
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 20 July 2023
Sec. Nutrigenomics
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1215559
This article is part of the Research Topic Diet, Nutritional Status, and Their Interaction with Genetics/Epigenetics in Different Stages of Carcinogenesis View all 4 articles
 Maryam Gholamalizadeh1
Maryam Gholamalizadeh1 Mona Jonoush2
Mona Jonoush2 Khadijeh Abbasi Mobarakeh3
Khadijeh Abbasi Mobarakeh3 Arezoo Amjadi4
Arezoo Amjadi4 Farkhondeh Alami5
Farkhondeh Alami5 Neda Valisoltani6
Neda Valisoltani6 Seyed Ali Askarpour7
Seyed Ali Askarpour7 Ghasem Azizi-Tabesh8
Ghasem Azizi-Tabesh8 Mohammad Keshavarz Mohammadian9
Mohammad Keshavarz Mohammadian9 Mohammad Esmail Akbari1
Mohammad Esmail Akbari1 Masoumeh Rajabibazl10
Masoumeh Rajabibazl10 Mahdi Alemrajabi11
Mahdi Alemrajabi11 Jafar Poodineh12
Jafar Poodineh12 Hossein Sadeghi13
Hossein Sadeghi13 Payam Hosseinzadeh14
Payam Hosseinzadeh14 Samaneh Mirzaei Dahka15
Samaneh Mirzaei Dahka15 Mostafa Badeli16
Mostafa Badeli16 Seyed Alireza Mosavi Jarrahi17*
Seyed Alireza Mosavi Jarrahi17* Saeid Doaei18*
Saeid Doaei18*Background: FTO gene is associated with obesity, dietary intake, and the risk of colorectal cancer (CRC). In this study, patients with colorectal cancer were assessed for the interactions between FTO gene polymorphisms and dietary intake.
Methods: This case–control study was carried out on 450 participants aged 35–70 years including 150 patients with colorectal cancer and 300 healthy controls. Blood samples were collected in order to extract DNA and genotyping of FTO gene for rs9939609 polymorphism. A validated 168-item food frequency questionnaire (FFQ) and the Nutritionist-IV software were used to assess dietary intake.
Results: In the participants with the TT genotype of FTO rs9939609 polymorphism, CRC risk was significantly associated with higher intake of dietary fat (OR:1.87 CI95%:1.76–1.99, p = 0.04), vitamin B3 (OR:1.20 CI95%:1.08–1.65, p = 0.04), and vitamin C (OR:1.06 CI95%:1.03–1.15, p = 0.04) and lower intake of β-carotene (OR:0.98 CI95%:0.97–0.99, p = 0.03), vitamin E (OR:0.77 CI95%:0.62–0.95, p = 0.02), vitamin B1 (OR:0.15 CI95%:0.04–0.50, p < 0.01), and biotin (OR:0.72 CI95%:0.0.57–0.92, p = 0.01). No significant association was found between CRC and dietary intake in carriers of AA/AT genotypes after adjustments for the confounders.
Conclusion: CRC risk may be decreased by β-carotene, vitamins E, B1, and biotin only in those without the risk allele of the FTO gene. The association of CRC and diet may be influenced by FTO genotype. Further studies are warranted.
Adenomas or adenomatous polyps in the colon and rectum cause colorectal cancer (CRC) (1). Globally, CRC is ranked third (10%) and the second (9%) most common cancer, according to GLOBOCAN 202 (2, 3). There are 7 and 8 cases of CRC per 100,000 Iranians, respectively, which is the second and third most common cancers (4, 5). Until 2025, CRC will remain a malignant cancer with a growth rate of 54.1% (6).
Colorectal cancer is caused by a variety of factors, including demographic, genetic, lifestyle, and environmental factors. People with a family history of CRC are more likely to develop CRC than those under 50 (4). Non-hereditary CRCs are the most common types (7, 8) resulting from somatic mutations caused by lifestyle factors including drinking alcohol, smoking, not exercising, obesity, and eating a diet high in red meat and processed meats and low in fruits, vegetables, and calcium (6, 9–11). Obesity is one of the most important risk factors for CRC (12), which can be influenced both genetically and by lifestyle factors (13). In recent studies, it has been found that people with polymorphisms in genes that encode enzymes involved in nutrient metabolism may be at greater risk of CRC due to the excessive intake of calories and processed meat, which alters the level of expression of CRC-related genes (4).
CRC, as well as breast, pancreatic, and prostate cancers, are associated with the rs9939609 polymorphism of the fat mass and obesity-associated (FTO) gene (14–17). FTO is associated with food intake control, energy balance, and basal metabolic rate (BMR) (18); hypothalamic FTO gene expression was correlated with macronutrient intake (19, 20). Diet-related chronic diseases may be prevented by modifying FTO genotype in order to determine nutritional requirements (19). FTO rs9939609 polymorphism may influence both dietary intake and risk of colorectal cancer, according to some studies. African-Americans with the FTO rs9939609 polymorphism have a higher body mass index (BMI) and colorectal adenomas (21) Adipokines and FTO gene polymorphisms interact to promote colorectal cancer, according to Yamaji et al. Other studies have, however, failed to find a link between FTO rs9939609 polymorphism and CRC (22, 23). Dietary components may influence the association between FTO genotype and CRC. The polymorphisms in genes may alter the requirement for certain nutrients, thus preventing carcinogenesis. Nutrients may have a limited effect on CRC risk only among individuals who carry risk alleles in CRC genes. Therefore, the purpose of this study was to determine whether FTO gene polymorphisms and dietary intake interact in patients with CRC.
A case–control study was conducted on 450 randomly selected participants between January 2020 and June 2021, which included 150 CRC patients and 300 healthy individuals. Participants were referred to three hospitals (Firoozgar, Shohadaye Tajrish, and Taleghani) in Tehran, Iran between January 2020 and June 2021. Participants were selected based on their willingness to participate in this study, confirmation of histological CRC in their tissues, a minimum of 6 months after the first diagnosis of CRC, and age between 35 and 70 years old. Participants in the healthy control group had to be willing to take part in this study without malignancies and be between 35 and 70 years old. Among the exclusion criteria were inability to gather required data (n = 9), drugs affecting food intake (n = 2), or diseases associated with diabetes and fatty liver (n = 10). There were ultimately 429 participants (135 case and 294 control). In face-to-face interviews, data on demographic variables such as age, sex, marital status, and ethnicity were gathered.
The protocol of the study was approved by ethics committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.CRC.REC.1398.028). The objectives of the study were explained verbally to all the patients and the control group and all participants signed the written consent form before participation in the study.
A blood sample (5 mL) was collected from each participant in EDTA tubes (EDTA K3, Shandong Weigao Group Medical Polymer Co., Ltd., China) at the beginning of the study. Deoxyribonucleic acid (DNA) was extracted from 200 μL of whole blood using the salting out method. PCR amplification was performed using a PCR amplification instrument (GeneQ; Hangzhou Bioer Technology Co., Ltd., Hangzhou, China) and master mix DNA polymerase (cat. no A180301; Ampliqon, Denmark). To determine the genotype of the FTO gene rs9939609 polymorphism, the tetra-primer amplification refractory mutation system-polymerase chain reaction (T-ARMS-PCR) method was used. The sequences for the primers are presented in Table 1.
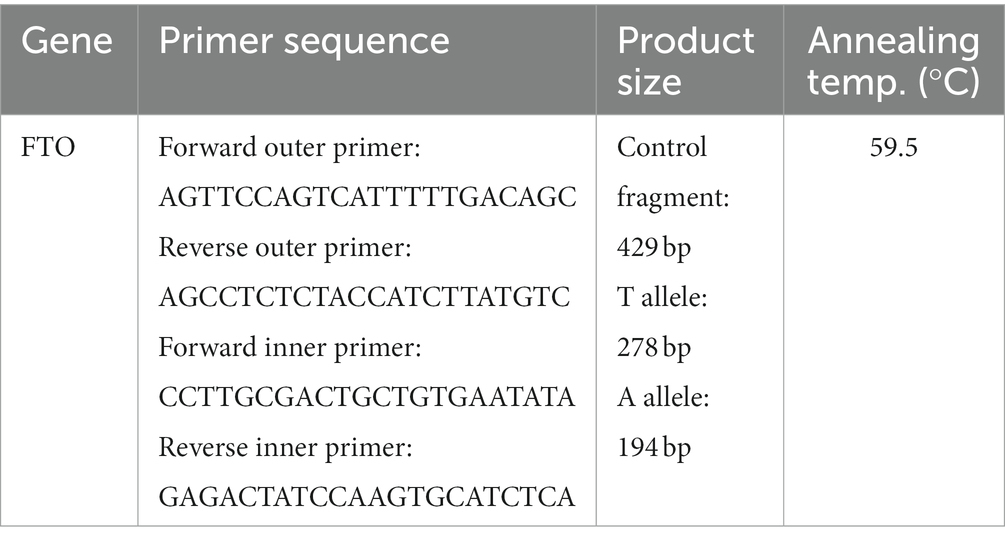
Table 1. The sequences of the primers used for tetra-primer amplification refractory mutation system-polymerase chain reaction (T-ARMS-PCR).
Physical activity data were assessed using the International Physical Activity Questionnaire (IPAQ), which was previously validated in Iran (24). All IPAQ results were expressed and analyzed as metabolic equivalents per minute (MET-minutes per week).
Dietary intake of the participants were assessed by a dietitian through a face-to-face interview (25). A validated 168-item semi-quantitative food frequency questionnaire (FFQ) was used included a list of foods with a standard serving size commonly consumed by Iranians. Participants were asked to report their frequency of consumption of a given serving of each food item during the previous year, on a daily (e.g., bread), weekly (e.g., meat) or monthly (e.g., fish) basis. Portion sizes of consumed foods were converted to grams per day using household measures (26). Then, the Nutritionist-IV software (First Databank Inc., Hearst Corp., San Bruno, CA) was used to analyze the intake of different types of dietary components including macronutrients and micronutrients. Each person’s daily calorie intake was calculated using the US Department of Agriculture food consumption database, which was modified for Iranian foods (27).
An evaluation of genotype distribution was conducted using the Hardy–Weinberg equilibrium. Hardy–Weinberg equilibrium (HWE) is the state of the genotypic frequency of two alleles of one autosomal gene locus after one discrete generation of random mating in an indefinitely large population (28). Using chi-square and independent t-test methods, we compared general characteristics and the frequency of FTO rs9939609 polymorphisms between case and control groups. A binary logistic regression model based on the dominant genetic model (TT vs. AT+AA) was used to assess the association between CRC and the risk allele in different models including a crude model (Model 1), adjusted for age and sex (Model 2), adjusted for age, sex, physical activity, alcohol use, and smoking (Model 3), adjusted for age, sex, physical activity, alcohol use, smoking, calorie intake and BMI (Model 4). SPSS software version 21 (SPSS Inc., Chicago, United States) and p-value < 0.05 were used for all statistical analyses.
All measurements were normally distributed. The genotype distribution of the study population was in Hardy–Weinberg equilibrium. All of the participants had Persian ethnicity. Regarding FTO rs9939609 genotype, about 36% (n = 154) of the participants had TT wild type genotype and 46% (n = 275) had AA/AT genotype. In people with TT genotype, the cases had higher BMI (27.67 ± 3.31 vs. 29.18 ± 3.9, p = 0.02) compared with the healthy controls. In the AA/AT group, the cases had higher age (52.5 ± 17.19 vs. 48.07 ± 11.19, p < 0.01), weight (69.62 ± 9.08 vs. 70.03 ± 10.89, p = 0.03) and BMI (27.58 ± 3.25 vs. 28.68 ± 4.06, p = 0.04) and lower height (158.65 ± 7.81 vs. 156.2 ± 5.71, p = 0.02). General characteristics of the participants according to the FTO rs9939609 genotype are presented in Table 2.
Regarding to the association of dietary intake and CRC, the cases with TT genotype of FTO rs9939609 polymorphism had lower intake of copper (1.49 ± 0.64 vs. 1.76 ± 0.71 g/d, p = 0.02), selenium (56.15 ± 22.97 vs. 67.26 ± 15.11 g/d, p < 0.01), β-carotene (2189.73 ± 474.3 vs. 2461.75 ± 772.57 g/d, p = 0.01), vitamin E (10.58 ± 4.14 vs. 13.99 ± 6.4 g/d, p < 0.01), tocopherol (8.46 ± 2.91 vs. 9.79 ± 4.53 g/d, p = 0.032), vitamin B1 (1.91 ± 0.87 vs. 2.3 ± 0.82 g/d, p = 0.01), folate (528 ± 0.61 vs. 574.39 ± 95.19 g/d, p = 0.01), biotin (26.76 ± 3.75 vs. 29.33 ± 6.61 g/d, p < 0.01) and higher intake of calorie (2500.48 ± 165.87 vs. 2594.64 ± 333.4 g/d, p = 0.021), fat (86.57 ± 10.38 vs. 93.25, ± 17.13 p < 0.01), fluoride (13967.59 ± 5662.25 vs. 11112.32 ± 3051.44 g/d, p < 0.01), vitamin A (819.7 ± 251.03 vs. 712.76 ± 113.86 g/d, p = 0.01), and vitamin K (157.9 ± 30.4 vs. 146.74 ± 21.64 g/d, p = 0.03). The cases with rs9939609 AA/AT genotype had lower intake of selenium (49.74 ± 26.83 vs. 68.41 ± 16.53 g/d, p < 0.01) and folate (512.65 ± 99.73 vs. 566.94 ± 68.2, g/d p < 0.01), and higher intake of sodium (6465.85 ± 1840.38 vs. 6071.8 ± 1064.48 g/d, p < 0.01), fluoride (16708.7 ± 11263.98 vs. 10806.85 ± 3489.56 g/d, p < 0.01), chromium (0.12 ± 0.17 vs. 0.09 ± 0.15 g/d, p = 0.03), molybdenum (50.93 ± 10.29 vs. 50.88 ± 4.72 g/d, p < 0.01), vitamin A (850.94 ± 307.34 vs. 688.77 ± 172.6 g/d, p < 0.01), and vitamin K (162.51 ± 55.33 vs. 143.9 ± 27.75 g/d, p < 0.01) (Table 3).
Table 4 presents the results of logistic regression on the association between CRC and dietary intake among FTO rs9939609 TT genotype carriers. The risk of CRC was positively associated with higher intake of dietary fat (OR:1.87 CI95%:1.76–1.99, p = 0.04), vitamin B3 (OR:1.20 CI95%:1.08–1.65, p = 0.04) and vitamin C (OR:1.06 CI95%:1.03–1.15, p = 0.04) and lower intake of β-carotene (OR:0.98, CI95%:0.97–0.99, p = 0.03), vitamin E (OR:0.77 CI95%:0.62–0.95, p = 0.02), vitamin B1 (OR:0.15 CI95%:0.04–0.50, p < 0.01), and biotin (OR:0.72 CI95%:0.0.57–0.92, p = 0.01) (Model 1). The results remained significant after adjustment for age and sex (Model 2). Further adjustments for physical activity, alcohol use, and smoking (Model 3) did not change the results. The association between CRC risk and higher intake of dietary fat (OR:1.65 CI95%:1.43–1.99, p = 0.04), vitamin B3 (OR:4.38 CI95%:1.06–18.12, p = 0.04) and vitamin C (OR:1.23 CI95%:1.01–1.50, p = 0.04) and lower intake of β-carotene (OR:0.97, CI95%:0.94–0.99, p = 0.04), vitamin E (OR:0.56, CI95%:0.35–0.89, p = 0.01), vitamin B1 (OR:0.11 CI95%:0.05–0.24, p = 0.01), and biotin (OR:0.30 CI95%:0.11–0.81, p = 0.02) remained significant after additional adjustments for calorie intake and BMI (Model 4). There was no significant association between CRC with the other nutrients in the carriers of TT genotype of FTO rs9939609 polymorphism.
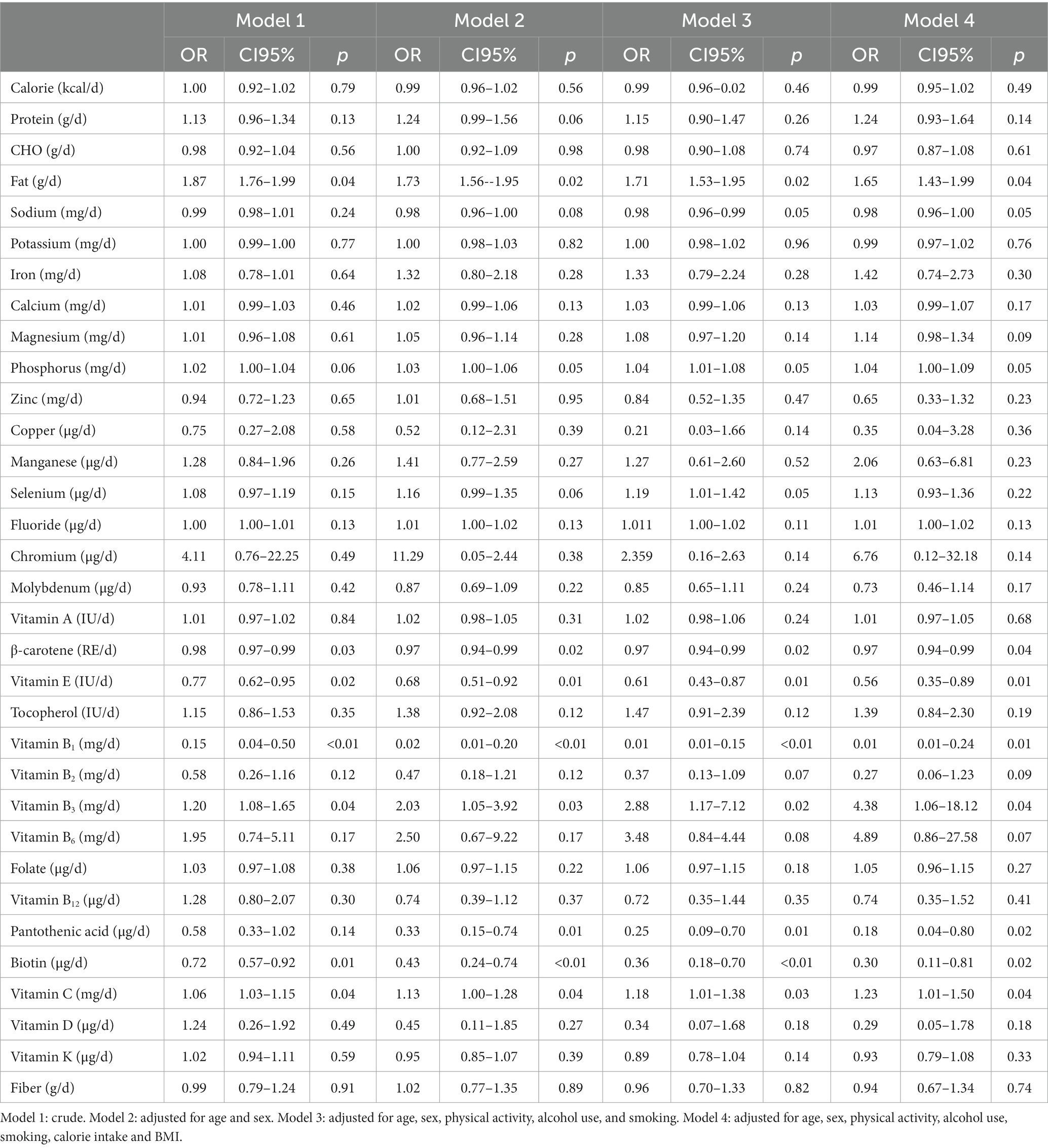
Table 4. Logistic regression of the association between colorectal cancer and dietary intake among people with TT genotype of FTO rs9939609 polymorphism.
The results of logistic regression on the association between CRC and dietary intake among people with AA/AT FTO rs9939609 genotype are presented in Table 5. Higher intake of chromium (OR:10.12 CI95%:1.20–85.18, p = 0.03) and pantothenic acid (OR:1.24 CI95%:1.01–1.52, p = 0.04) were associated with the higher risk of CRC. However, the associations were disappeared after adjustments for age and sex (Model 2). No significant association was found between CRC and dietary intake in carriers of AA/AT genotypes after further adjustments for physical activity, alcohol use, and smoking (Model 3), and additional adjustments for calorie intake and BMI.
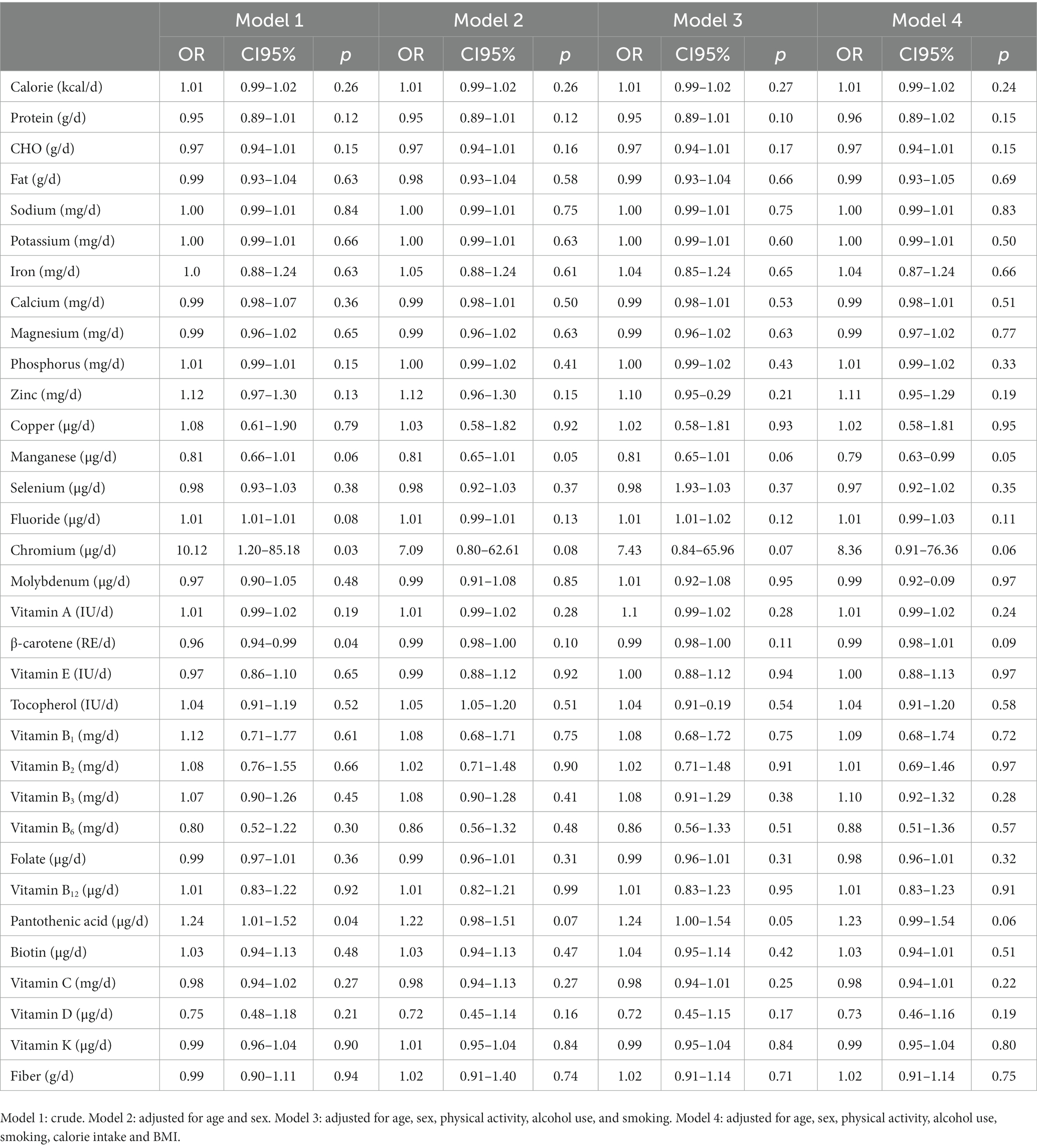
Table 5. Logistic regression of the association between colorectal cancer and dietary intake among people with AA/AT FTO rs9939609 genotype.
As far as the authors know, this is the first study to explore the interaction between the risk allele of the FTO rs9939609 polymorphism and dietary intake of different types of nutrients in CRC patients. Participants with the TT genotype had a significantly higher risk of CRC when they had higher intake of fat, vitamin B3, and vitamin C and lower intake of β-carotene, vitamin E, vitamin B1, and biotin. A higher intake of chromium, pantothenic acid, and lower intake of β-carotene was associated with the higher CRC risk among patients carrying the AA/AT genotype. However, no significant association was found between CRC risk and nutrients in carriers of FTO gene risk allele (A) after adjustments for the confounders.
The effects of dietary components on the risk of CRC are frequently reported (29–31). The results of this study on the association of CRC and dietary fat was in line with some other studies (32, 33). For example, one study reported a possible association between CRC with total fat, polyunsaturated fatty acids, and trans fatty acids (34). In line with the results of the present study, recent studies indicated that the association between CRC risk and dietary components can be influenced by FTO genotype (35, 36). For example, a case–control study found an inverse association between CRC and total dietary fiber intake only among people with AA/AT FTO rs9939609 genotype (35). Previous studies (37–40) have shown that dietary fiber can reduce the risk of CRC by promoting fermentation by gut bacteria as a prebiotic and producing short chain fatty acids (SCFAs) (37). Butyrate, a type of SCFA, has been found to inhibit neuropilin-1, a receptor commonly found in CRC cells (41). Additionally, butyrate may induce apoptosis and suppress the proliferation and invasion of CRC cells by regulating microRNAs such as miR-92a and miR-203 (42, 43). Butyrate may also inhibit the motility of CRC cells by blocking the Akt/ERK signaling pathway, suggesting its potential in preventing metastatic CRC (44).
In current study, there was an interaction between some micronutrients and the FTO rs9939609 genotype regarding CRC risk. Higher intake of dietary fat, vitamin B3, and vitamin C intake may increase the risk of CRC, whereas vitamin E, vitamin B1, and biotin intake may reduce the risk only among carriers of the FTO SNP rs9939609. Regarding the association of CRC with vitamin B3, a recent study found that niacin inhibits tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through activation of autophagic flux in human CRC cells (45). Using the HCT116 human colon carcinoma cell line, this study showed that niacin activates autophagy, and that the autophagy activation protects tumor cells from TRAIL-induced mitochondrial membrane dysfunction and tumor cell death. Interestingly, one recent study reported vitamin C and niacin have a dose-dependent effect on CRC risk. High doses of vitamin C and niacin (30 mg/kg body weight) killed CRC stem cells, whereas low doses induced proliferation of HT-29 and HCT-15 CRC stem cells (46). So, the effects of niacin and vitamin C vitamins on CRC risk may be influenced by FTO polymorphism due to the possible effects of the FTO gene polymorphisms on the level of the requirements to these vitamins (47, 48).
According to the present study, carriers of both TT and AT and AA genotypes of rs9939609 polymorphism may be protected against CRC by higher intakes of carriers. The protective antioxidant effects of carotenoids found in fruits and vegetables are frequently reported (49–52). In line with this study, previous studies reported that β-carotene intake was inversely associated with CRC risk (53–55). A cohort study found an inverse association between the risk of CRC and β-carotene intake only in male current smokers (56). A possible mechanism for the chemo-preventive and antiproliferative effects of carotenoid in cancer cells is downregulation of COX-2 but not that of COX-1 in LS-174 cells, indicating that this is a specific effect in either transcriptional activity or in RNA stability. In addition, the carotenoid was able to downregulate baseline and heregulin-α-induced expression of COX-2 and PGE2 content in CRC cells (57).
A higher intake of pantothenic acid was associated with a lower risk of CRC among TT genotype carriers of the FTO SNP rs9939609, while with a higher risk of CRC among A allele carriers of the FTO SNP rs9939609. The body uses pantothenic acid to utilize carbohydrates, proteins, and lipids. Pantothenic acid plays a key role as a cofactor for the synthesis of CoA (58) which is required for the tricarboxylic acid cycle, fatty acid metabolism, and acylation reactions. As a result of CoA catabolism, pantothenate and cysteamine are produced, the latter of which potentiates inflammation. This chemical breaks down disulfide bonds, inactivating proteins and directly inhibiting glutathione synthase. During inflammation, cysteamine is thought to generate oxidative stress (ROS) (59). However, the role of pantothenic acid on the risk of CRC have not yet been examined. The availability of pantothenic acid may modulate tumorigenesis according to epidemiological and laboratory animal studies (59). Genetic polymorphisms and their impact on dietary requirements determine whether nutrients are carcinogenic or anti-carcinogenic (60).
The strengths of the present study are the large number of the participants and adjustment for potential confounding factors, including age, sex, physical activity, alcohol use, smoking, calorie intake and BMI. Moreover, to our knowledge, this is the first study to investigate the effects of FTO gene rs9939609 polymorphism on the association between CRC and dietary components. However, this study had some limitations. The FFQ which was used for dietary assessment is a self-reporting tool and may contain measuring errors such as underreporting of dietary intake in obese and overweight participants. Also, the interactions between CRC, FTO gene, and diet were only assessed in the dominant genetic model and it was not possible to determine the effect of the number of risk alleles on the relationship between dietary intake and CRC in patients with CRC due to the limited study sample size. Future longitudinal studies with larger sample size of different FTO genotypes are needed to explore the exact interactions between CRC, FTO gene, and dietary intake.
The results of the present study showed that the FTO gene genotype is significantly effective on the relationship between diet and CRC. Some nutrients including dietary fat, vitamin B3, and vitamin C may act as risk factors and some other nutrients including β-carotene, vitamin E, vitamin B1, and biotin may be protective against the risk of CRC only in people without the risk allele of the FTO gene. There is a need for future longitudinal research to understand the mutual effects of dietary factors and FTO gene polymorphisms on the risk of CRC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by: IR.SBMU.CRC.REC.1398.028. The patients/participants provided their written informed consent to participate in this study.
SD, MGH, MJ, KhAM, AA, FA, NV, SAA, MEA, JP, GHAT and SAMJ designed the study, and were involved in the data collection, analysis, and drafting of the manuscript. MKM, MR, MA, HS, PH, SMD, MB and SD were involved in the design of the study, analysis of the data, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding for this study was provided by Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code 15784).
We thank all the participants in this study for their good cooperation. This paper was taken from the approved research project of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code 15784).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Saad El Din, K, Loree, JM, Sayre, EC, Gill, S, Brown, CJ, Dau, H, et al. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. (2020) 20:288. doi: 10.1186/s12885-020-06766-9
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Siegel, RL, Miller, KD, Goding Sauer, A, Fedewa, SA, Butterly, LF, Anderson, JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. (2020) 70:145–64. doi: 10.3322/caac.21601
4. Doaei, S, Hajiesmaeil, M, Aminifard, A, Mosavi-Jarrahi, SA, Akbari, ME, and Gholamalizadeh, M. Effects of gene polymorphisms of metabolic enzymes on the association between red and processed meat consumption and the development of colon cancer; a literature review. J Nutr Sci. (2018) 7:e26. doi: 10.1017/jns.2018.17
5. Aziz, R, Shahin, N, Fateme, M, Asma, RK, and Rafat, B. The economic burden of cancer in Iran during 1995-2019: a systematic review. Iran J Public Health. (2020) 50:35–45. doi: 10.18502/ijph.v50i1.5070
6. Roshandel, G, Ferlay, J, Ghanbari-Motlagh, A, Partovipour, E, Salavati, F, Aryan, K, et al. Cancer in Iran 2008 to 2025: recent incidence trends and short-term predictions of the future burden. Int J Cancer. (2021) 149:594–605. doi: 10.1002/ijc.33574
7. Baidoun, F, Elshiwy, K, Elkeraie, Y, Merjaneh, Z, Khoudari, G, Sarmini, MT, et al. Colorectal cancer epidemiology: recent trends and impact on outcomes. Curr Drug Targets. (2020) 22:998–1009. doi: 10.2174/1389450121999201117115717
8. Aran, V, Victorino, AP, Thuler, LC, and Ferreira, CG. Colorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer. (2016) 15:195–203. doi: 10.1016/j.clcc.2016.02.008
9. Farhood, B, Raei, B, Malekzadeh, R, Shirvani, M, Najafi, M, and Mortezazadeh, T. A review of incidence and mortality of colorectal, lung, liver, thyroid, and bladder cancers in Iran and compared to other countries. Contemp Oncol (Pozn). (2019) 23:7–15. doi: 10.5114/wo.2019.84112
10. Islami, F, Goding Sauer, A, Miller, KD, Siegel, RL, Fedewa, SA, Jacobs, EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. (2018) 68:31–54. doi: 10.3322/caac.21440
11. Safari, A, Shariff, ZM, Kandiah, M, Rashidkhani, B, and Fereidooni, F. Dietary patterns and risk of colorectal cancer in Tehran Province: a case-control study. BMC Public Health. (2013) 13:222. doi: 10.1186/1471-2458-13-222
12. Ye, P, Xi, Y, Huang, Z, and Xu, P. Linking obesity with colorectal cancer: epidemiology and mechanistic insights. Cancers. (2020) 12:1408. doi: 10.3390/cancers12061408
13. Heianza, Y, and Qi, L. Gene-diet interaction and precision nutrition in obesity. Int J Mol Sci. (2017) 18:787. doi: 10.3390/ijms18040787
14. Yamaji, T, Iwasaki, M, Sawada, N, Shimazu, T, Inoue, M, and Tsugane, S. Fat mass and obesity-associated gene polymorphisms, pre-diagnostic plasma adipokine levels and the risk of colorectal cancer: the Japan public health center-based prospective study. PLoS One. (2020) 15:e0229005. doi: 10.1371/journal.pone.0229005
15. Doaei, S, Gholamalizadeh, M, Akbari, ME, Akbari, S, Feradova, H, Rahimzadeh, G, et al. Dietary carbohydrate promotes cell survival in cancer via the up-regulation of fat mass and obesity-associated gene expression level. Malays J Med Sci. (2019) 26:8–17. doi: 10.21315/mjms2019.26.2.2
16. Khella, MS, Salem, AM, Abdel-Rahman, O, and Saad, AS. The association between the FTO rs9939609 variant and malignant pleural mesothelioma risk: a case-control study. Genet Test Mol Biomarkers. (2018) 22:79–84. doi: 10.1089/gtmb.2017.0146
17. Zeng, X, Ban, Z, Cao, J, Zhang, W, Chu, T, Lei, D, et al. Association of FTO mutations with risk and survival of breast cancer in a Chinese population. Dis Markers. (2015) 2015:101032:1–6. doi: 10.1155/2015/101032
18. Doaei, S, Kalantari, N, Mohammadi, NK, Tabesh, GA, and Gholamalizadeh, M. Macronutrients and the FTO gene expression in hypothalamus; a systematic review of experimental studies. Indian Heart J. (2017) 69:277–81. doi: 10.1016/j.ihj.2017.01.014
19. Mehrdad, M, Doaei, S, Gholamalizadeh, M, and Eftekhari, MH. The association between FTO genotype with macronutrients and calorie intake in overweight adults. Lipids Health Dis. (2020) 19:197. doi: 10.1186/s12944-020-01372-x
20. Vahid, F, Hekmatdoost, A, Mirmajidi, S, Doaei, S, Rahmani, D, and Faghfoori, ZJ. Association between index of nutritional quality and nonalcoholic fatty liver disease: the role of vitamin D and B group. Am J Med Sci. (2019) 358:212–8. doi: 10.1016/j.amjms.2019.06.008
21. Nock, NL, Plummer, SJ, Thompson, CL, Casey, G, and Li, L. FTO polymorphisms are associated with adult body mass index (BMI) and colorectal adenomas in African-Americans. Carcinogenesis. (2011) 32:748–56. doi: 10.1093/carcin/bgr026
22. Yang, B, Thrift, AP, Figueiredo, JC, Jenkins, MA, Schumacher, FR, Conti, DV, et al. Common variants in the obesity-associated genes FTO and MC4R are not associated with risk of colorectal cancer. Cancer Epidemiol. (2016) 44:1–4. doi: 10.1016/j.canep.2016.07.003
23. Gholamalizadeh, M, Tabrizi, R, Bourbour, F, Rezaei, S, Pourtaheri, A, Badeli, M, et al. Are the FTO gene polymorphisms associated with colorectal cancer? A meta-analysis. J Gastrointest Cancer. (2021) 52:846–53. doi: 10.1007/s12029-021-00651-9
24. Vasheghani-Farahani, A, Tahmasbi, M, Asheri, H, Ashraf, H, Nedjat, S, and Kordi, R. The Persian, last 7-day, long form of the international physical activity questionnaire: translation and validation study. Asian J Sports Med. (2011) 2:106–16. doi: 10.5812/asjsm.34781
25. Esfahani, FH, Asghari, G, Mirmiran, P, and Azizi, F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. J Epidemiol. (2010) 20:150–8. doi: 10.2188/jea.JE20090083
26. Mirmiran, P, Esfahani, FH, Mehrabi, Y, Hedayati, M, and Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2010) 13:654–62. doi: 10.1017/S1368980009991698
27. Haytowitz, DB, and Pehrsson, PR. USDA’s National Food and Nutrient Analysis Program (NFNAP) produces high-quality data for USDA food composition databases: two decades of collaboration. Food Chem. (2018) 238:134–8. doi: 10.1016/j.foodchem.2016.11.082
28. Mayo, O. A century of hardy–Weinberg equilibrium. Twin Res Hum Genet. (2008) 11:249–56. doi: 10.1375/twin.11.3.249
29. Sharma, R, Abbasi-Kangevari, M, Abd-Rabu, R, Abidi, H, Abu-Gharbieh, E, Acuna, JM, et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol. (2022) 7:627–47. doi: 10.1016/S2468-1253(22)00044-9
30. Jonoush, M, Fathi, S, Hassanpour Ardekanizadeh, N, Khalatbari Mohseni, G, Majidi, N, Keshavarz, SA, et al. The association between different types of dietary carbohydrates and colorectal cancer: a case-control study. Front Nutr. (2022) 9:9. doi: 10.3389/fnut.2022.898337
31. Gholamalizadeh, M, Behrad Nasab, M, Ahmadzadeh, M, Doaei, S, Jonoush, M, Shekari, S, et al. The association among calorie, macronutrient, and micronutrient intake with colorectal cancer: a case–control study. Food Sci Nutr. (2022) 10:1527–36. doi: 10.1002/fsn3.2775
32. West, DW, Slattery, ML, Robison, LM, Schuman, KL, Ford, MH, Mahoney, AW, et al. Dietary intake and colon cancer: sex-and anatomic site-specific associations. Am J Epidemiol. (1989) 130:883–94. doi: 10.1093/oxfordjournals.aje.a115421
33. Chiu, BC, Ji, B-T, Dai, Q, Gridley, G, McLaughlin, JK, Gao, Y-T, et al. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. (2003) 12:201–8.
34. Slattery, ML, Benson, J, Ma, K-N, Schaffer, D, and Potter, JD. Trans-fatty acids and colon cancer. Nutr Cancer. (2001) 39:170–5. doi: 10.1207/S15327914nc392_2
35. Fathi, S, Ahmadzadeh, M, Vahdat, M, Afsharfar, M, Roumi, Z, Ardekanizadeh, NH, et al. The effect of FTO rs9939609 polymorphism on the association between colorectal cancer and dietary fiber. Frontiers. Nutrition. (2022) 9:9. doi: 10.3389/fnut.2022.891819
36. Doaei, S, Gholamalizadeh, M, Akbari, ME, Akbari, S, Feradova, H, and Rahimzadeh, G. Dietary carbohydrate promotes cell survival in cancer via the up-regulation of fat mass and obesity-associated gene expression level. Malays J Med Sci. (2019) 26:8–17. doi: 10.21315/mjms2019.26.2.2
37. Loke, YL, Chew, MT, Ngeow, YF, Lim, WWD, and Peh, SC. Colon carcinogenesis: the interplay between diet and gut microbiota. Front Cell Infect Microbiol. (2020) 10:603086. doi: 10.3389/fcimb.2020.603086
38. Dahm, CC, Keogh, RH, Spencer, EA, Greenwood, DC, Key, TJ, Fentiman, IS, et al. Dietary fiber and colorectal cancer risk: a nested case–control study using food diaries. J Natl Cancer Inst. (2010) 102:614–26. doi: 10.1093/jnci/djq092
39. Luo, W-P, Fang, Y-J, Lu, M-S, Zhong, X, Chen, Y-M, and Zhang, C-X. High consumption of vegetable and fruit colour groups is inversely associated with the risk of colorectal cancer: a case–control study. Br J Nutr. (2015) 113:1129–38. doi: 10.1017/S0007114515000331
40. Gianfredi, V, Salvatori, T, Villarini, M, Moretti, M, Nucci, D, and Realdon, S. Is dietary fibre truly protective against colon cancer? A systematic review and meta-analysis. Int J Food Sci Nutr. (2018) 69:904–15. doi: 10.1080/09637486.2018.1446917
41. Yu, DC, Bury, JP, Tiernan, J, Waby, JS, Staton, CA, and Corfe, BM. Short-chain fatty acid level and field cancerization show opposing associations with enteroendocrine cell number and neuropilin expression in patients with colorectal adenoma. Mol Cancer. (2011) 10:27. doi: 10.1186/1476-4598-10-27
42. Hu, S, Liu, L, Chang, EB, Wang, J-Y, and Raufman, J-P. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol Cancer. (2015) 14:1–15. doi: 10.1186/s12943-015-0450-x
43. Han, R, Sun, Q, Wu, J, Zheng, P, and Zhao, G. Sodium butyrate upregulates miR-203 expression to exert anti-proliferation effect on colorectal cancer cells. Cell Physiol Biochem. (2016) 39:1919–29. doi: 10.1159/000447889
44. Li, Q, Ding, C, Meng, T, Lu, W, Liu, W, Hao, H, et al. Butyrate suppresses motility of colorectal cancer cells via deactivating Akt/ERK signaling in histone deacetylase dependent manner. J Pharmacol Sci. (2017) 135:148–55. doi: 10.1016/j.jphs.2017.11.004
45. Kim, S-W, Lee, J-H, Moon, J-H, Nazim, UM, Lee, Y-J, Seol, J-W, et al. Niacin alleviates TRAIL-mediated colon cancer cell death via autophagy flux activation. Oncotarget. (2016) 7:4356–68. doi: 10.18632/oncotarget.5374
46. Sen, U, Shenoy, PS, and Bose, B. Opposing effects of low versus high concentrations of water soluble vitamins/dietary ingredients vitamin C and niacin on colon cancer stem cells (CSCs). Cell Biol Int. (2017) 41:1127–45. doi: 10.1002/cbin.10830
47. Popović, A-M, Huđek Turković, A, Žuna, K, Bačun-Družina, V, Rubelj, I, and Matovinović, M. FTO gene polymorphisms at the crossroads of metabolic pathways of obesity and epigenetic influences. Food Technol Biotechnol. (2023) 61:14–26. doi: 10.17113/ftb.61.01.23.7594
48. Stover, PJ. Influence of human genetic variation on nutritional requirements. Am J Clin Nutr. (2006) 83:436S–42S. doi: 10.1093/ajcn/83.2.436S
49. Cheng, K, and Day, N. Nutrition and esophageal cancer. Cancer Causes Control. (1996) 7:33–40. doi: 10.1007/BF00115636
50. McGuire, S. US Department of Agriculture and US Department of Health and Human Services, Dietary Guidelines for Americans, 2010. Washington, DC: US Government Printing Office, January 2011. Adv Nutr. (2011) 2:293–4. doi: 10.3945/an.111.000430
51. Negri, E, La Vecchia, C, Franceschi, S, D'Avanzo, B, and Parazzini, F. Vegetable and fruit consumption and cancer risk. Int J Cancer. (1991) 48:350–4. doi: 10.1002/ijc.2910480307
52. Zheng, W, Sellers, TA, Doyle, TJ, Kushi, LH, Potter, JD, and Folsom, AR. Retinol, antioxidant vitamins, and cancers of the upper digestive tract in a prospective cohort study of postmenopausal women. Am J Epidemiol. (1995) 142:955–60. doi: 10.1093/oxfordjournals.aje.a117743
53. Koushik, A, Hunter, DJ, Spiegelman, D, Beeson, WL, Van Den Brandt, PA, Buring, JE, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst. (2007) 99:1471–83. doi: 10.1093/jnci/djm155
54. Lu, M-S, Fang, Y-J, Chen, Y-M, Luo, W-P, Pan, Z-Z, Zhong, X, et al. Higher intake of carotenoid is associated with a lower risk of colorectal cancer in Chinese adults: a case–control study. Eur J Nutr. (2015) 54:619–28. doi: 10.1007/s00394-014-0743-7
55. Slattery, ML, Benson, J, Curtin, K, Ma, K-N, Schaeffer, D, and Potter, JD. Carotenoids and colon cancer. Am J Clin Nutr. (2000) 71:575–82. doi: 10.1093/ajcn/71.2.575
56. Park, S-Y, Nomura, AM, Murphy, SP, Wilkens, LR, Henderson, BE, and Kolonel, LN. Carotenoid intake and colorectal cancer risk: the multiethnic cohort study. J Epidemiol. (2009) 19:63–71. doi: 10.2188/jea.JE20080078
57. Palozza, P, Serini, S, Maggiano, N, Tringali, G, Navarra, P, Ranelletti, FO, et al. β-Carotene downregulates the steady-state and heregulin-α–induced COX-2 pathways in colon cancer cells. J Nutr. (2005) 135:129–36. doi: 10.1093/jn/135.1.129
58. Leonardi, R, and Jackowski, S. Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus. (2007) 2:111–125. doi: 10.1128/ecosalplus.3.6.3.4
59. Peterson, CT, Rodionov, DA, Osterman, AL, and Peterson, SN. B vitamins and their role in immune regulation and cancer. Nutrients. (2020) 12. doi: 10.3390/nu12113380
Keywords: colorectal cancer, FTO gene, diet, nutrient, FTO
Citation: Gholamalizadeh M, Jonoush M, Mobarakeh KA, Amjadi A, Alami F, Valisoltani N, Askarpour SA, Azizi-Tabesh G, Mohammadian MK, Akbari ME, Rajabibazl M, Alemrajabi M, Poodineh J, Sadeghi H, Hosseinzadeh P, Dahka SM, Badeli M, Jarrahi SAM and Doaei S (2023) The effects of FTO gene rs9939609 polymorphism on the association between colorectal cancer and dietary intake. Front. Nutr. 10:1215559. doi: 10.3389/fnut.2023.1215559
Received: 02 May 2023; Accepted: 21 June 2023;
Published: 20 July 2023.
Edited by:
Shaokang Wang, Southeast University, ChinaReviewed by:
Dina Keumala Sari, Universitas Sumatera Utara, IndonesiaCopyright © 2023 Gholamalizadeh, Jonoush, Mobarakeh, Amjadi, Alami, Valisoltani, Askarpour, Azizi-Tabesh, Mohammadian, Akbari, Rajabibazl, Alemrajabi, Poodineh, Sadeghi, Hosseinzadeh, Dahka, Badeli, Jarrahi and Doaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saeid Doaei, RG9hZWlAZ3Vtcy5hYy5pcg==; Seyed Alireza Mosavi Jarrahi, cm1vc2F2aUB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.