were assessed using Selectra 2 Autoanalyzer (Vital Scientific, Spankeren, the Netherlands) employing commercial kits (Pars-Azmoon, Tehran, Iran) with the intra- and inter-assay coefficients of variation (CV) < 3%. The concentration of serum endothelin-1 was examined via ELISA kits (Biomedica, Vienna, Austria), with an intra- and inter-assay CV of 8.5%. Serum concentrations of malondialdehyde (MDA) and nitric oxide (NO) were measured using a colorimetric approach via commercial kits (Cayman Chemical, Ann Arbor, MI, United States), with the intra- and inter-assay CV of 4.6% and 7.8%, respectively. The serum concentrations of sE-selectin, sVCAM-1, and sICAM-1 were measured via ELISA kits (Diaclone, Besancon, France) with the intra- and inter-assay CV of 6.7, 6.3, and 3.5%, respectively. The serum concentration of hs-CRP was determined using ELISA kits (Diagnostics Biochem Canada, London, Canada) with an intra- and inter-assay CV of 4.6%.
Assessment of confounders
The body mass index (BMI) was calculated using participants’ weight and height, measured at the end of their dialysis session. Dialysis vintage was outlined as the time that each patient was on HD and stated as a year. Dialysis adequacy was calculated, based on the Kt/V index, using dialysis length, post-dialysis weight, ultrafiltration volume, and pre-and post-dialysis serum urea concentration (26).
Statistical analysis
Using a suggested formula for sample size calculation of cross-sectional studies from small populations and with α = 0.05 and d = 0.05, a total sample of 292 was calculated (27). The statistical analysis was done using SPSS version 26 (IBM Corp., Armonk, NY, United States). The normality distribution of continuous variables was assessed using the skewness statistic, Q-Q plot, and Kolmogorov–Smirnov test. Continuous and categorical variables are presented as mean ± standard error (SE) and number (percent), respectively. The differences in quantitative variables across tertiles of E-DII were assessed via the one-way analysis of variance (ANOVA). The distribution of qualitative variables across tertiles of E-DII was examined using the Chi-squared test. A residual approach was implemented to calculate energy-adjusted values of food items. Multiple linear regression analysis was utilized to investigate the link between E-DII and selected biomarkers of inflammation and oxidative stress in three different models. In the first model, gender, and age (continuous) were entered. In the second model, further adjustment was made for albumin (continuous), serum urea (continuous), serum creatinine (continuous), dialysis vintage (continuous), and dialysis adequacy (continuous). BMI (continuous) was adjusted in the final model. Value of p < 0.05 was considered statistically significant and all analyses were done two-tailed.
Results
A total of 291 HD patients (127 females and 164 males) with a mean (SE) age of 57.73 (0.88) years, BMI of 24.11 (0.26) kg/m2, dialysis adequacy of 1.23 (0.02) Kt/V, and dialysis vintage of 4.27 (0.25) years make up our study population. Of 291 HD patients, 261 participants had arteriovenous (AV) fistula and others had a central venous catheter. Participants of the highest tertile of E-DII compared to the lowest tertile significantly had higher sVCAM-1 and were more likely to be younger (p < 0.05). No significant differences were observed in terms of other baseline variables across tertiles of E-DII score (p > 0.05; Table 1).
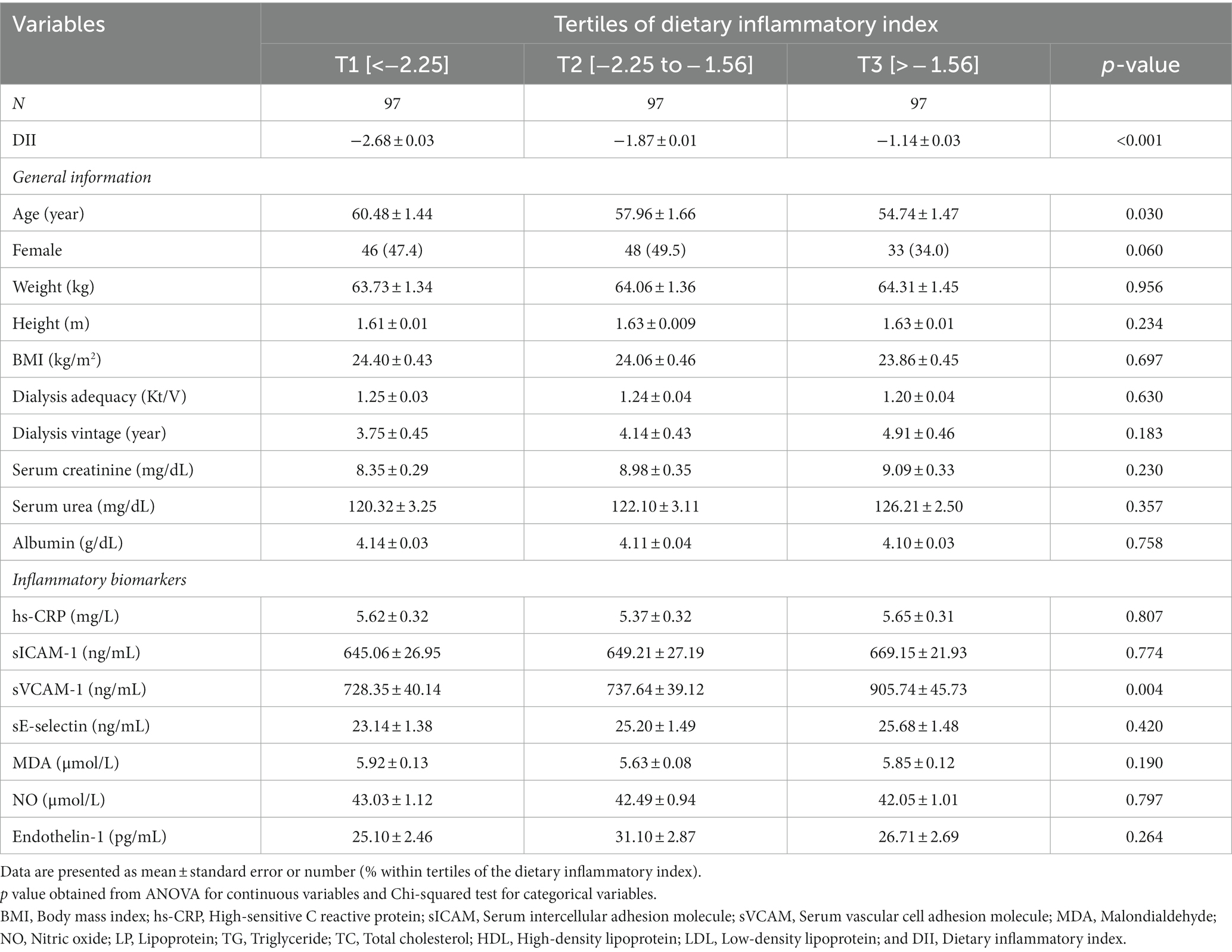
Table 1. Characteristics of the study population stratified by tertiles of dietary inflammatory index.
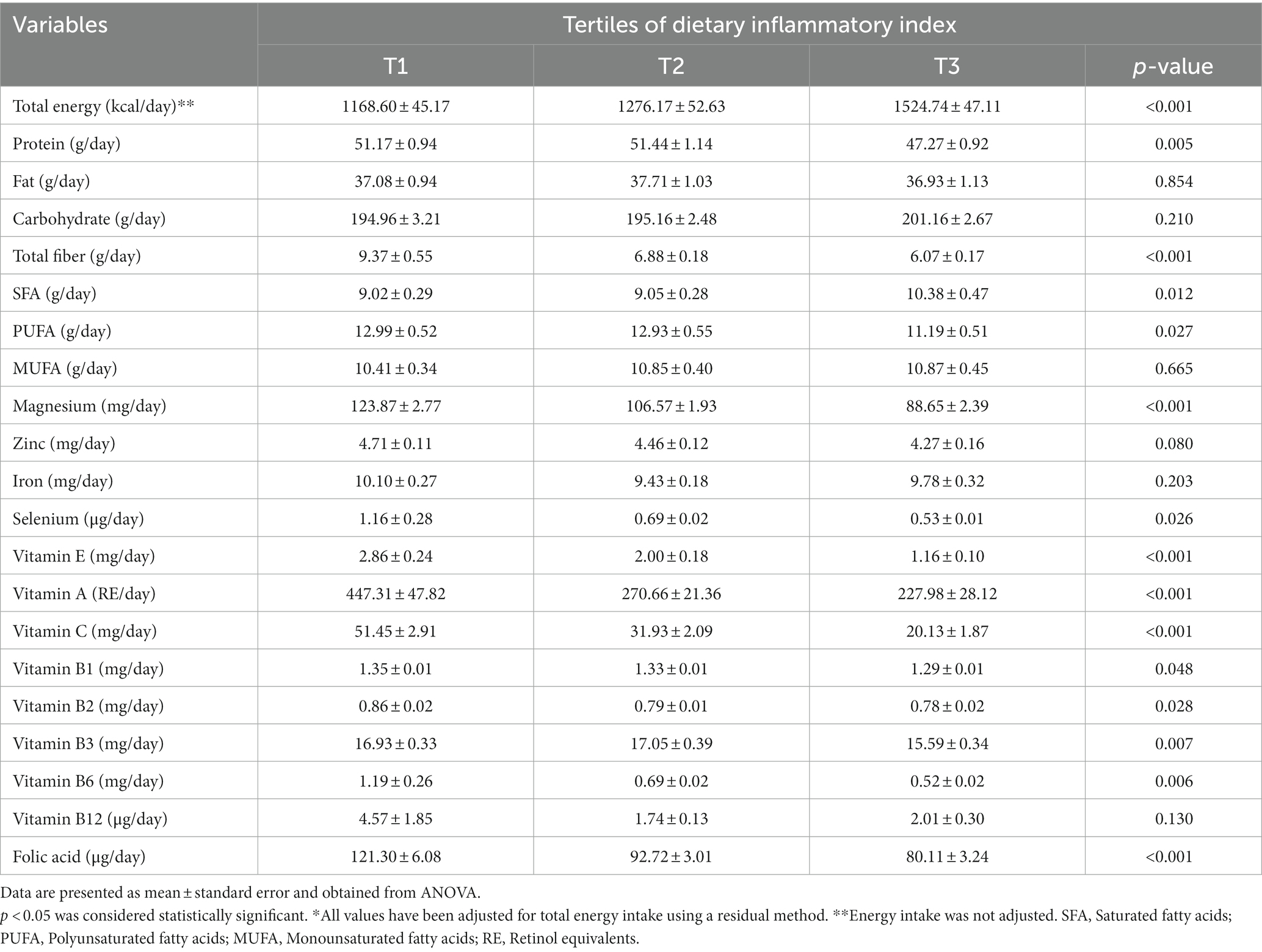
Dietary intakes of HD patients according to tertiles of E-DII are presented in Table 2. As can be seen, participants in the greatest tertile of E-DII consumed higher amounts of total energy and SFA, as well as lower amounts of protein, total fiber, PUFA, magnesium, selenium, vitamin E, A, C, B1, B2, B3, B6, and folic acid (p < 0.05).
The relation between E-DII and selected biomarkers of inflammation and oxidative stress is represented as beta estimates and the corresponding 95% CIs and can be seen in Table 3. In the crude model, the E-DII score was positively associated with a higher sVCAM-1 (β = 177.39; 95% CI: 60.51, 294.26; ptrend = 0.003) in individuals in the highest tertile of E-DII as compared to those in the lowest tertile. Adjustments for sex and age attenuated the findings (β = 15.64; 95% CI: 47.01, 284.28; ptrend = 0.007). Further adjustment for dialysis adequacy, dialysis vintage, serum creatinine, serum urea, and albumin attenuated the findings more, however, E-DII was still significantly associated with sVCAM-1 in participants in the third tertile of E-DII as compared to those in the first tertile (β = 128.63; 95% CI: 13.48, 243.78; ptrend = 0.029). After controlling for BMI, an increase of 128.72 in the sVCAM-1 was observed when the E-DII score increased from −2.68 to −1.14 (95% CI: 13.50, 243.94; ptrend = 0.029) There was no significant association between sE-selectin and E-DII in the crude model (β = 2.54; 95% CI: −1.45, 6.53; ptrend = 0.212) or after adjustment for age and sex (β = 3.53; 95% CI: −0.47, 7.54; ptrend = 0.085) for those in the highest tertile of E-DII compared to lowest tertile. However, further adjustment for dialysis adequacy, dialysis vintage, serum creatinine, serum urea, albumin, and BMI made this association significant (β = 4.11; 95% CI: 0.22, 8.00; ptrend = 0.039). No significant relationship was detected between E-DII and hs-CRP, sICAM-1, MDA, NO, and Endothelin-1 (Table 3).
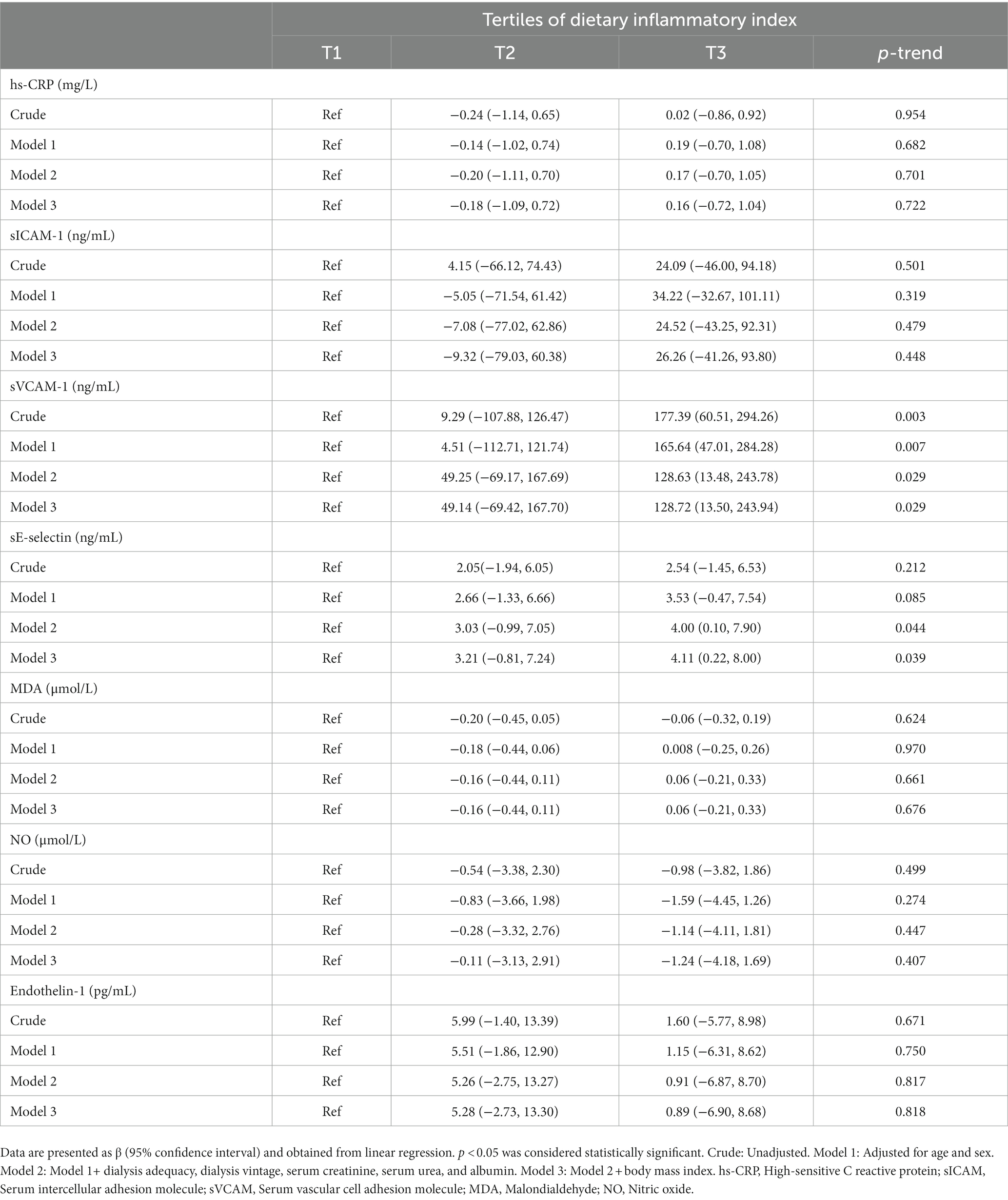
Table 3. Beta (β) and 95% confidence interval for biomarkers of inflammation and oxidative stress across tertiles of the dietary inflammatory index.
Discussion
A population of Iranian HD patients was recruited to examine the association between E-DII and markers of endothelial and systemic inflammation as novel risk factors for CVD. We found that E-DII can be a predictor of higher sVCAM-1 and sE-selectin. Our findings suggest the importance of the inflammatory potential of diet in the context of endothelial and systemic inflammation. New and substantial information regarding the association of E-DII with novel CVD risk factors in a sample of Iranian HD patients can be added to the literature by these findings.
In the current study, E-DII and sVCAM-1 were associated independently of age and sex. However, the inclusion of other confounders (i.e., dialysis adequacy, dialysis vintage, serum creatinine, serum urea, albumin, and BMI) attenuated the findings, indicating that the potential effect of E-DII may be related to body composition, residual kidney function, and other aspects of dialysis, as evidenced by previous literature (28–30). Moreover, patients in the highest tertile of E-DII tended to have higher sE-selectin that was independent of assessed confounders. The findings regarding sVCAM-1 and sE-selectin also imply that a larger sample size may amplify the observed association. Therefore, further studies with a larger sample size are needed to elucidate this issue.
According to the available literature, no research has been carried out to investigate the association between E-DII and markers of endothelial and systemic inflammation as novel risk factors for CVD among Iranian HD patients. A cross-sectional study on 221 patients (mean age of 56.57 years) diagnosed with CKD among the Iranian population proposed that compliance with a pro-inflammatory diet was associated with a higher risk of CKD progression (odds ratio: 2.12; 95%CI: 1.05, 4.26) (13). Another population-based study among 21,649 subjects (47.3 years) also concluded that a diet with higher inflammatory potentials is associated with higher CKD prevalence and declining kidney function (12). Another cross-sectional study was done on 105 HD patients (57.5 years) at Turkish dialysis centers and suggested that DII was significantly correlated with the markers of malnutrition and inflammation (e.g., CRP) (16). A cohort study of 137 patients undergoing HD (61.7 years) revealed a direct association between DII and mortality during the 2-year follow-up (14). Another study among 532 adolescents (12–17 years old) in European countries also suggested a positive association between DII and sVCAM-1 (31). There are some points worthy of mention. There is no previous study that can be used to confirm the present findings in HD patients. Moreover, the available literature is heterogeneous in terms of the study population, age, ethnicity, background comorbidities, sample size, DII calculation, and methodological settings that make the comparison difficult. Furthermore, previous studies used various approaches to assess patients’ dietary intakes (e.g., food frequency questionnaire, food record, and food recall) which adds to the existing heterogeneity. Overall, this study, similar to other available evidence, confirms the possible link between diet and inflammatory status (32).
As evidenced by a majority of previous documents, CKD is accompanied by a low-grade systemic inflammation that increases the secretion of inflammatory cytokines which led to an inflammatory state (33). The etiology of inflammation among HD patients is multifactorial and complex, including increased oxidative stress, malnutrition, subclinical or clinical infection of the vascular access port, decreased clearance and increased release of inflammatory cytokines, oxidative and carbonyl stress, frequent contact between blood mononuclear cells and dialysis tubes and dialyzer membranes, and impure dialysis water/solution (34). However, as evidenced by the findings of the current study, a pro-inflammatory diet, as shown by higher E-DII values, might be another reason for inflammation. The findings of the current study reinforce the idea that a diet poor in anti-inflammatory parameters, and rich in pro-inflammatory parameters may increase inflammation among HD patients. As a new approach to represent the inflammatory potential of the diet, DII can be implemented in a diverse population with available dietary data (11, 35). The DII can also be used to assess individuals’ dietary inflammatory status and subsequently guide them in reducing inflammation levels to decrease the risk of certain chronic conditions (11). Dietary components with anti-oxidative and anti-inflammatory properties are proposed to be effective in reducing inflammation and CVD risk (36). Potential agents with anti-oxidative and anti-inflammatory properties that have been studied in CKD patients to decrease the levels of inflammation are fish oil, vitamin A/carotenoids, vitamin C, and vitamin E (37). Similarly, in the current study, dietary vitamin E (p < 0.001), vitamin C (p < 0.001), and vitamin A (p < 0.001) intakes were higher in the first tertile (more anti-inflammatory diet). Moreover, it has been reported that high glycemic index foods and SFA are associated with elevated levels of inflammation (38). In line with this, patients with the highest E-DII consumed higher amounts of SFA (p = 0.012) and lower amounts of PUFA (p = 0.027).
The total energy intakes of our study population ranged from 1168.60 to 1524.74 kcal/day across tertiles of E-DII. This finding implies that the included participants consumed lower amounts of energy as indicated by their BMI status which was 18.33–23.70 kcal/kg/day. A previous study by Bossola et al. also proposed that 70.2% of their HD patients had inadequate energy intake (39). A dietary energy intake of 30–35 kcal/kg/day is generally recommended for HD patients; however, various reports revealed that the actual energy intake of HD patients is lower than recommended values (40–42). Psychosocial modifiers, low diet quality, poor appetite, and low dialysis adequacy are among the most suggested factors contributing to lower dietary intake and malnutrition among HD patients (42). In agreement with improper total energy intake, dietary consumption of other nutrients and food groups also failed to reach recommended values contributing to the lower diet quality and possibly higher inflammatory potential of a diet (43). Previous reports suggested a higher intake of fruits, vegetables, whole grains, nuts, seeds, legumes, and spices as well as a lower intake of red meat, processed meat, refined grains, added sugars, and fried foods. Moreover, healthy fats should be chosen including olive oil, avocado, fish oil, and flaxseed oil (44). However, all of these recommendations are not practical for those undergoing HD, and a personalized dietary recommendation is strongly suggested for this population.
Strengths and limitations
This is the first study among Iranian HD patients that examined the association between E-DII and comprehensive markers of endothelial and systemic inflammation as novel risk factors for CVD, which can be the main strength of the current survey.
The present study has several limitations. We used a 4-day dietary recall to obtain the dietary intakes of participants, however, this approach mainly relies on subjects’ memory and therefore might be prone to over−/underestimation of usual dietary intakes. Therefore, it is strongly suggested that future studies use multiple pass 24-h recall to overcome some limitations of the classical 24-h approach. A total of 28 out of 45 dietary items were available for calculating E-DII in which herbs and spices were overlooked including ginger, saffron, turmeric, pepper, rosemary, thyme, and oregano. Likewise, a cause-and-effect conclusion cannot be obtained due to the cross-sectional design of this paper. All patients suffered from CKD; however, the underlying causes were ignored in collecting data and therefore might be another limitation of the current study. Moreover, other co-existing diseases including diabetes, hypertension, thyroid disorders, etc. should also be considered in collecting data in future studies.
Conclusion
The present findings suggest that adherence to a pro-inflammatory diet among HD patients is associated with a higher inflammatory status as evidenced by sVCAM-1 and sE-selectin; however, bidirectionality may exist and the role of residual confounders should be taken into account. Therefore, more longitudinal investigations are needed to elucidate the role of diet on the inflammatory status of HD patients. Likewise, further studies with a larger sample size are also needed to confirm our findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The National Nutrition and Food Technology Research Institute of Iran Ethics Committee accepted the study protocol as satisfactory (IR.SBMU.NNFTRI.REC.1387.319). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AtA and HT: conception and design and acquisition of data. ArA: analysis and interpretation of data. ArA, EK, AtA, and MN: drafting. ArA, EK, AtA, and HT: intellectual content revision. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to show appreciation to all patients who generously contributed to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fox, CS , Matsushita, K , Woodward, M , Bilo, HJ , Chalmers, J , Heerspink, HJL, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. (2012) 380:1662–73. doi: 10.1016/S0140-6736(12)61350-6
2. Mahmoodi, BK , Matsushita, K , Woodward, M , Blankestijn, PJ , Cirillo, M , Ohkubo, T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. (2012) 380:1649–61. doi: 10.1016/S0140-6736(12)61272-0
3. Cozzolino, M , Mangano, M , Stucchi, A , Ciceri, P , Conte, F , and Galassi, A . Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. (2018) 33:iii28–34. doi: 10.1093/ndt/gfy174
4. Demir, M , Kucuk, A , Sezer, MT , Altuntas, A , and Kaya, S . Malnutrition-inflammation score and endothelial dysfunction in hemodialysis patients. J Ren Nutr. (2010) 20:377–83. doi: 10.1053/j.jrn.2010.03.002
5. Tabibi, H , As'habi, A , Mahdavi-Mazdeh, M , Hedayati, M , and Nozary-Heshmati, B . Comparison of novel risk factors for cardiovascular disease between hemodialysis patients with and without protein-energy wasting. Int Urol Nephrol. (2014) 46:2015–20. doi: 10.1007/s11255-014-0750-x
6. Sun, J , Axelsson, J , Machowska, A , Heimbürger, O , Bárány, P , Lindholm, B, et al. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin J Am Soc Nephrol. (2016) 11:1163–72. doi: 10.2215/CJN.10441015
7. van Dooren, FE , Schram, MT , Schalkwijk, CG , Stehouwer, CD , Henry, RM , Dagnelie, PC, et al. Associations of low grade inflammation and endothelial dysfunction with depression–the Maastricht study. Brain Behav Immun. (2016) 56:390–6. doi: 10.1016/j.bbi.2016.03.004
8. Mohammadifard, N , Sarrafzadegan, N , Nouri, F , Sajjadi, F , Alikhasi, H , Maghroun, M, et al. Using factor analysis to identify dietary patterns in Iranian adults: Isfahan healthy heart program. Int J Public Health. (2012) 57:235–41. doi: 10.1007/s00038-011-0260-x
9. Biruete, A , Jeong, JH , Barnes, JL , and Wilund, KR . Modified nutritional recommendations to improve dietary patterns and outcomes in hemodialysis patients. J Ren Nutr. (2017) 27:62–70. doi: 10.1053/j.jrn.2016.06.001
10. Heyland, DK , Novak, F , Drover, JW , Jain, M , Su, X , and Suchner, U . Should immunonutrition become routine in critically ill patients?: a systematic review of the evidence. JAMA. (2001) 286:944–53. doi: 10.1001/jama.286.8.944
11. Shivappa, N , Steck, SE , Hurley, TG , Hussey, JR , and Hébert, JR . Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
12. Mazidi, M , Shivappa, N , Wirth, MD , Hebert, JR , and Kengne, AP . Greater dietary inflammatory index score is associated with higher likelihood of chronic kidney disease. Br J Nutr. (2018) 120:204–9. doi: 10.1017/S0007114518001071
13. Rouhani, MH , Najafabadi, MM , Surkan, PJ , Esmaillzadeh, A , Feizi, A , and Azadbakht, L . Dietary inflammatory index and its association with renal function and progression of chronic kidney disease. Clin Nutr ESPEN. (2019) 29:237–41. doi: 10.1016/j.clnesp.2018.09.001
14. Balbino, KP , Juvanhol, LL , Wendling, AL , Bressan, J , Shivappa, N , Hebert, JR, et al. Dietary inflammatory index and mortality in hemodialysis patients by path analysis approach (NUGE-HD study). Nutrition. (2021) 89:111239. doi: 10.1016/j.nut.2021.111239
15. Yaseri, M , Alipoor, E , Hafizi, N , Maghsoudi-Nasab, S , Shivappa, N , Hebert, JR, et al. Dietary inflammatory index is a better determinant of quality of life compared to obesity status in patients with hemodialysis. J Ren Nutr. (2021) 31:313–9. doi: 10.1053/j.jrn.2020.07.006
16. Kizil, M , Tengilimoglu-Metin, MM , Gumus, D , Sevim, S , Turkoglu, İ , and Mandiroglu, F . Dietary inflammatory index is associated with serum C-reactive protein and protein energy wasting in hemodialysis patients: a cross-sectional study. Nutr Res Pract. (2016) 10:404–10. doi: 10.4162/nrp.2016.10.4.404
17. Pocovi-Gerardino, G , Correa-Rodríguez, M , Callejas-Rubio, J-L , Ríos-Fernández, R , Martín-Amada, M , Cruz-Caparros, M-G, et al. Dietary inflammatory index score and cardiovascular disease risk markers in women with systemic lupus erythematosus. J Acad Nutr Diet. (2020) 120:280–7. doi: 10.1016/j.jand.2019.06.007
18. Ruiz-Canela, M , Bes-Rastrollo, M , and Martínez-González, MA . The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int J Mol Sci. (2016) 17:1265. doi: 10.3390/ijms17081265
19. Garcia-Arellano, A , Ramallal, R , Ruiz-Canela, M , Salas-Salvadó, J , Corella, D , Shivappa, N, et al. Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED study. Nutrients. (2015) 7:4124–38. doi: 10.3390/nu7064124
20. Shivappa, N , Godos, J , Hébert, JR , Wirth, MD , Piuri, G , Speciani, AF, et al. Dietary inflammatory index and cardiovascular risk and mortality—a Meta-analysis. Nutrients. (2018) 10:200. doi: 10.3390/nu10020200
21. Nurmohamed, S , and Nube, M . Reverse epidemiology: paradoxical observations in haemodialysis patients. Neth J Med. (2005) 63:376–81.
22. Kopple, JD . The phenomenon of altered risk factor patterns or reverse epidemiology in persons with advanced chronic kidney failure. Am J Clin Nutr. (2005) 81:1257–66. doi: 10.1093/ajcn/81.6.1257
23. Therrien, M , Byham-Gray, L , and Beto, J . A review of dietary intake studies in maintenance dialysis patients. J Ren Nutr. (2015) 25:329–38. doi: 10.1053/j.jrn.2014.11.001
24. Ahuja, J. , Montville, J.B. , Omolewa-Tomobi, G. , Heendeniya, K.Y. , Martin, C.L. , Steinfeldt, L.C., et al. USDA food and nutrient database for dietary studies, 5.0–documentation and user guide. US Department of Agriculture, Agricultural Research Service, food surveys research group: Beltsville, MD, USA. (2012).
25. Willett, W , and Stampfer, MJ . Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. (1986) 124:17–27. doi: 10.1093/oxfordjournals.aje.a114366
26. Daugirdas, JT . “Physiologic principles and urea kinetic modeling,” in Handbook of Dialysis 4th Ed. Eds. Daugirdas J. T., Blake P. G., Ing, T. S. New Delhi, India: Wolters Kluwer (India) pvt. Ltd (2007).
27. Nayak, BK . Understanding the relevance of sample size calculation. Indian J Ophthalmol. (2010) 58:469. doi: 10.4103/0301-4738.71673
28. Mandic, A , Cavar, I , Skoro, I , Tomic, I , Ljubic, K , Coric, S, et al. Body composition and inflammation in hemodialysis patients. Ther Apher Dial. (2017) 21:556–64. doi: 10.1111/1744-9987.12575
29. Wang, AY-M , Wang, M , Woo, J , Lam, CW-K , Lui, S-F , Li, PK-T, et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. (2004) 15:2186–94. doi: 10.1097/01.ASN.0000135053.98172.D6
30. Ayus, JC , Mizani, MR , Achinger, SG , Thadhani, R , Go, AS , and Lee, S . Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J Am Soc Nephrol. (2005) 16:2778–88. doi: 10.1681/ASN.2005040392
31. Shivappa, N , Hebert, JR , Marcos, A , Diaz, LE , Gomez, S , Nova, E, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. (2017) 61:1600707. doi: 10.1002/mnfr.201600707
32. Galland, L . Diet and inflammation. Nutr Clin Pract. (2010) 25:634–40. doi: 10.1177/0884533610385703
33. DeAngelis, RA , Reis, ES , Ricklin, D , and Lambris, JD . Targeted complement inhibition as a promising strategy for preventing inflammatory complications in hemodialysis. Immunobiology. (2012) 217:1097–105. doi: 10.1016/j.imbio.2012.07.012
34. Dai, L , Golembiewska, E , Lindholm, B , and Stenvinkel, P . End-stage renal disease, inflammation and cardiovascular outcomes. Expand Hemodialy. (2017) 191:32–43. doi: 10.1159/000479254
35. Cavicchia, PP , Steck, SE , Hurley, TG , Hussey, JR , Ma, Y , Ockene, IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. (2009) 139:2365–72. doi: 10.3945/jn.109.114025
36. Cardozo, LF , Pedruzzi, LM , Stenvinkel, P , Stockler-Pinto, MB , Daleprane, JB , Leite, M Jr, et al. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie. (2013) 95:1525–33. doi: 10.1016/j.biochi.2013.04.012
37. Kalantar-Zadeh, K , Stenvinkel, P , Bross, R , Khawar, OS , Rammohan, M , Colman, S, et al. Kidney insufficiency and nutrient-based modulation of inflammation. Curr Opin Clin Nutr Metabol Care. (2005) 8:388–96. doi: 10.1097/01.mco.0000172578.56396.9e
38. Raphael, W , and Sordillo, LM . Dietary polyunsaturated fatty acids and inflammation: the role of phospholipid biosynthesis. Int J Mol Sci. (2013) 14:21167–88. doi: 10.3390/ijms141021167
39. Bossola, M , Muscaritoli, M , Tazza, L , Panocchia, N , Liberatori, M , Giungi, S, et al. Variables associated with reduced dietary intake in hemodialysis patients. J Ren Nutr. (2005) 15:244–52. doi: 10.1053/j.jrn.2005.01.004
40. Daugirdas, JT , Depner, TA , Inrig, J , Mehrotra, R , Rocco, MV , Suri, RS, et al. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. (2015) 66:884–930. doi: 10.1053/j.ajkd.2015.07.015
41. Bellizzi, V , Di Iorio, BR , Terracciano, V , Minutolo, R , Iodice, C , De Nicola, L, et al. Daily nutrient intake represents a modifiable determinant of nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant. (2003) 18:1874–81. doi: 10.1093/ndt/gfg239
42. Sahathevan, S , Khor, B-H , Ng, H-M , Abdul Gafor, AH , Mat Daud, ZA , Mafra, D, et al. Understanding development of malnutrition in hemodialysis patients: a narrative review. Nutrients. (2020) 12:3147. doi: 10.3390/nu12103147
43. Alipoor, E , Karimbeiki, R , Shivappa, N , Yaseri, M , Hebert, JR , and Hosseinzadeh-Attar, MJ . Dietary inflammatory index and parameters of diet quality in normal weight and obese patients undergoing hemodialysis. Nutrition. (2019) 61:32–7. doi: 10.1016/j.nut.2018.09.036
Keywords: inflammation, diet, hemodialysis, Iran, dietary inflammatory index
Citation: Arab A, Karimi E, Nazari M, Tabibi H and As’habi A (2023) Association between the dietary inflammatory index and markers of endothelial and systemic inflammation in hemodialysis patients. Front. Nutr. 10:1230747. doi: 10.3389/fnut.2023.1230747
Edited by:
Fabiana Baggio Nerbass, Fundacão Pró-Rim, BrazilReviewed by:
Jeanette Mary Andrade, University of Florida, United StatesPiergiorgio Messa, University of Milan, Italy
Copyright © 2023 Arab, Karimi, Nazari, Tabibi and As’habi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atefeh As’habi, YXNoYWJpX251dHJpdGlvbkB5YWhvby5jb20=
 Arman Arab
Arman Arab Elham Karimi
Elham Karimi Maryam Nazari4
Maryam Nazari4 Hadi Tabibi
Hadi Tabibi Atefeh As’habi
Atefeh As’habi