- 1Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Iran
- 2Department of Biostatistics and Epidemiology, Kashan University of Medical Sciences, Kashan, Iran
Background: Both sleep time and quality can be associated with overweight or obesity. In obese people, visceral fat tissue develops, which results in an increment in the production of cytokines. The increased production of inflammatory cytokines can disturb the sleep/wake cycle. Therefore, weight loss by reducing fat tissue can improve sleep disorders. Intermittent fasting diets are popular and effective diets that can decrease body weight and improve anthropometric data and body composition. The present study aimed to evaluate the effect of Alternate-day Modified Fasting (ADMF) on sleep quality, body weight, and daytime sleepiness.
Methods: Classification of 56 obese or overweight women, based on age and body mass index (BMI), was done using stratified randomization. Then individuals were assigned to the ADMF group (intervention) or Daily Calorie Restriction (CR) group (control) using the random numbers table for 8 weeks. We measured the Pittsburgh sleep quality Index (PSQI), weight, BMI, and the Epworth sleepiness scale (ESS) as primary outcomes and assessed subjective sleep quality (SSQ), sleep latency, sleep disturbances, habitual sleep efficiency, daytime dysfunction, and sleep duration as secondary outcomes at baseline and after the study.
Results: Following an ADMF diet resulted in a greater decrease in weight (kg) [−5.23 (1.73) vs. −3.15 (0.88); P < 0.001] and BMI (kg/m2) [−2.05 (0.66) vs. −1.17 (0.34); P < 0.001] compared to CR. No significant differences were found in the changes of PSQI [−0.39 (1.43) vs. −0.45 (1.88); P = 0.73] and ESS [−0.22 (1.24) vs. −0.54 (1.67); P = 0.43] between two groups. Also, following the ADMF diet led to significant changes in SSQ [−0.69 (0.47) vs. −0.08 (0.40); P = <0.001], and daytime dysfunction [−0.65 (0.57) vs. 0.04 (0.75); P: 0.001] in compare with CR diet.
Conclusion: These results suggested that an ADMF could be a beneficial diet for controlling body weight and BMI. The ADMF diet didn’t affect PSQI and ESS in women with overweight or obesity but significantly improved SSQ and daytime dysfunction.
Clinical Trial Registration: The Iranian Registry of Clinical Trials (IRCT20220522054958N3), https://www.irct.ir/trial/64510.
Background
Sleep disorders affect nearly one-third of adults. The association between sleep quality and food intake has been shown in studies (1). Both people with severe and moderate obesity are affected by low sleep quality (2, 3). The increment of visceral adipose tissue results in the release of inflammatory cytokines that may lead to a disturbance of the sleep-wake cycle (3). Current studies have shown a bidirectional relation between sleep and oxidative stress and inflammation. It has been shown that extremely long sleep duration and sleep disturbances could be related to increased levels of IL-6 and c-reactive protein, while insufficient sleep duration with IL-6 (4). Therefore, both duration and sleep quality can ameliorate with a decrease in weight (5). Gangwisch et al. showed that higher BMI is related to lower sleep duration (6). The first-line therapy for the reduction of weight in individuals with obesity or overweight is calorie restriction (7). Adherence to conventional diets for weight loss is low because of daily energy restriction (8). In recent years, the fasting diet has been proposed as an unconventional diet for losing weight [17]. In addition to losing body weight, it improves metabolic health (9). Among the different fasting methods that have been investigated, the ADMF diet is known to be an effective diet to lose weight. ADMF comprises intermittent periods of feasting and fasting, on alternate days. Some studies have shown a 3–7% decrease in weight under an ADMF diet during 8–12 weeks (10). Compared to CR, intermittent fasting diets have exhibited greater participant compliance over longer periods (11). What is not clear is whether calorie restriction or a fasting diet will further ameliorate body weight (7) and, after that, affect the quality of sleep. Several studies suggest that alternate-day fasting compared with CR could preserve muscle mass and reduce visceral adipose area (12). A systematic review reported that alternate-day fasting diets reduced body weight similar to CR (7, 13). Hutchison et al. have shown that alternate-day fasting leads to greater weight loss compared with CR (14). Recent evidence has reported that calorie restriction increases the quality of sleep (15–17), but studies on the effect of intermittent fasting diets on the quality of sleep are limited. Teong et al. showed that a significant change wasn’t found between CR and intermittent fasting diet on the sleep quality of women with overweight or obesity (18). Therefore, to achieve more definitive results in this field, the effect of a method of fasting “ADMF” on body weight, sleep quality, and daytime sleepiness was investigated in this trial.
Methods
Design of study
This study was a randomized, controlled, trial to investigate the effect of an ADMF diet and CR diet on body weight, daytime sleepiness, and sleep quality in overweight/obese women for 8 weeks. Individuals were recruited from several health centers located in Kashan, Iran by simple random sampling. Then participants were randomly assigned into groups control (CR) and intervention (ADMF). The study protocol was registered at the Iranian Registry of Clinical Trials (IRCT20220522054958N3) and was approved by the Ethics Committee of Kashan University of Medical Sciences (IR.KAUMS.MEDNT.REC.1401.046). All patients gave written consent to participate in the study. Inclusion criteria included women between 18 and 50 years old and 40 >BMI ≥25. Exclusion criteria included pregnancy, breastfeeding, having a chronic disease such as hypertension, diabetes, gastrointestinal disorders, and heart disease, losing 1–2 kg of weight in the past month, the habit of smoking, alcohol abuse, taking specific medication or following a specific diet, taking dietary supplements for weight loss, overnight shifts, having psychological and mental disorders.
In this study, 56 women were recruited by Simple Random Sampling from some health centers located in Kashan considering the inclusion and exclusion criteria. Eligible individuals were stratified based on age and BMI to make sure homogeneity of between-group. Individuals per stratum were placed into an ADMF group or CR group after baseline investigations. The allocation sequences were generated by using random numbers table by an independent statistician. The statistician was blinded throughout the entire trial.
Diet protocol
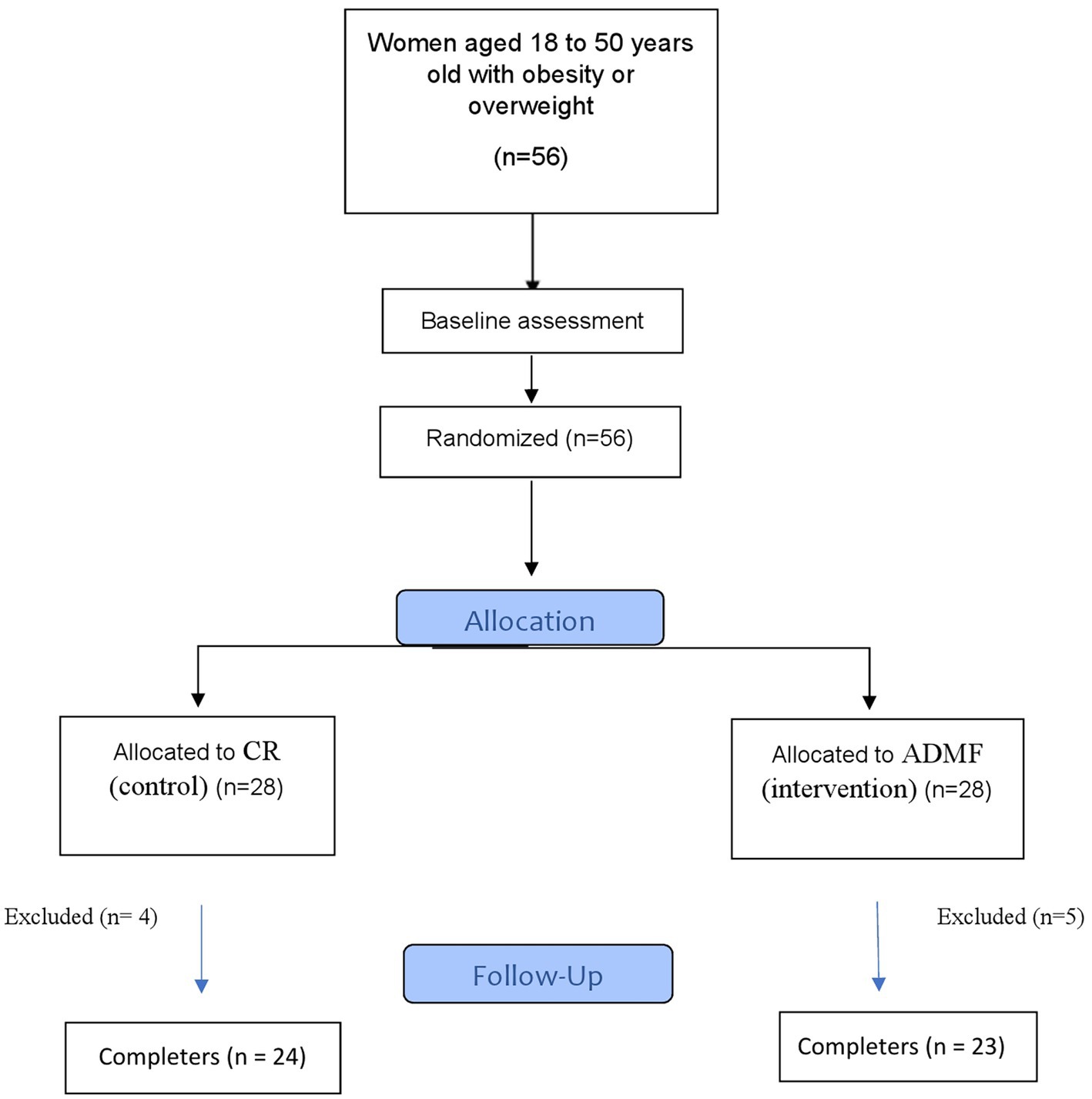
The flow diagram of the study has been presented in Figure 1. Individuals per stratum were placed into an ADMF group or CR group after baseline investigations. All individuals were needed to follow diets that were given to them based on daily energy requirements and their group for 8 weeks. The daily energy requirements of the participants were estimated by using the Mifflin equation (19). ADMF involved intermittent periods of fasting and feeding, every-other-day (on the fasting days, participants consumed only 25% of the daily recommended calorie and then on feeding days they consumed 100% of the estimated daily energy requirements). The fast and feed days started at midnight. All meals of the fast days were eaten as lunch between 12.00 p.m. to 2.00 p.m. to make sure that each participant was sustaining the same time of fasting. The consumption of non-starchy veggies (green leaf, cucumber, tomato, and lettuce) as well as energy-free beverages like water, tea, and coffee without sugar (less than 400 mg caffeine daily) was permitted. Participants were encouraged to drink plenty of water. The individuals consumed 100% of their daily energy needs (three main meals and three snacks) on feeding days and were asked to intake breakfast at 8:00 a.m., lunch at 13:00 and dinner at 8:00 p.m. Also, they were asked to eat their snacks at 10:00, 16:00, and 22:00. Participants of the CR group daily consumed 63% of their calculated calories (three main meals and three snacks) and were asked to take their main meals at 8:00, 13:00, and 20:00, respectively. Also, they were asked to take their snacks at 10:00, 16:00, and 22:00. The duration of the diet for both groups was 8 weeks. All participants in intervention and control groups needed to cook all their meals in their houses. Daily dietary protein, fat, and carbohydrates, accounted for 15, 30, and 55% of energy needs, respectively. Intervention and control group patients were required to keep their regular physical activity over the trial. All participants had the same number of calls to the dietician.
Adherence to the diet
Participants’ adherence to the regimen was monitored every two weeks by completing food record questionnaires three times a week (two normal days and one day off) (20) The questionnaires were compared to the prescribed diet to assess adherence. To ensure accurate completion of the questionnaires, all participants received instructions on how to complete the forms, which included selecting appropriate days and units of measurement. The information obtained from the questionnaires was converted to grams using a home scale guide and analyzed with N4 software (First Databank Inc.; Hearst Corporation), which was adapted for Iranian foods. The software calculated the amount of energy and macronutrients received. Adequate adherence was defined as a total caloric intake between 80 and 110% of the prescribed amount (21). Participants whose daily calorie or macronutrient intake was less than 80% or more than 110% of the recommended amount were excluded from the study. Additionally, participants were contacted by phone every two weeks and monitored to answer any questions and encourage adherence to the diet and study protocol.
Assessment of variables
The impact of ADMF and CR on primary outcomes (body weight, BMI, PSQI, and ESS) and secondary outcomes (SSQ, sleep latency, sleep duration, sleep efficiency, daytime dysfunctions, sleep disturbances) was assessed by change from baseline to end of the intervention.
Weight and BMI
Participants’ weight, without shoes and with light clothes, was evaluated using the scale with an accuracy of 0.1 kg. A stadiometer with an accuracy of 0.5 cm was used to measure the height of patients. BMI was computed as weight (in kilograms) divided by height (in meters squared).
Sleep quality
The sleep quality in the previous 4 weeks is evaluated self-reportedly by the PSQI. The PSQI questionnaire has 19 items and assesses 7 components of sleep: SSQ, sleep latency, sleep duration, use of sleeping medication, sleep disturbance, habitual sleep efficiency, and daytime dysfunction (22). The items are rated on a 4-point Likert scale in terms of the severity of the problem or frequency. The score range of each item is from 0 to 3. The total PSQI score has a range between 0 and 21. Higher scores express lower sleep quality (22).
Daytime sleepiness
In this study, daytime sleepiness was measured by the ESS, a self-reported questionnaire. Participants answered items based on how likely they were to fall asleep or doze off during sedentary activities. ESS is an eight-item questionnaire and includes a respondent format “high chance of dozing” = 3, “moderate chance of dozing” = 2, “slight chance of dozing” = 1, and “would never doze” = 0. The total ESS score was calculated by summing the total scores of eight items. The total score range was from 0 to 24. Higher values indicate higher levels of sleepiness. The change scores were evaluated as the changes between the total ESS scores at the beginning and the end of the study (23).
Physical activity record questionnaire
The physical activity questionnaire, based on metabolic equivalents (MET), was used to evaluate physical activity in this trial. It consisted of nine activity levels, ranging from rest and sleep with a metabolic equivalent of 0.9 to intense activity with a metabolic equivalent of ≥6 (24). Each level (A: 0.9 MET, such as sleep and rest; B: 1.0 MET, such as sitting quietly; C: 1.5 METs, such as working at a computer; D: 2.0 METs, such as standing or washing dishes; E: 3.0 METs, such as light cleaning; F: 4.0 METs, such as bicycling; G: 5.0 METs, such as gardening; H: 6.0 METs, such as aerobics; and I: >6 METs, such as running) was described with examples of activities of that particular MET level, and by a drawing. The physical activity scale was created so that the amount of time spent on each MET activity level (15, 30, or 45 min, and 1–10 h) on an average 24-h weekday could be recorded. The participants indicated the number of times per day they participated in each of the nine levels of activities, numbered 1–9. The Physical Activity score was evaluated by multiplying the MET level of the activity by the number of times per day and summing all the activity scores together (24).
Statistical assessment
The Kolmogorov-Smirnov test was applied to investigate the normality of data distribution. A Chi-square test was used to compare qualitative data between the two groups. An independent t-test was used to evaluate between-group differences in quantitative data. To compare the mean of the quantitative data (within the group) at the beginning and end of the study, the paired t-test was used in parametric conditions and the Wilcoxon test was used in non-parametric conditions. To compare the mean between the two groups, a t-test was used in parametric conditions and the Mann-Whitney test was used in non-parametric conditions.
Results
As shown in the study diagram (Figure 1), 56 women were eligible. Participants were randomly allocated to the intervention and control groups. During the study, 5 women from intervention [pregnancy (n = 1), discontinued intervention (n = 3), and migration (n = 1)] and 4 patients from the control group [discontinued intervention (n = 4)] were excluded. Finally, 47 participants completed the study and were consisted in the final analysis. The baseline characteristics of the participants in the present analysis were shown in Table 1. No significant changes were found between groups in demographic characteristics, age, BMI, and physical activity.
Primary outcomes
After an 8-week follow-up, a higher, significant, decrease in body weight (kg) [−5.23 (1.73) vs. −3.15 (0.88); P <0.001] and BMI (kg/m2) [−2.05 (0.66) vs. −1.17 (0.34); P< 0.001] was observed in ADMF group in comparison to CR. The significant differences weren’t observed in the change of PSQI [−0.39 (1.43) vs. −0.45 (1.88); P= 0.73] and ESS [−0.22 (1.24) vs. −0.54 (1.67); P= 0.43] between 2 groups. Also, no significant differences were found in physical activity before and after the intervention, in the ADMF group [26.65 (8.19) vs. 26.91 (8.60); P = 0.309] and CR group [28.67 (6.17) vs. 28.83 (6.24); P = 0.553] (Table 2). The adverse effects related to following the diets weren’t reported among the intervention or control group all over the study.
Secondary outcomes
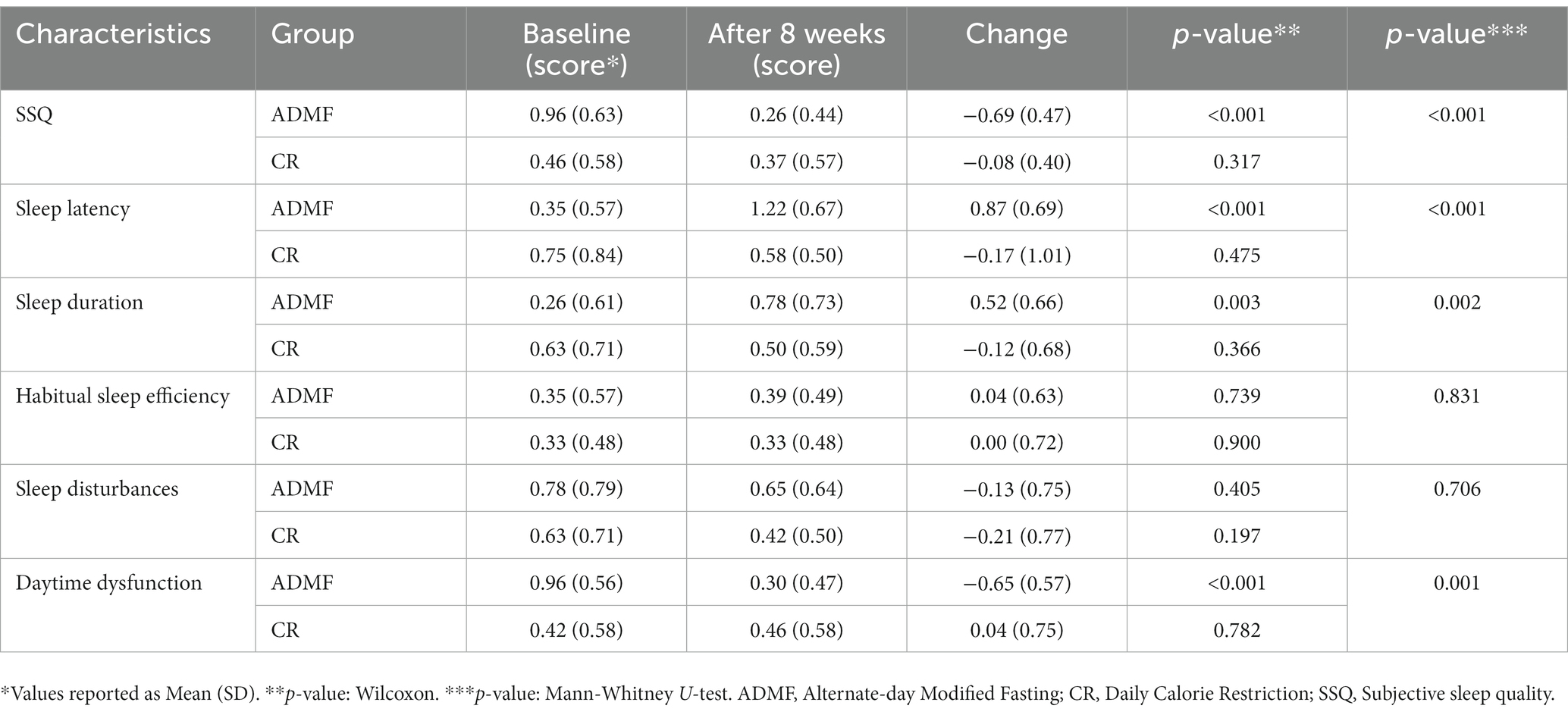
Following the ADMF diet led to significant positive changes in SSQ [−0.69 (0.47) vs. −0.08 (0.40); P= <0.001] and daytime dysfunction [−0.65 (0.57) vs. 0.04 (0.75); P: 0.001] in compare with CR diet. However, significant negative changes were seen in sleep latency [0.87 (0.69) vs. −0.17 (1.01); P= <0.001] and sleep duration [0.52 (0.66) vs. −0.12 (0.68); P= 0.002] and in parameters of habitual sleep efficiency [0.04 (0.63) vs. 0.00 (0.72); P= 0.83] and sleep disturbances [−0.13 (0.75) vs. −0.21 (0.77); P= 0.71] no significant changes were observed between groups (Table 3).
Discussion
The present study indicated that following the ADMF diet for 8 weeks among women with obesity or overweight improved BMI, body weight, SSQ, and daytime dysfunction compared to CR, however, had no effect on PSQI, ESS, habitual sleep efficiency, and sleep disturbances and harmed sleep duration and sleep latency. Our research indicated that although both diets (ADMF and CR) could result in weight loss after 8 weeks; the effect of the ADMF diet on body weight and BMI loss was higher than the CR. This finding was consistent with several previous studies, for example, Johnson et al. (25) and Razavi et al. (26). In contrast, the study by Trepanowski et al., which lasted for 6 months, did not show any significant beneficial effect on weight loss. While short-term fasting may promote greater weight loss than traditional diets, this effect may not be significant in longer interventions. In fact, other studies have suggested that longer intermittent fasting diets can lead to greater weight loss, and researchers have proposed that intermittent fasting may be a useful dietary method for obese individuals (27, 28). However, the reasons for the discrepancies in anthropometric indices observed in these studies are not well understood, and more long- and short-term trials are needed to fully evaluate the effectiveness of the intermittent fasting. ADMF has exhibited greater participant compliance compared to daily calorie restriction, for longer periods (29). In conventional CR diets, calorie intake must be restricted every day (30), however, the ADMF diet requires food restriction every other day which increases compliance with the diet (29). A decrease in body weight is directly associated with the degree of adherence to the diet (27) and the high adherence rate to the ADMF diet leads to significant weight loss. It has been reported that fasting-induced weight loss is mainly from body fat tissue reduction, while muscle mass is usually preserved during a fasting diet (31). As subjects in ADMF require to fast 3–4 days a week, more decrease in weight is often observed in such diets in comparison with CR (32). The energy balance manages body weight changes (33). During fasting hours, less glucose is available to the body, hence, fat and ketones are considered the main source of energy, and therefore, a decrease in weight and fat tissue will occur (34, 35). The result of several studies indicated that participants in the fasting group reported lower appetite after the intervention. The changes in appetite-regulating hormones may have changed people’s appetite. In animal trials, treatment with alternate-day fasting increased adiponectin concentrations, while decreasing leptin and resistin (36).
Sleep disturbances are common findings in obese people (37), which affects not only people with extreme obesity but also people with medium obesity. Excessive fat tissue especially visceral adipose plays a main role in this relation (38). Indeed, visceral adipose tissue increases the secretion of inflammatory cytokines (TNF-α, IL-1, and IL-6). These cytokines cause low-grade chronic inflammation (39). The studies reported that some pro-inflammatory cytokines could have a role in sleep regulation (40). Especially, IL-1β and TNF-α have circadian secretion, with the maximum TNF-α and IL-6 secretion between 01:00 to 02:00 a.m., thus they can regulate the physiology of sleep in both humans and animals (41). In individuals with obesity, IL-6 and TNF-α have more secretion in the morning instead of the night and are related to BMI and sleep disorder (42). Therefore, these findings propose a hypothetical vicious circle including pro-inflammatory cytokines, obesity, and sleep disorder (3). Although our intervention did not affect the total PSQI score and ESS score, subscales of PSQI such as daytime dysfunction and SSQ improved significantly in the ADMF group. While the subscales of sleep latency and duration significantly worsened in the intervention group. It can be said that ADMF has increased sleep quality and daily function. However, the findings of various studies are inconsistent and further research is needed to better understand the possibility of a relationship between adherence to the ADMF diet and sleep quality. There are some limitations in our study. The sample size and duration of the study were relatively short. Nevertheless, in this short follow-up, we showed the effects of ADMF on body weight and some sleep indices. Another limitation of our study is that the assessment of sleep quality and daytime sleepiness was based only on self-reported questionnaires, which might have resulted in misstatements. We determined compliance with the prescribed diet using a food record questionnaire. Forms were completed by the patients three days a week, once every two weeks. Also, we monitored individuals via phone interviews all over the study.
Conclusion
Since obesity has been suggested as one of the main causes of sleep disorders, weight loss diets may play a role in sleep quality. The present study suggests that ADMF is an efficacious dietary method for decreasing body weight and managing BMI in women with obesity or overweight. In addition, we indicated that an ADMF diet can increase SSQ and improve daytime dysfunction, in comparison with a CR diet. These results give us better insight into the ability of ADMF versus CR for the management of weight and BMI. However, more studies are required to address the direction of causality and generalize the results to other diverse population groups with different health statuses. Moreover, more research is required to study the long-term effects of ADMF on holistic and metabolic health. The findings of this study will increase our information on fasting diets, which can be applied to ameliorate dietary recommendations. The addressing of the public health effect of sleep behaviors in the prevention of chronic diseases corroborates this call for further research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Kashan University of Medical Sciences (IR.KAUMS.MEDNT.REC.1401.046). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SJ conceived the trial, designed the experiment, and was the Chief Investigator. AY analyzed the data and was responsible for the statistical design of the study. SH assisted with the conduction of the study and wrote the manuscript. All study authors read and approved the final version of the manuscript.
Funding
This work was financially supported by the Kashan University of Medical Sciences and Health Services (Kashan, Iran).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, Body mass index; ADMF, Alternate-day Modified Fasting; CR, Daily calorie restriction; ESS, Epworth sleepiness scale; PSQI, Pittsburgh sleep quality index; SSQ, Subjective sleep quality; MET, Metabolic equivalents.
References
1. Hashimoto, A, Inoue, H, and Kuwano, T. Low energy intake and dietary quality are associated with low objective sleep quality in young Japanese women. Nutr Res. (2020) 80:44–54. doi: 10.1016/j.nutres.2020.06.002
2. Tan, X, Alén, M, Cheng, SM, Mikkola, TM, Tenhunen, J, Lyytikäinen, A, et al. Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle-aged men. J Sleep Res. (2015) 24:414–24. doi: 10.1111/jsr.12283
3. Muscogiuri, G, Barrea, L, Aprano, S, Framondi, L, di Matteo, R, Laudisio, D, et al. Sleep quality in obesity: does adherence to the mediterranean diet matter? Nutrients. (2020) 12:1364. doi: 10.3390/nu12051364
4. Godos, J, Grosso, G, Castellano, S, Galvano, F, Caraci, F, and Ferri, R. Association between diet and sleep quality: a systematic review. Sleep Med Rev. (2021) 57:101430. doi: 10.1016/j.smrv.2021.101430
5. Alfaris, N, Wadden, TA, Sarwer, DB, Diwald, L, Volger, S, Hong, P, et al. Effects of a 2-year behavioral weight loss intervention on sleep and mood in obese individuals treated in primary care practice. Obesity. (2015) 23:558–64. doi: 10.1002/oby.20996
6. Gangwisch, JE, Malaspina, D, Boden-Albala, B, and Heymsfield, SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. (2005) 28:1289–96. doi: 10.1093/sleep/28.10.1289
7. Trepanowski, JF, Kroeger, CM, Barnosky, A, Klempel, M, Bhutani, S, Hoddy, KK, et al. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin Nutr. (2018) 37:1871–8. doi: 10.1016/j.clnu.2017.11.018
8. Moreira, EAM, Most, M, Howard, J, and Ravussin, E. Dietary adherence to long-term controlled feeding in a calorie-restriction study in overweight men and women. Nutr Clin Pract. (2011) 26:309–15. doi: 10.1177/0884533611405992
9. Hoddy, KK, Marlatt, KL, Çetinkaya, H, and Ravussin, E. Intermittent fasting and metabolic health: from religious fast to time-restricted feeding. Obesity. (2020) 28:S29–37. doi: 10.1002/oby.22829
10. Bhutani, S, Klempel, MC, Kroeger, CM, Trepanowski, JF, and Varady, KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity. (2013) 21:1370–9. doi: 10.1002/oby.20353
11. Anton, S, and Leeuwenburgh, C. Fasting or caloric restriction for healthy aging. Exp Gerontol. (2013) 48:1003–5. doi: 10.1016/j.exger.2013.04.011
12. Gotthardt, JD, Verpeut, JL, Yeomans, BL, Yang, JA, Yasrebi, A, Roepke, TA, et al. Intermittent fasting promotes fat loss with lean mass retention, increased hypothalamic norepinephrine content, and increased neuropeptide Y gene expression in diet-induced obese male mice. Endocrinology. (2016) 157:679–91. doi: 10.1210/en.2015-1622
13. Trepanowski, J, Kroeger, C, Klempel, M, Calvo, Y, and Varady, K. Alternateday fasting versus daily calorie restriction for weight loss and cardio-protection (120.6). FASEB J. (2014) 28:120.6. doi: 10.1096/fasebj.28.1_supplement.120.6
14. Hutchison, AT, Liu, B, Wood, RE, Vincent, AD, Thompson, CH, O’Callaghan, NJ, et al. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity. (2019) 27:50–8. doi: 10.1002/oby.22345
15. Heilbronn, LK, De Jonge, L, Frisard, MI, DeLany, JP, Larson-Meyer, DE, Rood, J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. (2006) 295:1539–48. doi: 10.1001/jama.295.13.1539
16. Chaput, J-P, Drapeau, V, Hetherington, M, Lemieux, S, Provencher, V, and Tremblay, A. Psychobiological impact of a progressive weight loss program in obese men. Physiol Behav. (2005) 86:224–32. doi: 10.1016/j.physbeh.2005.07.014
17. Martin, CK, Bhapkar, M, Pittas, AG, Pieper, CF, Das, SK, Williamson, DA, et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: the CALERIE 2 randomized clinical trial. JAMA Int Med. (2016) 176:743–52. doi: 10.1001/jamainternmed.2016.1189
18. Teong, XT, Hutchison, AT, Liu, B, Wittert, GA, Lange, K, Banks, S, et al. Eight weeks of intermittent fasting versus calorie restriction does not alter eating behaviors, mood, sleep quality, quality of life and cognitive performance in women with overweight. Nutr Res. (2021) 92:32–9. doi: 10.1016/j.nutres.2021.06.006
19. Mifflin, MD, St Jeor, ST, Hill, LA, Scott, BJ, Daugherty, SA, and Koh, YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. (1990) 51:241–7. doi: 10.1093/ajcn/51.2.241
20. Parvaresh, A, Razavi, R, Abbasi, B, Yaghoobloo, K, Hassanzadeh, A, Mohammadifard, N, et al. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: a randomized clinical trial. Complement Ther Med. (2019) 47:102187. doi: 10.1016/j.ctim.2019.08.021
21. Razo-Olvera, D, Mabel, F, Martin-Vences, AJ, Brito-Córdova, GX, Elías-López, D, Landa-Anell, MV, et al. Primary barriers of adherence to a structured nutritional intervention in patients with dyslipidemia. Nutrients. (2021) 13:1744. doi: 10.3390/nu13061744
22. Raniti, MB, Waloszek, JM, Schwartz, O, Allen, NB, and Trinder, J. Factor structure and psychometric properties of the Pittsburgh Sleep Quality Index in community-based adolescents. Sleep. (2018) 41:1–12. doi: 10.1093/sleep/zsy066
23. Johns, MW . Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. (1993) 103:30–6. doi: 10.1378/chest.103.1.30
24. Aadahl, M, and Jørgensen, T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. (2003) 35:1196–202. doi: 10.1249/01.MSS.0000074446.02192.14
25. Johnson, JB, Summer, W, Cutler, RG, Martin, B, Hyun, D-H, Dixit, VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Rad Biol Med. (2007) 42:665–74. doi: 10.1016/j.freeradbiomed.2006.12.005
26. Razavi, R, Parvaresh, A, Abbasi, B, Yaghoobloo, K, Hassanzadeh, A, Mohammadifard, N, et al. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vit Nutr Res. (2020) 91:242–50. doi: 10.1024/0300-9831/a000623
27. Varady, KA, Bhutani, S, Church, EC, and Klempel, MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nut. (2009) 90:1138–43. doi: 10.3945/ajcn.2009.28380
28. Eshghinia, S, and Mohammadzadeh, F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diab Metab Disord. (2013) 12:1–4. doi: 10.1186/2251-6581-12-4
29. Varady, KA, and Hellerstein, MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. (2007) 86:7–13. doi: 10.1093/ajcn/86.1.7
30. Malik, VS, and Hu, FB. Popular weight-loss diets: from evidence to practice. Nat Clin Pract Cardiovasc Med. (2007) 4:34–41. doi: 10.1038/ncpcardio0726
31. Alhamdan, B, Garcia-Alvarez, A, Alzahrnai, A, Karanxha, J, Stretchberry, D, Contrera, K, et al. Alternate-day versus daily energy restriction diets: which is more effective for weight loss? A systematic review and meta-analysis. Obes Sci Pract. (2016) 2:293–302. doi: 10.1002/osp4.52
32. Barnosky, AR, Hoddy, KK, Unterman, TG, and Varady, KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. (2014) 164:302–11. doi: 10.1016/j.trsl.2014.05.013
33. Kacimi, S, Ref’at, A, Fararjeh, MA, Bustanji, YK, Mohammad, MK, and Salem, ML. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. (2012) 32:947–55. doi: 10.1016/j.nutres.2012.06.021
34. El Ati, J, Beji, C, and Danguir, J. Increased fat oxidation during Ramadan fasting in healthy women: an adaptative mechanism for body-weight maintenance. Am J Clin Nutr. (1995) 62:302–7. doi: 10.1093/ajcn/62.2.302
35. De Cabo, R, and Mattson, MP. Effects of intermittent fasting on health, aging, and disease. New Engl J Med. (2019) 381:2541–51. doi: 10.1056/NEJMra1905136
36. Varady, KA, Allister, CA, Roohk, DJ, and Hellerstein, MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem. (2010) 21:188–95. doi: 10.1016/j.jnutbio.2008.11.001
37. Pugliese, G, Barrea, L, Laudisio, D, Salzano, C, Aprano, S, Colao, A, et al. Sleep apnea, obesity, and disturbed glucose homeostasis: epidemiologic evidence, biologic insights, and therapeutic strategies. Curr Obes Rep. (2020) 9:30–8. doi: 10.1007/s13679-020-00369-y
38. Moreno-Vecino, B, Arija-Blázquez, A, Pedrero-Chamizo, R, Gómez-Cabello, A, Alegre, L, Pérez-López, F, et al. Sleep disturbance, obesity, physical fitness and quality of life in older women: EXERNET study group. Climacteric. (2017) 20:72–9. doi: 10.1080/13697137.2016.1264934
39. Hotamisligil, GS . Inflammation and metabolic disorders. Nature. (2006) 444:860–7. doi: 10.1038/nature05485
40. Perrini, S, Cignarelli, A, Quaranta, VN, Falcone, VA, Kounaki, S, Porro, S, et al. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. JCI Insight. (2017) 2:e94379. doi: 10.1172/jci.insight.94379
41. Opp, M, Kapas, L, and Toth, L. Cytokine involvement in the regulation of sleep. Proc Soc Exp Biol Med. (1992) 201:16–27. doi: 10.3181/00379727-201-43474
Keywords: alternate-day modified fasting, intermittent fasting, calorie restriction, sleep quality, Pittsburgh
Citation: Hooshiar SH, Yazdani A and Jafarnejad S (2023) Alternate-day modified fasting diet improves weight loss, subjective sleep quality and daytime dysfunction in women with obesity or overweight: a randomized, controlled trial. Front. Nutr. 10:1174293. doi: 10.3389/fnut.2023.1174293
Edited by:
Miroslava Rossenova Atanassova, Møreforsking AS, NorwayReviewed by:
Benjamin D. Horne, Intermountain Healthcare, United StatesSusan Racette, Arizona State University, United States
Copyright © 2023 Hooshiar, Yazdani and Jafarnejad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sadegh Jafarnejad, c2phZmFybmVqYWRAYWx1bW51cy50dW1zLmFjLmly
 Saeedeh Hosseini Hooshiar1
Saeedeh Hosseini Hooshiar1 Sadegh Jafarnejad
Sadegh Jafarnejad