
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Nutr., 27 September 2023
Sec. Nutrition and Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1224740
This article is part of the Research TopicBiochemical Biomarkers of Nutritional StatusView all 7 articles
 Kentaro Nakamura1†
Kentaro Nakamura1† Keisuke Hagihara2*†
Keisuke Hagihara2*† Naoko Nagai3
Naoko Nagai3 Ryuichiro Egashira2
Ryuichiro Egashira2 Mariko Takeuchi2
Mariko Takeuchi2 Mai Nakano2
Mai Nakano2 Hitomi Saito2
Hitomi Saito2 Misaki Moriguchi2
Misaki Moriguchi2 Satoko Tonari2
Satoko Tonari2 Hisako Fujii4
Hisako Fujii4 Akimitsu Miyake5
Akimitsu Miyake5 Yusuke Omae1
Yusuke Omae1 Kinya Ashida1
Kinya Ashida1The efficacy of low-carbohydrate, high-fat diets, such as ketogenic diets, for cancer patients is of research interest. We previously demonstrated the efficacy of the ketogenic diet in a case study in which medium-chain triglycerides (MCTs) or MCT-containing formula (ketogenic formula) was used as a supplement to increase blood ketone bodies. However, little is known about the amounts needed to induce ketogenic effects and about the usefulness of monitoring of breath acetone. To investigate the pharmacokinetics of MCTs and their metabolites, blood ketone bodies and breath acetone, 24 healthy subjects received one of four single oral doses of the ketogenic formula (equivalent to 0, 10, 20, and 30 g of MCTs) under fasting conditions. Total blood ketone bodies, β-hydroxybutyrate, octanoic acid, and decanoic acid were increased in a dose-dependent manner. The ketogenic effect was considered to depend on octanoic and decanoic acids, because a positive correlation was observed between them. A strong positive correlation was also observed between total serum ketone bodies and breath acetone at each time points. Therefore, monitoring breath acetone levels seems a less invasive method to predict blood concentrations of ketone bodies during ketogenic diet therapy.
Clinical trial registration:https://rctportal.niph.go.jp/en/detail?trial_id=UMIN000032634, UMIN-CTR UMIN000032634.
The ketogenic diet is a high-fat, low-carbohydrate diet with adequate amounts of protein that is designed to increase blood ketone bodies (1). The ketogenic diet is a nutrition therapy for the treatment of epilepsy (2). Recently, this diet has been reported to exert various effects on our health, such as losing weight, improving lipid profiles and enhancing cognitive functions (3–6). In addition, the ketogenic diet and ketone bodies have been shown to have a therapeutic potential in several pathological conditions, such as diabetes, polycystic ovary syndrome, acne, neurological diseases, respiratory and cardiovascular diseases, and cancer (7–9). Although the ketogenic diet is inexpensive, fairly easy to implement, it is not well tolerated in the long term (10). Dietary modifications improve the tolerability of the ketogenic diet. Besides carbohydrate restriction, ingesting medium-chain triglycerides (MCTs) is a common method to increase blood ketone bodies (11). MCTs in commercial products are mainly composed of medium-chain fatty acids, octanoic acid (C8) and decanoic acid (C10), and are rapidly digested and absorbed after ingestion and are converted to ketone bodies in the liver (12). A ketogenic diet using MCTs could allow a higher carbohydrate content in meals and achieve sufficient ketone body production (10).
We previously reported a case study suggesting that the ketogenic diet may be a promising supportive therapy for patients with various types of advanced cancer (13). In this ketogenic diet, the ketogenic formula, which contains MCTs, was used as supplements to enhance blood ketone bodies. The ketogenic formula is a special infant formula with a high-fat, low-carbohydrate composition, which has long been used for ketogenic dietary therapy for infants with congenital metabolic disorders and refractory epilepsy in Japan (2, 14). However, it has not been clear how much formula is needed to increase blood ketone body levels in adults. In our ketogenic diet regimen, the amount of ketogenic formula used was clinically determined based on practical experience, while referring to blood levels of ketone bodies (13). In this study, to establish an effective ketogenic diet regimen for adult patients, we investigated the pharmacokinetics of blood ketone bodies in healthy individuals supplemented with a single administration of ketogenic formula. In addition, we investigated the correlation between blood ketone bodies and breath acetone to evaluate the monitoring breath acetone.
This study was conducted with the approval of the ethical review committees at each facility of Osaka University, Osaka City University, medical institution for clinical trial implementation, and Meiji Co., Ltd., which is a joint research institute (approval numbers 17443, 4060, and 2017-022, respectively), and was registered in the University Hospital Information Network (UMIN) clinical trial system prior to enrollment of subjects (UMIN000032634).
The inclusion criteria were as follows: the subject signed a written informed consent form to participate in the study, was a male aged between 20 and 40, and had a body mass index between 18.5 and 25. The exclusion criteria were as follows: the subject was a smoker; was receiving drug treatment; had digestive abnormalities; was allergic to milk, soybeans, or pork; had lactose intolerance; was currently on a low-carbohydrate diet or a ketogenic diet; or had an abnormal value in blood biochemical tests (fasting blood glucose, hemoglobin A1c, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, γ-glutamyl transferase, blood urea nitrogen, uric acid, creatinine, triglycerides, high-density lipoprotein cholesterol, C-reactive protein, red blood cells, white blood cells, hemoglobin, hematocrit, and platelet count).
The subjects were provided the ketogenic formula (Ketonformula® 817-B, Meiji Co., Ltd., Tokyo, Japan) and the placebo formula. The ketogenic formula is a special infant formula with a high-fat, low-carbohydrate content, which is formulated with MCTs with a ketogenic ratio of 3:1. In a placebo formula, the MCTs contained in the ketogenic formula were replaced with long-chain triglycerides (LCTs). Seventy-five grams of powder was dissolved in water on the test day, and the total volume was prepared to 300 mL. The compositions of the formulas are listed in Table 1.
This study was conducted in a double-blind, randomized, parallel-group comparative manner. After consent was obtained, the subjects were randomly allocated using the block method. Twenty-four subjects were randomly assigned to four groups: (1) placebo (placebo formula 75 g; the amount of MCTs was 0 g); (2) low-dose (ketogenic formula 25 g + placebo formula 50 g; the amount of MCTs was 10 g); (3) medium-dose (ketogenic formula 50 g + placebo formula 25 g; the amount of MCTs was 20 g); and (4) high-dose (ketogenic formula 75 g; the amount of MCTs was 30 g).
The subjects visited the hospital without breakfast on the test day. Medical interviews and blood sampling were performed, and breath acetone concentration, vital signs, and the Gastrointestinal Symptom Rating Scale (GSRS) were measured as baseline data. Next, the test formula (about 300 mL) was ingested once. Blood was collected, and breath acetone concentration was measured at 0.5, 1, 1.5, 2, 3, 4, and 6 h after ingestion of the test formula. In addition, a medical interview was conducted at 3 and 6 h, and the GSRS score and vital signs were measured at 6 h. The GSRS score is a validated questionnaire for measurement of the quality of life of gastrointestinal symptoms regarding reflux, abdominal pain, indigestion, diarrhea, and constipation. The subjects were restricted from taking a meal in addition to the formula until the study was completed.
The blood samples were sent to LSI Medience Corporation1 for measurements of the concentrations of β-hydroxybutyrate (BHB), acetoacetic acid (AcAc), and glucose. The term “serum total ketone bodies (serum TKB)” represents the sum of BHB and AcAc concentrations in serum. We also measured the serum concentrations of medium-chain fatty acids: octanoic (C8), decanoic (C10), and dodecanoic (C12) acid. Serum samples were methyl esterificated using a Fatty Acid Methylation Kit (Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s protocol. Methylated medium-chain fatty acids were quantified using gas chromatography–mass spectrometry (GC–MS) (SHIMADZU, Kyoto, Japan). We measured breath acetone concentration using a breath acetone-measuring device (NTT Docomo, Japan).
The sample size was calculated based on our previous study, in which changes in plasma ketone bodies were tracked after a single administration of 50 g of the ketogenic formula to healthy aged subjects without cognitive impairment (5). We calculated that enrollment of six subjects in each group was required with an α of 5% and a power of 80%.
The data presented in the tables and figures are expressed as means ± standard deviation. Statistical analysis software R3.4.4 was used. The dose proportionality of maximum concentration (Cmax) and the area under the curve (AUC) were evaluated using a power model. The point estimate of the slope of the regression line and the confidence interval (CI) were calculated, and the confidence coefficient was 95%. Dose proportionality was concluded if the 95% CI included 1. Correlations between total serum ketone bodies and medium-chain fatty acids and breath acetone were investigated by Pearson’s correlation coefficient test. Differences were considered significant at p < 0.05.
Twenty-eight subjects were recruited, and four did not meet the inclusion criteria; therefore, 24 subjects were enrolled in the study (Supplementary Figure S1). The subjects were randomly divided into the placebo group (n = 6), low-dose group (n = 6), medium-dose group (n = 6), and high-dose group (n = 6). One subject assigned to the medium-dose group did not participate on the test day, so that the medium-dose group was investigated at n = 5. The demographic characteristics of the subjects at baseline are summarized in Table 2. There were no differences between the groups in height, weight, body mass index, muscle mass, or hematological values.
As shown in Figure 1A, in the high-dose group, serum TKB began to increase from 30 min after ingestion, and reached the peak concentration at 3–4 h (Table 3; Tmax: 3.7 ± 1.4 h). In addition, an increase in serum TKB was observed in the medium-dose group from 30 min after ingestion, which reached the peak concentration at 2 h after ingestion. However, the increase in serum TKB was delayed and reached the peak concentration only at 4–6 h in the low-dose and placebo groups. Next, using a power model, we analyzed whether the ketogenic formula induced serum TKB in a dose-dependent manner. Dose proportionality was demonstrated, as the 95% CI for AUC and Cmax included 1 (Table 3).
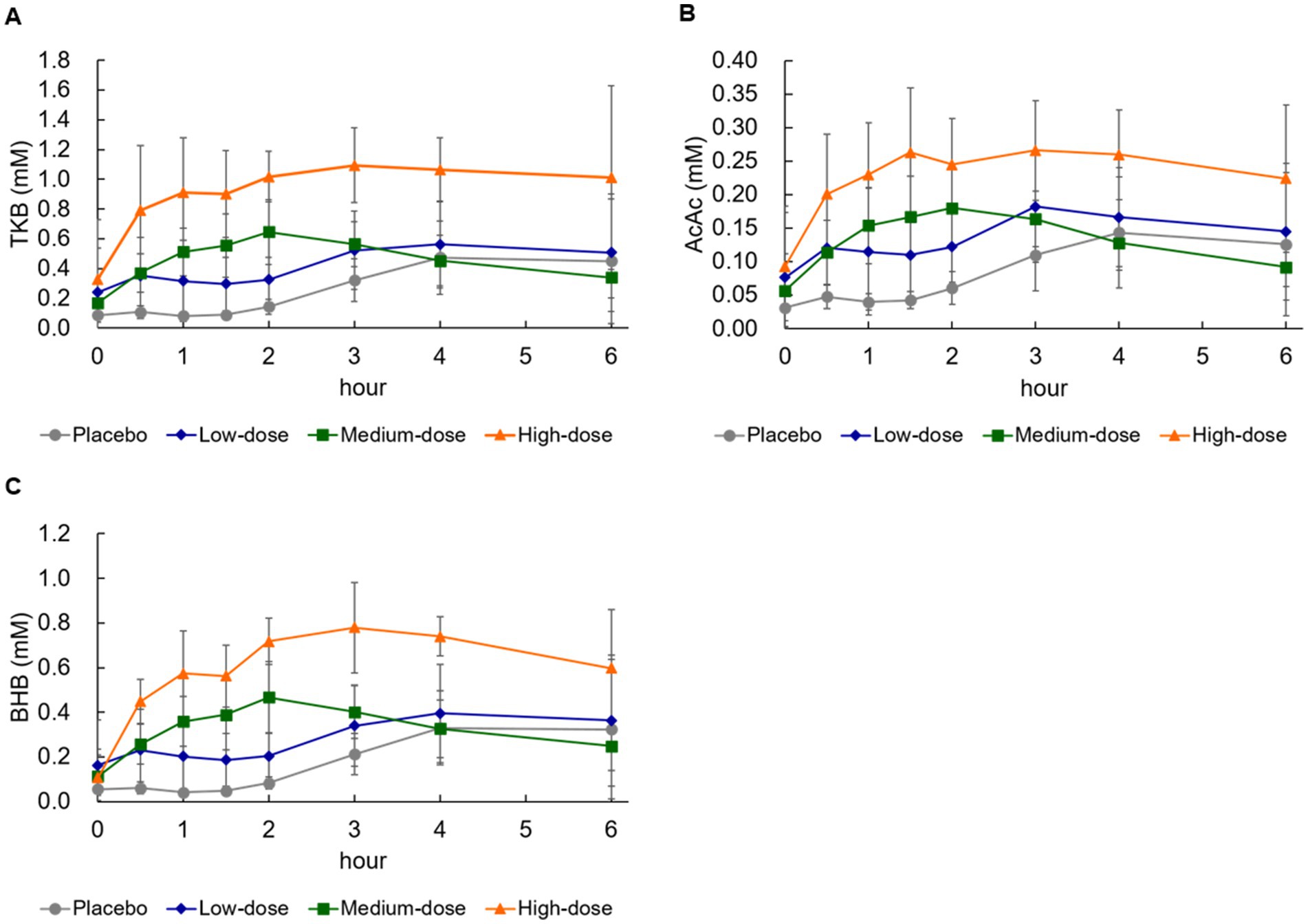
Figure 1. Changes in blood concentration of ketone bodies and glucose. (A) Total ketone bodies (TKB), (B) acetoacetic acid (AcAc), (C) β-hydroxybutyrate (BHB). Values are means ± standard deviation.
An increase in serum AcAc was noted from 30 min after ingestion in the high-dose and medium-dose groups, which reached the peak concentration at 3 h and 1.5–2 h, respectively (Table 4). On the contrary, the serum AcAc reached the peak concentration at 4–6 h in the low-dose and placebo groups. As shown in Table 4, neither the Cmax nor the AUC of AcAc was dose-proportional, because the 95% CI did not include 1 (Table 4).
The serum BHB concentration also began to increase from 30 min after ingestion in the high-dose and medium-dose groups, and the BHB reached the peak concentration at approximately 3 h and 2 h, respectively (Table 5). On the contrary, the increase in serum AcAc reached the peak concentration at 4–6 h in the low-dose and placebo groups. When evaluated using a power model, as shown in Table 5, the CI included 1, so it was judged that both Cmax and AUC of BHB were dose-proportional (Table 5).
Changes in serum octanoic acid concentration were detected 30 min after ingestion in the high-, medium-, and low-dose groups compared with the placebo group (Figure 2A). When evaluated using the power model, Cmax and AUC were judged to be dose-proportional (Table 6). Changes in serum decanoic acid concentration showed similar results, indicating dose proportionality (Figure 2B; Table 6). On the other hand, changes in serum concentrations of dodecanoic acid, which is abundant in the placebo formula, was detected 1 h after ingestion in the placebo group, but no dose proportionality was observed (Figure 2C; Table 6).
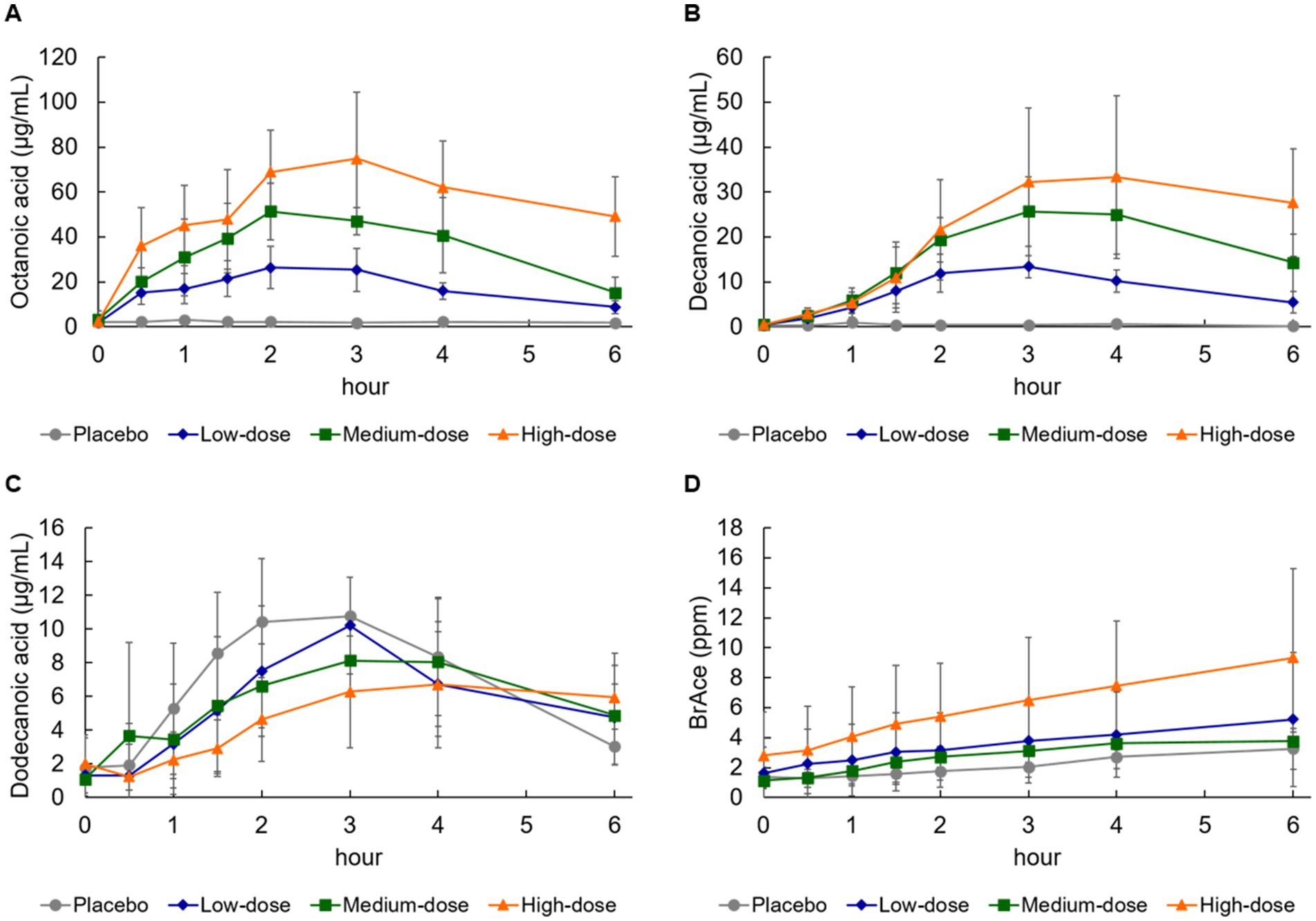
Figure 2. Changes in blood medium-chain fatty acid and breath acetone concentration. (A) Octanoic acid, (B) decanoic acid, (C) dodecanoic acid, and (D) breath acetone (BrAce). Values are means ± standard deviation.
Breath acetone gradually increased and reached the peak concentration at 4–6 h after ingestion of the formula in all groups (Figure 2D; Table 7). Breath acetone had a delayed peak (Figure 2D), unlike serum ketone bodies that reached the peak concentration at 0.5–4 h (Figures 1A–C). When evaluated using a power model, both Cmax and AUC were dose-proportional, although the amount of proportionality was weak (Table 7).
A statistically significant (p < 0.001) positive correlation was observed between serum TKB and octanoic and decanoic acid concentrations, but not with dodecanoic acid concentration (Figures 3A–C). TKB was also significantly correlated with breath acetone, which consists of metabolites of medium-chain fatty acids other than BHB and AcAc and is rapidly exhaled through the lungs (p < 0.001, Figure 3D). As a result of analysis at each time point, a significant positive correlation was also observed between TKB and breath acetone from 0.5 h to 6 h (Supplementary Figure S2).
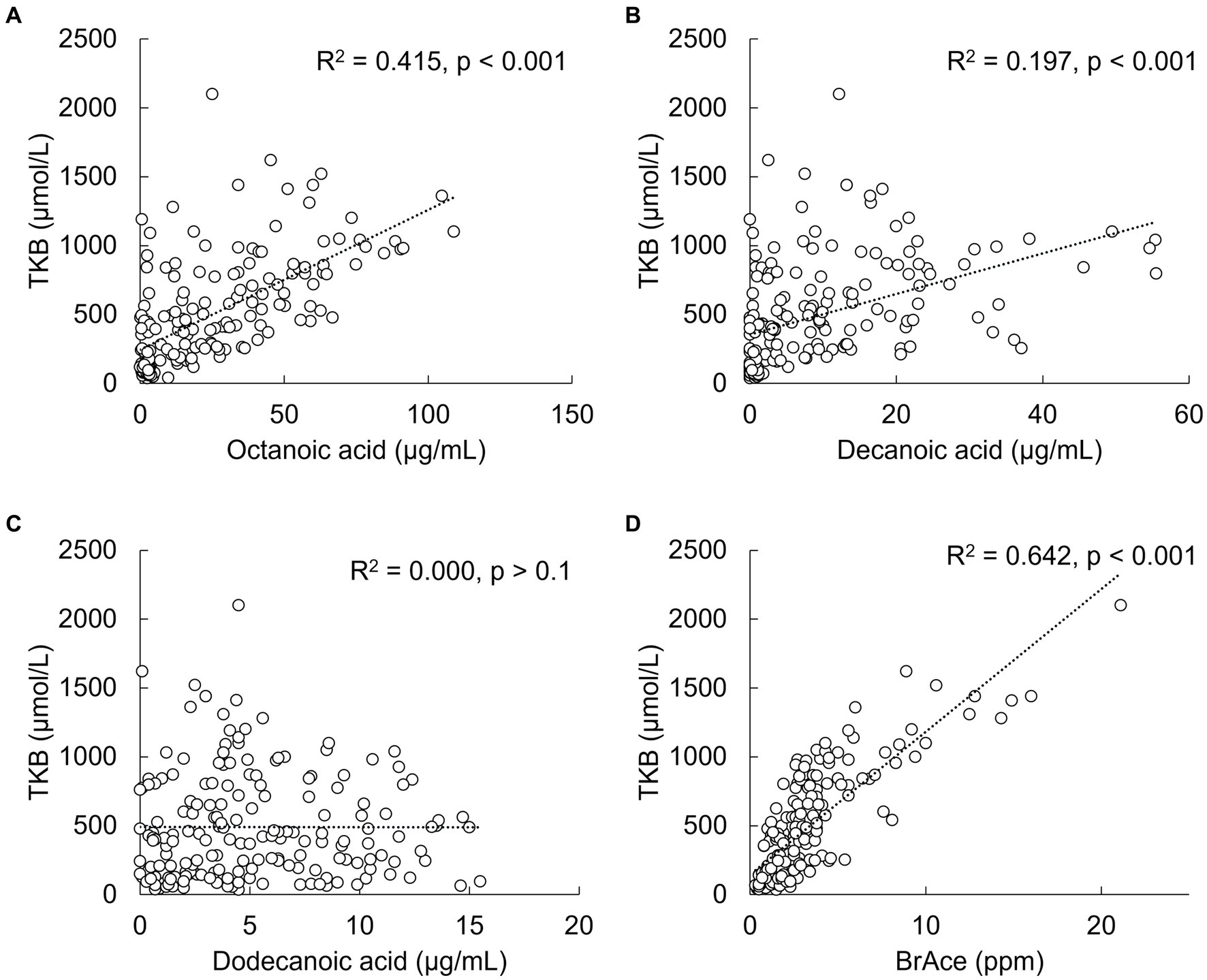
Figure 3. Correlations between total ketone bodies (TKB) and medium-chain fatty acid or breath acetone (BrAce) concentrations. (A) Octanoic acid, (B) decanoic acid, (C) dodecanoic acid, and (D) breath acetone.
No adverse events were observed from administration of ketogenic formula, and there were no significant changes in vital signs or GSRS scores (Supplementary Tables S1, S2). No significant changes in blood glucose were observed in any group after ingestion of ketogenic formula (Supplementary Figure S3).
Ketogenic formula has long been used in the ketogenic diet for children with refractory epilepsy or congenital metabolic disorders (15). On the other hand, it was not clear how much formula was needed to increase the blood concentration of ketone bodies in adults. In this study, we found that ketogenic formula increased total serum ketone bodies, β-hydroxybutyrate, and medium-chain fatty acids (octanoic acid and decanoic acid) in a dose-dependent manner in healthy adults. Ketogenic formula also gradually increased breath acetone in all groups.
From previous studies, it is considered that these results are largely due to the contribution of MCTs contained in ketogenic formula (16–19). MCTs are rapidly digested and absorbed after ingestion and are converted to ketone bodies in the liver (12). It has been shown that the blood concentration of ketone bodies increases, depending on the intake of MCTs (20), and is elevated at low doses of MCT (17). Moreover, blood ketone bodies were shown to be increased after MCT ingestion compared with after LCT ingestion (18, 19). In another study, an MCT-supplemented ketogenic diet increased blood β-hydroxybutyrate and led to nutritional ketosis compared with an LCT-based ketogenic diet (16). Competitive interactions of MCT and LCT were also reported (21). The presence of LCT does not affect the ketogenic effect of MCT, and adding LCT to MCT increases blood ketone body levels compared with MCT alone. Among MCTs, octanoic acid has the highest ketogenic effect compared with decanoic and dodecanoic acid, and decanoic acid has a higher ketogenic effect than dodecanoic acid (11). In the present study, ketogenic formula increased blood octanoic and decanoic acids as well as ketone bodies. Moreover, a positive correlation was observed between serum TKB and octanoic and decanoic acid concentrations. Therefore, the keto-inducing effect was considered to depend on the blood concentrations of octanoic and decanoic acids.
To our knowledge, this study is the first to investigate the changes in blood levels of medium-chain fatty acids and their metabolites, ketone bodies, and breath acetone levels after ingestion of MCT, whereas previous studies investigated only changes in blood ketone bodies and medium-chain fatty acids (11, 22, 23). In this study, blood ketone bodies after ketogenic formula ingestion were paralleled by breath acetone concentration. In addition, a strong correlation was observed between serum TKB and breath acetone concentration at each time point. Although some devices for measuring breath acetone are highly variable, the device used in this study has been reported to show a high correlation with the measurement using gas chromatography (24). We further confirmed that the intra-measurement variability of the device used in this study was 4.0% via a preliminary experiment (Supplementary Tables S3). Monitoring breath acetone levels is a less invasive method than measuring ketone bodies in the blood. Therefore, it seems beneficial to predict blood ketone body concentrations from breath acetone.
In this study, no adverse events were observed with the administration of ketogenic formula in healthy adults. Our findings are consistent with those of previous studies. No severe side effects were observed after a single dose of ketogenic formula in a previous study (5). On the other hand, it has been reported that MCT induces abdominal symptoms, such as diarrhea (22). It has also been reported that emulsification of MCT causes mild symptoms (22). MCT in the ketogenic formula is emulsified with LCT and milk protein during the manufacturing process. This may have reduced abdominal symptoms even with intake of ketogenic formula in this study. Hypoglycemia, which has been consistently observed in ketogenic interventions (25), was not observed in this study. The first possible reason is that hypoglycemia was suppressed by the effect of carbohydrates contained in the ketogenic formula. Another possible reason is that the amino acid alanine derived from protein in the ketogenic formula moderated the drop in blood glucose associated with ketosis (25).
The mechanism of the ketogenic diet has been thought to be due to the combined effects of ketone bodies and glucose restriction (26). It has been thought that the ketogenic diet induces metabolic changes in vivo by switching the energy source from carbohydrates to fatty acids and ketones bodies, resulting in therapeutic effects (26). Recently, cellular signaling functions of β-hydroxybutyrate have been reported (26, 27), and the administration of β-hydroxybutyrate as well as the ketogenic diet has been reported to prevent or improve several diseases such as neurological disorders, cardiovascular disease, diabetic kidney disease, and cancer (7, 28–30). Ingestion of exogenous ketone salts or esters is another method for increasing blood ketone bodies. It has reported that the ingestion of approximately 14 g of exogenous ketone salts could elevate blood ketone body levels to >1 mM, which corresponds to the ingestion of 30 g of MCT (31). Also, exogenous ketone salt has not been reported to cause noticeable adverse effects such as abdominal symptoms which were noted as side effects in MCT (31). On the contrary, when exogenous ketone salts are ingested, blood ketone bodies rise rapidly, but converge in approximately 2 h (31). In the present study, the ketogenic formula was able to maintain blood ketone body levels for 6 h throughout the study period without any noticeable side effects. From the findings of this study, it is considered desirable for adults to take ≥50 g of the ketogenic formula to maintain blood ketone body concentration for a prolonged duration.
One of the limitations of this study is that the number of subjects was small. With a small number of subjects, individual differences might affect the results more than differences in the test foods. Therefore, it is possible that some indicators did not detect significant differences due to the small number of subjects. Another limitation is that this study was performed in healthy participants instead of participants with cancer, which is the population receiving the ketogenic diet. Clinical and in vivo studies have shown that the ketogenic diet in combination with standard therapy has the potential to enhance the antitumor effects of classic cancer therapy and increase patients’ quality of life (10, 32). The ketogenic formula may contribute to the treatment or amelioration of these conditions. In addition, the participants were limited to men in this study. The reasons for this were to adjust the body sizes of the participants and to eliminate the influence of the menstrual cycle. It has been reported that the menstrual cycle affects the energy metabolism (33), and it is possible that the menstrual cycle affects the ketone body production. Maher et al. investigated the energy metabolism with women participants to consider the estrous cycle (34). To accumulate further evidence, it is essential to acquire knowledge about the utilization of the ketogenic formula and to establish a ketogenic diet regimen that can be safely implemented for various individuals, such as women and patients with cancer and other diseases. In this study, the participants were administered only the ketogenic formula in the fasting state. The ketogenesis of MCT is suppressed by consuming carbohydrate with MCT (35). Although we have previously demonstrated that the ketogenic formula increased blood ketone bodies under conditions of a ketogenic diet (36), further studies are required to investigate how much the ketogenesis of MCT decreases depending on the amount of carbohydrates ingested at the same time.
In conclusion, the ketogenic formula was increased serum ketone bodies in a dose-dependent manner. Breath acetone was also increased in correlation with blood ketone bodies, which indicates the benefit of monitoring breath acetone levels in order to predict blood ketone body concentrations during ketogenic diet therapy. The results of this study provide new insight to establish the ketogenic diet regimen in adults.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethical review committees at each facility of Osaka University, Osaka City University, medical institution for clinical trial implementation, and Meiji Co., Ltd. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
KN, KH, NN, HF, YO, and KA contributed to the conception and design of the study. KH, KN, MT, MN, HS, MM, ST, and HF performed the study and organized the database. AM performed the statistical analysis. KN wrote the first draft of the manuscript. KH and RE wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that this study received funding from Meiji Holdings Co., Ltd., and The Nisshin OilliO Group. The funder had the following involvement in the study: the study design, the writing of this article, and the decision to submit it for publication.
We appreciate Takehiro Yamaguchi, Akina Sasayama, and Yumi Yokoyama for their technical support.
KN, YO, and KA are employees of Meiji Holdings Co., Ltd. KH holds the position of Joint Research Chair, and RE is a member of the Joint Research group.
The authors declare that this study received funding from Meiji Holdings Co., Ltd., and The Nisshin OilliO Group. The funder had the following involvement in the study: the study design, the writing of this article, and the decision to submit it for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1224740/full#supplementary-material
1. Allen, BG, Bhatia, SK, Anderson, CM, Eichenberger-Gilmore, JM, Sibenaller, ZA, Mapuskar, KA, et al. Ketogenic diets as an adjuvant Cancer therapy: history and potential mechanism. Redox Biol. (2014) 2:963–70. doi: 10.1016/j.redox.2014.08.002
2. Takahashi, Y, Imai, K, Yamaguchi, T, Oboshi, T, Ikeda, H, Yoshitomi, S, et al. Therapeutic effects of ketone formula in patients with intractable epilepsy. No To Hattatsu. (2018) 50:44–9. doi: 10.11251/ojjscn.50.44
3. Drabińska, N, Wiczkowski, W, and Piskuła, MK. Recent advances in the application of a ketogenic diet for obesity management. Trends Food Sci Technol. (2021) 110:28–38. doi: 10.1016/j.tifs.2021.01.080
4. Dowis, K, and Banga, S. The potential health benefits of the ketogenic diet: a narrative review. Nutrients. (2021) 13:1654. doi: 10.3390/nu13051654
5. Ota, M, Matsuo, J, Ishida, I, Hattori, K, Teraishi, T, Tonouchi, H, et al. Effect of a ketogenic meal on cognitive function in elderly adults: potential for cognitive enhancement. Psychopharmacology. (2016) 233:3797–802. doi: 10.1007/s00213-016-4414-7
6. Yomogida, Y, Matsuo, J, Ishida, I, Ota, M, Nakamura, K, Ashida, K, et al. An Fmri investigation into the effects of ketogenic medium-chain triglycerides on cognitive function in elderly adults: a pilot study. Nutrients. (2021) 13:2134. doi: 10.3390/nu13072134
7. Cavaleri, F, and Bashar, E. Potential synergies of Β-Hydroxybutyrate and butyrate on the modulation of metabolism, inflammation, cognition, and general health. J Nutr Metab. (2018) 2018:7195760. doi: 10.1155/2018/7195760
8. Paoli, A, Rubini, A, Volek, JS, and Grimaldi, KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. (2013) 67:789–96. doi: 10.1038/ejcn.2013.116
9. Ota, M, Matsuo, J, Ishida, I, Takano, H, Yokoi, Y, Hori, H, et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer's disease. Neurosci Lett. (2019) 690:232–6. doi: 10.1016/j.neulet.2018.10.048
10. Weber, DD, Aminzadeh-Gohari, S, Tulipan, J, Catalano, L, Feichtinger, RG, and Kofler, B. Ketogenic diet in the treatment of Cancer – where do we stand? Mol Metabol. (2020) 33:102–21. doi: 10.1016/j.molmet.2019.06.026
11. St-Pierre, V, Vandenberghe, C, Lowry, C-M, Fortier, M, Castellano, C-A, Wagner, R, et al. Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day. Front Nutr. (2019) 6:46. doi: 10.3389/fnut.2019.00046
12. Odle, J . New insights into the utilization of medium-chain triglycerides by the neonate: observations from a piglet model. J Nutr. (1997) 127:1061–7. doi: 10.1093/jn/127.6.1061
13. Hagihara, K, Kajimoto, K, Osaga, S, Nagai, N, Shimosegawa, E, Nakata, H, et al. Promising effect of a new ketogenic diet regimen in patients with advanced Cancer. Nutrients. (2020) 12:1473. doi: 10.3390/nu12051473
14. Kumada, T, Imai, K, Takahashi, Y, Nabatame, S, and Oguni, H. Ketogenic diet using a Japanese ketogenic Milk for patients with epilepsy: a multi-institutional study. Brain Dev. (2018) 40:188–95. doi: 10.1016/j.braindev.2017.11.003
15. Hayashi, A, Kumada, T, Nozaki, F, Hiejima, I, Miyajima, T, and Fujii, T. Changes in serum levels of selenium, zinc and copper in patients on a ketogenic diet using Ketonformula. No To Hattatsu. (2013) 45:288–93. doi: 10.11251/ojjscn.45.288
16. Harvey, CJDC, Schofield, GM, Williden, M, and McQuillan, JA. The effect of medium chain triglycerides on time to nutritional ketosis and symptoms of keto-induction in healthy adults: a randomised controlled clinical trial. J Nutr Metab. (2018) 2018:2630565–9. doi: 10.1155/2018/2630565
17. Courchesne-Loyer, A, Fortier, M, Tremblay-Mercier, J, Chouinard-Watkins, R, Roy, M, Nugent, S, et al. Stimulation of mild, sustained Ketonemia by medium-chain Triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition (Burbank, Los Angeles County, Calif). (2013) 29:635–40. doi: 10.1016/j.nut.2012.09.009
18. Seaton, TB, Welle, SL, Warenko, MK, and Campbell, RG. Thermic effect of medium-chain and long-chain triglycerides in man. Am J Clin Nutr. (1986) 44:630–4. doi: 10.1093/ajcn/44.5.630
19. Van Wymelbeke, V, Himaya, A, Louis-Sylvestre, J, and Fantino, M. Influence of medium-chain and long-chain Triacylglycerols on the control of food intake in men. Am J Clin Nutr. (1998) 68:226–34. doi: 10.1093/ajcn/68.2.226
20. Cunnane, SC, Courchesne-Loyer, A, Vandenberghe, C, St-Pierre, V, Fortier, M, Hennebelle, M, et al. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front Mol Neurosci. (2016) 9:53. doi: 10.3389/fnmol.2016.00053
21. Cotter, R, Johnson, RC, Young, SK, Lin, LI, and Rowe, WB. Competitive effects of long-chain-triglyceride emulsion on the metabolism of medium-chain-triglyceride emulsions. Am J Clin Nutr. (1989) 50:794–800. doi: 10.1093/ajcn/50.4.794
22. Courchesne-Loyer, A, Lowry, CM, St-Pierre, V, Vandenberghe, C, Fortier, M, Castellano, CA, et al. Emulsification increases the acute ketogenic effect and bioavailability of medium-chain triglycerides in humans: protein, carbohydrate, and fat metabolism. Curr Dev Nutr. (2017) 1:e000851. doi: 10.3945/cdn.117.000851
23. Vandenberghe, C, St-Pierre, V, Fortier, M, Castellano, CA, Cuenoud, B, and Cunnane, SC. Medium chain triglycerides modulate the ketogenic effect of a metabolic switch. Front Nutr. (2020) 7:3. doi: 10.3389/fnut.2020.00003
24. Toyooka, T, Hiyama, S, and Yamada, Y. A prototype portable breath acetone analyzer for monitoring fat loss. J Breath Res. (2013) 7:036005. doi: 10.1088/1752-7155/7/3/036005
25. Soto-Mota, A, Norwitz, NG, Evans, RD, and Clarke, K. Exogenous D-Β-Hydroxybutyrate lowers blood glucose in part by decreasing the availability of L-alanine for gluconeogenesis. Endocrinol Diabet Metabol. (2022) 5:e00300. doi: 10.1002/edm2.300
26. Boison, D . New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol. (2017) 30:187–92. doi: 10.1097/wco.0000000000000432
27. Newman, JC, and Verdin, E. Β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. (2017) 37:51–76. doi: 10.1146/annurev-nutr-071816-064916
28. Han, YM, Ramprasath, T, and Zou, MH. Β-Hydroxybutyrate and its metabolic effects on age-associated pathology. Exp Mol Med. (2020) 52:548–55. doi: 10.1038/s12276-020-0415-z
29. Tomita, I, Kume, S, Sugahara, S, Osawa, N, Yamahara, K, Yasuda-Yamahara, M, et al. Sglt2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced Mtorc1 inhibition. Cell Metab. (2020) 32:404–19.e6. doi: 10.1016/j.cmet.2020.06.020
30. Poff, AM, Ari, C, Arnold, P, Seyfried, TN, and D'Agostino, DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic Cancer. Int J Cancer. (2014) 135:1711–20. doi: 10.1002/ijc.28809
31. Cuenoud, B, Hartweg, M, Godin, J-P, Croteau, E, Maltais, M, Castellano, C-A, et al. Metabolism of exogenous D-Beta-Hydroxybutyrate, an energy substrate avidly consumed by the heart and kidney. Front Nutr. (2020) 7:7. doi: 10.3389/fnut.2020.00013
32. Klement, RJ . The emerging role of ketogenic diets in Cancer treatment. Curr Opin Clin Nutr Metab Care. (2019) 22:129–34. doi: 10.1097/mco.0000000000000540
33. Benton, MJ, Hutchins, AM, and Dawes, JJ. Effect of menstrual cycle on resting metabolism: a systematic review and Meta-analysis. PLoS One. (2020) 15:e0236025. doi: 10.1371/journal.pone.0236025
34. Maher, T, Deleuse, M, Thondre, S, Shafat, A, and Clegg, ME. A comparison of the satiating properties of medium-chain triglycerides and conjugated linoleic acid in participants with healthy weight and overweight or obesity. Eur J Nutr. (2021) 60:203–15. doi: 10.1007/s00394-020-02235-y
35. Heidt, C, Fobker, M, Newport, M, Feldmann, R, Fischer, T, and Marquardt, T. Beta-Hydroxybutyrate (Bhb), glucose, insulin, Octanoate (C8), and Decanoate (C10) responses to a medium-chain triglyceride (Mct) oil with and without glucose: a single-center study in healthy adults. Nutrients. (2023) 15:1148. doi: 10.3390/nu15051148
36. Nakamura, K, Hagihara, K, Nagai, N, Egashira, R, Takeuchi, M, Nakano, M, et al. Ketogenic effects of multiple doses of a medium chain triglycerides enriched ketogenic formula in healthy men under the ketogenic diet: a randomized, double-blinded, placebo-controlled study. Nutrients. (2022) 14:1199. doi: 10.3390/nu14061199
Keywords: medium chain triglycerides, ketogenic diet, single dose study, dose response, breath acetone
Citation: Nakamura K, Hagihara K, Nagai N, Egashira R, Takeuchi M, Nakano M, Saito H, Moriguchi M, Tonari S, Fujii H, Miyake A, Omae Y and Ashida K (2023) Ketogenic effects of medium chain triglycerides containing formula and its correlation to breath acetone in healthy volunteers: a randomized, double-blinded, placebo-controlled, single dose-response study. Front. Nutr. 10:1224740. doi: 10.3389/fnut.2023.1224740
Received: 18 May 2023; Accepted: 23 August 2023;
Published: 27 September 2023.
Edited by:
Cheng Zheng, University of Nebraska Medical Center, United StatesReviewed by:
Adrian Soto-Mota, National Institute of Medical Sciences and Nutrition Salvador Zubirán, MexicoCopyright © 2023 Nakamura, Hagihara, Nagai, Egashira, Takeuchi, Nakano, Saito, Moriguchi, Tonari, Fujii, Miyake, Omae and Ashida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keisuke Hagihara, ay5oYWdpaGFyYUBrYW5wb3UubWVkLm9zYWthLXUuYWMuanA=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.