- 1Division of Food Science and Technology, SKUAST, Shalimar, India
- 2Sheri Kashmir Institute of Medical Sciences, Srinagar, India
- 3Department of Biotechnology, School of Life Sciences, Central University of Kashmir, Ganderbal, India
- 4Department of Biotechnology, Yeungnam University, Gyeongsan, Republic of Korea
Humans are constantly facing multiple health challenges from both communicable and non-communicable diseases that significantly affect their health. Additionally, drug resistance or failure has made the situation even worse and poses serious challenges for researchers to develop new drugs. Hence, to address these problems, there is an urgent need to discover and develop timely and long-term-based therapeutic treatments from different sources. One such approach is harnessing the potential of plant secondary metabolites. Plants have been utilized for therapeutic purposes in addition to being used for nutritional benefits. In the last two decades, plant-based drug developments have been one of the effective means of treating human diseases owing to their multiple functions. More recently, anti-nutritional factors (ANFs) have emerged as one of the important targets for novel plant-based drug development due to their multifaceted and potential pharmacological properties. However, their anti-nutritional properties have been the major setback for their limited success in the pharmacological sector. In this review, we provide an overview of ANFs and their beneficial roles in preventing human diseases with multiple case studies. We also highlight the recent developments and applications of ANFs in the food industry, agriculture, and pharmaceutics with future perspectives. Furthermore, we evaluate meta-analyses on ANFs from the last 30 years in relation to their function in human health benefits. This review is an endeavor to reevaluate the merit of these natural compounds and explore their potential for both human and animal health.
1. Introduction
Plants and their products have been major sources of nutrients in both human and animal diets. They have also been utilized for therapeutic purposes in addition to being used for nutritional benefits. Biologically active constituents are distributed widely in the plant kingdom, particularly in plants consumed by humans and animals (1). These compounds have both positive and negative effects, depending primarily on their concentration. Among them are anti-nutritional factors (ANFs) or non-beneficial compounds that can affect human and animal growth as well as reduce their nutrient intake, absorption, and utilization. These include phytic acid, saponins, alkaloids, certain oligosaccharides, protease inhibitors, glucosinolates, tannins, and cyanogenic glycosides (2, 3). ANfs are known to alter the absorption of nutrients such as vitamins, minerals, and proteins in addition to inhibiting enzyme activities. There are a plethora of studies that have proven the negative impact of ANFs on nutrient bioavailability in different living organisms (2, 3). However, the deleterious effects of ANFs on nutrient metabolism vary according to age, species, concentration of ANFs, processing, and interactions with other nutrients. Recently, ANFs have attracted considerable interest among researchers owing to their incredible and broad spectrum of biological activities that may be useful to humans (4). For instance, ANFs like saponins are known to possess an array of beneficial effects such as lowering plasma cholesterol levels in humans and possessing anticancer properties, as well as being crucial in reducing the risk of various chronic diseases (5). Similarly, phytic acid has also been shown to be effective in the prevention and treatment of a variety of pathological diseases and cancer through in vitro and in vivo assays (6). Tannins are polyphenols that, in addition to being antinutrients, have positive effects on humans. Glucosinolates and their companions, such as isothiocyanates (ITCs), on the other hand, have been shown to reduce the risk of cardiovascular and neurological diseases, as well as being anticancer and anti-inflammatory (7). However, there is a glaring information vacuum about how ANFs simultaneously alter food absorption and treat human disorders because earlier studies have mostly concentrated on their detrimental effects on human nutrition and other related features. Indeed, ANFs are potential candidates for future plant-based drug development in humans; however, how they elicit beneficial or detrimental effects on them remains enigmatic at the molecular level. Therefore, more comprehensive biochemical, molecular, and physiological studies are required in different model systems to understand their negative or beneficial roles which will provide novel insights into their implications in human disease therapy. Also, understanding how ANFs contribute to preventing or lowering human disease and the identification of cellular targets or receptors are crucial for the development of ANF-based remedial toolkits for treating human diseases. In this context, integration of multiomics with additional chemical, cellular, drug-designing, and in silico methods is necessary to decipher the molecular mechanism and identify the various differentially expressed genes, proteins, metabolites, and ionomes regulated by ANFs in different model systems.
ANFs are classified in two ways: as elements that lower nutrient intake in both humans and animals and as compounds that can be found in human or animal diets that affect immunological function and reproductive function (8). They are produced by different metabolic pathways that alter the overall normal nutrition metabolism. The highest levels of antinutrients can be found in legumes, grains, and nuts, but they can also be found in leaves, roots, and fruits of some plant species. ANFs are crucial for protecting plants from herbivores, insects, and diseases, as well as unfavorable growing circumstances (9). Similarly, they can also serve as useful tools to manage various diseases (Figure 1). Previous studies showed that, if consumed in adequate amounts, anti-nutrients can influence nutritional physiology and even act as a natural cure to improve human health (9). A study based on controlled-case trials and epidemiological research reported that many ANFs available in lower concentrations have favorable effects for averting coronary diseases and various cancers [Figure 2; (10)]. For this reason, ANFs are often referred to as plant-bioactive or non-nutritive compounds (2, 11). Many factors influence their activity, for instance, their chemical nature, amount present, and interrelationship with other dietary components. In recent years, a lot of literature has been published on the ANFs of food products which mainly highlighted their negative role in animals or humans. However, the aim of this review is to assess diversified scientific information on the potential health benefits and inimical effects of major ANFs in plant foods. In this review, we provided recent updates on ANFs and their beneficial role in combating human diseases. We also provided an overview of different ANFs found in plants. We also focused on the applications of ANFs in the food industry, agriculture, and pharmaceutics with future perspectives. Further, we examined the meta-analyses on the role of ANFs and their human health benefits in medical science in Web of Science from the past two decades (1999 to 2022) from top-publishing countries for a total number of publication categories and countries in core clinical journals and specific publication types (review articles, articles, and proceeding papers) (Figure 2).
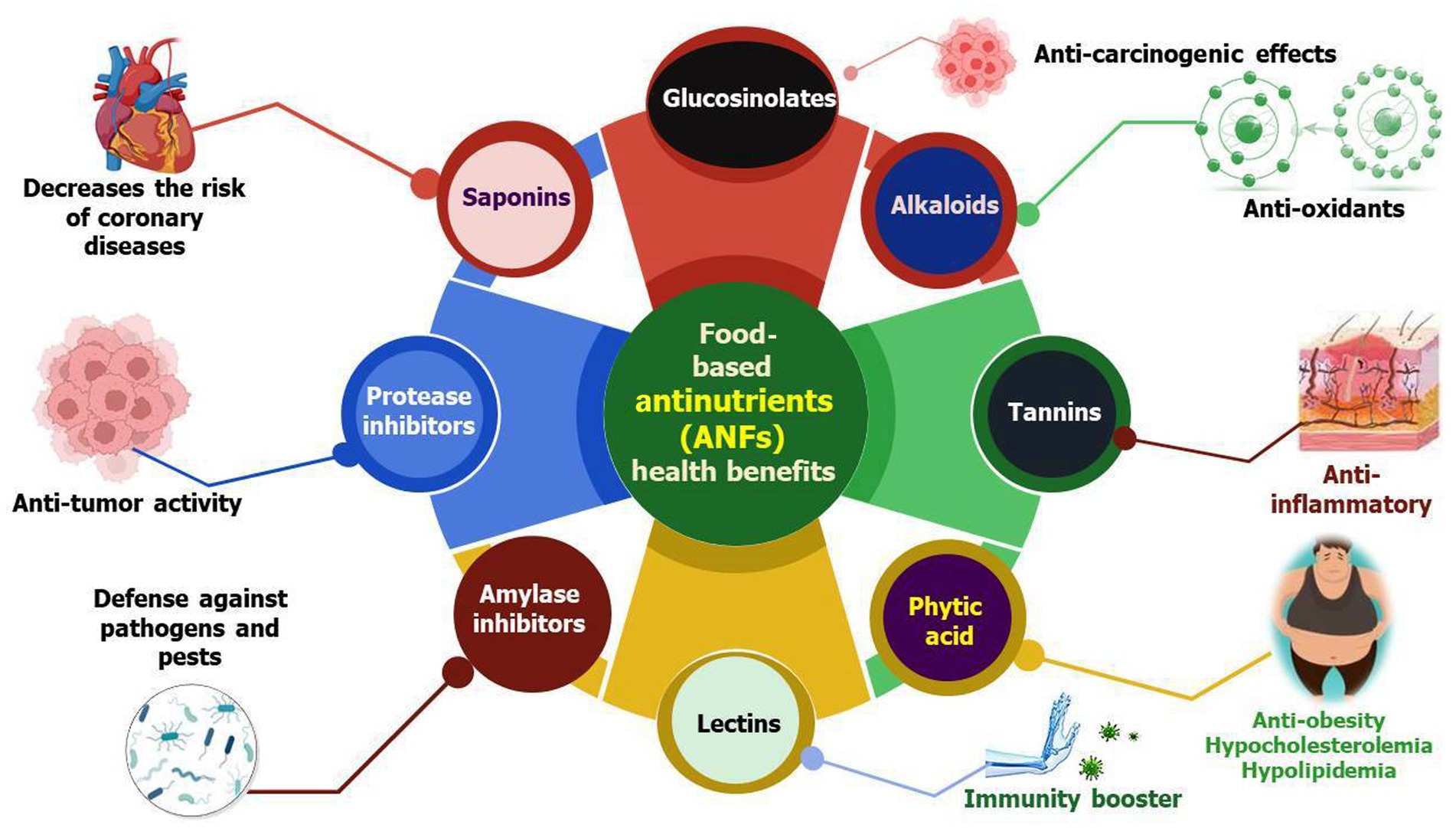
Figure 1. Schematic representation of critical health benefits possessed by ANFs. The figure displays the role of ANFs in the prevention of serious life-threatening human diseases that negatively affect the quality of life. These diseases include cancers, diabetes, bacterial and fungal infections, certain metabolic diseases, hypertension, and cardiovascular ailments.
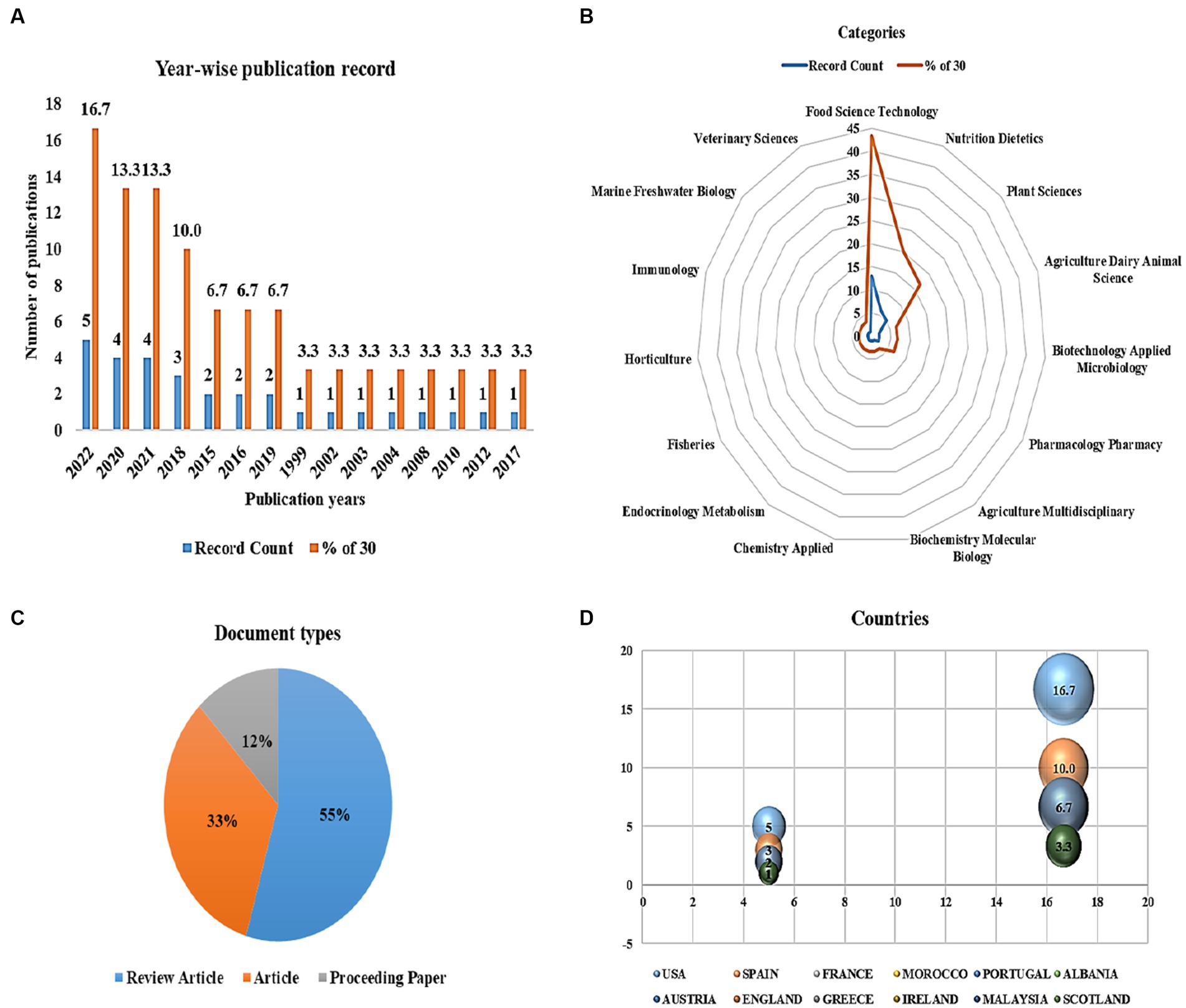
Figure 2. Meta-analyses on the role of anti-nutritional factors and their human health benefits in medical science. (A) Year-wise publication records, (B) web of science categories, (C) document types, and (D) countries with the most journal articles published from the past two decades.
1.1. Diversity of anti-nutritional factors and their functions
In plants, different types of ANFs have been found with diverse activities. In this review, we discussed some of the important ANFs and their medical importance. There are several reports on ANFs; however, the majority of them focus on their anti-nutritional characteristics, leaving wide knowledge gaps regarding their beneficial functions. Here, we systematically discuss the beneficial role of ANFs in treating human disorders and also highlight key points for their future research.
1.2. Protease inhibitors
Protease inhibitors are ubiquitous in the kingdom Plantae, including in the seeds of legumes and cereals. Protease inhibitors are known to subdue the function of all four classes of proteolytic enzymes, namely, serine, cysteine, aspartyl, and metalloproteinases in the gastrointestinal tract of animals (12–15). Their antinutrient activity is linked to growth inhibition and hypertrophy of the pancreas. In animal studies, it was found that protease inhibitors are associated with pancreatic cancer and, hence, possess anti-carcinogenic effects and a wide range of therapeutic applications [Table 1; (29)]. Protease inhibitors act like competitive inhibitors, i.e., they bind to the active site of the enzyme and form a complex with a very low dissociation constant (107–1014 M at neutral pH). The inhibitor imitates the substrate and therefore creates an inhibitor–enzyme complex which cannot be detached through the usual mechanism. This inhibits the active site of the enzyme and, in turn, the protease activity of the enzyme is silenced effectively (30). Inhibitors of serine proteases are the largest group of protease inhibitors. Two large families of protease inhibitors have been identified in legumes: the Bowman-Birk type (BBI) and the Kunitz-type inhibitors. BBI is the most widely categorized family of inhibitors in legume seeds and is composed of small peptide molecules and contains 71 amino acids. These inhibitors contain high levels of cysteine and seven disulfide bridges. The BBI peptide molecules are typically double-headed and so block two protease molecules simultaneously, which may be the same (such as trypsin) or different (such as one chymotrypsin and one elastase or trypsin). The BBI may be resistant to breakdown by heat (31). The Kunitz inhibitors are another class of inhibitors having a molecular weight of approximately 20 kDa. They are peptides of 181 amino acids, commonly found in soya beans and winged beans containing two disulfide bridges. These molecules are single-headed that inhibit the active site of one enzyme molecule (usually trypsin or chymotrypsin) simultaneously (32, 33). According to a recent study, protease inhibitors (PIs) operate as anticarcinogenic agents under in vivo and in vitro studies; however, the precise mechanism underlying PIs’ anticarcinogenic effect is not yet fully understood (34). It was found that PIs can affect both the early and later stages of carcinogenesis, but they have no impact on cells that have already undergone transformation (34). PIs have the ability to reverse the initial events, most likely by inhibiting a cellular activity that was initiated by carcinogen exposure. The ability of a PI to affect the expression of specific oncogenes and the amounts of specific types of proteolytic activity is central to its role in the repression of carcinogenesis (34). PIs with antineoplastic action have no discernible effect on normal cells, but they are capable of negating carcinogen-induced cellular changes in different studies (35, 36). The inhibitory profiles of PIs that affect transformation have been examined since not all PIs have the ability to prevent the transformation in vivo (37, 38). For instance, chymostatin - a very common and efficient chymotrypsin inhibitor is one of the most effective PI that suppresses the transformation. It has the ability to eliminate radiation–induced transformation in vitro with only picomolar concentrations in the medium (38). BBI family inhibitors are effective in suppressing transformation with nanomolar concentrations (39). It has been confirmed that BBIs from soybeans have the ability to inhibit prostate cancer cells’ formation of oxygen radicals and trigger DNA repair through a p53-dependent mechanism (40, 41). Moreover, experiments have shown that BBIs have impeded prostate tumor development by promoting the production of connexin 43 expressions in transgenic rats via its antiproliferative activity (42, 43).
PIs commonly found in raw legume seeds are trypsin and chymotrypsin inhibitors (44). Trypsin inhibitors (TIs) have been connected to increased pancreatic secretory activity, increased pancreatic hypertrophy, and decreased protein digestibility. Additionally, feeding studies including the administration of raw soybeans supplemented with partially purified trypsin inhibitors to rats and chickens resulted in considerable pancreatic hypertrophy and plethoric enzyme secretion (45, 46). Interestingly, a 22 kDa trypsin TI protein from the storage roots of sweet potato (Ipomoea batatas L.) has been shown in prior research to have an anti-proliferative activity and works by inhibiting the growth of NB4 promyelocytic leukemia cells (47). According to this study, TI causes NB4 cells to undergo apoptosis by impairing the cell cycle at the G1 phase and activating the caspase-3 and -8 cascades (Figure 3). Trypsin, chymotrypsin, and elastase were all inhibited by protease inhibitors (LC-pi I, II, III, and IV) that were isolated from the seeds of Lavatera cashmeriana and were recognized as Kunitz-type inhibitors based on their molecular size (48, 49). Moreover, all four classes of inhibitors exhibited anticarcinogenic activity under in vitro conditions. Among all, LC-pi I and II were treated as potential anticancer agents (50). Moreover, under in vitro conditions, a strong inhibitory effect of LC-pi I was observed at the initiation stage of cancer in the prostate (PC-3) and breast (MCF-7) cancer cell lines because of the presence of the protease inhibitor activity of trypsin, chymotrypsin, and elastase (49).
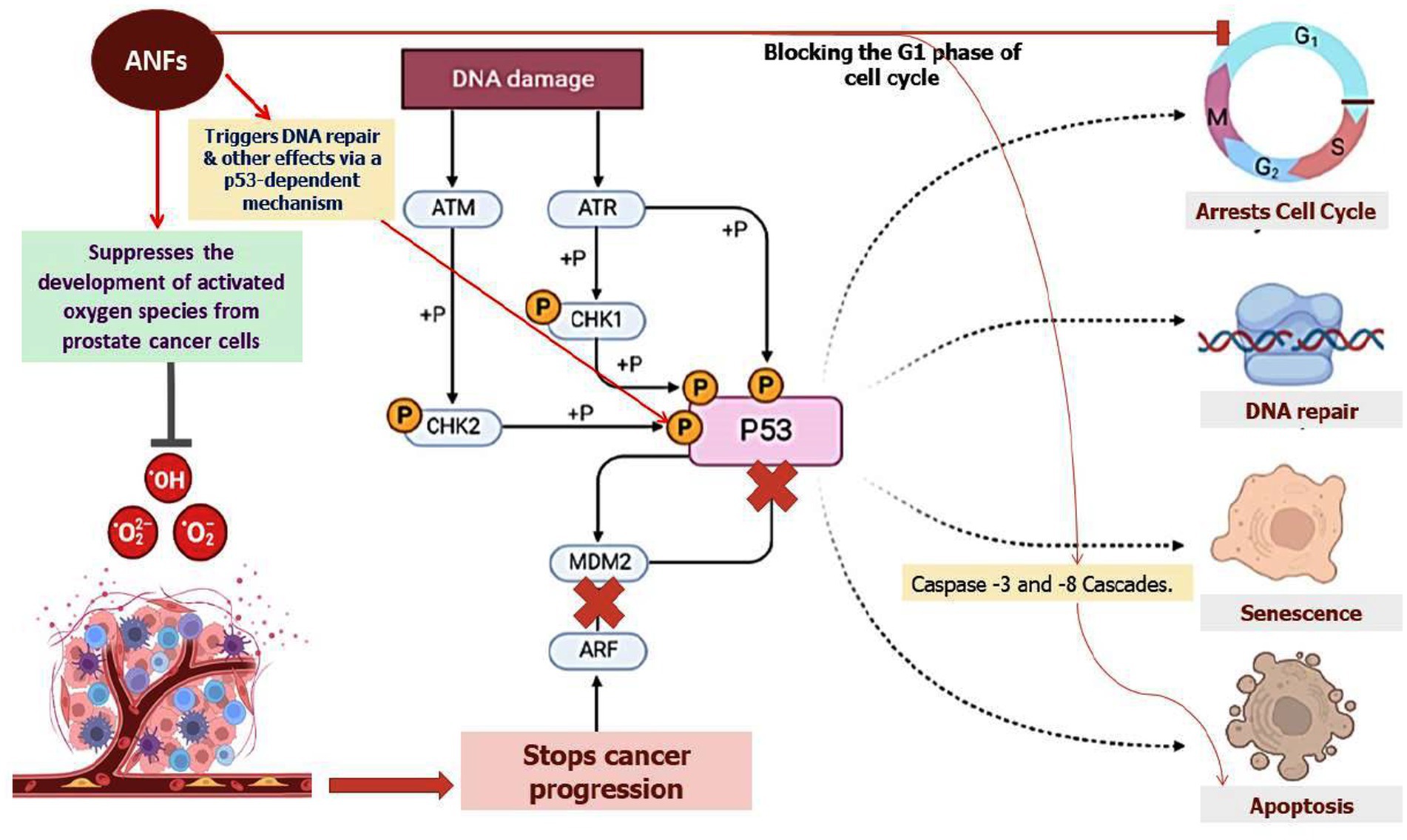
Figure 3. The role of ANFs in altering the cell cycle at the G1 phase. The representation also shows the role of ANFs in repressing the formation of activated oxygen species from prostate cancer cells. ANFs such as TI protein were shown to trigger apoptosis by hindering cell growth and eliciting the courses of caspase-3 and -8 cascades as shown in the figure.
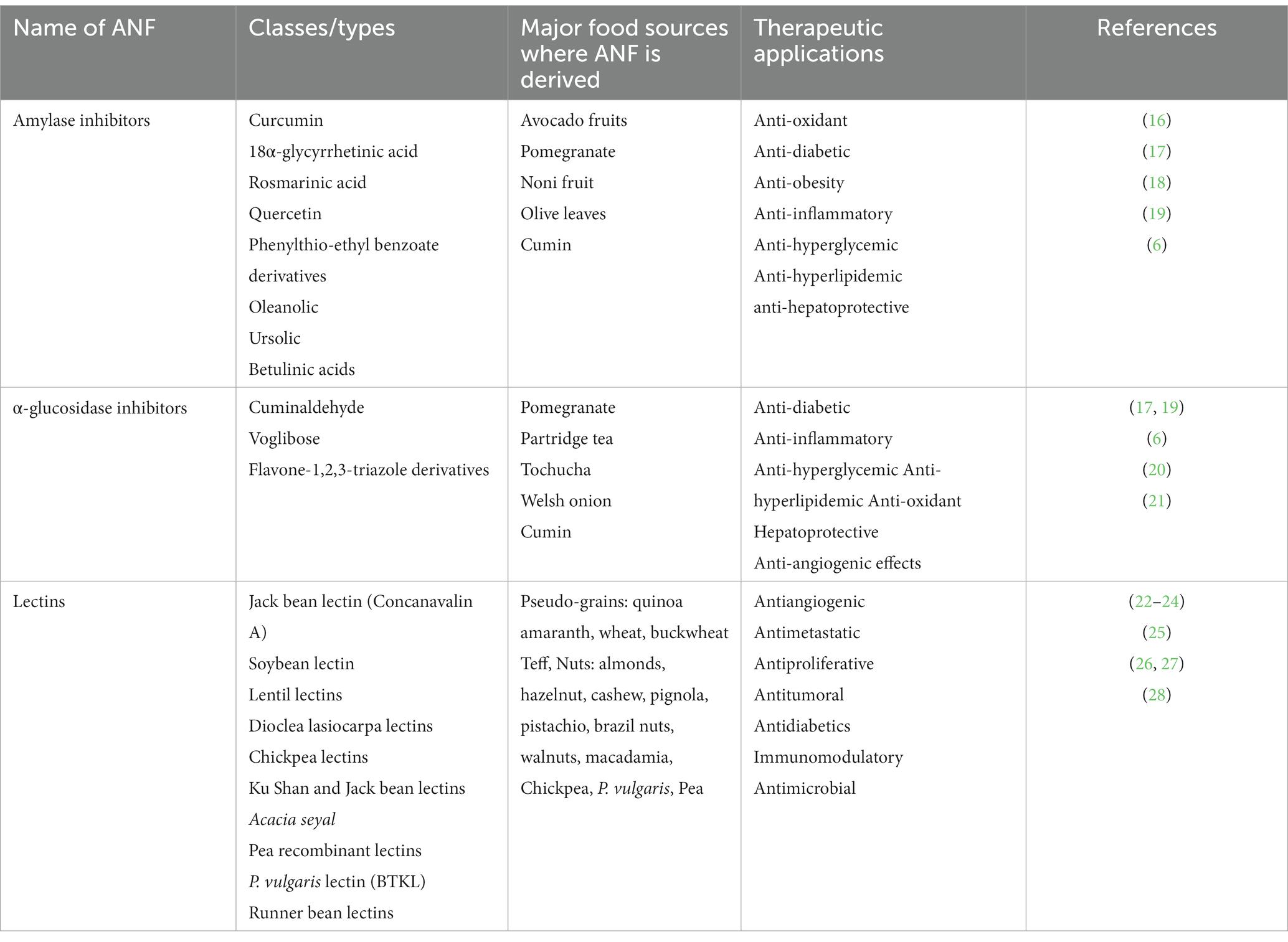
2. Amylase and α-glucosidase inhibitors: a natural gateway to cure hyperglycemic patients
Many plants have amylase enzyme inhibitors, which are important for controlling endogenous-amylase activity as well as defense against pathogens and pests (51). They are essential for humans because they help convert dietary carbs into glucose molecules, which prevent the body from absorbing dietary starches. They are commonly found in pigeon peas and effectively function within a pH range of 4.5–9.5 (52). Amylase inhibitors are known to be heat-labile and decrease the action of bovine pancreatic amylase, but they have no effect on the activity of bacterial or fungal amylase. The use of natural enzyme inhibitors is of dire requirement due to the large side effects imparted by synthetic enzymes. Inhibitors such as derivatives from phlorotannin including fucodiphloroethol G, dieckol, 6,6′-bieckol, 2-phloroeckol, 7-phloroeckol, phlorofucofuroeckol A, 2,7′-phloroglucinol-6,6′-bieckol, 2-O-(2,4,6-trihydroxyphenyl)-6,6′-bieckol, 8,8′-bieckol, 6,8′-bieckol, and eckol possess strong inhibitory activity against α-amylase and α-glucosidase (53–55). All these inhibitors have been reported to be potential therapeutic agents against diabetes, and they have been recorded as possessing hypoglycemic effects. However, their unsteadiness in the gastrointestinal pathway led to the failure in reduction of insulin response and enhanced the caloric output of victuals by adopting them as starch-blocker pills. The α-amylase inhibitor from white beans (Phaseolus vulgaris) has been reported to potentially induce weight loss and lower blood sugar levels brought on by a diet high in carbohydrates (56, 57). Furthermore, renin and angiotensin-I-converting enzyme activity is inhibited by the penta- and hexapeptides produced from pigeon peas, which also have strong antioxidant characteristics (58). Amylase inhibitors are attributed to possessing two functions: protecting the seeds from microbial infections and pests and also inhibiting endogenous amylase (59). The inhibitors of α-amylase play a critical role in treating type-2 Diabetes mellitus by slowing down the absorption of glucose molecules in the body (60, 61). Moreover, fucoidan derived from several species of the genus Sargassum is shown to possess anti-diabetic activity by inhibiting the enzymes α-amylase and α-glucosidase (62–69). Moreover, several inhibitors such as octaphlorethol A (OPA), diphlorethohydroxycarmalol (DPHC), and ishophloroglucin A (IPA) derived from Ishige okamurae and Ishige foliacea possess strong inhibitory activity against α-amylase (70, 71). The latter may serve as possible medications to treat non-insulin-dependent D. mellitus. Plant-based phytochemicals comprising of phenol are artless inhibitors of α-amylase and α-glucosidase with a much stronger effect on α-glucosidase and moderate repressive impact on α-amylase. Thus, with little side effects, these phenolic phytochemicals can be employed as an effective strategy for preventing postprandial hyperglycemia (72). Equally, consuming a diet high in mixed carbohydrates and inhibiting α-amylase and α-glucosidase through the action of phenolic antioxidants will minimize postprandial hyperglycemia and may be a successful method of managing type-2 diabetes. Flavan-3-ols are believed to intervene in the incipience of cardiovascular disease through different mechanisms such as antioxidative, anti-thrombogenic, and anti-inflammatory processes. Proanthocyanidins (PAs) and flavan-3-ol monomers, specifically, contribute to reducing the cholesterol levels in blood plasma, prevent LDL oxidation, and activate endothelial nitric oxide synthase to avoid platelet adhesion and aggregation that can lead to blood clot formation (73, 74). All these reports clearly indicate that amylase inhibitors have great potential in treating diseases, especially type I diabetes, and the findings will pave the way in the design of dietary guidelines that can aid in maintaining good human health. Further, we have shown the potential targets of food-based amylase class of ANFs on the prevention of hyperglycemic conditions in diabetic patients (Figure 4).
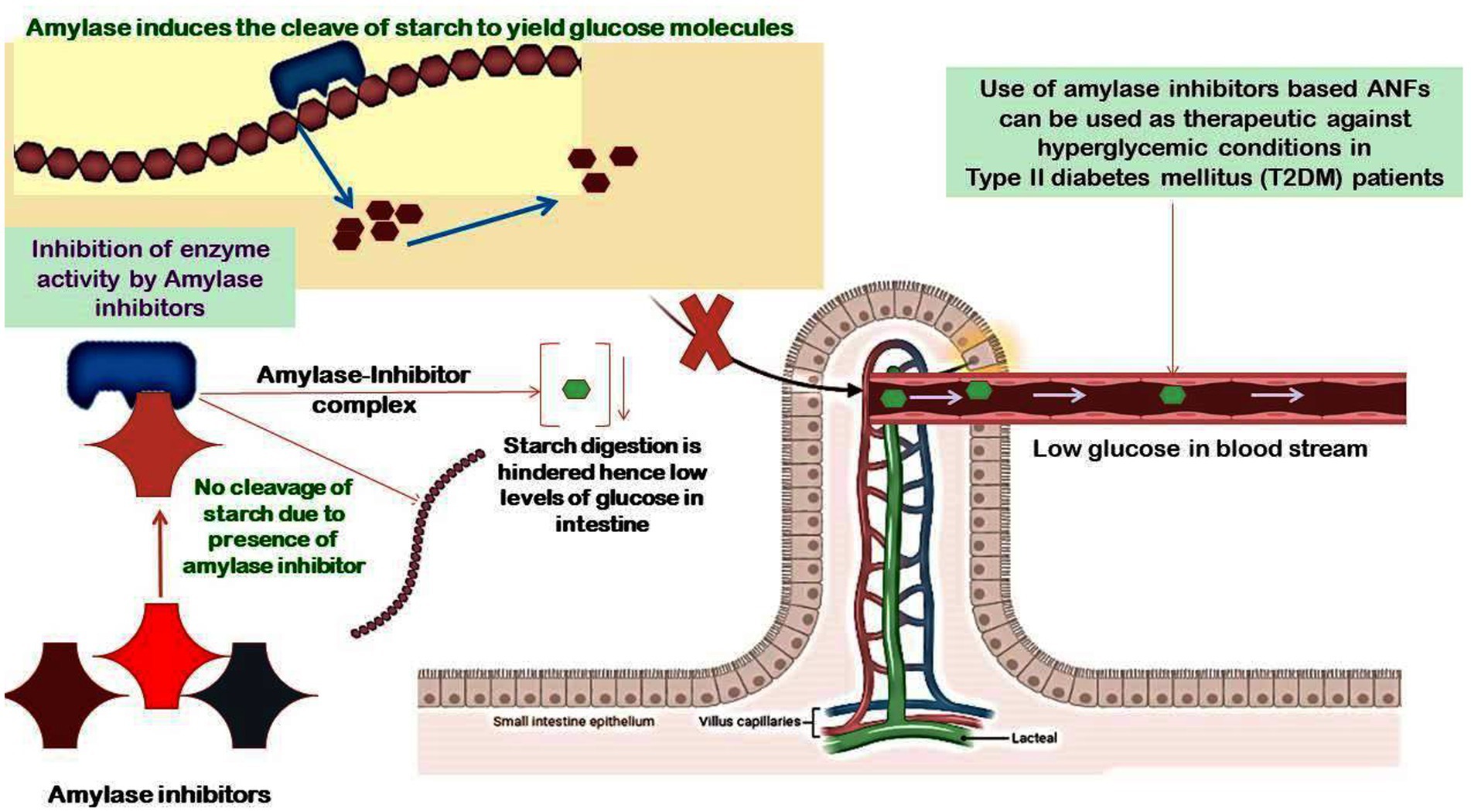
Figure 4. Potential targets of food-based amylase class of ANFs on the prevention of hyperglycemic conditions in diabetic patients. The α-amylase inhibitors in whole food grains are usually released during the process of digestion in the GI tract, and these inhibitors form a complex with the α-amylase, hence blocking its activity to limit the hydrolysis of starch and lowering the production of glucose molecules. This mechanism paves the way for amylase inhibitors-based therapeutics in individuals suffering from diabetes to maintain a low glycemic index.
3. Lectins
Proteins that can conglomerate RBCs with specified sugar selectivity are referred to as “lectins” (75). They are also called hemagglutinins when the sugar specificity is not known. Lectins are glycated proteins extensively present in legumes and certain oil seeds such as soya beans (76). They possess at least one non-catalytic domain, which is reversibly bound to monosaccharides or oligosaccharides. They can agglutinate the erythrocytes by binding to the carbohydrate moieties present on the surface of erythrocytes without modifying the carbohydrate properties (77). Lectins can damage the intestinal cells by binding to intestinal epithelial cells and impair nutrient absorption leading to infiltration of bacteria into the blood stream (78). Lectins can bind to RBCs, causing hemagglutination and anemia. Lectins are the hot spot for biologists, especially for agricultural and medical research applications (77). Studies have revealed new evidence of potential light in lectins, particularly cereal lectins in the etiology of human disorders like rheumatoid arthritis and cardio vascular diseases (CVDs) (79). Plant Lectins administered orally may have an extensive impact on varied types of tumors. For instance, a study showed that galactose-specific peanut agglutinin, PNA, activates cell proliferation in colonic explants in vitro, and eating peanuts enhanced rectal proliferation in persons with a mucosal expression of the peanut lectin receptor (80). It has been reported that the lectin from kidney beans prevented the development of tumors in NMR and BaLB/c mice (81). Many lectins are employed as therapeutic agents because they preferentially bind to cancer cell membranes or their receptors, producing cytotoxicity, apoptosis, and prevention of tumor growth. These lectins have been proven to have anticancer characteristics in vitro, in vivo, and in human case studies (80, 81). Lectins have the potential to enter cells and cause cancer cell agglutination or aggregation. The beneficial impact of lectin is complex due to a number of factors including dispossession of nutrients of the growing tumor by the altitudinous nutrient and polyamine sine qua non for lectin-induced compulsory gut growth, suppression of angiogenesis by kidney bean lectin in the growing tumor, and stimulation of the immune system to counter the tumor growth. Consequently, lectins have great potential to act as therapeutic molecules within limited dose and route.
4. Naturally derived ANF compounds
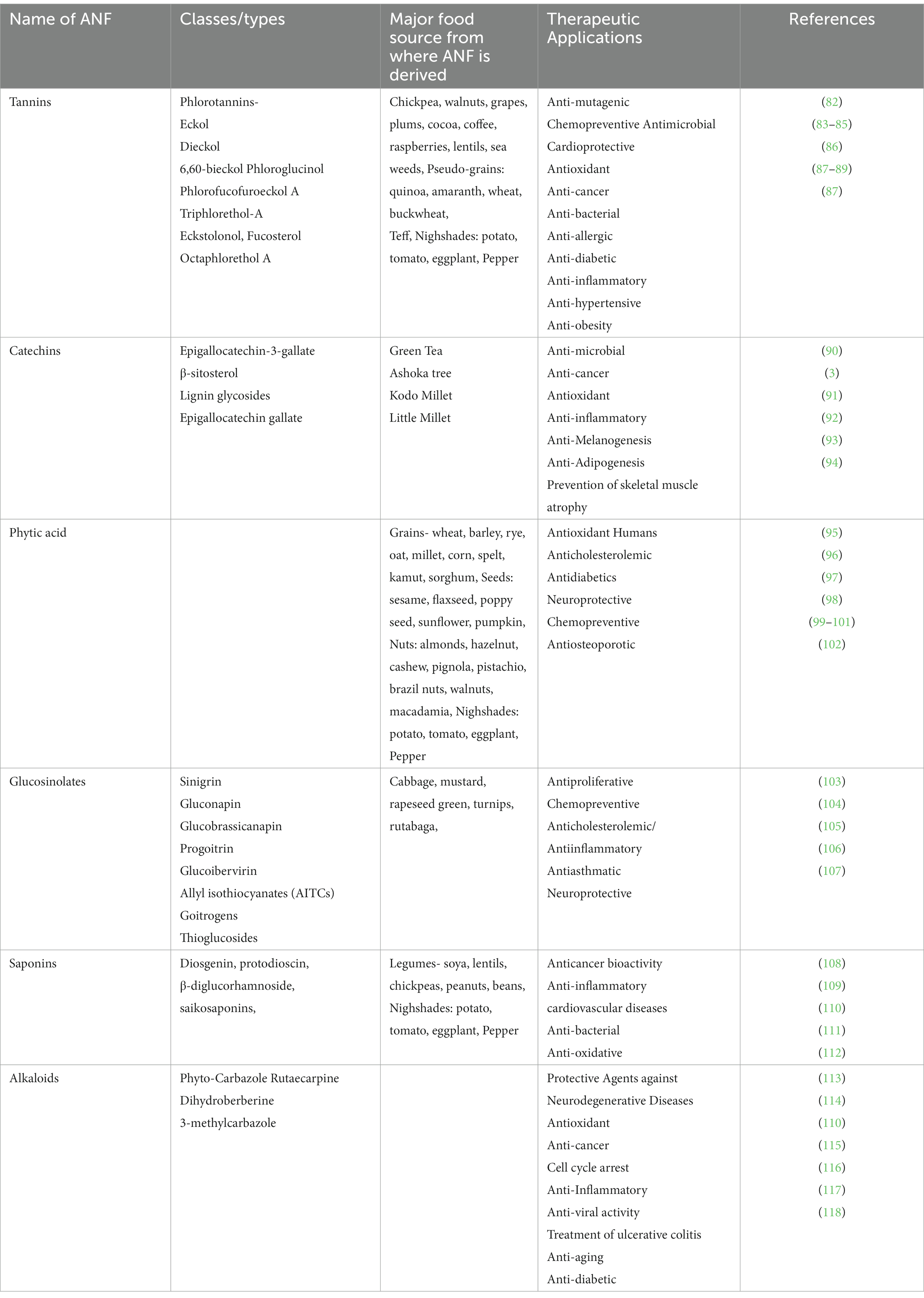
Several other plant-derived ANFs have been reported to possess both beneficial and toxic effects on human health. For instance, certain compounds interfere with the solubility or absorption of minerals. These compounds include glucosinolates, phytic acid, oxalic acid (thioglucosides), and gossypol. In general, plant-derived compounds have been attributed to several therapeutic applications (refer to Table 2). These compounds include tannins, catechins, phytic acid, glucosinolates, saponins, and alkaloids. In this context, we have detailed the therapeutic applications of these ANFs in the preceding sections of this review.
4.1. Tannins: a functionally multifaceted ANF for enhancing human health
Tannins are polyphenolic substances with molecular weights ranging from 500 to 3,000 daltons. Chemically, they are classified as condensed tannins/pro-anthocyanidins and hydrolyzable tannins (119). Hydrolyzable tannins (HT) are compounds with a central polyol (D-glucose) core. The hydroxyl groups in these carbohydrate molecules are in part or completely esterified with phenol moiety-containing compounds, such as gallic acid (gallotannin) or ellagic acid (ellagitannin). HTs frequently occur in less quantity within plants and are easily hydrolyzed by mild acids and bases into carbohydrate and phenolic acids. Condensed tannins are naturally present in polyphenolic bioflavonoids, typically in the conformation of oligomers or polymers of polyhydroxy flavan-3-ol units, like (+)-catechin, (−)-epicatechin, flavan-3,4-diols, leucoanthocyanidins, or a combination of the two (120). Condensed tannins are commonly present in tree barks as barriers against microbes and are also found in other tissues of plants including buds, leaves, roots, seeds, and stems. The anti-nutritional effects of tannins are determined by their chemical conformation, dosage, and tolerable daily intake (29). Tannins have been documented to affect the digestibility of proteins and influence the bioavailability of non-heme iron, leading to less absorption of iron and calcium. They have an effect on carbohydrate digestibility, contributing to the reduced energy value of a diet (121).
Tannins are known to bind proteins through hydrogen bonds and hydrophobic interactions. They are known to interact with digestive enzymes, which exhibit anti-trypsin and anti-amylase activities, thus rendering them unavailable for digestion. It has been confirmed that condensed tannins triggered the testa-bound trypsin inhibitor activity (45). Tannins can also form a complex with vitamin B. They can also contribute to decreased palatability and lower growth rate (76). The implication of food tannins on human health is a common worry although they do have some beneficial effects as well. Some tannins possess antioxidant activity and are considered to be cardio-protective, anti-inflammatory, anti-carcinogenic, and anti-mutagenic (122, 123). Tannins are reported to inhibit lipid peroxidation and lipoxygenases under in vitro conditions. Recent studies indicate they have the ability to scavenge radicals, such as hydroxyl, superoxide, and peroxyl, which are considered to be important cellular prooxidants (124). According to an in vitro study using the human colon cancer cell line HT29, proanthocyanidin-rich apple polyphenol extract prevented the phosphorylation of epidermal growth factor (EGF), which in turn suppressed the development of these cells (125). Additionally, it has been shown that these substances inhibit the growth of cancer cells under in vivo conditions (126). Moreover, it is reported that tannins have the potential to enhance glucose absorption and prevent adipogenesis (127, 128). A class of tannins known as phlorotannins has been found to provide significant health benefits, including anti-oxidant, anti-inflammatory, anti-cancer, anti-proliferative, anti-bacterial, anti-mutagenic, anti-allergic, anti-diabetic, anti-obesity, and anti-hypertensive properties (83, 86–89). For instance, phlorotannins such as pyrogallol-phloroglucinol-6,6-bieckol, dieckol, 2,7-phloroglucinol-6,6-bieckol, and phlorofucofuroeckol A profoundly inhibit the dysfunction of suppressing monocyte migration, death of monocyte-associated endothelial cell, and inhibition of inflammation by monocyte-associated vesicles (129). All these studies strongly suggest that tannins are potential therapeutic agents against a wide range of diseases. Henceforth, it is critical to investigate the proper protocol for standardizing the dosage and troubleshooting its therapeutic effects through in vitro and in vivo studies.
4.2. Catechins
Catechins (flavan-3-ol) are a group of secondary metabolites of flavonoids that have two benzene rings and a dihydropyran heterocycle. They are known to protect both involuntary and radiation-induced cancers as well as chemically generated mutations. Catechins promote phase I and II metabolic enzymes that enhance the formation and excretion of detoxified carcinogenic metabolites, inhibit cell division, and, thus, slow the emergence and spread of cancer. Catechins are strong antioxidants that prevent the oxidation of LDL cholesterol, lower cholesterol, and reduce body fat, all of which help to lower the risk of cardiovascular disease (122). Moreover, they help to reduce high blood pressure-induced strokes (122). Catechins and polyphenols present in tea leaves are efficient scavengers of reactive oxygen species (ROS) and act as indirect antioxidants by influencing transcription factors and enzyme activities. In humans, mild improvements in plasma antioxidant ability were observed following the consumption of catechins from green tea. Catechins present in tea and green tea have a very positive effect on the biomarkers of oxidative stress, particularly on the oxidative damage caused to the DNA in animal models, but human data on biomarkers of in vivo oxidative stress is limited. Greater human research studies examining the impact of tea and the catechins present in them on the biomarkers of oxidative damage to lipids, proteins, and DNA are required (130). Owing to its multiple therapeutic properties, it is vital to investigate the role of catechins in driving complex networks and unravel the underlying mechanisms to regulate the wide range of disorders.
4.3. Phytic acid
Phytic acid is a myoinositol 1,2,3,4,5,6-hexakis dihydrogen phosphate found in plants, animals, and soil in the form of a salt of the mono and divalent cations K+, Mg2+, and Ca2+. It is the major cache of phosphorous encompassing 1–5% by weight in cereals, legumes, oil seeds, and nuts (131). Phytate promptly proliferates in the seeds during their ripening period. They are hoarded within leguminous seeds and oil seeds inside the globoid crystal in the protein bodies. Phytates also serve as reservoirs of cations, which are high-energy phosphoryl groups, and by chelating free iron, act as an efficient natural antioxidant (132). Monogastric animals like poultry and humans cannot break down phytic acid due to the absence of an adequate quantity of phytate-reducing enzymes in their alimentary tract (133–135). Phytates act as potent anions in a broad pH range and thus have an unfavorable impact on the bioavailability of divalent and trivalent mineral ions such as Zn2+, Fe2+/3+, Ca2+, Mg2+, Mn2+, and Cu2+ in diet (29). The impact of an increased intake of a phytate-containing diet on mineral deficiency depends on what else is being consumed. The intake of phytate is a problem in regions of the world where cereal proteins are a significant and preponderant dietic component (25). Phytates substantially reduce the bioavailability of calcium, and the molar ratio of Ca2+: phytate has been recommended as a marker of Ca2+ bioavailability. The critical molar ratio of Ca2+: phytate is specified to be 6:1 (136). It has been reported that the interaction between phytate and carbohydrates (starch) reduces the bioavailability and breakdown of carbohydrates as the formation of the phytate-carbohydrate complex affects the rate of metabolism of starch (137). Nonetheless, recent data reveals positive health effects of phytic acid on hypocholesterolemia and hypolipidemia as an anticancer agent. The prophylaxis and suppression of tumor evolution and progression are primarily associated with the ability of IP6 to regulate the segregation, multiplication, and programmed cell death of tumor-forming cells (138, 139). This IP6-conferred anticarcinogenic protection is associated with its suppression of the generation of oxygen-free radicals by the preclusion of the Fenton reaction in iron chelation (140). Reports suggest that phytate inhibits the growth of human cell lines such as leukemic hematopoietic K-562 cell line (141), colon cancer HT-29 cell line (142), breast cancer cell lines (143), cervical cancer cell lines (144), prostate cancer cell lines, and HepG2 hepatoma cell line (145) in a dose- and time-dependent manner. Nonetheless, the phytate sensitivity of cells from different origins varies, indicating that phytate can affect different cell types by specific mechanisms of action. Phytates’ effectiveness as an anticancer agent was also shown in carcinogen DMBA-induced breast cancer rats. The animals treated with phytate showed a significant decline in proliferation. The study also compared the oncogenic indicators like serum total sialic acid (TSA), and documented grades of tissue nitric oxide to reflect carcinoma activity after administration of DMBA. Phytic acid administration in comparison to the control group considerably decreased the generation of TSA, increased programmed cell death, and suppressed oxidative stress linked with the generation of oxygen-free radicals from the carcinoma, therefore implying a probable medicinal value of phytic acid in breast oncogenesis. Phytic acid reduces the rate of cell multiplication in breast carcinoma, functions as an antioxidant, and also enhances programmed cell death (138). Dietary phytate may also aid diabetic patients as it suppresses the blood glucose levels by reducing the rate of metabolism of starch, hence slowing gastric emptiness. Similarly, phytate has also been proved to modulate the release of insulin (146). Across the western countries, cardiovascular disease is considered a leading cause of death. Elevated plasma cholesterol or elevated LDL-cholesterol concentrations have been shown to be one of the risk factors of heart diseases. Phytate, which is a component of fiber, is believed to affect the etiology of heart diseases. It has been shown that the reduction in serum cholesterol and triglyceride levels arises as a result of dietary phytate supplementation (147, 148). The decrease in serum Zn level and Zn-Cu ratio was correlated with this effect, and an imbalance in Zn-Cu metabolism is related with coronary heart disease. Phytic acid has been accepted as a superior preservative for juices (149) and meat products (150, 151). In addition, apple extract tempered with IP6 during distillation and packaging have exhibited a substantial diminution in the discoloration (brown color formation) due to polyphenol oxidase inhibition by IP6, whereas pigs fed with diet containing IP6 exhibited an enhanced shelf life of meat. The impact of IP6 on unsaturated fatty acids helped in maintaining the quality of meat and also enhanced the shelf life by suppressing lipid peroxidation (150–152). All these studies clearly approve the therapeutic potential of phytic acid and hence demands further investigation to unravel its proper use and applications.
4.4. Glucosinolates
Glucosinolates are an important class of phytochemicals present in Brassica vegetables within the range of 1.5–2.0 mg/g. They are primarily found in cabbage, broccoli, and Brussels sprouts. (153–155). Most glucosinolates are chemically and thermally stable but their hydrolytic derivatives are physiologically active (154). In raw vegetables, enzymatic hydrolysis occurs when cells are disrupted by masticating or refining the release of β-thioglucosidase (156). Glucosinolates are digested by microflora in the human GI tract and can therefore be utilized biologically in cooked vegetables even though cooking vegetables inactivates thioglucosidase. (153, 157). When plant tissue is damaged, the enzyme thioglucosidase breaks down the thioglucosidic bond, resulting in the creation of glucose and thiohydrosimate-O-sulphonate (an unstable aglycone) (155, 156). Depending on the reaction parameters such as pH and glucosinolate structure, different products can be obtained including isothiocyanates, nitriles, sulfides, thiocyanates, epithiitriles, oxazolidin-2-thiones, and indolyl compounds (154, 156). Hydrolytic decomposition products of glucosinolates, glucoraphanin, gluconasturtiin, and glucobrassicin exhibit antioncogenic properties. Furthermore, indol-3-carbinol, a component of glucobrasicin has the ability to inhibit human breast and ovarian cancers (158–160). Vegetables from the Brassica family comprise thioglycosides that are broken down into thiocyanates, which, in turn, impede the transport of iron and integration of iodine in thyroglobulin, ameliorating the augmentation of TSH secretion and thyroid cells. There is little epidemiological proof that the goitrogenic effects of glucosinolate degradation products lead to significant causes of human disease. There is significant doubt about the positive and negative repercussions of Brassica vegetables on health since there are major concerns regarding the breakdown products of glucosinolate and their antioncogenic effects. (161). Over 130 different glucosinolates have been identified as having antioxidant, anticancer, fungicide, and bactericidal properties (162, 163). For instance, isothiocyanates decrease redox imbalance levels in the system, alter chemokine function based on the reaction of the immune system, prompt programmed cell death, hinder the advancement of the cell cycle, prevent the formation and differentiation of blood vessels, and also show anti-bacterial, anti-viral, and anti-carcinogenic properties (160, 164–166). There are primarily two routes that have been proposed for isothiocyanates’ anticarcinogenic effects. First, phase II enzymes are activated concurrently with the inactivation of the phase I enzyme cytochrome P450s, which binds to isothiocyanate. Another mechanism involves the start of programmed cell death, which eliminates genetically compromised cells and halts the cell cycle (167–169). It has been proven that sulphoraphane from broccoli inhibits the growth of tumors by acting as the main inducer of cell-defensive phase II enzymes. As broccoli and Brussels sprouts are highly consumed, the extracts obtained from them are regarded as an appropriate tool for supplying sulphoraphane to humans (170). In another study, it was shown that 4-methylthiobutyl isothiocyanate specifically induced cytotoxicity in tumor-initiating cells via the p53-independent pathway; however, no apoptosis or necrosis was detected when used on normal liver cells. This compound was produced by the enzymatic breakdown of glucoerucin isolated from plants of the rocket species or by catabolism of sulphoraphane of isothiocyanate (165). In a clinical trial, the consumption of 250 g/day of broccoli and 250 g/day of Brussels sprouts significantly increased the clearance of the potentially cancer-causing chemical 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) found in properly cooked meat. It has been demonstrated that excess intake of vegetables from the Brassica family can minimize the risk of colorectal cancer by augmenting the excretion of PhIP and diet-containing heterocyclic amine carcinogens (171).
4.5. Saponins
Saponins are non-volatile surface-active secondary metabolites that are typically found in plants. They are made up of a sugar moiety coupled with a steroid (or triterpene) group. These surface-active compounds are present in legumes in addition to some spices and herbs (172, 173). High concentrations of saponins impart astringency and a bitter flavor to food plants. They have historically been considered to be antinutrients due to their negative effects, for instance, growth degradation and their bitter taste, and their throat-irritating nature has led to their reduced consumption and is a key limiting factor in their application (174). Saponins have been shown to decrease the physiological availability of nutrients and enzymes and they also hinder the activity of certain metabolic catalysts such as trypsin and chymotrypsin and thus affect protein digestibility (175). Recent studies demonstrate saponins’ ability to lower cholesterol, boost the immune system, and prevent cancer (176). They also decrease the peril of coronary heart disease in humans. Foods rich in saponin are essential for regulating the level of cholesterol in blood plasma, preventing peptic ulcers and osteoporosis, and decreasing the risk of heart diseases (177). Saponins are used as adjuvants in viral (e.g., Quillaja saponaria-21) and bacterial vaccine (e.g., Quillaja saponins) applications (174). A diet high in saponins can be used to treat acute lead poisoning, prevent dental cavities, inhibit platelet aggregation, and cure hypercalciuria in people (178).
4.6. Alkaloids
Alkaloids are secondary metabolites found in a wide range of plant species that possess therapeutic properties. Alkaloids, which primarily represent themselves as specialized molecules participating in metabolism, are nitrogen-containing chemicals having the capacity to react with acids to produce salts (179). Alkaloids appear to be present in distillate from roots, seeds, leaves, or barks of affiliates of at least 40% of plant families. Families including Amaryllidaceae, Compositae, Leguminosae, Liliaceae, Papaveraceae, and Solanaceae are especially rich in alkaloids. Solanine and tomatine are common examples. Solanine is found in little concentrations in potatoes, whereas tomatine is present in tomatoes. Alkaloids are premeditated to be anti-nutrients due to their effect on the nervous system, hindering or erroneously enhancing electrochemical transmission. The glycoalkaloids, solanine, and chaconine found in potato and Solanum spp. (180) are hemolytically active and harmful to fungi and humans. There have been studies on the infertility effects of certain plant alkaloids (181) although less intake of alkaloids arbitrate pivotal therapeutic functions, such as pain reduction, blood pressure control, tumor cell destruction, and stimulation of circulation and respiration (175).
5. Applications of ANF in the food industry, agriculture, and pharmaceutics
ANFs are generally considered harmful biologically active compounds due to their negative effect on nutrition availability and absorption. However, recent studies have shown that they have an incredibly beneficial role in preventing human diseases, and hence have become one of the important targets for plant-based drug development. However, plant-based diet also contains an array of ANFs or non-beneficial compounds that can affect human and animal growth as well as reduce their nutrient intake, absorption, and utilization. These include phytic acid, saponins, alkaloids, certain oligosaccharides, protease inhibitors, glucosinolates, tannins, and cyanogenic glycosides. Refined saponins or their concentrates have been used as additives in the production of food and beverages mainly as foaming agents or as emulsion stabilizers. In addition to foods, saponins are also used as anti-oxidants (182). Saponins are utilized in the manufacturing of lyophilized powders with vitamin E for the enhancement of foods, drinks, and animal feeds. The benefit of isoflavones is due to their oestrogenic activity and possible application as enhancers of growth in the fodder industry (183). Flavonoids have been shown to prevent thermal or chemically caused lipid peroxidation as well as chelating metallic and super oxide ions in the food processing industry (184). Flavones and leucoanthocyanidins impart scrumptious zing to the foods after processing (185). Using flavones as additives in many soft drinks and lemon brands imparts a peculiar bitter taste to them. The suppression of the growth of many plant species by saponins from alfalfa and Vernonia amygdalina (Compositae) leaves have been reported (186–188). Flavonoids are also known to be used as seed germinators and plant growth regulators. Tannins have also been known to be useful in forages (189). It has also been suggested that animal food with relatively higher amounts of proanthocyanidins (2–4% digestible matter) affects protein metabolism in a positive way, delaying the breakdown of dietary proteins into ammonia by micro-organisms present in the rumen and improving the outflow of protein from the rumen, thus improving the intestinal absorption of amino acid in animals. Condensed tannins in several plants used as animal food such as Lotus corniculatus and Hedysarum coronarium have been shown to be beneficial for ruminants and have contributed to the production of more milk, increased growth of wool, ovulation rate, and lambing percentage while lessening the risk of bloating and also decreasing the burden of parasites. This is likely to be associated with increased absorption of essential amino acids from the small intestine by condensed tannins. Owing to their astringent nature, tannins are known to be constituents of several drugs. They are used in the management and cure of hemorrhoids, diarrhoa, dysentery, and leucorrhoea and as a helpful pharmaceutical for throat infections (190). Flavonoid medications have been widely used to ameliorate circulatory disorders involving capillary dysfunction. They are also known to be efficient in averting and mitigating capillary fragility and permeability (191). Legumes contain primary and secondary metabolites and other phytochemicals such as nutraceuticals, pharmaceuticals, pesticides, and industrial products (192). The use of natural products, particularly plants, for the treatment of a variety of disorders is as old as mankind (193). Numerous researchers documented that antimicrobial activity is associated with plant secondary metabolites such as saponins, tannins, alkaloids, flavonoids, quinines, and phenolic compounds (194–196). Alkaloids have been used for skin infection treatments (196, 197).
6. Conclusion and future directions
ANFs are widely found in daily foods and have been laced with tremendous health benefits. Vast literature supports the negative impacts of ANFs on human health and little emphasis is placed on the beneficial effects of ANFs. Growing evidence as discussed in this review article clearly indicates that regulated consumption of ANFs may help to overcome a wide range of human diseases and are beneficial for regulating several physiological and metabolic processes. While many of the potentially harmful compounds from plants have been isolated and explored, less significance has been given to the positive impact when compared to their antinutritive or interfering effects. As plant breeders and nutritionists are finding ways to minimize the presence of these antinutrients in plant-based foods, endeavors should be aimed at maximizing the salutary and therapeutic worth of these entities. Chemically synthesized drugs are costly, and micro-organisms are developing resistance to them in the form of drug resistance. In this regard, it is worth exploring natural plant-based compounds to tackle the issue of drug resistance encountered in the management and treatment of various diseases. We believe that the current account on the positive benefits of ANFs will provide a strong platform for promoting future investigation and exploration of ANFs to devise proper strategies to incorporate them and use them as therapeutic tools for human aliments.
Author contributions
RS, IBN, OMB, SA, AT, and RAM: conceptualization. RS, IBN, OMB, SA, AT, and RAM: writing—original draft preparation. OMB, SA, AT, and RAM: review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Prado, J, and Rostagno, M. Natural product extraction: principles and applications. Cambridge: Royal Society of Chemistry (2022).
2. Ali, A, Devarajan, S, Manickavasagan, A, and Ata, A. Antinutritional factors and biological constraints in the utilization of plant protein foods. Plant Protein Foods: Springer (2022). 407–438.
3. Dey, S, Saxena, A, Kumar, Y, Maity, T, and Tarafdar, A. Understanding the antinutritional factors and bioactive compounds of kodo millet (Paspalum scrobiculatum) and little millet (Panicum sumatrense). J Food Qual. (2022) 2022:1–19. doi: 10.1155/2022/1578448
4. Pihlanto, A, Mattila, P, Makinen, S, and Pajari, AM. Bioactivities of alternative protein sources and their potential health benefits. Food Funct. (2017) 8:3443–58. doi: 10.1039/C7FO00302A
5. Singh, B, Singh, JP, Singh, N, and Kaur, A. Saponins in pulses and their health promoting activities: a review. Food Chem. (2017) 233:540–9. doi: 10.1016/j.foodchem.2017.04.161
6. Silva, FS, Oliveira, PJ, and Duarte, MF. Oleanolic, ursolic, and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: promise or illusion? J Agric Food Chem. (2016) 64:2991–3008. doi: 10.1021/acs.jafc.5b06021
7. Jaafaru, MS, Abd Karim, NA, Enas, ME, Rollin, P, Mazzon, E, and Abdull Razis, AF. Protective effect of glucosinolates hydrolytic products in neurodegenerative diseases (NDDs). Nutrients. (2018) 10:580. doi: 10.3390/nu10050580
8. Soetan, K, and Oyewole, O. The need for adequate processing to reduce the anti-nutritional factors in plants used as human foods and animal feeds: a review. Afr J Food Sci. (2009) 3:223–32.
9. Nath, H, Samtiya, M, and Dhewa, T. Beneficial attributes and adverse effects of major plant-based foods anti-nutrients on health: a review. Human. Nutr Metab. (2022):200147. doi: 10.1016/j.hnm.2022.200147
10. Redden, R, Chen, W, and Sharma, B. Chickpea breeding and management. United Kingdom: CABI (2005).
11. Muzquiz, M, Burbano, C, Cuadrado, C, and Martin, M. Analytical methods for determination of compounds with no nutritive value. Handbook on common bean related laboratory methods Galicia, Spain. (2001), 11–26
12. Mareš, M, Meloun, B, Pavlik, M, Kostka, V, and Baudyš, M. Primary structure of cathepsin D inhibitor from potatoes and its structure relationship to soybean trypsin inhibitor family. FEBS Lett. (1989) 251:94–8. doi: 10.1016/0014-5793(89)81435-8
13. Otto, H-H, and Schirmeister, T. Cysteine proteases and their inhibitors. Chem Rev. (1997) 97:133–72. doi: 10.1021/cr950025u
14. Christeller, JT, Farley, PC, Ramsay, RJ, Sullivan, PA, and Laing, WA. Purification, characterization and cloning of an aspartic proteinase inhibitor from squash phloem exudate. Eur J Biochem. (1998) 254:160–7. doi: 10.1046/j.1432-1327.1998.2540160.x
15. Haq, SK, Atif, SM, and Khan, RH. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: natural and engineered phytoprotection. Arch Biochem Biophys. (2004) 431:145–59. doi: 10.1016/j.abb.2004.07.022
16. Patwekar, M, Quazi, A, Faheem, I, Kamal, MA, Mukim, M, Rather, GA, et al. In vitro inhibitory effect on alpha amylase enzyme by polyherbal dip tea in diabetes. Indo Global Journal of Pharmaceutical Sciences (IGJPS). (2022) 12:156–65. doi: 10.35652/IGJPS.2022.12018
17. Laaraj, N, Bouhrim, M, Kharchoufa, L, Tiji, S, Bendaha, H, Addi, M, et al. Phytochemical analysis, α-glucosidase and α-amylase inhibitory activities and acute toxicity studies of extracts from pomegranate (Punica granatum) bark, a valuable agro-industrial by-product. Foods. (2022) 11:1353. doi: 10.3390/foods11091353
18. Jaradat, N, Khasati, A, Hawi, M, Hawash, M, Shekfeh, S, Qneibi, M, et al. Antidiabetic, antioxidant, and anti-obesity effects of phenylthio-ethyl benzoate derivatives, and molecular docking study regarding α-amylase enzyme. Sci Rep. (2022) 12:1–9. doi: 10.1038/s41598-022-07188-2
19. Deng, X-Y, Ke, J-J, Zheng, Y-Y, Li, D-L, Zhang, K, Zheng, X, et al. Synthesis and bioactivities evaluation of oleanolic acid oxime ester derivatives as α-glucosidase and α-amylase inhibitors. J Enzyme Inhib Med Chem. (2022) 37:451–61. doi: 10.1080/14756366.2021.2018682
20. Nakagawa, K. Studies targeting α-glucosidase inhibition, antiangiogenic effects, and lipid modification regulation: background, evaluation, and challenges in the development of food ingredients for therapeutic purposes. Biosci Biotechnol Biochem. (2013) 77:900–8. doi: 10.1271/bbb.120908
21. Agrawal, N, Sharma, M, Singh, S, and Goyal, A. Recent advances of α-glucosidase inhibitors: a comprehensive review. Curr Top Med Chem. (2022) 22:2069–86. doi: 10.2174/1568026622666220831092855
22. Panda, PK, Naik, PP, Praharaj, PP, Meher, BR, Gupta, PK, Verma, RS, et al. Abrus agglutinin stimulates BMP-2-dependent differentiation through autophagic degradation of β-catenin in colon cancer stem cells. Mol Carcinog. (2018) 57:664–77. doi: 10.1002/mc.22791
23. Bhutia, SK, Panda, PK, Sinha, N, Praharaj, PP, Bhol, CS, Panigrahi, DP, et al. Plant lectins in cancer therapeutics: targeting apoptosis and autophagy-dependent cell death. Pharmacol Res. (2019) 144:8–18. doi: 10.1016/j.phrs.2019.04.001
24. Sinha, N, Meher, BR, Naik, PP, Panda, PK, Mukhapadhyay, S, Maiti, TK, et al. p73 induction by Abrus agglutinin facilitates snail ubiquitination to inhibit epithelial to mesenchymal transition in oral cancer. Phytomedicine. (2019) 55:179–90. doi: 10.1016/j.phymed.2018.08.003
25. von Schoen-Angerer, T, Goyert, A, Vagedes, J, Kiene, H, Merckens, H, and Kienle, GS. Disappearance of an advanced adenomatous colon polyp after intratumoural injection with Viscum album (European mistletoe) extract: a case report. J Gastrointestin Liver Dis. (2014) 23:449–52. doi: 10.15403/jgld.2014.1121.234.acpy
26. Sawant, SS, Randive, VR, and Kulkarni, SR. Lectins from seeds of Abrus precatorius: evaluation of antidiabetic and antihyperlipidemic potential in diabetic rats. Asian J. Pharmaceut Res. (2017) 7:71–80. doi: 10.5958/2231-5691.2017.00013.2
27. Mazalovska, M, and Kouokam, JC. Plant-derived lectins as potential cancer therapeutics and diagnostic tools. Biomed Res Int. (2020) 2020:1631394. doi: 10.1155/2020/1631394
28. El-Araby, MM, El-Shatoury, EH, Soliman, MM, and Shaaban, HF. Characterization and antimicrobial activity of lectins purified from three Egyptian leguminous seeds. AMB Express. (2020) 10:1–14. doi: 10.1186/s13568-020-01024-4
29. Gemede, HF, and Ratta, N. Antinutritional factors in plant foods: potential health benefits and adverse effects. Int J Nutr Food Sci. (2014) 3:284–9. doi: 10.11648/j.ijnfs.20140304.18
30. Lawrence, PK, and Koundal, KR. Plant protease inhibitors in control of phytophagous insects. Electron J Biotechnol. (2002) 5:5–6. doi: 10.2225/vol5-issue1-fulltext-3
31. Aoki-Shioi, N, Nagai, Y, Deshimaru, M, and Terada, S. Precursor genes of Bowman-Birk-type serine proteinase inhibitors comprise multiple inhibitory domains to promote diversity. Biochim Biophys Acta Gen Subj. (2023) 1867:130248. doi: 10.1016/j.bbagen.2022.130248
32. Takács, K, Szabó, EE, Nagy, A, Cserhalmi, Z, Falusi, J, and Gelencsér, É. The effect of radiofrequency heat treatment on trypsin inhibitor activity and in vitro digestibility of soybean varieties (Glycine max.(L.) Merr.). J Food Sci Technol. (2022) 59:4436–45. doi: 10.1007/s13197-022-05523-z
33. Zubko, V, Plavynska, S, Plavynskyi, V, Plavynska, O, Saienko, A, and Roubík, H. Inactivation of anti-nutrients in soybeans via micronisation. Res Agric Eng. (2022) 68:157–67. doi: 10.17221/2/2021-RAE
34. Cid-Gallegos, M, Corzo-Ríos, L, Jiménez-Martinez, C, and Sánchez-Chino, X. Protease inhibitors from plants as therapeutic agents-a review. Plant Foods Hum Nutr. (2022) 77:20–9. doi: 10.1007/s11130-022-00949-4
35. Venkatachalam, P, and Nadumane, VK. Purification and characterization of a protease inhibitor with anticancer potential from Bacillus endophyticus JUPR15. Curr Cancer Ther Rev. (2019) 15:74–82. doi: 10.2174/1573394714666180321150605
36. Song, R, Qiao, W, He, J, Huang, J, Luo, Y, and Yang, T. Proteases and their modulators in cancer therapy: challenges and opportunities. J Med Chem. (2021) 64:2851–77. doi: 10.1021/acs.jmedchem.0c01640
37. Marathe, KR, Patil, RH, Vishwakarma, KS, Chaudhari, AB, and Maheshwari, VL. Protease inhibitors and their applications: an overview. Stud Nat Prod Chem. (2019) 62:211–42. doi: 10.1016/B978-0-444-64185-4.00006-X
38. Kennedy, AR. Proteases, protease inhibitors and radiation carcinogenesis. Int J Radiat Biol. (2023) 99:882–890. doi: 10.1080/09553002.2021.1962567
39. Gitlin-Domagalska, A, Maciejewska, A, and Dębowski, D. Bowman-Birk inhibitors: insights into family of multifunctional proteins and peptides with potential therapeutical applications. Pharmaceuticals. (2020) 13:421. doi: 10.3390/ph13120421
40. Hellinger, R, and Gruber, CW. Peptide-based protease inhibitors from plants. Drug Discov Today. (2019) 24:1877–89. doi: 10.1016/j.drudis.2019.05.026
41. Lucena, SV, Rufino, FP, de Dantas Moura, GED, Rabêlo, L, Monteiro, NK, Ferreira, AT, et al. The Kunitz chymotrypsin inhibitor from Erythrina velutina seeds displays activity against HeLa cells through arrest in cell cycle. 3 Biotech. (2022) 12:1–10. doi: 10.1007/s13205-021-03084-0
42. McCormick, DL, Johnson, WD, Bosland, MC, Lubet, RA, and Steele, VE. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr Cancer. (2007) 57:184–93. doi: 10.1080/01635580701277478
43. Tang, M, Asamoto, M, Ogawa, K, Naiki-Ito, A, Sato, S, Takahashi, S, et al. Induction of apoptosis in the LNCap human prostate carcinoma cell line and prostate adenocarcinomas of SV40T antigen transgenic rats by the Bowman–Birk inhibitor. Pathol Int. (2009) 59:790–6. doi: 10.1111/j.1440-1827.2009.02445.x
44. Abu Hafsa, SH, Hassan, AA, Elghandour, MM, Barbabosa-Pliego, A, Mellado, M, and Salem, AZ. Dietary anti-nutritional factors and their roles in livestock nutrition. V. K. Yata, A. K. Mohanty, and E Lichtfouse. (eds) Sustainable agriculture reviews 57. Sustainable Agriculture Reviews, vol 57. Springer, Cham (2022)
45. Kumar, Y, Basu, S, Goswami, D, Devi, M, Shivhare, US, and Vishwakarma, RK. Anti-nutritional compounds in pulses: implications and alleviation methods. Legume Sci. (2022) 4:e111. doi: 10.1002/leg3.111
46. Kwon, D, Son, SW, Kim, SH, Bae, JE, Lee, Y-H, and Jung, Y-S. Effects of dietary restriction on hepatic sulfur-containing amino acid metabolism and its significance in acetaminophen-induced liver injury. J Nutr Biochem. (2022) 108:109082. doi: 10.1016/j.jnutbio.2022.109082
47. Huang, G-J, Sheu, M-J, Chen, H-J, Chang, Y-S, and Lin, Y-H. Growth inhibition and induction of apoptosis in NB4 promyelocytic leukemia cells by trypsin inhibitor from sweet potato storage roots. J Agric Food Chem. (2007) 55:2548–53. doi: 10.1021/jf063008m
48. Rakashanda, S, Ishaq, M, Masood, A, and Amin, S. Antibacterial activity of a trypsin-chymotrypsin-elastase inhibitor isolated from Lavatera cashmeriana camb. seeds. J Anim Plant Sci. (2012) 22:983–6.
49. Rakashanda, S, Qazi, AK, Majeed, R, Rafiq, S, Dar, IM, Masood, A, et al. Antiproliferative activity of Lavatera cashmeriana-protease inhibitors towards human cancer cells. Asian Pac J Cancer Prev. (2013) 14:3975–8. doi: 10.7314/APJCP.2013.14.6.3975
50. Rakashanda, S, Mubashir, S, Qurishi, Y, Hamid, A, Masood, A, and Amin, S. Trypsin inhibitors from Lavatera cashmeriana Camb. Seeds: isolation, characterization and in-vitro cytoxicity activity. Int J Pharm Sci Invent. (2013) 2:55–65.
51. Dang, L, and Van Damme, EJ. Toxic proteins in plants. Phytochemistry. (2015) 117:51–64. doi: 10.1016/j.phytochem.2015.05.020
52. Marshall, JJ, and Lauda, CM. Purification and properties of phaseolamin, an inhibitor of alpha-amylase, from the kidney bean, Phaseolus vulgaris. J Biol Chem. (1975) 250:8030–7. doi: 10.1016/S0021-9258(19)40811-9
53. Lee, SH, Karadeniz, F, Kim, MM, and Kim, SK. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J Sci Food Agric. (2009) 89:1552–8. doi: 10.1002/jsfa.3623
54. Lee, S-H, Park, M-H, Heo, S-J, Kang, S-M, Ko, S-C, Han, J-S, et al. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem Toxicol. (2010) 48:2633–7. doi: 10.1016/j.fct.2010.06.032
55. Lee, H-A, Lee, J-H, and Han, J-S. A phlorotannin constituent of Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Pharm Biol. (2017) 55:1149–54. doi: 10.1080/13880209.2017.1291693
56. Daboné, C, Delisle, HF, and Receveur, O. Poor nutritional status of schoolchildren in urban and peri-urban areas of Ouagadougou (Burkina Faso). Nutr J. (2011) 10:1–8. doi: 10.1186/1475-2891-10-34
57. Peddio, S, Padiglia, A, Cannea, FB, Crnjar, R, Zam, W, Sharifi-Rad, J, et al. Common bean (Phaseolus vulgaris L.) α-amylase inhibitors as safe nutraceutical strategy against diabetes and obesity: an update review. Phytother Res. (2022). doi: 10.1002/ptr.7480
58. Olagunju, AI, Alashi, AM, Omoba, OS, Enujiugha, VN, and Aluko, RE. Pigeon pea penta-and hexapeptides with antioxidant properties also inhibit renin and angiotensin-I-converting enzyme activities. J Food Biochem. (2022):e14485. doi: 10.1111/jfbc.14485
59. Henry, R, McKinnon, G, Haak, I, and Brennan, P. Use of alpha-amylase inhibitors to control sprouting. Pre Harvest Sprout Cereals. (1993) 1992:232–5.
60. Ali, AM. Anti-diabetic potential of phenolic compounds: a review. Int J Food Prop. (2013) 16:91–103. doi: 10.1080/10942912.2011.595864
61. Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: finding needle in the haystack. Eur J Med Chem. (2015) 103:133–62. doi: 10.1016/j.ejmech.2015.08.043
62. Wang, J, Jin, W, Zhang, W, Hou, Y, Zhang, H, and Zhang, Q. Hypoglycemic property of acidic polysaccharide extracted from Saccharina japonica and its potential mechanism. Carbohydr Polym. (2013) 95:143–7. doi: 10.1016/j.carbpol.2013.02.076
63. Kim, K-T, Rioux, L-E, and Turgeon, SL. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. (2014) 98:27–33. doi: 10.1016/j.phytochem.2013.12.003
64. Kim, K-T, Rioux, L-E, and Turgeon, SL. Molecular weight and sulfate content modulate the inhibition of α-amylase by fucoidan relevant for type 2 diabetes management. PharmaNutrition. (2015) 3:108–14. doi: 10.1016/j.phanu.2015.02.001
65. Kumar, TV, Lakshmanasenthil, S, Geetharamani, D, Marudhupandi, T, Suja, G, and Suganya, P. Fucoidan–a α-d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int J Biol Macromol. (2015) 72:1044–7. doi: 10.1016/j.ijbiomac.2014.10.013
66. Senthil, SL, Kumar, TV, Geetharamani, D, Suja, G, Yesudas, R, and Chacko, A. Fucoidan–an α-amylase inhibitor from Sargassum wightii with relevance to NIDDM. Int J Biol Macromol. (2015) 81:644–7. doi: 10.1016/j.ijbiomac.2015.08.065
67. Shan, X, Liu, X, Hao, J, Cai, C, Fan, F, Dun, Y, et al. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int J Biol Macromol. (2016) 82:249–55. doi: 10.1016/j.ijbiomac.2015.11.036
68. Raghu, C, Arjun, H, and Anantharaman, P. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr Polym. (2019) 209:350–5. doi: 10.1016/j.carbpol.2019.01.039
69. Daub, CD, Mabate, B, Malgas, S, and Pletschke, BI. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int J Biol Macromol. (2020) 151:412–20. doi: 10.1016/j.ijbiomac.2020.02.161
70. Lee, S-H, Kang, S-M, Ko, S-C, Moon, S-H, Jeon, B-T, Lee, DH, et al. Octaphlorethol a: a potent α-glucosidase inhibitor isolated from Ishige foliacea shows an anti-hyperglycemic effect in mice with streptozotocin-induced diabetes. Food Funct. (2014) 5:2602–8. doi: 10.1039/C4FO00420E
71. Ryu, B, Jiang, Y, Kim, H-S, Hyun, J-M, Lim, S-B, Li, Y, et al. Ishophloroglucin A, a novel phlorotannin for standardizing the anti-α-glucosidase activity of Ishige okamurae. Mar Drugs. (2018) 16:436. doi: 10.3390/md16110436
72. Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit Rev Oral Biol Med. (2002) 13:184–96. doi: 10.1177/154411130201300208
73. Bagchi, D, Sen, CK, Ray, SD, Das, DK, Bagchi, M, Preuss, HG, et al. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res. (2003) 523:87–97. doi: 10.1016/s0027-5107(02)00324-x
74. Aron, PM, and Kennedy, JA. Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res. (2008) 52:79–104. doi: 10.1002/mnfr.200700137
75. Fereidoon, S. Beneficial health effects and drawbacks of antinutrients and phytochemicals in foods. Appl Microbiol Biotechnol. (2014) 97:45–55. doi: 10.1021/BK-1997-0662
76. Kiranmayi, P. Is bio active compounds inplantsacts as anti nutritonal factors. Int J Curr Pharm Res. (2014) 6:36–8.
77. Lam, SK, and Ng, TB. Lectins: production and practical applications. Appl Microbiol Biotechnol. (2011) 89:45–55. doi: 10.1007/s00253-010-2892-9
78. Bora, P. Anti-nutritional factors in foods and their effects. J Acad Industr Res. (2014) 3:285–90.
79. Cordain, L, Toohey, L, Smith, M, and Hickey, M. Modulation of immune function by dietary lectins in rheumatoid arthritis. Br J Nutr. (2000) 83:207–17. doi: 10.1017/S0007114500000271
80. Ryder, SD, Jacyna, MR, Levi, AJ, Rizzi, PM, and Rhodes, JM. Peanut ingestion increases rectal proliferation in individuals with mucosal expression of peanut lectin receptor. Gastroenterology. (1998) 114:44–9. doi: 10.1016/S0016-5085(98)70631-6
81. Pryme, IF, Bardocz, S, Pusztai, A, Ewen, SW, and Pfüller, U. A mistletoe lectin (ML-1)-containing diet reduces the viability of a murine non-Hodgkin lymphoma tumor. Cancer Detect Prev. (2004) 28:52–6. doi: 10.1016/j.cdp.2003.10.003
82. Serrano, J, Puupponen-Pimiä, R, Dauer, A, Aura, AM, and Saura-Calixto, F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. (2009) 53:S310–29. doi: 10.1002/mnfr.200900039
83. Wijesekara, I, Yoon, NY, and Kim, SK. Phlorotannins from Ecklonia cava (Phaeophyceae): biological activities and potential health benefits. Biofactors. (2010) 36:408–14. doi: 10.1002/biof.114
84. Dolara, P, Luceri, C, De Filippo, C, Femia, AP, Giovannelli, L, Caderni, G, et al. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat Res. (2005) 591:237–46. doi: 10.1016/j.mrfmmm.2005.04.022
85. Barszcz, M, Taciak, M, Tuśnio, A, and Skomiał, J. Effects of dietary level of tannic acid and protein on internal organ weights and biochemical blood parameters of rats. PLoS One. (2018) 13:e0190769. doi: 10.1371/journal.pone.0190769
86. Meng, W, Mu, T, Sun, H, and Garcia-Vaquero, M. Phlorotannins: a review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. (2021) 60:102484. doi: 10.1016/j.algal.2021.102484
87. Wijesinghe, W, and Jeon, Y-J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: a review. Int J Food Sci Nutr. (2012) 63:225–35. doi: 10.3109/09637486.2011.619965
88. Seca, AM, and Pinto, DC. Overview on the antihypertensive and anti-obesity effects of secondary metabolites from seaweeds. Mar Drugs. (2018) 16:237. doi: 10.3390/md16070237
89. Shrestha, S, Zhang, W, and Smid, S. Phlorotannins: a review on biosynthesis, chemistry and bioactivity. Food Biosci. (2021) 39:100832. doi: 10.1016/j.fbio.2020.100832
90. Aragão, MGB, Aires, CP, and Corona, SAM. Effects of the green tea catechin epigallocatechin-3-gallate on S treptococcus mutans planktonic cultures and biofilms: systematic literature review of in vitro studies. Biofouling. (2022) 38:687–95. doi: 10.1080/08927014.2022.2116320
91. Ntamo, Y, Jack, B, Ziqubu, K, Mazibuko-Mbeje, SE, Nkambule, BB, Nyambuya, TM, et al. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit Rev Food Sci Nutr. (2022):1–23. doi: 10.1080/10408398.2022.2104805
92. Ahmad, SR, and Ghosh, P. A systematic investigation on flavonoids, catechin, β-sitosterol and lignin glycosides from Saraca asoca (ashoka) having anti-cancer & antioxidant properties with no side effect. J Indian Chem Soc. (2022) 99:100293. doi: 10.1016/j.jics.2021.100293
93. Wandee, R, Sutthanut, K, Songsri, J, Sonsena, S, Krongyut, O, Tippayawat, P, et al. Tamarind seed coat: a catechin-rich source with anti-oxidation, anti-melanogenesis, anti-adipogenesis and anti-microbial activities. Molecules. (2022) 27:5319. doi: 10.3390/molecules27165319
94. Yadav, A, Yadav, SS, Singh, S, and Dabur, R. Natural products: potential therapeutic agents to prevent skeletal muscle atrophy. Eur J Pharmacol. (2022):174995. doi: 10.1016/j.ejphar.2022.174995
95. Sanchis, P, Rivera, R, Berga, F, Fortuny, R, Adrover, M, Costa-Bauza, A, et al. Phytate decreases formation of advanced glycation end-products in patients with type II diabetes: randomized crossover trial. Sci Rep. (2018) 8:1–13. doi: 10.1038/s41598-018-27853-9
96. Zajdel, A, Wilczok, A, Węglarz, L, and Dzierżewicz, Z. Phytic acid inhibits lipid peroxidation in vitro. Biomed Res Int. (2013) 2013:147307. doi: 10.1155/2013/147307
97. Onomi, S, Okazaki, Y, and Katayama, T. Effect of dietary level of phytic acid on hepatic and serum lipid status in rats fed a high-sucrose diet. Biosci Biotechnol Biochem. (2004) 68:1379–81. doi: 10.1271/bbb.68.1379
98. Omoruyi, FO, Stennett, D, Foster, S, and Dilworth, L. New frontiers for the use of IP6 and inositol combination in treating diabetes mellitus: a review. Molecules. (2020) 25:1720. doi: 10.3390/molecules25071720
99. Anekonda, TS, Wadsworth, TL, Sabin, R, Frahler, K, Harris, C, Petriko, B, et al. Phytic acid as a potential treatment for Alzheimer’s pathology: evidence from animal and in vitro models. J Alzheimers Dis. (2011) 23:21–35. doi: 10.3233/JAD-2010-101287
100. Xu, Q, Kanthasamy, AG, and Reddy, MB. Phytic acid protects against 6-hydroxydopamine-induced dopaminergic neuron apoptosis in normal and iron excess conditions in a cell culture model. Parkinson’s Dis. (2011) 2011:431068. doi: 10.4061/2011/431068
101. Abdulwaliyu, I, Arekemase, SO, Adudu, JA, Batari, ML, Egbule, MN, and Okoduwa, SIR. Investigation of the medicinal significance of phytic acid as an indispensable anti-nutrient in diseases. Clin Nutr Experiment. (2019) 28:42–61. doi: 10.1016/j.yclnex.2019.10.002
102. Fernandez-Palomeque, C, Grau, A, Perello, J, Sanchis, P, Isern, B, Prieto, RM, et al. Relationship between urinary level of phytate and valvular calcification in an elderly population: a cross-sectional study. PLoS One. (2015) 10:e0136560. doi: 10.1371/journal.pone.0136560
103. Chatterjee, S, Rhee, Y, Chung, P-S, Ge, R-F, and Ahn, J-C. Sulforaphene enhances the efficacy of photodynamic therapy in anaplastic thyroid cancer through Ras/RAF/MEK/ERK pathway suppression. J Photochem Photobiol B Biol. (2018) 179:46–53. doi: 10.1016/j.jphotobiol.2017.12.013
104. Tahata, S, Singh, SV, Lin, Y, Hahm, E-R, Beumer, JH, Christner, SM, et al. Evaluation of biodistribution of sulforaphane after administration of oral broccoli sprout extract in melanoma patients with multiple atypical nevi. Cancer Prev Res. (2018) 11:429–38. doi: 10.1158/1940-6207.CAPR-17-0268
105. Traka, MH, Melchini, A, Coode-Bate, J, Al Kadhi, O, Saha, S, Defernez, M, et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention—results from the effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am J Clin Nutr. (2019) 109:1133–44. doi: 10.1093/ajcn/nqz012
106. Valdivia, M, Soto-Becerra, P, Laguna-Barraza, R, Rojas, PA, Reyes-Mandujano, I, Gonzáles-Reyes, P, et al. Effect of a natural supplement containing glucosinolates, phytosterols and citrus flavonoids on body weight and metabolic parameters in a menopausal murine model induced by bilateral ovariectomy. Gynecol Endocrinol. (2020) 36:1106–11. doi: 10.1080/09513590.2020.1821639
107. Miękus, N, Marszałek, K, Podlacha, M, Iqbal, A, Puchalski, C, and Świergiel, AH. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules. (2020) 25:3804. doi: 10.3390/molecules25173804
108. Zou, Y. Naturally occurring steroidal Saponins as potential anticancer agents: current developments and mechanisms of action. Curr Top Med Chem. (2022). doi: 10.2174/1568026622666220330011047
109. Liu, J, Wang, Y, Qiu, L, Yu, Y, and Wang, C. Saponins of Panax notoginseng: chemistry, cellular targets and therapeutic opportunities in cardiovascular diseases. Expert Opin Investig Drugs. (2014) 23:523–39. doi: 10.1517/13543784.2014.892582
110. Khan, H, Alam, W, Alsharif, KF, Aschner, M, Pervez, S, and Saso, L. Alkaloids and colon cancer: molecular mechanisms and therapeutic implications for cell cycle arrest. Molecules. (2022) 27:920. doi: 10.3390/molecules27030920
111. Smułek, W, Rojewska, M, Pacholak, A, Machrowicz, O, Prochaska, K, and Kaczorek, E. Co-interaction of nitrofurantoin and saponins surfactants with biomembrane leads to an increase in antibiotic’s antibacterial activity. J Mol Liq. (2022) 364:120070. doi: 10.1016/j.molliq.2022.120070
112. Golmohammadi, MG, Banaei, S, and Abedi, A. Saponin protects against cyclophosphamide-induced kidney and liver damage via antioxidant and anti-inflammatory actions. Physiol Int. (2022) 110:108–20. doi: 10.1556/2060.2023.00190
113. Tan, M, Sharma, N, and An, S. Phyto-carbazole alkaloids from the rutaceae family as potential protective agents against neurodegenerative diseases. Antioxidants. (2022) 11:493. doi: 10.3390/antiox11030493
114. Pandrangi, SL, Chalumuri, SS, and Garimella, S. Emerging therapeutic efficacy of alkaloids as anticancer agents. Ann Roman Soc Cell Biol. (2022) 26:64–74.
115. Jayakumar, T, Yang, C-M, Yen, T-L, Hsu, C-Y, Sheu, J-R, Hsia, C-W, et al. Anti-inflammatory mechanism of an alkaloid rutaecarpine in LTA-stimulated RAW 264.7 cells: pivotal role on NF-κB and ERK/p38 signaling molecules. Int J Mol Sci. (2022) 23:5889. doi: 10.3390/ijms23115889
116. Abookleesh, FL, Al-Anzi, BS, and Ullah, A. Potential antiviral action of alkaloids. Molecules. (2022) 27:903. doi: 10.3390/molecules27030903
117. Bjørklund, G, Shanaida, M, Lysiuk, R, Butnariu, M, Peana, M, Sarac, I, et al. Natural compounds and products from an anti-aging perspective. Molecules. (2022) 27:7084. doi: 10.3390/molecules27207084
118. Singh, S, Bansal, A, Singh, V, Chopra, T, and Poddar, J. Flavonoids, alkaloids and terpenoids: a new hope for the treatment of diabetes mellitus. J Diabetes Metab Disord. (2022) 21:941–50. doi: 10.1007/s40200-021-00943-8
119. Akande, K, Doma, U, Agu, H, and Adamu, H. Major antinutrients found in plant protein sources: their effect on nutrition. Pak J Nutr. (2010) 9:827–32. doi: 10.3923/pjn.2010.827.832
120. Porter, LJ. 11—Tannins In: JB Harborne, editor. Methods in plant biochemistry, plant phenolics, vol. 1. Cambridge, MA: Academic Press (2012). 389–419.
121. Adeparusi, E. Effect of processing on the nutrients and anti-nutrients of lima bean (Phaseolus lunatus L.) flour. Food Nahrung. (2001) 45:94–6. doi: 10.1002/1521-3803(20010401)45:2<94::AID-FOOD94>3.0.CO;2-E
122. Kumari, M, and Jain, S. Tannins: An antinutrient with positive effect to manage diabetes. Res J Rec Sci ISSN. (2012) 2277:2502.
123. Ojo, MA. Tannins in foods: nutritional implications and processing effects of hydrothermal techniques on underutilized hard-to-cook legume seeds–a review. Prevent Nutr Food Sci. (2022) 27:14. doi: 10.3746/pnf.2022.27.1.14
124. Gyamfi, MA, and Aniya, Y. Antioxidant properties of Thonningianin A, isolated from the African medicinal herb, Thonningia sanguinea. Biochem Pharmacol. (2002) 63:1725–37. doi: 10.1016/S0006-2952(02)00915-2
125. Fridrich, D, Kern, M, Pahlke, G, Volz, N, Will, F, Dietrich, H, et al. Apple polyphenols diminish the phosphorylation of the epidermal growth factor receptor in HT29 colon carcinoma cells. Mol Nutr Food Res. (2007) 51:594–601. doi: 10.1002/mnfr.200600189
126. Barth, SW, Faehndrich, C, Bub, A, Watzl, B, Will, F, Dietrich, H, et al. Cloudy apple juice is more effective than apple polyphenols and an apple juice derived cloud fraction in a rat model of colon carcinogenesis. J Agric Food Chem. (2007) 55:1181–7. doi: 10.1021/jf063078t
127. Muthusamy, V, Anand, S, Sangeetha, K, Sujatha, S, Arun, B, and Lakshmi, B. Tannins present in Cichorium intybus enhance glucose uptake and inhibit adipogenesis in 3T3-L1 adipocytes through PTP1B inhibition. Chem Biol Interact. (2008) 174:69–78. doi: 10.1016/j.cbi.2008.04.016
128. Li, Y, Zhu, L, Guo, C, Xue, M, Xia, F, Wang, Y, et al. Dietary intake of hydrolyzable tannins and condensed tannins to regulate lipid metabolism. Mini Rev Med Chem. (2022) 22:1789–802. doi: 10.2174/1389557522666211229112223
129. Oh, S, Son, M, Lee, HS, Kim, H-S, Jeon, Y-J, and Byun, K. Protective effect of pyrogallol-phloroglucinol-6, 6-bieckol from Ecklonia cava on monocyte-associated vascular dysfunction. Mar Drugs. (2018) 16:441. doi: 10.3390/md16110441
130. Higdon, JV, and Frei, B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. (2003) 43:89–143. doi: 10.1080/10408690390826464
131. Vats, P, and Banerjee, UC. Production studies and catalytic properties of phytases (myo-inositolhexakisphosphate phosphohydrolases): an overview. Enzym Microb Technol. (2004) 35:3–14. doi: 10.1016/j.enzmictec.2004.03.010
132. Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim Feed Sci Technol. (2001) 91:3–20. doi: 10.1016/S0377-8401(01)00227-9
133. Maenz, DD, and Classen, HL. Phytase activity in the small intestinal brush border membrane of the chicken. Poult Sci. (1998) 77:557–63. doi: 10.1093/ps/77.4.557
134. Boling, SD, Douglas, M, Johnson, M, Wang, X, Parsons, C, Koelkebeck, K, et al. The effects of dietary available phosphorus levels and phytase on performance of young and older laying hens. Poult Sci. (2000) 79:224–30. doi: 10.1093/ps/79.2.224
135. Singh, B, Kunze, G, and Satyanarayana, T. Developments in biochemical aspects and biotechnological applications of microbial phytases. Biotechnol Mol Biol Rev. (2011) 6:69–87.
136. Oladimeji, M, Akindahunsi, A, and Okafor, A. Investigation of the bioavailability of zinc and calcium from some tropical tubers. Nahrung. (2000) 44:136–7. doi: 10.1002/(SICI)1521-3803(20000301)44:2<136::AID-FOOD136>3.0.CO;2-7
137. Dost, K, and Tokul, O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal Chim Acta. (2006) 558:22–7. doi: 10.1016/j.aca.2005.11.035
138. Vucenik, I, Ramakrishna, G, Tantivejkul, K, Anderson, LM, and Ramljak, D. Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCδ-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res Treat. (2005) 91:35–45. doi: 10.1007/s10549-004-6456-5
139. Vucenik, I, Zhang, Z, and Shamsuddin, A. IP6 in treatment of liver cancer. II. Intra-tumoral injection of IP6 regresses pre-existing human liver cancer xenotransplanted in nude mice. Anticancer Res. (1998) 18:4091–6.
140. Graf, E, and Eaton, JW. Dietary suppression of colonic cancer fiber or phytate? Cancer. (1985) 56:717–8. doi: 10.1002/1097-0142(19850815)56:4<717::AID-CNCR2820560402>3.0.CO;2-4
141. Deliliers, GL, Servida, F, Fracchiolla, NS, Ricci, C, Borsotti, C, Colombo, G, et al. Effect of inositol hexaphosphate (IP6) on human normal and leukaemic haematopoietic cells. Br J Haematol. (2002) 117:577–87. doi: 10.1046/j.1365-2141.2002.03453.x
142. Shamsuddin, AM, Baten, A, and Lalwani, ND. Effects of inositol hexaphosphate on growth and differentiation in K-562 erythroleukemia cell line. Cancer Lett. (1992) 64:195–202. doi: 10.1016/0304-3835(92)90043-U
143. Shamsuddin, AM, and Yang, G-Y. Inositol hexaphosphate inhibits growth and induces differentiation of PC-3 human prostate cancer cells. Carcinogenesis. (1995) 16:1975–9. doi: 10.1093/carcin/16.8.1975
144. Ferry, S, Matsuda, M, Yoshida, H, and Hirata, M. Inositol hexakisphosphate blocks tumor cell growth by activating apoptotic machinery as well as by inhibiting the Akt/NFκB-mediated cell survival pathway. Carcinogenesis. (2002) 23:2031–41. doi: 10.1093/carcin/23.12.2031
145. Saied, I, and MsHAMsUDDIN, A. In HepG2 human liver cancer cell line. Anticancer Res. (1998) 18:4083–90.
146. Shamsuddin, AM. Anti-cancer function of phytic acid. Int J Food Sci Technol. (2002) 37:769–82. doi: 10.1046/j.1365-2621.2002.00620.x
147. Jariwalla, R, Sabin, R, Lawson, S, and Herman, Z. Lowering of serum cholesterol and triglycerides and modulation of divalent cations by dietary phytate. J Appl Nutr. (1990) 42:18–28.
148. Potter, SM. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr. (1995) 125:606S–11S.
149. Du, Y, Dou, S, and Wu, S. Efficacy of phytic acid as an inhibitor of enzymatic and non-enzymatic browning in apple juice. Food Chem. (2012) 135:580–2. doi: 10.1016/j.foodchem.2012.04.131
150. Ghiretti, G, Zanardi, E, Novelli, E, Campanini, G, Dazzi, G, Madarena, G, et al. Comparative evaluation of some antioxidants in salame Milano and mortadella production. Meat Sci. (1997) 47:167–76. doi: 10.1016/S0309-1740(97)00059-4
151. Md, COSTA, Cd, SILVA, Bridi, A, Fonseca, N, Oba, A, Silva, R, et al. Estabilidade lipídica do pernil e da linguiça frescal de suínos tratados com dietas com alta concentração de ácido fítico. Semin Ciênc Agrár. (2011) 32:1863–72. doi: 10.5433/1679-0359.2011v32Suplp1863
152. Monteiro, RT, Silva, CA, Bridi, AM, Obra, A, Lozano, AP, Peres, LM, et al. Efeito do ácido fítico e do ferro inorgânico dietéticos na qualidade da carne suina refrigerada. B Indústr Anim. (2015) 72:261–70. doi: 10.17523/bia.v72n3p261
153. Kristal, AR, and Lampe, JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. (2002) 42:1–9. doi: 10.1207/S15327914NC421_1
154. Holst, B, and Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep. (2004) 21:425–47. doi: 10.1039/b204039p
155. Ehlers, A, Florian, S, Schumacher, F, Meinl, W, Lenze, D, Hummel, M, et al. The glucosinolate metabolite 1-methoxy-3-indolylmethyl alcohol induces a gene expression profile in mouse liver similar to the expression signature caused by known genotoxic hepatocarcinogens. Mol Nutr Food Res. (2015) 59:685–97. doi: 10.1002/mnfr.201400707
156. Hanschen, FS, Herz, C, Schlotz, N, Kupke, F, Bartolomé Rodríguez, MM, Schreiner, M, et al. The Brassica epithionitrile 1-cyano-2, 3-epithiopropane triggers cell death in human liver cancer cells in vitro. Mol Nutr Food Res. (2015) 59:2178–89. doi: 10.1002/mnfr.201500296
157. Dinkova-Kostova, AT, and Kostov, RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med. (2012) 18:337–47. doi: 10.1016/j.molmed.2012.04.003
158. Gupta, U. What’s new about crop plants: Novel discoveries of the 21st century. Boca Raton: CRC Press (2011).
159. Rodríguez-Cantú, LN, Gutiérrez-Uribe, JA, Arriola-Vucovich, J, Díaz-De La Garza, RI, Fahey, JW, and Serna-Saldivar, SO. Broccoli (Brassica oleracea var. italica) sprouts and extracts rich in glucosinolates and isothiocyanates affect cholesterol metabolism and genes involved in lipid homeostasis in hamsters. J Agric Food Chem. (2011) 59:1095–103. doi: 10.1021/jf103513w
160. Wu, QJ, Yang, Y, Wang, J, Han, LH, and Xiang, YB. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci. (2013) 104:1067–73. doi: 10.1111/cas.12195
161. Mithen, RF, Dekker, M, Verkerk, R, Rabot, S, and Johnson, IT. The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J Sci Food Agric. (2000) 80:967–84. doi: 10.1002/(SICI)1097-0010(20000515)80:7<967::AID-JSFA597>3.0.CO;2-V
162. Cartea, ME, Lema, M, Francisco, M, and Velasco, P. Basic information on vegetable Brassica crops. Genetics, genomics and breeding of vegetable Brassicas (2011) 1–33.
163. Giacoppo, S, Galuppo, M, Montaut, S, Iori, R, Rollin, P, Bramanti, P, et al. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases. Fitoterapia. (2015) 106:12–21. doi: 10.1016/j.fitote.2015.08.001
164. Fowke, JH, Gao, Y-T, Chow, W-H, Cai, Q, Shu, X-O, Li, H-l, et al. Urinary isothiocyanate levels and lung cancer risk among non-smoking women: a prospective investigation. Lung Cancer. (2011) 73:18–24. doi: 10.1016/j.lungcan.2010.10.024
165. Lamy, E, Hertrampf, A, Herz, C, Schüler, J, Erlacher, M, Bertele, D, et al. Preclinical evaluation of 4-methylthiobutyl isothiocyanate on liver cancer and cancer stem cells with different p53 status. PLoS One. (2013) 8:e70846. doi: 10.1371/journal.pone.0070846
166. Wu, Q-J, Wang, J, Gao, J, Zhang, W, Han, L-H, Gao, S, et al. Urinary isothiocyanates level and liver cancer risk: a nested case-control study in Shanghai, China. Nutr Cancer. (2014) 66:1023–9. doi: 10.1080/01635581.2014.936953
167. Tan, X-L, Shi, M, Tang, H, Han, W, and Spivack, SD. Candidate dietary phytochemicals modulate expression of phase II enzymes GSTP1 and NQO1 in human lung cells. J Nutr. (2010) 140:1404–10. doi: 10.3945/jn.110.121905
168. Pawlik, A, Szczepanski, MA, Klimaszewska, A, Gackowska, L, Zuryn, A, and Grzanka, A. Phenethyl isothiocyanate-induced cytoskeletal changes and cell death in lung cancer cells. Food Chem Toxicol. (2012) 50:3577–94. doi: 10.1016/j.fct.2012.07.043
169. Kalpana Deepa Priya, D, Gayathri, R, Gunassekaran, GR, Murugan, S, and Sakthisekaran, D. Apoptotic role of natural isothiocyanate from broccoli (Brassica oleracea italica) in experimental chemical lung carcinogenesis. Pharm Biol. (2013) 51:621–8. doi: 10.3109/13880209.2012.761242
170. Dinkova-Kostova, AT, Fahey, JW, Wade, KL, Jenkins, SN, Shapiro, TA, Fuchs, EJ, et al. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol Biomark Prev. (2007) 16:847–51. doi: 10.1158/1055-9965.EPI-06-0934
171. Walters, DG, Young, PJ, Agus, C, Knize, MG, Boobis, AR, Gooderham, NJ, et al. Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) in humans. Carcinogenesis. (2004) 25:1659–69. doi: 10.1093/carcin/bgh164
172. Hudson, B In: IE Liener, editor. Toxic constituents of plant foodstuffs. New York and London: Academic Press (1980) 502 pp. Price: $39 [middle dot] 50. Agricultural Systems. 1981;7(1):80-.
173. Scott, M, Goss-Sampson, M, and Bomford, R. Adjuvant activity of saponin: antigen localization studies. Int Arch Allergy Immunol. (1985) 77:409–12.
174. Shi, J, Arunasalam, K, Yeung, D, Kakuda, Y, Mittal, G, and Jiang, Y. Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food. (2004) 7:67–78. doi: 10.1089/109662004322984734
175. Wei, S. Isolation and determination of anti-nutritional compounds from root and shells of Peanut (Arachis hypogaea). A project report submitted to the Department of Chemical Science Faculty of Science University Tunku Abdul Rahman in partial fulfillment of the requirements for the degree of Bachelor of Science (Hons) Biochemistry (2011).
176. Oleszek, W. Chromatographic determination of plant saponins. J Chromatogr A. (2002) 967:147–62. doi: 10.1016/S0021-9673(01)01556-4
177. Kao, T, Huang, S, Inbaraj, BS, and Chen, B. Determination of flavonoids and saponins in Gynostemma pentaphyllum (Thunb.) Makino by liquid chromatography–mass spectrometry. Anal Chim Acta. (2008) 626:200–11. doi: 10.1016/j.aca.2008.07.049
178. Uematsu, Y, Hirata, K, Saito, K, and Kudo, I. Spectrophotometric determination of saponin in Yucca extract used as food additive. J AOAC Int. (2000) 83:1451–4. doi: 10.1093/jaoac/83.6.1451
179. Watkins, R, TurIey, D, and Chaudhry, Q. Useful chemicals from the main commercial tree species in the UK. Forestry Commision ConIine. (2004).
180. Saito, K, Horie, M, Hoshino, Y, Nose, N, and Nakazawa, H. High-performance liquid chromatographic determination of glycoalkaloids in potato products. J Chromatogr A. (1990) 508:141–7. doi: 10.1016/S0021-9673(00)91247-0
182. Takashi, I, Keiichi, U, and Hisayuki, R. Gourd saponins as antioxidants in oils, foods, cosmetics and pharmaceuticals. Chem Abstr. (1986) 105:151–81.
183. Bradbury, R, and White, D. Estrogens and related substances in plants. Vitam Horm. (1954) 12:207–33.
184. Kim, JY, Germolec, DR, and Luster, MI. Panax ginseng as a potential immunomodulator: studies in mice. Immunopharmacol Immunotoxicol. (1990) 12:257–76.
185. Harborne, J. Comparative biochemistry of the flavonoids. London: Academic Press Inc (1967). 1 p.
186. Oleszek, W, Jurzysta, M, and Gorski, P. Alfalfa saponins—the allelopathic agents. Allelopathy: Springer (1992). 151–167.
187. Waller, G, Jurzysta, M, and Thorne, R. Allelopathic activity of root saponins from alfalfa (Medicago sativa L.) on weeds and wheat. Bot Bull Acad Sin. (1993) 34.
188. Igile, G. Phytochemical and biological studies on some constituents of Vernonia amygdalina (1995).
189. Aerts, RJ, Barry, TN, and McNabb, WC. Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages. Agric Ecosyst Environ. (1999) 75:1–12. doi: 10.1016/S0167-8809(99)00062-6
191. Fahey, G Jr, and Jung, H. Phenolic compounds in forages and fibrous feedstuffs. Toxicants Plant Origin. (1989) 4:123–90.
192. Morris, B. Bio-functional legumes with nutraceutical, pharmaceutical, and industrial uses. Econ Bot. (2003) 57:254–61. doi: 10.1663/0013-0001(2003)057[0254:BLWNPA]2.0.CO;2
193. Ogbonna, A, Makut, M, Gyar, S, and Adamu, E. Antimicrobial activity of the ethanolic extract of the seeds of Ricinus communis. Nig J Biotech. (2007) 18:40–3. doi: 10.1016/S2221-1691(13)60004-0
194. Ekong, E. Medicinal plants research in Nigeria: retrospect and prospects In: A Sofowora, editor. The state of medicinal plants research in Nigeria. Nigeria: Ibadan University Press (1989)
195. Ebana, R, Madunagu, B, Ekpe, E, and Otung, I. Microbiological exploitation of cardiac glycosides and alkaloids from Garcinia kola, Borreria ocymoides, Kola nitida and Citrus aurantifolia. J Appl Bacteriol. (1991) 71:398–401. doi: 10.1111/j.1365-2672.1991.tb03807.x
196. Sofowora, A. Medicinal plants and traditional medicine in Africa Spectrum books LTD. Ibadan: Nigeria (1993). 289 p.
Keywords: anti-nutritional factors (ANF), nutritional deficiency, plant-based foods, secondary metabolites, human diseases
Citation: Salim R, Nehvi IB, Mir RA, Tyagi A, Ali S and Bhat OM (2023) A review on anti-nutritional factors: unraveling the natural gateways to human health. Front. Nutr. 10:1215873. doi: 10.3389/fnut.2023.1215873
Edited by:
Runqiang Yang, Nanjing Agricultural University, ChinaReviewed by:
Yao Tang, Tianjin University of Science and Technology, ChinaRadjassegarin Arumugam, A. V. C. College, India
Copyright © 2023 Salim, Nehvi, Mir, Tyagi, Ali and Bhat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakeeb Ahmad Mir, cmFrZWViYWhtYWRAZ21haWwuY29t; Anshika Tyagi, YW5zaGlrYWJpb0B5dS5hYy5rcg==; Sajad Ali, c2FqYWRtaWNyb0B5dS5hYy5rcg==; Owais M. Bhat, c3Vubnkub3dhaXNAZ21haWwuY29t
†These authors have contributed equally to this work
 Rehana Salim1†
Rehana Salim1† Iqra Bashir Nehvi
Iqra Bashir Nehvi Rakeeb Ahmad Mir
Rakeeb Ahmad Mir Sajad Ali
Sajad Ali Owais M. Bhat
Owais M. Bhat