- 1Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Nutrition, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 3Department of Community Nutrition, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
- 4Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
- 5Centre for Intelligent Healthcare, Coventry University, Coventry, United Kingdom
Background: Obesity affects body composition and anthropometric measurements. A Body Shape Index (ABSI) and Body Roundness Index (BRI) are reportedly associated with an increased risk of cardiovascular disease. However, the relationship between ABSI, BRI, cardiometabolic factors, and inflammatory elements is not well-elucidated. Therefore, this study sought to examine the mediatory effect of inflammatory markers on the association between ABSI and BRI with cardiometabolic risk factors in overweight and obese women.
Methods: This cross-sectional study was performed on 394 obese and overweight women. The typical food intake of individuals was assessed using a 147-item semi-quantitative Food Frequency Questionnaire (FFQ). Body composition was measured by bioelectrical impedance analysis (BIA). Biochemical parameters, such as inflammatory markers and anthropometric components, were also assessed. For each participant, all measurements were carried out on the same day.
Result: There was a significant positive association between ABSI and AC and CRI.I in subjects with higher ABSI scores before and after adjustment (P < 0.05). In addition, there was a significant positive association between BRI and FBS, TC, TG, AIP, AC, CRI.I, CRI.II, and TyG in participants with higher BRI scores before and after adjustment (P < 0.05). We found that hs-CRP, PAI-1, MCP-1, TGF-β, and Galectin-3 were mediators of these relationships (P < 0.05).
Conclusion: Inflammation can play an important role in the relationship between body shape indices and cardiometabolic risk factors among overweight and obese women.
1. Introduction
Worldwide, the incidence of overweight and obesity represents a significant public health issue. Globally, in 2016, 39% of men and 40% of women, aged 18 years and older, representing almost 2 billion adults, were overweight, and 11% of men and 15% of women, over half a billion, were obese (1). The prevalence of obesity tends to increase with age and is typically greater in the women population (2). Obesity, broadly speaking, occurs when energy intake goes beyond energy utilization for a long period of time, resulting in fat accumulation (3). Genetic, social, environmental, cultural, and biological elements play an important role in obesity development. One of the most plausible causes of global increases in weight is the biological response to low-cost and energy-rich food, combined with an extensive decrease in physical activity levels (4, 5). Being overweight and obese have been related to an increased risk of cardiovascular disease (CVD), metabolic syndrome (MetS), dyslipidemia, hypertension, stroke, type 2 diabetes, many types of cancer, such as colon and postmenopausal breast cancers, osteoarthritis, and other musculoskeletal disorders (6). CVD is one of the most evidence-based clinical implications of obesity (7); indeed, the CVD mortality rate is directly and indirectly influenced by obesity. Indirect impacts are due to concomitant CVD risk factors, such as insulin resistance, glucose intolerance, and high blood pressure, while direct effects are associated with inflammation (8–10).
Obesity and overweight directly affect body composition and, thus, anthropometric measurements. The body mass index (BMI), waist circumference (WC), and waist-to-height ratio (WHR) have been regularly used as anthropometric predictors of CVD for many years, and several studies have indicated distinct cardiovascular risk profiles in people with alike BMI. However, A Body Shape Index (ABSI) and Body Roundness Index (BRI) are reported to represent more accurate indicators (11–13). High ABSI and BRI are related to excess abdominal adipose tissue accumulation. Furthermore, BRI and ABSI are posited to be more sensitive to the presence of MetS, insulin resistance, and inflammatory factors as cardiovascular risk factors (14–16).
By considering the relationship between ABSI and BRI and cardiometabolic factors, inflammatory elements may play an effective role. Imbalanced lipid profiles and inflammatory factors, such as Galectin-3, monocyte chemotactic protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), and transforming growth factor β (TGF-β,) which are related to body composition and obesity, play a significant role in increasing the risk of cardiovascular diseases (17). Galectin-3, a β-galactoside-binding protein belonging to the lectin family, is a novel predictor of heart failure (HF) risk and mortality. Other key processes, in which Galectin-3 plays a role, include inflammation, tissue fibrosis, and angiogenesis. Normally, Galectin-3 has a low expression in the heart, and its synthesis and secretion increase in HF. Galectin-3 primarily protects the heart with anti-apoptotic and anti-necrosis functions, but its long-term expression results in fibrosis and unfavorable regeneration of the damaged tissue. Galectin-3-binding sites are mainly in the myocardial matrix, fibroblasts, and macrophages (18, 19). Additionally, MCP-1 plays a major role in CVD development, and this protein, through its chemotactic activity, causes diapedesis of monocytes to the subendothelial space, where they are converted into foam cells and begin fatty streaks and finally give rise to atherosclerotic plaque formation. In addition, inflammatory macrophages probably are involved in plaque rupture and ischemia caused by it, as well as restenosis after angioplasty. There is strong evidence for a major role of MCP-1 in myocarditis, ischemia/reperfusion injury in the heart, and transplant rejection. Moreover, MCP-1 has various effects on the types of cells involved in cardiac fibrotic remodeling. Despite the multiple functions of MCP-1 in cardio-pathobiology, the molecular mechanisms underlying these functions are poorly understood (20). The likelihood of ischemic cardiovascular events is associated with PAI-1 levels, and the increase in PAI-1 levels raises the risk of atherothrombotic events and may also lead to the progression of vascular disease (21). In addition, TGF-β is reported to have a significant effect on the cardiovascular system. TGF-β is a cytokine that exerts a wide range of different and often contradictory functions. Its effects on the cardiovascular system are also ambiguous because, on the one hand, there is strong evidence for the “protective cytokine hypothesis” that expresses TGF-β as an anti-atherogenic factor, and on the other hand, it has been proven to be involved in some pro-inflammatory effects. Moreover, in addition to the beneficial and restorative positive role of TGF-β, there are disadvantages as follows: TGF-β plays an important role in postangioplasty restenosis and postinfarction myocardial remodeling (leading to heart failure), and it also plays a role in many blood circulation disorders that are related to fibrosis and vascular regeneration (22). Despite the above evidence, the relationship between ABSI, BRI, cardiometabolic factors, and inflammatory elements is not well-elucidated. Therefore, this study sought to examine the mediatory effect of inflammatory markers on the association between ABSI and BRI with cardiometabolic risk factors in overweight and obese women.
2. Methods
2.1. Study design and population
This cross-sectional study was carried out on 394 non-postmenopausal, healthy, overweight, and obese (BMI = 25–40 kg/m2) women, aged 18–48 years, who were referred to healthcare centers of Tehran University of Medical Sciences (TUMS). The multi-stage cluster sampling method was used for sampling. Participants who had any acute or chronic disease background, such as high blood pressure, diabetes mellitus, cancer, polycystic ovary syndrome (PCOS), and hepatic and kidney disease, were excluded. Other exclusion criteria included alcohol consumption, pregnancy and lactation, adherence to special/non-normal dietary intake, and substantial body weight changes in the last year. Furthermore, women using medications that affect body weight and/or glucose and/or lipid-lowering drugs were not eligible to participate in this study. We also excluded subjects whose energy intake was <800 or more than 4,200 (kcal/day) (as under-reporters and over-reporters) (23, 24). Written consent forms were obtained from each participant before the study commencement. The protocol of this study was approved by the Ethics Committee of the TUMS (IR.TUMS.VCR.REC.1397.804).
2.2. General and anthropometric assessment
General information, including age, occupational and educational status, and marital status, were collected from all participants. The patient's height was determined by a digital stadiometer (Seca Germany) while barefoot and reported to be the nearest 0.1 cm. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by a standard mercury sphygmomanometer in the right arm after 15 min of rest in a sitting position. The first and fifth Korotkoff sounds were taken as SBP and DBP, respectively. The average of the two measurements is considered as the participant's blood pressure. Anthropometric measurements, such as weight, WC, and hip circumferences (HC), were evaluated by skilled technicians who followed standard guidelines. The midpoint between the lowest rib and iliac crest was used for measuring WC. WHR was computed by dividing WC by HC, and BMI was obtained by Equation 1 as follows:
Where, Wt is the weight (kg) and Ht is the height (m).
Finally, BMI = 25–29.9 kg/m2 was considered overweight, and obesity was described as BMI = 30–40 kg/m2. A bioelectrical impedance analyzer (BIA) device (In Body 770, Korea) was used to assess body composition variables (25). BRI was calculated by equation 2, with the help of an online calculator (26):
where WC is the waist circumference (cm) and Ht is the height (cm).
In addition, ABSI was computed by Equation 3 (27) as follows:
Where, WC is the waist circumference (cm), BMI is the body mass index (kg/m2), and Ht is the height (cm).
2.3. Biochemical assessment
Venous blood samples were collected from all participants following 12 h fasting. After centrifuging, liquating, and storing samples at −80°C at the TUMS nutrition laboratory, the following parameters were measured: fasting blood sugar (FBS) by using the Glucose Oxidase phenol 4-Aminoantypyrine Peroxidase (GOD/PAP) method, serum insulin values by utilizing radio-immune assay, lipid profile inclusive high-density lipoprotein cholesterol ( HDL), low-density lipoprotein cholesterol (LDL), total cholesterol (TC), and triglyceride (TG) by applying enzymatic procedure (Pars Azmun Co, Tehran, Iran). Hypersensitive C-reactive protein (hs-CRP), TGF-β, PAI-1, MCP-1, and Galectin-3 levels were measured by the enzyme-linked immunosorbent assay (ELISA) method. The atherogenic index of plasma (AIP), a useful parameter in clinical practice, was determined as the logarithm of the TG/HDL ratio. Additionally, we compute the lipid ratio based on the definition by Olamoyegun et al. (28), calculated as follows:
Moreover, the triglyceride glucose (TGy) index was calculated as follows (29):
2.4. Physical activity assessment
The validated international physical activity questionnaire (IPAQ) was applied to collect data about participants' physical activities. Finally, metabolic equivalent (MET) scores and MET-minutes per week (MET-min/wk) were calculated as a summation of all activity categories and reported as mild (<600), moderate (600–3,500), and severe (>3,500) (MET-h/wk.) (30).
2.5. Dietary assessment
A 147-item Food Frequency Questionnaire (FFQ) was applied to assess usual dietary intake. The validity and reliability of FFQ were confirmed in Esmaillzadeh et al. study (31). The questionnaires were administered by an expert nutritionist, and all subjects were asked to report the frequency of different food items consumed during the previous year. Daily (e.g., bread), weekly (e.g., rice, meat), or monthly (e.g., fish) categories were considered for each item's report. By using household measurements, the frequency of each food consumption was changed into grams per day. In the next step, the Iranian Food Composition Table and N4 program were used to assess dietary nutrients and total energy intake.
2.6. Statistical analysis
The Kolmogorov–Smirnov test was used to examine the data's normal distribution. Quantitative and qualitative differences between the ABSI and BRI groups were described using independent t-test and chi-square test, respectively. In this study, participants were dichotomized based on their median ABSI and BRI scores (0.791 and 5.642, respectively). To describe the characteristics of the study population among the ABSI and BRI groups, we used analysis of covariance (ANCOVA), considering age and total energy intake as cofounders. We also used linear regression to investigate the association between ABSI and BRI with metabolic markers and atherogenic indices. BRI group <5.642 and ABSI group <0.791 were considered the reference groups. In this model, we also examined the mediating effects of inflammatory markers (hs-CRP, PAI-1, MCP-1, TGF-β, and Galectin-3). We created several models to examine the mediating role of these inflammatory indicators. First, we examined the crude and adjusted model, including age and energy intake. Then, in the final model, we separately included each of the inflammatory markers as a confounding variable along with age and energy intake. Finally, we considered the mediating effect of the variables. Data analysis was conducted using SPSS software (version 25, SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. Study population characteristics
This cross-sectional study was performed on 394 overweight and obese Iranian women. The mean age, weight, BMI, ABSI, and BRI of participants were 36.67 ± 9.10, 80.28 ± 11.05 kg, 30.98 ± 3.90 kg/m2, 0.79 ± 0.02, and 5.85 ± 1.35, respectively. Among the study population, 286 (72.4%) were married, 138 (35.1%) were employed, and 189 (47.8%) had a bachelor's degree or higher. It should be noted that 127 (49.2%), 119 (46.1%), and 12 (4.7%) participants had low, moderate, and high physical activity, respectively.
3.2. Characteristics of the study population among the ABSI and BRI groups
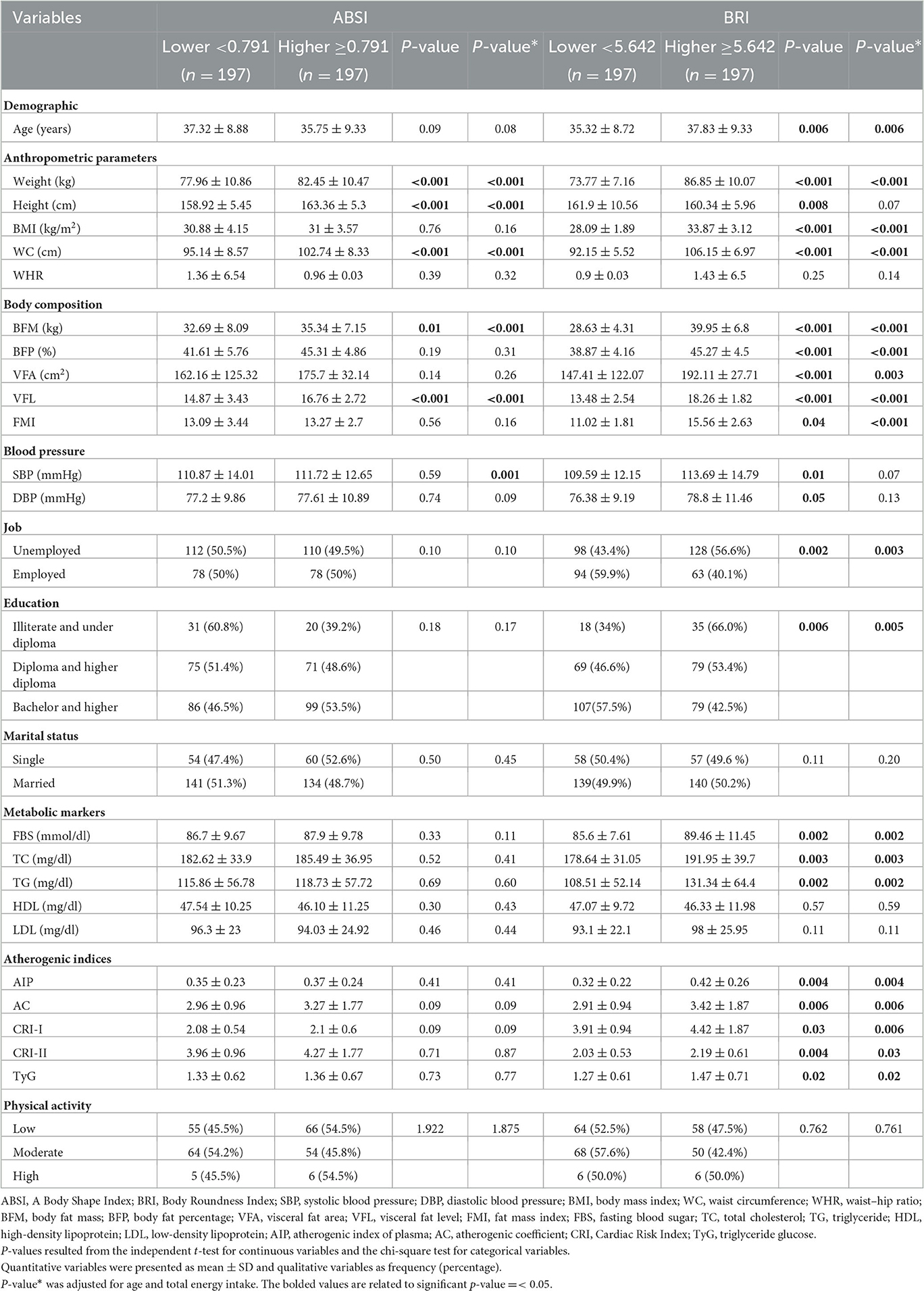
The mean and standard deviation (SD) of quantitative and qualitative variables among the ABSI and BRI groups are shown in Table 1.
Based on our findings, the means of some variables in the crude model, including weight, height, WC, VFL (P < 0.001), and BFM (P = 0.001), were significantly higher in subjects with higher ABSI scores than subjects with lower ABSI scores. The means of SBP (P = 0.01), height (P = 0.008), weight, BMI, WC, BFM, BFP, VFA, VFL (P < 0.001), FMI (P = 0.04), FBS (P = 0.002), TC (P = 0.003), TG (P = 0.002), AIP (P = 0.004), AC (P = 0.006), CRI.I (P = 0.03), CRI.II (P = 0.04), and TyG (P = 0.02) were significantly higher in subjects with higher BRI scores compared to those with lower BRI scores.
After we adjusted age and total energy intake as confounding variables, weight, height, WC, BFM, and VFL remained significantly higher in subjects with higher ABSI scores than in subjects with lower ABSI scores. The mean of weight, BMI, WC, BFM, BFP, VFL, VFA (P = 0.003), FMI (P = 0.04), FBS (P = 0.002), TC (P = 0.003), TG (P = 0.002), AIP (P = 0.004), AC (P = 0.006), CRI.I (P = 0.006), CRI.II (P = 0.032), and TyG (P = 0.02) remained significantly higher in participants with higher BRI scores compared to those with lower BRI scores. However, the mean height and systolic blood pressure (SBP), after adjustment for confounding factors, were not significantly different between subjects with higher BRI scores compared to subjects with lower BRI scores.
3.3. Dietary intakes among ABSI and BRI groups
The mean and SD of energy and dietary intakes of the participants among the ABSI and BRI groups are shown in Table 2. Although energy intake was higher in subjects with higher ABSI and BRI scores compared to those with lower scores, this difference was not significant. After adjusting for total energy intake as a confounding variable, we observed that selenium (P = 0.01) and B12 (P = 0.01) intakes were significantly higher in participants with higher ABSI scores, but vitamin C (P = 0.03) and sucrose (P = 0.02) intakes were significantly higher in participants with lower ABSI score. Among the BRI group, calcium (P = 0.03) and iron (P = 0.02) intakes were significantly higher in women with higher BRI scores. The mean intake of other macronutrients and micronutrients was not significantly different between the study groups (P > 0.05).
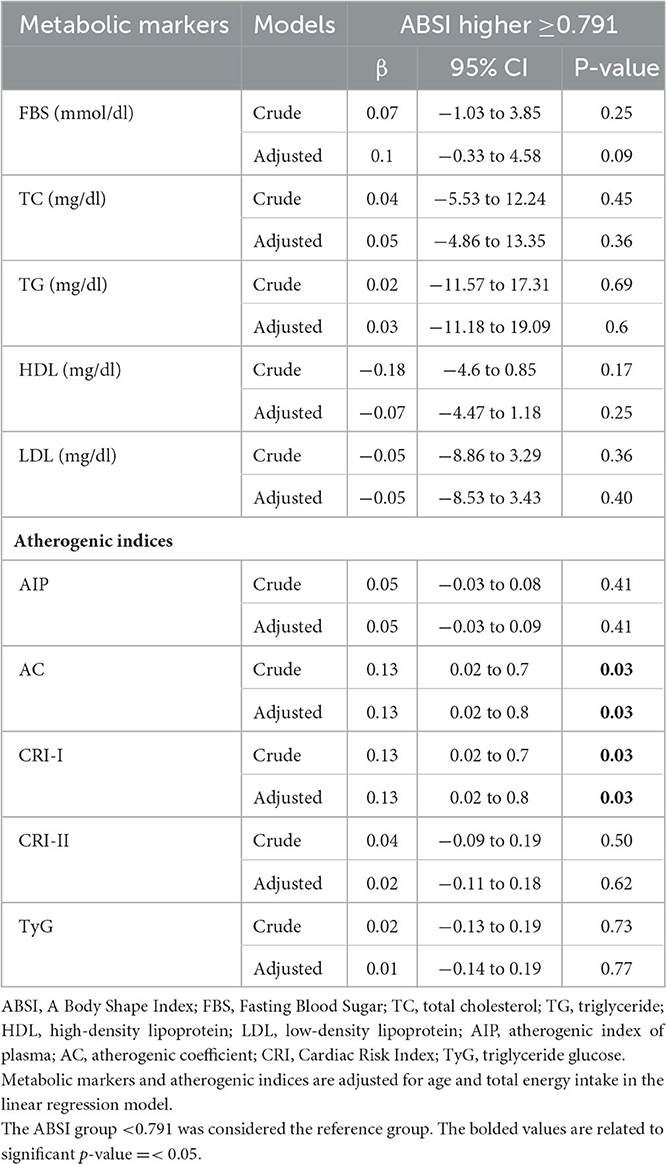
3.4. The association of ABSI with metabolic markers and atherogenic indices
The association between ABSI with metabolic markers and atherogenic indices is demonstrated in the crude and adjusted models in Table 3. In the crude model, there was a significant association between ABSI with AC (β = 0.13, 95% CI = 0.02–0.07, P = 0.03) and CRI-I (β = 0.13, 95% CI = 0.02–0.07, P = 0.03) in subjects with higher ABSI scores, as compared to those with lower ABSI scores. In addition, after adjusting for age and total energy intake, as confounding variables, in a general linear model, this association remained significant (β = 0.13, 95% CI = 0.02–0.07, P = 0.03). No significant association was observed between ABSI scores with other metabolic markers and atherogenic indices before and after adjustment (P > 0.05).
3.5. The evaluation of the mediating effect of inflammatory markers in the relationship between ABSI and atherogenic indices
We investigated the effect of inflammatory markers including hs-CRP, PAI-1, MCP-1, TGF-β, and Galectin-3 as mediators for the association between ABSI and atherogenic indices (Table 4). Each of these inflammatory markers was included in the final model as a confounding variable along with age and total energy intake. We observed that some of these inflammatory markers attenuated the relationship between ABSI and atherogenic indices, so they can be considered mediator markers.
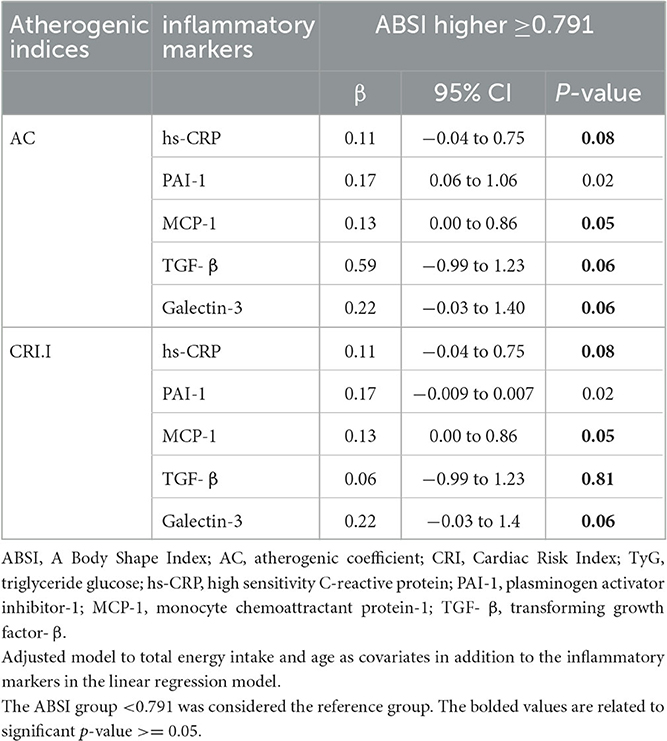
Table 4. Evaluation of the mediating effect of inflammatory markers in the relationship between ABSI and atherogenic indices.
hs-CRP was an intermediate for ABSI and AC (β = 0.11, 95%CI = −0.04 to 0.75, P = 0.08) and CRI-I (β = 0.17, 95%CI = 3.94 to 21.95, P = 0.53). MCP-1 was an intermediate agent for ABSI and AC and CRI-I (β = 0.13, 95%CI = 0.00 to 0.86, P = 0.05). TGF-β was an intermediate agent for ABSI and AC (β = 0.59, 95%CI = −0.99 to 1.23, P = 0.06) and CRI-I (β = 0.06, 95%CI = −0.99 to 1.23, P = 0.81). Galectin-3 was an intermediate agent for ABSI and AC (β = 0.22, 95%CI = −0.03 to 1.40, P = 0.06) and CRI-I (β = 0.22, 95%CI = −0.03 to 1.4, P = 0.06).
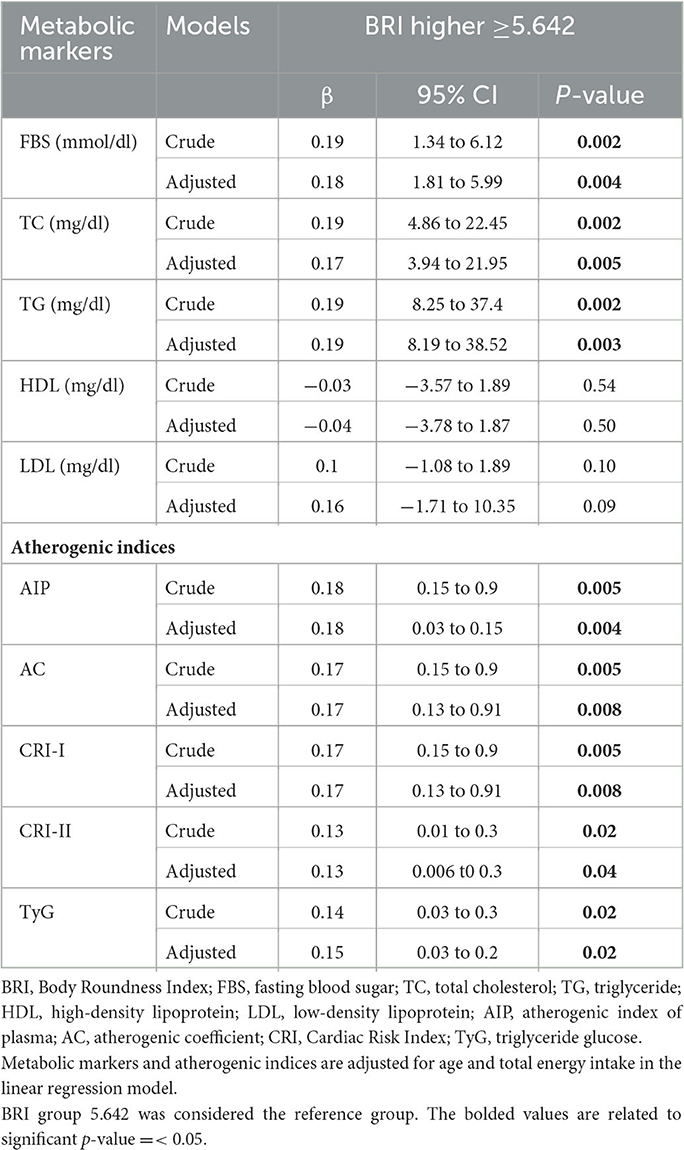
3.6. The association of BRI with metabolic markers and atherogenic indices
The association between BRI with metabolic markers and atherogenic indices is represented in the crude and adjusted models in Table 5.
In the crude model, there was a significant association between BRI with FBS (β = 0.19, 95%CI = 1.34 to 6.12, P = 0.002), TC (β = 0.19, 95%CI = 4.86 to 22.45, P = 0.002), TG (β = 0.19, 95%CI = 8.25 to 37.4, P = 0.002), AIP (β = 0.18, 95%CI = 0.15 to 0.9, P = 0.005), AC and CRI-I (β = 0.17, 95%CI = 0.15 to 0.9, P = 0.005), CRI-II (β = 0.13, 95%CI = 0.01 to 0.3, P = 0.02), and TyG (β = 0.14,95% CI = 0.03 to 0.3, P = 0.02).
After we adjusted for age and energy intake, there was a significant association between BRI with FBS (β = 0.18, 95%CI = 1.81 to 5.99, P = 0.004), TC (β = 0.17, 95%CI = 3.94 to 21.95, P = 0.005), TG (β = 0.19, 95%CI = 8.19 to 38.52, P = 0.003), and atherogenic risk factors including AIP (β = 0.18, 95%CI = 0.03 to 0.15, P = 0.004), AC and CRI.I (β = 0.17, 95%CI = 0.13 to 0.91, P = 0.008), CRI-II (β = 0.13, 95%CI = 0.006 t0 0.3, P = 0.04), and TyG (β = 0.13,95% CI = 0.03 to 0.2, P = 0.02) in participants with higher BRI scores compared to participants with lower BRI scores.
3.7. The evaluation of the mediating effect of inflammatory markers in the relationship between BRI with metabolic markers and atherogenic indices
We evaluated the effect of inflammatory markers including hs-CRP, PAI-1, MCP-1, TGF-β, and Galectin-3 as mediators for the association between BRI with metabolic markers and atherogenic indices (Table 6). Each of these inflammatory markers was included in the final model as a confounding variable, along with age and total energy intake. We observed that some of these inflammatory markers attenuated the relationship between BRI with metabolic markers and atherogenic indices, such that they can be considered as mediating markers.

Table 6. Evaluation of the mediating effect of inflammatory markers in the relationship between BRI with metabolic markers and atherogenic indices.
Regarding BRI, hs-CRP was an intermediate marker of this index and TG (β = 1.06, 95%CI = −5.99 to 144.83, P = 0.06), AIP (β = 0.11, 95%CI = −0.01 to 0.12, P = 0.10), AC (β = 0.17, 95%CI = 3.94 to 21.95, P = 0.05), CRI-I (β = 0.11, 95%CI = −0.04 to 0.81, P = 0.09), CRI-II (β = 0.02, 95%CI = −0.12 to 0.19, P = 0.67), and TyG (β = 0.11, 95%CI = −0.02 to 0.35, P = 0.09).
PAI-1 was an intermediate for BRI and FBS (β = 0.1, 95%CI = −0.85 to 4.59, P = 0.17), TG (β = 0.15, 95%CI = 3.94 to 21.95, P = 0.05), AC (β = 0.05, 95%CI = 0.001 to 1.01, P = 0.05), CRI-I (β = 0.15, 95%CI = 0.001 to 1.01, P = 0.05), CRI-II (β = 0.06, 95%CI = −0.09 to 0.25, P = 0.38), and TyG (β = 0.13, 95%CI = −0.02 to 0.39, P = 0.08).
MCP-1 was an intermediate for BRI and AC (β < 0.001, 95%CI = 3.94 to 21.95, P = 0.68). TGF-β was an intermediate agent for BRI and TC (β = 0.59, 95%CI= −35.78 to 93.51, P = 0.33), AIP (β=0.88, 95%CI = −0.08 to 0.82, P = 0.09), AC (β = 0.003, 95%CI = −0.001 to 0.007, P = 0.15), CRI-I (β = 0.68, 95%CI = −0.5 to 2.63, P = 0.15), CRI-II (β = 0.81, 95%CI = −0.47 to 1.89, P = 0.¬20), and TyG (β = 0.85, 95%CI = −0.23 to 1.22, P = 0.15).
Galectin-3 was an intermediate for BRI and FBS (β = 0.1, 95%CI = −2.73 to 7.53, P = 0.35), TC (β = 0.16, 95%CI = −4.23 to 27.01, P = 0.15), and CRI-II (β = 0.22, 95%CI = −0.01 to 0.67, P = 0.05).
4. Discussion
In the current cross-sectional study, we investigated inflammatory markers' mediatory effect on the association between ABSI and BRI with cardiometabolic risk factors in overweight and obese women. To the best of our knowledge, this study is the first to investigate such a mediatory effect. The present study revealed that, after adjusting for age and total energy intake, there was a significant positive association between ABSI and AC and CRI-I. We also revealed that this association could be influenced by the mediation of inflammatory markers, such as hs-CRP, MCP-1, TGF-β, and Galectin-3. Moreover, we observed a significant positive association between BRI and FBS, TC, TG, and atherogenic indices, after adjusting for confounding variables. In this regard, the association between BRI and AC, CRI-I, CRI-II, and TyG appears to be influenced by hs-CRP, PAI-1, hs-CRP, and TGF-β.
The results of previous studies have shown a significant relationship between ABSI and BRI with cardiometabolic risk factors, which are more accurate indicators than traditional measures, e.g., BMI (11–13). For ABSI, we observed a positive relationship with AC and CRI-I, two atherogenic indices. Concordant with our results, some studies concluded that high ABSI is associated with an increased risk of CVD (32, 33). The positive association of ABSI with small dense LDL, TG, and FBS and the negative association with HDL have been shown in previous studies (27, 34). Indeed, one study highlighted that ABSI can be a valuable index for evaluating central obesity in cardiometabolic risk (27); however, another study did not show this (14). The correlation between ABSI and visceral adipose tissue (VAT) was demonstrated in another study (35), and it was also reported that higher ABSI is associated with higher adipose tissue accumulation around the abdomen (36). A retrospective study concluded that using ABSI and BMI together is more associated with VAT thickness than BMI alone (27). Considering the relationship between ABSI and cardiometabolic risk factors, inflammation may play an effective role. As our study demonstrated, inflammatory markers, including hs-CRP, MCP-1, TGF-β, and Galectin-3, have a mediatory effect on the association of ABSI with AC and CRI-I. Indeed, central obesity can decrease plasma adiponectin via an increase in the pro-inflammatory adipokines (37). Leptin and adiponectin, as energy-regulating hormones, are released from adipose tissues, and their secretion increases and decreases, respectively, in MetS/obese patients. Leptin is negatively associated with HDL and adiponectin (38), while, in obese patients, increased levels of hs-CRP have been observed. High hs-CRP is associated with low adiponectin, and thus, reduced adiponectin production has been suggested to cause vascular and systemic inflammation in obese patients (39).
In addition to CVD, some evidence has suggested that BRI could be a good predictor for metabolic syndrome and insulin resistance (11, 14, 15, 26). One study stated that BRI can predict both CVD and CVD risk factors (11). We observed a significant association between BRI and cardiometabolic risk factors in the present study. BRI is established as an index of body fat distribution, has been reported to be a good predictor of body fat and VAT percent, and is more accurate than BMI, WC, and WHR because of the higher health status reflection (40). Recently, a study showed that BRI, as an index of adipose tissue, can indicate the presence of left ventricular hypertrophy (41) and may be a more helpful predictor of MetS and IR than BMI (15). The results of the aforementioned study demonstrated a significant association between BRI with TG, HDL, LDL, BP, FBS, and inflammatory markers (15). Both BRI and ABSI, as well as WC, can predict inflammation levels, based on hs-CRP levels, in obese patients (15). Similar to ABSI, we observed that inflammatory markers can also mediate the relationship between BRI and cardiometabolic risk factors. We observed that inflammation mediates the association between BRI and FBS, TC, TG, and atherogenic indices. Among the mechanisms that can be mentioned are the following: first, the breakdown of VAT through many steps can increase gluconeogenesis in the liver, hence reducing the very low-density lipoprotein (VLDL) output; this leads to impaired glucose tolerance and increased TG (42–45). Second, in inflammatory conditions, adipose tissue begins to release fat and produce cortisol and pro-inflammatory cytokines, such as adipokines, which itself affects metabolic health (42, 43).
The present study has some strengths that should be noted. First, to the best of our knowledge, this is the first study to evaluate the mediatory effect of inflammatory markers on the association between ABSI and BRI with cardiometabolic risk factors in overweight and obese women. Second, this study was conducted on obese and overweight Iranian women, yielding a detailed insight into this population. However, this study also has limitations that must be acknowledged. First, the relatively small sample size in this study clearly represents an avenue for improvement in future studies. Second, the cross-sectional design of the study precludes causal inferences being made. Third, to obtain the usual dietary intake, we used the FFQ, which is based on participants' memory, and may be subjected to recall bias. Fourth, some errors outside the operational control of the study while measuring may have occurred.
5. Conclusion
In summary, the present study found a significant positive association between ABSI and atherogenic indices. Moreover, we observed a significant positive association between BRI and FBS, TC, TG, and atherogenic indices. We revealed that these associations could be influenced by the mediation of inflammatory markers, including hs-CRP, MCP-1, TGF-β, and Galectin-3. However, the cross-sectional nature of our study does not allow us to attribute causality among these relationships. Further prospective studies with larger sample sizes are needed to better clarify this issue.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available from KM but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of KM. Requests to access these datasets should be directed to bWlyemFlaV9raEBzaW5hLnR1bXMuYWMuaXI=.
Ethics statement
The studies involving human participants were reviewed and approved by the study protocol was approved by the Ethics Committee of Tehran University of medical sciences (IR.TUMS.VCR.REC.1397.804) and is acknowledged by authors. All participants signed a written informed consent. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AM contributed to the conception and design. NV, MG, DK, CC, MR, and AM contributed to all experimental work. FA contributed to data and statistical analysis. KM supervised the whole project. All authors performed editing, approved the final version of this study for submission, participated in the finalization of the manuscript, approved the final draft and read and approved the final manuscript.
Funding
This study was supported by the Tehran University of Medical Sciences (Grant No: 96-01-161-34479/95-04-161-33893).
Acknowledgments
We thank all the participants in this study, as well as the School of Nutritional and Dietetics at Tehran University of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANOVA, analysis of variance; ANCOVA, analysis of covariance; AIP, atherogenic index of plasma; ABSI, A Body Shape Index; β, beta; BFP, body fat percentage; BFM, body fat mass; BRI, Body Roundness Index; BMI, body mass index; CVD, cardiovascular diseases; ELISA, enzyme-linked immunosorbent assay; FBS, fasting blood sugar; FFQ, Food Frequency Questionnaire; FFM, fat free mass; FMI, fat mass index; HC, hip circumference; HDL, high-density lipoprotein cholesterol; HF, heart failure; IPAQ, International Physical Activity Questionnaires; LDL, low-density lipoprotein cholesterol; LBM, lean body mass; MCP-1, monocyte chemotactic protein; MET, metabolic equivalent; PBF, percentage body fat mass; PAI-1, plasminogen activator inhibitor-1; PCOS, polycystic ovary syndrome; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglyceride; TyG, triglyceride and glucose; TGF-β, transforming growth factor-β ; VLDL, very low-density lipoprotein; VAT, visceral adipose tissue; VFA, visceral fat area; VFL, visceral fat level; WC, waist circumference; WHR, waist–hip ratio.
References
2. Van Itallie TB. Health implications of overweight and obesity in the United States. Ann Int Med. (1985) 103(Pt 2):983–8. doi: 10.7326/0003-4819-103-6-983
3. Racette SB, Deusinger SS, Deusinger RH. Obesity: overview of prevalence, etiology, and treatment. Phys Ther. (2003) 83:276–88. doi: 10.1093/ptj/83.3.276
4. Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. (2015) 4:363–70. doi: 10.1007/s13679-015-0169-4
5. Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol. (2021) 12:706978. doi: 10.3389/fendo.2021.706978
6. Office of the Surgeon G, Office of Disease P, Health P, Centers for Disease C, Prevention, National Institutes of H. Publications and Reports of the Surgeon General. The Surgeon General's Call To Action To Prevent and Decrease Overweight and Obesity. Rockville (MD): Office of the Surgeon General (US) (2001).
7. Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO. Obesity and cardiovascular diseases. Minerva Med. (2017) 108:212–28. doi: 10.23736/S0026-4806.17.05022-4
8. Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. (2003) 138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008
9. Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American heart association council on nutrition, physical activity, and metabolism: endorsed by the American college of cardiology foundation. Circulation. (2004) 110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E
10. Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. (2019) 92:98–107. doi: 10.1016/j.metabol.2018.10.011
11. Maessen MF, Eijsvogels TM, Verheggen RJ, Hopman MT, Verbeek AL, de Vegt F. Entering a new era of body indices: the feasibility of a body shape index and body roundness index to identify cardiovascular health status. PLoS ONE. (2014) 9:e107212. doi: 10.1371/journal.pone.0107212
12. Chang Y, Guo X, Chen Y, Guo L, Li Z, Yu S, et al. A body shape index and body roundness index: two new body indices to identify diabetes mellitus among rural populations in Northeast China. BMC Public Health. (2015) 15:794. doi: 10.1186/s12889-015-2150-2
13. Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. (2018) 61:103–13. doi: 10.1016/j.pcad.2018.06.004
14. Xu J, Zhang L, Wu Q, Zhou Y, Jin Z, Li Z, et al. Body roundness index is a superior indicator to associate with the cardio-metabolic risk: evidence from a cross-sectional study with 17,000 Eastern-China adults. BMC Cardiovasc Disord. (2021) 21:97. doi: 10.1186/s12872-021-01905-x
15. Li G, Wu HK, Wu XW, Cao Z, Tu YC, Ma Y, et al. The feasibility of two anthropometric indices to identify metabolic syndrome, insulin resistance and inflammatory factors in obese and overweight adults. Nutrition. (2019) 57:194–201. doi: 10.1016/j.nut.2018.05.004
16. Ji M, Zhang S, An R. Effectiveness of A Body Shape Index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev. (2018) 19:737–59. doi: 10.1111/obr.12666
17. Ito T, Ikeda U. Inflammatory cytokines and cardiovascular disease. Curr Drug Targets Inflamm Aller. (2003) 2:257–65. doi: 10.2174/1568010033484106
18. Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. (2012) 60:1249–56. doi: 10.1016/j.jacc.2012.04.053
19. Blanda V, Bracale UM, Di Taranto MD, Fortunato G. Galectin-3 in cardiovascular diseases. Int J Mol Sci. (2020) 21:232. doi: 10.3390/ijms21239232
20. Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci. (2009) 117:95–109. doi: 10.1042/CS20080581
21. Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. (2005) 3:1879–83. doi: 10.1111/j.1538-7836.2005.01420.x
22. Dabek J, Kułach A, Monastyrska-Cup B, Gasior Z. Transforming growth factor beta and cardiovascular diseases: the other facet of the 'protective cytokine'. Pharmacol Rep. (2006) 58:799–805.
23. Farhadnejad H, Teymoori F, Jahromi MK, Mokhtari E, Asghari G, Mirmiran P, et al. Correction: high dietary and lifestyle inflammatory scores are associated with increased risk of chronic kidney disease in Iranian adults. Nutr J. (2023) 22:6. doi: 10.1186/s12937-023-00839-8
24. Rouhani P, Amoushahi M, Keshteli AH, Saneei P, Afshar H, Esmaillzadeh A, et al. Dietary riboflavin intake in relation to psychological disorders in Iranian adults: an observational study. Sci Rep. (2023) 13:5152. doi: 10.1038/s41598-023-32309-w
25. Yarizadeh H, Mirzababaei A, Ghodoosi N, Pooyan S, Djafarian K, Clark CCT, et al. The interaction between the dietary inflammatory index and MC4R gene variants on cardiovascular risk factors. Clin Nutri. (2021) 40:488–95. doi: 10.1016/j.clnu.2020.04.044
26. Liu B, Liu B, Wu G, Yin F. Relationship between body-roundness index and metabolic syndrome in type 2 diabetes. Diabetes Metab Syndr Obes. (2019) 12:931–5. doi: 10.2147/DMSO.S209964
27. Bertoli S, Leone A, Krakauer NY, Bedogni G, Vanzulli A, Redaelli VI, et al. Association of body shape index (ABSI) with cardio-metabolic risk factors: a cross-sectional study of 6,081 Caucasian adults. PLoS ONE. (2017) 12:e0185013. doi: 10.1371/journal.pone.0185013
28. Olamoyegun MA, Oluyombo R, Asaolu SO. Evaluation of dyslipidemia, lipid ratios, and atherogenic index as cardiovascular risk factors among semi-urban dwellers in Nigeria. Ann Afr Med. (2016) 15:194–9. doi: 10.4103/1596-3519.194280
29. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Preventive medicine. (2016) 86:99–105. doi: 10.1016/j.ypmed.2016.01.022
30. Assessment of Physical Activity of Adolescents in Isfahan. J Shahrekord Uuniv Med Sci. (2001) 3:27–33.
31. Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain intake and the prevalence of hypertriglyceridemic waist phenotype in Tehranian adults. Am J Clin Nutr. (2005) 81:55–63. doi: 10.1093/ajcn/81.1.55
32. Dhana K, Ikram MA, Hofman A, Franco OH, Kavousi M. Anthropometric measures in cardiovascular disease prediction: comparison of laboratory-based versus non-laboratory-based model. Heart. (2015) 101:377–83. doi: 10.1136/heartjnl-2014-306704
33. Corbatón-Anchuelo A, Krakauer JC, Serrano-García I, Krakauer NY, Martínez-Larrad MT, Serrano-Ríos M, et al. Body shape index (ABSI) and hip index (HI) adjust waist and hip circumferences for body mass index, but only ABSI predicts high cardiovascular risk in the Spanish Caucasian population. Metab Syndr Relat Disord. (2021) 19:352–7. doi: 10.1089/met.2020.0129
34. Gentile M, Iannuzzo G, Mattiello A, Rubba F, Panico S, Rubba P. Association between body shape index and small dense LDL particles in a cohort of mediterranean women: findings from Progetto ATENA. J Clin Biochem Nutr. (2017) 61:130–4. doi: 10.3164/jcbn.17-13
35. Krakauer NY, Krakauer JC, A. new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. (2012) 7:e39504. doi: 10.1371/journal.pone.0039504
36. He S, Chen X. Could the new body shape index predict the new onset of diabetes mellitus in the Chinese population? PLoS ONE. (2013) 8:e50573. doi: 10.1371/journal.pone.0050573
37. El-Wakkad A, Hassan Nel M, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine. (2013) 61:682–7. doi: 10.1016/j.cyto.2012.11.010
38. Borges MD, Franca EL, Fujimori M, Silva SMC, de Marchi PGF, Deluque AL, et al. Relationship between proinflammatory cytokines/chemokines and adipokines in serum of young adults with obesity. Endocr Metab Immune Disord Drug Targets. (2018) 18:260–7. doi: 10.2174/1871530318666180131094733
39. Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes. (2003) 52:942–7. doi: 10.2337/diabetes.52.4.942
40. Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. (2013) 21:2264–71. doi: 10.1002/oby.20408
41. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart rhythm. (2019) 16:e301–e72. doi: 10.1016/j.hrthm.2019.05.007
42. Goran MI, Ball GDC, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metabol. (2003) 88:1417–27. doi: 10.1210/jc.2002-021442
43. Jialal I, Jialal G, Devaraj S, Adams-Huet B. The effect of increasing tertiles of waist circumference on cardio-metabolic risk, adipokines and biomarkers of inflammation and oxidative stress in nascent metabolic syndrome. J Diab Complicat. (2018) 32:379–83. doi: 10.1016/j.jdiacomp.2018.01.008
44. Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: association for weight management and obesity prevention) NAASO, the obesity society) the American society for nutrition) and the American diabetes association. Am J Clin Nutr. (2007) 85:1197–202. doi: 10.1093/ajcn/85.5.1197
Keywords: a body shape index, body roundness index, inflammation markers, obesity, cardiometabolic
Citation: Mirzababaei A, Abaj F, Khosravinia D, Ghorbani M, Valisoltani N, Clark CCT, Radmehr M and Mirzaei K (2023) The mediatory effect of inflammatory markers on the association between a body shape index and body roundness index with cardiometabolic risk factor in overweight and obese women: a cross-sectional study. Front. Nutr. 10:1178829. doi: 10.3389/fnut.2023.1178829
Received: 14 March 2023; Accepted: 09 May 2023;
Published: 09 June 2023.
Edited by:
Roberto Fernandes Da Costa, Federal University of Rio Grande do Norte, BrazilReviewed by:
Anna Kȩska, Józef Piłsudski University of Physical Education in Warsaw, PolandMaria Consuelo Velazquez-Alva, Autonomous Metropolitan University Xochimilco Campus, Mexico
Stephan Garcia Andrade Silva, Federal University of São Paulo, Brazil
Copyright © 2023 Mirzababaei, Abaj, Khosravinia, Ghorbani, Valisoltani, Clark, Radmehr and Mirzaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khadijeh Mirzaei, bWlyemFlaV9raEBzaW5hLnR1bXMuYWMuaXI=
 Atieh Mirzababaei1
Atieh Mirzababaei1 Darya Khosravinia
Darya Khosravinia Neda Valisoltani
Neda Valisoltani Cain C. T. Clark
Cain C. T. Clark Mina Radmehr
Mina Radmehr