
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 15 August 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1207751
This article is part of the Research Topic Clinical Utilization of Plant-based Nutrition and Fasting Protocols as Novel Therapies View all 6 articles
Introduction: Dyslipidemia is a major cardiovascular disease risk factor associated with increased mortality. The intake of plant food-derived bioactive compounds is associated with beneficial cardiovascular effects, including decreased blood lipid levels and cardiovascular risk. We aimed to evaluate the effects of anthocyanin intake on blood lipid levels by analyzing relevant randomized controlled trials.
Methods: We searched the PubMed and Embase databases using the “Patient/Population, Intervention, Comparison, and Outcomes” format to determine whether anthocyanin supplementation intervention affected blood lipid levels compared with placebo supplementation in human participants.
Results: A total of 41 studies with 2,788 participants were included in the meta-analysis. Anthocyanin supplementation significantly reduced triglyceride [standardized mean difference (SMD) = −0.10; 95% confidence interval [CI], −0.18, −0.01) and low-density lipoprotein-cholesterol (SMD = −0.16; 95% CI −0.26, −0.07) levels and increased high-density lipoprotein-cholesterol levels (SMD = 0.42; 95% CI 0.20, 0.65).
Discussion: Anthocyanin supplementation significantly improved blood lipid component levels in the included studies. Larger, well-designed clinical trials are needed to further investigate the effects of anthocyanin intake on blood lipid levels and the safety of anthocyanin supplementation for treating dyslipidemia.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021257087, identifier: CRD42021257087.
Dyslipidemia, one of the key components of metabolic syndrome, is a major risk factor for cardiovascular disease (CVD) and increased mortality (1, 2). Globally, CVD is a leading cause of morbidity and mortality; hence, the priority of developing effective means to reduce CVD risk cannot be overstated. Lifestyle changes, such as eating a healthy diet and increasing physical activity, have been shown to reduce CVD risk. Various bioactive compounds derived from plant foods are also associated with beneficial cardiovascular effects and CVD risk reduction.
Water-soluble anthocyanin pigment supplementation, which is a secondary metabolite belonging to the flavonoids family, has antioxidant and antiinflammatory properties and many health benefits including protection against carcinoma and diabetes. The positive effects of anthocyanin supplementation on dyslipidemia have been reported in several clinical trials. However, although meta-analyses of randomized controlled trials have been conducted, the relationship between anthocyanin supplementation and dyslipidemia has not been consistently reviewed to date (3–5).
Cyanidin, a type of anthocyanin, is a pigment mainly found in red-skinned or red-fleshed fruits, including apples, hawthorn berries, bilberries, cranberries, chokeberries or aronia berries, and lingonberries (6). Numerous in vitro and animal studies have reported that cyanidin may modulate the lipid metabolism (7–9); however, systematic reviews and meta-analyses on the lipid profile improvement effect of cyanidin in human are lacking.
In the present study, we aimed to analyze the effects of anthocyanin supplementation on blood lipid levels by conducting a systematic review and meta-analysis of relevant randomized control trials.
This systematic review and meta-analysis were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Appendix 1) (10). The protocol was registered in PROSPERO (CRD42021257087).
The PubMed and Embase databases were searched from their inception to June 2023 using the search terms “Anthocyanin OR Cyanidin” and “Black food” to identify relevant articles. Only studies conducted in clinical settings and published in the English language were considered. Additional articles were identified via a manual search of the reference lists of the original articles, reviews, and meta-analyses. The duplicate results were eliminated using EndNote software, and the titles and abstracts were screened by two authors (H-HJ and Y-ML) using Rayyan QCRI online software (https://www.rayyan.ai). The relevant studies then underwent dual full-text screening.
The meta-analysis was performed using the “Patient/Population, Intervention, Comparison, and Outcomes” format to determine whether an intervention with anthocyanin supplementation (I) had any effect on blood lipids (O) compared with placebo supplementation (C) among participants (P). The outcomes of interest were levels of triglyceride (TG), total cholesterol, low-density lipoprotein (LDL)-cholesterol, and high-density lipoprotein (HDL)-cholesterol. Parallel or crossover randomized control trials were included, whereas observational studies and review articles were excluded. The intervention duration and dose were at least 2 weeks and 10 mg/day, respectively. Two authors (H-HJ and Y-ML) independently reviewed data from all the studies that fulfilled the inclusion criteria, and any conflicts were consensually resolved.
Two reviewers (H-HJ and Y-ML) independently extracted the following data from the included studies: authors, year of publication, study design, place, study population (age, number, proportion of women, and health status), and intervention (sources, dose, type, duration, and concentration of anthocyanin and cyanidin) (Table 1). Two reviewers (H-HJ and Y-ML) independently assessed the risk of bias using the Jadad scale (52). This scale considers randomization, blinding, and accountability of all patients. If all these items are regarded as appropriate, a score of 5 is assigned. The Jadad scale scores of ≥3 and <3 were considered to have a low and high risk of bias, respectively. Any disagreements were consensually resolved.
We used Egger's regression test for funnel plot asymmetry to assess the potential publication bias of the included studies (53). P-values of <0.05 were considered significant.
We used the standardized mean difference (SMD) with the 95% confidence interval (CI) as effect size measures. In studies where the mean difference was not reported, the mean differences were calculated by subtracting the baseline mean from the post-intervention mean; the standard deviation (SD) differences were estimated using the following formula:
where SDB is the baseline SD and SDF is the SD of the final measures in the study (54).
The correlation value was conservatively set at 0.5 to calculate the change in SD (55). Owing to the clinical heterogeneity of the studies, including differences in study design, doses, and intervention, a random-effects model was used for the meta-analysis of quantitative data. A forest plot was mapped to indicate the pooled SMD and 95% CI. Between-study heterogeneity was assessed by forest plot visualization. Subsequently, the Q-test and I2 statistic were used to quantitatively evaluate the statistical heterogeneity. In general, a P-value of <0.1 for Q statistics and an I2 > 50% indicate considerable heterogeneity (54). Sensitivity analysis was conducted to investigate the effect of each study on the pooled effect size if the I2 values were >50%. Furthermore, a subgroup analysis was performed to explore the possible source of heterogeneity for the following subsets: participants (number and healthy status), studies (design and area), or interventions (duration and dosage). All analyses were performed using R statistical software (Foundation for Statistical Computing, Vienna, Austria).
A total of 440 studies were initially identified: 429 from the database search and 11 from the manual search. A total of 104 duplicate studies were removed, and the titles and abstracts of 336 studies were screened by two authors. Subsequently, 276 studies were excluded. The remaining 60 studies underwent double full-text review. Subsequently, 19 studies were excluded: 4 were excluded because of insufficient data presentation (56–59) and 15 were excluded because of inappropriate interventions (60–74). Finally, 41 studies were included in the systematic review. The PRISMA flowchart for the selection process is presented in Figure 1.
A total of 41 studies that enrolled 2,788 participants were included in the systematic review and meta-analysis. The characteristics of the included studies are summarized in Table 1. Among the 41 studies, 34 were parallel-arm studies (11, 12, 14–21, 23, 26–40, 42–46, 48–50), whereas 7 were crossover studies (13, 22, 24, 25, 41, 47, 51). Most studies included both sexes, three studies included only females (13, 17, 21) and six included only males (14, 24, 25, 27, 43, 46). All studies were conducted on adults, and the average age of the participants ranged from 24 to 67 years. Among the 41 studies, 14 were conducted in the United States (13, 15, 19–22, 25, 26, 29, 33, 35, 40, 42, 46), 11 in China (18, 23, 28, 32, 36, 38, 39, 45, 47, 49, 50), 5 in the UK (17, 30, 41, 44, 51), 2 in Iran (31, 34), and 1 each in Austria (11), Denmark (12), Spain (14), Finland (16), Norway (24), Australia (27), Korea (37), Germany (43), and Japan (48). Twenty-six studies were conducted in individuals with diseases such as chronic obstructive pulmonary disease (14), dyslipidemia (23, 28, 31, 38, 49), hypertension (24), obesity (20, 27, 37), type 2 diabetes mellitus (32, 34, 39, 46, 47, 50), CVDs (16, 22, 26), non-alcoholic fatty liver disease (36), and metabolic syndrome (19, 21, 29, 35, 42, 44), and 15 studies were conducted in healthy individuals (11–13, 15, 17, 18, 25, 30, 33, 40, 41, 43, 45, 48, 51). In total, 15 studies focused on an intervention with bilberry and black currant (15, 16, 18, 23, 24, 28, 32, 36, 39, 45, 47–51); 15 on elderberry (11, 17), blueberry (19, 20, 25, 35, 43, 46), cranberry (21, 22, 26, 33), whortleberry (31), açaí berry (42), or mixed fruits (43) supplementation; and 10 on grapes (12, 13), pomegranates (14), purple carrot (27), tart cherry (30), cornelian cherry (34), black soybean (37), aronia berries (40), strawberry (29), blood orange (41), and black rice (51) supplementation. One study (38) that reported on anthocyanin supplementation did not mention the food source. The duration of the interventions in the included studies varied (2–24 weeks). Among the 41 studies, 22 used supplements of concentrated anthocyanins in capsule (11, 15, 17, 18, 23, 24, 28, 31, 32, 34, 36–40, 45, 47–51) or tablet (12) form, 9 studies (14, 21, 22, 26, 30, 33, 41–43) used juice form supplements, and 9 other studies (13, 19, 20, 25, 27, 29, 35, 44, 46) used dried powder form supplements. One study (16) used mixed-type supplements, including juice and puree forms. The anthocyanin concentration ranged from a minimum of 20 mg/day to a maximum of 742 mg/day, with an average of 238.5 mg/day. Among the 41 studies, 21 presented intake levels of cyanidin with total anthocyanins. The total anthocyanin intake was 215.3 mg/day in 21 studies, which was marginally lower than the intake of 238.5 mg/day in all 41 studies. The cyanidin concentration of supplements ranged from a minimum of 6.6 mg/day to a maximum of 515 mg/day, with an average of 116.5 mg/day.
The risk of bias assessments for individual studies are presented in Supplementary Table 1. Thirty-five of the included studies had a low risk of bias (Jadad score ≥ 3) and six had a high risk of bias (Jadad score <3). All 41 studies were randomized; however, only 20 appropriately described the method of randomization. Of the 33 studies that mentioned blinding, only 26 described the method of blinding. In 36 of the included 41 studies, the reasons for withdrawal or dropout of participants were described.
The forest plots for the overall random effects of anthocyanin-rich food supplementation on the levels of TG, total cholesterol, LDL-cholesterol, and HDL-cholesterol are illustrated in Figures 2–5. The overall pooled statistics revealed that the TG levels of participants (47 results from 38 studies) were significantly reduced (SMD = −0.10; 95% CI −0.18, −0.01), and a small degree of heterogeneity was observed in the analysis of TG (I2 = 34% and P = 0.01) (Figure 2). A total of 40 studies that included 50 effect sizes investigated the impact of anthocyanin supplementation on total cholesterol levels (Figure 3). The pooled statistics revealed that the SMD of total cholesterol was −0.05 (95% CI −0.12, 0.01), and a small degree of between-study heterogeneity was observed in the analysis (I2 = 28% and P = 0.04). Overall, 44 effect sizes from 35 studies were included in the analysis of the effect of anthocyanin supplements on LDL-cholesterol levels and revealed a significant effect (SMD = −0.16; 95% CI −0.26, −0.07) (Figure 4). The forest plot for the overall effect of anthocyanin supplements on HDL-cholesterol levels, which included the results of 39 studies, is presented in Figure 5. The SMD of the overall pooled HDL-cholesterol levels was 0.42 (95% CI 0.20, 0.65), which was a considerable increase. Statistical heterogeneity was observed in the analysis of LDL-cholesterol (I2 = 38% and P = 0.01) and HDL-cholesterol (I2 = 81% and P < 0.01) levels. Sensitivity analysis of the effect of anthocyanin supplementation on HDL-cholesterol levels revealed that the removal of any study did not alter the significance of the pooled effect size (Supplementary Figure 1). Note that, for the results of the meta-analysis of the 21 studies that included cyanidin intake, the effect on blood lipids had a greater impact than the results of all 41 studies (Supplementary Figures 2–5); moreover, cyanidin significantly reduced TG (SMD = −0.10; 95% CI −0.19, −0.01) and LDL-cholesterol (SMD = −0.23; 95% CI −0.37, −0.10) levels and increased HDL-cholesterol (SMD = 0.50; 95% CI 0.18, 0.82) levels.
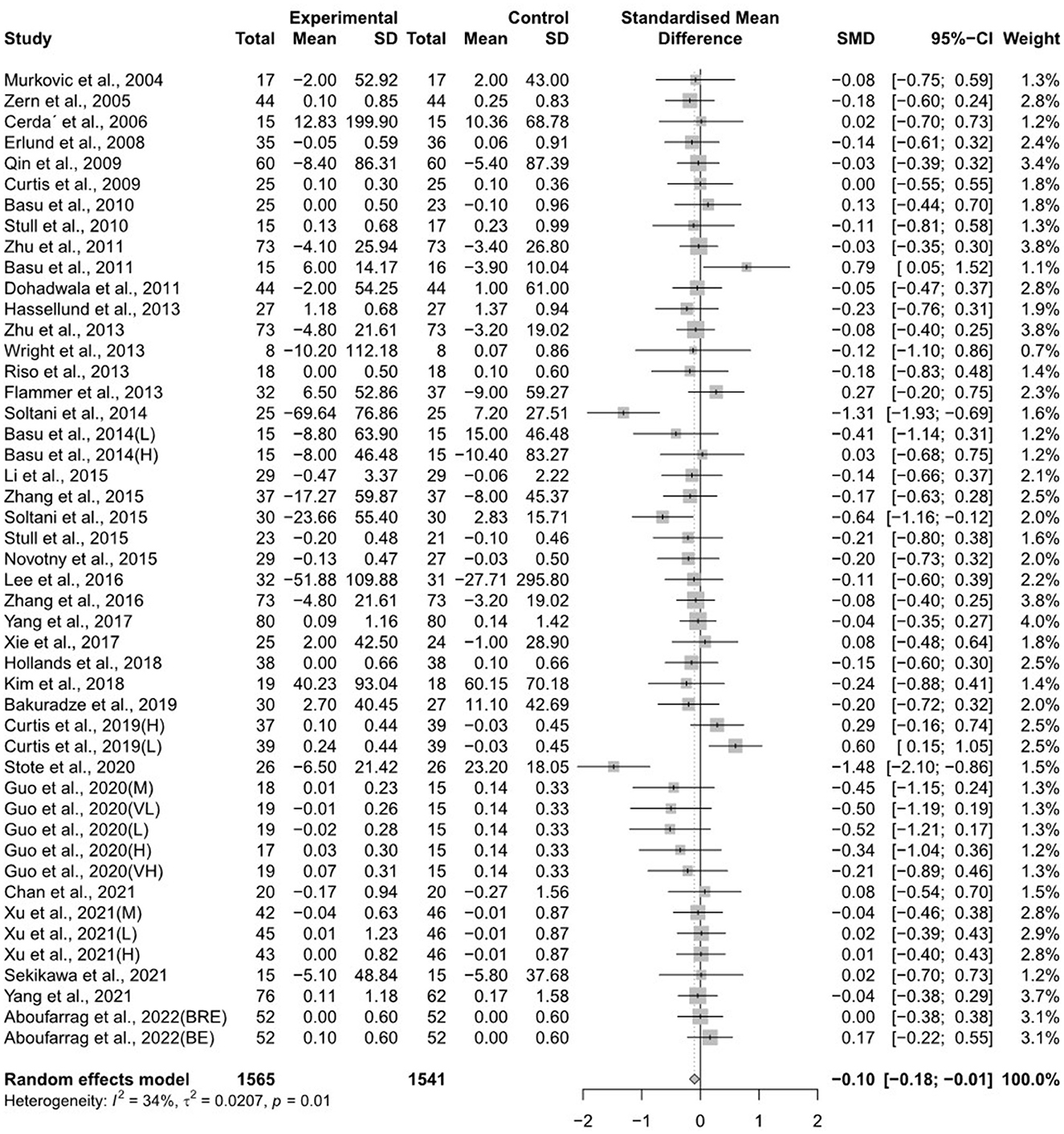
Figure 2. A forest plot of the change in the standardized mean differences (with 95% confidence intervals) of triglycerides in participants administered anthocyanin supplements compared with the control.
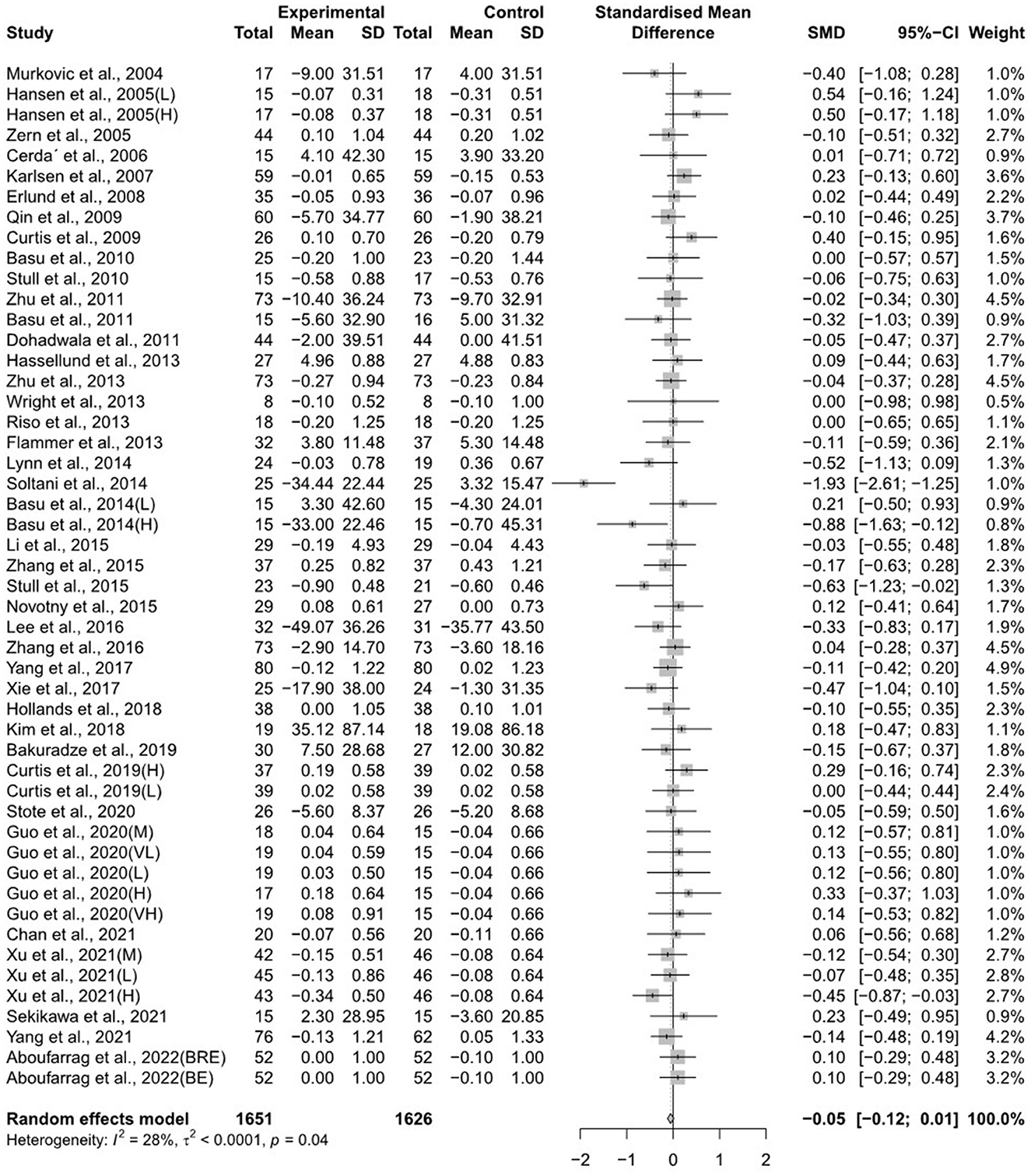
Figure 3. A forest plot of the change in the standardized mean differences (with 95% confidence intervals) of total cholesterol in participants administered anthocyanin supplements compared with the control.
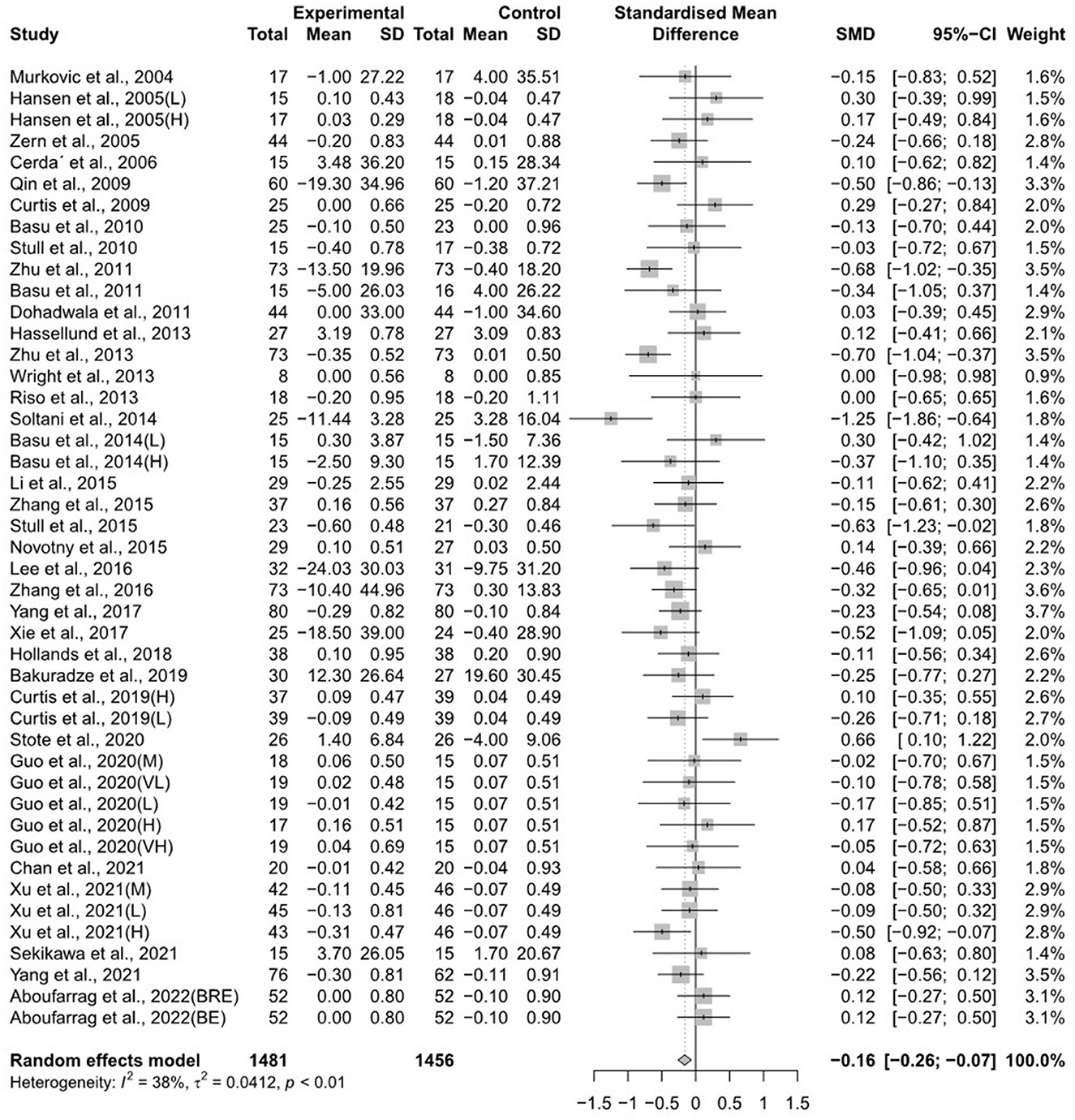
Figure 4. A forest plot of the change in the standardized mean differences (with 95% confidence intervals) of low-density lipoprotein-cholesterol in participants administered anthocyanin supplements compared with the control.
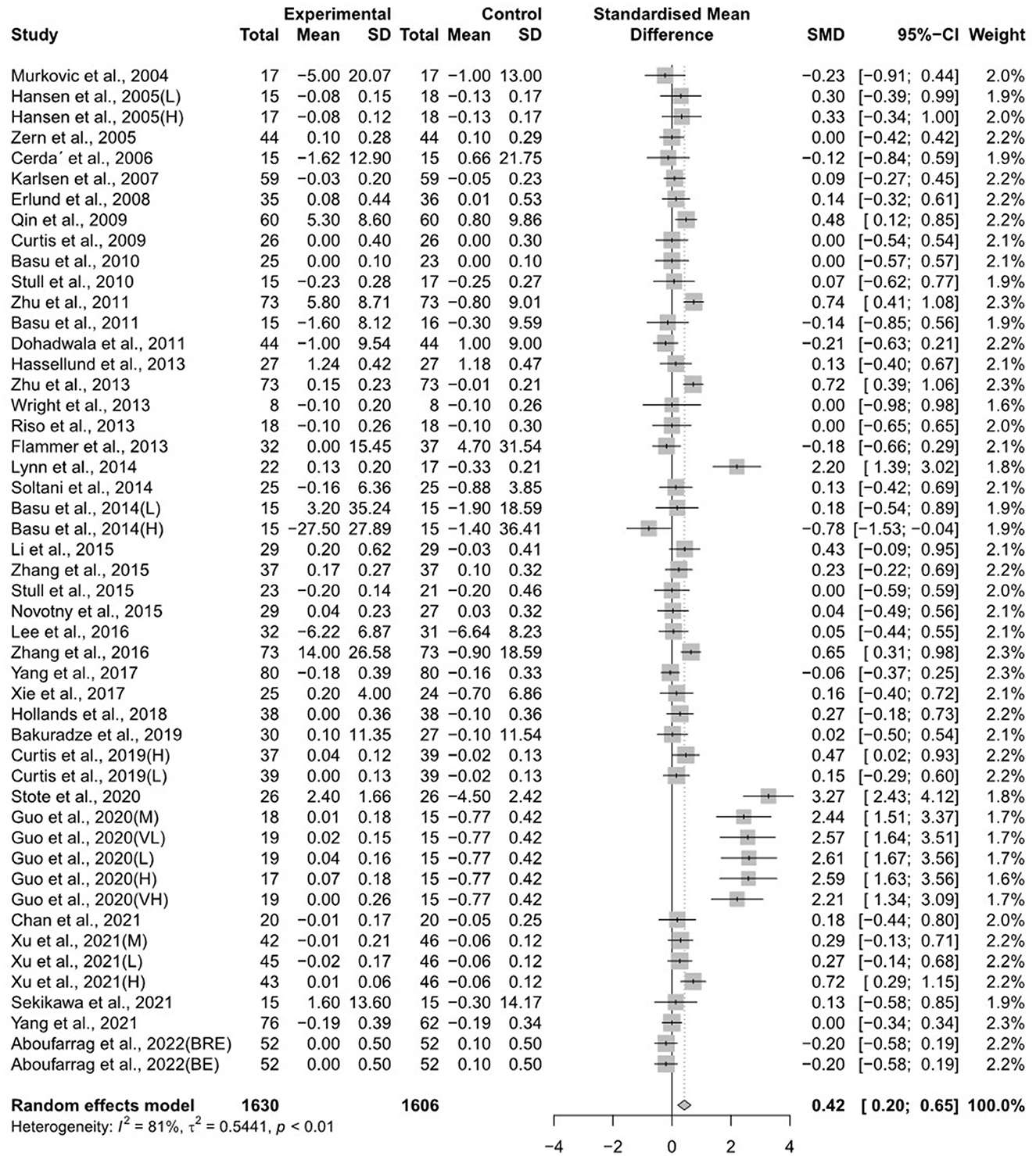
Figure 5. A forest plot of the change in the standardized mean differences (with 95% confidence intervals) of high-density lipoprotein-cholesterol in participants administered anthocyanin supplements compared with the control.
Furthermore, we performed subgroup analyses to explore the possible source of heterogeneity among the studies (Table 2). The effect of anthocyanin supplementation on HDL-cholesterol was significantly greater in the subgroup with a normal cholesterol level <200 mg/dL than in the subgroup with a higher cholesterol level ≥ 200 mg/dL at the baseline (P = 0.020). However, heterogeneity (I2) was 51% in hypercholesterolemic participants (total cholesterol ≥ 200) and lower than 91% in normal-level participants. The effect on HDL-cholesterol exhibited a higher increase in the subgroup with an average age <40 years (SMD = 1.30; 95% CI 0.54, 2.05) than in the subgroup with an average age ≥40 years (SMD = 0.21; 95% CI 0.07, 0.35). In particular, the effect of anthocyanin supplementation on HDL-cholesterol was significantly higher in studies with a low risk of bias than in the groups with a high risk of bias (P = 0.002). Subgroup analysis among study design, anthocyanin dosage, and risk of bias indicated decreased heterogeneity within subgroups. However, substantial unexplained heterogeneity was observed between these subgroups.
In addition, the difference in the effect of anthocyanin supplementation on lipid improvement was compared by dividing healthy participants and participants with dyslipidemia into subgroups (Supplementary Table 2). As a result of performing subgroup analysis according to dyslipidemia status, there was no significant difference between the two groups in TG and TC levels. However, the effect of anthocyanin supplementation on LDL-cholesterol (P = 0.027) and HDL-cholesterol (P = 0.020) was significantly different between the two groups. In particular, LDL-cholesterol showed a significant reduction only in dyslipidemia participants.
Publication bias was observed according to the results of Egger's test and demonstrated significant bias for LDL-cholesterol (P = 0.0253) and HDL-cholesterol (P = 0.0087) levels. Despite signs of publication bias using Egger's test, the Duval and Tweedie trim and fill method (75) revealed that the adjusted estimate remained significant (Supplementary Table 3 and Supplementary Figure 6). LDL-cholesterol (SMD = −0.13; 95% CI −0.21, −0.05) on anthocyanin supplementation still showed a significant reduction effect with zero heterogeneity (I2 = 0%) except for four outlier studies (23, 28, 31, 46). Excluding 10 outlier studies (29, 30, 45, 46, 51), the effect size of HDL-cholesterol decreased compared with the overall study results but showed low heterogeneity (I2= 31%) and a significantly increasing effect (SMD = 0.20; 95% CI 0.10, 0.30). There was no demonstrable publication bias for TG (P = 0.0697) and total cholesterol (P = 0.5943).
Among the 41 studies, 37 studies for anthocyanin supplements reported no serious adverse events leading to withdrawal. In four studies (19, 24, 40, 44), adverse events leading to withdrawal were reported in the anthocyanin supplement groups. In total, 6 studies (24, 37, 40, 47, 49, 50) reported mild adverse events, including dark stools, headache, insomnia, and diarrhea, and 17 studies (12–16, 20–22, 25–27, 33, 35, 39, 42, 43, 51) did not report any adverse events. The distribution of adverse events in the treatment groups and placebo is presented in Supplementary Table 4.
The present study aimed to investigate the effects of anthocyanin supplements on blood lipid levels by focusing on the results of randomized controlled trials. A total of 41 studies that enrolled 2,788 participants were included in the meta-analysis, which revealed that anthocyanin supplements had significantly improved blood lipid levels; they reduced TG and LDL-cholesterol levels and increased HDL-cholesterol levels.
A meta-analysis of 12–13 randomized controlled trials reported that anthocyanin supplements did not significantly improve blood lipid levels; however, in the subgroup analyses, decreased total cholesterol and LDL-cholesterol levels were observed when anthocyanin supplementation exceeded 300 mg/day (3). Another meta-analysis of 27 trials indicated that anthocyanin supplements were associated with decreased total cholesterol and LDL-cholesterol levels and marginally increased HDL-cholesterol levels in both healthy subjects and in those with cardiometabolic disease; however, no significant effects were observed on TG levels (4). Shah and Shah (5) reported that anthocyanin supplements significantly reduced TG and LDL-cholesterol levels and increased HDL-cholesterol levels in both the healthy and patient populations, with no significant effect on total cholesterol levels through a meta-analysis of 9–13 randomized controlled trials. The present meta-analysis included 41 studies (2,788 participants) with inconsiderable heterogeneity and publication bias. Therefore, our results are expected to provide more reliable and integrative insight into the effect of anthocyanin supplements on blood lipid levels in both healthy and patient populations.
Several studies have elucidated the mechanisms responsible for the beneficial effects of anthocyanins on blood lipid levels. Anthocyanin reportedly increases cholesterol efflux from macrophages, contributing to reverse cholesterol transport (18, 76, 77). Moreover, it decreases the mass and activity of plasma cholesteryl ester transfer protein, which is associated with increased efficiency of reverse cholesterol transport (18). Anthocyanins can activate AMP-activated protein kinase, which inhibits cholesterol and TG synthesis by HMG-CoA and acetyl-CoA carboxylase inhibition, respectively (78). Furthermore, anthocyanin dose-dependently reduces the micellar solubility of cholesterol and exhibits a significant reduction in cholesterol uptake in Caco-2 cells (79–81). It reportedly increased the excretion of fecal neutral and acidic sterols in experimental animals fed a cholesterol-enriched diet (82, 83).
In the present study, anthocyanin supplements did not have a significant effect on total cholesterol levels. This was consistent with the report of Shah and Shah (5). However, anthocyanin supplementation led to significant improvements in the lipid profiles (total cholesterol, TG, HDL-cholesterol, and LDL-cholesterol) of patients with dyslipidemia (84). In addition, Daneshzad (3) showed that anthocyanin supplementation had significant effects on total cholesterol for more than 300 mg/day for more than 12 weeks. However, our subgroup analysis did not indicate significant differences between subgroups according to cholesterol levels (≥ 200 mg/dL or <200 mg/dL) or dosage or duration (data not shown). Meanwhile, the total cholesterol level did not predict the risk of CVD and coronary heart disease compared with TG, HDL-cholesterol, and lipid ratios (85, 86). Further studies are needed to identify the protective effects of anthocyanins on increased morbidity or mortality using lipid ratios such as the total cholesterol:HDL-cholesterol and TG:HDL-cholesterol ratio.
CVD can be prevented by appropriately addressing the major risk factors such as dyslipidemia, hypertension, oxidative stress, and inflammatory stress (87). The present study revealed that anthocyanins could help reduce CVD risk by decreasing blood TG and LDL-cholesterol levels and increasing HDL-cholesterol levels. Several studies have reported that anthocyanins may have a significant blood-pressure-lowering activity (88). Furthermore, anthocyanins have potent antioxidant and antiinflammatory effects (89). In addition, anthocyanin supplementation improved vascular function, which is a strong predictor for CVD (90). Thus, increased intake of anthocyanin-rich foods may effectively reduce CVD risk.
Among the included studies, 21 presented intake levels of cyanidin. The average of total anthocyanin intake was 215.3 mg/day in those studies, and the cyanidin concentration was 116.5 mg/day (6.6~515.0 mg/day). The effect of cyanidin on blood lipids had a greater impact than the results of total anthocyanin. One recent study (51) failed to compare the effect of the two anthocyanin types (cyanidin-type vs. delphinidin-type) on blood lipid levels. Further study is needed to identify and clarify the mechanism of anthocyanin's structure on each bioactivity.
The strengths of the present study are the inclusion of all clinical trials that investigated the effects of anthocyanin on blood lipid levels. However, this study has some limitations. First, only articles in the English language were included in the meta-analysis, which raised concerns regarding the identification and selection of relevant studies. Second, significant between-study heterogeneity unexplained by differences in the methods of anthocyanin intervention, study design, and study population was observed.
In conclusion, we evaluated the effects of anthocyanin supplementation on blood lipid levels via a systematic review and meta-analysis of randomized controlled trials. Our results revealed that anthocyanin supplementation had a significant effect on TG, LDL-cholesterol, and HDL-cholesterol levels. Larger, well-designed clinical trials are needed to investigate the efficacy and safety of anthocyanin supplementation for the treatment of dyslipidemia.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
H-HJ and Y-ML: conceptualization, data curation, and writing—original draft preparation. Y-ML and I-GH: writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
This research was supported by the research program for Agricultural Science & Technology Development under the National Institute of Agricultural Science (NAS)-Rural Development Administration (RDA), South Korea, under grant numbers PJ01420101 and PJ01703102.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1207751/full#supplementary-material
CI, Confidence interval; CVD, Cardiovascular disease; HDL, High-density lipoprotein; MetS, Metabolic syndrome; MI, Myocardial Infraction; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RDA, Rural Development Administration; SD, Standard deviation; SMD, Standardized mean difference.
1. Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. (2005) 28:1769–78. doi: 10.2337/diacare.28.7.1769
2. Cornier M-A, Dabelea d, hernandez tl, lindstrom rc, steig aj, stob nr, et al. the metabolic Syndrome. Endocr Rev. (2008) 29:777–822. doi: 10.1210/er.2008-0024
3. Daneshzad E, Shab-Bidar S, Mohammadpour Z, Djafarian K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. (2019) 38:1153–65. doi: 10.1016/j.clnu.2018.06.979
4. Yang L, Ling W, Du Z, Chen Y, Li D, Deng S, et al. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. (2017) 8:684–93. doi: 10.3945/an.116.014852
5. Shah K, Shah P. Effect of anthocyanin supplementations on lipid profile and inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Cholesterol. (2018) 2018:93. doi: 10.1155/2018/8450793
6. Stintzing FC, Stintzing AS, Carle R, Frei B, Wrolstad RE. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J Agric Food Chem. (2002) 50:6172–81. doi: 10.1021/jf0204811
7. Jia Y, Hoang MH, Jun H-J, Lee JH, Lee S-J. Cyanidin, a natural flavonoid, is an agonistic ligand for liver × receptor alpha and beta and reduces cellular lipid accumulation in macrophages and hepatocytes. Bioorg Med Chem Lett. (2013) 23:4185–90. doi: 10.1016/j.bmcl.2013.05.030
8. Wang Z, Zhang M, Wang Z, Guo Z, Wang Z, Chen Q. Cyanidin-3-O-glucoside attenuates endothelial cell dysfunction by modulating Mir-204-5p/Sirt1-mediated inflammation and apoptosis. Biofactors. (2020) 46:803–12. doi: 10.1002/biof.1660
9. Li X, Shi Z, Zhu Y, Shen T, Wang H, Shui G, et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting pink1-mediated mitophagy in mice. Br J Pharmacol. (2020) 177:3591–607. doi: 10.1111/bph.15083
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
11. Murkovic M, Abuja PM, Bergmann AR, Zirngast A, Adam U, Winklhofer-Roob BM, et al. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: a randomized, double-blind, placebo-controlled study. Eur J Clin Nutr. (2004) 58:244–9.doi: 10.1038/sj.ejcn.1601773
12. Hansen AS, Marckmann P, Dragsted LO, Finne Nielsen IL, Nielsen SE, Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur J Clin Nutr. (2005) 59:449–55. doi: 10.1038/sj.ejcn.1602107
13. Zern TL, Wood RJ, Greene C, West KL, Liu Y, Aggarwal D, et al. Grape polyphenols exert a cardioprotective effect in pre-and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. (2005) 135:1911–7. doi: 10.1093/jn/135.8.1911
14. Cerda B, Soto C, Albaladejo MD, Martinez P, Sanchez-Gascon F, Tomas-Barberan F, et al. Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. (2006) 60:245–53. doi: 10.1038/sj.ejcn.1602309
15. Karlsen A, Retterstøl L, Laake P, Paur I, Kjølsrud-Bøhn S, Sandvik L, et al. Anthocyanins inhibit nuclear factor-κB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. (2007) 137:1951–4. doi: 10.1093/jn/137.8.1951
16. Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. (2008) 87:323–31. doi: 10.1093/ajcn/87.2.323
17. Curtis PJ, Kroon PA, Hollands WJ, Walls R, Jenkins G, Kay CD, et al. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J Nutr. (2009) 139:2266–71. doi: 10.3945/jn.109.113126
18. Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. (2009) 90:485–92. doi: 10.3945/ajcn.2009.27814
19. Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. (2010) 140:1582–7. doi: 10.3945/jn.110.124701
20. Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. (2010) 140:1764–8. doi: 10.3945/jn.110.125336
21. Basu A, Betts NM, Ortiz J, Simmons B, Wu M, Lyons TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutrition Research. (2011) 31:190–6. doi: 10.1016/j.nutres.2011.02.003
22. Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. (2011) 93:934–40. doi: 10.3945/ajcn.110.004242
23. Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, et al. Purified anthocyanin supplementation improves endothelial function via no-CGMP activation in hypercholesterolemic individuals. Clin Chem. (2011) 57:1524–33. doi: 10.1373/clinchem.2011.167361
24. Hassellund SS, Flaa A, Kjeldsen SE, Seljeflot I, Karlsen A, Erlund I, et al. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens. (2013) 27:100–6. doi: 10.1038/jhh.2012.4
25. Riso P, Klimis-Zacas D, Del Bo C, Martini D, Campolo J, Vendrame S, et al. Effect of a wild blueberry (vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. (2013) 52:949–61. doi: 10.1007/s00394-012-0402-9
26. Flammer AJ, Martin EA, Gössl M, Widmer RJ, Lennon RJ, Sexton JA, et al. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur J Nutr. (2013) 52:289–96. doi: 10.1007/s00394-012-0334-4
27. Wright OR, Netzel GA, Sakzewski AR. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: the quench trial. Can J Physiol Pharmacol. (2013) 91:480–8. doi: 10.1139/cjpp-2012-0349
28. Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. (2013) 23:843–9. doi: 10.1016/j.numecd.2012.06.005
29. Basu A, Betts NM, Nguyen A, Newman ED, Fu D, Lyons TJ. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J Nutr. (2014) 144:830–7. doi: 10.3945/jn.113.188169
30. Lynn A, Mathew S, Moore CT, Russell J, Robinson E, Soumpasi V, et al. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Hum Nutr. (2014) 69:122–7. doi: 10.1007/s11130-014-0409-x
31. Soltani R, Hakimi M, Asgary S, Ghanadian SM, Keshvari M, Sarrafzadegan N. Evaluation of the effects of vaccinium arctostaphylos L. Fruit extract on serum lipids and hs-crp levels and oxidative stress in adult patients with hyperlipidemia: a randomized, double-blind, placebo-controlled clinical trial evid based complement. Alternat Med. (2014) 2014:217451. doi: 10.1155/2014/217451
32. Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. (2015) 145:742–8. doi: 10.3945/jn.114.205674
33. Novotny JA, Baer DJ, Khoo C, Gebauer SK, Charron CS. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating c-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr. (2015) 145:1185–93. doi: 10.3945/jn.114.203190
34. Soltani R, Gorji A, Asgary S, Sarrafzadegan N, Siavash M. Evaluation of the effects of Cornus mas L. fruit extract on glycemic control and insulin level in type 2 diabetic adult patients: a randomized double-blind placebo-controlled clinical trial. Evid Based Complement Alternat Med. (2015) 2015:740954. doi: 10.1155/2015/740954
35. Stull AJ, Cash KC, Champagne CM, Gupta AK, Boston R, Beyl RA, et al. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. (2015) 7:4107–23. doi: 10.3390/nu7064107
36. Zhang PW, Chen FX Li D, Ling WH, Guo HH. A consort-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine. (2015) 94:e758. doi: 10.1097/MD.0000000000000758
37. Lee M, Sorn SR, Park Y, Park HK. Anthocyanin rich-black soybean testa improved visceral fat and plasma lipid profiles in overweight/obese Korean adults: a randomized controlled trial. J Med Food. (2016) 19:995–1003. doi: 10.1089/jmf.2016.3762
38. Zhang X, Zhu Y, Song F, Yao Y, Ya F, Li D, et al. Effects of purified anthocyanin supplementation on platelet chemokines in hypocholesterolemic individuals: a randomized controlled trial. Nutr Metab. (2016) 13:86. doi: 10.1186/s12986-016-0146-2
39. Xie L, Vance T, Kim B, Lee SG, Caceres C, Wang Y, et al. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: a randomized controlled trial. Nutr Res. (2017) 37:67–77. doi: 10.1016/j.nutres.2016.12.007
40. Yang L, Ling W, Yang Y, Chen Y, Tian Z, Du Z, et al. Role of purified anthocyanins in improving cardiometabolic risk factors in chinese men and women with prediabetes or early untreated diabetes-a randomized controlled trial. Nutrients. (2017) 9:1104. doi: 10.3390/nu9101104
41. Hollands WJ, Armah CN, Doleman JF, Perez-Moral N, Winterbone MS, Kroon PA. 4-Week consumption of anthocyanin-rich blood orange juice does not affect ldl-cholesterol or other biomarkers of cvd risk and glycaemia compared with standard orange juice: a randomised controlled trial. Br J Nutr. (2018) 119:415–21. doi: 10.1017/S0007114517003865
42. Kim H, Simbo SY, Fang C, McAlister L, Roque A, Banerjee N. et al. Acai (euterpe oleracea mart) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled. Clin Trial Food Funct. (2018) 9:3097–103. doi: 10.1039/C8FO00595H
43. Bakuradze T, Tausend A, Galan J, Groh IAM, Berry D, Tur JA, et al. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic Res. (2019) 53:1045–55. doi: 10.1080/10715762.2019.1618851
44. Curtis PJ, van der Velpen V, Berends L, Jennings A, Feelisch M, Umpleby AM, et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am J Clin Nutr. (2019) 109:1535–45. doi: 10.1093/ajcn/nqy380
45. Guo Y, Zhang P, Liu Y, Zha L, Ling W, Guo H, et al. Dose-response evaluation of purified anthocyanins on inflammatory and oxidative biomarkers and metabolic risk factors in healthy young adults: a randomized controlled trial. Nutrition. (2020) 74:110745. doi: 10.1016/j.nut.2020.110745
46. Stote KS, Wilson MM, Hallenbeck D, Thomas K, Rourke JM, Sweeney MI, et al. Effect of blueberry consumption on cardiometabolic health parameters in men with type 2 diabetes: an 8-week, double-blind, randomized, placebo-controlled trial. Curr Dev Nutr. (2020) 4:nzaa030. doi: 10.1093/cdn/nzaa030
47. Chan SW, Chu TTW, Choi SW, Benzie IFF, Tomlinson B. Impact of short-term bilberry supplementation on glycemic control, cardiovascular disease risk factors, and antioxidant status in chinese patients with type 2 diabetes. Phytother Res. (2021) 35:3236–45. doi: 10.1002/ptr.7038
48. Sekikawa T, Kizawa Y, Takeoka A, Sakiyama T, Li Y, Yamada T. The effect of consuming an anthocyanin-containing supplement derived from bilberry (vaccinium myrtillus) on eye function: a randomized, double-blind, placebo-controlled parallel study. Funct Foods Health Dis. (2021) 11:782. doi: 10.31989/ffhd.v11i3.782
49. Xu Z, Xie J, Zhang H, Pang J, Li Q, Wang X, et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia-a randomized controlled trial. Eur J Clin Nutr. (2021) 75:345–54. doi: 10.1038/s41430-020-0609-4
50. Yang L, Qiu Y, Ling W, Liu Z, Yang L, Wang C, et al. Anthocyanins regulate serum adipsin and visfatin in patients with prediabetes or newly diagnosed diabetes: a randomized controlled trial. Eur J Nutr. (2021) 60:1935–44. doi: 10.1007/s00394-020-02379-x
51. Aboufarrag H, Hollands WJ, Percival J, Philo M, Savva GM, Kroon PA. No effect of isolated anthocyanins from bilberry fruit and black rice on ldl cholesterol or other biomarkers of cardiovascular disease in adults with elevated cholesterol: a randomized, placebo-controlled, cross-over trial. Mol Nutr Food Res. (2022) 66:2101157. doi: 10.1002/mnfr.202101157
52. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
53. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
54. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. London: John Wiley & Sons (2019).
55. Fukuta H, Goto T, Wakami K, Ohte N. Effects of drug and exercise intervention on functional capacity and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Eur J Prev Cardiol. (2016) 23:78–85. doi: 10.1177/2047487314564729
56. de Mello VD, Lankinen MA, Lindström J, Puupponen-Pimiä R, Laaksonen DE, Pihlajamäki J, et al. Fasting serum hippuric acid is elevated after bilberry (vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol Nutr Food Res. (2017) 61:1700019. doi: 10.1002/mnfr.201700019
57. Davinelli S, Bertoglio JC, Zarrelli A, Pina R, Scapagnini G. A randomized clinical trial evaluating the efficacy of an anthocyanin–maqui berry extract (Delphinol®) on oxidative stress biomarkers. J Am Coll Nutr. (2015) 34:28–33. doi: 10.1080/07315724.2015.1080108
58. Emamat H, Zahedmehr A, Asadian S, Nasrollahzadeh J. The effect of barberry (berberis integerrima) on lipid profile and systemic inflammation in subjects with cardiovascular risk factors: a randomized controlled trial. BMC Compl Med Therap. (2022) 22:59. doi: 10.1186/s12906-022-03539-8
59. Jung AJ, Sharma A, Lee S-H, Lee S-J, Kim J-H, Lee H-J. Efficacy of black rice extract on obesity in obese postmenopausal women: a 12-week randomized, double-blind, placebo-controlled preliminary clinical trial. Menopause. (2021) 28:1391–9. doi: 10.1097/GME.0000000000001862
60. Vidlar A, Vostalova J, Ulrichova J, Student V, Stejskal D, Reichenbach R, et al. The effectiveness of dried cranberries ( Vaccinium Macrocarpon) in men with lower urinary tract symptoms. Br J Nutr. (2010) 104:1181–9. doi: 10.1017/S0007114510002059
61. Kianbakht S, Abasi B, Hashem Dabaghian F. Improved lipid profile in hyperlipidemic patients taking vaccinium arctostaphylos fruit hydroalcoholic extract: a randomized double-blind placebo-controlled clinical trial. Phytother Res. (2014) 28:432–6. doi: 10.1002/ptr.5011
62. Gamel TH, Abdel-Aal EM, Tucker AJ, Pare SM, Faughnan K, O'Brien CD, et al. Consumption of whole purple and regular wheat modestly improves metabolic markers in adults with elevated high-sensitivity c-reactive protein: a randomised, single-blind parallel-arm study. Br J Nutr. (2020) 124:1179–89. doi: 10.1017/S0007114520002275
63. Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. (2006) 45:113–22. doi: 10.1007/s00394-005-0572-9
64. Edirisinghe I, Banaszewski K, Cappozzo J, Sandhya K, Ellis CL, Tadapaneni R, et al. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. (2011) 106:913–22. doi: 10.1017/S0007114511001176
65. Cook MD, Myers SD, Gault ML, Edwards VC, Willems MET. Dose effects of new zealand blackcurrant on substrate oxidation and physiological responses during prolonged cycling. Eur J Appl Physiol. (2017) 117:1207–16. doi: 10.1007/s00421-017-3607-z
66. Jokioja J, Linderborg KM, Kortesniemi M, Nuora A, Heinonen J, Sainio T, et al. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. (2020) 310:125797. doi: 10.1016/j.foodchem.2019.125797
67. Herrera-Arellano A, Flores-Romero S, Chavez-Soto M, Tortoriello J. Effectiveness and tolerability of a standardized extract from hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine. (2004) 11:375–82. doi: 10.1016/j.phymed.2004.04.001
68. Dallas C, Gerbi A, Tenca G, Juchaux F, Bernard F-X. Lipolytic Effect of a polyphenolic citrus dry extract of red orange, grapefruit, orange (Sinetrol) in human body fat adipocytes. Mechanism of action by inhibition of camp-phosphodiesterase (PDE). Phytomedicine. (2008) 15:783–92. doi: 10.1016/j.phymed.2008.05.006
69. de Liz S, Cardoso AL, Copetti CLK, de Fragas Hinnig P, Vieira FGK, da Silva EL. et al. Açaí (euterpe oleracea mart) and juçara (euterpe edulis mart) juices improved hdl-c levels and antioxidant defense of healthy adults in a 4-week randomized cross-over study. Clin Nutr. (2020) 39:3629–36. doi: 10.1016/j.clnu.2020.04.007
70. Naruszewicz M, Łaniewska I, Millo B, Dłuzniewski M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (Mi). Atherosclerosis. (2007) 194:e179–e84. doi: 10.1016/j.atherosclerosis.2006.12.032
71. Gurrola-Díaz CM, García-López PM, Sánchez-Enríquez S, Troyo-Sanromán R, Andrade-González I, Gómez-Leyva J. Effects of hibiscus sabdariffa extract powder and preventive treatment (Diet) on the lipid profiles of patients with metabolic syndrome (Mesy). Phytomedicine. (2010) 17:500–5. doi: 10.1016/j.phymed.2009.10.014
72. Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytotherapy Res. (2014) 28:193–9. doi: 10.1002/ptr.4977
73. Cardoso AL, Teixeira LL, Hassimotto NMA, Baptista SL, Copetti CLK, Rieger DK, et al. Kinetic profile of urine metabolites after acute intake of a phenolic compounds-rich juice of Juçara (euterpe edulis mart) and antioxidant capacity in serum and erythrocytes: a human study. Int J Mol Sci. (2023) 24:9555. doi: 10.3390/ijms24119555
74. Hunt JE, Coelho MO, Buxton S, Butcher R, Foran D, Rowland D, et al. Consumption of New Zealand blackcurrant extract improves recovery from exercise-induced muscle damage in non-resistance trained men and women: a double-blind randomised trial. Nutrients. (2021) 13:2875. doi: 10.3390/nu13082875
75. Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
76. Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. (2012) 111:967–81. doi: 10.1161/CIRCRESAHA.112.266502
77. Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor Γ-Liver × receptor α-Abca1 pathway. J Biol Chem. (2005) 280:36792–801. doi: 10.1074/jbc.M505047200
78. Wallace TC, Slavin M, Frankenfeld CL. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients. (2016) 8:32. doi: 10.3390/nu8010032
79. Yao S-L, Xu Y, Zhang Y-Y, Lu Y-H. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. (2013) 4:1602–8. doi: 10.1039/c3fo60196j
80. Thilavech T, Adisakwattana S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement Altern Med. (2019) 19:1–10. doi: 10.1186/s12906-019-2664-8
81. Chamnansilpa N, Aksornchu P, Adisakwattana S, Thilavech T, Mäkynen K, Dahlan W, et al. Anthocyanin-rich fraction from thai berries interferes with the key steps of lipid digestion and cholesterol absorption. Heliyon. (2020) 6:e05408. doi: 10.1016/j.heliyon.2020.e05408
82. Liang Y, Chen J, Zuo Y, Ma KY, Jiang Y, Huang Y, et al. Blueberry anthocyanins at doses of 05 and 1% lowered plasma cholesterol by increasing fecal excretion of acidic and neutral sterols in hamsters fed a cholesterol-enriched diet. Eur J Nutr. (2013) 52:869–75. doi: 10.1007/s00394-012-0393-6
83. Wang L, Zhu H, Zhao Y, Jiao R, Lei L, Chen J, et al. Cranberry anthocyanin as an herbal medicine lowers plasma cholesterol by increasing excretion of fecal sterols. Phytomedicine. (2018) 38:98–106. doi: 10.1016/j.phymed.2017.11.008
84. Liu C, Sun J, Lu Y, Bo Y. Effects of anthocyanin on serum lipids in dyslipidemia patients: a systematic review and meta-analysis. PLoS ONE. (2016) 11:e0162089. doi: 10.1371/journal.pone.0162089
85. Collaboration APCS. A comparison of lipid variables as predictors of cardiovascular disease in the asia pacific region. Ann Epidemiol. (2005) 15:405–13. doi: 10.1016/j.annepidem.2005.01.005
86. Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. (2007) 298:776–85. doi: 10.1001/jama.298.7.776
87. WHO. Cardiovascular Diseases (Cvds). (2016). Available online at: http://www.who.int/mediacentre/factsheets/fs317/en (accessed April 1, 2021).
88. Vendrame S, Klimis-Zacas D. Potential factors influencing the effects of anthocyanins on blood pressure regulation in humans: a review. Nutrients. (2019) 11:1431. doi: 10.3390/nu11061431
89. Miguel MG. Anthocyanins: antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. (2011) 5:7–15. doi: 10.3390/molecules15129252
Keywords: metabolic syndrome, dyslipidemia, food supplementation, triglyceride, HDL cholesterol
Citation: Jang H-H, Hwang I-G and Lee Y-M (2023) Effects of anthocyanin supplementation on blood lipid levels: a systematic review and meta-analysis. Front. Nutr. 10:1207751. doi: 10.3389/fnut.2023.1207751
Received: 18 April 2023; Accepted: 25 July 2023;
Published: 15 August 2023.
Edited by:
Humaira Jamshed, Habib University, PakistanReviewed by:
Jasmina Debeljak Martacic, University of Belgrade, SerbiaCopyright © 2023 Jang, Hwang and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young-Min Lee, eW1sZWVAZ2ludWUuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.