
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 15 September 2023
Sec. Sport and Exercise Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1183973
 Lijiao Xiong1,2†
Lijiao Xiong1,2† Zhaohao Zeng2,3†
Zhaohao Zeng2,3† Shuojia Wang1,2,4
Shuojia Wang1,2,4 Tingfeng Liao1,2
Tingfeng Liao1,2 Xiaohao Wang1,2
Xiaohao Wang1,2 Xinyu Wang1,2
Xinyu Wang1,2 Guangyan Yang1,2
Guangyan Yang1,2 Yanchun Li1,2
Yanchun Li1,2 Lixing Li1,2
Lixing Li1,2 Jing Zhu1,2
Jing Zhu1,2 Pengfei Zhao1,2
Pengfei Zhao1,2 Shu Yang1,2*
Shu Yang1,2* Lin Kang1,2*
Lin Kang1,2* Zhen Liang1,2*
Zhen Liang1,2*Objective: To investigate the association between handgrip strength (HGS) with all-cause and cardiovascular disease (CVD) mortality in US adults.
Method: We analyzed data from the National Health and Nutrition Examination Survey (NHANES) prospective cohort study (2011–2014) with 10,470 participants. The cox regression analysis, Kaplan–Meier survival curves, fitted curves, ROC curves, and propensity score-matched analysis (PSM) with inverse probability of treatment weighting (IPTW), SMRW (PSM with repeated weights), PA (pairwise algorithm), and OW (overlap weighting) regression analysis were performed to assess the relationship between HGS and all-cause and CVD mortality.
Results: The low HGSs (men <37.4 kg, women <24 kg), was found to be associated with higher all-cause and CVD mortality in a reverse J-shaped curve (p < 0.05). Adjusting for multiple covariates including age, BMI, race, education level, marriage status, smoking and alcohol use, and various comorbidities, the hazard ratio (HR) for all-cause mortality in the lowest HGS quintile 1 (Q1) was 3.45 (2.14–5.58) for men and 3.3 (1.88–5.79) for women. For CVD mortality, the HR was 2.99 (1.07–8.37) for men and 10.35 (2.29–46.78) for women. The area under the curve (AUC) for HGS alone as a predictor of all-cause mortality was 0.791 (0.768–0.814) for men and 0.780 (0.752–0.807) for women (p < 0.05), while the AUC for HGS and age was 0.851 (0.830–0.871) for men and 0.848 (0.826–0.869) for women (p < 0.05). For CVD mortality, the AUC for HGS alone was 0.785 (95% CI 0.738–0.833) for men and 0.821 (95% CI 0.777–0.865) for women (p < 0.05), while the AUC for HGS and age as predictors of all-cause mortality was 0.853 (0.861–0.891) for men and 0.859 (0.821–0.896) for women (p < 0.05). The HGS Q1 (men <37.4 kg and women <24 kg) was matched separately for PSM. After univariate, multivariate Cox regression models, PSM, IPTW, SMRW, PA, and OW analyses, women had 2.37–3.12 and 2.92–5.12 HRs with low HGS for all-cause and CVD mortality, while men had 2.21–2.82 and 2.33–2.85 for all-cause and CVD mortality, respectively (p < 0.05).
Conclusion: Adults with low HGS exhibited a significantly increased risk of both all-cause and CVD mortality, regardless of gender. Additionally, low HGS served as an independent risk factor and predictor for both all-cause and CVD mortality.
The decline in skeletal muscle strength is a common phenomenon associated with aging, which significantly affects physical performance and increases the risk of disability and mortality (1, 2). The assessment of handgrip strength (HGS) serves as a facile and impartial metric of muscle strength and a harbinger of adverse health outcomes (3–5). Research in the past has demonstrated a decrement of 1% per annum in HGS beyond midlife (6). The maintenance of high HGS during middle age has been postulated to augment the capacity for healthy aging (7).
Many previous studies had indicated that individuals with low levels of HGS are at an increased risk for mortality and cardiovascular disease (CVD) mortality (3, 7). However, the association between HGS and mortality risk remains controversial, particularly when considering gender differences. A prospective cohort study of individuals aged 75 and above in Taiwan showed no association between HGS and overall or CVD mortality (8). HGS generally tends to be higher in men, often exceeding 10 kilograms. Interestingly, stratified analyses based on gender reveal divergent findings. Some studies report significant associations between lower HGS in both men and women who had higher all-cause and CVD mortality (9). In contrast, a Korean study found that lower HGS in men was significantly associated with all-cause mortality, while not in women (10). Conversely, a study in the UK found an association between HGS and all-cause mortality in women, but not in men (11). Similarly, a study conducted on Japanese patients with type 2 diabetes indicated a significant association between HGS and all-cause mortality in men, whereas not in women (12). Another study conducted within the UK Biobank revealed a correlation between HGS in men and CVD disease mortality, while no significant association was found in women (13).
Given the ongoing debate surrounding the relationship between HGS and mortality for different genders, it is necessary to conduct separate analyses for men and women. HGS is influenced by various factors, including age and comorbidities (14), which may confound the relationship between HGS and mortality. A study across 28 countries showed that low HGS in older adults, regardless of gender, was associated with all-cause mortality (5). However, this study only included general demographic data as confounding factors and did not consider the impact of comorbidities. Propensity score matching (PSM) analysis is a valuable approach that can effectively match individuals across different groups, controlling for confounding factors and reducing selection bias, thereby enabling more robust causal inferences (15). Therefore, this study aims to conduct separate analyses for males and females of HGS and its association with all-cause and CVD mortality among the adult population in the United States. By utilizing propensity score matching, we hope to minimize the impact of confounding factors and obtain more reliable results.
The population was from the National Health and Nutrition Examination Survey (NHANES, 2011–2014), which is a comprehensive cross-sectional survey conducted across all 50 states and the District of Columbia in the United States (16). Informed consent was obtained from all participants after approval by the Institutional Review Board of the National Center for Health Statistics (NCHS) (16). Through a multilevel stratified probability design, 5,000 participants were sampled annually, and each performed a standardized questionnaire and physical examination. This representative survey’s data have been published online every 2 years since 1999. Online data sets are accessible for public use at https://www.cdc.gov/nchs/nhanes/index.htm (16). Given that this study utilized publicly available deidentified data and waived informed consent, the institutional review board at the Shenzhen People’s Hospital deemed the study exempt. All research findings were reported in compliance with STROBE guidelines throughout.
This research used publicly accessible NHANES data from 2011–2014, including 19,931 individuals. The following exclusion criteria were used to restrict our analysis: missing data for mortality (n = 7,982); missing data for HGS (n = 1,375); missing data for weight (n = 86); missing data for height (n = 18). In the final, 10,470 participants were enrolled in this research (Figure 1).
In this study, muscle strength estimation was achieved by utilizing HGS data from both hands, as detailed in the NHANES Muscle Strength—Grip Test protocol (16). Participants were instructed to firmly hold the dynamometer with either their dominant or non-dominant hand while exhaling to avoid raising intrathoracic pressure, unless physically unable to stand. During the measurement process, the procedure was explained and demonstrated to the participant by trained examiners, and the grip size of the dynamometer was adjusted to the participant’s hand size. Testing was conducted three times on the same hand alternately. In this study, the HGS was defined as the highest value obtained using either hand.
The all-cause mortality rate was the number of participants who died from any cause after the date of the baseline survey but before December 31, 2018 (16). Data on mortality follow-up using the ICD-10 from the NHANES are publicly available in the Public-use Linked Mortality Files.1 The CVD death was classified as ICD 054-068.
A variety of clinical and demographic factors were considered as covariates., including age, sex, BMI, race and ethnicity, educational level, marital status, diabetes, asthma, congestive heart failure, coronary heart disease, stroke, cancer, high blood pressure, smoking status, and alcohol drinking status. In NHANES, these informations were collected from survey responses. Participants were classified as Mexican American, other Hispanic, non-Hispanic Asian, White, Black, or Other (including multiracial). The education level of participants was categorized as Less than 9th grade, 9th–11th grade (includes 12th grade with no diploma), High school graduate or equivalent, college graduate or above, and others. The categories for marital status were described as married, widowed, divorced, separated, never married, living with a partner, and other. Asthma, congestive heart failure, coronary heart disease, stroke, cancer, high blood pressure, and diabetes were diagnosed by a physician or other health professional. The smoking and drinking behaviors were categorized as never, past, and current use. The formula for calculating BMI as weight (kg)/[height (m2) × height (m2)].
For continuous variables, 95% confidence intervals (CIs) were provided, while for categorical variables, percentage frequencies were provided. T-tests and χ2 tests were used to compare continuous and categorical data. No imputation approach was applied because all variables had low missing data rates. The mortality risk is calculated using Cox proportional hazards regression models. Curve fitting of restricted cubic spline plot and Kaplan–Meier curves are visually illustrated. HGS alone and models that included HGS and age were used as predictors for mortality. An analysis of propensity score matching (PSM) with the following covariates was conducted to reduce potential selection bias. After calculating individual propensity scores using a Cox regression model, the nearest-neighbor matching algorithm with a caliper width of 0.2 standard deviations of the propensity score was utilized to match patients among the lowest HGS and other groups. We then utilized four distinct weighting techniques: the inverse probability of treatment weighting (IPTW) regression analysis (14), the standardized mortality ratio weighting (SMRW) regression analysis (14), the pairwise algorithm (PA) weighted regression analysis (5) and the overlay weight (OW) regression analysis (15). Statistical analyses were carried out using the R software package (http://www.R-project.org, The R Foundation) and the Free Statistics software version 1.7. Statistical significance was determined by a two-sided p-value <0.05.
The final sample included 10,470 participants in the study, with 5,175 (49.4%) men and 5,295 (50.6%) women with a mean age of 37.1 ± 11.6 years (Table 1). There were 1,230 patients with diabetes mellitus (11.7%), 1,595 patients with asthma (15.2%), 308 patients with congestive hypertension (2.9%), 366 patients with coronary heart disease (3.5%), 339 patients with stroke (3.2%), 886 patients with cancer (8.5%), and 3,604 patients with high blood pressure (34.4%). The mean follow-up period was 81.2 (range 70–95) months for all causes and cause-specific mortality. The follow-up period ended with 847 (8.1%) deaths, 222 (26.2%) deaths from cardiovascular, 201 (23.7%) deaths from cancer, 45 (5.3%) deaths from chronic respiratory diseases, 35 (4.1%) deaths from accidents, 48 (5.7%) deaths from cerebrovascular disease, 15 (1.8%) deaths from Alzheimer’s disease, 40 (4.7%) deaths from diabetes mellitus, 14 (1.7%) deaths from influenza and pneumonia, 22 (2.6%) deaths from renal disease, and 205 (24.2%) deaths from all other causes.
According to curve fitting, men and women with higher HGS had a lower risk of all-cause mortality, approaching the reverse J shape (p < 0.05) (Figure 2). For CVD mortality, similar reverse J-shaped curves were observed between HGS and CVD mortality, with a significant difference for men (p < 0.05) but not for women (p > 0.05) (Figure 2). Kaplan–Meier survival curves indicated that low HGS (men <37.4 kg, women <24 kg) was associated with an increased all-cause and CVD mortality risk (p < 0.05) (Figure 3).
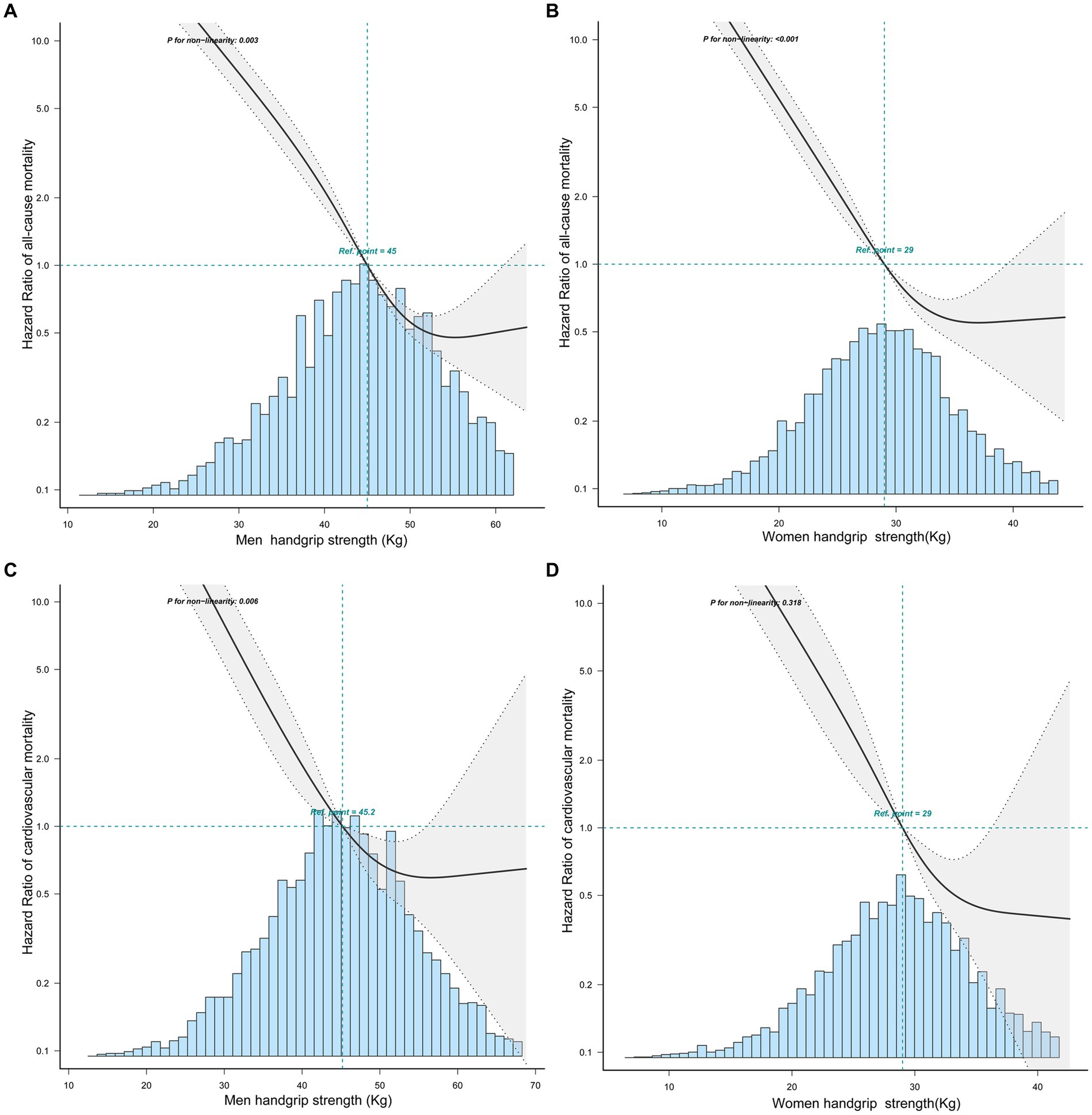
Figure 2. The relationship between handgrip strength with all-cause and CVD mortality in men and women by curve fitting. (A) The relationship between HGS and all-cause mortality in men. (B) The relationship between HGS and all-cause mortality in women. (C) The relationship between HGS and CVD mortality in men. (D) The relationship between HGS and CVD mortality in women.
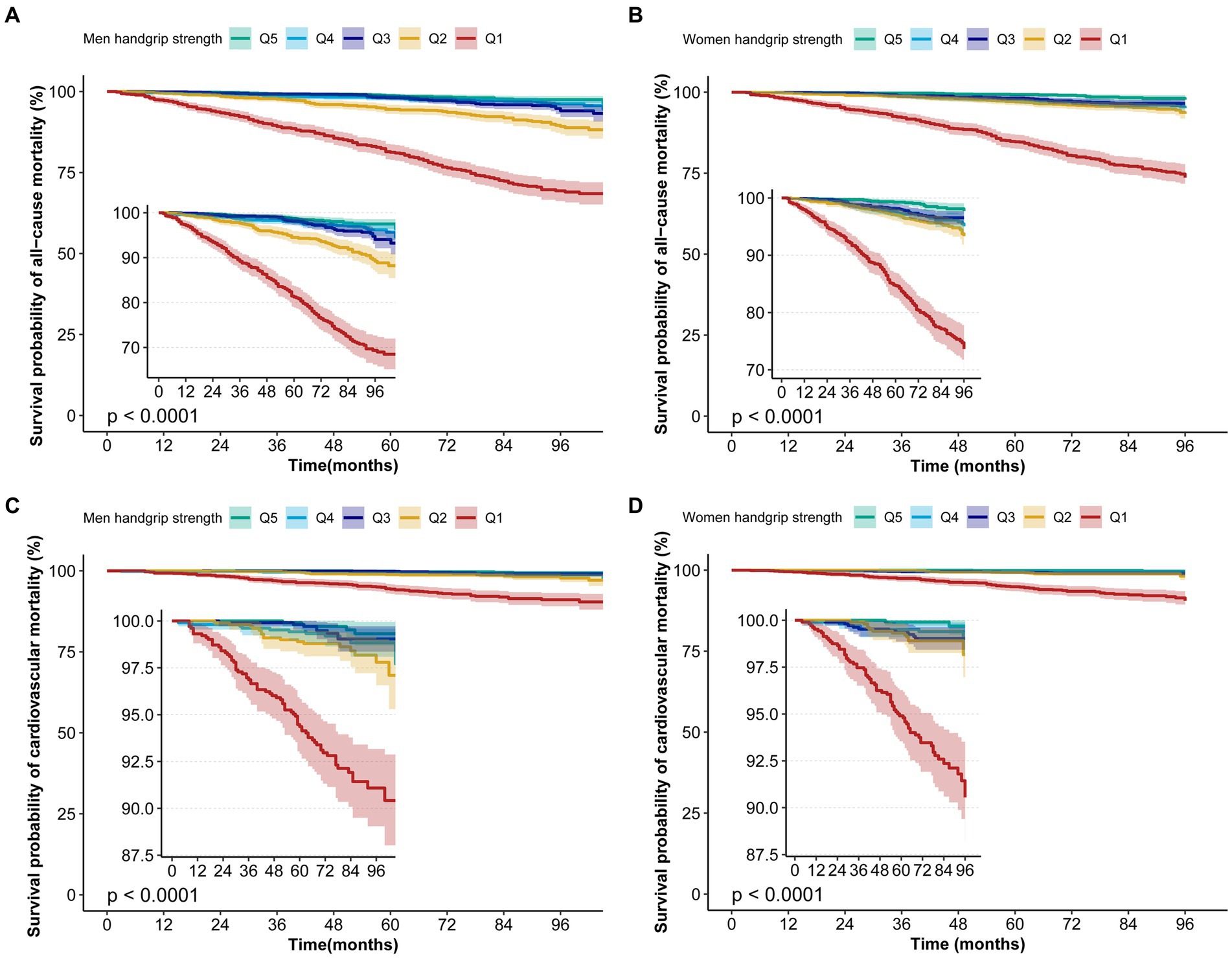
Figure 3. Kaplan–Meier survival curves for HGS associated with all-cause and CVD mortality risk. (A) The Kaplan–Meier survival curves for HGS with all-cause mortality in men. (B) The Kaplan–Meier survival curves for HGS with all-cause mortality in women. (C) The Kaplan–Meier survival curves for HGS with CVD mortality in men. (D) The Kaplan–Meier survival curves for HGS with CVD mortality in women.
The results from multivariable Cox regression analyses are presented in Table 2. Compared to Q5, the unadjusted hazard ratio (HR) of HGS Q1 for all-cause mortality (model 1) was 14.19 (95% CI 9.28–21.71) for men and 15.88 (95% CI 9.71–25.98) for women. The HR for CVD mortality was 17.44 (95% CI 7.05–43.13) for men and 42.18 (95% CI 10.35–171.82) for women (model 1). After adjusting for age and BMI (model 2), the HR of HGS Q1 for all-cause mortality was 3.39 (95% CI 2.13–5.39) for men and 3.22 (95% CI 1.88–5.53) for women, whereas for CVD mortality it was 3.13 (95% CI 1.16–8.39) for men and 9.59 (95% CI 2.21–41.63) for women. Further adjustment for age, BMI, race, education level, marriage status, drinking, and smoking (model 3), the HR of HGS Q1 for all-cause mortality was 3.85 (95% CI 2.39–6.19) for men and 3.78 (95% CI 2.16–6.6) for women, whereas for CVD mortality it was 3.9 (95% CI 1.42–10.76) for men and 12.36 (95% CI 2.77–55.21) for women. Finally, after adjusting for age, BMI, race, education level, marriage status, drinking, smoking, asthma, congestive heart failure, coronary heart disease, stroke, cancer, high blood pressure, and diabetes (model 4), the HR of HGS Q1 for all-cause mortality was 3.45 (95% CI 2.14–5.58) for men and 3.3 (95% CI 1.88–5.79) for women, whereas for CVD mortality was 2.99 (95% CI 1.07–8.37) for men and 10.35 (95% CI 2.29–46.78) for women. All of the unadjusted and adjusted HR for HGS Q1 were statistically significant (p < 0.05), regardless of gender.
HGS shows significant predictive capacity for both all-cause and CVD mortality. When HGS is considered as a standalone predictor, it exhibits notable predictive power. In men, the area under the curve (AUC) for HGS in predicting all-cause mortality is 0.791 (95% CI 0.768–0.814) (blue line in Figure 4A). A similar trend is observed in women, with an AUC of 0.780 (95% CI 0.752–0.807) (blue line in Figure 4B) (p < 0.05).
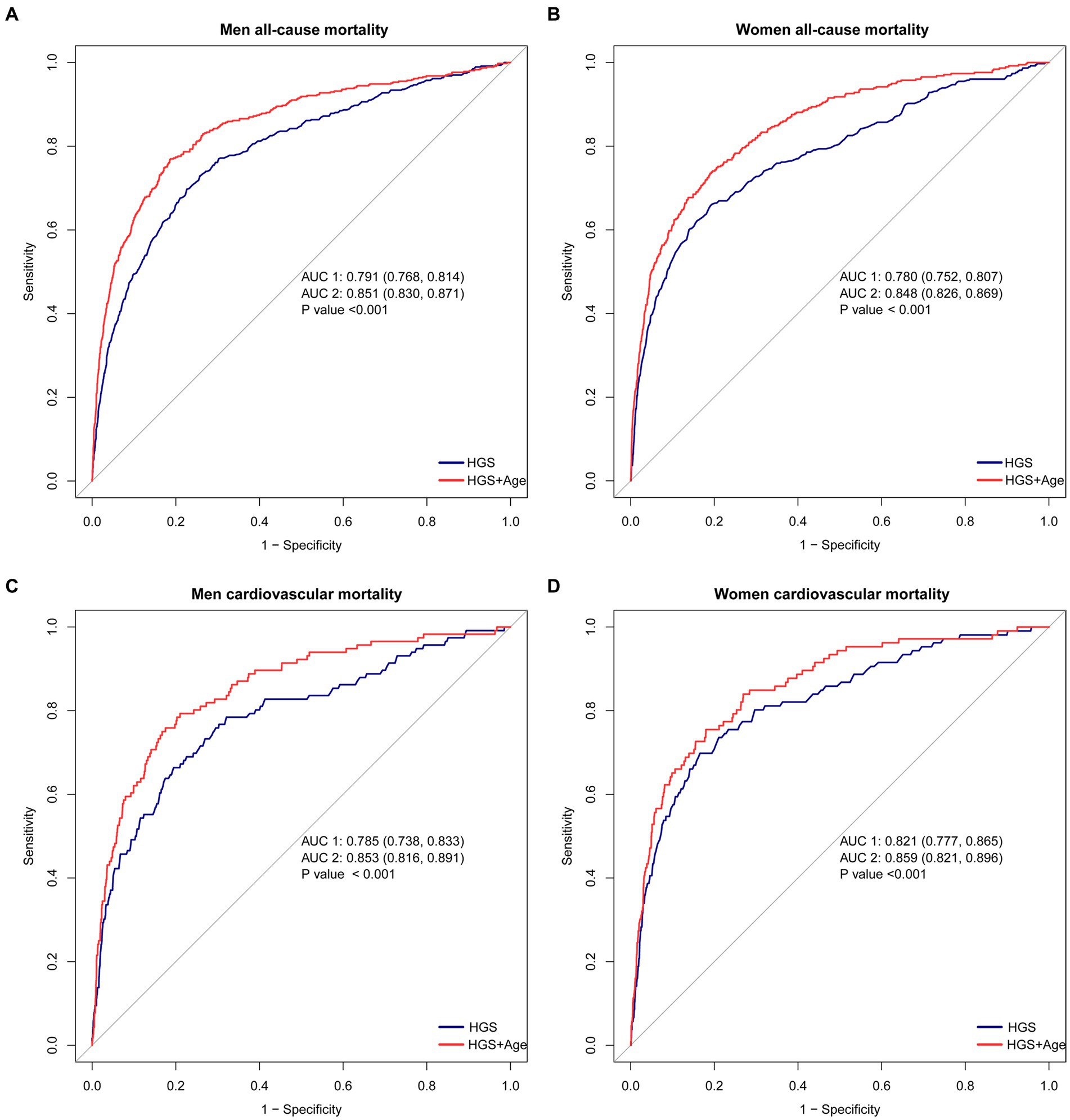
Figure 4. The ROC curves for HGS and age in predicting all-cause and CVD mortality. (A) The ROC curves for HGS and age in predicting all-cause mortality in men. (B) The ROC curves for HGS and age in predicting all-cause mortality in women. (C) The ROC curves for HGS and age in predicting CVD mortality in men. (D) The ROC curves for HGS and age in predicting CVD mortality in women. ##The blue lines represent the model using HGS as a single predictive factor, while the red lines represent the model using both HGS and Age as predictive factors.
Furthermore, HGS demonstrates robust predictive capability for CVD mortality. In men, the AUC for HGS as a predictor is 0.785 (95% CI 0.738–0.833) (blue line in Figure 4C). Likewise, women display strong predictive ability, with an AUC of 0.821 (95% CI 0.777–0.865) (blue line in Figure 4D) (p < 0.05).
The predictive accuracy of all-cause mortality can be significantly enhanced by combining HGS with age. When HGS and age are jointly considered, the AUC improves substantially. In men, the AUC for this joint prediction increases to 0.851 (95% CI 0.830–0.871) (red line in Figure 4A). Similarly, in women, the AUC rises to 0.848 (95% CI 0.826–0.869) (red line in Figure 4B) (p < 0.05).
Moreover, the combination of HGS and age showcases remarkable performance in predicting CVD mortality. In men, the AUC for this combination rises to 0.853 (95% CI 0.861–0.891) (red line in Figure 4C). Similarly, in women, an outstanding AUC of 0.859 (95% CI 0.821–0.896) is displayed (red line in Figure 4D) (p < 0.05).
The lowest quintile of HGS (men <37.4 kg and women <24 kg) was matched as a separate group for PSM. After PSM, the number of male participants decreased from 1,034 to 774, and the number of female participants decreased from 1,059 to 788. There were no statistically significant differences in baseline characteristics after PSM, including age, BMI, race, education, marital status, smoking, alcohol use, and comorbidities, between the low HGS group and the high HGS group among both males and females (Table 1; Supplementary Figure S1). In both men and women with low HGS (men <37.4 kg and women <24 kg), there were significantly higher hazard ratios (HRs) for all-cause and CVD mortality by various sensitivity analyses (p < 0.05). For men with low HGS, the HRs for all-cause and CVD mortality ranged from 2.21 to 2.82 and 2.33 to 2.85, respectively (p < 0.05). For women with low HGS, the HRs for all-cause and CVD mortality ranged from 2.37 to 3.12 and 2.92 to 5.12, respectively (p < 0.05) (Figure 5).
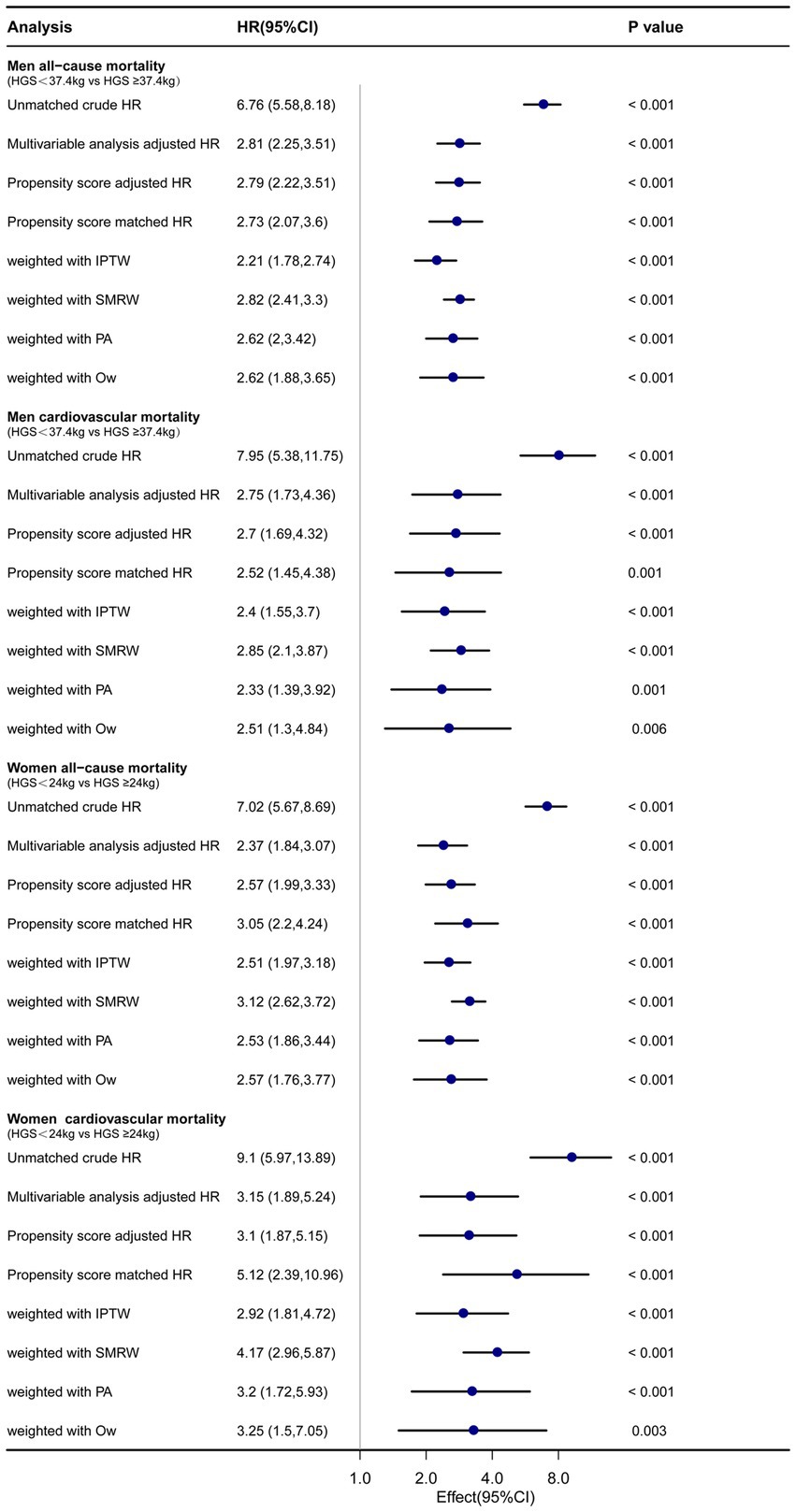
Figure 5. Forest plot shows HRs of all-cause and CVD mortality in men and women with low HGS using a variety of models. IPTW: the inverse probability of treatment weighting regression analysis. SMRW: the standardized mortality ratio weighting regression analysis. PA: pairwise algorithm weighted regression analysis. OW: overlay weight regression analysis.
Low HGS has been associated with adverse health outcomes. However, there is still ongoing debate about gender differences in this relationship between HGS and mortality (10–12). Our study revealed a reverse J-shape association between HGS and death from all-causes and CVD disease. The results indicated that men and women with lower HGS faced higher risks of both all-cause and CVD mortality, even after rigorous adjustments for demographic factors and comorbidities. Moreover, the group differences and the impact of confounding factors were minimized by PSM, IPTW, SMRW, PA, and OW weighting. These methods yielded robust results: low HGS significantly predicts all-cause and CVD mortality both in men and women. Our finding further confirms the importance of HGS as an independent risk factor in predicting all-cause and CVD mortality.
The findings of this research have significant implications for public health. Lower HGS is closely linked to increased risks of mortality and CVD, indicating the importance of maintaining optimal muscle strength for overall health (14, 17–20). The underlying mechanisms behind this association are multifaceted. HGS serves as a surrogate marker for early-stage sarcopenia (21), physical fitness, and functional capacity, with lower HGS potentially indicating reduced physical activity levels, elevated inflammation, and compromised cardiovascular and metabolic function (18, 20, 22–25). Additionally, low handgrip strength may reflect an overall decline in muscle mass and strength, which is frequently associated with age-related conditions and an elevated risk of adverse health outcomes (26–28).
Compared to previous studies, this research conducted separate analyses for men and women, recognizing potential gender differences. Inconsistencies in previous research results may stem from several factors. Firstly, variations in the selected study populations, including differences in age, gender, race, and geographical distribution, could contribute to divergent findings (6). Secondly, disparities in study design and methodology, such as sample size, duration of study, statistical approaches, and adjustments for potential confounding factors, may influence the consistency of results (5, 18, 20). Additionally, discrepancies may arise from variances in the instruments and standardized procedures used for grip strength measurements across studies (20). Furthermore, it is important to consider potential gender differences and confounding variables (7). Additionally, the study employed PSM techniques, minimizing the impact of confounding variables and strengthening the validity of the results. By utilizing PSM, this study overcomes some limitations of prior research designs.
The study has several strengths, including large sample size, a long follow-up period, and adjustment for various potential confounders. Limitations inherent in our investigation include the absence of data concerning alterations in HGS over time. Moreover, despite adjusting for a broad range of confounding variables, it remains possible that there exist additional unmeasured factors that influence the results. Additionally, as our study solely comprised American adults, our findings may not be universally applicable to other populations. Consequently, future research should center on longitudinal studies, which can examine the link between HGS and mortality risk across time and in heterogeneous populations. Despite these limitations, the findings of this study have important clinical implications. HGS is a simple and noninvasive measure that can be easily obtained in clinical settings. Identifying individuals with low HGS may allow earlier interventions to prevent or manage chronic diseases and ultimately improve health outcomes.
In conclusion, our study highlights the importance of low HGS as an independent risk factor and a significant predictor for all-cause and CVD mortality. These findings emphasized the importance of maintaining HGS as a potential means of reducing mortality risk and suggested that low HGS may serve as a valuable predictor of mortality risk, with potential implications for clinical practice and public health interventions.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors. Publicly available datasets were analyzed in this study. The NHANES data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
The National Center for Health Statistics (NCHS) Institutional Review Board approved all study procedures, and participants provided written informed consent. This representative survey’s data have been published online every two years since 1999. Online data sets are accessible for public use at https://www.cdc.gov/nchs/nhanes/index.htm. Because the study used publicly available deidentified data and informed consent was waived. Based on a publicly accessible database, this study did not require ethical approval or informed consent. The patients/participants provided their written informed consent to participate in this study.
LX and ZZ: conceiving the protocol, data analysis and interpretation, acquisition of data, statistical analysis and interpretation of data, and manuscript preparation. SW, TL, XHW, and XYW: study concept and design, statistical analysis, and interpretation of data. GY, YL, LL, JZ, and PZ: data analysis and data acquisition. SY, LK, and ZL: concept and design, final drafting of the manuscript, and study supervision. All authors contributed to the article and approved the submitted version.
This research was supported by grants from the Science and Technology Planning Project of Shenzhen City, Guangdong Province, China (Nos. KCXFZ20201221173600001 and JCYJ20220818102605013).
The authors express their gratitude to the NHANSE research team and all of the respondents who participated in this study. Additionally, the authors thank the Free Statistics team for providing technical support, as well as valuable data analysis and visualization tools.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1183973/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | “Absolute standardized differences before and after propensity score matching comparing covariates for participants with and without low HGS in men (HGS < 37.4Kg) and women (HGS < 24 kg).”
1. Ramirez-Velez, R, Correa-Bautista, JE, Garcia-Hermoso, A, Cano, CA, and Izquierdo, M. Reference values for handgrip strength and their association with intrinsic capacity domains among older adults. J Cachexia Sarcopenia Muscle. (2019) 10:278–86. doi: 10.1002/jcsm.12373
2. Cruz-Jentoft, AJ, and Sayer, AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
3. Soysal, P, Hurst, C, Demurtas, J, Firth, J, Howden, R, Yang, L, et al. Handgrip strength and health outcomes: umbrella review of systematic reviews with meta-analyses of observational studies. J Sport Health Sci. (2021) 10:290–5. doi: 10.1016/j.jshs.2020.06.009
4. Lee, J. Associations between handgrip strength and disease-specific mortality including cancer, cardiovascular, and respiratory diseases in older adults: a meta-analysis. J Aging Phys Act. (2020) 28:320–31. doi: 10.1123/japa.2018-0348
5. Lopez-Bueno, R, Andersen, LL, Calatayud, J, Casana, J, Grabovac, I, Oberndorfer, M, et al. Associations of handgrip strength with all-cause and cancer mortality in older adults: a prospective cohort study in 28 countries. Age Ageing. (2022) 51:1–11. doi: 10.1093/ageing/afac117
6. Dufner, TJ, Fitzgerald, JS, Lang, JJ, and Tomkinson, GR. Temporal trends in the handgrip strength of 2,592,714 adults from 14 countries between 1960 and 2017: a systematic analysis. Sports Med. (2020) 50:2175–91. doi: 10.1007/s40279-020-01339-z
7. Lopez-Bueno, R, Andersen, LL, Koyanagi, A, Nunez-Cortes, R, Calatayud, J, Casana, J, et al. Thresholds of handgrip strength for all-cause, cancer, and cardiovascular mortality: a systematic review with dose-response meta-analysis. Ageing Res Rev. (2022) 82:101778. doi: 10.1016/j.arr.2022.101778
8. Chen, PJ, Lin, MH, Peng, LN, Liu, CL, Chang, CW, Lin, YT, et al. Predicting cause-specific mortality of older men living in the veterans home by handgrip strength and walking speed: a 3-year, prospective cohort study in Taiwan. J Am Med Dir Assoc. (2012) 13:517–21. doi: 10.1016/j.jamda.2012.02.002
9. Celis-Morales, CA, Welsh, P, Lyall, DM, Steell, L, Petermann, F, Anderson, J, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. (2018) 361:k1651. doi: 10.1136/bmj.k1651
10. Park, S, Cho, J, Kim, D, Jin, Y, Lee, I, Hong, H, et al. Handgrip strength, depression, and all-cause mortality in Korean older adults. BMC Geriatr. (2019) 19:127. doi: 10.1186/s12877-019-1140-0
11. Smith, L, Yang, L, and Hamer, M. Handgrip strength, inflammatory markers, and mortality. Scand J Med Sci Sports. (2019) 29:1190–6. doi: 10.1111/sms.13433
12. Hamasaki, H, Kawashima, Y, Katsuyama, H, Sako, A, Goto, A, and Yanai, H. Association of handgrip strength with hospitalization, cardiovascular events, and mortality in Japanese patients with type 2 diabetes. Sci Rep. (2017) 7:7041. doi: 10.1038/s41598-017-07438-8
13. Yates, T, Zaccardi, F, Dhalwani, NN, Davies, MJ, Bakrania, K, Celis-Morales, CA, et al. Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur Heart J. (2017) 38:3232–40. doi: 10.1093/eurheartj/ehx449
14. Lu, Y, Li, G, Ferrari, P, Freisling, H, Qiao, Y, Wu, L, et al. Associations of handgrip strength with morbidity and all-cause mortality of cardiometabolic multimorbidity. BMC Med. (2022) 20:191. doi: 10.1186/s12916-022-02389-y
15. Doran, B, Guo, Y, Xu, J, Weintraub, H, Mora, S, Maron, DJ, et al. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: insight from the National Health and Nutrition Examination Survey III (NHANES-III). Circulation. (2014) 130:546–53. doi: 10.1161/CIRCULATIONAHA.114.010001
16. US Centers for Disease Control and Prevention; About the national health and nutrition examination survey. National Center for Health Statistics. (2020). Available at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. (Accessed April 7, 2021)
17. Fritz, NE, McCarthy, CJ, and Adamo, DE. Handgrip strength as a means of monitoring progression of cognitive decline—a scoping review. Ageing Res Rev. (2017) 35:112–23. doi: 10.1016/j.arr.2017.01.004
18. Lunt, E, Ong, T, Gordon, AL, Greenhaff, PL, and Gladman, J. The clinical usefulness of muscle mass and strength measures in older people: a systematic review. Age Ageing. (2021) 50:88–95. doi: 10.1093/ageing/afaa123
19. Kim, KK, Lee, KR, and Hwang, IC. Association between handgrip strength and cardiovascular risk factors among Korean adolescents. J Pediatr Endocrinol Metab. (2020) 33:1213–7. doi: 10.1515/jpem-2020-0167
20. Klawitter, L, Vincent, BM, Choi, BJ, Smith, J, Hammer, KD, Jurivich, DA, et al. Handgrip strength asymmetry and weakness are associated with future morbidity accumulation in Americans. J Strength Cond Res. (2022) 36:106–12. doi: 10.1519/JSC.0000000000004166
21. Xu, J, Wan, CS, Ktoris, K, Reijnierse, EM, and Maier, AB. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. (2022) 68:361–76. doi: 10.1159/000517099
22. Simmonds, SJ, Syddall, HE, Westbury, LD, Dodds, RM, Cooper, C, and Aihie, SA. Grip strength among community-dwelling older people predicts hospital admission during the following decade. Age Ageing. (2015) 44:954–9. doi: 10.1093/ageing/afv146
23. Gedmantaite, A, Celis-Morales, CA, Ho, F, Pell, JP, Ratkevicius, A, and Gray, SR. Associations between diet and handgrip strength: a cross-sectional study from UK Biobank. Mech Ageing Dev. (2020) 189:111269. doi: 10.1016/j.mad.2020.111269
24. Joo, YS, Jhee, JH, Kim, HW, Han, SH, Yoo, TH, Kang, SW, et al. Physical performance and chronic kidney disease development in elderly adults: results from a nationwide cohort study. Aging. (2020) 12:17393–417. doi: 10.18632/aging.103741
25. Wilkinson, TJ, Miksza, J, Yates, T, Lightfoot, CJ, Baker, LA, Watson, EL, et al. Association of sarcopenia with mortality and end-stage renal disease in those with chronic kidney disease: a UK Biobank study. J Cachexia Sarcopenia Muscle. (2021) 12:586–98. doi: 10.1002/jcsm.12705
26. He, P, Ye, Z, Liu, M, Li, H, Zhang, Y, Zhou, C, et al. Association of handgrip strength and/or walking pace with incident chronic kidney disease: a UK Biobank observational study. J Cachexia Sarcopenia Muscle. (2023) 14:805–14. doi: 10.1002/jcsm.13180
27. Duchowny, KA, Ackley, SF, Brenowitz, WD, Wang, J, Zimmerman, SC, Caunca, MR, et al. Associations between handgrip strength and dementia risk, cognition, and neuroimaging outcomes in the UK Biobank cohort study. JAMA Netw Open. (2022) 5:e2218314. doi: 10.1001/jamanetworkopen.2022.18314
Keywords: handgrip strength, all-cause mortality, CVD mortality, NHANES, propensity score-matched analysis
Citation: Xiong L, Zeng Z, Wang S, Liao T, Wang X, Wang X, Yang G, Li Y, Li L, Zhu J, Zhao P, Yang S, Kang L and Liang Z (2023) The association of handgrip strength with all-cause and cardiovascular mortality: results from the National Health and Nutrition Examination Survey database prospective cohort study with propensity score matching. Front. Nutr. 10:1183973. doi: 10.3389/fnut.2023.1183973
Received: 28 March 2023; Accepted: 29 August 2023;
Published: 15 September 2023.
Edited by:
David Christopher Nieman, Appalachian State University, United StatesReviewed by:
Brenda McGrath, OCHIN, Inc., United StatesCopyright © 2023 Xiong, Zeng, Wang, Liao, Wang, Wang, Yang, Li, Li, Zhu, Zhao, Yang, Kang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Yang, eWFuZy5zaHVAc3pob3NwdGlhbC5jb20=; Lin Kang, a2FuZy5saW5Ac3pob3NwaXRhbC5jb20=; Zhen Liang, bGlhbmcuemhlbkBzemhvc3BpdGFsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.