
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 08 June 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1175994
This article is part of the Research Topic Dietary Patterns in Cancer Prevention and Survival View all 17 articles
Background: Despite increasing evidence that has shown the association of ultra-processed foods (UPFs) with cancer risk, the results remain inconclusive. We, therefore, conducted the meta-analysis to clarify the association by including recently published studies.
Methods: A comprehensive search was conducted in PubMed, Embase, and Web of Science to identify all relevant studies from inception to January 2023. To pool data, fixed-effects or random-effects models were used where appropriate. Subgroup analyses, sensitivity analyses, and publication bias tests were performed.
Results: A total of 13 studies (4 cohort studies and 9 case–control studies) were included in the analysis, with a total of 625,738 participants. The highest UPFs consumption was associated with increased risk of colorectal cancer (OR = 1.23, 95% CI: 1.10–1.38), colon cancer (OR = 1.25, 95% CI: 1.14–1.36), and breast cancer (OR = 1.10, 95% CI: 1.00–1.20) but not rectal cancer (OR = 1.18, 95% CI: 0.97–1.43) and prostate cancer (OR = 1.03, 95% CI: 0.93–1.12). In addition, the subgroup analyses showed that a positive association between UPFs consumption and colorectal cancer was observed among men (OR = 1.31, 95% CI: 1.15–1.50), whereas no significant association was observed among women (OR = 1.10, 95% CI: 0.94–1.29).
Conclusion: The present meta-analysis suggests that high UPFs consumption is associated with a significantly increased risk of certain site-specific cancers, especially the digestive tract and some hormone-related cancers. However, further rigorously designed prospective and experimental studies are needed to better understand causal pathways.
Cancer is one of the leading causes of death worldwide (1). According to a report from the World Health Organization, cancer is responsible for almost 10 million deaths per year, and every sixth death in the world is attributed to cancer (2, 3). It is expected that new cases of cancer will increase to 28.4 million by 2040, and the burden of cancer will double in the next 20 years. Therefore, there is a need for more research on exploring and intervening in potential risk factors for cancer. It is reported that a substantial proportion of cancer cases could be prevented by eliminating risk factors (4). In addition to genetic predisposition, numerous modifiable factors have also been implicated in regulating tumorigenesis and cancer development, such as a sedentary lifestyle (5) and unhealthy dietary patterns (6). Thus, further study on lifestyle modification is warranted to better identify targets for the intervention of cancer.
Evidence of the link between the degree of food processing and increased cancer risk is emerging (7). Recent global estimates demonstrate dramatical changes in the processing of foodstuffs, which have witnessed a marked increase in processed food availability, especially during the historically unprecedented SARS-CoV-2 pandemic lockdown setting (8), with ultra-processed foods (UPFs) accounting for more than half of total energy intake (9). Indeed, UPFs are usually characterized by their poor nutritional composition, high energy density, and the presence of components derived from food processing or packaging, with potential carcinogenic properties. Previous studies have investigated the possible linkage between UPFs consumption and chronic non-communicable diseases (10, 11) and related morbidity (12) and mortality (13), including three systematic reviews on cancer (14–16). Nevertheless, existing systematic reviews evaluating the associations of UPFs consumption with cancer did not get quantitative synthesis results limited by the number of studies available for inclusion (14). In addition, although there is evidence suggesting the potential carcinogenic pathways underlying the association between UPFs and cancer risk, previous studies have only focused on the most common cancer sites, such as breast cancer, and no previous study has assessed the effect of UPFs on a comprehensive range of cancers. Furthermore, several additional studies have been published on the effect of UPFs consumption on various types of cancer (17); however, these results are conflicting, leading to insufficient generalizability of the findings.
To bridge the knowledge gap, in the present study, we conducted the current comprehensive and updated systematic review and further explored the association between UPFs consumption and different types of cancer.
The present systematic review and meta-analysis were carried out in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18).
The electronic databases of PubMed, Embase, and Web of Science were comprehensively searched for relevant studies from inception to January 2023. The following search terms were used: (ultra-processed OR processed food OR ultraprocessed) and (neoplasm OR tumor* OR cancer* OR malignant* OR carcinoma OR adenocarcinoma OR neoplasia). There were no restrictions on language. Further studies and relevant gray literature were manually searched by checking the references of the potentially eligible articles.
This review included observational studies (cross-sectional, cohort, and case–control) that investigated the association between UPFs consumption and cancer risk and reported the results as relative risks (RRs) or odds ratios (ORs) with 95% confidence intervals (CIs). The UPFs were defined by the NOVA food classification system. The outcome of interest is specific cancer type, and non-malignant abnormalities (e.g., adenomas) were not considered. We excluded experimental studies, review articles, letters, editorials, and abstracts without full texts.
Data extraction was carried out from eligible articles using a predefined checklist. The following information was extracted: the first author's name, year of publication, country, design, follow-up time (for cohort studies), total subjects, the number of cases, type of cancer, age, gender, exposure, methods of exposure assessment, ORs, or RRs (95% CIs), and adjusted (confounding) variables. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used to assess the quality of the included studies (19). Scores ranged from 0 to 9 with a score of ≥7 being considered as of high quality. Data collection and quality assessment processes were independently performed by YL and G-PW. Any discrepancies in data extraction and quality assessment were resolved by discussion with the third author.
All data were analyzed using STATA version 14.0 (StataCorp, College Station, TX, USA). The ORs with 95%CIs for UPF consumption and cancer risk were pooled using fixed-effects or random-effects models where appropriate. Heterogeneity was assessed using the I2 value and Q-test (P-heterogeneity). If the P-heterogeneity of the Q-test ≤0.10 or I2 ≥50% indicated a high degree of heterogeneity among studies, then a random-effects model was used. UPFs consumption was analyzed as a continuous variable (per 10% increment) and as a categorical variable. Subgroup analyses were conducted according to a series of key variables that might influence the association between UPFs and cancer, including tumor subtype, sex (for colorectal), and menopausal status (for breast cancer). Sensitivity analyses were carried out by removing each study and recalculating the pooled effect estimates (i.e., one study removed analysis). Publication bias was assessed by formal testing by Egger's test and Begg's test.
The flow chart of the literature screening and selection process is presented in Figure 1. A total of 13 studies met our inclusion criteria and were included in the present systemic review (17, 20–31). All the studies with a total sample size of 625,738 participants were published from 2018. Of these studies, four were cohort studies and nine were case–control designs. In total, five studies were conducted in America, five in Europe, two in Africa, and one in Asia. Of the 13 eligible studies, six focused on colorectal cancer, six on breast cancer, four on prostate cancer, and two on pancreatic cancer, chronic lymphocytic leukemia, and central nervous system tumors. The degree of processing of foods was classified according to the NOVA classification system. The general characteristics of included studies are described in Table 1.
In total, three prospective cohort studies with a total of 508,654 participants and three case–control studies with a total of 8,424 participants reported the association between UPFs consumption and the risk of colorectal cancer. The highest consumption of UPFs was found to be associated with an increased risk of colorectal cancer. The pooled OR was 1.23 (95% CI: 1.10–1.38), with moderate evidence of heterogeneity (I2 = 67.2%, P = 0.01, Figure 2A). There was no evidence of significant publication bias with Begg's test (P = 0.54) and Egger's test (P = 0.27). Sensitivity analyses suggested that the pooled estimate of colorectal cancer risk did not apparently modify any one study, confirming the stability of the present results. Each 10% increase in UPFs consumption was associated with a 4% higher risk of colorectal cancer (OR = 1.04, 95% CI: 1.01–1.07; I2= 55.9%, P=0.06, Figure 2B). Subgroup analyses showed that a positive association between UPF consumption and colorectal cancer was observed among men (OR = 1.31, 95% CI: 1.15–1.50), whereas no significant association was observed among women (OR = 1.10, 95% CI: 0.94–1.29). The subgroup analyses are presented in Table 2.
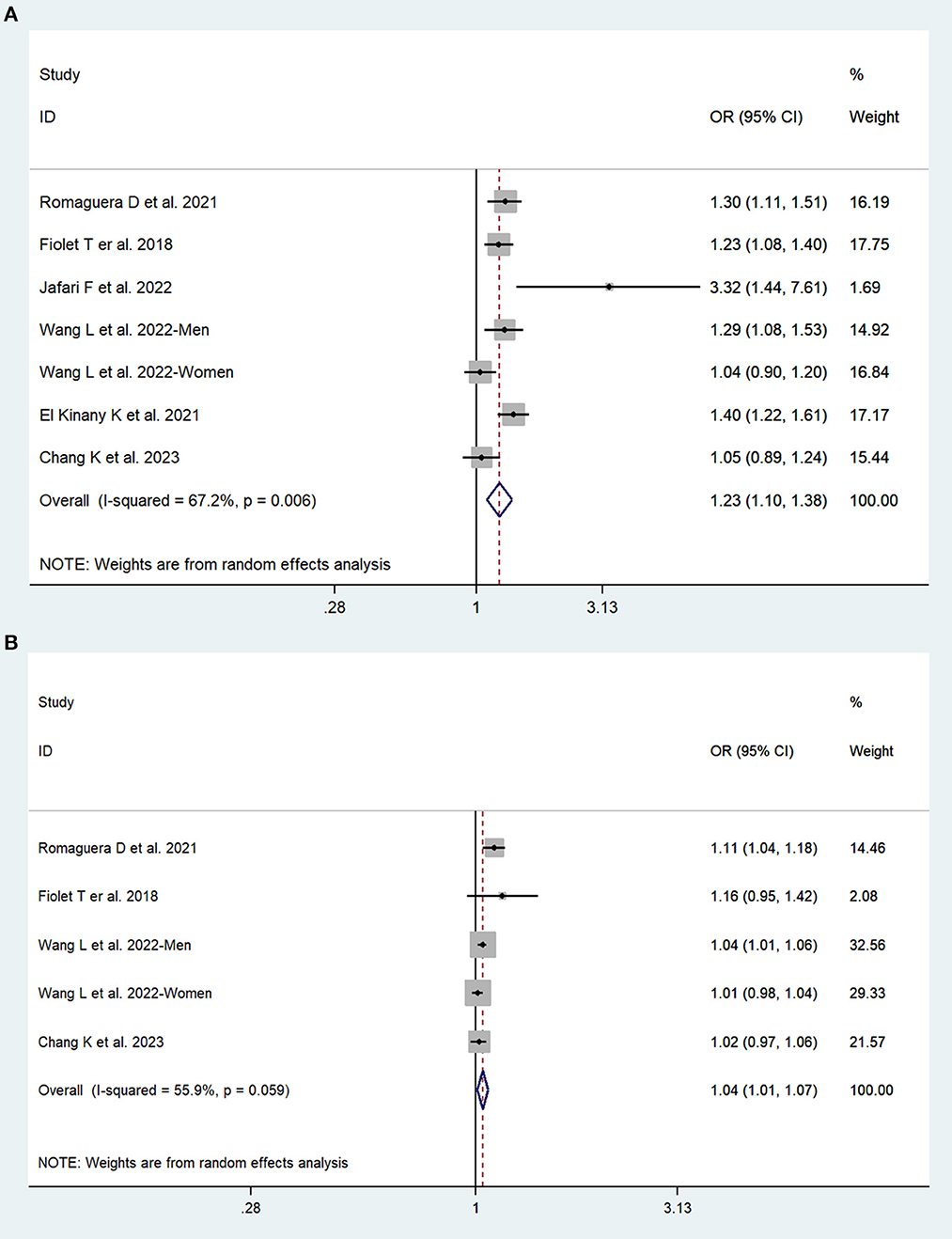
Figure 2. Forest plots of pooled ORs for UPFs and colorectal cancer. (A) The highest category UPFs compared with the lowest category UPFs. (B) 10% increase in UPFs consumption.
In total, two cohort studies with a total of 279,585 participants and four case–control studies with a total of 5,059 participants assessed the link between UPFs consumption and breast cancer risk. This meta-analysis showed that greater UPFs consumption was associated with higher odds of breast cancer (OR: 1.10, 95%CI: 1.00–1.20). Heterogeneity between studies was not significant (I2 = 45.4%, P = 0.10, Figure 3A). Publication bias was tested by Egger's test (P = 0.03) and Begg's test (P = 0.19). Each 10% increase in UPFs consumption was not associated with the risk of breast cancer (OR = 1.03, 95% CI: 0.98–1.09, I2= 58.8%, P = 0.09, Figure 3B).
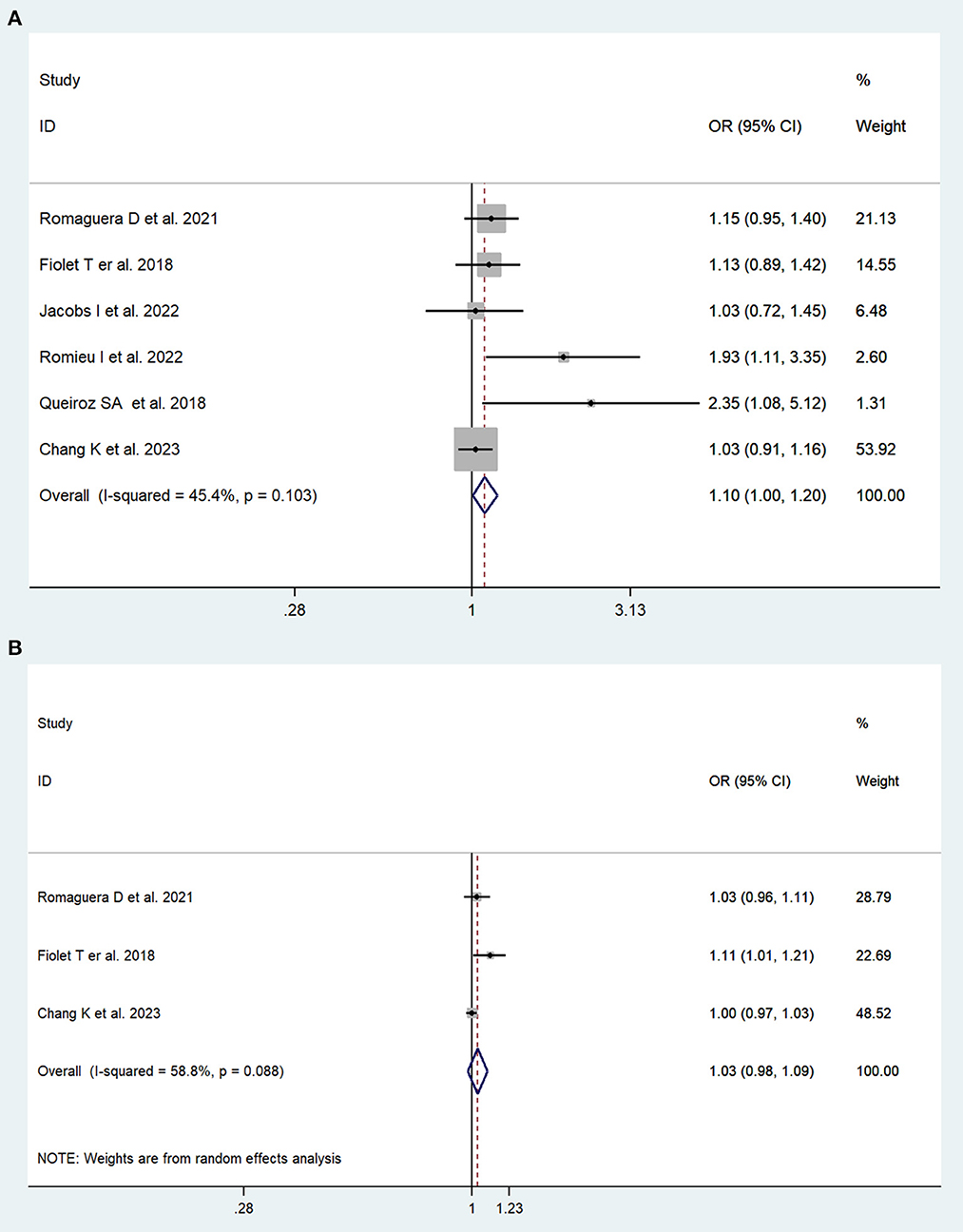
Figure 3. Forest plots of pooled ORs for UPFs and breast cancer. (A) The highest category UPFs compared with the lowest category UPFs. (B) 10% increase in UPFs consumption.
In total, two cohort studies with a total of 220,247 participants and two case–control studies with a total of 6,123 participants reported the association between UPFs consumption and prostate cancer risk. There was no significant association between UPFs consumption and prostate cancer. The pooled OR (95%CI) for the highest UPFs consumption was 1.03 (0.93–1.12), with no significant heterogeneity between studies (I2 = 0%, P = 0.75, Figure 4).
In total, two studies were available regarding three other types of cancer including pancreatic cancer, chronic lymphocytic leukemia, and central nervous system tumors. One study investigated the link between UPFs consumption and other multiple cancer sites with the findings shown in Figure 4.
The present systematic review comprehensively quantified the association between UPFs consumption and various types of cancer risk integrating four prospective cohort studies and nine case–control studies. Our findings indicated that greater intake of UPFs was associated with increased odds of colorectal and breast cancer. Every 10% increase in the proportion of UPFs in the diet was associated with a 4% higher risk of colorectal cancer. In addition, the results of subgroup analyses proposed that a significant association of UPFs consumption with an increased risk of colorectal cancer was noted in men but not among women.
Our findings provided robust evidence that a high intake of processed foods increases the risk of colorectal cancer, which has been previously reported in recent systematic reviews (32). For example, a meta-analysis of prospective cohort studies showed that compared with the lowest category of processed meat intake, the highest category was associated with higher overall colorectal cancer risk (33). Similarly, significant positive associations were also observed for colon cancer. In addition, our results are also consistent with previous meta-analyses and broaden whole of dietary pattern analyses. The systematic reviews found that the dietary inflammatory index characterized by excess consumption of processed foods, including processed meats, sweets, fried foods, and refined grains appears to be associated with cancer risk (34), while Mediterranean-style diets, which are rich in fruits, vegetables, extra virgin olive oil, whole grains, and other unprocessed or minimally processed foods, reduce the risk of colorectal cancer by 17% (35). However, these dietary patterns are often unable to determine the industrial processing level of foods. The objective and standardized criteria of the NOVA classification system were used in all the included studies to distinguish UPFs from other foods based on the nature, extent, and purpose of food processing (36–38), which can provide novel insights into understanding the role of food processing level in the development of cancer (39). Of note, the stratified analyses showed a positive association between UPFs consumption and increased risk of colorectal cancer in men but not among women. The findings are somewhat concordant with another previous ultra-processed food inflammation study, which suggests that men are more predisposed to the carcinogenic effects of diet (40). Potential explanations for such different sex patterns may involve the effect of sex hormones or genetics (41). Further studies are required to clarify these findings.
In the analysis of breast cancer, a positive association was found between higher UPFs consumption and breast cancer risk, which is consistent with those from the prior meta-analyses. Previously, a meta-analysis combining data from 15 studies showed that the highest processed meat intake was related to a 9% increased risk of breast cancer compared with the lowest intake (32). In another previous analysis, a similar magnitude positive association was found between processed meat intake and breast cancer risk by comparing the highest with the lowest category (42). It seems that menopausal status may influence the association between UPFs consumption and breast cancer risk. It was found that higher processed meat consumption was associated with a 9% greater risk of postmenopausal breast cancer; however, such a positive association was not observed for premenopausal breast cancer (42). The present study examining the association by menopausal status suggested no significant associations with the intake of UPFs for breast cancer in premenopausal and postmenopausal women. In addition, breast cancer is a heterogeneous disease, with potentially distinct etiology for different hormone receptor statuses, and it has been suggested that estrogen receptor-positive breast tumors (ER+) are more strongly associated with hormone-related factors than estrogen receptor-negative tumors (ER-) (43); therefore, assessing risk factors for breast cancer incorporating molecular pathological information may confer even greater insights (44). ER status was reported in one included study; it shows a significant association for UPFs among ER + breast cancer, while no association was observed in ER-tumor risk (27). Thus, further studies are required to understand the heterogeneity of this relationship by molecular subtypes according to the menopausal status of breast cancer.
The present meta-analysis has some strengths. This is the first meta-analysis comprehensively quantitatively summarizing the evidence on the association between UPFs consumption and various types of cancer, providing strong implications for dietary policies and guidelines. In addition, the present meta-analysis included large sample size and high-quality epidemiological data, with the standardized assessment of processed diet intake using the NOVA system, along with sensitivity analyses and detailed subgroup analyses, ensuring greater precision and reliability of the results. Despite the interesting results of the present meta-analysis, some limitations should be considered. First, although we include several prospective cohorts with large sample sizes, some of the included studies are case–control designs, which does not allow for the identification of a causal link between the exposure and outcome. Second, cancer is often described as the result of complex interactions between biological, social, and psychological factors, although most included studies have adjusted for a wide range of potential confounders, other unmeasured or inadequately measured factors, for example, genetic and environmental factors, may result in residual confounding. Third, of the articles included, UPFs intake was generally evaluated through food frequency questionnaires or food records that were not specifically designed to identify UPFs, which can result in some degree of misclassification error, thus leading to bias associations. Further well-designed studies that address such limitations are warranted to confirm the associations.
Although the underlying pathways of our findings have not yet been fully elucidated, several mechanisms have been proposed to account for the potential carcinogenicity of UPFs. First, UPFs often have a poorer nutritional quality compared to minimally processed foods, which tend to be rich in unfavorable nutritional components, such as saturated fat, added sugar, energy density, and salt, along with lower fiber and vitamins. Meanwhile, a randomized controlled trial conducted in inpatients found that more ultra-processed diet intake could lead to excess calorie intake and substantial weight gain (45). Poor diet quality together with obesogenic properties are all important factors in driving their detrimental impact on cancer. Second, food additives in the processing or packaging of UPFs, such as emulsifiers, preservatives, colors, and flavors, have also been suggested as potential mechanisms linking UPFs to higher cancer risk. Some contaminants in UPFs have been linked to proinflammation potential (46), endocrine-disrupting effects (47), and dysbiosis (48), which have been proven to promote carcinogenesis in epidemiological, clinical, and experimental studies. For example, it is notably suggested that consumption of UPFs was associated with an elevated level of inflammatory biomarkers, such as IL-6 concentration, which are involved in tumor progression at almost every step including initiation, progression, and metastasis (49). Moreover, consumption of UPFs may increase exposure to endocrine-disrupting chemicals, including bisphenol A and phthalates, leading to a persistent epigenetic change in genes and subsequently stimulating the proliferation of hormone-sensitive tissues in a tumor sense. In addition, UPFs could also alter gut microbiota composition and function unfavorably (50), which, in turn, increase cancer risk through multiple molecular signals, including inhibiting T-cell activity and promoting DNA damage (51). Further investigation into the mechanistic pathways is warranted to better identify targets for intervention.
The present systematic review showed that the high consumption of UPFs was associated with an increased risk of certain site-specific cancers, especially the digestive tract and some hormone-related cancers including colorectal and breast, providing a more comprehensive understanding of the potential implications in the development of cancer associated with processed diet. The findings support the importance of public health by boosting prevention policies to limit UPFs consumption and promoting healthier nutritional status for primary cancer prevention. Furthermore, well-designed studies are needed to better strengthen the evidence of the association between UPFs and cancer risk.
G-YZ conceived and designed the study. G-PW, H-NC, G-QC, and YL analyzed the data. G-PW and YL wrote the manuscript. All authors provided critical revisions of the manuscript and approved the final manuscript.
This study was supported by the Shandong Provincial Key Research and Development Program (grant no. 2019GSF108196), the Science and Technology Project of Jinan (grant no. 2019-1-17, 2022-1-25), the Center of China–US Sports Economics and Health Engineering of Shandong (grant no. SDCA20191013), and the Academic Promotion Programme of Shandong First Medical University (grant no. 2019QL013). The funding sources had no role in study design, data analysis and interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. (2018) 4:1553–68. doi: 10.1200/JCO.2018.36.15_suppl.1568
2. Cancer Research UK. Worldwide Cancer Statistics. Available online at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer
3. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. (2021) 127:3029–30. doi: 10.1002/cncr.33587
4. WHO. Available online at: https://www.who.int/news-room/fact-sheets/detail/cancer
5. Dixon-Suen SC, Lewis SJ, Martin RM, English DR, Boyle T, Giles GG, et al. Physical activity, sedentary time and breast cancer risk: a Mendelian randomisation study. Br J Sports Med. (2022) 56:1157–70. doi: 10.1136/bjsports-2021-105132
6. Grosso G, Bella F, Godos J, Sciacca S, Del Rio D, Ray S, et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev. (2017) 75:405–19. doi: 10.1093/nutrit/nux012
7. Kazemi A, Barati-Boldaji R, Soltani S, Mohammadipoor N, Esmaeilinezhad Z, Clark CCT, et al. Intake of various food groups and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. (2021) 12:809–49. doi: 10.1093/advances/nmaa147
8. De Nucci S, Zupo R, Castellana F, Sila A, Triggiani V, Lisco G, et al. Public health response to the SARS-CoV-2 pandemic: concern about ultra-processed food consumption. Foods. (2022) 11:950. doi: 10.3390/foods11070950
9. Kelly B, Jacoby E. Public health nutrition special issue on ultra-processed foods. Public Health Nutr. (2018) 21:1–4. doi: 10.1017/S1368980017002853
10. Wang M, Du X, Huang W, Xu Y. Ultra-processed foods consumption increases the risk of hypertension in adults: a systematic review and meta-analysis. Am J Hypertens. (2022) 35:892–901. doi: 10.1093/ajh/hpac069
11. Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol. (2022) 7:1128–40. doi: 10.1016/S2468-1253(22)00169-8
12. Crimarco A, Landry MJ, Gardner CD. Ultra-processed foods, weight gain, and co-morbidity risk. Curr Obes Rep. (2022) 11:80–92. doi: 10.1007/s13679-021-00460-y
13. Chen X, Chu J, Hu W, Sun N, He Q, Liu S, et al. Associations of ultra-processed food consumption with cardiovascular disease and all-cause mortality: UK Biobank. Eur J Public Health. (2022) 32:779–85. doi: 10.1093/eurpub/ckac104
14. Chen X, Zhang Z, Yang H, Qiu P, Wang H, Wang F, et al. Consumption of ultra-processed foods and health outcomes: a systematic review of epidemiological studies. Nutr J. (2020) 19:86. doi: 10.1186/s12937-020-00604-1
15. Elizabeth L, Machado P, Zinöcker M, Baker P, Lawrence M. Ultra-processed foods and health outcomes: a narrative review. Nutrients. (2020) 12:1955. doi: 10.3390/nu12071955
16. Kliemann N, Al Nahas A, Vamos EP, Touvier M, Kesse-Guyot E, Gunter MJ, et al. Ultra-processed foods and cancer risk: from global food systems to individual exposures and mechanisms. Br J Cancer. (2022) 127:14–20. doi: 10.1038/s41416-022-01749-y
17. Wang L, Du M, Wang K, Khandpur N, Rossato SL, Drouin-Chartier J-P, et al. Association of ultra-processed food consumption with colorectal cancer risk among men and women: results from three prospective US cohort studies. BMJ. (2022) 378:e068921. doi: 10.1136/bmj-2021-068921
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
20. Romaguera D, Fernández-Barrés S, Gracia-Lavedán E, Vendrell E, Azpiri M, Ruiz-Moreno E, et al. Consumption of ultra-processed foods and drinks and colorectal, breast, and prostate cancer. Clin Nutr. (2021) 40:1537–45. doi: 10.1016/j.clnu.2021.02.033
21. Fiolet T, Srour B, Sellem L, Kesse-Guyot E, Allès B, Méjean C, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. Bmj. (2018) 360:k322. doi: 10.1136/bmj.k322
22. Trudeau K, Rousseau MC, Parent M. Extent of food processing and risk of prostate cancer: the PROtEuS study in Montreal, Canada. Nutrients. (2020) 12:637. doi: 10.3390/nu12030637
23. Jacobs I, Taljaard-Krugell C, Wicks M, Cubasch H, Joffe M, Laubscher R, et al. Degree of food processing and breast cancer risk in black urban women from Soweto, South African: the South African Breast Cancer study. Br J Nutr. (2022) 128:2278–89. doi: 10.1017/S0007114522000423
24. El Kinany K, Huybrechts I, Hatime Z, El Asri A, Boudouaya HA, Deoula MMS, et al. Food processing groups and colorectal cancer risk in Morocco: evidence from a nationally representative case-control study. Eur J Nutr. (2022) 61:2507–15. doi: 10.1007/s00394-022-02820-3
25. Solans M, Fernández-Barrés S, Romaguera D, Benavente Y, Marcos-Gragera R, Gracia-Lavedan E, et al. Consumption of ultra-processed food and drinks and chronic lymphocytic Leukemia in the MCC-Spain study. Int J Environ Res Public Health. (2021) 18:5457. doi: 10.3390/ijerph18105457
26. Zhong GC, Zhu Q, Cai D, Hu JJ Dai X, Gong JP, et al. Ultra-processed food consumption and the risk of pancreatic cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. (2023) 152:835–44. doi: 10.1002/ijc.34290
27. Romieu I, Khandpur N, Katsikari A, Biessy C, Torres-Mejía G, Ángeles-Llerenas A, et al. Consumption of industrial processed foods and risk of premenopausal breast cancer among Latin American women: the PRECAMA study. BMJ Nutr Prev Health. (2022) 5:1–9. doi: 10.1136/bmjnph-2021-000335
28. Esposito S, Bonaccio M, Ruggiero E, Costanzo S, Di Castelnuovo A, Gialluisi A, et al. Food processing and risk of central nervous system tumours: a preliminary case-control analysis from the MEditerranean DIet in relation to CancEr of brAin (MEDICEA) study. Clin Nutr. (2023) 42:93–101. doi: 10.1016/j.clnu.2022.11.016
29. Jafari F, Yarmand S, Nouri M, Nejad ET, Ramezani A, Sohrabi Z, et al. Ultra-processed food intake and risk of colorectal cancer: a matched case-control study. Nutr Cancer. (2022) 2022:1–10. doi: 10.1080/01635581.2022.2125990
30. Queiroz SA. Sousa IMd, Silva F, Lyra CdO, Fayh APT. Nutritional and environmental risk factors for breast cancer: a case-control study. Social Sci Med. (2018) 28:28723. doi: 10.15448/1980-6108.2018.2.28723
31. Chang K, Millett C, Rauber F, Levy RB, Huybrechts I, Kliemann N, et al. Ultra-processed food consumption, cancer risk, and cancer mortality: a prospective cohort study of the UK Biobank. Lancet. (2022) 400:S31. doi: 10.1016/S0140-6736(22)02241-3
32. Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. (2021) 36:937–51. doi: 10.1007/s10654-021-00741-9
33. Händel MN, Rohde JF, Jacobsen R, Nielsen SM, Christensen R, Alexander DD, et al. Processed meat intake and incidence of colorectal cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Clin Nutr. (2020) 74:1132–48. doi: 10.1038/s41430-020-0576-9
34. Li D, Hao X, Li J, Wu Z, Chen S, Lin J, et al. Dose-response relation between dietary inflammatory index and human cancer risk: evidence from 44 epidemiologic studies involving 1,082,092 participants. Am J Clin Nutr. (2018) 107:371–88. doi: 10.1093/ajcn/nqx064
35. Morze J, Danielewicz A, Przybyłowicz K, Zeng H, Hoffmann G, Schwingshackl L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur J Nutr. (2021) 60:1561–86. doi: 10.1007/s00394-020-02346-6
36. Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. (2018) 21:5–17. doi: 10.1017/S1368980017000234
37. Monteiro CA, Cannon G, Lawrence M, Costa Louzada ML, Pereira Machado P. FAO Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System. (2019).
38. Monteiro CA, Cannon G, Levy RB, Moubarac JC, Jaime P, et al. A NOVA The star shines bright World Nutrition. (2016) p. 28–38.
39. Sneed NM, Ukwuani S, Sommer EC, Samuels LR, Truesdale KP, Matheson D, et al. Reliability and validity of assigning ultraprocessed food categories to 24-h dietary recall data. Am J Clin Nutr. (2023) 117:182–90. doi: 10.1016/j.ajcnut.2022.10.016
40. Petimar J, Smith-Warner SA, Fung TT, Rosner B, Chan AT, Hu FB, et al. Recommendation-based dietary indexes and risk of colorectal cancer in the nurses' health study and health professionals follow-up study. Am J Clin Nutr. (2018) 108:1092–103. doi: 10.1093/ajcn/nqy171
41. Kim H, Giovannucci EL. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. (2017) 28:1–4. doi: 10.1007/s10552-016-0831-5
42. Anderson JJ, Darwis NDM, Mackay DF, Celis-Morales CA, Lyall DM, Sattar N, et al. Red and processed meat consumption and breast cancer: UK Biobank cohort study and meta-analysis. Eur J Cancer. (2018) 90:73–82. doi: 10.1016/j.ejca.2017.11.022
43. Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. (2004) 13:1558–68. doi: 10.1158/1055-9965.1558.13.10
44. Larsson SC, Bergkvist L, Wolk A. Long-term meat intake and risk of breast cancer by oestrogen and progesterone receptor status in a cohort of Swedish women. Eur J Cancer. (2009) 45:3042–6. doi: 10.1016/j.ejca.2009.04.035
45. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. (2019) 30:226. doi: 10.1016/j.cmet.2019.05.020
46. Lane MM, Lotfaliany M, Forbes M, Loughman A, Rocks T, O'Neil A, et al. Higher ultra-processed food consumption is associated with greater high-sensitivity C-reactive protein concentration in adults: cross-sectional results from the melbourne collaborative cohort study. Nutrients. (2022) 14:3309. doi: 10.3390/nu14163309
47. Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ Int. (2019) 131:105057. doi: 10.1016/j.envint.2019.105057
48. Cuevas-Sierra A, Milagro FI, Aranaz P, Martínez JA, Riezu-Boj JI. Gut microbiota differences according to ultra-processed food consumption in a Spanish population. Nutrients. (2021) 13:2710. doi: 10.3390/nu13082710
49. Martínez Leo EE, Peñafiel AM, Hernández Escalante VM, Cabrera Araujo ZM. Ultra-processed diet, systemic oxidative stress, and breach of immunologic tolerance. Nutrition. (2021) 91–92:111419. doi: 10.1016/j.nut.2021.111419
50. Fernandes AE, Rosa PWL, Melo ME, Martins RCR, Santin FGO, Moura A, et al. Differences in the gut microbiota of women according to ultra-processed food consumption. Nutr Metab Cardiovasc Dis. (2023) 33:84–9. doi: 10.1016/j.numecd.2022.09.025
Keywords: ultra-processed foods (UPFs), colorectal cancer, breast cancer, systematic review, meta-analysis
Citation: Lian Y, Wang G-P, Chen G-Q, Chen H-N and Zhang G-Y (2023) Association between ultra-processed foods and risk of cancer: a systematic review and meta-analysis. Front. Nutr. 10:1175994. doi: 10.3389/fnut.2023.1175994
Received: 28 February 2023; Accepted: 16 May 2023;
Published: 08 June 2023.
Edited by:
Raul Zamora-Ros, Institut d'Investigacio Biomedica de Bellvitge (IDIBELL), SpainReviewed by:
Roberta Zupo, University of Bari Aldo Moro, ItalyCopyright © 2023 Lian, Wang, Chen, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang-Yong Zhang, Z3Vhbmd5b25nemhhbmdAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.