- 1Department of Neurology, Peking University Third Hospital, Beijing, China
- 2Peking University Health Science Center, Beijing, China
- 3Beijing Key Laboratory of Biomarker and Translational Research in Neurodegenerative Diseases, Beijing, China
- 4Key Laboratory for Neuroscience, National Health Commission/Ministry of Education, Peking University, Beijing, China
Background: Previous studies have suggested a potential association between nutrients and cerebral small vessel disease (CSVD), but this association has not been fully addressed.
Object: We intended to clarify the causal associations between four categories of essential nutrients (amino acids, polyunsaturated fatty acids, minerals and vitamins) and two acute manifestations of CSVD (intracerebral hemorrhage and small vessel stroke) using two-sample Mendelian randomization (MR) analysis.
Method: We obtained European-based large-scale genome-wide association studies (GWASs) related to CSVD (6,255 cases and 233,058 controls) and nutrient concentrations. Causality evaluation mainly included the results of the inverse variance-weighted (IVW) method. The simple median method, the weighted median method and the MR–Egger method were adopted for sensitivity analyses.
Results: For ICH or SVS, increased levels of phenylalanine (OR = 1.188, p < 0.001) and dihomo-gamma-linolenic acid (DGLA) (OR = 1.153, p = 0.001) showed risk effects, while docosapentaenoic acid (DPA) (OR = 0.501, p < 0.001), zinc (OR = 0.919, p < 0.001), and arachidonic acid (OR = 0.966, p = 0.007) showed protective effects. For lobar hemorrhage or SVS, AA (OR = 0.978, p < 0.001), zinc (OR = 0.918, p < 0.001), and retinol (OR = 0.753, p < 0.001) showed risk effects; DPA (OR = 0.682, p = 0.022), gamma-linolenic acid (OR = 0.120, p = 0.033) and 25(OH)D (OR = 0.874, p = 0.040) showed protective effects. For nonlobar hemorrhage or SVS, DGLA (OR = 1.088, p < 0.001) and phenylalanine (OR = 1.175, p = 0.001) showed risk effects.
Conclusion: Our study analyzed the effect of nutrients on CSVD risk from a genetic perspective, with implications for CSVD prevention through nutrient supplementation.
1. Introduction
Cerebral small vessel disease (CSVD) is a group of clinical syndromes that affect perforated arterioles, capillaries, and venules supplying the white matter and deep structures of the brain (1). Neuroimaging findings resulting from CSVD include recent small subcortical infarcts, lacunar infarction, white matter hyperintensity (WMH) and cerebral microbleed (CMB) (2), which are considered the main vascular factors of dementia, cognitive decline, gait disturbance, mood disturbance, and stroke (3). CSVD causes small vessel stroke (SVS), which accounts for approximately 25% of ischemic stroke cases (4). Most intracerebral hemorrhages (ICHs) are also caused by CSVD. ICH and SVS are acute manifestations of CSVD caused by the rupture or occlusion of the small vessels (3).
Circulating nutrients include essential amino acids, essential polyunsaturated fatty acids, essential vitamins and essential minerals. Supplementation with essential nutrients is considered a promising preventive measure against CSVD due to their ability to maintain metabolic homeostasis and reduce oxidative stress, which plays a central role in tissue function (5). However, we only found cohort or observational studies examining dietary interventions that affect cardiovascular and cerebrovascular outcomes (6–8), but specific nutrient categories and effects were not addressed. Observational studies are more likely to be influenced by selection bias and confounders. Randomized controlled trials (RCTs) are able to overcome the limitations of observational studies and provide the highest level of evidence, but they come with a high cost and are not always possible due to ethical or other concerns (9). To overcome the limitations of RCTs, Mendelian randomization (MR) was introduced to mimic RCTs to perform causal inference. According to Mendelian Laws of Inheritance, genotypes are randomly assigned from one generation to the next generation and they are unaffected by confounding factors. Thus, we performed a MR study to decipher the causal association between essential nutrients (including essential amino acids, essential polyunsaturated fatty acids, essential minerals and essential vitamins) and CSVD (including ICH and SVS).
2. Materials and methods
2.1. Data collection and instrumental variable selection
The design of our study was shown in Figure 1. We extracted single nucleotide polymorphisms (SNPs) for each essential nutrient (exposure) from the most recently published genome-wide association study (GWAS) with the largest sample size on PubMed. SNPs related to essential amino acids were extracted from datasets including valine, tryptophan, phenylalanine, isoleucine, leucine, lysine and methionine (10, 11). For the essential polyunsaturated fatty acids, eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), arachidonic acid, dihomo-gamma-linolenic acid (DGLA), alpha-linolenic acid (ALA), gamma-linolenic acid and linoleic acid were included (10, 12, 13). Calcium (14), copper, iron (15), magnesium (16), zinc (17), and phosphorus (18) were included in the essential minerals. To assess the effects of vitamins on our outcomes, we examined vitamin A (retinol) (19), beta-carotene (20), lycopene (19), vitamin B (B6 and B12) (21, 22), vitamin C (23), vitamin D [25(OH)D] (24), and vitamin E (alpha-tocopherol) (25).
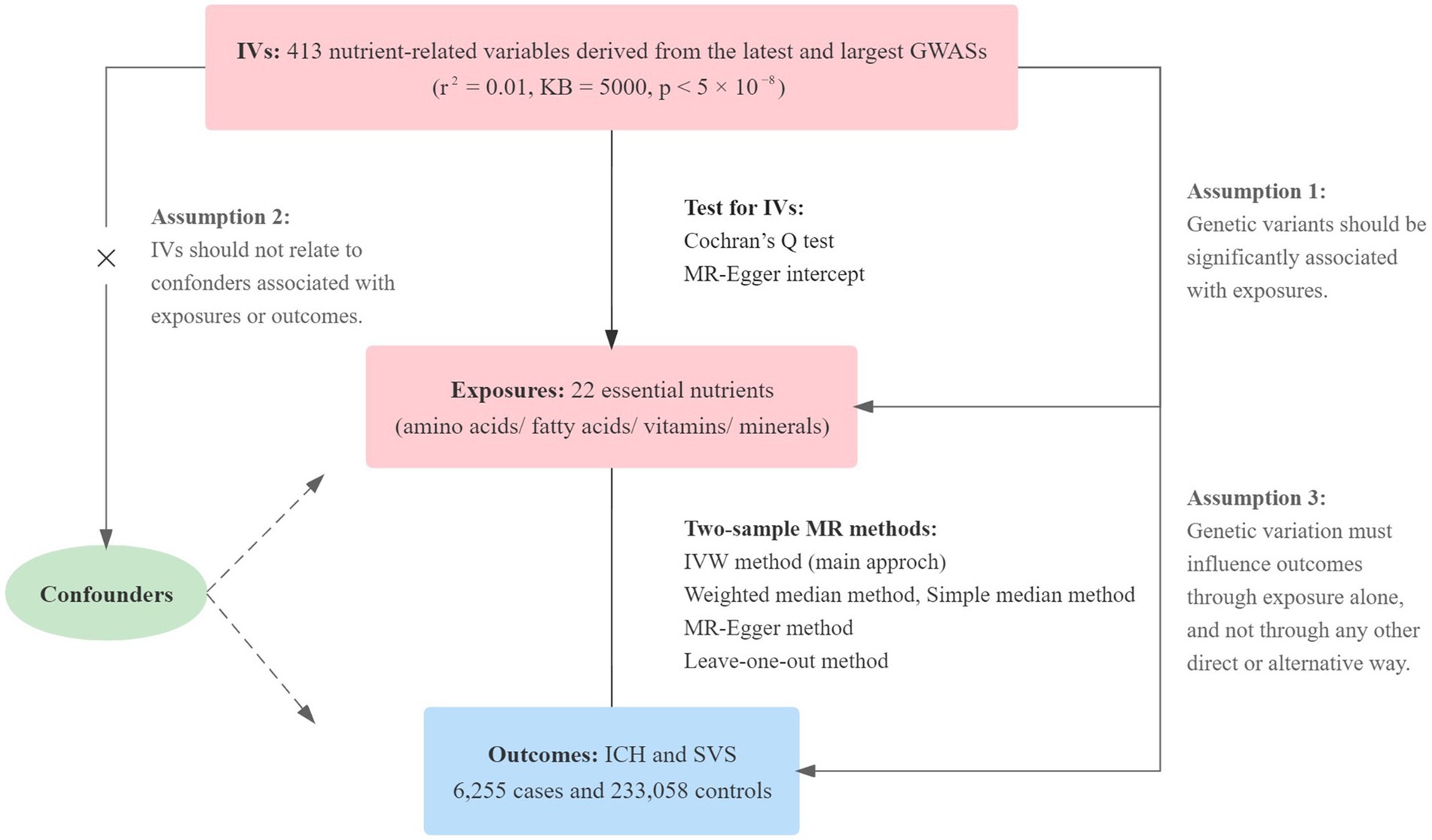
Figure 1. The process of our MR analyses is shown in this flow chart. Three key assumptions for our two-sample MR study. Assumption 1: The selected instrumental variables (IVs) should be significantly related to nutrients. Assumption 2: The selected IVs are not associated with other confounders. Assumption 3: There is no independent causal pathway between IVs and the outcome other than through nutrients. IVs, instrumental variables; GWASs, genome-wide association studies; MR, Mendelian randomization; IVW, inverse variance-weighted; ICH, intracerebral hemorrhage; SVS, small vessel stroke; KB, kilo base.
We selected the eligible SNPs as the instrumental variables (IVs) for the exposures. And we set strict selection condition on IVs: (1) SNPs should have significant associations with exposures (p < 5 × 10−8), with no linkage disequilibrium (r2 = 0.01, region size = 5,000 kb); (2) SNPs should be not rare (MAF ≥ 0.01). SNPs not found in outcome GWASs were replaced with proxies searched in the online tool SNIPA (r2 > 0.8) (26). We deleted those SNPs without available proxies.
The GWAS summary level data of outcomes were obtained from a recently published meta-analysis study, including 6,255 ICH or SVS cases and 233,058 controls from European ancestry. In this GWAS, three ICH datasets and one SVS dataset were integrated into a cross-trait dataset and generated as three outcomes, namely, ICH or SVS, lobar hemorrhage or SVS and nonlobar hemorrhage or SVS (27).
Due to the limitations of the MR methods, only exposures with at least two IVs could be used to perform the following analyses. Isoleucine, lysine, methionine, EPA, ALA, vitamin B6 and beta-carotene were removed. In total, we obtained 2-171 IVs related to 22 nutrient biomarkers (the characteristics of all the exposure SNPs were listed in Supplementary Table S1).
2.2. Mendelian randomization
The inverse variance-weighted (IVW) method was the main approach we used to analyze the causality; it combines the SNP-outcome effects on SNP-exposure effects by a weighted linear regression and allows the existence of overdispersion (28). We chose the multiplicative random effects model of IVW for our analysis, as it can obtain the average value of real estimates. To strengthen the robustness of our results, we used some additional sensitivity analyses including the simple median method, the weighted median method and the MR–Egger method. The result of the weighted median method is the median of a weighted empirical density function of the ratio estimates (29). It can obtain a more effective estimate when at least 50% of IVs are valid compared to the simple median method. It is sensitive when IVs are added or deleted (30). Using the MR–Egger method, we can obtain an unbiased estimate even if the SNPs have pleiotropy (31).
We used Cochran’s Q test to measure the heterogeneity of SNPs, and the MR–Egger intercept method was performed to detect horizontal pleiotropy. In Cochran’s Q test, significant heterogeneity (p < 0.05) indicates that there may be some genetic variants that violate key assumptions of IVs (32). For the MR–Egger regression intercept, when the y-intercept has no significant difference from the origin (p > 0.05), IVs can be considered to have no horizontal pleiotropy. From the principal of the MR–Egger intercept method, exposures with less than three SNPs cannot be tested for horizontal pleiotropy.
We adopted scatterplots and leave-one-out plots to describe the effect of a single SNP on the outcomes. The scatterplot showed the effect of each SNP on the exposure and outcome. The leave-one-out (LOO) plot was used to assess the influential IVs by sequentially excluding each genetic variant and recalculating the MR-IVW estimate.
The Bonferroni correction was employed for multiple-testing correction, which changed the significance level of the p value from 0.05 to 7.58 × 10−4 (p value = 0.05/(22*3)). All analyses were performed in R software version 4.2.0 by the “TwoSampleMR” package (version 0.5.6).
3. Results
We evaluated 22 possible risk factors for ICH and SVS, including four amino acids, six polyunsaturated fatty acids, six minerals and six vitamins.
3.1. Amino acids
When analyzing the relationship between amino acids and ICH or SVS, the results of the IVW method showed the risk effect of phenylalanine on ICH or SVS (OR: 1.188; 95% CI: 1.083–1.303, p < 0.001) and the suggestive risk effect on nonlobar hemorrhage or SVS (OR: 1.175; 95% CI: 1.069–1.293, p = 0.001) (Figure 2, Supplementary Figures S1, S2). The simple median, the weighted median and the MR–Egger method all illustrated similar trends (Table 1). No SNP heterogeneity was detected via Cochran’s Q test. Similarly, no horizontal pleiotropy was found by the MR–Egger intercept test (Table 2). The LOO method showed that no single SNP of phenylalanine had a significant effect on ICH or SVS (Supplementary Figure S2D). Through the LOO method, we found that the effect of phenylalanine on nonlobar hemorrhage and SVS did not remain significant after removing a SNP (rs4253238) (Supplementary Figure S2L). Leucine, tryptophan and valine were not significantly associated with the three outcomes at the genetic level (Figure 2), and the heterogeneity assessment of SNPs showed that the results were relatively reliable (Table 2).
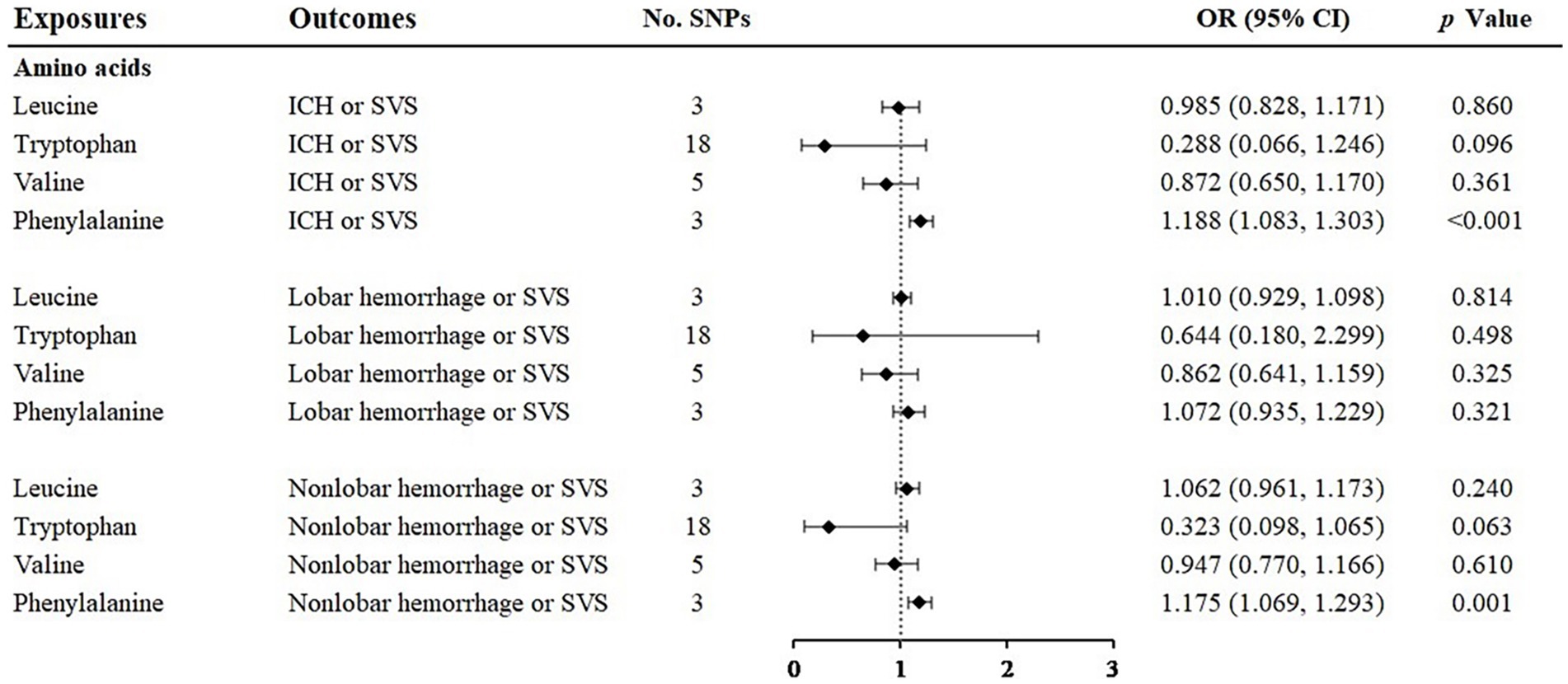
Figure 2. Associations of amino acids and the risk of cerebral hemorrhage or SVS using the IVW method. For the image in the figure, the black diamond represents the OR value, the straight line represents 95% CI, and the dotted line represents OR = 1. IVW, inverse variance-weighted; OR, odds ratio; 95% CI, 95% confidence interval; ICH, intracerebral hemorrhage; SVS, small vessel stroke; SNPs, single nucleotide polymorphisms.
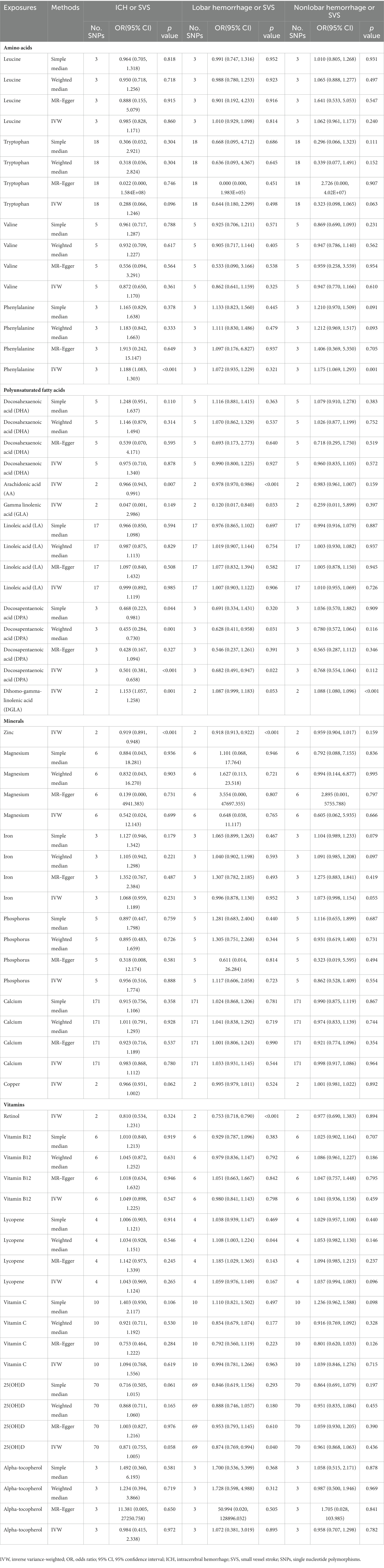
Table 1. Summary of the causal effects of each trait on intracerebral hemorrhage or small vessel stroke via different Mendelian randomization methods.
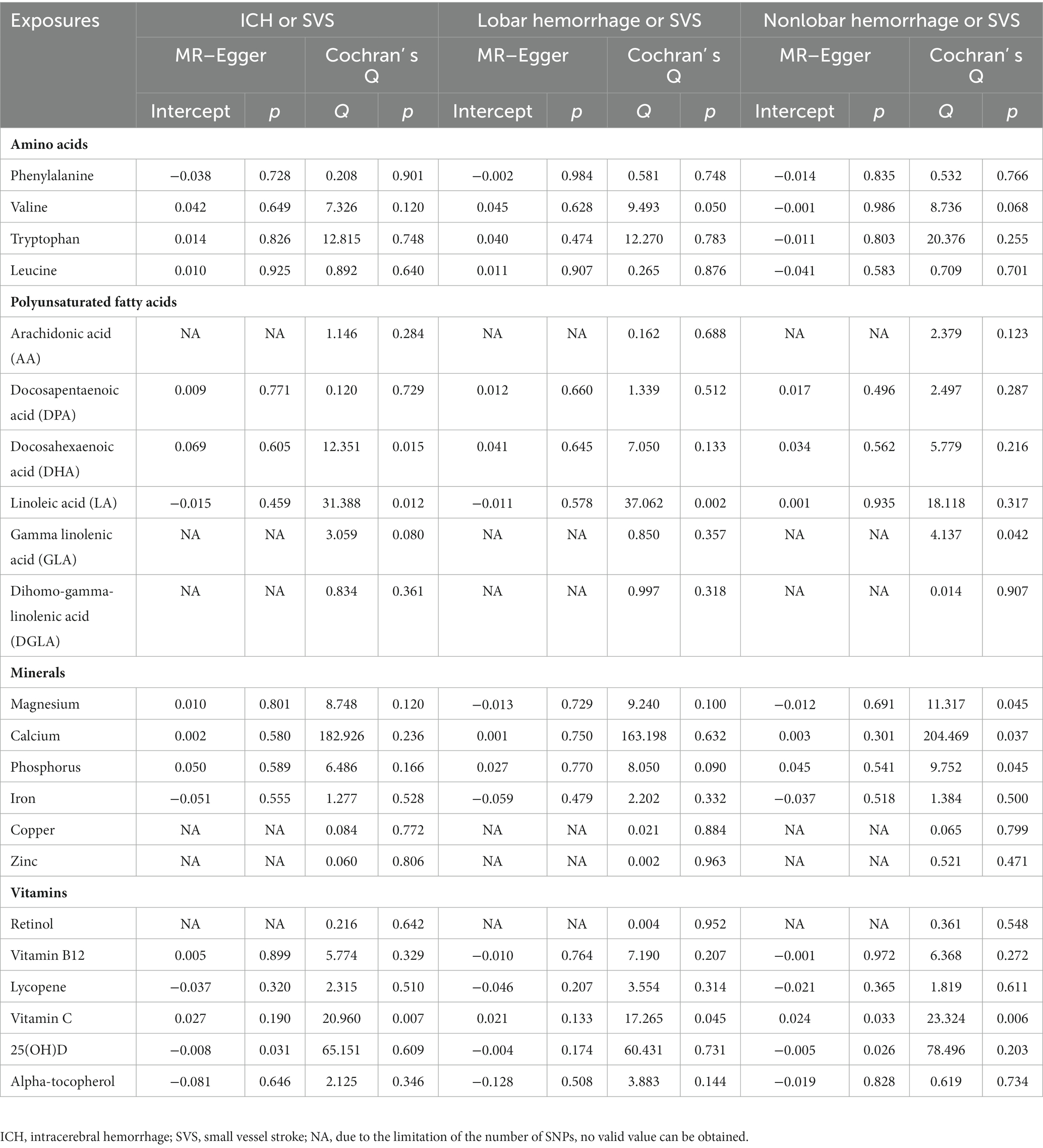
Table 2. Summary of the additional Mendelian randomization analysis for the effect of each trait on intracerebral hemorrhage or small vessel stroke.
3.2. Polyunsaturated fatty acids
With regard to the effect of ω3 polyunsaturated fatty acids including DPA and DHA, we found that DPA had a protective effect on ICH or SVS (OR: 0.501; 95% CI: 0.381–0.658, p < 0.001) and a suggestive protective effect on lobar hemorrhage or SVS (OR: 0.682; 95% CI: 0.491–0.947, p = 0.022) (Figure 3, Supplementary Figures S3, S4) by IVW method. The simple median method and the weighted median method provided robust evidence for the risk of DPA on ICH or SVS. The MR–Egger analysis also showed a similar trend. The weight median method demonstrated a protective trend for DPA on lobar hemorrhage or SVS (Table 1). The SNPs of DPA had no heterogeneity or horizontal pleiotropy (Table 2). DHA was not causally associated with any of the three outcomes.
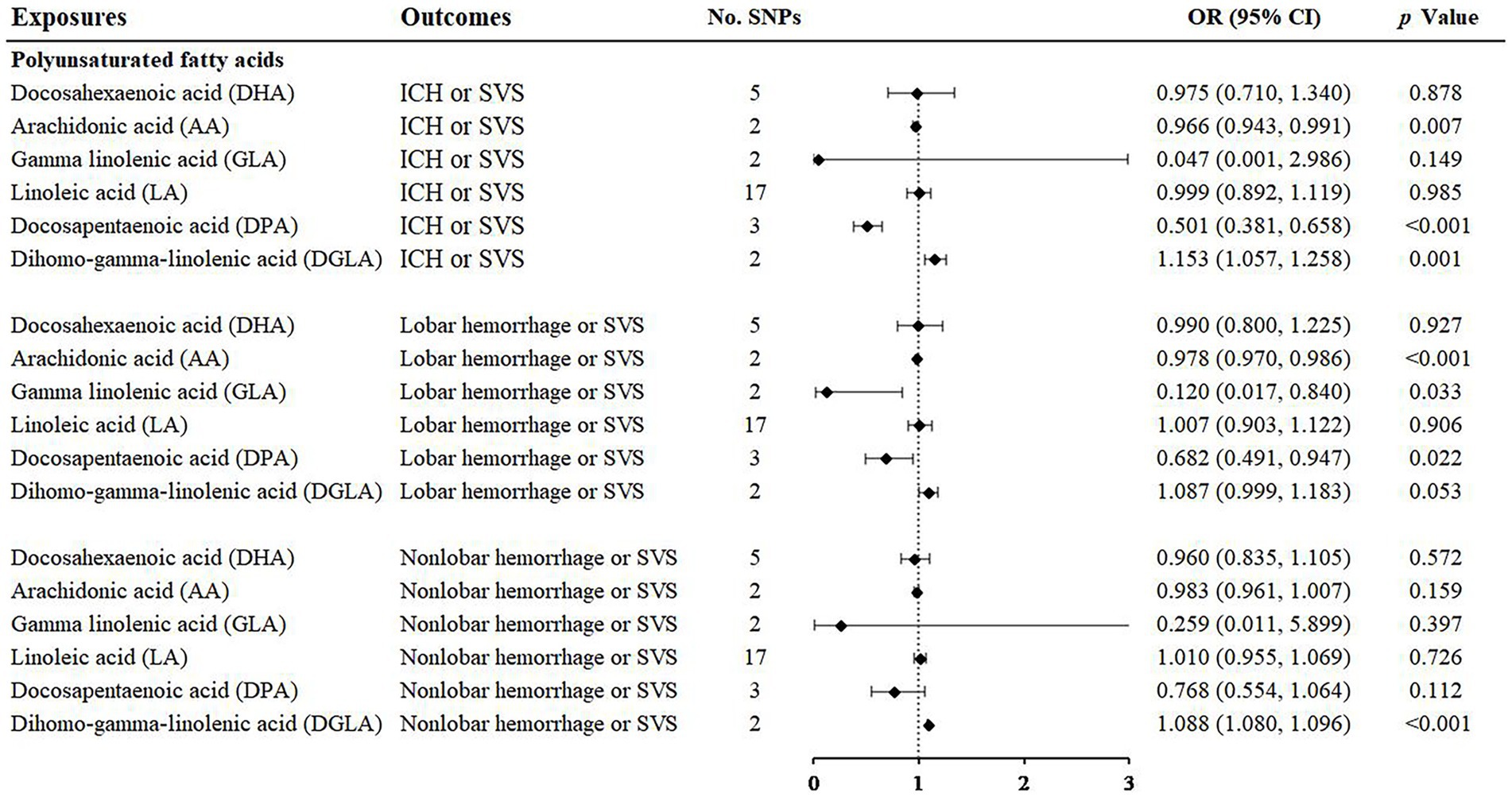
Figure 3. Associations of polyunsaturated fatty acids and the risk of cerebral hemorrhage or SVS using the IVW method. For the image in the figure, the black diamond represents the OR value, the straight line represents 95% CI, and the dotted line represents OR = 1. IVW, inverse variance-weighted; OR, odds ratio; 95% CI, 95% confidence interval; SVS, small vessel stroke; SNPs, single nucleotide polymorphisms.
For the ω6 polyunsaturated fatty acids, AA, DGLA, GLA and LA were included in our analysis. AA showed protective effects on ICH or SVS (OR: 0.966; 95% CI: 0.943–0.991, p = 0.007) and lobar hemorrhage or SVS (OR: 0.978; 95% CI: 0.970–0.986, p < 0.001). GLA had a suggestive protective relationship with lobar hemorrhage or SVS (OR: 0.120; 95% CI: 0.017–0.840, p = 0.033) (Figure 3, Supplementary Figures S3, S4). DGLA showed risk effects on ICH or SVS (OR: 1.153; 95% CI: 1.057–1.258, p = 0.001) and nonlobar hemorrhage or SVS (OR: 1.088; 95% CI: 1.080–1.096, p < 0.001) (Figure 3). However, there are only two available IVs for AA, as well as GLA and DGLA, which hindered the sensitivity analyses. We did not find causal association between LA and the three outcomes, which may be due to the heterogeneity in IVs of LA revealed by Cochran’s Q test (Table 2).
3.3. Minerals
Among the minerals we were interested in, only zinc showed a protective relationship with ICH or SVS and lobar hemorrhage or SVS. For one standard deviation unit increase in the concentration of zinc, the risk of ICH or SVS decreased by 8.1% (OR: 0.919; 95% CI: 0.891–0.948, p < 0.001) and lobar hemorrhage or SVS decreased by 8.2% (OR: 0.918; 95% CI: 0.913–0.922, p < 0.001) (Figure 4, Supplementary Figures S5, S6). We could not perform the sensitivity analysis on zinc due to the limited number of IVs. None of the other minerals, namely, magnesium, iron, phosphorus, calcium, and copper, were associated with any of the three outcomes.
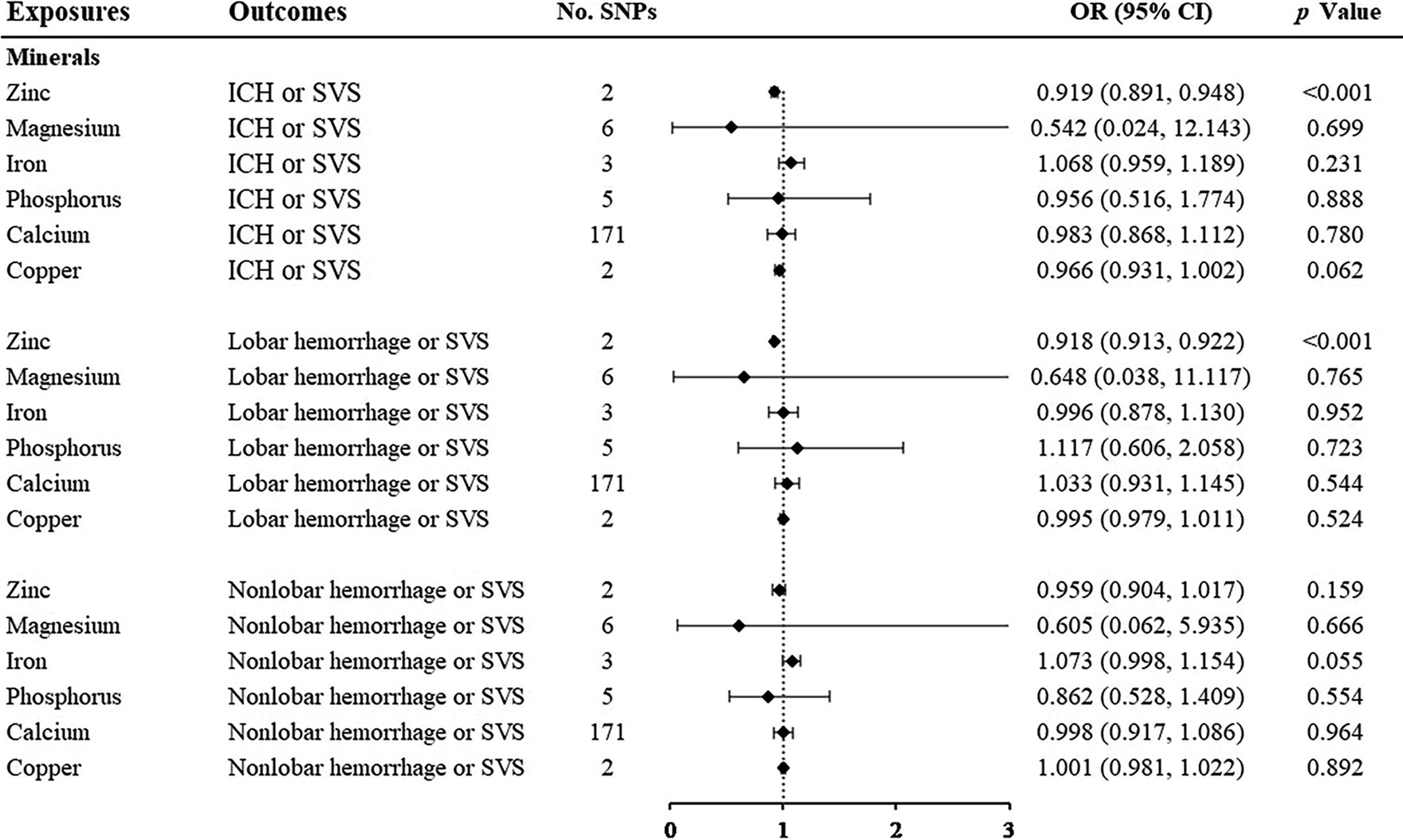
Figure 4. Associations of minerals and the risk of cerebral hemorrhage or SVS using the IVW method. For the image in the figure, the black diamond represents the OR value, the straight line represents 95% CI, and the dotted line represents OR = 1. IVW, inverse variance-weighted; OR, odds ratio; 95% CI, 95% confidence interval; SVS, small vessel stroke; SNPs, single nucleotide polymorphisms.
3.4. Vitamins
Among the vitamins or provitamins, 25(OH)D showed a protective effect on lobar hemorrhage or SVS (OR: 0.874; 95% CI: 0.769–0.994, p = 0.040) (Figure 5, Supplementary Figures S7, S8). Sensitivity analyses presented similar trends (Table 1). Additional tests confirmed that 25(OH)D was not influenced by heterogeneity and horizontal pleiotropy (Table 2). Retinol showed a protective relationship with lobar hemorrhage or SVS (OR: 0.753; 95% CI: 0.718–0.790, p < 0.001) and sensitivity analysis could not be performed due to the limited number of IVs. None of other vitamins, namely, vitamin B12, lycopene, vitamin C, and alpha-tocopherol, were causally associated with the three outcomes.
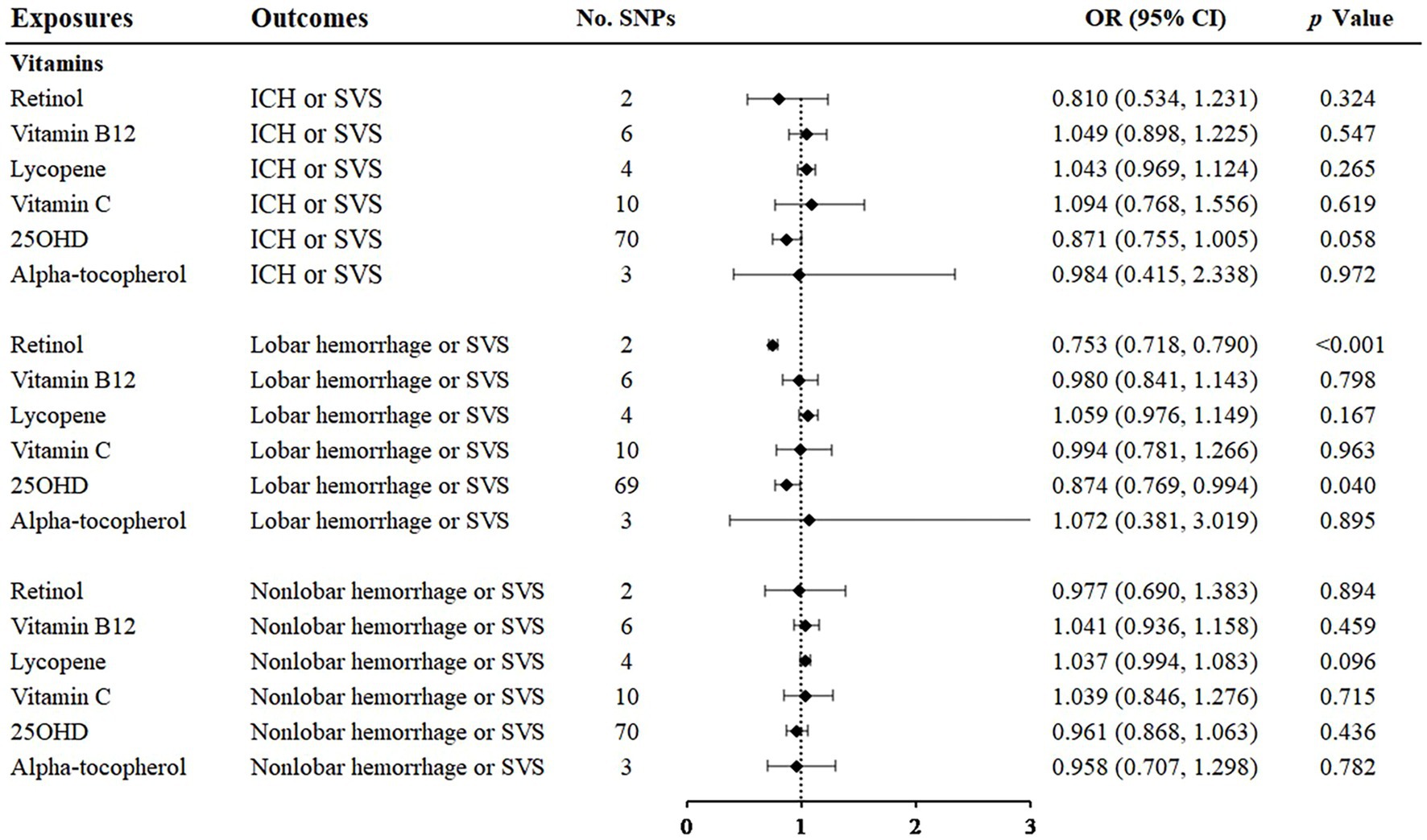
Figure 5. Associations of vitamins and the risk of cerebral hemorrhage or SVS using the IVW method. For the image in the figure, the black diamond represents the OR value, the straight line represents 95% CI, and the dotted line represents OR = 1. IVW, inverse variance-weighted; OR, odds ratio; 95% CI, 95% confidence interval; SVS, small vessel stroke; SNPs, single nucleotide polymorphisms.
4. Discussion
In our study, we performed MR analyses on essential circulating nutrients and CSVD using GWAS summary data. We found that for ICH or SVS, a higher genetically predicted level of phenylalanine was a risk factor, while DPA was a protective factor. For lobar hemorrhage or SVS, DPA and 25(OH)D were suggestive protective factors. For nonlobar hemorrhage or SVS, phenylalanine was a suggestive risk. The studies of Delgado-Lista et al. (7) and Sarfo et al. (8) have revealed some relationships between nutrients and cerebrovascular diseases, but our MR study provided more reliable evidence of CSVD by using GWAS summary data with the largest sample size by far.
Nonlobar hemorrhage is mostly caused by traditional vascular risk factors such as hypertension, and lobar hemorrhage in elderly individuals is mostly caused by cerebral amyloid angiopathy (CAA) (33). It was shown that nutrients had different effects according to the location of cerebral hemorrhage, which suggested that these nutrients may be related to different pathophysiological processes that eventually act on the brain parenchyma or cerebral blood vessels.
In our study of amino acids, phenylalanine had a hazardous predictive effect on ICH or SVS and nonlobar hemorrhage or SVS. A 12-year follow-up study of 9,584 Finnish men found that phenylalanine was associated with an increased risk of ischemic stroke and other cardiovascular events (34). A meta-analysis of seven prospective cohort studies also showed that phenylalanine was associated with a significantly increased risk of ischemic stroke (35), although ischemic stroke was not subdivided into subtypes such as SVS. There have been no previous studies of the effect of phenylalanine on the risk of ICH. Phenylalanine is an important aromatic amino acid. Excess phenylalanine can reduce cell membrane density, increase fluidity and permeability, and prompt cytotoxic effects (36). Phenylalanine may cause CSVD because it is associated with important vascular risk factors such as BMI, low-density cholesterol, systolic blood pressure, and blood glucose level (37). Phenylacetylglutamine, an intestinal microbial metabolite downstream of phenylalanine, can also increase the risk of cardiovascular disease (38). Future research is needed to explore the mechanism underlying phenylalanine and CSVD.
Regarding polyunsaturated fatty acids, previous studies based on Western populations have shown that dietary polyunsaturated fatty acid intake can reduce total cholesterol and low-density lipoprotein cholesterol, which in turn can reduce the risk of stroke (39, 40). Polyunsaturated fatty acids were defined as ω3 and ω6. The public generally recognizes that ω3 polyunsaturated fatty acid supplementation is beneficial for cardiovascular and cerebrovascular diseases. It can reduce the level of triglycerides in plasma and improve the status of coagulation, inflammation and fibrinolysis in patients (41). This protective relationship has also been confirmed by large epidemiological studies (42). However, in recent years, a growing number of meta-studies have shown that people could not obtain significant benefit for cerebrovascular outcomes from ω3 supplementation (43). Even if there is a benefit, it is only a very slight protective effect (44) and is no longer recommended clinically (45). Previous clinical studies on ω3 supplementation have varied in certain characteristics, including but not limited to dose, formulation, study population, clinical setting (primary and secondary prevention), and background therapy (46), which can all lead to bias. MR studies are known to reduce the likelihood of bias. In the ω3 category of our study, DPA showed a protective effect on ICH or SVS and a suggestive protective effect on lobar hemorrhage or SVS. There was a lack of data on the association between DPA and CSVD in the past, which should be confirmed in the future.
The circulating concentration of 25(OH)D is considered the best measurement of plasma vitamin D status (47). A meta-analysis showed that vitamin D intake and circulating vitamin D were associated with ischemic stroke but were not associated with hemorrhagic stroke (48). However, the number of original studies on hemorrhagic stroke was limited which may cause insignificant results. An RCT recruited 5,110 community participants and revealed that supplementation with vitamin D in high doses once a month did not prevent participants from cardiovascular diseases (49). One reason for this may be that vitamin D supplements have a short half-life, so the monthly dosing cycle may not achieve effective blood concentrations. Moreover, the average follow-up in the studies was only 3.3 years, and vitamin D may benefit longer-term observations. We also found some previous MR studies about the association between 25(OH)D and stroke. One study found that higher plasma 25(OH)D concentrations were not associated with ischemic stroke and its subtypes, including SVS. However, there was only 6 SNPs associated with 25OHD concentrations in this study, which was 69 in our study. Another previous MR study showed that vitamin D was a protective factor of ICH (50), which was consistent with our results. In addition, 25(OH)D was proven to correlate inversely with the neuroimaging markers of CSVD, including lacunar infarction, WMH and deep CMB (51). The outcome of our study was the cross trait of ICH and SVS, which can reveal the comprehensive associations between 25(OH)D and CSVD. Thus, our result may be more powerful due to the larger sample size of GWAS and larger number of SNPs. Vitamin D plays an important role in the occurrence of various cardiometabolic features, including diabetes (52), metabolic syndrome (53), renin-angiotensin system activation (54) and inhibition of the production of some inflammatory factors (55). These mechanisms supported the biological plausibility for our research.
We also focused on vitamin B12. Vitamin B12 is generally recognized to have a protective effect on cerebrovascular risk through homocysteine metabolic pathways, but the results were nonsignificant in our analyses. This may be due to the limited effect of vitamin B12 supplementation on lowering serum homocysteine levels (56). Additionally, a RCT study showed that vitamin B12 did not appear to be as effective as placebo in reducing the incidence of major vascular events (57). Moreover, an MR study showed that vitamin B12 was not significantly related to SVS (58). In summary, vitamin B12 supplementation may not protect people from CSVD.
In addition to focusing on the effects of nutrients on CSVD, we considered the effects of nutrients that exhibit a protective trend toward lobar hemorrhage or SVS on cerebral amyloid angiopathy (CAA), including DPA and vitamin D. CAA is generally caused by the deposition of amyloid proteins in cerebral blood vessels. For fatty acids, their aggregates can cobind to peptide oligomers in the early stages of amyloid fibrosis and inhibit the formation of amyloids. DPA may use this pathway to prevent lobar hemorrhage. Vitamin D plays an important role in the maintenance of calcium homeostasis, the reduction in calcium influx caused by amyloid deposition and the reduction in neuronal damage (59). This suggests that these nutrients might affect the occurrence of CAA by influencing pathophysiological processes similar to amyloid deposition in AD.
Our research used cross-trait GWAS data of ICH and SVS to avoid the problem of reduced efficiency due to the small sample size of ICH GWAS data alone (60). This method effectively utilized the large-scale SVS GWAS data and was able to better reflect the comprehensive effect of exposures on the common features of CSVD. Previous prospective cohort studies focusing on nutrients and CSVD have been hampered due to the difficulties in collecting accurate blood concentrations of single nutrients, individual fluctuations in blood drug levels, and the need for a long observation period after the intervention. Our MR study avoided these difficulties and provided high-quality evidence for clinical studies in the future.
Nevertheless, our study had some limitations. As the GWAS outcomes were cross-featured, it was not possible to distinguish exposures that were independently associated with ICH and SVS, which obscured the contradictions between ICH and SVS. Moreover, some nutrients including AA, GLA, DGLA, zinc, and retinol have limited number of IVs, which may reduce the statistical power. However, the GWAS data we used were the largest by far and it may provide some insight into this area. Finally, in terms of the interpretation of the results, our analyses reflected the impact of lifetime nutrient accumulation, and the short-term efficacy of nutrient supplementation should be interpreted cautiously. Further validations by clinical studies are still needed.
5. Conclusion
Our results showed that for ICH or SVS, a higher genetically predicted level of phenylalanine was a risk factor, while DPA was a protective factor. For lobar hemorrhage or SVS, DPA and 25(OH)D were suggestive protective factors. For nonlobar hemorrhage or SVS, phenylalanine was a suggestive risk factor. Our study analyzed the effects of essential nutrients on the risk of ICH and SVS from the perspective of gene–environment interactions, which could benefit intervention strategies for CSVD in the general population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KX: data curation and methodology. JL: formal analysis. DF and QY: supervision. ZW, YL, YT, YW, YZ, LZ, and LT: validation. JL and KX: writing—original draft. JL, KX, LT, QY, and DF: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 81901204) and the Beijing Municipal Science and Technology Commission (grant number D141100000114005).
Acknowledgments
The authors would like to thank the MEGASTROKE study for providing outcome GWAS and thank all nutrient GWAS studies for making summary data publicly available, and the authors appreciate all participants who made a contribution to those studies. The authors also thank the National Natural Science Foundation of China for support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1172587/full#supplementary-material
References
1. Wardlaw, JM, Smith, C, and Dichgans, M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. (2019) 18:684–96. doi: 10.1016/S1474-4422(19)30079-1
2. Wardlaw, JM, Smith, EE, Biessels, GJ, Cordonnier, C, Fazekas, F, Frayne, R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
3. Ter Telgte, A, van Leijsen, EMC, Wiegertjes, K, Klijn, CJM, Tuladhar, AM, and de Leeuw, FE. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. (2018) 14:387–98. doi: 10.1038/s41582-018-0014-y
4. Traylor, M, Malik, R, Nalls, MA, Cotlarciuc, I, Radmanesh, F, Thorleifsson, G, et al. Genetic variation at 16q24.2 is associated with small vessel stroke. Ann Neurol. (2017) 81:383–94. doi: 10.1002/ana.24840
5. Shenkin, A. The key role of micronutrients. Clin Nutr. (2006) 25:1–13. doi: 10.1016/j.clnu.2005.11.006
6. Nassir, C, Ghazali, MM, Hashim, S, Idris, NS, Yuen, LS, Hui, WJ, et al. Diets and cellular-derived microparticles: weighing a plausible link with cerebral small vessel disease. Front Cardiovasc Med. (2021) 8:632131. doi: 10.3389/fcvm.2021.632131
7. Delgado-Lista, J, Alcala-Diaz, JF, Torres-Peña, JD, Quintana-Navarro, GM, Fuentes, F, Garcia-Rios, A, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. (2022) 399:1876–85. doi: 10.1016/S0140-6736(22)00122-2
8. Sarfo, FS, Ovbiagele, B, Gebregziabher, M, Akpa, O, Akpalu, A, Wahab, K, et al. Unraveling the risk factors for spontaneous intracerebral hemorrhage among west Africans. Neurology. (2020) 94:e998–e1012. doi: 10.1212/WNL.0000000000009056
9. Lee, K, and Lim, CY. Mendelian randomization analysis in observational epidemiology. J Lipid Atheroscler. (2019) 8:67–77. doi: 10.12997/jla.2019.8.2.67
10. Kettunen, J, Demirkan, A, Würtz, P, Draisma, HHM, Haller, T, Rawal, R, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. (2016) 7:11122. doi: 10.1038/ncomms11122
11. The Multiple Tissue Human Expression Resource (MuTHER) ConsortiumShin, SY, Fauman, EB, Petersen, AK, Krumsiek, J, Santos, R, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. (2014) 46:543–50. doi: 10.1038/ng.2982
12. Guan, W, Steffen, BT, Lemaitre, RN, Wu, JHY, Tanaka, T, Manichaikul, A, et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ Cardiovasc Genet. (2014) 7:321–31. doi: 10.1161/CIRCGENETICS.113.000208
13. Lemaitre, RN, Tanaka, T, Tang, W, Manichaikul, A, Foy, M, Kabagambe, EK, et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE consortium. PLoS Genet. (2011) 7:e1002193. doi: 10.1371/journal.pgen.1002193
14. Sinnott-Armstrong, N, Tanigawa, Y, Amar, D, Mars, N, Benner, C, Aguirre, M, et al. Genetics of 35 blood and urine biomarkers in the UK biobank. Nat Genet. (2021) 53:185–94. doi: 10.1038/s41588-020-00757-z
15. Benyamin, B, Esko, T, Ried, JS, Radhakrishnan, A, Vermeulen, SH, Traglia, M, et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun. (2014) 5:4926. doi: 10.1038/ncomms5926
16. Meyer, TE, Verwoert, GC, Hwang, SJ, Glazer, NL, Smith, AV, van Rooij, FJ, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. (2010) 6:e1001045. doi: 10.1371/journal.pgen.1001045
17. Evans, DM, Zhu, G, Dy, V, Heath, AC, Madden, PA, Kemp, JP, et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum Mol Genet. (2013) 22:3998–4006. doi: 10.1093/hmg/ddt239
18. Kestenbaum, B, Glazer, NL, Köttgen, A, Felix, JF, Hwang, S-J, Liu, Y, et al. Common genetic variants associate with serum phosphorus concentration. J Am Soc Nephrol. (2010) 21:1223–32. doi: 10.1681/ASN.2009111104
19. D’Adamo, CR, D’Urso, A, Ryan, KA, Yerges-Armstrong, LM, Semba, RD, Steinle, NI, et al. A common variant in the SETD7 gene predicts serum lycopene concentrations. Nutrients. (2016) 8:82. doi: 10.3390/nu8020082
20. Ferrucci, L, Perry, JR, Matteini, A, Perola, M, Tanaka, T, Silander, K, et al. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. (2009) 84:123–33. doi: 10.1016/j.ajhg.2008.12.019
21. Hazra, A, Kraft, P, Lazarus, R, Chen, C, Chanock, SJ, Jacques, P, et al. Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum Mol Genet. (2009) 18:4677–87. doi: 10.1093/hmg/ddp428
22. Grarup, N, Sulem, P, Sandholt, CH, Thorleifsson, G, Ahluwalia, TS, Steinthorsdottir, V, et al. Genetic architecture of vitamin B12 and folate levels uncovered applying deeply sequenced large datasets. PLoS Genet. (2013) 9:e1003530. doi: 10.1371/journal.pgen.1003530
23. Zheng, J-S, Luan, J’a, Sofianopoulou, E, Imamura, F, Stewart, ID, Day, FR, et al. Plasma vitamin C and type 2 diabetes: genome-wide association study and Mendelian randomization analysis in European populations. Diabetes Care. (2021) 44:98–106. doi: 10.2337/dc20-1328
24. Manousaki, D, Mitchell, R, Dudding, T, Haworth, S, Harroud, A, Forgetta, V, et al. Genome-wide association study for vitamin D levels reveals 69 independent loci. Am J Hum Genet. (2020) 106:327–37. doi: 10.1016/j.ajhg.2020.01.017
25. Major, JM, Yu, K, Wheeler, W, Zhang, H, Cornelis, MC, Wright, ME, et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum Mol Genet. (2011) 20:3876–83. doi: 10.1093/hmg/ddr296
26. Matthias, A. SNiPA: an interactive, genetic variant-centered annotation browser. Bioinformatics (Oxford, England). (2015) 31:1334–6. doi: 10.1093/bioinformatics/btu779
27. Chung, J, Marini, S, Pera, J, Norrving, B, Jimenez-Conde, J, Roquer, J, et al. Genome-wide association study of cerebral small vessel disease reveals established and novel loci. Brain. (2019) 142:3176–89. doi: 10.1093/brain/awz233
28. Burgess, S, Bowden, J, Fall, T, Ingelsson, E, and Thompson, SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28:30–42. doi: 10.1097/EDE.0000000000000559
29. Zheng, J, Baird, D, Borges, MC, Bowden, J, Hemani, G, Haycock, P, et al. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep. (2017) 4:330–45. doi: 10.1007/s40471-017-0128-6
30. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
31. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
32. Bowden, J, del Greco M, F, Minelli, C, Zhao, Q, Lawlor, DA, Sheehan, NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. (2019) 48:728–42. doi: 10.1093/ije/dyy258
33. Jolink, WMT, Wiegertjes, K, Rinkel, GJE, Algra, A, de Leeuw, FE, and Klijn, CJM. Location-specific risk factors for intracerebral hemorrhage: systematic review and meta-analysis. Neurology. (2020) 95:e1807–18. doi: 10.1212/WNL.0000000000010418
34. Jauhiainen, R, Vangipurapu, J, Laakso, A, Kuulasmaa, T, Kuusisto, J, and Laakso, M. The association of 9 amino acids with cardiovascular events in Finnish men in a 12-year follow-up study. J Clin Endocrinol Metabol. (2021) 106:3448–54. doi: 10.1210/clinem/dgab562
35. Vojinovic, D, Kalaoja, M, Trompet, S, Fischer, K, Shipley, MJ, Li, S, et al. Association of circulating metabolites in plasma or serum and risk of stroke: Meta-analysis from seven prospective cohorts. Neurology. (2020) 96:e1110–23. doi: 10.1212/WNL.0000000000011236
36. Erimban, S, and Daschakraborty, S. How does excess phenylalanine affect the packing density and fluidity of a lipid membrane? Phys Chem Chem Phys. (2021) 23:27294–303. doi: 10.1039/D1CP05004D
37. Vangipurapu, J, Stancáková, A, Smith, U, Kuusisto, J, and Laakso, M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 Finnish men. Diabetes. (2019) 68:1353–8. doi: 10.2337/db18-1076
38. Nemet, I, Saha, PP, Gupta, N, Zhu, W, Romano, KA, Skye, SM, et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cells. (2020) 180:862–77.e22. doi: 10.1016/j.cell.2020.02.016
39. Sacks, FM, Lichtenstein, AH, Wu, JHY, Appel, LJ, Creager, MA, Kris-Etherton, PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. (2017) 136:e1–e23. doi: 10.1161/CIR.0000000000000510
40. Schwab, U, Lauritzen, L, Tholstrup, T, Haldorsson, TI, Riserus, U, Uusitupa, M, et al. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Nutr Res. (2014) 58:25145. doi: 10.3402/fnr.v58.25145
41. Derosa, G, Maffioli, P, D'Angelo, A, Salvadeo, S, Ferrari, I, Fogari, E, et al. Effects of long chain ω-3 fatty acids on metalloproteinases and their inhibitors in combined dyslipidemia patients. Expert Opin Pharmacother. (2009) 10:1239–47. doi: 10.1517/14656560902865601
42. Kris-Etherton, PM, Harris, WS, and Appel, LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. (2002) 106:2747–57. doi: 10.1161/01.CIR.0000038493.65177.94
43. Zheng, T, Zhao, J, Wang, Y, Liu, W, Wang, Z, Shang, Y, et al. The limited effect of omega-3 polyunsaturated fatty acids on cardiovascular risk in patients with impaired glucose metabolism: a meta-analysis. Clin Biochem. (2014) 47:369–77. doi: 10.1016/j.clinbiochem.2013.11.025
44. Abdelhamid, AS, Brown, TJ, Brainard, JS, Biswas, P, Thorpe, GC, Moore, HJ, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. (2020) 2020:CD003177. doi: 10.1002/14651858.CD003177.pub5
45. Sheikh, O, Vande Hei, AG, Battisha, A, Hammad, T, Pham, S, and Chilton, R. Cardiovascular, electrophysiologic, and hematologic effects of omega-3 fatty acids beyond reducing hypertriglyceridemia: as it pertains to the recently published REDUCE-IT trial. Cardiovasc Diabetol. (2019) 18:84. doi: 10.1186/s12933-019-0887-0
46. Siscovick, DS, Barringer, TA, Fretts, AM, Wu, JH, Lichtenstein, AH, Costello, RB, et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. (2017) 135:e867–84. doi: 10.1161/CIR.0000000000000482
48. Zhou, R, Wang, M, Huang, H, Li, W, Hu, Y, and Wu, T. Lower vitamin D status is associated with an increased risk of ischemic stroke: a systematic review and Meta-analysis. Nutrients. (2018) 10:277. doi: 10.3390/nu10030277
49. Scragg, R, Stewart, AW, Waayer, D, Lawes, CMM, Toop, L, Sluyter, J, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol. (2017) 2:608–16. doi: 10.1001/jamacardio.2017.0175
50. Szejko, N, Acosta, JN, Both, CP, Leasure, A, Matouk, C, Sansing, L, et al. Genetically-Proxied levels of vitamin D and risk of intracerebral hemorrhage. J Am Heart Assoc. (2022) 11:e024141. doi: 10.1161/JAHA.121.024141
51. Chung, PW, Park, KY, Kim, JM, Shin, DW, Park, MS, Chung, YJ, et al. 25-hydroxyvitamin D status is associated with chronic cerebral small vessel disease. Stroke. (2015) 46:248–51. doi: 10.1161/STROKEAHA.114.007706
52. Dadrass, A, Mohamadzadeh Salamat, K, Hamidi, K, and Azizbeigi, K. Anti-inflammatory effects of vitamin D and resistance training in men with type 2 diabetes mellitus and vitamin D deficiency: a randomized, double-blinded, placebo-controlled clinical trial. J Diabetes Metab Disord. (2019) 18:323–31. doi: 10.1007/s40200-019-00416-z
53. Park, JE, Pichiah, PBT, and Cha, YS. Vitamin D and metabolic diseases: growing roles of vitamin D. J Obes Metab Syndr. (2018) 27:223–32. doi: 10.7570/jomes.2018.27.4.223
54. Cui, C, Xu, P, Li, G, Qiao, Y, Han, W, Geng, C, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. (2019) 26:101295. doi: 10.1016/j.redox.2019.101295
55. Krivoy, A, Satz, J, Hornfeld, SH, Bar, L, Gaughran, F, Shoval, G, et al. Low levels of serum vitamin D in clozapine-treated schizophrenia patients are associated with high levels of the proinflammatory cytokine IL-6. Int Clin Psychopharmacol. (2020) 35:208–13. doi: 10.1097/YIC.0000000000000303
56. Collaboration HLT. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine lowering trialists' collaboration. BMJ. (1998) 316:894–8. doi: 10.1136/bmj.316.7135.894
57. VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO prevent stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. (2010) 9:855–65. doi: 10.1016/S1474-4422(10)70187-3
58. Larsson, SC, Traylor, M, and Markus, HS. Homocysteine and small vessel stroke: a mendelian randomization analysis. Ann Neurol. (2019) 85:495–501. doi: 10.1002/ana.25440
59. Przybelski, RJ, and Binkley, NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. (2007) 460:202–5. doi: 10.1016/j.abb.2006.12.018
60. Woo, D, Falcone, GJ, Devan, WJ, Brown, WM, Biffi, A, Howard, TD, et al. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet. (2014) 94:511–21. doi: 10.1016/j.ajhg.2014.02.012
Glossary
Keywords: cerebral small vessel disease, intracerebral hemorrhage, small vessel stroke, nutrient, Mendelian randomization
Citation: Li J, Xia K, Wang Z, Liu Y, Tong Y, Wang Y, Zhou Y, Zhang L, Tang L, Fan D and Yang Q (2023) Essential nutrients and cerebral small vessel diseases: a two-sample Mendelian randomization study. Front. Nutr. 10:1172587. doi: 10.3389/fnut.2023.1172587
Edited by:
George Lagoumintzis, University of Patras, GreeceReviewed by:
Kariofyllis Karamperis, University of Patras, GreeceNikolaos A. Afratis, National and Kapodistrian University of Athens, Greece
Copyright © 2023 Li, Xia, Wang, Liu, Tong, Wang, Zhou, Zhang, Tang, Fan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongsheng Fan, ZHNmYW4yMDEwQGFsaXl1bi5jb20=; Qiong Yang, eWFuZ3Fpb25ncHV0aEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Jiayi Li
Jiayi Li Kailin Xia
Kailin Xia Zhengrui Wang1,2
Zhengrui Wang1,2 Yicheng Tong
Yicheng Tong Linjing Zhang
Linjing Zhang Lu Tang
Lu Tang Dongsheng Fan
Dongsheng Fan