- 1The First Clinical College, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Department of Vascular Surgery, The First Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 3The Traditional Chinese Medicine College, Shandong University of Traditional Chinese Medicine, Jinan, China
Background: Calcium and magnesium are essential minerals that have significant roles in nerve function and regulation. There may be a correlation between dietary calcium and magnesium intake and peripheral neuropathy. However, this relationship remains unclear and requires further study.
Methods: Data from 7,726 participants in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2004 were analyzed in this study. The relationship between total dietary calcium and magnesium intake, as well as each quantile, and peripheral neuropathy was analyzed using a multifactor logistic regression model. To illustrate the dose–response relationship between calcium and magnesium intake and peripheral neuropathy, we utilized a restricted cubic spline (RCS) plot.
Results: Our analysis found a positive correlation between dietary intake of calcium and magnesium and peripheral neuropathy (calcium: OR 1.000, 95% CI 1.000–1.000; magnesium: OR 1.001, 95% CI 1.00–1.002). Participants in the first and third quantiles of dietary calcium intake had a significantly higher incidence of peripheral neuropathy than those in the second quantile (OR 1.333, 95% CI 1.034–1.719, OR 1.497, 95% CI 1.155–1.941). Those in the first and third quantiles of dietary magnesium intake also had a significantly higher incidence of peripheral neuropathy than those in the second quantile (OR 1.275, 95% CI 1.064–1.528, OR 1.525, 95% CI 1.231–1.890). The restricted cubic spline analysis revealed a U-shaped nonlinear relationship between dietary intake of calcium and magnesium and peripheral neuropathy.
Conclusion: The study found a U-shaped non-linear relationship between dietary calcium and magnesium intake levels and peripheral neuropathy, indicating that both excessive and insufficient intake of calcium and magnesium can increase the incidence of peripheral neuropathy.
Introduction
Peripheral neuropathy (PN) is marked by various symptoms associated with sensory and motor dysfunction, like numbness, pain, burning sensation, and muscle atrophy (1). It typically starts in the feet and gradually progresses towards the proximal end, rarely affecting the upper limbs alone (2). Peripheral neuropathy is associated with prolonged and stubborn ulcers, higher amputation, and all-cause mortality rates (3). Its incidence is increasing, especially among the elderly, posing a significant public health challenge (4). The disease is linked to various pathological factors, including diabetes, abnormal immune function, exposure to toxins (such as chemotherapy, drugs, and environmental pollution), and abnormal nutritional status (5). Currently, there are no specific drugs or treatments for peripheral neuropathy, and preventing its onset is a focus of ongoing research (6).
Calcium and magnesium are essential micronutrients that play a vital role in regulating nerve function and muscle conduction (7, 8). Studies suggest that an imbalance of calcium and magnesium homeostasis in the body can cause peripheral nerve degeneration and pain (9–11). However, there is limited research on the relationship between dietary calcium and magnesium intake and peripheral neuropathy. Therefore, we aimed to investigate the potential association between dietary calcium and magnesium intake and peripheral neuropathy in the general adult population using NHANES data from 1999 to 2004.
Methods
Data source
The NHANES is a nationwide survey organized by the National Center for Health Statistics (NCHS) in the US. It evaluates the health and nutritional status of Americans through a variety of physical exams, laboratory tests, and interviews. The data analyzed in our study was gathered during the 1999–2004 NHANES cycles. The NHANES 1999–2004 research protocol was approved by the NCHS, and no further institutional review committee approval is necessary for this secondary analysis.
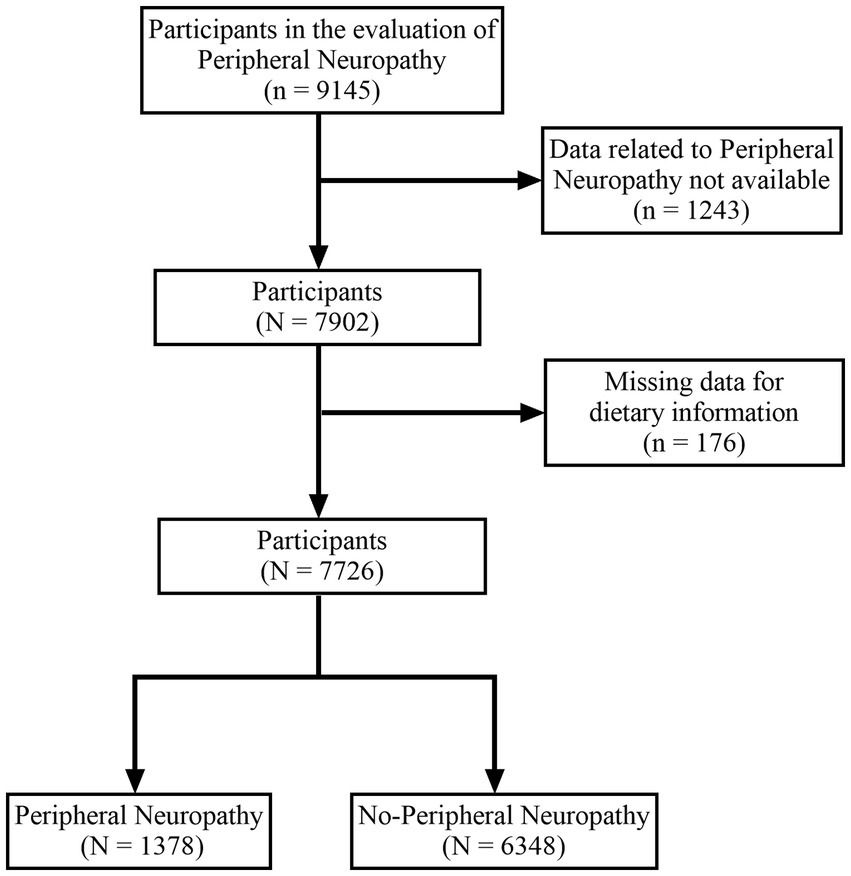
A total of 9,145 subjects participated in the evaluation of peripheral neuropathy. We excluded subjects who could not provide information on PN (n = 1,243) and lacked relevant dietary data (n = 176). Ultimately, our study included 7,726 subjects, comprising 1,378 subjects with peripheral neuropathy and 6,348 subjects without peripheral neuropathy (Figure 1).
Peripheral neuropathy
The health technician used a standard monofilament (Semmes-Weinstein nylon, size 5.07) to apply pressure on three sites of each foot to test the participant’s foot sensation. In case the respondent could not correctly answer or was unsure of the monofilament’s location, the site was considered non-sensory. The presence of peripheral neuropathy was determined as having at least one non-sensory site on both feet (12).
Dietary intake of calcium and magnesium
The participants’ dietary intake of calcium and magnesium was estimated using the 24 h dietary recall method. This method involves collecting a short and precise list of foods and beverages consumed by the individual over 24 h. The calcium and magnesium content in food is determined by the United States Department of Agriculture’s National Nutrient Database. Trained technicians collect dietary data and conduct nutrient composition analysis to ensure the information’s authenticity and accuracy. The NHANES Dietary Interviewer Procedures Manual provides detailed instructions for this process.
Covariates
Covariates considered in the analysis were age, gender, race (white, black, Mexican American, or other races), smoking status (now, former, or never), body mass index (BMI), and the presence of cardiovascular disease (CVD), hyperlipidemia, hypertension, diabetes, and chronic kidney disease (CKD). We divided patients with CKD into three groups based on the prognostic risk in the KDIGO 2021 Clinical Practice Guidelines for Glomerular Disease Management: medium risk, high risk, and extremely high risk (13). The diagnostic criteria for the diseases included in the covariates can be found in the attachment (Supplementary material).
Statistical analysis
We utilized R studio (4.2.1) to analyze NHANES data from 1999 to 2004. All statistical analyses were weighted. Continuous variables were presented as mean ± standard deviation, while categorical variables were expressed as numbers (percentages). We divided the population into three quantiles based on their dietary intake of calcium and magnesium. For calcium intake, the critical values of the three quantiles were: Q1 ≤ 481 mg, 481 mg < Q2 ≤ 843 mg, and Q3 > 843 mg. For magnesium intake, the critical values of the three quantiles were: Q1 ≤ 196 mg, 196 mg < Q2 ≤ 299 mg, and Q3 > 299 mg. The relationship between total dietary calcium and magnesium intake, as well as each quantile, and peripheral neuropathy was analyzed using a multifactor logistic regression model. To illustrate the dose–response relationship between calcium and magnesium intake and peripheral neuropathy, we utilized a restricted cubic spline (RCS) plot.
Results
Baseline information
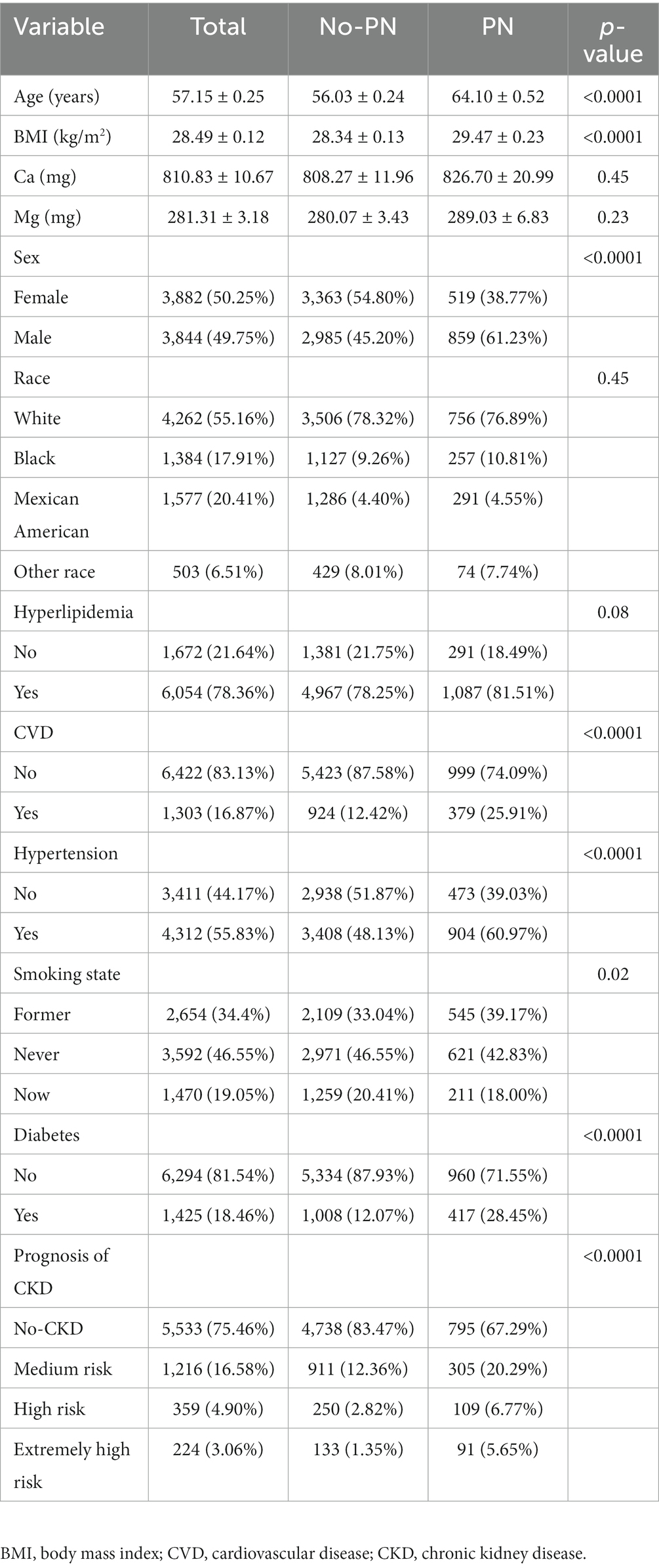
Table 1 presents a comprehensive overview of the characteristics of 7,726 eligible participants, with an average age of 57.15 years, an average dietary calcium intake of 810.83 mg, and an average dietary magnesium intake of 281.31 mg. The participants were categorized into two groups based on the presence or absence of PN. Significant differences were observed between the two groups in terms of age, gender, BMI, smoking status, and the prevalence of hypertension, diabetes, CVD, and prognosis of CKD (p < 0.05). However, there was no significant difference between the two groups in terms of race, dietary calcium and magnesium intake, and the prevalence of hyperlipidemia (p > 0.05). Analysis of the three quantiles of dietary calcium and magnesium intake (Tables 2, 3) revealed a lower incidence rate of peripheral neuropathy in the second quantile of dietary calcium and magnesium intake compared to the first and third quantiles.
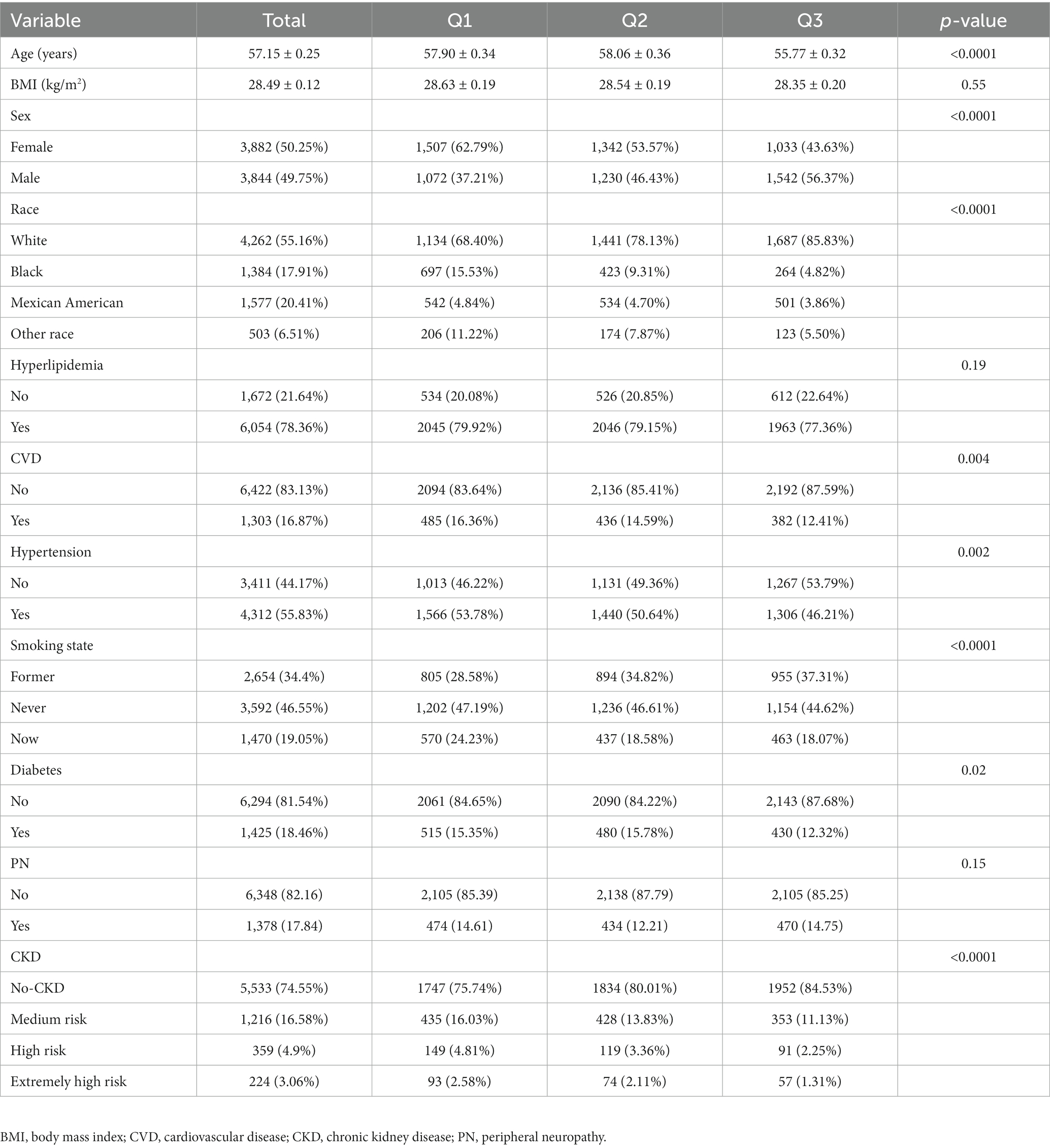
Table 2. Baseline characteristics of all subjects, stratified by the triple quantile of calcium intake.
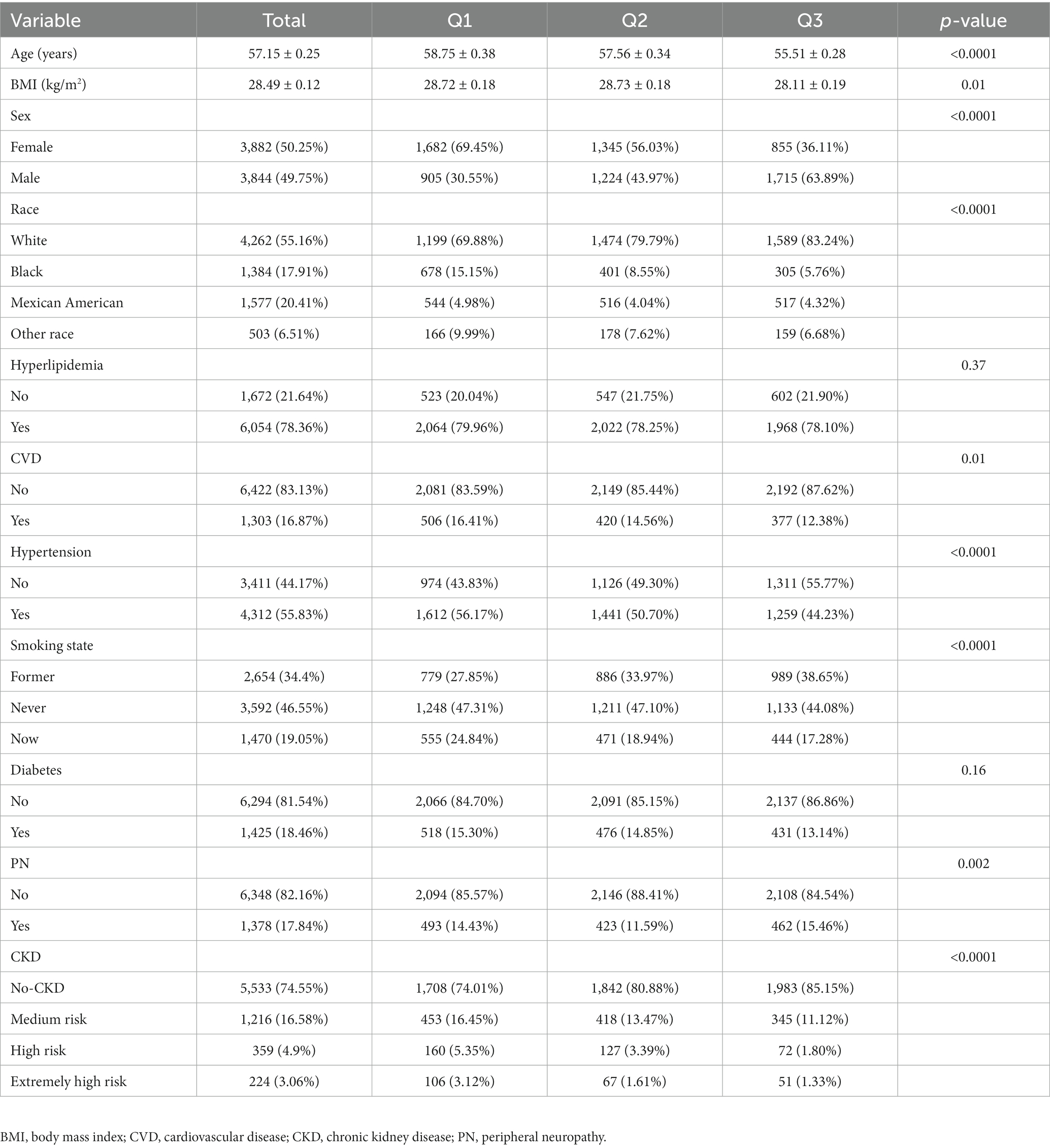
Table 3. Baseline characteristics of all subjects, stratified by the triple quantile of magnesium intake.
The relationship between dietary calcium and magnesium intake and peripheral neuropathy
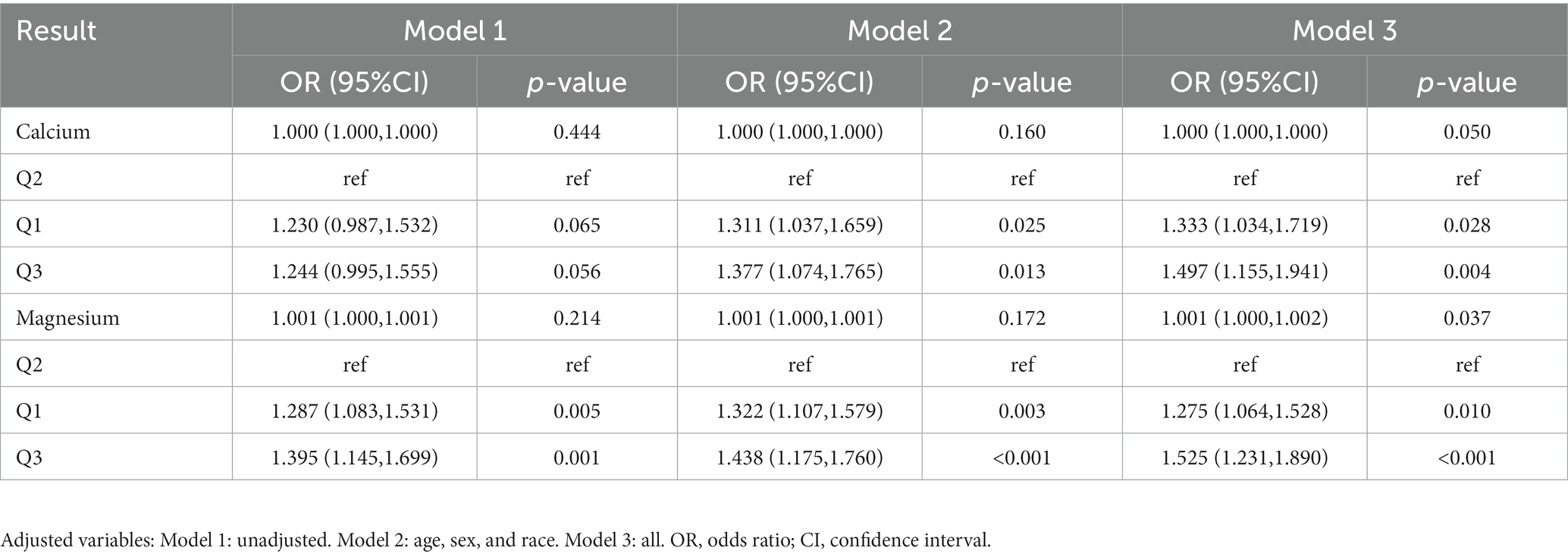
After adjusting for all covariates, the multifactor logistic regression analysis (Table 4) revealed a positive correlation between dietary calcium and magnesium intake and peripheral neuropathy (calcium: OR: 1.000, 95%CI: 1.000–1.000; magnesium: OR: 1.001, 95%CI: 1.000–1.002). When dietary calcium and magnesium intake were divided into three quantiles, it was observed that the incidence rate of peripheral neuropathy was significantly higher in the first and third quantiles of dietary calcium intake compared to the second quantile (OR 1.333, 95% CI 1.034–1.719, OR 1.497, 95% CI 1.155–1.941). The incidence of peripheral neuropathy in the first and third quantiles of dietary magnesium intake was also significantly higher than that in the second quantiles (OR 1.275, 95% CI 1.064–1.528, OR 1.525, 95% CI 1.231–1.890). This suggests the possibility of a non-linear relationship between dietary calcium and magnesium intake and peripheral neuropathy.
RCS
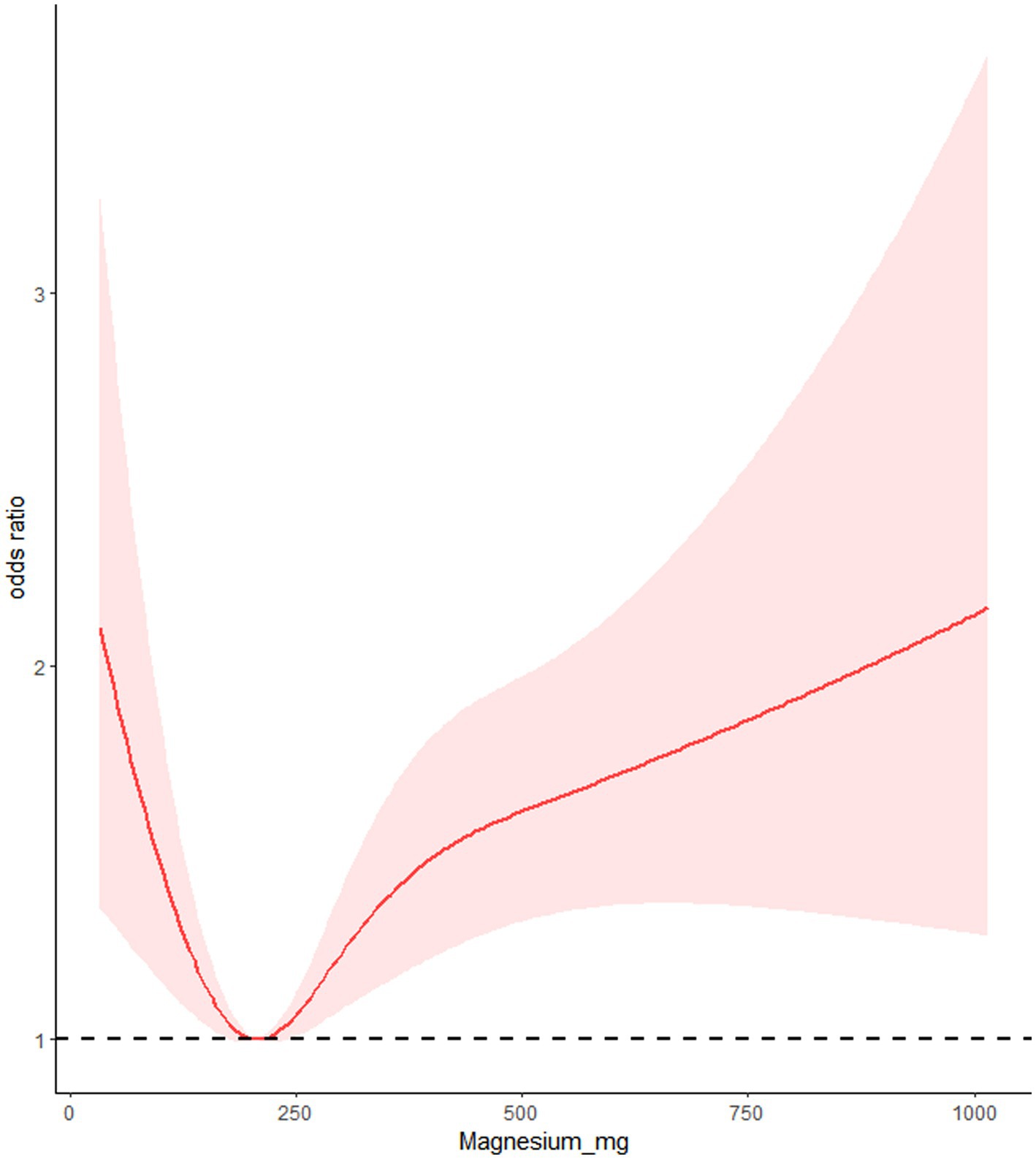
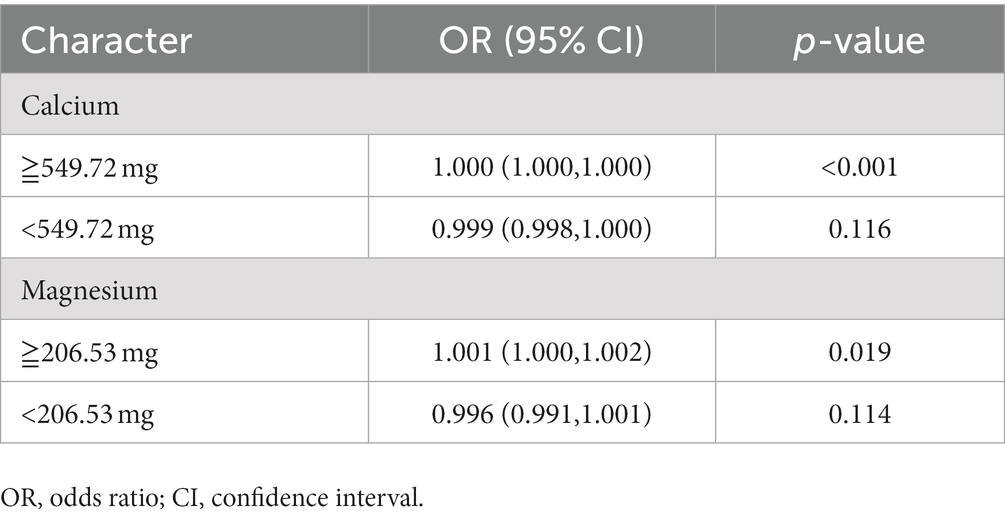
We utilized RCS to analyze the relationship between dietary calcium and magnesium intake and peripheral neuropathy. The RCS results revealed a U-shaped nonlinear relationship between dietary calcium and magnesium and peripheral neuropathy (p for nonlinear <0.001). The critical point of the U-shaped curve for dietary calcium intake and peripheral neuropathy was determined to be 549.72 mg. When dietary calcium intake was below 549.72 mg, there was a negative correlation between dietary calcium intake and the incidence of peripheral neuropathy. However, when dietary calcium intake exceeded 549.72 mg, there was a positive correlation between dietary calcium intake and the incidence of peripheral neuropathy. The critical point of the U-shaped curve for dietary magnesium intake and peripheral neuropathy was determined to be 206.53 mg. When magnesium intake was below 206.53 mg, there was a negative correlation between dietary magnesium intake and the incidence of peripheral neuropathy. When magnesium intake exceeded 206.53 mg, there was a positive correlation between dietary magnesium intake and the incidence of peripheral neuropathy (Figures 2, 3).
Threshold effect analysis
After adjusting for all covariates, the results of the stratification analysis (Table 5) showed an overall negative correlation trend between dietary calcium and magnesium intake and peripheral neuropathy when dietary calcium and magnesium intake did not exceed the critical values (calcium: OR: 0.999, 95%CI: 0.998–1.000; magnesium: OR: 0.996, 95%CI: 0.991–1.001). When dietary calcium and magnesium intake exceeded the critical value, there was a significant positive correlation between dietary calcium and magnesium intake and peripheral neuropathy (calcium: OR: 1.000, 95%CI: 1.000–1.000; magnesium: OR: 1.001, 95%CI: 1.000–1.002).
Discussion
In this cross-sectional study, we aimed to investigate the relationship between dietary intake levels of calcium and magnesium and peripheral neuropathy in the general population of the United States. Our findings showed a U-shaped nonlinear relationship between dietary intake levels of calcium and magnesium and peripheral neuropathy, indicating that both excessive and insufficient intake of these minerals can increase the incidence of peripheral neuropathy. To our knowledge, this is the first study to explore the association between dietary intake levels of calcium and magnesium and peripheral neuropathy in the general population.
Previous research on the relationship between calcium and magnesium and peripheral neuropathy has not been consistent, especially in cancer patients (14–16). While some studies have reported that calcium and magnesium infusion can reduce the incidence and severity of peripheral neuropathy after chemotherapy (15, 17), more studies have reported negative results (18–20). In addition, Wesselink et al. found that magnesium intake is associated with the incidence of chemotherapy-induced peripheral neuropathy in cancer patients, while calcium intake is not (21). But, research on the relationship between calcium and magnesium and peripheral neuropathy in non-cancer populations is limited, with no reports on the relationship between dietary intake of calcium and magnesium and peripheral neuropathy in the general population.
Calcium and magnesium are both essential minerals that play important roles in the function and regulation of peripheral nerves (22–24). Calcium and magnesium play a critical role in maintaining the stability and excitability of the nerve cell (25, 26). Calcium and magnesium homeostasis helps maintain appropriate membrane potential, thus ensuring the transmission of nerve impulses. They also regulate signal transduction within nerve cells, with calcium ions controlling the permeability of potassium and sodium ions, and magnesium ions potentially affecting calcium ion signal transduction (27). In addition, calcium and magnesium participate in various neural cell metabolic processes, including protein synthesis and decomposition, enzyme activation, and energy metabolism, which impact the survival and function of neural cells (28, 29). Proper levels of calcium and magnesium also help maintain the pH value within nerve cells. Therefore, deficiency or excess of calcium or magnesium can affect the function and regulation of peripheral nerves.
Peripheral neuropathy is commonly associated with diabetes (30), but our research suggests that diabetes accounts for only 28.45% of all cases of peripheral neuropathy. This suggests that neuropathy caused by other factors may be significantly underdiagnosed or overlooked (31). In addition, it is important to note that the co-occurrence of peripheral neuropathy with other chronic diseases such as hypertension, CVD, and CKD could potentially complicate the management and treatment of peripheral neuropathy. In this study, 60.97% of the subjects with peripheral neuropathy also suffered from hypertension, 25.91% of the patients also suffered from CVD. It also indicates that peripheral neuropathy may be related to other chronic diseases except diabetes.
We must acknowledge the study’s limitations. One of the primary constraints is that this is a cross-sectional study, and as such, it cannot establish the underlying reasons for the observed non-linear relationship between dietary calcium and magnesium intake and peripheral neuropathy. Additionally, the use of the 24 h recall method to determine dietary calcium and magnesium intake may not accurately reflect long-term calcium and magnesium intake.
Conclusion
Our study revealed a U-shaped-nonlinear correlation between dietary calcium and magnesium intake levels and peripheral neuropathy in the general population of the United States. These findings suggest that maintaining appropriate levels of calcium and magnesium intake could potentially aid in preventing peripheral neuropathy. Nevertheless, further research is required to validate these outcomes and examine the potential mechanisms underlying the association between dietary calcium and magnesium intake and peripheral neuropathy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
NHANES is a nationwide survey organized by the National Center for Health Statistics (NCHS) in the US. The NHANES 1999–2004 research protocol was approved by the NCHS, and no further institutional review committee approval is necessary for this secondary analysis. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZW led the study design and data collection. XY, ZR, and LL contributed to the interpretation of the results. BW and JW contributed to the manuscript writing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1217465/full#supplementary-material
References
1. Castelli, G, Desai, KM, and Cantone, RE. Peripheral neuropathy: evaluation and differential diagnosis. Am Fam Physician. (2020) 102:732–9.
2. Watson, JC, and Dyck, PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. (2015) 90:940–51. doi: 10.1016/j.mayocp.2015.05.004
3. Doughty, CT, and Seyedsadjadi, R. Approach to peripheral neuropathy for the primary care clinician. Am J Med. (2018) 131:1010–6. doi: 10.1016/j.amjmed.2017.12.042
4. Barrell, K, and Smith, AG. Peripheral neuropathy. Med Clin North Am. (2019) 103:383–97. doi: 10.1016/j.mcna.2018.10.006
5. Siao, P, and Kaku, M. A clinician’s approach to peripheral neuropathy. Semin Neurol. (2019) 39:519–30. doi: 10.1055/s-0039-1694747
6. Jones, MR, Urits, I, Wolf, J, Corrigan, D, Colburn, L, Peterson, E, et al. Drug-induced peripheral neuropathy: a narrative review. Curr Clin Pharmacol. (2020) 15:38–48. doi: 10.2174/1574884714666190121154813
7. Chen, Y-Y, Feng, L-M, Xu, D-Q, Yue, S-J, Fu, R-J, Zhang, M-M, et al. Combination of paeoniflorin and liquiritin alleviates neuropathic pain by lipid metabolism and calcium signaling coordination. Front Pharmacol. (2022) 13:944386. doi: 10.3389/fphar.2022.944386
8. Cruz-Jentoft, AJ, Dawson Hughes, B, Scott, D, Sanders, KM, and Rizzoli, R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: a narrative review. Maturitas. (2020) 132:57–64. doi: 10.1016/j.maturitas.2019.11.007
9. Zhang, J, Zhang, B, Zhang, J, Lin, W, and Zhang, S. Magnesium promotes the regeneration of the peripheral nerve. Front Cell Dev Biol. (2021) 9:717854. doi: 10.3389/fcell.2021.717854
10. Dolati, S, Rikhtegar, R, Mehdizadeh, A, and Yousefi, M. The role of magnesium in pathophysiology and migraine treatment. Biol Trace Elem Res. (2020) 196:375–83. doi: 10.1007/s12011-019-01931-z
11. Guo, C, and Ma, Y-Y. Calcium permeable-AMPA receptors and excitotoxicity in neurological disorders. Front Neural Circuits. (2021) 15:711564. doi: 10.3389/fncir.2021.711564
12. Hicks, CW, Wang, D, Windham, BG, Matsushita, K, and Selvin, E. Prevalence of peripheral neuropathy defined by monofilament insensitivity in middle-aged and older adults in two US cohorts. Sci Rep. (2021) 11:19159. doi: 10.1038/s41598-021-98565-w
13. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the Management of Glomerular Diseases. Kidney Int. (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
14. Selvy, M, Pereira, B, Kerckhove, N, Busserolles, J, Farsi, F, Guastella, V, et al. Prevention, diagnosis and management of chemotherapy-induced peripheral neuropathy: a cross-sectional study of French oncologists’ professional practices. Support Care Cancer. (2021) 29:4033–43. doi: 10.1007/s00520-020-05928-6
15. Colvin, LA. Chemotherapy-induced peripheral neuropathy: where are we now? Pain. (2019) 160:S1–S10. doi: 10.1097/j.pain.0000000000001540
16. Jordan, B, Jahn, F, Beckmann, J, Unverzagt, S, Müller-Tidow, C, and Jordan, K. Calcium and magnesium infusions for the prevention of oxaliplatin-induced peripheral neurotoxicity: a systematic review. Oncology. (2016) 90:299–306. doi: 10.1159/000445977
17. Gamelin, L, Boisdron-Celle, M, Delva, R, Guérin-Meyer, V, Ifrah, N, Morel, A, et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. (2004) 10:4055–61. doi: 10.1158/1078-0432.CCR-03-0666
18. Wu, Z, Ouyang, J, He, Z, and Zhang, S. Infusion of calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in colorectal cancer: a systematic review and meta-analysis. Eur J Cancer. (2012) 48:1791–8. doi: 10.1016/j.ejca.2012.03.018
19. Loprinzi, CL, Qin, R, Dakhil, SR, Fehrenbacher, L, Flynn, KA, Atherton, P, et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol. (2014) 32:997–1005. doi: 10.1200/JCO.2013.52.0536
20. Xu, XT, Dai, ZH, Xu, Q, Qiao, YQ, Gu, Y, Nie, F, et al. Safety and efficacy of calcium and magnesium infusions in the chemoprevention of oxaliplatin-induced sensory neuropathy in gastrointestinal cancers. J Dig Dis. (2013) 14:288–98. doi: 10.1111/1751-2980.12050
21. Wesselink, E, Winkels, RM, van Baar, H, Geijsen, AJMR, van Zutphen, M, van Halteren, HK, et al. Dietary intake of magnesium or calcium and chemotherapy-induced peripheral neuropathy in colorectal cancer patients. Nutrients. (2018) 10:398. doi: 10.3390/nu10040398
22. Yan, Y, Shen, F-Y, Agresti, M, Zhang, L-L, Matloub, HS, LoGiudice, JA, et al. Best time window for the use of calcium-modulating agents to improve functional recovery in injured peripheral nerves-an experiment in rats. J Neurosci Res. (2017) 95:1786–95. doi: 10.1002/jnr.24009
23. Volpe, SL. Magnesium and the athlete. Curr Sports Med Rep. (2015) 14:279–83. doi: 10.1249/JSR.0000000000000178
24. Mert, T, Gunes, Y, Guven, M, Gunay, I, and Ozcengiz, D. Effects of calcium and magnesium on peripheral nerve conduction. Pol J Pharmacol. (2003) 55:25–30.
26. Yamanaka, R, Shindo, Y, and Oka, K. Magnesium is a key player in neuronal maturation and neuropathology. Int J Mol Sci. (2019) 20:3439. doi: 10.3390/ijms20143439
27. Romero, PJ, and Whittam, R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. (1971) 214:481–507. doi: 10.1113/jphysiol.1971.sp009445
28. Nemoto, T, Tagashira, H, Kita, T, Kita, S, and Iwamoto, T. Functional characteristics and therapeutic potential of SLC41 transporters. J Pharmacol Sci. (2023) 151:88–92. doi: 10.1016/j.jphs.2022.12.003
29. Del Arco, A, González-Moreno, L, Pérez-Liébana, I, Juaristi, I, González-Sánchez, P, Contreras, L, et al. Regulation of neuronal energy metabolism by calcium: role of MCU and Aralar/malate-aspartate shuttle. Biochim Biophys Acta, Mol Cell Res. (2023) 1870:119468. doi: 10.1016/j.bbamcr.2023.119468
30. Andersen, H. Motor dysfunction in diabetes. Diabetes Metab Res Rev. (2012) 28:89–92. doi: 10.1002/dmrr.2257
Keywords: calcium, magnesium, peripheral neuropathy, restricted cubic spline, nonlinear relationship
Citation: Wu Z, Yang X, Ruan Z, Li L, Wu J and Wang B (2023) Nonlinear relationship between dietary calcium and magnesium intake and peripheral neuropathy in the general population of the United States. Front. Nutr. 10:1217465. doi: 10.3389/fnut.2023.1217465
Edited by:
Mahban Rahimifard, Tehran University of Medical Sciences (TUMS), IranReviewed by:
Ge Luo, Zhejiang University School of Medicine, ChinaTakahisa Deguchi, Kagoshima University, Japan
Copyright © 2023 Wu, Yang, Ruan, Li, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Wang, d2FuZ2JpbjE5Nzk1NEAxNjMuY29t; Jianlin Wu, d3VqaWFubGluMjAxOEAxNjMuY29t
 Zhe Wu
Zhe Wu Xuesong Yang2
Xuesong Yang2 Zhishen Ruan
Zhishen Ruan