
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 21 November 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1215220
This article is part of the Research Topic Dietary Patterns in Cancer Prevention and Survival View all 17 articles
Introduction: Epidemiological studies have revealed a link between dietary habits and the breast cancer risk. The causality of the association between food consumption and breast cancer requires further investigation.
Methods: Using Mendelian randomization, we assessed the causal effects of 10 dietary habits on the risks of breast cancer and its subtypes (estrogen receptor [ER] + and ER- breast cancer). We obtained dietary pattern data in 2018 (number of single-nucleotide polymorphisms [SNPs] = 9,851,867) and breast cancer data in 2017 (number of SNPs = 10,680,257) from IEU OpenGWAS. Rigorous sensitivity analyses were conducted to ensure that the study results were credible and robust.
Results: We identified that genetic predisposition to higher dried fruit intake was linked to a reduced risk of overall breast cancer (inverse variance-weighted [IVW] odds ratio [OR] = 0.55; 95% confidence interval [CI]: 0.43–0.70; p = 1.75 × 10−6), ER+ breast cancer (IVW OR = 0.62; 95% CI: 0.47–0.82; p = 8.96 × 10−4) and ER− breast cancer (IVW OR = 0.48; 95% CI: 0.34–0.68; p = 3.18 × 10−5), whereas genetic predisposition to more oily fish intake was linked to a lower risk of ER+ breast cancer (IVW OR = 0.73; 95% CI: 0.53–0.99; p = 0.04).
Discussion: Our findings suggest that a genetic predisposition for dried fruit and oily fish consumption may be protective against breast cancer; however, further investigation is required.
Among women, breast cancer is the most common type of cancer, accounting for 11.7% of all cancer cases, and the leading cause of cancer-related deaths (1). The existing epidemiological research broadly supports a link between nutrition and the cancer risk (2, 3). Elucidating the relationship between dietary factors and the breast cancer risk can help prevent breast cancer.
Several epidemiological studies have investigated the relationship between dietary patterns and breast cancer with conflicting findings. A series of prospective cohort studies have suggested an association between a reduced breast cancer risk and diets abundant in fruits, vegetables, cereals, fish, nuts, and olive oil (4–10). However, other studies have shown that these diets are not associated with the breast cancer risk (11–13). Considering the limitations of observational studies, the validity of these findings is subject to random and systematic errors, including the effects of cohort design bias, potential selection bias, small sample size, missed follow-ups, and the presence of reverse causality between outcomes and exposure (14). Randomized controlled trials are limited by ethical issues, cost, and long follow-ups (15). Whether or not these dietary patterns play a causal role in breast cancer remains unknown.
Mendelian randomization (MR) is an emerging method that infers the causality of exposure and outcome using single-nucleotide polymorphisms (SNPs) as instrumental variables (IV) for risk factors (16, 17). Random assignment of SNPs during meiosis and recombination mimic the random grouping of the randomized controlled trial experiments. Thus, in theory, this genetic tool is less susceptible to potential reverse causality and confounding bias (e.g., potential environmental factors). Using MR techniques, we investigated the potential link between 10 dietary patterns and breast cancer susceptibility (overall breast cancer and breast cancer stratified by estrogen receptor [ER] status).
We performed a two-sample MR analysis to investigate the causal impact of the risk factors on the outcomes. MR has the advantages of avoiding reverse causality and minimizing confounding effects in observational studies. Figure 1 shows a graphical flow of the experimental design.
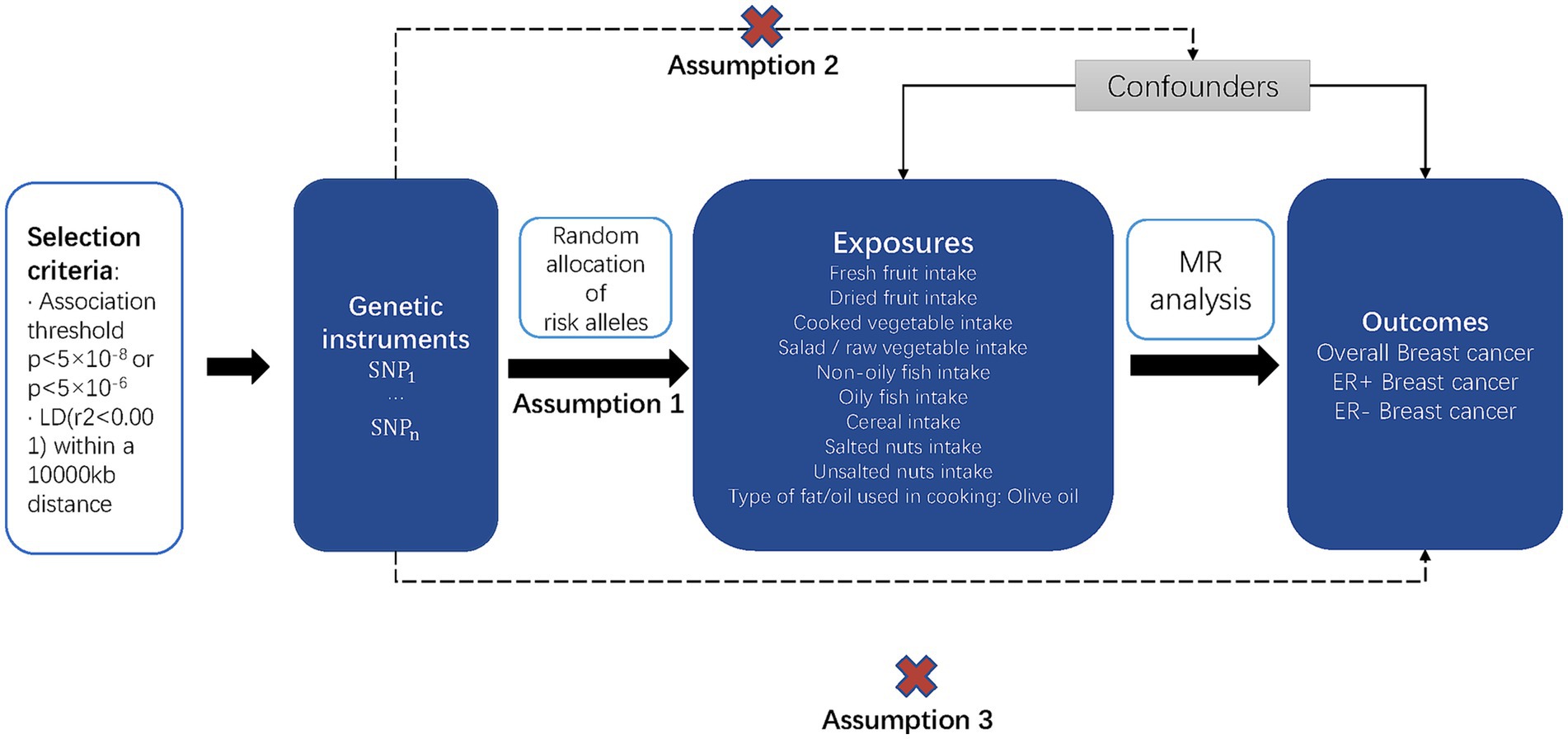
Figure 1. Overview of the study design and the three key assumptions of MR. Possible causal relationships between factors that might go against Mendelian randomization assumptions are shown by the dotted arrows.
As shown in Figure 1, the MR design is predicated on three key assumptions: assumption 1, IVs are strongly associated with exposure factors, indicated by the solid arrow in the figure; assumption 2, IVs are not associated with confounding factors, indicated by the dotted arrow in the figure; and assumption 3, the chosen IVs affect the outcome only via exposure, indicated by the dotted arrow in the figure (18).
We collected exposure and outcome data from the IEU OpenGWAS database1, which contains 126 billion genetic associations from 14,582 complete GWAS datasets, representing human phenotypes and disease outcomes across different populations (19, 20). Table 1 shows the data source, sample size, number of SNPs, and strength of IVs for each exposure factor.
Exposure data were obtained from the United Kingdom Biobank GWAS database and aggregated using the IEU OpenGWAS (21). The consortium collected the 2018 statistics for fresh fruit (n = 446,462 participants), dried fruit (n = 421,764 participants), cooked vegetable (n = 448,651 participants), salad/raw vegetable (n = 435,435 participants), non-oily fish (n = 460,880 participants), oily fish (n = 460,443 participants), cereal (n = 441,640 participants), salted nut (n = 64,949 participants), unsalted nut (n = 64,949 participants), and olive oil (n = 64,949 participants) intake in the European population. Data on dietary patterns were obtained from the participants by answering a touchscreen questionnaire.
Outcome data, which are publicly available GWAS summary datasets of breast cancer, were obtained from the Breast Cancer Association Consortium. The consortium pooled statistics for the overall (n = 122,977 cases, 105,974 non-cases), ER+ (n = 69,501 cases), and ER- (n = 21,468 cases) breast cancer populations of European ancestry and adjusted the main covariates, including country and 10 principal components (22).
First, to extract SNPs genetically linked to the traits, we performed rigorous screening (p < 5 × 10−8; minor allele frequency [MAF] > 0.01) of SNPs associated with the dietary patterns. For the three dietary patterns of salted nut, unsalted nut, and olive oil intake, we did not select enough valid SNPs using the threshold of p < 5 × 10−8. To explore more associations between those three dietary patterns and breast cancer, we used the relatively relaxed threshold of p < 5 × 10−6 (23). Second, to remove linkage disequilibrium IVs, we excluded SNPs with r2 > 0.001, with the most significant SNPs within a clumping distance of 10,000 kb. Third, to remove incompatible and palindromic SNPs whose direction could not be determined, we harmonized the data. Data harmonization helps avoid redundant calculations of the same allele across datasets. Palindromic variants were removed by eliminating alleles with frequencies close to 50% (24). Fourth, to exclude weak IVs, we calculate the F-values using the formula (25). R2 in the formula was calculated using (26). When the F-statistic was <10, we determined that genetic variation to be a weak IV (27). We removed one SNP with an F-value <10 for fresh fruit intake (rs586346, F = 9.91). Table 1 lists the F- and R2 values for each exposure. Supplementary Table S1 shows the F and R2 values of all SNPs. Finally, to assess the statistical power of the results, we used http://cnsgenomic.com/shiny/mRnd/ (accessed on March 2, 2023) (28). Table 2 shows the calculated statistical power estimates.
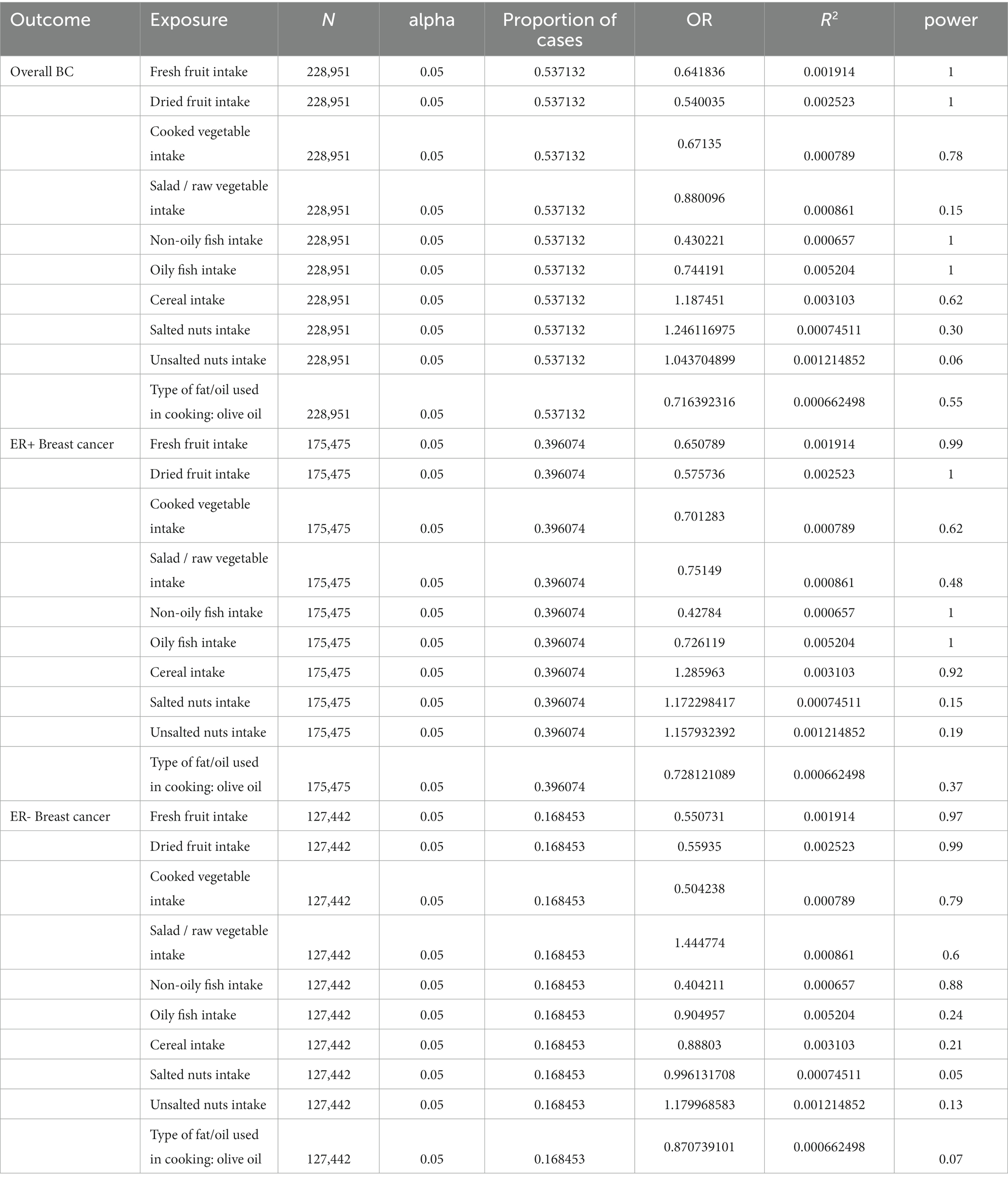
Table 2. Estimation of power for Mendelian randomization analyses for breast cancer risk based on the total sample size and proportion of phenotypic variance of nutrients explained by instruments.
This study was guided by the Strengthening the Reporting of Observational Research in Epidemiology using MR (29). We mainly used the inverse variance-weighted (IVW) model to evaluate the causal relationship between 10 dietary patterns and the breast cancer risk (including total breast cancer, ER+ breast cancer, and ER- breast cancer). The results of the MR-Egger and weighted median (WM) methods are also presented, which help interpret the results from multiple perspectives. To evaluate the sensitivity of the results, we performed Cochran’s Q, MR-Egger intercept, MR-PRESSO, and MR-Steiger filtering tests and plotted leave-one-out, scatter, and funnel plots. These plots are available in the Supplementary file. To assess the heterogeneity of the IVs, we performed Cochran’s Q test using IVW and MR-Egger’s methods. Heterogeneity was indicated when the p-value of the Cochran’s Q test was <0.05. When heterogeneity was strong, we used a random-effects model rather than a fixed-effects model (30). To test for the presence of horizontal pleiotropy, we calculated the difference between the MR-Egger intercept term and 0 (Pintercept). A significant difference (Pintercept <0.05) indicated the presence of horizontal pleiotropy. Supplementary Table S2 shows the results of Cochran’s Q and MR-Egger intercept tests. The MR-PRESSO method was used to exclude the influential outliers. To assess the direction of the effects of IV on the exposure and outcomes, we performed the MR-Steiger filtering test (31). Table 3 lists the outliers excluded by the MR-PRESSO and MR-Steiger filtering tests. To assess the robustness of the results, we constructed scatter plots and leave-one-out analyses.
Statistical analyses were performed using R 4.2.1 software using the “Two-sample MR” and “MR-PRESSO” packages. We adopted a Bonferroni-corrected threshold of p = 0.00167 (0.05/30) as a sign of a significant effect and 0.00167 < p < 0.05, as a sign of a suggestive association.
Supplementary Table S1 shows the SNPs screened for strong associations with the 10 dietary factors. Table 3 shows the outliers excluded by the MR-PRESSO and MR-Steiger analyses. Heterogeneity was detected in all studies except for the dried fruit intake and ER- breast cancer study, salad/raw vegetable intake and ER- breast cancer study, and cereal intake and ER- breast cancer study; therefore, we applied a random-effects model in the IVW analysis. From the results of the pleiotropy test, we can assume the presence of no horizontal pleiotropy in terms of statistical significance because the p-values of the MR-Egger intercept were > 0.05. Figures 2–4 show leave-one-out, scatter, and funnel plots of MR for dietary patterns associated with the breast cancer risk based on the IVW analysis.
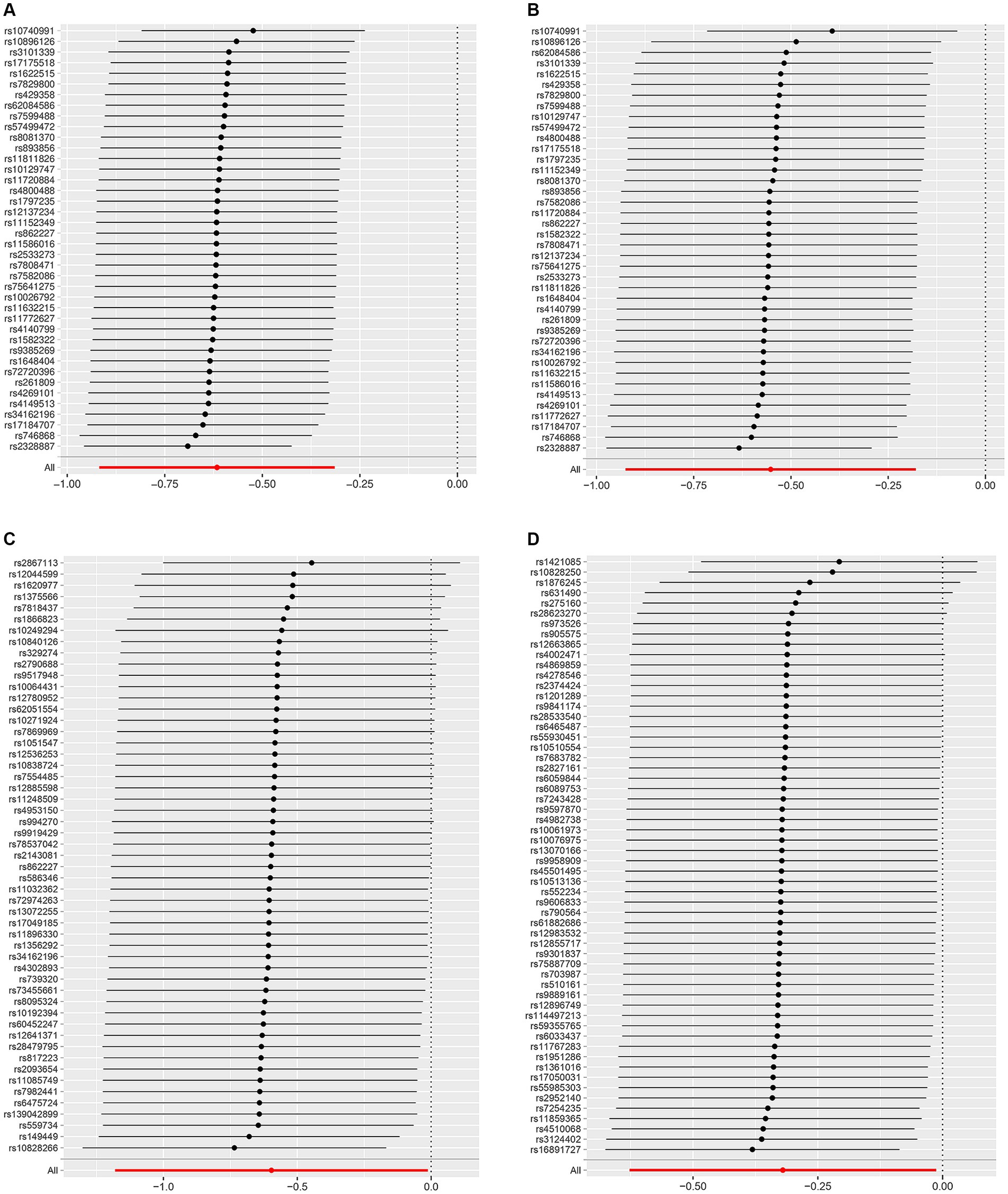
Figure 2. Leave-one-out plots of the MR results of (A) genetically predicted dried fruit intake with overall breast cancer; (B) genetically predicted dried fruit intake with ER+ breast cancer; (C) genetically predicted dried fruit intake with ER− breast cancer; (D) genetically predicted oily fish intake with ER+ breast cancer.
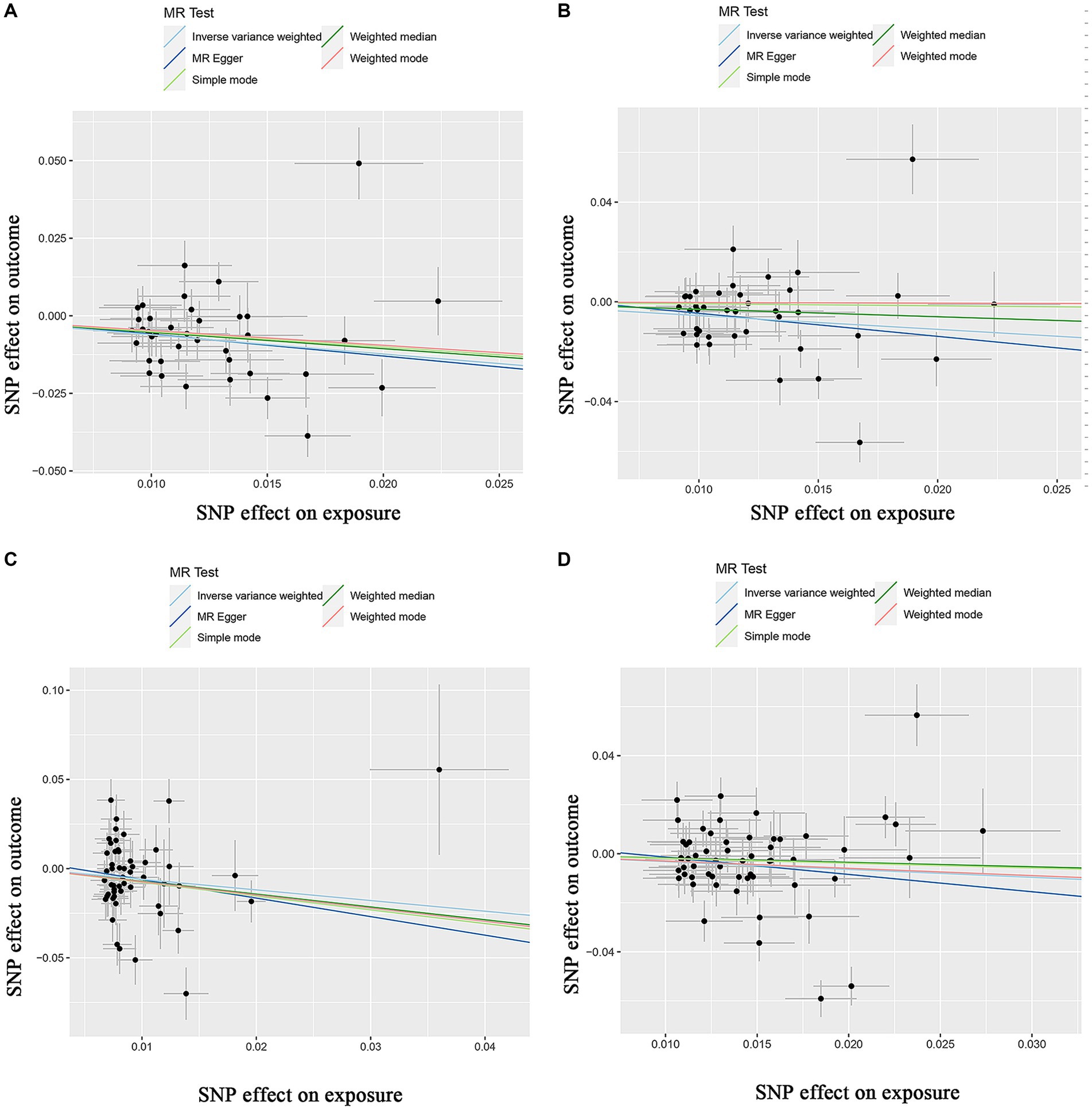
Figure 3. Scatter plots of the MR results of (A) genetically predicted dried fruit intake with overall breast cancer; (B) genetically predicted dried fruit intake with ER+ breast cancer; (C) genetically predicted dried fruit intake with ER− breast cancer; (D) genetically predicted oily fish intake with ER+ breast cancer.
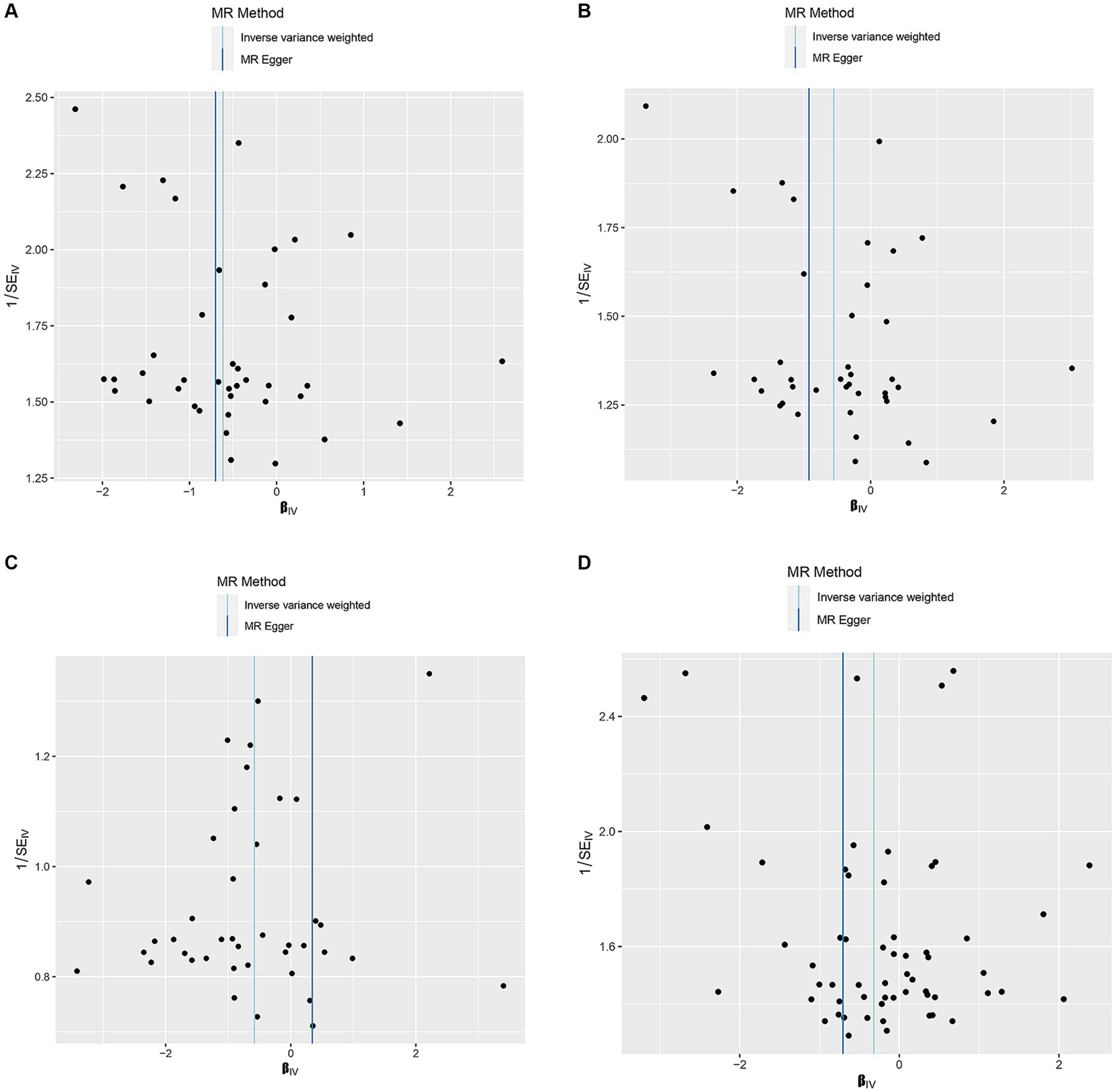
Figure 4. Funnel plots of the MR results of (A) genetically predicted dried fruit intake with overall breast cancer; (B) genetically predicted dried fruit intake with ER+ breast cancer; (C) genetically predicted dried fruit intake with ER− breast cancer; (D) genetically predicted oily fish intake with ER+ breast cancer.
As shown in Table 4 and Figure 5, after eliminating outliers based on MR-PRESSO and MR-Steiger filtering test results, of all dietary factors studied, only genetic tendency to intake dried fruit was found to be significantly associated with a reduced overall breast cancer risk (IVW odds ratio [OR] = 0.55; 95% confidence interval [CI]: 0.43–0.70; p = 1.75 × 10−6), ER+ breast cancer (IVW OR = 0.62; 95% CI: 0.47–0.82; p = 8.96 × 10−4) and ER- breast cancer (IVW OR = 0.48; 95% CI: 0.34–0.68; p = 3.18 ×10−5). In the WM model, dried fruit intake was associated with the overall breast cancer risk (WM OR = 0.59; 95% CI: 0.44–0.80; p = 6.68 × 10−4), and ER- breast cancer (WM OR = 0.50; 95% CI: 0.31–0.82; p = 6.22 × 10−3). However, in the WM model, dried fruit intake was not associated with ER+ breast cancer (WM OR = 0.74; 95% CI: 0.53–1.05; p = 0.09). Genetic tendency to intake oily fish, failing to pass the Bonferroni correction, was suggestive to be associated with a reduced risk of ER+ breast cancer (IVW OR = 0.73; 95% CI: 0.53–0.99; p = 0.04). In the WM model, oily fish intake was not associated with ER+ breast cancer (WM OR = 0.84; 95% CI: 0.65–1.07; p = 0.16).
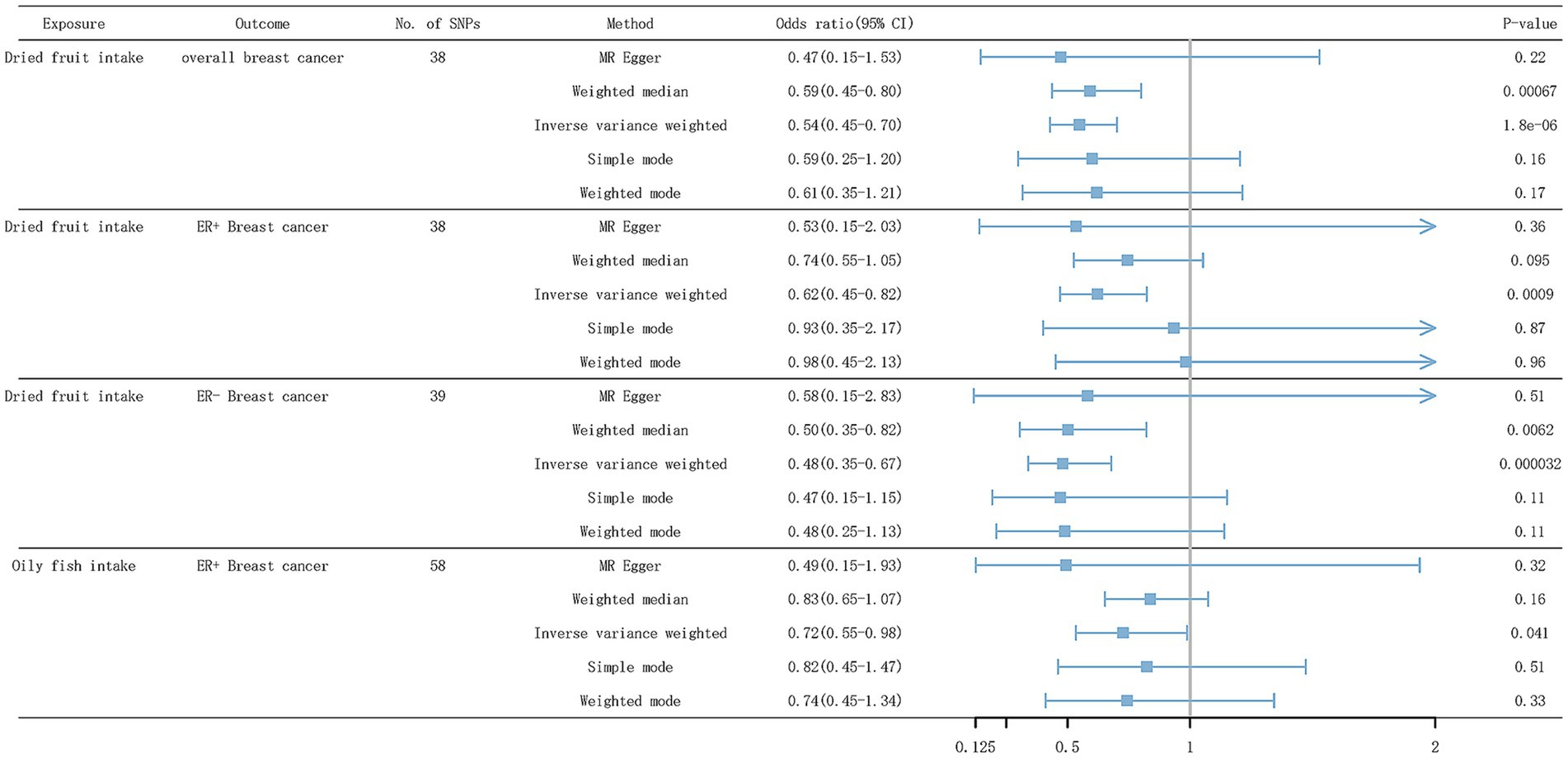
Figure 5. Forest plot of IVW results for dietary patterns associated with BC risk. SNP, single nucleotide polymorphism; ER+ breast cancer, Estrogen receptor-positive breast cancer; ER− breast cancer, Estrogen receptor-negative breast cancer.
Fresh fruit (IVW OR = 0.77; 95% CI: 0.58–1.00; p = 0.05), cooked vegetable (IVW OR = 0.67; 95% CI: 0.28–1.59; p = 0.36), salad/raw vegetable (IVW OR = 1.08; 95% CI: 0.70–1.69; p = 0.72), non-oily fish (IVW OR = 0.71; 95% CI: 0.41–1.23; p = 0.22), oily fish (IVW OR = 0.85; 95% CI: 0.71–1.03; p = 0.09), cereal (IVW OR = 1.02; 95% CI: 0.82–1.29; p = 0.83), salted nut (IVW OR = 1.24; 95% CI: 0.87–1.77; p = 0.21), unsalted nut (IVW OR = 1.04; 95% CI: 0.74–1.45; p = 0.79), or olive oil (IVW OR = 0.71; 95% CI: 0.47–1.08; p = 0.11) was not associated with the overall breast cancer risk (Figure 6).
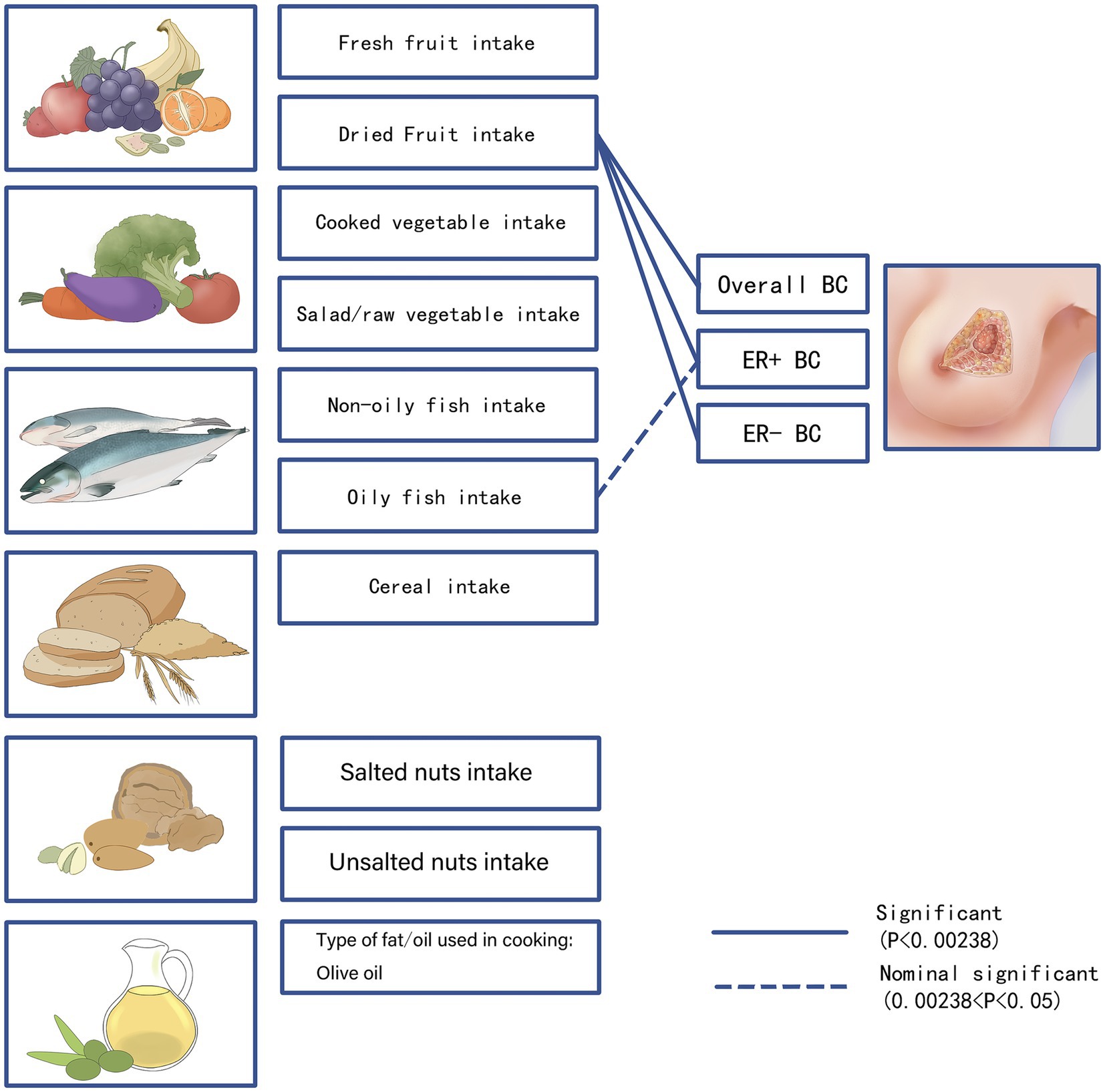
Figure 6. We visualized the association of 10 dietary patterns with breast cancer risk. p-values <0.00238 for the IVW method were considered as significant results, which we show as solid lines in the figure. 0.00238 <p-value<0.05 for the IVW method were considered as potentially significant results, which we show as dashed lines in the figure. BC, breast cancer; ER+ BC, Estrogen receptor-positive breast cancer; ER− BC, Estrogen receptor-negative breast cancer.
Fresh fruit (IVW OR = 0.80; 95% CI: 0.59–1.08; p = 0.15), cooked vegetable (IVW OR = 0.70; 95% CI: 0.30–1.63; p = 0.41), salad/raw vegetable (IVW OR = 1.02; 95% CI: 0.63–1.66; p = 0.90), non-oily fish (IVW OR = 0.78; 95% CI: 0.43–1.40; p = 0.41), cereal (IVW OR = 1.13; 95% CI: 0.87–1.47; p = 0.32), salted nut (IVW OR = 1.17; 95% CI: 0.76–1.80; p = 0.46), unsalted nut (IVW OR = 1.15; 95% CI: 0.72–1.84; p = 0.53), or olive oil (IVW OR = 0.72; 95% CI: 0.45–1.17; p = 0.19) intake was not associated with ER+ breast cancer.
Fresh fruit (IVW OR = 0.65; 95% CI: 0.42–1.00; p = 0.05), cooked vegetable (IVW OR = 0.84; 95% CI: 0.42–1.66; p = 0.62), salad/raw vegetable (IVW OR = 1.44; 95% CI: 0.74–2.78; p = 0.27), non-oily fish (IVW OR = 0.93; 95% CI: 0.45–1.90; p = 0.84), oily fish (IVW OR = 1.04; 95% CI: 0.76–1.41; p = 0.79), cereal (IVW OR = 0.88; 95% CI: 0.64–1.22; p = 0.46), salted nut (IVW OR = 0.99; 95% CI: 0.47–2.09; p = 0.99), unsalted nut (IVW OR = 1.17; 95% CI: 0.67–2.06; p = 0.56), or olive oil (IVW OR = 0.87; 95% CI: 0.45–1.67; p = 0.67) intake was not associated with ER- breast cancer.
The full MR results for the 10 dietary habits and overall, ER+, and ER- breast cancer risks, including results of the MR-Egger, WM, IVW, simple model, and WM methods, can be viewed in Supplementary Tables S3–S5, respectively.
Observational studies lack correction for risk factors and have a range of biases, such as study design and population, which may lead to inconsistent conclusions. In a previous study, the causal relationship between dietary patterns and the breast cancer risk was not fully elucidated. Previous studies have not been uniformly conclusive regarding the association between fruit intake and the breast cancer risk in European populations. A subset of observational studies showed no significant association between fruit intake and the breast cancer risk (4, 5, 32). A European Prospective Investigation into Cancer and Nutrition study involving 285,526 women showed no significant association between fruit intake (including dried and canned fruits) and the breast cancer risk (33). However, a prospective nurses’ health study involving 182,145 women with >20 years of follow-up showed that a higher total fruit intake was associated with a reduced breast cancer risk (34). Clinical observational evidence is limited on the effect of dried fruit intake on the breast cancer risk. A prospective cohort study involving women in the United Kingdom found no association between dried fruit consumption and breast cancer incidence (35). The relationship between vegetable intake and the breast cancer risk has been controversial in previous observational studies. Previous epidemiological studies suggested that vegetable intake is not associated with the breast cancer risk (12, 36), whereas traditional studies have shown that vegetable intake is associated with the breast cancer risk (4, 5, 34, 37). The results of previous conventional studies on the relationship between grain intake and the breast cancer risk were conflicting. Some studies have found a negative association (38–42), while others have found no clear association (11, 43–45). Xiao et al. (6) previously conducted a meta-analysis of four cohort studies and seven case–control studies and observed a negative association between cereal intake and breast cancer only in the case–control study, while no negative association was observed in the cohort studies.
In this study, we used a large-scale GWAS database for a two-sample MR analysis and found that genetically predicted dried fruit intake plays a critical role in breast cancer susceptibility. In addition to dried fruit intake, we found evidence that genetic predisposition to greater intake of oily fish may reduce the risk of ER+ breast cancer. Our study had several strengths. First, we used a large-scale GWAS database, which allowed us to use a much larger sample size compared to traditional studies, thus minimizing bias. Second, our study population was from Europe, effectively limiting population heterogeneity bias. Third, using genetic variables associated with a single phenotype as IVs, we largely reduced the bias caused by common genetic effects between phenotypes. Fourth, we performed rigorous sensitivity analyses to assess the effects of outliers and pleiotropy. Fifth, our MR analysis assessed the causal effects of exposure and outcomes, thus reducing the reverse causality associated with the outcome. Sixth, the MR study allowed us to identify risk factors at the genetic level, and thus, early identification of high-risk groups, which had implications for disease screening. However, our study had certain limitations. First, all samples were from Europe, thus reducing the generalizability to other populations. Second, this study relied heavily on self-reporting and may have been subject to a reporting bias. Third, the MR results only suggest possible genetic correlations and causal associations at the genetic level, and more mechanism-based experiments are required to further confirm this biological plausibility (46). Although our findings clarify a causal relationship between some dietary patterns and the breast cancer risk, the causal relationships derived from the MR experiments cannot be fully equated to the expected impact of risk factors on outcomes in a clinical setting (47). Causal relationships in MR reflect a genetic-level predisposition to risk factors, which makes interventions for risk factors potentially clinically meaningful (46). Although guiding clinical interventions for risk factors based on MR results is not appropriate, causal inferences using MR designs may be useful for screening specific populations susceptible to disease and could provide some guidance for conducting randomized controlled trials. Fourth, for the three dietary patterns of salted nut, unsalted nut, and olive oil intake, we used relatively more relaxed threshold of p < 5 × 10−6, and the F-values of the SNPs were < 10, which may led to conclusions that would be relatively weakly IV biased, i.e., genetic variants may not be strongly correlated with exposure factors. This is due to the limited sample size of the exposures. In future studies, databases with larger sample sizes could help screen for more representative IVs. Fifth, the GWAS data on dietary patterns, particularly complex dietary patterns like the Mediterranean diet, are relatively limited. This represents a valuable direction for future GWAS databases and Mendelian randomization studies.
The potential mechanisms by which dried fruit and oily fish intake were associated with the breast cancer risk in this study should be discussed. Owing to thermal degradation and oxidation reactions, dried fruits contain higher amounts of nutrients and phytochemicals compared to fresh fruits. 5-hydroxymethylfurfural, a compound commonly found in dried fruits, exhibits beneficial biological properties including in vitro antioxidant activity (48) and anti-hypoxic effects (49). The processing of dried fruit may also reduce the cancer risk. Mycotoxins are exogenous toxins that may be generated during the processing of dried fruits. Low-dose mycotoxin consumption can activate physiological responses, thereby counteracting chronic inflammation (50). A recent review suggested that dried fruits can reduce the impact of carcinogens by inducing the detoxification of enzymes (51). Dried fruit was prepared by removing water from the fruit and had a nutrient profile similar to that of the equivalent fresh fruit but at higher concentrations. A comparative study on raisins and grapes showed that drying concentrated polyphenol content and thus increased antioxidant activity (52). Thus, microbiome metabolites of polyphenols and other phytonutrients may be beneficial to health (53). Polyphenols exert antioxidant effects that reduce the proliferation of cancer cells and protect the DNA from damage caused by carcinogens (54). In conclusion, dried fruit intake prevents breast cancer; however, the mechanism requires further investigation. Contrary to the relationship between fish intake and the breast cancer risk, previous studies have shown that fish intake was not associated with the breast cancer risk (13), unlike the findings of this experiment. A possible explanation is that although fish are rich in omega-3 polyunsaturated fatty acids, which retard breast cancer growth according to in vitro and animal studies (55). They may also be contaminated with dioxins, methylmercury, and PCBs (56, 57). The possible dangers of dioxins or other contaminants were outweighed by the health risks of not eating fish (58).
Our study revealed that distinct histological subtypes of breast cancer are affected by the genetic propensity to dry fruit and oily fish intake to varying degrees. These findings suggest that individuals who do not include dried fruits and oily fish in their diets may benefit from considering earlier or more frequent breast cancer screenings, such as breast ultrasound and mammography, to facilitate early breast cancer detection. Additionally, at the genetic level, our results indicate an association between dietary habits involving dried fruit and oily fish intake and a reduced breast cancer risk, thereby contributing to the value of dietary recommendations for breast cancer prevention.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
XW and YD: conceptualization. XW: formal analysis and writing– original draft. YD: funding acquisition and supervision. XW and LC: software. LC, RC, RM, YL, and QZ: validation. XW, RC, RM, YL, and QZ: visualization. LC and YD: writing – review and editing. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Grant No. 82203558) and the Science and Technology Development Project of Jilin Province (Grant No. 20220204091YY). The funders played no role in study design, data collection, analysis, interpretation, or paper writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1215220/full#supplementary-material
1. ^https://gwas.mrcieu.ac=/, accessed on February 1, 2023.
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Kerr, J, Anderson, C, and Lippman, SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. (2017) 18:e457–71. doi: 10.1016/S1470-2045(17)30411-4
3. Key, TJ, Bradbury, KE, Perez-Cornago, A, Sinha, R, Tsilidis, KK, and Tsugane, S. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ. (2020) 368:m511. doi: 10.1136/bmj.m511
4. Emaus, MJ, Peeters, PH, Bakker, MF, Overvad, K, Tjønneland, A, and Olsen, A. Vegetable and fruit consumption and the risk of hormone receptor-defined breast cancer in the EPIC cohort. Am J Clin Nutr. (2016) 103:168–77. doi: 10.3945/ajcn.114.101436
5. Jung, S, Spiegelman, D, Baglietto, L, Bernstein, L, Boggs, DA, van den Brandt, PA, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. (2013) 105:219–36. doi: 10.1093/jnci/djs635
6. Xiao, Y, Ke, Y, Wu, S, Huang, S, Li, S, Lv, Z, et al. Association between whole grain intake and breast cancer risk: a systematic review and meta-analysis of observational studies. Nutr J. (2018) 17:87. doi: 10.1186/s12937-018-0394-2
7. Aune, D, Chan, DS, Greenwood, DC, Vieira, AR, Rosenblatt, DA, Vieira, R, et al. Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. (2012) 23:1394–402. doi: 10.1093/annonc/mdr589
8. Haraldsdottir, A, Steingrimsdottir, L, Valdimarsdottir, UA, Aspelund, T, Tryggvadottir, L, Harris, TB, et al. Early life residence, fish consumption, and risk of breast cancer. Cancer Epidemiol Biomark Prev. (2017) 26:346–54. doi: 10.1158/1055-9965.EPI-16-0473-T
9. Zhang, D, Dai, C, Zhou, L, Li, Y, Liu, K, Deng, YJ, et al. Meta-analysis of the association between nut consumption and the risks of cancer incidence and cancer-specific mortality. Aging. (2020) 12:10772–94. doi: 10.18632/aging.103292
10. Moral, R, and Escrich, E. Influence of olive oil and its components on breast Cancer: molecular mechanisms. Molecules. (2022) 27:477. doi: 10.3390/molecules27020477
11. Makarem, N, Nicholson, JM, Bandera, EV, McKeown, NM, and Parekh, N. Consumption of whole grains and cereal fiber in relation to cancer risk: a systematic review of longitudinal studies. Nutr Rev. (2016) 74:353–73. doi: 10.1093/nutrit/nuw003
12. Peng, C, Luo, WP, and Zhang, CX. Fruit and vegetable intake and breast cancer prognosis: a meta-analysis of prospective cohort studies. Br J Nutr. (2017) 117:737–49. doi: 10.1017/S0007114517000423
13. Engeset, D, Braaten, T, Teucher, B, Kühn, T, Bueno-de-Mesquita, HB, Leenders, M, et al. Fish consumption and mortality in the European prospective investigation into cancer and nutrition cohort. Eur J Epidemiol. (2015) 30:57–70. doi: 10.1007/s10654-014-9966-4
14. Klungel, OH, Martens, EP, Psaty, BM, Grobbee, DE, Sullivan, SD, and Stricker, BH. Methods to assess intended effects of drug treatment in observational studies are reviewed. J Clin Epidemiol. (2004) 57:1223–31. doi: 10.1016/j.jclinepi.2004.03.011
15. Evans, DM, and Davey, SG. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet. (2015) 16:327–50. doi: 10.1146/annurev-genom-090314-050016
16. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
17. Li, C, Ou, R, and Shang, H. Rheumatoid arthritis decreases risk for Parkinson’s disease: a Mendelian randomization study. NPJ Parkinsons Dis. (2021) 7:17. doi: 10.1038/s41531-021-00166-x
18. Burgess, S, Butterworth, A, and Thompson, SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
19. Elsworth, B, Lyon, M, Alexander, T, Liu, Y, Matthews, P, and Hallett, J. The MRC IEU OpenGWAS data infrastructure. bioRxiv. (2020) 10:244293. doi: 10.1101/2020.08.10.244293
20. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The MR-base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:7. doi: 10.7554/eLife.34408
21. Canela-Xandri, O, Rawlik, K, and Tenesa, A. An atlas of genetic associations in UK biobank. Nat Genet. (2018) 50:1593–9. doi: 10.1038/s41588-018-0248-z
22. Michailidou, K, Lindström, S, Dennis, J, Beesley, J, Hui, S, Kar, S, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. (2017) 551:92–4. doi: 10.1038/nature24284
23. Yang, J, He, X, Qian, L, Zhao, B, Fan, Y, Gao, F, et al. Association between plasma proteome and childhood neurodevelopmental disorders: a two-sample Mendelian randomization analysis. EBioMedicine. (2022) 78:103948. doi: 10.1016/j.ebiom.2022.103948
24. Burgess, S, Davey Smith, G, Davies, NM, Dudbridge, F, Gill, D, Glymour, MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.1
25. You, R, Chen, L, Xu, L, Zhang, D, Li, H, Shi, X, et al. High level of uromodulin increases the risk of hypertension: a Mendelian randomization study. Front Cardiovasc Med. (2021) 8:8. doi: 10.3389/fcvm.2021.736001
26. Palmer, TM, Lawlor, DA, Harbord, RM, Sheehan, NA, Tobias, JH, Timpson, NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. (2012) 21:223–42. doi: 10.1177/0962280210394459
27. Burgess, S, and Thompson, SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
28. Brion, MJ, Shakhbazov, K, and Visscher, PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
29. Skrivankova, VW, Richmond, RC, Woolf, BAR, Davies, NM, Swanson, SA, and VanderWeele, TJ. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. (2021) 375:n2233. doi: 10.1136/bmj.n2233
30. Bowden, J, Spiller, W, Del Greco, MF, Sheehan, N, Thompson, J, Minelli, C, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int J Epidemiol. (2018) 47:2100. doi: 10.1093/ije/dyy265
31. Hemani, G, Tilling, K, and Davey, SG. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081
32. George, SM, Park, Y, Leitzmann, MF, Freedman, ND, Dowling, EC, Reedy, J, et al. Fruit and vegetable intake and risk of cancer: a prospective cohort study. Am J Clin Nutr. (2009) 89:347–53. doi: 10.3945/ajcn.2008.26722
33. van Gils, CH, Peeters, PHM, Bueno-de-Mesquita, HB, Boshuizen, HC, Lahmann, PH, and Clavel-Chapelon, F. Consumption of vegetables and fruits and risk of breast cancer. JAMA. (2005) 293:183–93. doi: 10.1001/jama.293.2.183
34. Farvid, MS, Chen, WY, Rosner, BA, Tamimi, RM, Willett, WC, and Eliassen, AH. Fruit and vegetable consumption and breast cancer incidence: repeated measures over 30 years of follow-up. Int J Cancer. (2019) 144:1496–510. doi: 10.1002/ijc.31653
35. Dunneram, Y, Greenwood, DC, and Cade, JE. Diet and risk of breast, endometrial and ovarian cancer: UK Women’s cohort study. Br J Nutr. (2019) 122:564–74. doi: 10.1017/S0007114518003665
36. Aune, D, Chan, DS, Vieira, AR, Rosenblatt, DA, Vieira, R, and Greenwood, DC. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. (2012) 134:479–93. doi: 10.1007/s10549-012-2118-1
37. Kazemi, A, Barati-Boldaji, R, Soltani, S, Mohammadipoor, N, Esmaeilinezhad, Z, Clark, CCT, et al. Intake of various food groups and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. (2021) 12:809–49. doi: 10.1093/advances/nmaa147
38. Shin, WK, Lee, HW, Shin, A, Lee, JK, Lee, SA, Lee, JE, et al. Multi-grain rice diet decreases risk of breast cancer in Korean women: results from the health examinees study. Nutrients. (2020) 12:2273. doi: 10.3390/nu12082273
39. Farvid, MS, Cho, E, Eliassen, AH, Chen, WY, and Willett, WC. Lifetime grain consumption and breast cancer risk. Breast Cancer Res Treat. (2016) 159:335–45. doi: 10.1007/s10549-016-3910-0
40. Mourouti, N, Kontogianni, MD, Papavagelis, C, Psaltopoulou, T, Kapetanstrataki, MG, Plytzanopoulou, P, et al. Whole grain consumption and breast cancer: a case-control study in women. J Am Coll Nutr. (2016) 35:143–9. doi: 10.1080/07315724.2014.963899
41. Yun, SH, Kim, K, Nam, SJ, Kong, G, and Kim, MK. The association of carbohydrate intake, glycemic load, glycemic index, and selected rice foods with breast cancer risk: a case-control study in South Korea. Asia Pac J Clin Nutr. (2010) 19:383–92. doi: 10.6133/APJCN.2010.19.3.13
42. Chatenoud, L, Tavani, A, La Vecchia, C, Jacobs, DR Jr, Negri, E, and Levi, F. Whole grain food intake and cancer risk. Int J Cancer. (1998) 77:24–8. doi: 10.1002/(SICI)1097-0215(19980703)77:1<24::AID-IJC5>3.0.CO;2-1
43. Egeberg, R, Olsen, A, Loft, S, Christensen, J, Johnsen, NF, Overvad, K, et al. Intake of whole grain products and risk of breast cancer by hormone receptor status and histology among postmenopausal women. Int J Cancer. (2009) 124:745–50. doi: 10.1002/ijc.23992
44. Nicodemus, KK, Jacobs, DR, and Folsom, AR. Whole and refined grain intake and risk of incident postmenopausal breast cancer (United States). Cancer Causes Control. (2001) 12:917–25. doi: 10.1023/A:1013746719385
45. La Vecchia, C, Decarli, A, Franceschi, S, Gentile, A, Negri, E, and Parazzini, F. Dietary factors and the risk of breast cancer. Nutr Cancer. (1987) 10:205–14. doi: 10.1080/01635588709513958
46. Burgess, S, Swanson, SA, and Labrecque, JA. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur J Epidemiol. (2021) 36:253–7. doi: 10.1007/s10654-021-00726-8
47. VanderWeele, TJ, Tchetgen Tchetgen, EJ, Cornelis, M, and Kraft, P. Methodological challenges in mendelian randomization. Epidemiology. (2014) 25:427–35. doi: 10.1097/EDE.0000000000000081
48. Zhao, L, Chen, J, Su, J, Li, L, Hu, S, Li, B, et al. In vitro antioxidant and Antiproliferative activities of 5-Hydroxymethylfurfural. J Agric Food Chem. (2013) 61:10604–11. doi: 10.1021/jf403098y
49. Li, M-M, Wu, L-Y, Zhao, T, Xiong, L, Huang, X, Liu, Z-H, et al. The protective role of 5-HMF against hypoxic injury. Cell Stress Chaperones. (2011) 16:267–73. doi: 10.1007/s12192-010-0238-2
50. Mossine, VV, Mawhinney, TP, and Giovannucci, EL. Dried fruit intake and cancer: a systematic review of observational studies. Adv Nutr. (2020) 11:237–50. doi: 10.1093/advances/nmz085
51. Bolling, BW, Aune, D, Noh, H, Petersen, KS, and Freisling, H. Dried fruits, nuts, and Cancer risk and survival: a review of the evidence and future research directions. Nutrients. (2023) 15:1443. doi: 10.3390/nu15061443
52. Sadler, MJ, Gibson, S, Whelan, K, Ha, MA, Lovegrove, J, and Higgs, J. Dried fruit and public health - what does the evidence tell us? Int J Food Sci Nutr. (2019) 70:675–87. doi: 10.1080/09637486.2019.1568398
53. Cardona, F, Andrés-Lacueva, C, Tulipani, S, Tinahones, FJ, and Queipo-Ortuño, MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. (2013) 24:1415–22. doi: 10.1016/j.jnutbio.2013.05.001
54. Braakhuis, AJ, Campion, P, and Bishop, KS. Reducing breast cancer recurrence: the role of dietary polyphenolics. Nutrients. (2016) 8:547. doi: 10.3390/nu8090547
55. Terry, P, Rohan, TE, Wolk, A, Maehle-Schmidt, M, and Magnusson, C. Fish consumption and breast cancer risk. Nutr Cancer. (2002) 44:1–6. doi: 10.1207/S15327914NC441_1
56. Sanzo, JM, Dorronsoro, M, Amiano, P, Amurrio, A, Aguinagalde, FX, Azpiri, MA, et al. Estimation and validation of mercury intake associated with fish consumption in an EPIC cohort of Spain. Public Health Nutr. (2001) 4:981–8. doi: 10.1079/PHN2001170
57. Sjödin, A, Hagmar, L, Klasson-Wehler, E, Björk, J, and Bergman, A. Influence of the consumption of fatty baltic sea fish on plasma levels of halogenated environmental contaminants in Latvian and Swedish men. Environ Health Perspect. (2000) 108:1035–41. doi: 10.1289/ehp.108-1240159
Keywords: breast cancer, Mendelian randomization, dietary patterns, genome-wide association study (GWAS), nutrition
Citation: Wang X, Chen L, Cao R, Ma R, Liu Y, Zhao Q and Du Y (2023) Associations of 10 dietary habits with breast cancer: a Mendelian randomization study. Front. Nutr. 10:1215220. doi: 10.3389/fnut.2023.1215220
Received: 01 May 2023; Accepted: 06 November 2023;
Published: 21 November 2023.
Edited by:
Esther Molina-Montes, University of Granada, SpainReviewed by:
Jinyi Zhou, Jiangsu Provincial Center for Disease Control and Prevention, ChinaCopyright © 2023 Wang, Chen, Cao, Ma, Liu, Zhao and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Du, amR5eWR5QGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.