- 1College of Horticulture, Jilin Agricultural University, Changchun, China
- 2International Cooperation Research Center of China for New Germplasm Breeding of Edible Mushrooms, Jilin Agricultural University, Changchun, China
- 3Institute of Economical Plants Research, Academy of Agricultural Science of Jilin Province, Gongzhuling, China
- 4College of Forestry and Grassland, Jilin Agricultural University, Changchun, China
- 5Jiangxi Academy of Agricultural Sciences Nanchang, Nanchang, China
- 6Food Science and Technology Program, Department of Life Sciences, BNU-HKBU United International College, Zhuhai, China
Background: Mushrooms are considered as next-generation healthy food components. Owing to their low-fat content, high-quality proteins, dietary fiber, and rich source of nutraceuticals. They are ideally preferred in formulation of low-caloric functional foods. In this view, the breeding strategies of mushroom Auricularia cornea (A. cornea) focusing on high yield and higher quality with rich nutritional values and health benefits are still needed.
Materials and methods: A total of 50 strains of A. cornea were used to analyze the bio efficiency and the time required for fruiting body formation following the cultivation experiment. The calorimetric method was used to evaluate the antioxidant activity and quantify the crude polysaccharides and minerals content thereafter.
Results: The results showed that the time required for fruiting body formation and biological efficiency varied significantly among the selected strains. Noticeably, the wild domesticated strain Ac13 of A. cornea mushroom showed the shortest fruit development time (80 days). Similarly, the hybrid strains including Ac3 and Ac15 possessed the highest biological efficiency (82.40 and 94.84%). Hybrid strains Ac18 (15.2%) and cultivated strains Ac33 (15.6%) showed the highest content of crude polysaccharides, while cultivated strains Ac1 and Ac33, demonstrated the highest content of total polysaccharides in the fruiting body (216 mg. g−1 and 200 mg. g−1). In the case of mineral content, the highest zinc contents were observed from the cultivated strain Ac46 (486.33 mg·kg−1). The maximum iron content was detected from the hybrid strain Ac3 (788 mg·kg−1), and the wild domesticated strain Ac28 (350 mg·kg−1). The crude polysaccharides of the A. cornea strain showed significant antioxidant potential, and the ability of Ac33 and Ac24 to scavenge DPPH radicals and ABTS, which was significantly improved compared to other strains, respectively. Principal component analysis was applied to examine the agronomic traits and chemical compounds of various strains of A. cornea mushrooms. The results revealed that cultivated, wild domesticated, and hybrid strains of A. cornea exhibited distinct characteristics in terms of growth, yield, and nutritional properties.
Conclusion: The crude polysaccharides from A. cornea mushroom strains act as natural antioxidants, the wild, hybrid, and commercial A. cornea mushroom strains can achieve rapid growth, early maturation, and high yields. The evaluation of biochemical indexes and nutritional characteristics of strains with excellent traits provided a scientific basis for initiating high-quality breeding, provided germplasm resources for the production of “functional food” with real nutritional and health value.
Introduction
Mushrooms are the only treasure trove of biologically active metabolites that are extremely rich in high-quality carbohydrates, minerals, proteins, and dietary fiber (1, 2). Under the current world scenario, people eagerly await novel natural, non-toxic functional foods that provide nutritional, and medicinal value (3). With their unique aroma, mushrooms become a delicious food, a step ahead of other natural resources.
Mushroom polysaccharides are considered suitable biological response regulators because of their wide range of health benefits (4, 5). Among all mushroom-derived substances, polysaccharides have been shown to have potential biological activities, including anti-inflammatory, antioxidant, anticancer, antitumor, and significant effects on innate and adaptive immunity (6). One of the effective bioactive compounds of edible fungi is polysaccharides, and another important feature of mushroom polysaccharides is their potential to scavenge free radicals (7, 8). Prior studies revealed that free radicals are the key factor behind several chronic diseases, including neurological disorders, cancer, arthritis, diabetes, and others (9). Although many synthetic antioxidants such as butylated hydroxyanisole, tert-butyled hydroxyquinine, and butylated hydroxytoluene are readily available in the market, the main concerns are increasing about the safety of these synthetic products used in conventional foods. The regular intake of a substance has been found to have detrimental effects on the health of individuals, and prolonged utilization may result in severe adverse reactions. In this regard, mushroom polysaccharides may be recommended as a good alternative to the food industry as a novel potential antioxidant and low-toxicity alternative.
Auricularia cornea is a medicinal and edible mushroom. It belongs to the fungi Basida, Auriculariaceae, mainly distributed in China, Korea and other East Asian countries (10). Auricularia is the fourth most widely cultivated mushroom worldwide and a popular ingredient in Chinese cuisine and medicine. Fungus is one of the most widely cultivated mushrooms in China (11). According to the China Edible Mushroom Association, the annual production of A. auricularia and A. cornea reached 75.2 million tons and 16.9 million tons, respectively, in 2017 (12). It is also cultivated in other parts of the world. The advantage of A. cornea mushroom is its long shelf life (13). Due to its ability to undergo desiccation, this particular species presents a viable option for growers seeking to propagate mushrooms in contrast to alternative varieties (14). A. cornea is a low-calorie food that is rich in nutrients including good source of complex carbohydrates, low-fat source of protein, B vitamins, including riboflavin, niacin, and pantothenic acid, minerals such as iron, potassium, and phosphorus, polysaccharides, and consumed in moderation as part of a balanced diet (11). Though the determination of amino acids is important for assessing the nutritional value of a food or dietary supplement, it may not always be necessary in studies of A. cornea as it is already been shown to be a good source of protein, containing all essential amino acids (15). Therefore, we focused on other aspects of its nutritional composition, such as its mineral content, carbohydrate content, or antioxidant activity, rather than determining the amino acid profile. The mineral content and antioxidants of A. cornea can help determine its potential as a dietary supplement for maintaining optimal health, reduction of oxidative stress and inflammation in the body. However, the polysaccharides, minerals, and antioxidant properties of A. cornea have not been studied using many germplasm resources. Therefore, this study focused on the time required for fruiting body formation, biological efficiency, polysaccharide content, antioxidant activity, and mineral element content of 50 A. cornea mushroom strains from China and Japan and newly developed A. cornea varieties.
Materials and methods
Mushroom strains
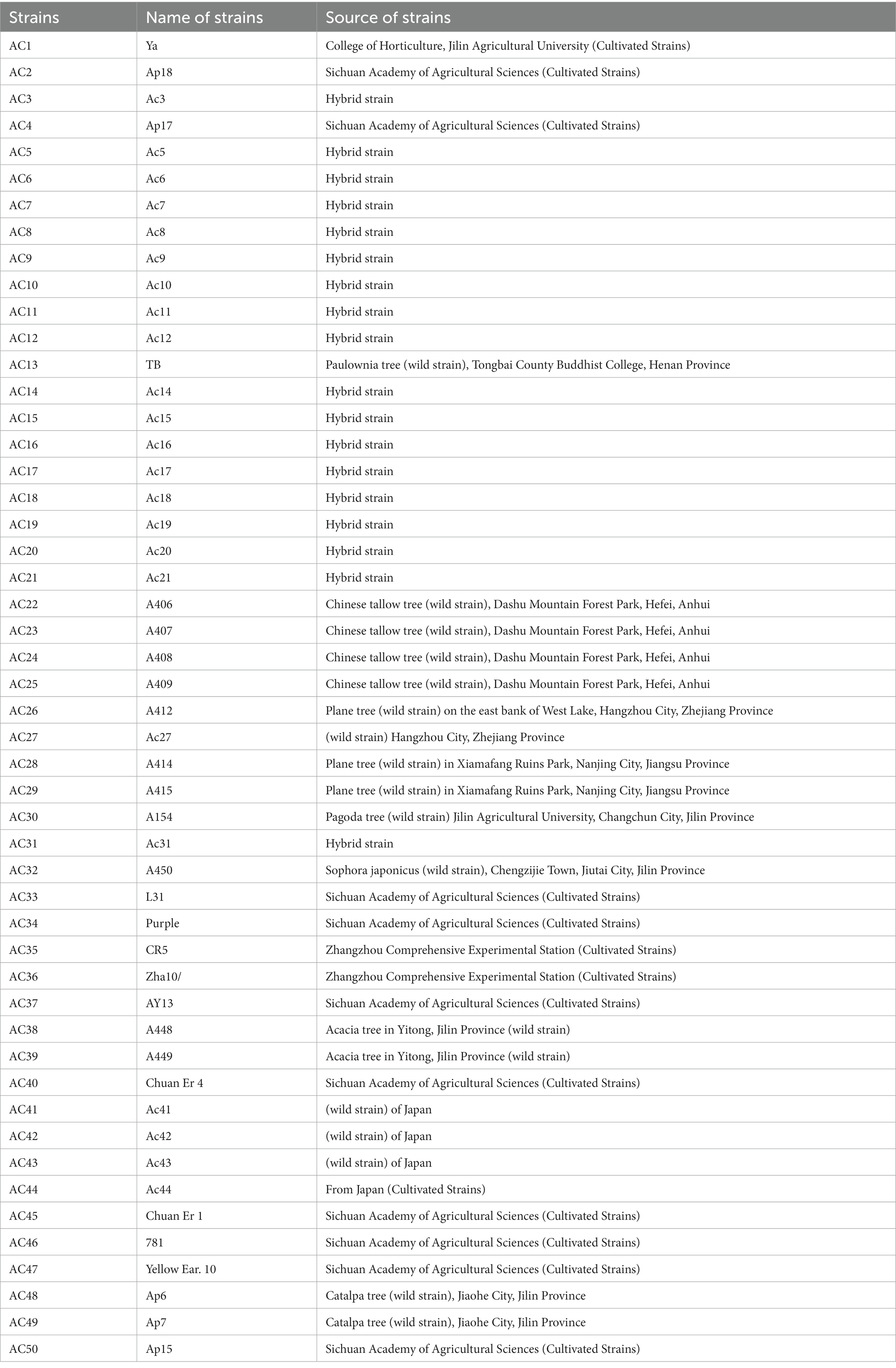
In this study, a total of 50 strains of A. cornea were used (listed in Table 1). Among these strains, 14 commercial cultivated strains were obtained from distinct regions of China, namely Sichuan Academy of Agricultural Sciences, Zhangzhou Comprehensive Experimental Station, College of Horticulture at Jilin Agricultural University, as well as from Japan. Furthermore, the study also included 18 wild A. cornea strains from five different provinces in China, namely Jiaohe City, Jilin Province; Yitong City, Jilin Province; Chengzijie Town, Jiutai City, Jilin Province, Jilin Agricultural University Campus, Changchun City, Jilin Province, Xiamafang Ruins Park, Nanjing City, Jiangsu Province; East bank of West Lake, Hangzhou City, Zhejiang Province; Dashushan Forest Park, Hefei City, Anhui Province; Tongbai County Buddhist College, Henan Province, as well as strains from Japan. In addition, the study involved 14 hybrid A. cornea strains.
Cultivation experiment
Mix the pre-wet sawdust substrate (78%) with wheat bran (20%), CaCO3 (1%), and CaSO4 (1%). Adjust the water content of the sawdust mixture to about 55 to 60%. Each polyethylene bag (height 30 cm, diameter 10 cm) was filled with 0.5 kg sawdust-based substrate, sterilized (121°C, 120 min), and then inoculated with 4 g/bag of prepared spawn. Inoculation bags were kept in the spawning chamber under dark conditions (temperature 25 ± 1°C, relative humidity 80%). When the mycelium was fully colonized, transfer the bags to the growing chamber (20 ± 2°C, relative humidity > 90%) to stimulate primordium formation. The fruiting bodies were then harvested by twisting method with clean hands for further analysis when fully grown to reveal waveform edge (16).
Evaluation of agronomic traits
The agronomic characteristics enlisted as the time required for fruiting body formation and biological efficiency. The total number of days from inoculation bag to harvest fruiting bodies. Harvested fruiting bodies from the first and second flushes were weighed in fresh form for analysis of biological efficiency. Bioefficiency is the ratio of weight (g) of fresh fruiting body/dry weight of substrate (g), expressed as a percentage.
Extraction of crude polysaccharides
According to the method proposed by Cai et al. (17) and Skalicka-Wozniak et al. (18), crude polysaccharides were extracted and purified from A. cornea strains and slightly modified. Five grams of dry powdered sample was extracted thrice with 200 mL hot water (80°C) for 3 hours. The water extract was filtered with fiber gauze, combined and then adjusted the volume up to 50 mL. Afterward, 150 mL of chilled ethanol (96%) was added and placed in a refrigerator at 4°C overnight to induce precipitation. After centrifugation (15,000 rpm, 6 min) the precipitate was collected, washed with ethanol, dried in the oven at 45°C and then grinded with a pestle and mortar for further analysis. The following equation has been used to measure the crude polysaccharide yield.
Yield (%) = m1/m2 × 100%, where m1 is the weight of crude polysaccharide, and m2 is the weight of A. cornea powder.
Total polysaccharides
The total polysaccharide content in crude polysaccharides obtained from the fruiting body of the A. cornea strain was determined by phenol-sulfuric acid method (18). An equal weight of 1 mg sample of crude polysaccharide dissolved in 10 mL sterilized water. Then, mix 1 mL of this solution with 1 mL of phenol solution (5%) and 5 mL of concentrated sulfuric acid. The mixture was kept in the dark shaker at 25°C for 30 min, and measure the absorbance at 490 nm with a spectrophotometer. The polysaccharide content of A. cornea strains was determined by utilizing the glucose standard curve to calculate the total amount. We then set up nine glucose solutions from different sources, each with a different concentration of (1 mg·mL−1) glucose stock solution (i.e., (20, 40, 60, 80, 100, 120, 140, 160, and 180 μL) in a 10 mL centrifuge tube, adjust the volume to 2 mL by distilled water, then add 1 mL of 5% phenol solution and 5 mL of concentrated sulfuric acid, and repeat the same steps as above.
Mineral quantification
The minerals present in the fruiting bodies of the A. cornea mushroom strain were measured using the method of (19) (cite the reference). The fruiting body samples are oven-dried at 35°C for 24 h and ground into a powder that can pass through a 1 mm sieve. Weighted 1 g of mushrooms in each sample to determine the mineral content. Afterward, “wet” digestion with a 3:1 mixture of nitric and perchloric acid. Finally, zinc, copper, manganese, and iron in solution were quantified using an atomic absorption spectrometer (19).
Determination of antioxidant activity
DPPH radical scavenging using the method described by (20) was performed by vigorously mixing 2 mL of crude polysaccharide solution (0.25, 0.50, 0.75, 1.00, 1.25, and 1.50 mg/mL) and 2 mL of DPPH solution (0.2 mmol/L) and kept in the dark for 30 min and 2 mL of absolute ethanol solution was used as a control. Vitamin C is used as a standard and is formulated at the same concentration as crude polysaccharide solutions. A spectrophotometer is used to calculate absorbance at 517 nm. The following equation determines the free radical scavenging effect of DPPH:
Scavenging rate (%) = (Ablank − Asample)/Ablank × 100.
A blank represents the absorbance of the control solution (no sample), while A sample represents the absorbance of the test sample.
ABTS radical scavenging assay
Free radical scavenging activity of crude polysaccharides was determined using the ABTS radical cation (ABTS+) test, a slightly modified Binsan’s method (17). The reaction of 7 mM ABTS solution with 2.45 mM potassium persulfate yields ABTS+, which was kept at room temperature for 16 h in the dark. At 734 mM, dilute the ABTS+ solution with ethanol to obtain an absorbance of 0.70 ± 0.02. Applied 1 mL of crude polysaccharide solution samples at different concentrations (0.25, 0.50, 0.75, 1.00, 1.25, and 1.50 mg/mL) to 3.9 mL of ABTS+ solution and mix vigorously. The absorbance at 734 nm was measured after 6 min of reaction at room temperature (17, 21).
Statistical analysis
Data were analyzed using a well-known statistical method: Fisher Analysis of Variance (ANOVA). Treatment means were compared using the Least Significant Difference (LSD) test at the 5% probability level.
Results and discussion
Evaluation of agronomic traits
The biological efficiency of A. cornea, including the results of the fruiting bodies’ maturation period from the spawning stage to the point of harvest were described in Table 2. The shortest time of matured fruiting bodies of the A. cornea mushroom strain was recorded from wild strains Ac13 (80 days) and A24 (90 days). In comparison, the duration of the cultivated strain Ac44 was extended (166 days). The number of days of maturity of fruiting bodies of cultivated, wild and hybrid strains ranged from spawning to harvesting fruiting bodies (166–80 days).
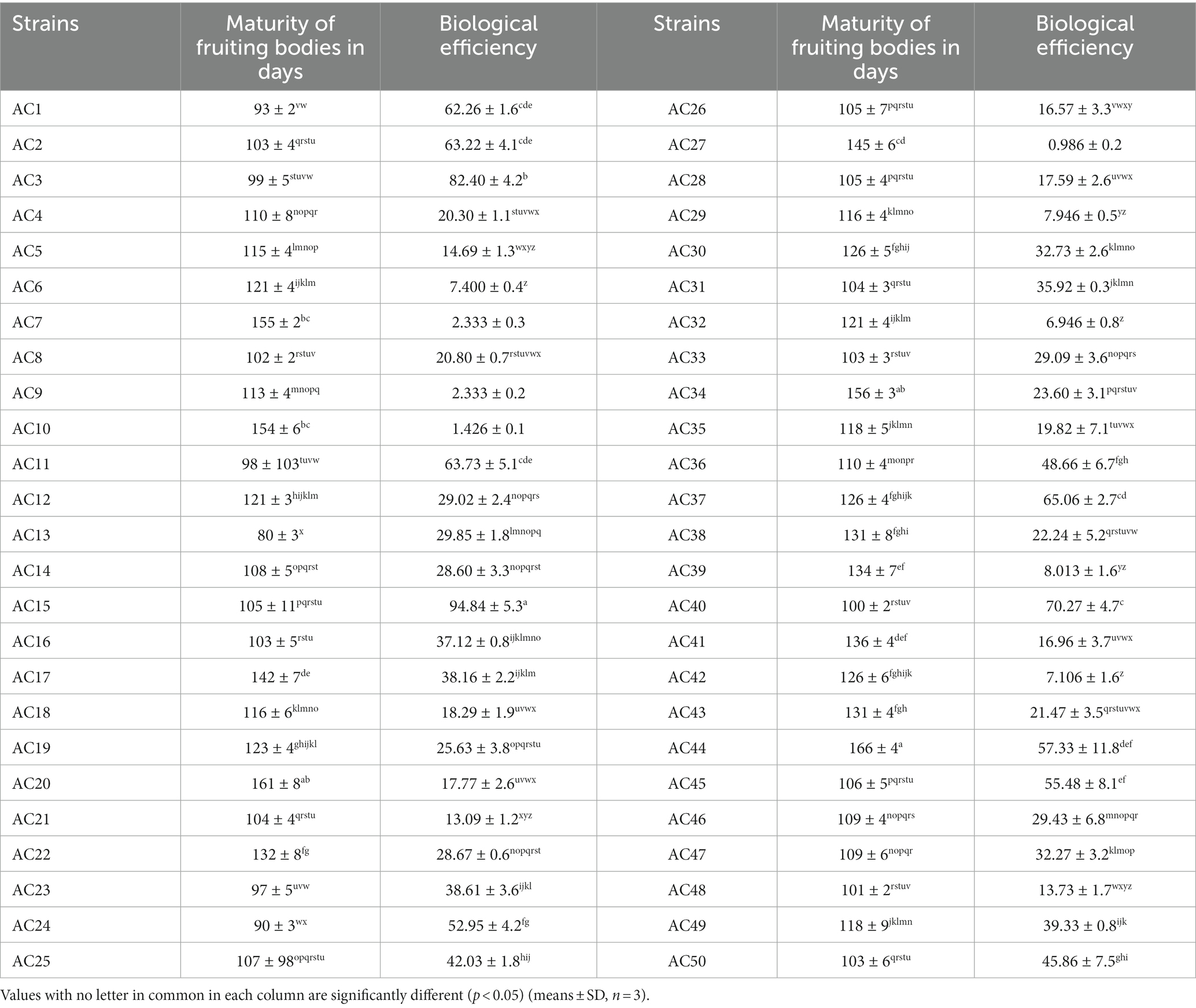
Table 2. Total number of days for the maturity of fruiting bodies and biological efficiency of A. cornea strains.
The time to complete fruiting bodies recorded from cultivated A. cornea strains was more than 90 days and longer for wild strains compared to cultivated strains (16). This study reports the attainment of the shortest duration for the completion of fruiting bodies from wild and domesticated strains, representing a novel finding. (i.e., Ac13 and Ac24). The variances in biological efficiency among A. cornea strains, including cultivated, wild, and hybrid varieties, have been observed. The hybrid strain Ac15 showed the highest biological efficiency (94.84%), while the wild strain Ac27 indicted the lowest biological efficiency (0.92%). The biological efficiency of A. cornea strains ranged from (94.84–0.986%) as shown in Table 2.
Crude polysaccharide content
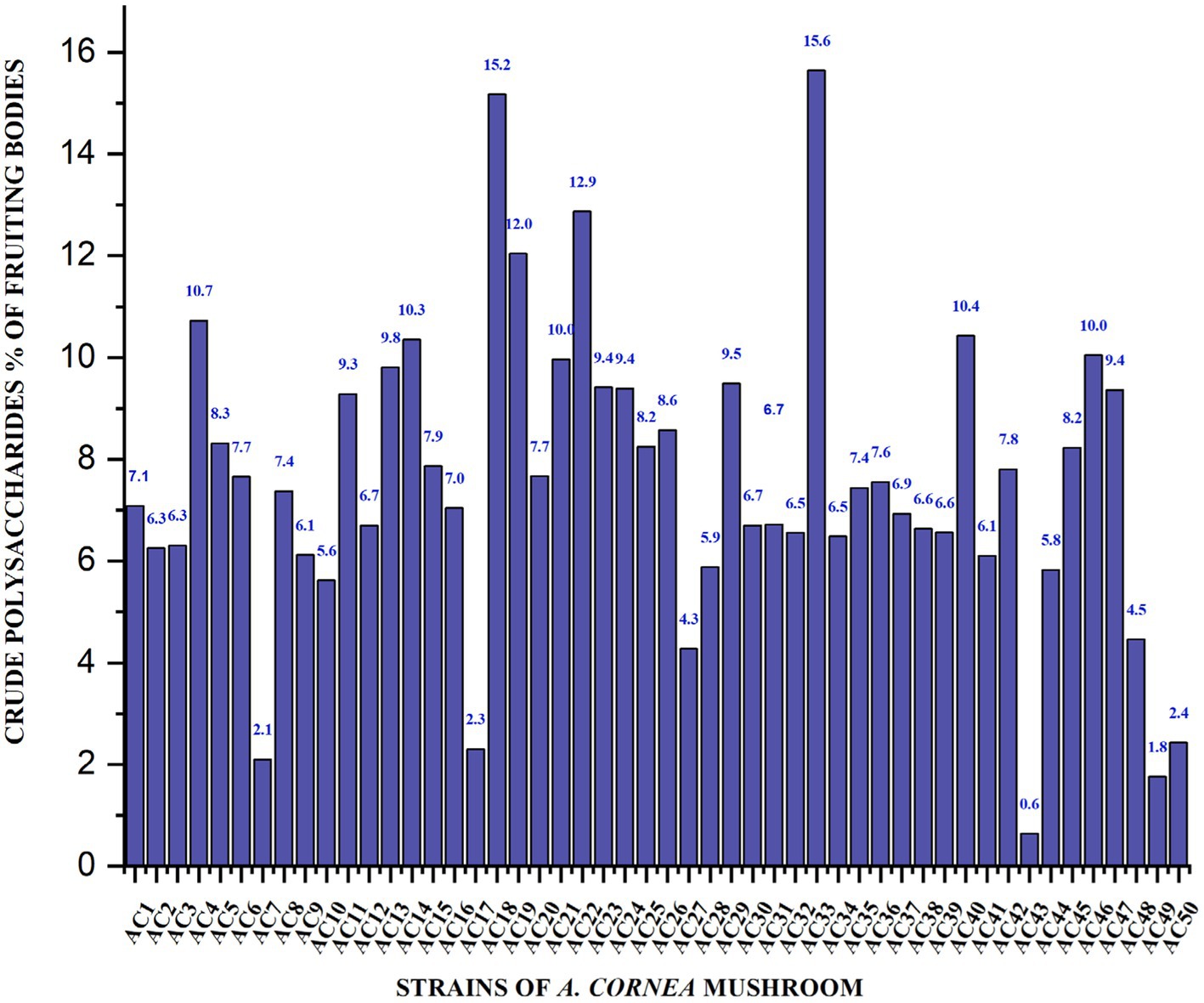
The crude polysaccharide content of cultivated, wild and hybrid A. cornea strain was thoroughly investigated. Crude polysaccharides extracted from the fruiting bodies of the A. cornea strain range from 15.64–0.63% (Figure 1). The cultivated A. cornea strain Ac33 demonstrated the highest crude polysaccharide content (15.64%). While the Japanese wild strain Ac43 indicted the lowest crude polysaccharide content (0.63%). In addition, the outcomes obtained from the crude polysaccharides derived from the hybrid strain Ac33 were found to be twice as much as the control utilized by Li et al. Specifically, the control group exhibited a dry weight of 7%, whereas the selenium supplementation in the control group resulted in a 23% dry weight (14).
Total polysaccharide content
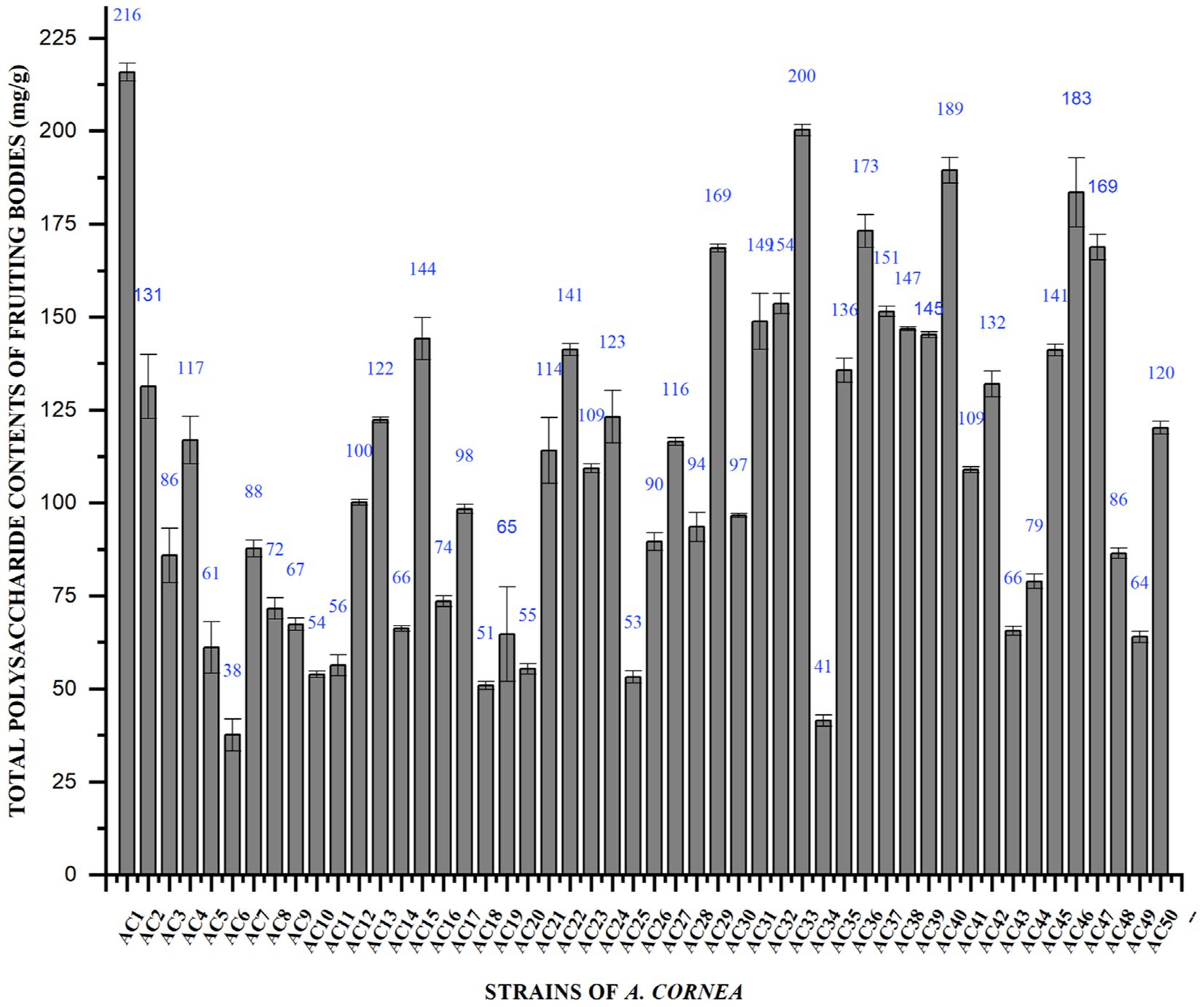
Total polysaccharide content of cultivated, wild and hybrid strains of A. cornea fruiting bodies was determined by the phenol-sulfuric acid method and the results were shown in Figure 2. The total polysaccharide content of fruiting bodies of the A. cornea mushroom strain varied considerably, from 215.88 ± 2.4 mg. g−1 to 37.63 ± 4.3 mg. g−1. The highest contents of total polysaccharides were obtained from cultivated strained Ac1 (215.88 mg. g−1). The lowest total polysaccharide content of fruiting bodies was observed in the cultivated strain Ac6 (37.63 mg. g−1). The study conducted by Su et al. revealed that the Auricularia species exhibited a range of 71.3 to 81.49 g in total polysaccharide content. The outcome of 100 g−1 is comparable to these results (22).
Mineral content
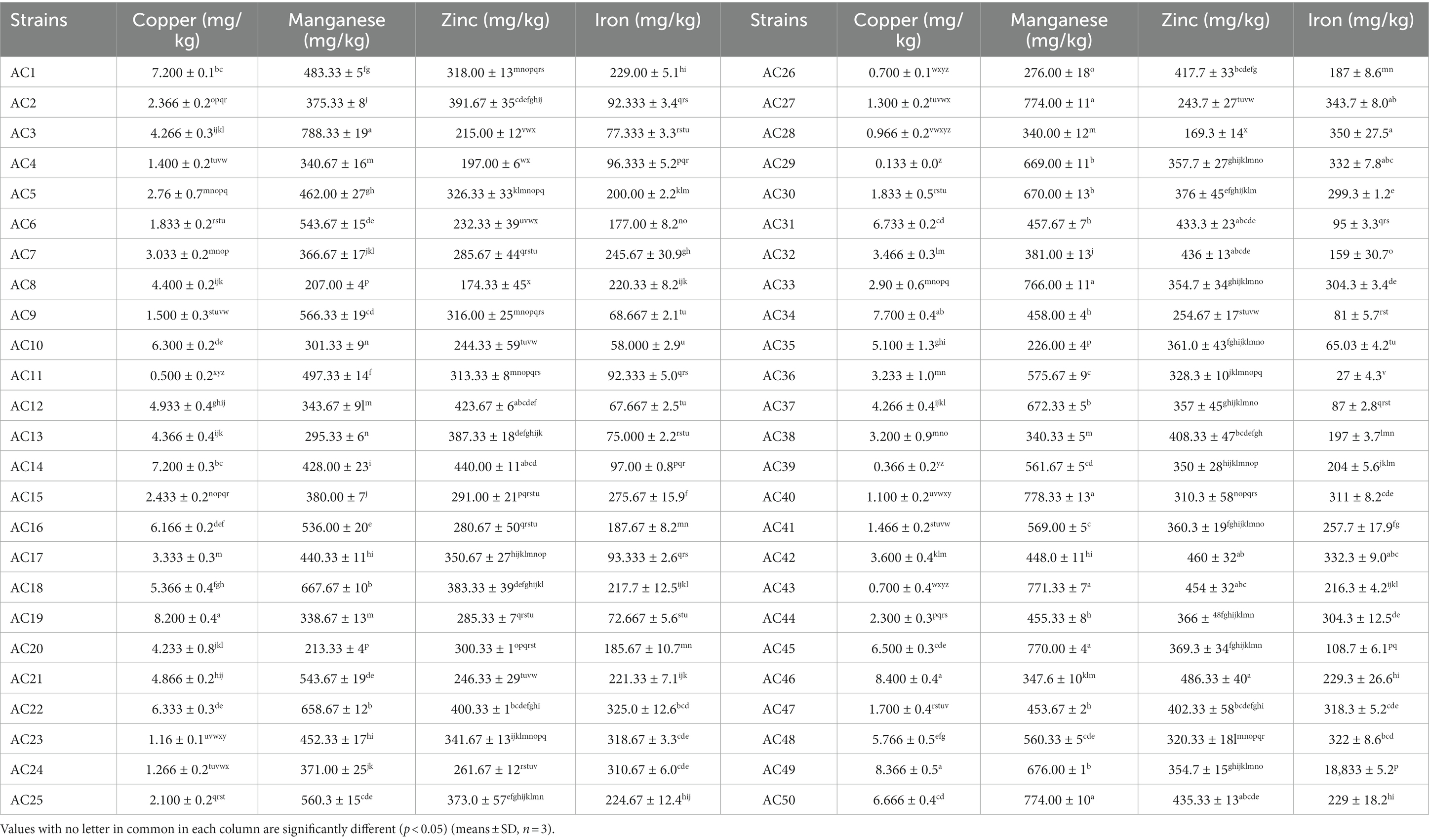
The mineral composition of the fruiting body of domesticated, wild, and hybrid A. cornea strains are shown in Table 3. The copper and manganese content of the A. cornea was 0.133 to 8.40 mg. kg−1 and 213 to 788 mg. kg−1, respectively. The cultivated strain Ac46 showed the highest copper content (8.40 mg. kg−1), followed by wild strain Ac49 (8.36 mg. kg−1) and hybrid strain Ac19 (8.2 mg. kg−1), while wild strain Ac29 contained the lowest copper content (0.133 mg. kg−1).
Significant differences were observed in the manganese content of A. cornea strains, with the highest manganese content in hybrid strains Ac3 (788 mg. kg−1), followed by cultivated strain Ac40 (778 mg. kg−1), and then wild strain Ac27 (774 mg. kg−1). In contrast, the hybrid strain Ac20 showed the lowest (213 mg. kg−1). Among the 50 A. cornea stains, the cultivated strain Ac46 showed the highest zinc content (486.33 mg. kg−1). At the same time, the wild strain Ac28 demonstrated the highest iron content (350 mg. kg−1). In contrast, the wild strain Ac28 contained the lowest zinc content (169 mg. kg−1), while the cultivated strain Ac36 showed the lowest iron content (27 mg. kg−1). In general, A. cornea was high in zinc and iron contents. Mushrooms are good natural zinc and iron accumulators and biologically essential for the human body. Our findings are consistent with the results of Wang et al., who investigated the mineral content of various A. cornea strains. In addition, prior study of Rebecca et al., showed lower results for iron, manganese, copper, and iron contents compare to our results (15).
In the current study, we found that A. cornea mushrooms can be used in various food items. Malnutrition is currently causing health issues for people all over the world. It is estimated that 17% of the population is at risk of zinc deficiency (12). This decline is due to insufficient levels of these elements (copper, manganese, zinc, and iron) in a healthy diet. Implementing effective strategies to prevent and regulate these nutrients in the human diet is therefore essentially required. Hence, there is a trend of increasing dietary supplements worldwide. While the increase in their chemical composition requires a natural process of absorption and accumulation, mushrooms are screened for their high nutritional and commercial value (23). The A. cornea mushrooms are considered to be a significant source of vital nutrients. Thus, the utilization of A. cornea mushrooms has the potential to efficiently mitigate nutritional insufficiencies prevalent in impoverished and malnourished populations. Furthermore, these findings establish the basis for novel germplasm advancements.
DPPH radical scavenging activity
DPPH radicals are static and are commonly used to assess the radical scavenging activity of biological compounds. Biological compounds can transfer an electron or a hydrogen atom to a DPPH radical, which is how they scavenge DPPH radical (24). This is a commonly used technique to determine the sensitivity, incompetence, and velocity of many samples to determine their antioxidant capacity (25). As shown in Figure 3A, the DPPH radical scavenging capacity of all crude polysaccharide A. cornea has a concentration-dependent connection. The concentration-dependent DPPH radical scavenging potential of strains of A. cornea is demonstrated in Figure 3A. When the concentration of crude polysaccharides increased from 0.25–1.50 mg/mL, the scavenging activity of crude polysaccharides on DPPH free radicals progressively increased. The highest scavenging rate appears at a crude polysaccharide concentration of 1.50 mg/mL. The strongest scavenging rates of Ac1, Ac3, Ac11, Ac13, Ac15, Ac24, and Ac33 were 32.1, 23.6, 19.2, 35.6, 26.3, 40.8, and 46.2%, respectively, weaker than Vc (vitamin C). Seven types of A.cornea mushroom strains crude polysaccharides scavenge free radicals: Ac33 > Ac24 > Ac13 > Ac1 > Ac15 > Ac3 > Ac11. The scavenging activity on DPPH radicles was similar to that of crude polysaccharides from Lepista nuda, and was relatively smaller than that of polysaccharides from Auricularia species (10, 22, 26).
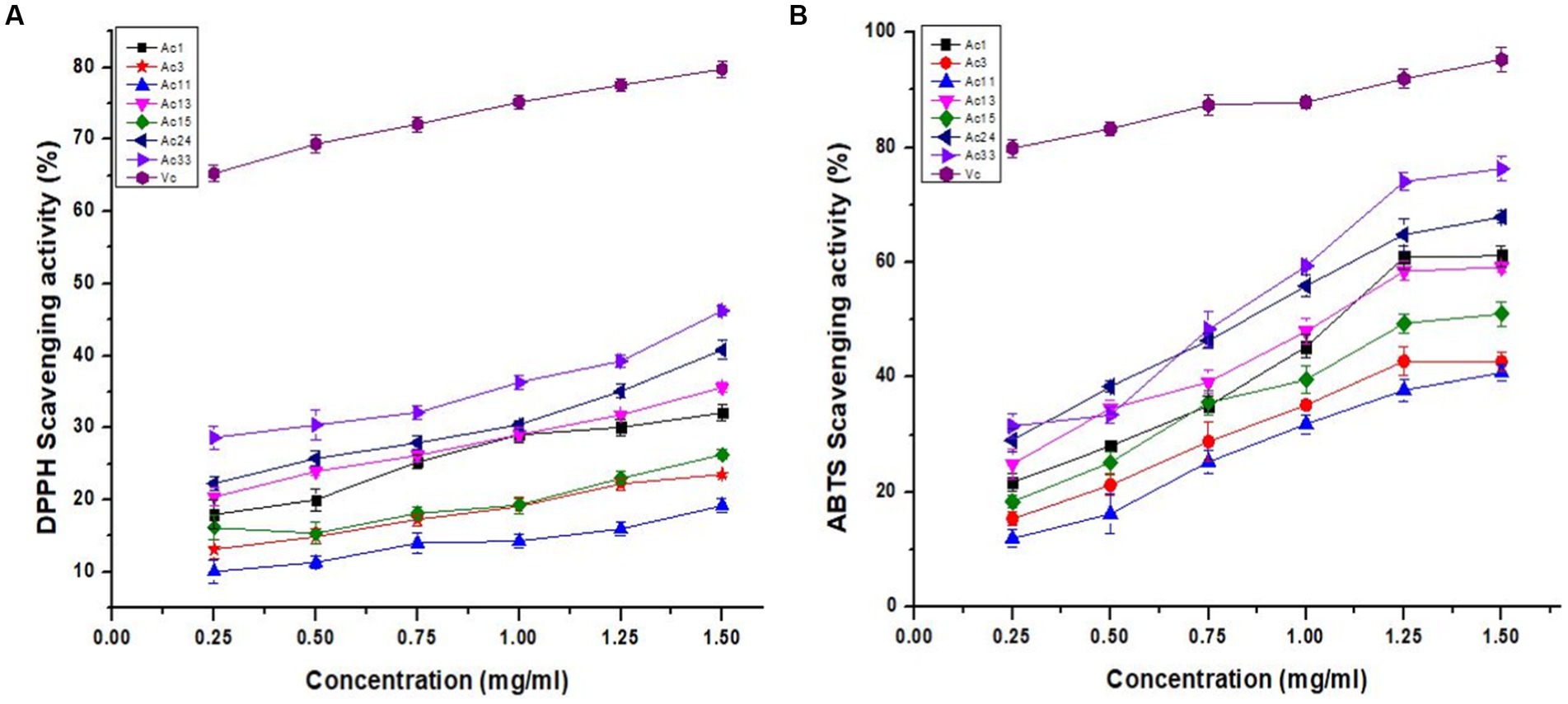
Figure 3. Antioxidant activities of crude polysaccharides of A. cornea strains. (A) DPPH radical scavenging and (B) ABTS scavenging activities.
ABTS radical scavenging activity
ABTS radical scavenging activity is a simple and commonly used procedure for measuring the antioxidant activity of natural ingredients. As shown in Figure 3B, crude polysaccharides from 7 A. cornea strains showed 11.9–76.3% ABTS radical scavenging activity at concentrations of 0.25–1.50 mg/mL. It was found that ABTS radical scavenging activity increased with increasing crude polysaccharide concentration. The crude polysaccharides of commercially cultivated strain Ac33 achieved 76.3% maximum ABTS radical scavenging activity at 1.50 mg/mL, while the hybrid strain Ac11 obtained 11.9% minimum ABTS radical scavenging activity at 0.25 mg/mL. However, at a maximum concentration of 1.50 mg/mL, standard vitamin C (Vc) had 95.3% ABTS radical scavenging activity. The potential of seven crude polysaccharides to scavenge ABTS free radicals was Ac33 > Ac24 > Ac1 > Ac13 > Ac15 > Ac3 > Ac11. Similarly, the polysaccharides obtained from Pleurotus sajor caju showed ABTS radical scavenging capacity of 16.01 to 70.09% at different 25 to125 μg/mL concentrations. In comparison, polysaccharides from Pleurotus sajor caju showed an ABTS scavenging capacity of 63.96% at 5 mg/mL concentrations (27). Similarly, at a 10 mg/mL concentration, the ABTS clearance efficiency of melanin extracted from black fungus was 95.6%29. Our results correlate significantly with previous work (28).
Principal components analysis
The differences and similarities between the chemical compounds and agronomic traits of A. cornea mushroom strains were subjected to principal component analysis (PCA) as shown in Figure 4. The study involved the analysis of six distinct components, namely the total number of days required for harvesting of fruiting bodies and biological efficiency, across a sample of 50 strains of A. cornea. Principal Component Analysis (PCA) is a commonly employed technique aimed at reducing a large number of variables to a limited set of principal components. These components are selected based on their ability to account for the maximum variance present in the data being analyzed. The distributions of all the samples are conveniently positioned around the center of the map, as shown by the results. The first two principal components (PC1 and PC2) accounted for 46.86% of the total variance (26.14 and 20.72%, respectively). The PC1 was associated with the contents of iron, zinc, manganese, total polysaccharides of fruiting bodies, biological efficiency whereas the PC2 was correlated with MFD and Copper. The components were closed to each other, positively correlated, such as iron, manganese, the total polysaccharides of fruiting bodies.
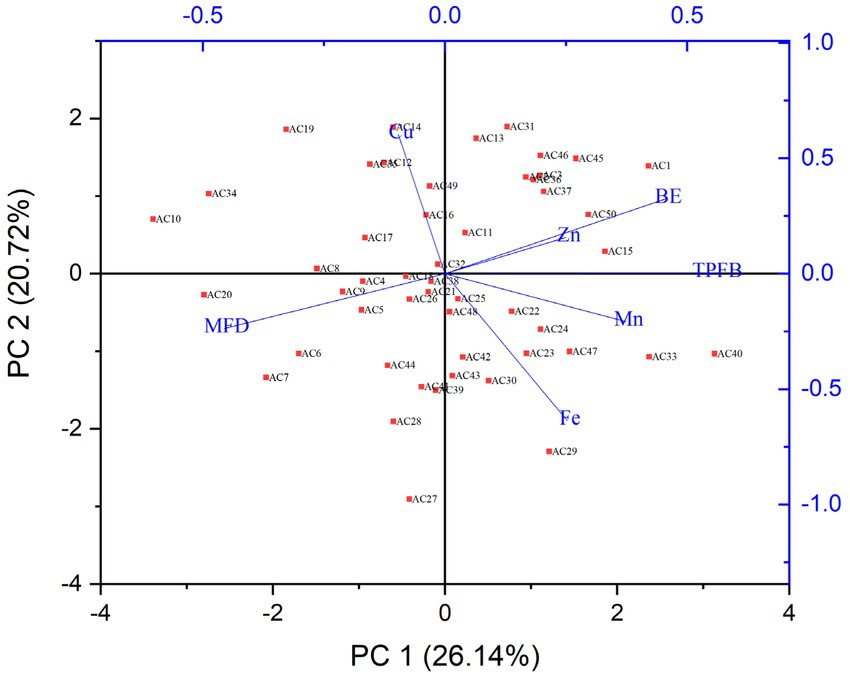
Figure 4. Biplot based on Principal Component Analysis of mushroom chemical composition and A. cornea strains arrangement. (Ac), mushroom strains; (MFD), numbers of days for harvesting of fruiting bodies; (BE), biological efficiency; (Zn), zinc; (Fe), iron; (Cu), copper; (Mn), manganese, and (TPB), total polysaccharides of fruiting bodies.
On the other hand, certain compounds such as zinc, crude polysaccharides and biological efficiency were not significantly correlated with iron, manganese, the total polysaccharides of fruiting bodies, but they were closely significant with each other. Some compounds were separated, they were negatively correlated, such as copper and days to maturity of fruiting bodies. The principal component analysis (PCA) results elucidated the interrelationships among different compounds and explicated their distinct and affirmative correlations.
Conclusion
The study determined that the crude polysaccharides derived from various strains of A. cornea mushrooms exhibit natural antioxidant properties. Additionally, the investigation revealed that wild, hybrid, and commercial strains of A. cornea mushrooms are capable of attaining accelerated growth, premature maturation, and elevated productivity. The scientific basis for commencing high-quality breeding and producing “functional food” with genuine nutritional and health value, as well as the development of innovative antioxidant food additives and active ingredients of different fungal strains, was provided by the assessment of biochemical indexes and nutritional characteristics of strains with exceptional traits. Additionally, the germplasm resources were established for this purpose, along with abundant resources of crude polysaccharides.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
AK, F-JY, MF, L-XL, and Y-MZ: conceptualization. PW and J-JM: methodology. AK and BX: writing and review. F-JY, MF, L-XL, and Y-hW: supervision. K-SS, X-XM, and QH: analysis. All authors contributed to the article and approved the submitted version.
Funding
The research was supported by China Agriculture Research System (No. CARS-20) and The project of “one thousand talents plan” in Jiangxi Province, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang, K, Zhang, S, Ying, Y, Li, Y, Cai, M, Guan, R, et al. Cultivated fruit body of Phellinus baumii: a potentially sustainable antidiabetic resource. ACS Omega. (2020) 5:8596–604. doi: 10.1021/acsomega.9b04478
2. Zeng, W-C, Zhang, Z, Gao, H, Jia, L-R, and Chen, W-Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr Polym. (2012) 89:694–700. doi: 10.1016/j.carbpol.2012.03.078
3. Thu, ZM, Myo, KK, Aung, HT, Clericuzio, M, Armijos, C, and Vidari, G. Bioactive phytochemical constituents of wild edible mushrooms from Southeast Asia. Molecules. (2020) 25:1972. doi: 10.3390/molecules25081972
4. Deveci, E, Çayan, F, Tel-Çayan, G, and Duru, ME. Structural characterization and determination of biological activities for different polysaccharides extracted from tree mushroom species. J Food Biochem. (2019) 43:e12965. doi: 10.1111/jfbc.12965
5. Ma, F, Wu, J, Li, P, Tao, D, Zhao, H, Zhang, B, et al. Effect of solution plasma process with hydrogen peroxide on the degradation of water-soluble polysaccharide from Auricularia auricula. II: solution conformation and antioxidant activities in vitro. Carbohydr Polym. (2018) 198:575–80. doi: 10.1016/j.carbpol.2018.06.113
6. Miao, J, Regenstein, JM, Qiu, J, Zhang, J, Zhang, X, Li, H, et al. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-a review. Int J Biol Macromol. (2020) 150:102–13. doi: 10.1016/j.ijbiomac.2020.02.054
7. Matkowski, A, Tasarz, P, and Szypuła, E. Antioxidant activity of herb extracts from five medicinal plants from Lamiaceae, subfamily Lamioideae. J Med Plants Res. (2008) 2:321–30.
8. Yuan, J-F, Zhang, Z-Q, Fan, Z-C, and Yang, J-X. Antioxidant effects and cytotoxicity of three purified polysaccharides from Ligusticum chuanxiong Hort. Carbohydr Polym. (2008) 74:822–7. doi: 10.1016/j.carbpol.2008.04.040
9. Shi, M, Zhang, Z, and Yang, Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP). Carbohydr Polym. (2013) 95:200–6. doi: 10.1016/j.carbpol.2013.02.081
10. Chen, Y, and Xue, Y. Purification, chemical characterization and antioxidant activities of a novel polysaccharide from Auricularia polytricha. Int J Biol Macromol. (2018) 120:1087–92. doi: 10.1016/j.ijbiomac.2018.08.160
11. Khan, AA, Yao, F, Idrees, M, Lu, L, Fang, M, Wang, P, et al. A comparative study of growth, biological efficiency, antioxidant activity and molecular structure in wild and commercially cultivated Auricularia cornea strains. Folia Horticulturae. (2020) 32:255–64. doi: 10.2478/fhort-2020-0023
12. Rzymski, P, Mleczek, M, Niedzielski, P, Siwulski, M, and Gąsecka, M. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J Sci Food Agric. (2017) 97:923–8. doi: 10.1002/jsfa.7816
13. Wang, P, Yao, F-J, Lu, L-X, Fang, M, Zhang, Y-M, Khan, AA, et al. Map-based cloning of genes encoding key enzymes for pigment synthesis in Auricularia cornea. Fungal Biol. (2019) 123:843–53. doi: 10.1016/j.funbio.2019.09.002
14. Li, X, Yan, L, Li, Q, Tan, H, Zhou, J, Miao, R, et al. Transcriptional profiling of Auricularia cornea in selenium accumulation. Sci Rep. (2019) 9:1–13. doi: 10.1038/s41598-019-42157-2
15. Rebecca, R, Zhang, J, Zhao, W, Liu, Y, Wasantha, K, and Wang, S. Compositional and nutritional inventory of naturally mutant strain Auricularia corneavar Li edible mushroom from China. Prog Nutr. (2020) 22:323–9.
16. Liang, C-H, Wu, C-Y, Lu, P-L, Kuo, Y-C, and Liang, Z-C. Biological efficiency and nutritional value of the culinary-medicinal mushroom Auricularia cultivated on a sawdust basal substrate supplement with different proportions of grass plants. Saudi J Biol Sci. (2019) 26:263–9. doi: 10.1016/j.sjbs.2016.10.017
17. Cai, M, Lin, Y, Luo, Y-L, Liang, H-H, and Sun, P. Extraction, antimicrobial, and antioxidant activities of crude polysaccharides from the wood ear medicinal mushroom Auricularia auricula-judae (higher basidiomycetes). Int J Med Mushrooms. (2015) 17:591–600. doi: 10.1615/IntJMedMushrooms.v17.i6.90
18. Skalicka-Wozniak, K, Szypowski, J, Los, R, Siwulski, M, Sobieralski, K, Glowniak, K, et al. Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P Karst strains cultivated on different wood type substrates. Acta Soc Botanicorum Poloniae. (2012) 81:17–21. doi: 10.5586/asbp.2012.001
19. Khan, AA, Muhammad, MJ, Muhammad, I, Jan, I, Samin, G, Zahid, A, et al. Modulation of agronomic and nutritional response of pleurotus eryngii strains by utilizing glycine betaine enriched cotton waste. J Sci Food Agric. (2019) 99:6911–21. doi: 10.1002/jsfa.9977
20. Xiao, H, Fu, X, Cao, C, Li, C, Chen, C, and Huang, Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int J Biol Macromol. (2019) 121:407–14. doi: 10.1016/j.ijbiomac.2018.09.197
21. Liu, J, Li, Y, Liu, W, Qi, Q, Hu, X, Li, S, et al. Extraction of polysaccharide from Dendrobium nobile Lindl. By subcritical water extraction. ACS Omega. (2019) 4:20586–94. doi: 10.1021/acsomega.9b02550
22. Su, Y, and Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr Polym. (2020) 229:115407. doi: 10.1016/j.carbpol.2019.115407
23. Pastori, GM, Kiddle, G, Antoniw, J, Bernard, S, Veljovic-Jovanovic, S, Verrier, PJ, et al. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell. (2003) 15:939–51. doi: 10.1105/tpc.010538
24. Naik, G, Priyadarsini, K, Satav, J, Banavalikar, M, Sohoni, D, Biyani, M, et al. Comparative antioxidant activity of individual herbal components used in ayurvedic medicine. Phytochemistry. (2003) 63:97–104. doi: 10.1016/S0031-9422(02)00754-9
25. Ma, Y, He, H, Wu, J, Wang, C, Chao, K, and Huang, Q. Assessment of polysaccharides from mycelia of genus Ganoderma by mid-infrared and near-infrared spectroscopy. Sci Rep. (2018) 8:1–10. doi: 10.1038/s41598-017-18422-7
26. Shu, X, Zhang, Y, Jia, J, Ren, X, and Wang, Y. Extraction, purification, and properties of water-soluble polysaccharides from mushroom Lepista nuda. Int J Biol Macromol. (2019) 128:858–69. doi: 10.1016/j.ijbiomac.2019.01.214
27. Seedevi, P, Ganesan, AR, Mohan, K, Raguraman, V, Sivakumar, M, Sivasankar, P, et al. Chemical structure and biological properties of a polysaccharide isolated from Pleurotus sajor-caju. RSC Adv. (2019) 9:20472–82. doi: 10.1039/C9RA02977J
Keywords: Auricularia cornea, wild mushroom, anti-oxidant activity, minerals, polysaccharides
Citation: Khan AA, Lu L-X, Yao F-J, Fang M, Wang P, Zhang Y-M, Meng J-J, Ma X-X, He Q, Shao K-S, Wei Y-h and Xu B (2023) Characterization, antioxidant activity, and mineral profiling of Auricularia cornea mushroom strains. Front. Nutr. 10:1167805. doi: 10.3389/fnut.2023.1167805
Edited by:
Yajie Zou, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Rui-Heng Yang, Shanghai Academy of Agricultural Sciences, ChinaJing-Kun Yan, Dongguan University of Technology, China
Copyright © 2023 Khan, Lu, Yao, Fang, Wang, Zhang, Meng, Ma, He, Shao, Wei and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang-Jie Yao, eWFvZmpAYWxpeXVuLmNvbQ==; Yun-hui Wei, d2VpeWhAMTI2LmNvbQ==; Baojun Xu, YmFvanVueHVAdWljLmVkdS5jbg==
†These authors have contributed equally to this work
 Asif Ali Khan
Asif Ali Khan Li-Xin Lu1†
Li-Xin Lu1† Baojun Xu
Baojun Xu