- 1Laboratory of Nutritional Sciences, IRCCS Orthopedic Institute Galeazzi, Milan, Italy
- 2Orthopaedic Research Institute, Bournemouth University, Bournemouth, United Kingdom
- 3University Hospitals Dorset, NHS Foundation Trust, Poole, United Kingdom
- 4Laboratory of Experimental Biochemistry and Molecular Biology, IRCCS Orthopedic Institute Galeazzi, Milan, Italy
- 5Department of Athletics, Strength and Conditioning, Poznań University of Physical Education, Poznań, Poland
The correct identification of malnourished patients in the context of hip, knee, or spine surgery research would enhance the quality of analytical studies investigating the prediction potential of preoperative nutritional disorders on postoperative recovery. However, accurate malnutrition screening and diagnostic assessment rely on parameters that were not routinely collected in routine practice until a few years ago. The authors of this article present substitute literature-based equations that can be built up using historical routinely collected data to classify patients that had been at risk of malnutrition or malnourished. For what concerns the risk screening, several methods are available to identify patients at risk of over- or undernutrition, encompassing the BWd (body weight difference from the ideal weight), GNRI (geriatric nutritional risk index), INA (instant nutritional assessment), LxA (combination of lymphocyte count and albumin), PMA (protein malnutrition with acute inflammation), PMAC (protein malnutrition with acute and chronic inflammation), IDM (iron deficit malnutrition), and VBD (vitamin B deficit malnutrition). Conversely, the GLIM (global leadership initiative on malnutrition) criteria can be used to assess malnutrition and diagnose subclasses of undernutrition. Rational use of these tools can facilitate the conduction of efficient prospective studies in the future, as well as bespoke retrospective cohort studies and database research.
1. We need tools to identify malnutrition in retrospective research studies
Malnutrition is a polyhedric condition whose etiology lies in the failure of the individual to meet the nutritional requirements, reduced or excessive food intake, or an unspecified alteration of the nutritional status from ailments or medications. As many as one in two patients undergoing joint or spine surgery is at risk of malnutrition, is malnourished, or will be after surgery (1–3). Malnutrition causes profound changes to the host’s anatome and physiome, undermining daily activities and resilience to cope with distressing events (4). Major orthopedic surgery initiates a surgical stress response, exposing malnourished patients to a greater risk of complications and slow/impaired recovery (5). The correction of malnutrition is proposed to be one of the key elements of prehabilitation in orthopedic surgery, with dietary interventions playing an important role in optimizing the nutritional status, preventing adverse events, and enhancing recovery (6). Nutrition screening relies on quick and validated screening tool, such as the malnutrition screening tool (MST) (7), nutritional risk screening-2002 (NRS-2002) (8), or the malnutrition universal screening tool (MUST) (9), which all investigate the risk of being malnourished according to patients’ replies. The diagnosis of malnutrition is achieved using the framework formulated by the global leadership initiative on malnutrition (GLIM) (10). This assessment, diagnosis, and grading scheme is performed in patients at risk of malnutrition, considering the presence of non-volitional weight loss, hypophagia, abnormal body composition, muscular weakness, and the disease state. The finding of these signs requires trained personnel and devices for body analysis and testing, which were not routinely performed until recently. Consequently, there exists a vast amount of historical data that lacks the necessary information to explore the incidence and role of malnutrition in orthopedic surgery research.
In this article, we present literature-based indicators of nutritional disorders (undernutrition, overnutrition, and micronutrient abnormalities) that can be calculated from variables commonly part of most orthopedic centers’ clinical practice. These equations can be used prospectively and in the context of bespoke retrospective cohort studies and database research on joint arthroplasty and spine surgery. Nutrition-related conditions like cachexia, sarcopenia, and frailty are not debated in this perspective.
2. Equations to calculate the risk of malnutrition from routinely-collected data
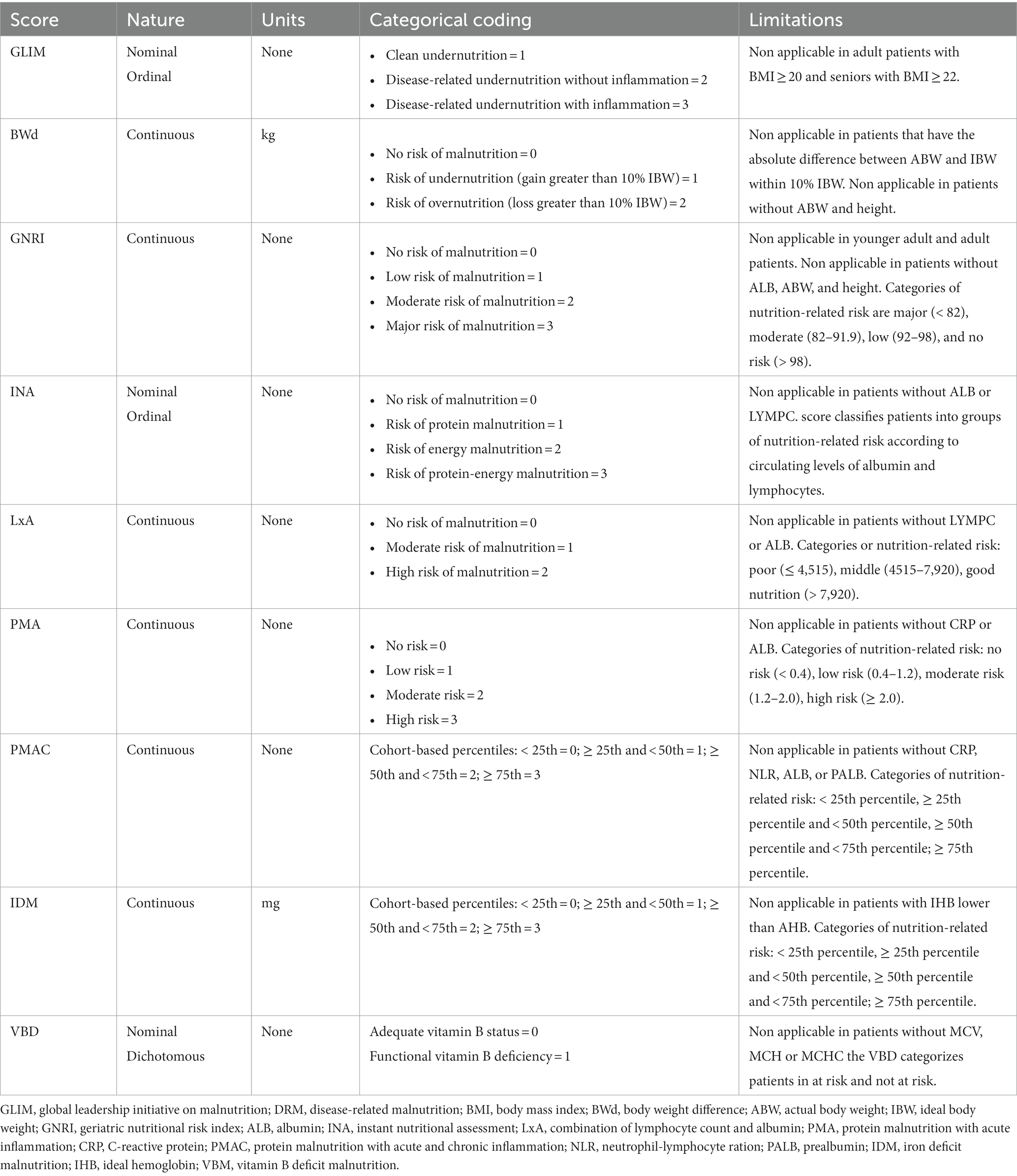
The risk of malnutrition can be inferred through equations shown in Table 1. First, we propose the calculation of the body weight difference (BWd) (11) from the ideal body weight (IBW), which informs the clinician how much the actual body weight (ABW) of the patient deviates from the reference value. If the difference is clinically relevant (usually identified as greater than 10%) then the subject may be considered at risk of undernutrition or overnutrition although it is not possible to know in retrospect whether the difference in weight is due to a non-volitional loss or gain. Second is the geriatric nutritional risk index (GNRI) (12), which combines one of the most used nutritional analytes (albumin) with a consideration of the patient’s weight similar to the BWd method. Third is the instant nutritional assessment (INA) (13), which was formulated in the second half of the nineties but is still relevant since it combines albumin (ALB) with total lymphocytic count (LYMPC), both being well-known indicators of nutritional status. Similarly, the numerical product of the two analytes (LxA) is the fourth nutrition-related score that we suggest for patient grouping (14). The fifth score defines patients at risk of protein malnutrition with acute inflammation (PMA) based on low ALB and high CRP (15) and, similarly, there is the sixth score that helps identify patients at risk of malnutrition based on elevated markers of acute/chronic inflammation and low proteins (PMAC). This latter has been adapted by the authors from the prognostic inflammatory and nutritional index (16), which contrariwise included the α1-acid glycoprotein as a second acute-phase reactant other than the CRP. The last two calculations that are proposed should theoretically signify the presence of certain specific nutritional deficits, being the deficit of iron (IDM) (17) or B vitamins (VBD) (18).
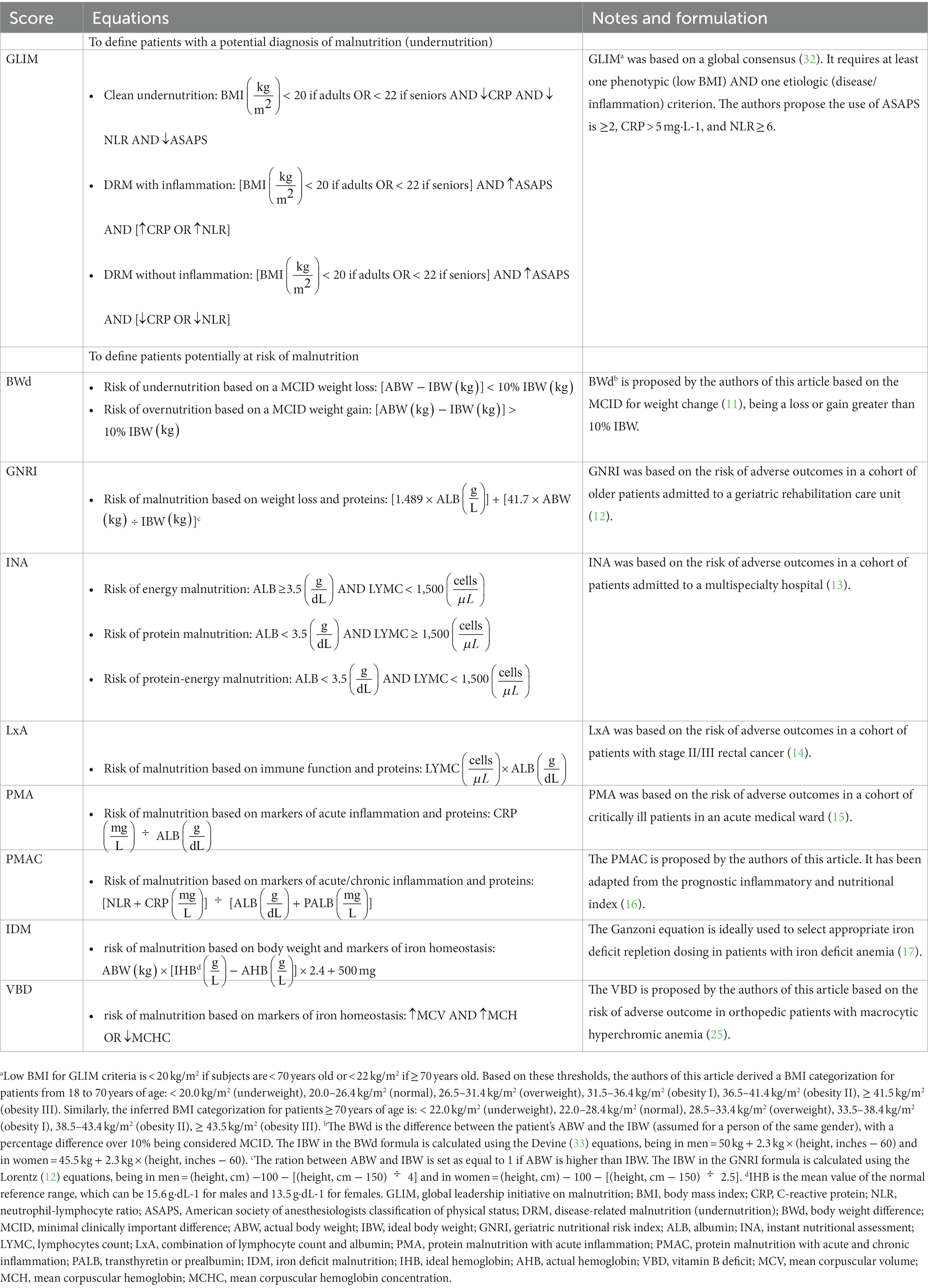
Table 1. Calculations built up with routinely-collected parameters to identify malnutrition in orthopedic surgery patients.
3. The GLIM equation from routinely-collected data to diagnose malnutrition
Even if the information necessary to apply the canonical GLIM diagnostic scheme is not available among the historical data, it is possible to infer a probable diagnosis of malnutrition using the substitute literature-based equation shown in Table 1. GLIM versatility has allowed its application in various clinical settings and study designs, and it has already been used in orthopedic surgery research (1, 2, 19) as an alternative framework to the classical diagnostic process (20). The GLIM equation characterizes patients according to different combinations of phenotypic (percentage of unintentional weight loss since last evaluation, low body mass index, reduced muscle mass) and etiological (reduced food intake or assimilation, inflammation, disease burden) criteria. In orthopedic surgery research, the phenotypic criterion accessible from clinical practice is often the body mass index (BMI), while diverse etiological criteria can be selected among different markers and indexes. We propose the use of the American society of anesthesiologists classification of physical status (ASAPS), C-reactive protein (CRP), and neutrophil-lymphocyte ratio (NLR) to discriminate clean malnutrition, disease-related malnutrition (DRM) with inflammation, and DRM without inflammation. The ASAPS is an indicator of disease burden and follows the next categorical coding: healthy = 1, mild disease = 2, severe disease = 3, threat to life = 4, moribund = 5, brain-dead = 6. The use of GLIM requires the sample study to be classified according to precise age ranges (< 40 years = younger adults; 40–70 years = adults; ≥ 70 years = older adults) and BMI categories that identify underweight as different than usual (< 20.0 kg/m2 if age < 70 years or < 22.0 kg/m2 if age ≥ 70 years). Although the BMI alone has not been offered in this article as an indicator of the nutritional status for its already acclaimed practice, it is important to highlight that its use according to the traditional labeling is archaic, especially in old patients whose height is profoundly changed and the aging process parallels with a shift in the health risk given by body compositional changes (21). Therefore, if it really were to be used, it would be worth correcting the Quetelet index (22) in agreement with the more recent knowledge on the protective role of fat. Consequently, in younger adults and adults, BMI < 18.5 kg/m2 is underweight, 18.5–24.9 kg/m2 is normal, 25.0–29.9 kg/m2 is overweight, 30.0–34.9 kg/m2 is obesity I, 35.0–39.9 kg/m2 is obesity II, and ≥ 40.0 kg/m2 is obesity III. In seniors, it might be appropriate to consider that BMI < 25.0 kg/m2 is underweight, 25.0–35.0 kg/m2 is normal, 35.1–40.0 kg/m2 is overweight, 40.1–45.0 kg/m2 is obesity I, 45.1–50.0 kg/m2 is obesity II, and ≥ 50.1 kg/m2 is obesity III (23). Concerning the CRP and NLR, their circulating levels are considered representative of acute and chronic inflammation, respectively, with the latter more accurately being able to differentiate a state of severe (≥ 8), moderate (6–7.99), mild (4–5.99), low (2–3.99), or normal (< 2) chronic inflammatory status.
4. What is the value of using these scores?
The incredible amount of historical data that lacks the information necessary for a correct diagnosis can nonetheless count on a set of non-diagnostic indicators each with its own drawbacks (Table 2) but having in common the fact that they couple at least two surrogate variables of nutritional interest. It is not meaningless to use a single laboratory analyte as an indicator of nutritional status, since they have long been used by themselves (especially albumin and hemoglobin) (21), and still provide remarkable findings in today’s orthopedic surgery research (24, 25). However, it is necessary to distinguish that risk screening and diagnosis are two distinct evaluations but part of the same two-step process. Therefore, the authors recommend the selection of at least one screening tool among those presented in this article together with the semi-gold standard GLIM when exploring the prevalence of malnutrition or its risk or the prediction potential on orthopedic surgery outcome in retrospective analytical studies. The concurrent use of machine learning techniques is also advised to further explore the actual weight of each anthropometric, biochemical, and disease-related variable used.
Concerning current clinical practice, the integration of valid and short tools, such as the four questions of the nutritional risk screening-2002 (NRS-2002-4Q) (26), the mini nutritional assessment-short form (MNA-SF) (27), or the patient generated-subjective global assessment short form (PG-SGA SF) (28) may be a valuable intermediate step to screen malnutrition in orthopedic surgery pathways. However, the much more valuable diagnostic frameworks that combine dietetic, anthropometric, biochemical, and functional variables like the GLIM (29) ought to be systematically incorporated as soon as feasible because of its cost-effectiveness (6, 18, 30).
5. Final considerations
Different criteria based on routinely collected data can be used to determine the prevalence of patients at risk of being malnourished or those suffering from malnutrition in the context of hip, knee, or spine surgery outcomes. When analyzing historical data, patients at risk of undernutrition can be identified using several equations, including the BWd based on the MCID weight loss, the GNRI, different combinations of laboratory parameters (INA, LxA, PMA, PMAC), and the IDM or VBD that determine the risk that the patient may suffer from an iron deficit or macrocytic hyperchromic anemia, respectively. Equally, the GLIM equation ought to be considered the reference calculation to diagnose undernutrition, while for the identification of overnutrition, we argue that the BWd calculation based on the MCID weight gain can be used. Our proposed literature-based equations come with flaws, being mere substitutes for the definition of the risk of being malnourished or the diagnosis of malnutrition. The lack of information regarding unintentional weight loss, muscle mass, food intake, and absorption might potentially misjudge the real prevalence of nutritional disorders. However, reported in tandem (31), a rational and cautious use of these tools will shed the light on the role of an unbalanced nutritional status in orthopedic patients and facilitate the conduction of prospective studies, bespoke retrospective cohort studies, and database research probing risk factors or prediction models.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MB formulated the first draft. TW and GL revised and integrated the manuscript. All authors agreed to be accountable for the content of the work and submitted the final version to this manuscript.
Funding
This work is part of the project “Ricerca Corrente” of the Italian Ministry of Health, which funded the article processing charge.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kobayashi, H, Inoue, T, Ogawa, M, Abe, T, Tanaka, T, and Kakiuchi, M. Malnutrition diagnosed by the global leadership initiative on malnutrition criteria as a predictor of gait ability in patients with hip fracture. Injury. (2022) 53:3394–400. doi: 10.1016/j.injury.2022.08.004
2. Johnson, KG, Alsoof, D, McDonald, CL, Berreta, RS, Cohen, EM, and Daniels, AH. Malnutrition, body mass index, and associated risk of complications after posterior lumbar spine fusion: a 3:1 matched cohort analysis. World Neurosurg. (2022) 163:e89–97. doi: 10.1016/j.wneu.2022.03.065
3. Briguglio, M, Crespi, T, Langella, F, Riso, P, Porrini, M, Scaramuzzo, L, et al. Perioperative anesthesia and acute smell alterations in spine surgery: a "sniffing impairment" influencing refeeding? Front Surg. (2022) 9:785676. doi: 10.3389/fsurg.2022.785676
4. Briguglio, M. The burdens of orthopedic patients and the value of the HEPAS approach (healthy eating, physical activity, and sleep hygiene). Front Med. (2021) 8:650947. doi: 10.3389/fmed.2021.650947
5. Briguglio, M. Nutritional orthopedics and space nutrition as two sides of the same coin: a scoping review. Nutrients. (2021) 13:483. doi: 10.3390/nu13020483
6. Briguglio, M, and Wainwright, TW. Nutritional and physical Prehabilitation in elective orthopedic surgery: rationale and proposal for implementation. Ther Clin Risk Manag. (2022) 18:21–30. doi: 10.2147/TCRM.S341953
7. Ferguson, M, Capra, S, Bauer, J, and Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. (1999) 15:458–64. doi: 10.1016/S0899-9007(99)00084-2
8. Kondrup, J, Allison, SP, Elia, M, Vellas, B, and Plauth, M. Educational and clinical practice committee ErSoPaENE. ESPEN guidelines for nutrition screening 2002. Clin Nutr. (2003) 22:415–21. doi: 10.1016/S0261-5614(03)00098-0
9. Stratton, RJ, Hackston, A, Longmore, D, Dixon, R, Price, S, Stroud, M, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br J Nutr. (2004) 92:799–808. doi: 10.1079/BJN20041258
10. Cederholm, T, Jensen, GL, Correia, MITD, Gonzalez, MC, Fukushima, R, Higashiguchi, T, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
11. Buzby, GP, Williford, WO, Peterson, OL, Crosby, LO, Page, CP, Reinhardt, GF, et al. A randomized clinical trial of total parenteral nutrition in malnourished surgical patients: the rationale and impact of previous clinical trials and pilot study on protocol design. Am J Clin Nutr. (1988) 47:357–65. doi: 10.1093/ajcn/47.2.357
12. Bouillanne, O, Morineau, G, Dupont, C, Coulombel, I, Vincent, JP, Nicolis, I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
13. Seltzer, MH, Bastidas, JA, Cooper, DM, Engler, P, Slocum, B, and Fletcher, HS. Instant nutritional assessment. JPEN J Parenter Enteral Nutr. (1979) 3:157–9. doi: 10.1177/014860717900300309
14. Yamamoto, T, Kawada, K, Hida, K, Matsusue, R, Itatani, Y, Mizuno, R, et al. Combination of lymphocyte count and albumin concentration as a new prognostic biomarker for rectal cancer. Sci Rep. (2021) 11:5027. doi: 10.1038/s41598-021-84475-4
15. Fairclough, E, Cairns, E, Hamilton, J, and Kelly, C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond). (2009) 9:30–3. doi: 10.7861/clinmedicine.9-1-30
16. Ingenbleek, Y, and Carpentier, YA. A prognostic inflammatory and nutritional index scoring critically ill patients. Int J Vitam Nutr Res. (1985) 55:91–101.
17. Ganzoni, AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr. (1970) 100:301–3.
18. Briguglio, M, Hrelia, S, Malaguti, M, de Vecchi, E, Lombardi, G, Banfi, G, et al. Oral supplementation with Sucrosomial ferric pyrophosphate plus L-ascorbic acid to ameliorate the martial status: a randomized controlled trial. Nutrients. (2020) 12:386. doi: 10.3390/nu12020386
19. Wu, H, Li, S, Lin, Y, Wang, J, Chekhonin, VP, Peltzer, K, et al. Association between malnutrition and leucopenia in patients with osteosarcoma. Front Nutr. (2022) 9:899501. doi: 10.3389/fnut.2022.899501
20. Duerksen, DR, Laporte, M, and Jeejeebhoy, K. Evaluation of nutrition status using the subjective global assessment: malnutrition, Cachexia, and sarcopenia. Nutr Clin Pract. (2021) 36:942–56. doi: 10.1002/ncp.10613
21. Briguglio, M, Gianola, S, Aguirre, MFI, Sirtori, P, Perazzo, P, Pennestri, F, et al. Nutritional support for enhanced recovery programs in orthopedics: future perspectives for implementing clinical practice. Nutr Clin Metab. (2019) 33:190–8. doi: 10.1016/j.nupar.2019.04.002
22. Nuttall, FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today. (2015) 50:117–28. doi: 10.1097/NT.0000000000000092
23. Kıskaç, M, Soysal, P, Smith, L, Capar, E, and Zorlu, M. What is the optimal body mass index range for older adults? Ann Geriatr Med Res. (2022) 26:49–57. doi: 10.4235/agmr.22.0012
24. Heimroth, J, Neufeld, EV, Sodhi, N, Walden, T, Willinger, ML, and Boraiah, S. Relationship between preoperative nutritional status and predicting short-term complications following revision Total hip arthroplasty. J Arthroplast. (2023) 38:1326–9. doi: 10.1016/j.arth.2023.02.077
25. Briguglio, M, Perazzo, P, Langella, F, Crespi, T, de Vecchi, E, Riso, P, et al. Prediction of long-term recovery from disability using hemoglobin-based models: results from a cohort of 1,392 patients undergoing spine surgery. Front Surg. (2022) 9:850342. doi: 10.3389/fsurg.2022.850342
26. Tangvik, RJ, Tell, GS, Eisman, JA, Guttormsen, AB, Henriksen, A, Nilsen, RM, et al. The nutritional strategy: four questions predict morbidity, mortality and health care costs. Clin Nutr. (2014) 33:634–41. doi: 10.1016/j.clnu.2013.09.008
27. Rubenstein, LZ, Harker, JO, Salvà, A, Guigoz, Y, and Vellas, B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. (2001) 56:M366–72. doi: 10.1093/gerona/56.6.M366
28. De Groot, LM, Lee, G, Ackerie, A, and van der Meij, BS. Malnutrition screening and assessment in the Cancer care ambulatory setting: mortality predictability and validity of the patient-generated subjective global assessment short form (PG-SGA SF) and the GLIM criteria. Nutrients. (2020) 12:2287. doi: 10.3390/nu12082287
29. Correia, MITD, Tappenden, KA, Malone, A, Prado, CM, Evans, DC, Sauer, AC, et al. Utilization and validation of the global leadership initiative on malnutrition (GLIM): a scoping review. Clin Nutr. (2022) 41:687–97. doi: 10.1016/j.clnu.2022.01.018
30. Briguglio, M, Gianturco, L, Stella, D, Colombo, C, Bonadies, M, Sala, O, et al. Correction of hypovitaminosis D improved global longitudinal strain earlier than left ventricular ejection fraction in cardiovascular older adults after orthopaedic surgery. J Geriatr Cardiol. (2018) 15:519–22. doi: 10.11909/j.issn.1671-5411.2018.08.005
31. Henriksen, C, Paur, I, Pedersen, A, Kværner, AS, Ræder, H, Henriksen, HB, et al. Agreement between GLIM and PG-SGA for diagnosis of malnutrition depends on the screening tool used in GLIM. Clin Nutr. (2022) 41:329–36. doi: 10.1016/j.clnu.2021.12.024
32. de van der Schueren, MAE, Keller, H, Consortium, GLIM, Cederholm, T, Barazzoni, R, Compher, C, et al. Global leadership initiative on malnutrition (GLIM): guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin Nutr. (2020) 39:2872–80. doi: 10.1016/j.clnu.2019.12.022
Keywords: orthopaedics, surgery, malnutrition, prehabilitation, enhanced recovery after surgery, health status, patient outcomes assessment, blood chemical analyses
Citation: Briguglio M, Wainwright TW and Lombardi G (2023) Definition of malnutrition from routinely-collected data for orthopedic surgery research: the global leadership initiative on malnutrition (GLIM) tool and others. Front. Nutr. 10:1200049. doi: 10.3389/fnut.2023.1200049
Edited by:
Carlo Pedrolli, Azienda Provinciale per i Servizi Sanitari (APSS), ItalyReviewed by:
Wei Chen, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2023 Briguglio, Wainwright and Lombardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matteo Briguglio, bWF0dGVvLmJyaWd1Z2xpb0BncnVwcG9zYW5kb25hdG8uaXQ=
 Matteo Briguglio
Matteo Briguglio Thomas W. Wainwright
Thomas W. Wainwright Giovanni Lombardi
Giovanni Lombardi