- Department of Food Science and Nutrition, University of Agricultural Sciences, Bengaluru, Karnataka, India
Rising incidences of life-style disorders like obesity, diabetes and cardiovascular diseases are a matter of concern coupled with escalated consumption of highly refined and high energy foods with low nutrient density. Food choices of consumers have witnessed significant changes globally with rising preference to highly processed palatable foods. Thus, it calls food scientists, researchers and nutritionists’ attention towards developing and promoting pleasant-tasting yet healthy foods with added nutritional benefits. This review highlights selected underutilized and novel ingredients from different food sources and their by-products that are gaining popularity because of their nutrient density, that can be employed to improve the nutritional quality of conventionally available empty-calorie foods. It also emphasizes on the therapeutic benefits of foods developed from these understudied grains, nuts, processing by-products of grains, fruits- and vegetable-byproducts and nutraceutical starches. This review aims to draw attention of food scientists and industrialists towards popularizing the utilization of these unconventional, yet nutrient rich foods sources in improving the nutritional profile of the conventional foods lacking in nutrient density.
1. Introduction
Recent decades across the globe and all the age groups, have shown marked increase in consumption of ultra-processed, processed, ready to eat convenience fried snacks, chips, and refined foods like desserts, bakery and confectionery. These foods lack nutrient density and are rich in calories, attributed to their high fat and/or sugar content, and salt (at certain instances). Thus, their excessive consumption combined with the sedentary lifestyle elevates risk of non-communicable diseases (NCDs) such as diabetes, obesity, cardiovascular disorders and so on (1). It was recorded in World Obesity Atlas (2) that by 2030 one in five women and one in seven men will have obesity (2). According to world health organization (WHO), globally there are 650 million adults, 340 million adolescent and 39 million children who are obese and it was estimated that 167 million people (including adults and children) will become overweight or obese by 2025 (3). WHO also indicated that kidney disease and diabetes caused around 2 million deaths in 2019 (4), globally there are 537 million adults suffering with diabetes, which is predicted to rise by 643 million by 2030 (5). Along with the higher intake of the calorie-rich foods, current diets and consumption patterns also lack dietary fiber, this can consequently contribute in escalating the prevalence of above said disorders, along with constipation and irritable bowel syndrome (6, 7).
Present situation with hiked incidences of these lifestyle disorders is creating health concerns amongst the consumers. Changes in dietary pattern and lifestyle modifications are major management keys to prevent and manage these NCDs. One such modification in dietary practices that can assist in prevention and management of NCDs include consumption of low glycemic index (GI), high fiber foods rich in bioactive compounds (8–10). Thus, buyers are now looking for, and choosing healthy, yet tasty snacks, indicating that their choices are shifting towards picking nutritious and palatable alternatives. This drives the food researchers, scientists and technologists in developing therapeutic, functional foods and low glycemic index foods (to reduce the calorie density) (11, 12). This growing interest in formulation of functional and healthy foods has created a recent research trend of applying novel food sources and processing by-products for formulation of therapeutic foods. These novel ingredients include, underutilized starches, by-products of grains, fruits and vegetables (such as brans, peel, skin, seeds, fiber) that have been reported as a concentrated source of nutrients having multiple health benefits. For instance, whole grain and dietary fiber (including resistance starch) consumption are linked to prevention in lifestyle associated disorders (6). Bran, including cereal bran (i.e., rice, wheat, oat), millet bran of both major (jowar, pearl-, finger-millet bran) and minor millet (barnyard-, foxtail-, little-, kodo-, proso-millet bran) are also reported to be a rich source of fiber, antioxidants, phytochemicals and minerals by several investigations documenting their nutritional richness (13–19). Vegetable and fruit wastes also have ample quantity of fiber, vitamins, minerals and phytochemicals thereby exhibiting health-promoting abilities (20–24).
Overall, these wastes or by-products are generated on a large-scale during food processing and their waste management is the major challenge (25). Despite the abundance of nutrients, usually, majority of these by-products are used as animal feed (26–28), however given their therapeutic benefits, they can also be used as functional ingredient in human food (29, 30). With this understanding, the current review highlights some of these selected underutilized sources based on their nutritional profile with special emphasis on applying these ingredients for formulation of low glycemic index (GI) foods. The review also tries to draw attention on sensory and therapeutic properties of the novel low GI foods.
2. Potential novel food sources and their nutritional profile
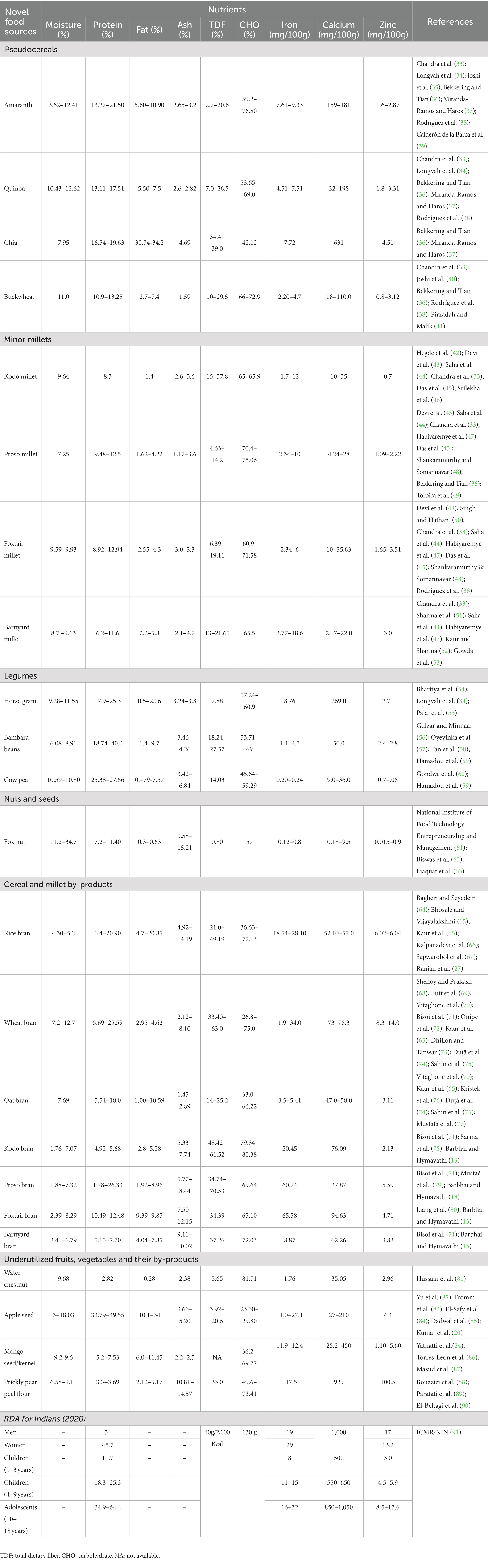
Underutilized cereals and grains, nuts and oilseeds, fruits and vegetables possess good nutritional profile and thereby find application in development of the low GI therapeutic foods. Apart from these underutilized foods, even their processing by-products like bran, vegetable stems, fruit pomace, skin, peel, seeds and so on have can be employed as functional ingredient in development of value added, nutrient dense, therapeutic foods (14, 20–27, 29, 31, 32). This section discusses the nutritional profile of some of selected potential underutilized ingredients and their by-products that can find application in development of low GI foods. Table 1 summarizes nutritional profile of these selected novel ingredients.
2.1. Underutilized grains (cereals/pseudocereals, millets), legumes and their by-products
2.1.1. Underutilized grains (cereals/pseudocereals, millets) and legumes
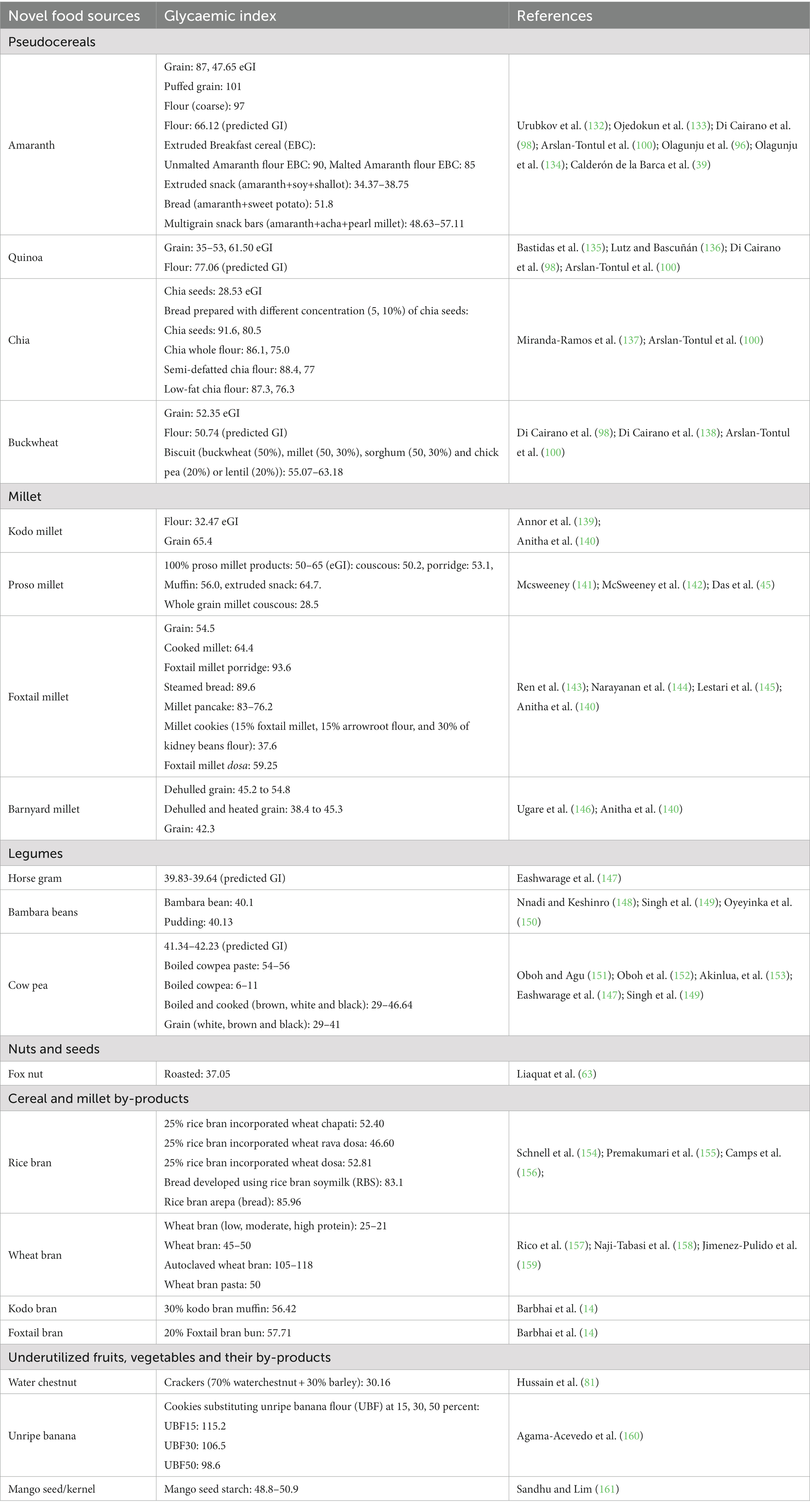
Pseudocereals, millets, and other underutilized grains need to be mainstreamed for providing dietary diversity and developing nutrient rich products apart from the major staple cereals (viz., rice, wheat and maize) and legumes (viz., pigeon peas, Bengal gram, green gram, chick peas, black matpe, kidney beans, peas, lentil) (92–94). Pseudocereals like amaranth (Amaranth caudatus) also called as ‘superfood’, quinoa (Chenopodium quinoa Willd) referred to as ‘Golden grains of Andes’, and millets as ‘nutri-cereals’ are good source of protein and essential amino acids (95, 96). Grain amaranth is known for its superior or equal protein and essential amino acid profile (viz., tryptophan, methionine, threonine, isoleucine, valine, phenylalanine) like staple cereals i.e., rice, wheat and maize (35, 97). Similarly, other pseudocereal namely buckwheat (Fagopyrum esculentum Moench.) has essential amino acids like lysine (5.9% of total protein), methionine (3.7% of total protein), tryptophan (1.4% of total protein) higher than rice (3.8%, 3.0%, 1.0% of total protein respectively), wheat (2.6%, 3.5%, 1.2% of total protein respectively), and maize (1.9%, 3.2%, 0.6% of total protein respectively) (40). Quinoa (17.51%) and chia (Salvia hispanica L.) seeds (19.63%) also contain higher protein than major staple cereals (37). Another nutritional benefit of consuming pseudocereals is absence of gluten, making it suitable for patients with celiac disease; and lower GI due to presence of total dietary fiber (TDF), resistance starch (RS) and SDS (slowly digestible starch) (98, 99). The TDF in quinoa was reported as 7–26.5%, while amaranth and buckwheat had 2.7–17.3 and 17.8 % respectively (35, 40); while Di Cairano et al. (98) revealed that the RS content of amaranth, buckwheat, and quinoa was 1.1g/100g, 0.5g/100g, 0.37g/100g, respectively. Thus, the pseudocereal like amaranth, chia seeds, buckwheat and quinoa, having ample TDF, RS and slow available glucose content, fall under low to moderate GI category. It was reported that expected glycemic index (eGI) of amaranth, chia seeds, buckwheat and quinoa ranged between low to moderate, with chia seeds having lowest eGI (28.53), followed by amaranth (47.65), buckwheat (52.35); while quinoa had moderate eGI (61.50). It was also reported that amaranth, chia, buckwheat and quinoa had RS content of 4.76, 0.08, 1.27, and 0.23%, respectively (100). In another study (98) the predicted GI for flours of amaranth, buckwheat and quinoa flours were indicated as 66.12, 50.74, 77.06 respectively. They also reported that the flours of amaranth, buckwheat and quinoa had total phenolic content (TPC) of ⁓ 1 mg gallic acid equivalents (GAE)/100 g, ⁓ 4.25 mg GAE/100g, ⁓ 1.70 mg GAE/100 g respectively; and TFC (total flavonoid content) of ⁓ 1.25 mg QE (quercetin equivalent)/100 g, ⁓ 3.25 mg QE/100 g, ⁓ 0.50 mg QE/100 g, respectively (98). Additionally, quinoa (184 μg/100 g) and amaranth (82 μg/100 g) have good folate content; while buckwheat (54 μg/100 g) and chia seeds (49 μg/100 g) had comparatively lower quantities of folate (36).
The protein content in minor millets namely foxtail (Setaria italica), kodo (Paspalum scrobiculatum), proso (Panicum miliaceum), and barnyard (Echinochloa esculenta) ranged between as 6–12.5% (33, 43–45, 47). In addition to protein, millets (major and minor) are also rich in dietary fiber, minerals and phenolic compounds, thus, they were termed as ‘Shree Anna’ during the presentation of Indian Budget, 2023 (101). Proso, kodo, foxtail and barnyard had TDF ranging from 8.5 to 37.8% with kodo millet having highest (37.8%) content and the mineral matter or ash content ranged between 1.9 and 4.4%. Minor millets have ample quantities of TPC ranging between 0.10 and 36.8 mg/100 g with kodo millet having highest quantities of phenols (36.8 mg/100 g) (33, 43–45, 47). Despite their nutrient density, millets are neglected grains, only major millets viz., sorghum (Sorghum bicolor), pearl millet (Pennisetum glaucum) and/or finger millet (Eleusine coracana) are consumed in many Indian states like Maharashtra, Karnataka, Orissa, Telangana and so on as staple grain after rice and wheat; however minor millets (kodo, proso, foxtail, barnyard, browntop) still remain greatly underutilized (13, 102–104).
Promising health benefits in preventing metabolic disorders upon consuming underutilized legumes like Bambara beans (Vigna subterranea (L.) Verdc.), Cowpea (Vigna unguiculata L. Walp.), horse gram [Macrotyloma uniflorum Lam. (Verdc.)], is attributed to their rich TDF, protein, and total phenolics, flavonoids and tannin profile (56, 59, 105). For Bambara beans and cowpea, the TDF, protein and fat ranged from 18.74–22.88 g/100 g, 14.03.27.57 g/100 g and 7.55–8.71 g/100 g, respectively. The authors described a strong positive correlation between frequent consumption of these underutilized grains and prevention of metabolic disorders like type II diabetes, obesity, high blood pressure and stroke (59). They also suggested that these legumes can assist in NCDs management and prevention due to the antioxidant activity of bioactive compounds viz., total phenolic content (TPC), total flavonoid content (TFC), and tannins apart from dietary fiber, that assist in lowering the oxidative stress associated with NCDs (59). Further, another study revealed that Bambara groundnut contained 61–69% CHO, 17–27% protein, 3.1–4.4% ash, 3.6–7.4% fat, and 3.3–6.4% fiber (106). In terms of horse gram, Prasad and Singh (105) reported that cotyledon had protein, fat, ash, TDF, CHO, soluble sugars, reducing sugars, viz., 22.6, 1.8, 2.9, 16.7, 66.9, 6.4 mg/100 g, and 538 mg/100 g, respectively. They also reported the TPC in horse gram cotyledon as 533.2 μg/g on dry weight basis, indicating the richness of bioactives present in horse gram.
Thus, reviving all these underutilized grains is essential for promoting health, wellbeing and developing low GI foods. The nutritional value of all these ingredients is summarized in Table 1.
2.1.2. Grain processing by-products
Apart from grains, the by-products like bran, husk, bran rich fractions, obtained from cereal and millets during household or industrial processing are also remarkably rich in their nutritional and phytochemical profile. These agro-wastes are generally used as animal feed; however, given their nutritional and therapeutic benefits, their use is proposed as novel ingredient in development of functional human foods (16, 25, 29). The preventive role of whole grain consumption against NCDs are attributed to the dietary fiber and phytochemicals concentrated in the grains outer cover and bran (70). Cereal and millet bran have a generous amount of TDF, that can assist in reducing the GI of the foods. Besides the TDF content, they are also a concentrated source of minerals and phytochemicals that play a role in enhancing their hypoglycemic effect. Different cereal brans have varied nutritional profiles due to the varietal difference and other environmental factors. It was noted that wheat bran had TDF of 36.5–52.4 g/100 g, while oat bran, maize bran and rice bran had 18.1–25.2 g/100 g, 86.7 g/100 g, and 38.90 g/100 g, respectively (65, 70). Another study revealed that wheat, rice and oat bran had protein levels of 9.40, 11.83, and 15.08%, respectively (65). Patel (107) highlighted that cereal brans can be used in management of obesity, diabetes as proteins, oligosaccharides, RS, phenolic acids, flavonoids, lignans and other bioactive compounds are concentrated in cereal bran (107). Cereal bran also has beta-glucans (especially in oat and barley bran), phytosterols that exhibit hypocholesterolaemic and hypoglycemic effects (16). Wolevers et al. (108) reported that supplementation of instant oatmeal value added with β-glucan extracted from oat bran reduced the blood glucose levels postprandially.
Similarly, a comparison between four minor millet brans (viz., kodo, proso, foxtail and barnyard), indicated that kodo millet bran was rich in TDF (61.52%), phenols (449.27 mg GAE/100 g), flavonoids (22.37 μg) RE (Rutin equivalent)/g, phytic acid (630 mg/100 g); while minerals like iron and calcium were highest in foxtail millet bran (65.58 mg/100 g; 94.63 mg/100 g, respectively) and comparatively proso millet bran had higher zinc (5.59 mg/100 g) and potassium (630.83 mg/100 g) levels. The protein content (g/100 g) of bran viz., kodo, proso, foxtail and barnyard were 5.68, 13.04, 10.49, 7.70 g, respectively, with proso bran having highest protein content (13). Thereby the authors concluded that millets brans can be used as functional ingredient in food and pharma industry just like cereal brans (13). Liang et al. (80) reported that foxtail millet bran had good protein (12.48%), fat (9.39%), crude fiber (51.69%), and ash (7.50%) content; and the oil extracted from foxtail bran had oleic acid (13.0%), linolenic (66.5%), α-tocopherol (15.53 ± 0.31 mg/100 g oil) and γ-tocopherol (48.79 ± 0.46 mg/100 g oil). While kodo millet bran had 4.92% protein, 79.84% carbohydrate, 2.83% fat, 5.33% ash, and 48.42% TDF (78). The phenolic compounds are concentrated in brans and it was reported that foxtail bran had phenolic content (510.53 mg/100g) higher than foxtail whole grain (132.76 mg/100 g) (109). Bisoi et al. (71) indicated that fibers extracted from proso, barnyard, kodo, sorghum, finger millet bound glucose molecules and lowered the starch digestibility demonstrating hypoglycemic effect. Another study demonstrated that a diet fed with kodo millet bran to mice for 16 weeks prevented the increase in serum cholesterol, lipids and glucose, thereby improving glucose tolerance. Kodo bran supplementation also increased the beneficial gut bacteria viz., Bifidobacteria, Lactobacillus sp., Roseburia spp. and A. muciniphila (78). Altogether, millet brans are also condensed source of nutrients with equal or superior nutritional profile like major cereal brans; even then millet brans remain underutilized in research and industrial sections. Thus, promoting their use in development of low GI foods can assist in providing new healthy and nutrient dense alternatives.
2.2. Underutilized nuts/seeds
Euryale ferox commonly called as ‘Fox nut—also known as ‘Gorgon nut’, ‘Phool Makhana’, ‘Makhana’, ‘lotus seeds’, black diamond’ or ‘black gems’, are small edible black seeds obtained from foxnut fruit grown largely in India, and some south-east Asian countries like Nepal, Bangladesh, China, Malaysia, Philippines, Thailand, and Japan. In India major cultivation is taken place in Bihar (especially north east of Bihar) while other states like Manipur, Assam, Orissa, Kashmir, West Bengal also cultivate fox nuts (110–112). Traditionally, in ayurveda, these seeds are known to have medicinal properties and are effective against Pitta (bile disorders) and Vata (rheumatic disorders), it is also known for medicinal benefits in Unani (110, 111). These underutilized seeds are further processed to make snacks like popcorn. They are powerpack nutrient dense seeds having nutritional profile with higher amounts of minerals, amino acids (lysine+ arginine/proline ratio of 4.74–7.6%; amino acid index: 89–93%), while low saturated fats, calories, and glycemic index making them good snack source for population suffering from diabetes and cardiovascular disorders (110, 111, 113). Yang et al. (114) indicated that the fox nut starch had 10.13% RS content. Studies have also been conducted to increase the dietary fiber, RS and reduce total soluble sugars in makhana by modifying makhana starch enzymatically using amylopullulanase. The authors recorded increase in TDF (from 0.80 to 1.4%) and decrease in total soluble sugars (from 62.55 to 50.13 % mg) after enzymatic treatment (62). Thus, foxnut can be used as low GI snack and its flour can also be used in developing other value-added products.
2.3. Underutilized fruits, vegetable and their processing by-products
Fruits and vegetable by-products are generated at every stage of processing at industrial as well as household level. For instance, stalks of green leafy vegetables, either pomace, skin/peel or seeds or both from different vegetables and fruits like tomato, moringa, onion, mango, orange, jamun, apple, papaya, pomegranate, banana, lotus and so on that are discarded during processing. Just like the fruits and vegetables their by-products are a rich source of micro nutrients including vitamins, minerals, TDF and starch. It is suggested that unripe banana flour contains complex carbohydrates especially RS (up to 68% w/w), phenolic compounds, phytosterols and β-carotene thereby is a novel and functional ingredient in prevention of NCDs (115, 116). Raw/unripe banana (Musa paradisiaca L.) flours have been reported to have 66.5% RS, while 5.9% of slowly digestible starch (SDS) and 2.5% rapidly digesting starch thereby suggesting their application in developing low GI foods (117). High moisture treatments and storage have shown to improve the slow digestible starch and RS in unripe banana flour (118). Thus, unripe banana flours containing RS and indigestible CHO, are an important alternative ingredient to reduce GI of foods.
Research studies have also depicted evidence of using mango (Mangifera indica) seed/kernel flours given their fiber, starch and phytochemical profile (24). It was reported that mango kernel flour was good source of macronutrients like protein (7.53 g), crude fiber (2.20 g), fat (11.45 g), carbohydrate (69.77 g) and minerals like iron (12.4 mg), calcium (170 mg), zinc (5.60 mg), sodium (2.90 mg), potassium (368 mg), magnesium (210 mg), and copper (8.60 mg) (24). Similarly, seeds from apple (Malus domestica)—a major waste discarded during processing, are good in macro- and micro-nutrients (protein: 33.79–49.55 g/100 g; fat: 12–34%; ash: 3.66–5.20%; CHO: 23.50–29.80 g/100 g; calcium: 27–210 mg/100 g; iron: 11.0–27.1 mg/100 g; zinc: 4.4 mg/100 g; magnesium: 51–510 mg/100 g; potassium: 65–650 mg/100 g; sodium: 214.1 mg/100 g; phosphorous: 666.5 mg/100 g). Further, apple seed also had good lipid, amino acid and bioactive compounds like TPC (14.56–15.92 mg/g), TFC (154.16 mg RE/g) with phloridzin as a major bioactive bestowing therapeutic properties viz. anti-obesity, cardioprotective, hypoglycemic and antimicrobial properties. Thus, the researchers proposed apple seeds use in therapeutic and functional foods (20, 82–85).
Other agro-wastes i.e., peel of Opuntia ficus indica commonly called as prickly pear in from of flour can be used in developing nutrient rich biscuits as prickly pear peel flour (PPF) has good nutritional profile, containing 14.57% ash indicating presence of more minerals, generous quantities of fiber (20.70 g/100 g), 49.6 g/100 g of total CHO, higher content of polyphenols (2,776 mg/100 g), 3.3 g/100 g protein and 2.7 g/100 g crude fat. The PPF also had total carotenoids (10.90 mg βcarotene equivalent (CAR)/100 g), betacyanins (336.8 mg/100 g) and betaxantins (250.0 mg/100 g). The presence of bioactive pigments and compounds in PPF contributed to its radical scavenging activity (274.7 mmol/g eq. Trolox) (88). El-Beltagi et al. (90) also reported that PPF can be used as nutraceutical flour considering its rich bioactive (phenolic, flavonoid content) and nutritional profile with generous amounts of fiber, minerals like calcium, iron and zinc. They also reported that the PPF contained phenolic compounds like piscidic acid (8.89%), Feruloyl-D-glucose (10.01%), kaempferol (14.07%), 3-O-Methylquercetin (13.7%), isorhamnetin (27.1%) and eucomic acid (19.6%).
2.4. Dietary fiber and resistant starch
Dietary fiber(DF; viz. cellulose, hemicellulose, pectin, gum, lignin and others) is resistant to enzymatic digestion in the intestine, but fermentable in the colonic region. It is a phytochemical compound majorly concentrated in whole grains, cereal/millet brans, fruits and vegetables (11, 16, 119). Whole grain and DF consumption are linked to reduced risk of metabolic disorders like obesity, diabetes, cardiovascular diseases and even colonic cancers; however, DF consumption is still less than the recommended allowances (7). In India the recommended dietary allowances (RDA) for DF is 40 g/2,000 Kcal (91) yet the consumption is way below the RDA. In the current situation given the increasing burden of NCDs, consumers are choosing healthy food alternatives, thus researchers and food industrialists are taking efforts to develop healthy, nutrient and DF rich snacking alternatives. For instance, cereal bran like rice, wheat, oat, barley having dietary fiber are added in bakery products like biscuit, buns, muffins, cakes bread, and so on (15, 65, 107, 120, 121). Further, resistant starch (RS)—also considered as a type of DF, resists starch digestion that takes place by α-amylase and pullanase in the intestine and ferments in the colonic area (122).
Resistant starch (RS) viz. RS1 (e.g., present in cell wall of whole grains), RS2 (e.g., unripe banana, potatoes, Hylon®VII), RS3 (e.g., retrograded starch, Novelose®330), RS4 (chemically modified starch), and RS5 (e.g., starch with complex helical structures of lipids and amylose) are recently gaining research interest considering their health benefits in preventing and managing obesity, diabetes, and hyperlipidemia; and maintaining good colon health by developing short chain fatty acids, thereby maintaining beneficial colonic microbiota. Thus, they have been used as functional ingredient in developing low GI alternatives for various food products like bakery items; and also find application in developing low fat fat-replacers, emulsifying and thickening agents (123–127). There is abundant research being conducted on developing high RS plant varieties by implementing breeding activities (128). Even different food processing techniques are applied like autoclaving, repeated cooking and cooling to increase the resistant starch content in foods. It was reported by Zheng et al. (129) that underutilized proso millets grains when treated with hydrothermal and autoclaving treatment, resulted in increase of RS content to 12–15% as compared to the untreated grains. Researchers are engaged in developing novel food processing techniques and treatments to enhance the RS content of cereal and millet grains (129). For developing low GI and no gluten foods, even commercially available RS brands (viz., Fibersym®, Actistar®) are employed (130). A study reported use of RS4 (commercially available cross-linked starch) having 91.9% TDF and 83.3% RS to prepare low GI nutrient bar. Thirty-four grams of RS4 was added in wheat germ bar making its TDF (20 g/8) higher when compared to control wheat germ bar made using puffed wheat (TDF: 5 g) (131). Thus, inclusion of DF and RS in recommended quantities can be a preventive measure to control NCDs like obesity, diabetes, cardiovascular disorders; also given the production of short chain fatty acids they can be use prebiotic agents and also help in preventing risk of some cancers like colonic cancer (16, 70, 119).
Overall, it can be concluded that DF and RS have therapeutic benefits and can be used as functional ingredients in formulating low GI therapeutic food products.
3. Application of novel ingredients and starch in low glycemic index (GI) food formulations
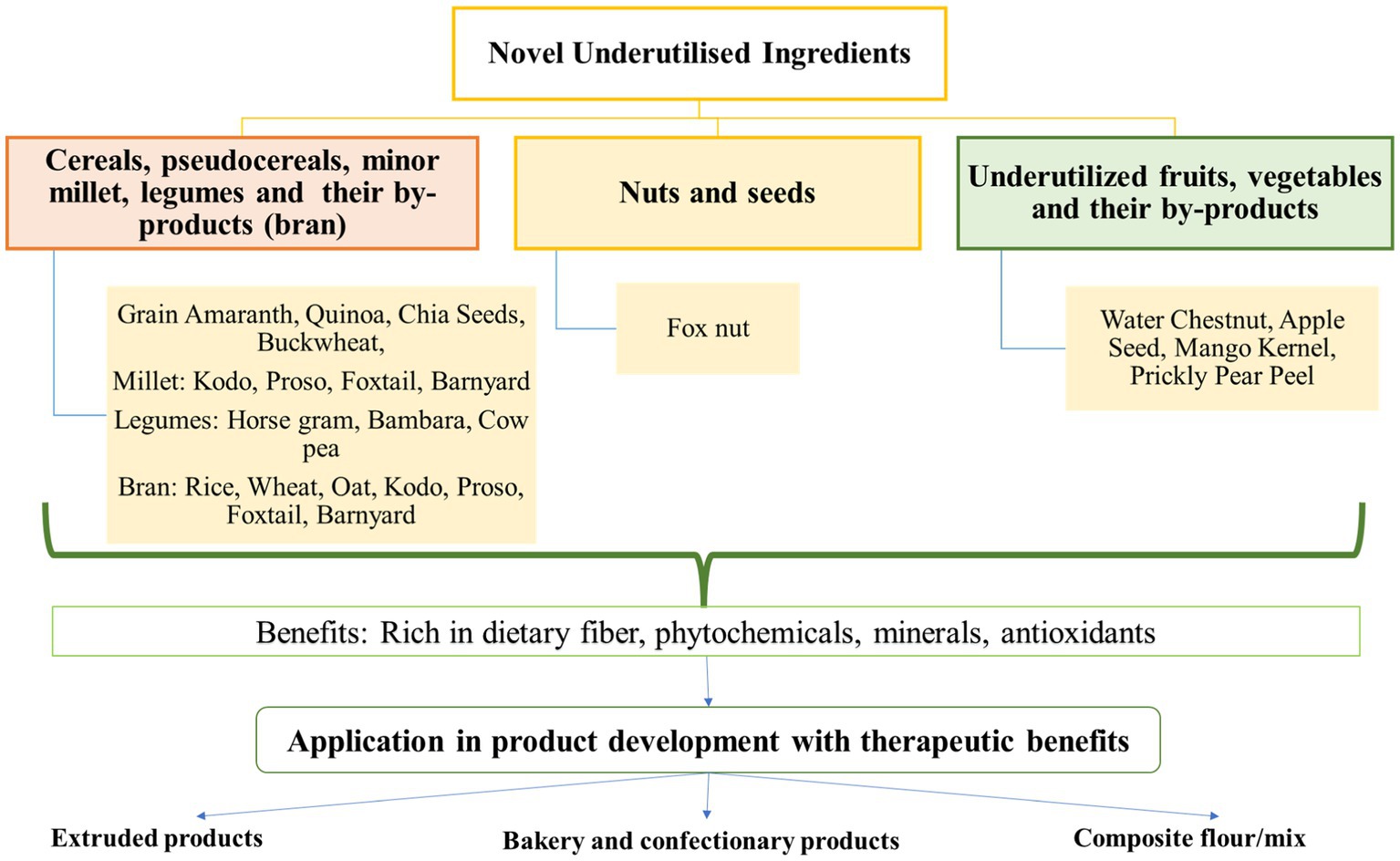
This section compiles scientific literature on developing low GI foods namely: extruded snacks, bakery and confectionary and composite mixes, with therapeutic benefits by supplementing the different above-mentioned novel ingredients. It also delineates the effects of value addition on their sensory, physical, nutritional and functional properties. Glycemic index of selected novel ingredients, their by-products and their products have been listed in Table 2. Figure 1 summarizes the application of novel ingredients in food formulations.
3.1. Extruded products
Research indicates that underutilized cereals are used in development of extruded flours and find utilization as novel flour for development of various extruded or baked products (162). Ojedokun et al. (133) used blends of malted amaranth and roasted sesame for developing extruded breakfast cereal and stated that formulation with 50% amaranth flour and 50% sesame were best acceptable. This formulation of breakfast cereal had the highest TDF (6.23 g/100 g) which reduced the GI and glycemic load (GL) of the product. Even the amino acid profile was better with addition of grain amaranth and sesame than control breakfast cereal (133). Optimized quantities of amaranth (55%), shallot (25%) and soycake (20%) were combined for preparing nutrient rich extruded snack; and their sensory acceptability was above 7. It was observed that the different variations of extruded snacks developed using amaranth, shallot and soycake had higher magnesium (42.00–46.10 mg/100 g) than wheat shallot market sample used as control (28.60 mg/100 g) and lower GI ranging between 34.37 and 38.75. Thus, the scientists indicated that these products find best suitability as alternative snacks for hyperglycemic patients (134).
Cassava-vegetable pasta using leafy vegetables namely amaranth leaves and fluted pumpkin leaf (at conc. 5, 10%) was prepared by Lawal et al. (163). It was observed that nutritional content improved with the addition of leafy vegetables than control. The protein content nearly doubled from 0.99 g/100 g (control) to 2.95 g/100 g (10% fluted pumpkin leaf-fortified cassava pasta), and dietary fiber increased from 9 g/100 g (control) to 10 g/100 g (10% fluted pumpkin leaf-fortified cassava pasta). The TPC, TFC and β-carotene ranged between 445–1,098 μg GAE/g; 58–61.6 μg RE/g and 2.11–7.82 μg/g in vegetable fortified pasta while control had only 226.6 μg GAE/g; 34.1 μg RE/g; and 0.48 μg/g, respectively. The resistant starch increased from 1.12% in control to 1.78 2.45% in vegetable fortified pasta thereby decreasing its GI from 71.72 (control) to 58.14–61.39 (vegetable fortified pasta). Thus, it was evident that underutilized leafy vegetable incorporation can reduce the GI of pasta and increase its nutritional value.
3.2. Bakery and confectionary products
Cereals, fruits and vegetable and their byproducts are used for value addition of baked products like biscuits, cookies, cakes and others (16, 25, 88, 164, 165). Underutilized roots and tubers like water chest nut serves as functional ingredient in reducing GI of crackers. Low GI crackers using combination of water chestnut (Eleocharis dulcis) and barley flour (100:0, 70:30, 50:50, 30:70, and 0:100, respectively) were developed by Hussain et al. (81). They concluded that 70% water chestnut flour and 30% barley flour (70, 30) was best accepted with overall acceptability of 4.55 on 5-point hedonic scale and had low GI of 30.16 and 13.47 glycemic load. Crackers developed with 70:30 (water chestnut: barley flour) combination had crude fiber content of 3.80%, 51.9% carbohydrate, 7.21% dietary fiber and 2.30% β-glucan; this could have reduced the GI of the crackers (81). Similarly, Agama-Acevedo et al. (160) lowered the glycemic index of cookies by addition UBF: unripe banana flour (at 15 g, 30 g, 50 g per 100 g), because UBF addition increased the resistant starch content of cookies to 3.1 g/100 g (UBF: 15 g/100 g), 5.44 g/100 g (UBF: 30 g/100 g) and 8.37 g/100 g (UBF: 50 g/100 g) while control cookies only had 2.3 g/100 g. The DF and SDS also enhanced in cookies with UBF (TDF: 6.6–10.9 g/100 g; SDS: 8.7–10.9 g/100 g) than control (DF: 4.8 g/100 g; SDS: 8.3 g/100 g) cookies. This lowered the predicted GI of cookies with UBF (UBF15: 115.2, UBF30: 106.5 and UBF50: 98.6) than control cookies (116.6).
Miranda-Ramos and Haros (37) optimized bread formulation by substituting combination flour containing underutilized pseudocereals i.e., amaranth (20%), chia (10%) and quinoa (4%) in wheat flour. Replacement of wheat flour with combination flour increased the protein (control: 14.3% optimized bread: 15.8%), and TDF (control: 5.4%; optimized bread: 15%) content. The increase TDF content resulted in decreased energy values of optimized bread when compared to control bread and it also decreased the loaf-specific volume. Addition of these pseudocereal made the optimized bread darker; however, the overall acceptability was similar for whole bread (8.1) and optimized bread (8.7). Calderón de la Barca et al. (39) also designed value added bread by substituting amaranth (22.7%) and sweet potato (8.6%) flours in wheat (68.7%) having sensory acceptability on par with control (wheat bread). This substitution of amaranth and sweet potato significantly enhanced nutritional and bioactive profile of bread compared to control bread with protein (10.15%), ash (2.44%), lipids (3.0%), dietary fiber (4.98 g/100 g), TPC (83.13mg GAE/100 g), DPPH (67.5%) higher than control bread. Especially, the beta-carotene content improved significantly from some traces in control bread to 1123.2 μg/100 g in value-added bread. The authors also confirmed reduction in GI of bread upon value addition with amaranth and sweet potato (51.8) when compared to control bread (GI: 72.0). In a study (96), multigrain snack bar (SB) was developed by addition of amaranth (A), acha (Digitaria exilis; DE) and pearl millet (PM) at different concentrations (viz. SB1 A90%:DE5%:PM 5% and SB2 A47.98%: DE26.68%: PM25.34%) and oat bar was used as control. All the snack bars were liked by the semi trained panel member, on 9-point hedonic scale with overall acceptability above 6–7 i.e., 6.75 (SB1), 7.15 (SB2) and 8.75 (control). The multigrain snack bar had higher nutritional profile for protein, ash, iron and zinc when compared to control; while calcium was higher in SB1 than control, but SB2 had lower calcium levels. Presence of phytate in large quantities can hinder the absorption of minerals, especially for calcium, iron, zinc and potassium due to binding of phytate with minerals. Usually, the phytate is not degraded easily, however, its levels can be reduced by using different processing techniques like soaking, roasting, milling, extrusion, fermentation, or use of phytase enzymes (166, 167). Despite the higher phytate and oxalates in SB1, and SB2 than control snack bar, the molar ratios for phytic acid with zinc, calcium, iron, potassium were well within the acceptable ratios for the multigrain snack bars (SB1 and SB2). The DPPH scavenging activity, ABTS activity, ferric reducing power, metal chelating ability were higher in SB1 and SB2 than control. Along with these nutritional benefits SB1 (48.63) and SB2 (57.11) also had lower GI than control (69.81) (96). Another study also indicated that combination of 15% foxtail millet, 15% arrowroot flour, and 30% of kidney beans flour for preparing cookies served best in terms of sensory acceptability and had low GI (37.6) owing to its high DF (14.48%) and RS (9.67%) content (145).
Cookies were developed using 4-6% grape pomace having higher total phenol of 3.42–4.03 mg GAE/g, protein (5.25–5.69%) and fiber (2.04-2.13%) than control cookies (TPC: 3.14 mg GAE/g; Protein: 3.10%; fiber: 1.74%). The cookies had darker color upon addition of grape pomace resulting in decreasing sensory scores of panel member; however up to 4–6% the grape pomace was acceptable (165). Similarly, de Toledo et al. (164) developed cookies using byproducts of melon (i.e., peels), pineapple (i.e., central axis) and apple (endocarp) at 5, 10, 15% concentrations. The nutritional composition became better as the concentration of the fruit byproducts increased. The cookies made with 15 % melon, pineapple and apple flours had higher fiber (soluble: 2.92, 1.05, 1.64% and insoluble: 3.54, 2.08, 2.52, respectively) than control (1.20%; 1.45% respectively). The protein content also increased from 8.54% (control) to 8.94% (15% melon); while carbohydrates (%) decreased from 19.55 (control) to 17.88 (15%pineapple); 19.06 (15% apple) and 18.81 (15% melon). Considering the sensory scores, 15% pineapple byproduct enriched cookies were highly acceptable, followed by 15% apple- and 15% melon-by products. Another nutritionally rich biscuits developed using prickly pear peel flour (20–30 g/100) scored higher overall sensory scores even when the color of biscuits darkened compared to control. The functional properties of flour like water absorption and holding capacities increased with increasing addition of prickly pear peel flour (PPF), due to their higher fiber content. However, the rheological properties of dough indicated that hardness increased with addition of PPF. Nutritional analysis of PPF biscuits (PPF: 30 g/100 g) showed improvement in ash (3.62 g/100 g), fiber (3.14 g/100 g), TPC (575 mg GAE/100 g), radical scavenging activity (236 mmol/g eq. Trolox), total carotenoid (3.54 mg CAR/100 g) and total betalains (176.7 mg/100 g), while decreased CHO (62.5 g/100 g), fat (15.3 g/100 g) and energy (1,726 Kcal) compared to control (ash:0.86 g; fiber: 0.77 g; TPC: 34 mg GAE/100 g; radical scavenging activity: 141 mmol/g eq. Trolox; total carotenoids: 0.90 mg CAR/100 g; total betalains: not detected; CHO: 63.5 g; fat: 16.4 g; energy:1,766 Kcal/100 g) respectively (88). Likewise, El-Beltagi et al. (90) also developed cakes with 5–15% PPF replacement in wheat flour and found that the fiber, mineral, phenols, and flavonoid content increased in cake resulting in higher antioxidant activity in the cake. They concluded that sensory scores were higher for 10% PPF enriched cake.
Research indicated use of commercial and laboratory-based RS in product development for reducing the GI or preparing low GI bakery products. Kahraman et al. (126) developed low GI cookies, by addition of different sources like wheat bran, lab-scale produced cross-linked starch (viz., cross-linked wheat starch: XL-W; cross-linked corn starch: XL-C), and commercial RS sources (viz., Hylon VII, Novelose330, Fibersym) at different concentration levels (0, 25, 50, and 75%). They indicated that addition of wheat bran, XL-C, Hylon VII, Novelose330, and Fibersym decreased the spread ratio and increased the thickness of the cookies when compared to control cookies (without any supplementation). In terms of TDF with increase in the supplementation levels of XL-W, XL-C, Fibersym and wheat bran, the TDF increased from 1.0 to 32.4%; 1.0 to 18.0%; 1.0 to 25.1%, and 1.0 to 27.2% respectively. This increase in TDF also in turn reduced the in vitro GI of the developed cookies (XL-C:78.8; XL-W: 77.2; Hylon VII: 83.0; Novelose 330: 86.2; Fibersym: 81.7; Wheat bran: 88.1) upon comparison with control (112.1). Rakmai et al. (1) developed low GI (49.3-51.9) pan cakes by partially replacing rice flour (Jasmine or Sangyod) with resistant maltodextrin (RM) at 10, 20 and 30% concentration and sucrose with sucralose (50 and 25%). They found that addition of RM decreased the chewiness and gumminess of pancakes. RM added pancakes were softer, however their firmness decreased as the concentration of RM increased above 20%. Thus, at level one they found that 10% RM replacement was most acceptable. At level two, the researchers partially replaced the sucrose used in making pancake with low calorie sugars –sucralose or stevioside. Their findings revealed that pan cakes (either made from Jasmine rice or Sangyod rice) with 25% sucralose replacing sucrose scored more (Jasmine + sucrose: 5.6; Sangyod + sucrose: 3.4) compared to stevioside pancakes (Jasmine + stevioside: 4.4; Sangyod + stevioside: 3.1) on 9-point hedonic scale. Further, the sensory score for overall acceptability was higher for Jasmine+10%RM+25%sucrose (6.7) than Sangyod + 10%RM + 25%sucrose (4.6) and control (Jasmine control: 5.7; Sangyod control: 3.5).
3.3. Composite flour/mix
Composite flour developed with addition of 20% wheat bran, enriched iron (29.2%), phytic acid (1.09 g/100 g), protein (12.49%), fat (2.45%), fiber (2.51%) and ash content (1.89%) indicating that nutritional profile enhanced with addition of bran (69). Naseer et al. (168) optimized instant phirni mix using 70% skimmed milk powder (SMP), 30% high amylose rice (HAR) and 0.8% CMC (carboxymethyl cellulose) and their overall sensory score was 8.39 on 9-point hedonic scale. The physio-chemical and nutritional profile of optimized phirni mix (per 100 g) had higher protein (25.12 g), ash (7.12 g), dietary fiber (3.10 g), and amylose (15.31 g) content than control (market phirni mix) sample (9.56 g; 6.08 g; 0.67 g; 5.27 g respectively). The addition of HAR, CMC also decreased the carbohydrate (60.58 g), sugars (30.00g), fat (1,30g), and energy (354.50 Kcal) than the control sample (77.91 g; 50.25 g; 2.14 g; 369.14 Kcal respectively). Upon reconstitution of optimized phirni mix, RS (4.38 g), hydrolysis index (15.31), predicted glycemic index (48.12) and glycemic load (7.50) were found decreased in the optimized mix than reconstituted control phirni mix (0.50 g; 37.32; 60.20; 9.78 respectively). Further, Di Cairano et al. (138) developed six variations of composite biscuit flour by combining buckwheat (50%), millet (50%, 30%), sorghum (50%, 30%) and chick pea (20%) or lentil (20%). The biscuits prepared from these composite flours were having RS content ranging between 0.30–0.73 g/100 g which was higher to control (0.40 g/100 g) and total starch ranging between 34.23–38.63 g/100 g which was lower to control (50.99 g/100 g). even the predicted glycemic index decreased in composite flours from 70.97 (control) to 55.07–63.18.
The above findings revealed that use of novel ingredients and unconventional food sources at different concentrations can be implemented to reduce GI of extruded, bakery products, and composite flours/mixes without affecting their sensory characteristics.
4. Therapeutic properties of products developed using novel ingredients and starch
This section provides brief insight on the therapeutic benefits of low GI products developed using the above mentioned selected novel ingredients and/or resistant starch. These low GI products can be considered as therapeutic alternative snacks for population suffering from NCDs given their hypoglycemic, hypocholesterolemic and anti-obesity properties.
4.1. Anti-obesity and hypolipidemic properties
Fu et al. (169) indicated that 6-week supplementation of banana resistant starch (BRS) at low (1.25 g/kg), medium (2.50 g/kg) and high (5.0 g/kg) dose along with high fat diet (HFD), fed to obese rats could prevent rise of glucose (4.16–3.78 mmol/L) when compared to control (fed with HFD only) obese rats (9.77 mmol/L). Even the triglyceride, total cholesterol, and low-density lipoprotein levels were lower in BRS + HFD obese rats (0.44–0.47 mmol/L; 1.45–1.61 mmol/L; 0.49–0.33 mmol/L respectively) than control obese rats (0.65; 1.79; 0.48 respectively). The authors also indicated that the BRS improved the gut microbiota by increasing the ratio of Bacteroidetes/Firmicutes microorganism. Even the serum level of leptin and insulin decrease in rats fed with BRS (leptin: 1.82–1.37 ng/ml; insulin: 11.51–9.29 U/L) than the control rats (leptin: 2.10 ng/ml; insulin: 15.15 U/L). The ghrelin hormone level which was less in obese rat (0.76 mU/L) was improved in rats fed with BRS (0.79–0.92 mU/L). Adiponectin a hormone related to anti-diabetic and cardioprotective activity was low in obese rats (23.60 ng/ml) which was increased to 24.71–34.44 ng/ml in BRS fed rats. Thus, it was concluded that BRS demonstrated anti-obesity effect by regulating the glucose and lipid mechanism, reducing the serum hormonal levels of leptin and insulin and increasing the ratio of beneficial gut microbiota (169).
The hypolipidemic properties of consuming RS4 enriched flours was delineated by Nichenametla et al. (170). They indicated that upon 12 weeks consumption of control (RS: 2 g/100 g) and RF4 enriched flours (RS: 25 g/100 g), RS4 enriched composite flour decreased total cholesterol by 7.2%, and the low-density lipoprotein by 5.5% in human subjects (male and female) with metabolic syndrome. Thus, the authors suggested that adding RS4 to daily regular diets can improve dyslipidemia and prevent the risk of metabolic syndrome and associated cardiovascular disorders (CVDs). This encourages the use of these novel ingredients and starches for harnessing therapeutic benefits.
4.2. Hypoglycemic properties
Low GI and GL foods are known to have hypoglycemic effect, thus are recommended for diabetic patients as part of their diets. Management of diet plays a major role in preventing and maintaining normal blood sugar levels of a diabetic person (171). The current trend of snacking includes consumption of highly processed, high calorie foods, mainly bakery products, fried foods that lack fiber or other essential nutrients and also fall into high GI category. However, efforts are being taken to reduce the GI of some of these snacks like bakery products, by value adding or enriching these products with nutrient dense novel ingredients that are rich source of fiber and bioactive compounds apart from normal nutrients. When white bread enriched with oat fiber (insoluble fiber 10.4 g per portion) was supplemented to overweight and obese women for 3 days, their insulin sensitivity improved. Serum insulin was noted as 29.7 in experimental group while it was 32.3 pmol/l in control (172). Miranda-Ramos and Haros (37) developed bread by addition of amaranth (20%), chia (10%) and quinoa (4%) in wheat flour; which reduced the GI of optimized bread (85%) upon comparison with control (95%). This reduction in GI can be correlated to the GI of quinoa, amaranth and chia in the formulation due to high dietary fiber and decreased starch content. Further, addition of pseudocereals also reduced the starch hydrolysis at 90 mins in optimized bread (68.1%) as compared to control bread (84.6%) thereby providing hypoglycemic effect.
Research indicates development of low GI foods by adding fruits and vegetable by-products. The fruits by products like mango peel were implemented for reducing GI of foods as they are rich in dietary fiber and phenols. Ajila et al. (173) revealed that value addition of mango peel (5–20%) in biscuits increased the TDF content to 20.7% while control only had 6.5%. Similarly, the polyphenol content was higher in 20% mango peel incorporated biscuits (4,500 μg GAE/g) than control (540 μg GAE/g) thereby, making it richer in antioxidant and bioactive compounds. Silva et al. (174) indicated that unripe banana pasta (75%) when fed to diabetic rats was able to prevent the hyperglycemia and also decreased cholesterol and triglyceride levels compared to the control diabetic group.
Addition of dietary fiber or RS has also proven to lower the GI of foods and provide hypoglycemic effect. A study has depicted that supplementation of nutri bar containing 34 g RS4, lowered the glucose and insulin response as compared to bar made with puffed wheat till 120 minutes after consumption (131). Rakmai et al. (1) indicated that pan cakes made by substituting rice flour (Jasmine or Sangyod) with 10% resistant maltodextrin (RM) and sucrose with 25% sucralose decreased the GI of pancakes to 51.9 (Jasmine + 10%RM + 25%sucrolase) and 49.3 (Sangyod+ 10%RM+ 25%sucrolase) when compared to control (Sangyod control: 58.2; Jasmine control: 60.8). The authors concluded that addition of dietary fiber and low-calorie sweetener reduced the calorific value (305.59 Kcal), carbohydrate (39.16g) and increased the dietary fiber (0.93 g), protein (6.39 g) content, compared to control pancakes (Protein: 5.75 g; TDF: 0 g; CHO: 46.59 g) thus making it an alternative for high GI pancakes.
Similarly, a promising low GI alternative for available milk desserts (phirni) for diabetic population was developed by Naseer et al. (168) combining skimmed milk powder (70%), high amylose rice (30%) and 0.8% carboxymethyl cellulose. Further, a study conducted by Kahraman et al. (126) also confirmed that addition of crosslinked (XL) corn (C) or wheat (W) starch and wheat bran reduced the GI of cookies (XL-C;78.8; XL-W: 77.2; wheat bran:88.1) than control (112.1) and thus, depicting the hypoglycemic properties of bran and crosslinked starch. Altogether, it is evident from these research studies that novel ingredients have complex carbohydrates and starch components thus, can be harnessed in developing low GI foods serving as alternatives for regular commercially available snacks consumed by the population suffering with NCDs.
5. Conclusion and future scope
With the increasing prevalence of NCDs across the globe, coupled with a sedentary life-style, it has become a pressing priority to develop low GI alternatives for regular snacking foods. This review brought to the foreground a few selected underutilized novel ingredients and unconventional starches that can be harnessed for lowering the GI of different bakery, extruded products or even composite mixes. Exploring different unconventional food sources and starch relates to a sustainable way of using underexploited grains, fruits, vegetables and agro-wastes/by-products. The review highlighted nutritional profile of such pseudocereals, millets and their by-products, fruits and vegetable by-products, delineating that they are rich sources of dietary fiber, complex slow digesting starches, RS and phytochemicals; thereby can aid in reducing/lowering the GI of foods. These novel foods can also provide therapeutic benefits like antidiabetic, anti-obesity, and hypoglycemic effects. Further, there is a need to commercialize and create an extensive market for popularizing such therapeutic low GI foods at commercial industrial level. Ample evidence is available on product development however, additional research on clinical trials is required for analyzing the complete nutritional and nutraceutical effect of these major ingredients upon consumption and assessing their glucose and/or lipid lowering mechanisms for gaining definitive results.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rakmai, J, Haruthaithanasan, V, Chompreeda, P, Chatakanonda, P, and Yonkoksung, U. Development of gluten-free and low glycemic index rice pancake: Impact of dietary fiber and low-calorie sweeteners on texture profile, sensory properties, and glycemic index. Food Hydrocoll Health. (2021) 1:100034. doi: 10.1016/j.fhfh.2021.100034
2. World Obesity Atlas (2022). One Billion People Globally Estimated to be Living with Obesity by 2030. Available at: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022. Accessed on March 12, 2023.
3. WHO (2022). World Obesity Day 2022 – Accelerating action to stop obesity. Available at: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity. Accessed on March 12, 2023.
4. WHO (2022). Diabetes. Available at: https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed on March 12, 2023.
5. IDF Diabetes Atlas (2021). Diabetes around the world. Available at: https://diabetesatlas.org/regional-factsheets/#.ZA4QvPyA9vp.gmaila. Accessed on March 12, 2023.
6. Barber, TM, Kabisch, S, Pfeiffer, AFH, and Weickert, MO. The health benefits of dietary fibre. Nutrients. (2020) 12:3209. doi: 10.3390/nu12103209
7. Lockyer, S, Spiro, A, and Stanner, S. Dietary fibre and the prevention of chronic disease–should health professionals be doing more to raise awareness? Nutr. Bull. (2016) 41:214–31. doi: 10.1111/nbu.12212
8. Das, A, Panneerselvam, A, Yannam, SK, and Baskaran, V. Shelf-life, nutritional and sensory quality of cereal and herb based low glycaemic index foods for managing diabetes. J. Food Process. Preserv. (2022) 46:e16162. doi: 10.1111/jfpp.16162
9. Toh, DWK, Koh, ES, and Kim, JE. Lowering breakfast glycemic index and glycemic load attenuates postprandial glycemic response: a systematically searched meta-analysis of randomized controlled trials. Nutrition. (2020) 71:110634. doi: 10.1016/j.nut.2019.110634
10. Vlachos, D, Malisova, S, Lindberg, FA, and Karaniki, G. Glycemic index (GI) or glycemic load (GL) and dietary interventions for optimizing postprandial hyperglycemia in patients with T2 diabetes: A review. Nutrients. (2020) 12:1561. doi: 10.3390/nu12061561
11. Lattimer, JM, and Haub, MD. Effects of dietary fiber and its components on metabolic health. Nutrients. (2010) 2:1266–89. doi: 10.3390/nu2121266
12. Plasek, B, Lakner, Z, and Temesi, Á. Factors that influence the perceived healthiness of food-review. Nutrients. (2020) 12:1881. doi: 10.3390/nu12061881
13. Barbhai, MD, and Hymavathi, TV. Nutrient, phytonutrient and antioxidant potential of selected underutilized nutri-cereal brans. J Food Meas Charact. (2022) 16:1952–66. doi: 10.1007/s11694-022-01301-9
14. Barbhai, MD, Hymavathi, TV, Kuna, A, Mulinti, S, and Voliveru, SR. Quality assessment of nutri-cereal bran rich fraction enriched buns and muffins. J. Food Sci. Technol. (2022) 59:2231–42. doi: 10.1007/s13197-021-05236-9
15. Bhosale, S, and Vijayalakshmi, D. Processing and nutritional composition of rice bran. Curr Res Nutr Food Sci J. (2015) 3:74–80. doi: 10.12944/CRNFSJ.3.1.08
16. Luithui, Y, Baghya Nisha, R, and Meera, MS. Cereal by-products as an important functional ingredient: effect of processing. J. Food Sci. Technol. (2019) 56:1–11. doi: 10.1007/s13197-018-3461-y
17. Makroo, HA, Naqash, S, Saxena, J, Sharma, S, Majid, D, and Dar, BN. Recovery and characteristics of starches from unconventional sources and their potential applications: a review. Appl Food Res. (2021) 1:100001. doi: 10.1016/j.afres.2021.100001
18. Onipe, OO, and Ramashia, SE. Finger millet seed coat—a functional nutrient-rich cereal by-product. Molecules. (2022) 27:7837. doi: 10.3390/molecules27227837
19. Steinert, RE, Raederstorff, D, and Wolevers, TMS. Effect of consuming oat bran mixed in water before a meal on glycemic responses in healthy humans—a pilot study. Nutrients. (2016) 8:524. doi: 10.3390/nu8090524
20. Kumar, M, Barbhai, MD, Esatbeyoglu, T, Zhang, B, Sheri, V, Dhumal, S, et al. Apple (Malus domestica Borkh.) seed: A review on health promoting bioactivities and its application as functional food ingredient. Food Biosci. (2022b) 50:102155. doi: 10.1016/j.fbio.2022.102155
21. Kumar, M, Barbhai, MD, Hasan, M, Dhumal, S, Singh, S, Pandiselvam, R, et al. Onion (Allium cepa L.) peel: A review on the extraction of bioactive compounds, its antioxidant potential, and its application as a functional food ingredient. J. Food Sci. (2022a) 87:4289–311. doi: 10.1111/1750-3841.16297
22. Kumar, M, Selvasekaran, P, Kapoor, S, Barbhai, MD, Lorenzo, JM, Saurabh, V, et al. Moringa oleifera Lam. seed proteins: Extraction, preparation of protein hydrolysates, bioactivities, functional food properties, and industrial application. Food Hydrocoll. (2022c) 131:107791. doi: 10.1016/j.foodhyd.2022.107791
23. Sawant, L, Singh, VK, Dethe, S, Bhaskar, A, Balachandran, J, Mundkinajeddu, D, et al. Aldose reductase and protein tyrosine phosphatase 1B inhibitory active compounds from Syzygium cumini seeds. Pharm. Biol. (2015) 53:1176–82. doi: 10.3109/13880209.2014.967784
24. Yatnatti, S, Vijayalakshmi, D, and Chandru, R. Processing and nutritive value of mango seed kernel flour. Curr Res Nutr Food Sci J. (2014) 2:170–5. doi: 10.12944/CRNFSJ.2.3.10
25. Zuñiga-Martínez, BS, Domínguez-Avila, JA, Robles-Sánchez, RM, Ayala-Zavala, JF, Villegas-Ochoa, MA, and González-Aguilar, GA. Agro-industrial fruit byproducts as health-promoting ingredients used to supplement baked food products. Foods. (2022) 11:3181. doi: 10.3390/foods11203181
26. Ganesh, KS, Sridhar, A, and Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities—A review. Chemosphere. (2022) 287:132221. doi: 10.1016/j.chemosphere.2021.132221
27. Ranjan, A, Kumar, S, Sahu, NP, Jain, KK, and Deo, AD. Strategies for maximizing utilization of de-oiled rice bran (DORB) in the fish feed. Aquac. Int. (2022) 30:99–114. doi: 10.1007/s10499-021-00791-6
28. Yunus, FUN, Nadeem, M, and Rashid, F. Single-cell protein production through microbial conversion of lignocellulosic residue (wheat bran) for animal feed. J. Inst. Brew. (2015) 121:553–7. doi: 10.1002/jib.251
29. Fărcaș, AC, Socaci, SA, Nemeș, SA, Pop, OL, Coldea, TE, Fogarasi, M, et al. An update regarding the bioactive compound of cereal by-products: Health benefits and potential applications. Nutrients. (2022) 14:3470. doi: 10.3390/nu14173470
30. Pathania, S, and Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact Carbohydr Diet Fibre. (2022) 27:100295. doi: 10.1016/j.bcdf.2021.100295
31. Ebert, A . Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income and more sustainable production systems. Sustainability. (2014) 6:319–35. doi: 10.3390/su6010319
32. Lau, KQ, Sabran, MR, and Shafie, SR. Utilization of vegetable and fruit by-products as functional ingredient and food. Front Nutr. (2021) 8:661693. doi: 10.3389/fnut.2021.661693
33. Chandra, D, Chandra, S, and Sharma, AK. Review of Finger millet (Eleusine coracana (L.) Gaertn): a power house of health benefiting nutrients. Food Sci. Human Wellness. (2016) 5:149–55. doi: 10.1016/j.fshw.2016.05.004
34. Longvah, T, Anantan, I, Bhaskarachary, K, and Venkaiah, K. Indian food composition tables. Hyderabad: National Institute of Nutrition, Indian Council of Medical Research (2017).
35. Joshi, DC, Sood, S, Hosahatti, R, Kant, L, Pattanayak, A, Kumar, A, et al. From zero to hero: the past, present and future of grain amaranth breeding. TAG Theor Appl Genet Theoretische und angewandte Genetik. (2018) 131:1807–23. doi: 10.1007/s00122-018-3138-y
36. Bekkering, CS, and Tian, L. Thinking outside of the cereal box: breeding underutilized (pseudo) cereals for improved human nutrition. Front. Genet. (2019) 10:1289. doi: 10.3389/fgene.2019.01289
37. Miranda-Ramos, KC, and Haros, CM. Combined effect of chia, quinoa and amaranth incorporation on the physico-chemical, nutritional and functional quality of fresh bread. Foods. (2020) 9:1859. doi: 10.3390/foods9121859
38. Rodríguez, JP, Rahman, H, Thushar, S, and Singh, RK. Healthy and resilient cereals and pseudo-cereals for marginal agriculture: Molecular advances for improving nutrient bioavailability. Front. Genet. (2020) 11:49. doi: 10.3389/fgene.2020.00049
39. Calderón de la Barca, AM, Mercado-Gómez, LE, Heredia-Sandoval, NG, Luna-Alcocer, V, Porras Loaiza, PMA, González-Ríos, H, et al. Highly nutritional bread with partial replacement of wheat by amaranth and orange sweet potato. Foods. (2022) 11:1473. doi: 10.3390/foods11101473
40. Joshi, DC, Chaudhari, GV, Sood, S, Kant, L, Pattanayak, A, Zhang, K, et al. Revisiting the versatile buckwheat: reinvigorating genetic gains through integrated breeding and genomics approach. Planta. (2019) 250:783–801. doi: 10.1007/s00425-018-03080-4
41. Pirzadah, TB, and Malik, B. Pseudocereals as super foods of 21st century: Recent technological interventions. J Agric Food Res. (2020) 2:100052. doi: 10.1016/j.jafr.2020.100052
42. Hegde, PS, Rajasekaran, NS, and Chandra, TS. Effects of the antioxidant properties of millet species on oxidative stress and glycemic status in alloxan-induced rats. Nutr. Res. (2005) 25:1109–20. doi: 10.1016/j.nutres.2005.09.020
43. Devi, PB, Vijayabharathi, R, Sathyabama, S, Malleshi, NG, and Priyadarisini, VB. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J. Food Sci. Technol. (2014) 51:1021–40. doi: 10.1007/s13197-011-0584-9
44. Saha, D, Gowda, MC, Arya, L, Verma, M, and Bansal, KC. Genetic and genomic resources of small millets. Crit. Rev. Plant Sci. (2016) 35:56–79. doi: 10.1080/07352689.2016.1147907
45. Das, S, Khound, R, Santra, M, and Santra, DK. Beyond bird feed: Proso millet for human health and environment. Agriculture. (2019) 9:64. doi: 10.3390/agriculture9030064
46. Srilekha, K, Kamalaja, T, Maheswari, KU, and Rani, RN. Evaluation of physical, functional and nutritional quality parameters of kodo millet flour. J Pharmacogn Phytochem. (2019) 8:192–5.
47. Habiyaremye, C, Matanguihan, JB, D’Alpoim Guedes, J, Ganjyal, GM, Whiteman, MR, Kidwell, KK, et al. Proso millet (Panicum miliaceum L.) and its potential for cultivation in the Pacific Northwest, US: a review. Front. Plant Sci. (2017) 7:1–17. doi: 10.3389/fpls.2016.01961
48. Shankaramurthy, KN, and Somannavar, MS. Moisture, carbohydrate, protein, fat, calcium, and zinc content in finger, foxtail, pearl, and proso millets. Indian J Health Sci Biomed Res (KLEU). (2019) 12:228. doi: 10.4103/kleuhsj.kleuhsj_32_19
49. Torbica, A, Belović, M, Popović, L, Čakarević, J, Jovičić, M, and Pavličević, J. Comparative study of nutritional and technological quality aspects of minor cereals. J food sci technol. (2021) 58:311–22. doi: 10.1007/s13197-020-04544-w
50. Singh, A, and Hathan, BS. Comparative Characterization of Foxtail Millet, Physico-Chemical Approach for its Suitability to Celiacs. J Food Process Technol. (2014) 5:1–4. doi: 10.4172/2157-7110.1000382
51. Sharma, S, Saxena, DC, and Riar, CS. Analysing the effect of germination on phenolics, dietary fibres, minerals and γ-amino butyric acid contents of barnyard millet (Echinochloa frumentaceae). Food Biosci. (2016) 13:60–8. doi: 10.1016/j.fbio.2015.12.007
52. Kaur, H, and Sharma, S. An overview of Barnyard millet (Echinochloa frumentacea). J Pharmacogn Phytochem. (2020) 9:819–22.
53. Gowda, NAN, Siliveru, K, Prasad, PVV, Bhatt, Y, Netravati, BP, and Gurikar, C. Modern processing of Indian millets: a perspective on changes in nutritional properties. Foods. (2022) 11:499. doi: 10.3390/foods11040499
54. Bhartiya, A, Aditya, JP, and Kant, L. Nutritional and remedial potential of an underutilized food legume horsegram (Macrotyloma uniflorum): a review. JAPS J Anim Plant Sci. (2015) 25:908–920.
55. Palai, JB, Jena, J, and Maitra, S. Prospects of underutilized food legumes in sustaining pulse needs in India–a review. Crop. Res. (2019) 54:82–8. doi: 10.31830/2454-1761.2019.014
56. Gulzar, M, and Minnaar, A. Underutilized protein resources from African legumes In: SR Nadathur, JPD Wanasundara, and L Scanlin, editors. Sustainable Protein Sources. Cambridge, MA: Academic Press (2017). 197–208.
57. Oyeyinka, AT, Pillay, K, and Siwela, M. Full title-in vitro digestibility, amino acid profile and antioxidant activity of cooked Bambara groundnut grain. Food Biosci. (2019) 31:100428. doi: 10.1016/j.fbio.2019.100428
58. Tan, XL, Azam-Ali, S, Goh, EV, Mustafa, M, Chai, HH, Ho, WK, et al. Bambara groundnut: an underutilized leguminous crop for global food security and nutrition. Front Nutr. (2020) 7:601496. doi: 10.3389/fnut.2020.601496
59. Hamadou, M, Alain, MMM, Obadias, FV, Hashmi, MZ, Başaran, B, Paul, BJ, et al. Consumption of underutilised grain legumes and the prevention of type II diabetes and cardiometabolic diseases: Evidence from field investigation and physicochemical analyses. Environ Challenges. (2022) 9:100621. doi: 10.1016/j.envc.2022.100621
60. Gondwe, TM, Alamu, EO, Mdziniso, P, and Maziya-Dixon, B. Cowpea (Vigna unguiculata (L.) Walp) for food security: an evaluation of end-user traits of improved varieties in Swaziland. Sci. Rep. (2019) 9:15991. doi: 10.1038/s41598-019-52360-w
61. National Institute of Food Technology Entrepreneurship and Management (2013). Available at: http://niftem.ac.in/site/pmfme/lmnew/makhanawriteup.pdf. Accessed on February 09, 2023.
62. Biswas, P, Das, M, Boral, S, Mukherjee, G, Chaudhury, K, and Banerjee, R. Enzyme mediated resistant starch production from Indian Fox Nut (Euryale ferox) and studies on digestibility and functional properties. Carbohydr. Polym. (2020) 237:116158. doi: 10.1016/j.carbpol.2020.116158
63. Liaquat, M, Pasha, I, Ahsin, M, and Salik, A. Roasted fox nuts (Euryale Ferox L.) contain higher concentration of phenolics, flavonoids, minerals and antioxidants, and exhibit lower glycemic index (GI) in human subjects. Food Production Process Nutr. (2022) 4:1. doi: 10.1186/s43014-021-00081-x
64. Bagheri, R, and Seyedein, SM. The effect of adding rice bran fibre on wheat dough performance and bread quality. World Appl. Sci. J. (2011) 14:121–5.
65. Kaur, BJ, Gupta, A, Bobade, H, Singh, B, and Sharma, S. Rheological profile and quality assessment of cereal Brans enriched buns, pizza base and flatbread. Int J Chem Stud. (2017) 5:1144–52.
66. Kalpanadevi, C, Singh, V, and Subramanian, R. Influence of milling on the nutritional composition of bran from different rice varieties. J. Food Sci. Technol. (2018) 55:2259–69. doi: 10.1007/s13197-018-3143-9
67. Sapwarobol, S, Saphyakhajorn, W, and Astina, J. Biological functions and activities of rice bran as a functional ingredient: a review. Nutr Metab Insights. (2021) 14:11786388211058559. doi: 10.1177/11786388211058559
68. Shenoy, AH, and Prakash, J. Wheat Bran (Triticum aestivum): composition, functionality and incorporation in unleavened bread. J. Food Qual. (2002) 25:197–211. doi: 10.1111/j.1745-4557.2002.tb01019.x
69. Butt, MS, Qamar, MI, Anjum, FM, Aziz, A, and Randhawa, MA. Development of minerals-enriched brown flour by utilizing wheat milling by-products. Nutr Food Sci. (2004) 34:161–5. doi: 10.1108/00346650410544855
70. Vitaglione, P, Napolitano, A, and Fogliano, V. Cereal dietary fibre: a natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. (2008) 19:451–63. doi: 10.1016/j.tifs.2008.02.005
71. Bisoi, PC, Sahoo, G, Mishra, SK, Das, C, and Das, KL. Hypoglycemic effects of insoluble fiber rich fraction of different cereals and millets. J. Food Process. Technol. (2012) 3:1–11. doi: 10.4172/2157-7110.1000191
72. Onipe, OO, Jideani, AI, and Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. (2015) 50:2509–18. doi: 10.1111/ijfs.12935
73. Dhillon, PK, and Tanwar, B. Muffins incorporated with multiple blend functional ingredients: development, sensory evaluation, proximate composition and total antioxidant activity. J Agric Eng Food Technol. (2018) 5:122–6.
74. Duţă, DE, Culeţu, A, and Mohan, G. Reutilization of cereal processing by-products in bread making In: CM Galanakis , editor. Sustainable Recovery and Reutilization of Cereal Processing By-products. Sawston: Woodhead Publishing (2018). 279–317.
75. Sahin, AW, Coffey, A, and Zannini, E. Functionalisation of wheat and oat bran using single-strain fermentation and its impact on techno-functional and nutritional properties of biscuits. Eur. Food Res. Technol. (2021) 247:1825–37. doi: 10.1007/s00217-021-03755-5
76. Kristek, A, Schär, MY, Soycan, G, Alsharif, S, Kuhnle, GGC, Walton, G, et al. The gut microbiota and cardiovascular health benefits: A focus on wholegrain oats. Nutr. Bull. (2018) 43:358–73. doi: 10.1111/nbu.12354
77. Mustafa, G, Arshad, MU, Saeed, F, Afzaal, M, Niaz, B, Hussain, M, et al. Comparative study of raw and fermented oat bran: nutritional composition with special reference to their structural and antioxidant profile. Fermentation. (2022) 8:509. doi: 10.3390/fermentation8100509
78. Sarma, SM, Khare, P, Jagtap, S, Singh, DP, Baboota, RK, Podili, K, et al. Kodo millet whole grain and bran supplementation prevents high-fat diet induced derangements in lipid profile, inflammatory status and gut bacteria in mice. Food Funct. (2017) 8:1174–83. doi: 10.1039/C6FO01467D
79. Mustač, NC, Novotni, D, Habuš, M, Drakula, S, Nanjara, L, Voučko, B, et al. Storage stability, micronisation, and application of nutrient-dense fraction of proso millet bran in gluten-free bread. J Cereal Sci. (2020) 7:102864. doi: 10.1016/j.jcs.2019.102864
80. Liang, S, Yang, G, and Ma, Y. Chemical characteristics and fatty acid profile of foxtail millet bran oil. J. Am. Oil Chem. Soc. (2010) 87:63–7. doi: 10.1007/s11746-009-1475-3
81. Hussain, SZ, Beigh, M, Qadri, T, Ahmad, I, and Naseer, B. Development of low glycemic index crackers from water chestnut and barley flour. Br. Food J. (2020) 122:1156–69. doi: 10.1108/BFJ-10-2019-0788
82. Yu, X, Van De Voort, FR, Li, Z, and Yue, T. Proximate composition of the apple seed and characterization of its oil. Int. J. Food Eng. (2007) 3. doi: 10.2202/1556-3758.1283
83. Fromm, M, Bayha, S, Carle, R, and Kammerer, DR. Characterization and quantitation of low and high molecular weight phenolic compounds in apple seeds. J. Agric. Food Chem. (2012) 60:1232–42. doi: 10.1021/jf204623d
84. El-Safy, FS, Salem, RH, and Abd El-Ghany, ME. Chemical and nutritional evaluation of different seed flours as novel sources of protein. World J. Dairy Food Sci. (2012) 7:59–65. doi: 10.5829/idosi.wjdfs.2012.7.1.61215
85. Dadwal, V, Agrawal, H, Sonkhla, K, Joshi, R, and Gupta, M. Characterization of phenolics, amino acids, fatty acids and antioxidant activity in pulp and seeds of high altitude Himalayan crab apple fruits (Malus baccata). J. Food Sci. Technol. (2018) 55:2160–9. doi: 10.1007/s13197-018-3133-y
86. Torres-León, C, Rojas, R, Contreras-Esquivel, JC, Serna-Cock, L, Belmares-Cerda, RE, and Aguilar, CN. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. (2016) 55:109–17. doi: 10.1016/j.tifs.2016.06.009
87. Masud, F, Akhmad, R, and Muhammad, S. Mango seed kernel flour (Mangifera indica): nutrient composition and potential as food. Malays. J. Nutr. (2020) 26:101–6. doi: 10.31246/mjn-2019-0082
88. Bouazizi, S, Montevecchi, G, Antonelli, A, and Hamdi, M. Effects of prickly pear (Opuntia ficus-indica L.) peel flour as an innovative ingredient in biscuits formulation. Lwt. (2020) 124:109155. doi: 10.1016/j.lwt.2020.109155
89. Parafati, L, Restuccia, C, Palmeri, R, Fallico, B, and Arena, E. Characterization of prickly pear peel flour as a bioactive and functional ingredient in bread preparation. Foods. (2020) 9:1189. doi: 10.3390/foods9091189
90. El-Beltagi, HS, Ahmed, AR, Mohamed, HI, Al-Otaibi, HH, Ramadan, KMA, and Elkatry, HO. Utilization of prickly pear peels flour as a natural source of minerals, dietary fiber and antioxidants: effect on cakes production. Agronomy. (2023) 13:439. doi: 10.3390/agronomy13020439
91. ICMR-NIN . ICMR-NIN Expert Group on Nutrient Requirement for Indians, Recommended Dietary Allowances (RDA) and Estimated Average Requirements (EAR). Hyderabad: National Institute of Nutrition, Indian Council of Medical Research (2020).
92. Mohanty, S., and Satyasai, K. J. (2015). Feeling the pulse–Indian pulses sector. NABARD Rural Pulse. Department of Economic Analysis and Research (DEAR), NABARD, Mumbai.
93. Nayak, SP, Lone, RA, Fakhrah, S, Chauhan, A, Sarvendra, K, and Mohanty, CS. Mainstreaming underutilized legumes for providing nutritional security In: R Bhat , editor. Future Foods. Cambridge, MA: Academic Press (2022). 151–63.
94. Singh, RK, Sreenivasulu, N, and Prasad, M. Potential of underutilized crops to introduce the nutritional diversity and achieve zero hunger. Funct Integr Genom. (2022) 22:1459–65. doi: 10.1007/s10142-022-00898-w
95. Balakrishnan, G, and Schneider, RG. The Role of amaranth, quinoa, and millets for the development of healthy, sustainable food products—A concise review. Foods. (2022) 11:2442. doi: 10.3390/foods11162442
96. Olagunju, AI, Arigbede, TI, Makanjuola, SA, and Oyebode, ET. Nutritional compositions, bioactive properties, and in-vivo glycemic indices of amaranth-based optimized multigrain snack bar products. Measurement: Food. (2022) 7:100039. doi: 10.1016/j.meafoo.2022.100039
97. Malik, AM, and Singh, A. Pseudocereals proteins-A comprehensive review on its isolation, composition and quality evaluation techniques. Food Chem Adv. (2022) 1:100001. doi: 10.1016/j.focha.2021.100001
98. Di Cairano, M, Condelli, N, Caruso, MC, Marti, A, Cela, N, and Galgano, F. Functional properties and predicted glycemic index of gluten free cereal, pseudocereal and legume flours. Lwt. (2020) 133:109860. doi: 10.1016/j.lwt.2020.109860
99. Punia Bangar, S, Sharma, N, Singh, A, Phimolsiripol, Y, and Brennan, CS. Glycaemic response of pseudocereal-based gluten-free food products: a review. Int. J. Food Sci. Technol. (2022) 57:4936–44. doi: 10.1111/ijfs.15890
100. Arslan-Tontul, S, Candal Uslu, C, Mutlu, C, and Erbaş, M. Expected glycemic impact and probiotic stimulating effects of whole grain flours of buckwheat, quinoa, amaranth and chia. J. Food Sci. Technol. (2022) 59:1460–7. doi: 10.1007/s13197-021-05156-8
101. The Economic Times . (2023). Say hello to millets, the 'mother of all grains' that FM Nirmala Sitharaman wants Indians to eat. Available at: https://economictimes.indiatimes.com/magazines/panache/say-hello-to-millets-the-mother-of-all-grains-that-fm-nirmala-sitharaman-wants-indians-to-eat/articleshow/97514701.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst. Accessed on 01-02-2023.
102. Barbhai, MD, Hymavathi, TV, Kuna, A, Sreedhar, M, and Rani, VS. Sensorial and functional properties of nutri-cereal bran enriched muffins and buns. Int Res J Pure Appl Chem. (2020) 21:36–47.
103. Dekka, S, Paul, A, Vidyalakshmi, R, and Mahendran, R. Potential processing technologies for utilization of millets: An updated comprehensive review. J. Food Process Eng. (2023):e14279. doi: 10.1111/jfpe.14279
104. Devisetti, R, Yadahally, SN, and Bhattacharya, S. Nutrients and antinutrients in foxtail and proso millet milled fractions: Evaluation of their flour functionality. LWT-Food Sci Technol. (2014) 59:889–95. doi: 10.1016/j.lwt.2014.07.003
105. Prasad, SK, and Singh, MK. Horse gram–an underutilized nutraceutical pulse crop: a review. J. Food Sci. Technol. (2015) 52:2489–99. doi: 10.1007/s13197-014-1312-z
106. Muhammad, I, Rafii, MY, Ramlee, SI, Nazli, MH, Harun, AR, Oladosu, Y, et al. Exploration of bambara groundnut (Vigna subterranea (L.) Verdc.), an underutilized crop, to aid global food security: Varietal improvement, genetic diversity and processing. Agronomy. (2020) 10:766. doi: 10.3390/agronomy10060766
107. Patel, S . Cereal bran fortified-functional foods for obesity and diabetes management: Triumphs, hurdles and possibilities. J. Funct. Foods. (2015) 14:255–69. doi: 10.1016/j.jff.2015.02.010
108. Wolevers, TMS, Jenkins, AL, Prudence, K, Johnson, J, Duss, R, Chu, Y, et al. Effect of adding oat bran to instant oatmeal on glycaemic response in humans—a study to establish the minimum effective dose of oat β-glucan. Food Funct. (2018) 9:1692–700. doi: 10.1039/C7FO01768E
109. Sridevi, (2007). Evaluation of antioxidant properties of whole grains and processed regional cereals of Karnataka. Master thesis. University of Agricultural Sciences, Dharwad.
111. Nehal, N, Mann, S, and Gupta, RK. Two promising under-utilized grains: a review. Indian J. Tradit. Knowl. (2015) 14:416–22.
112. Pawar, S, and Singh, SP. Functional bread fortified with foxnut flour and amaranth flour. Pharm Innov J. (2022) 11:528–35.
113. Kumar, N, Rani, S, Kuamr, G, Kumari, S, Singh, IS, Gautam, S, et al. Physiological and biochemical responses of makhana (Euryale ferox) to gamma irradiation. J. Biol. Phys. (2019) 45:1–12. doi: 10.1007/s10867-018-9511-x
114. Yang, Y, Chen, Q, Yu, A, Tong, S, and Gu, Z. Study on structural characterization, physicochemical properties and digestive properties of euryale ferox resistant starch. Food Chem. (2021) 359:129924. doi: 10.1016/j.foodchem.2021.129924
115. Dibakoane, SR, Du Plessis, B, Da Silva, LS, Anyasi, TA, Emmambux, MN, Mlambo, V, et al. Nutraceutical properties of unripe banana flour resistant starch: a review. Starch-Stärke. (2022) 2200041:2200041. doi: 10.1002/star.202200041
116. Ho, LH, and Wong, SY. Resistant starch from exotic fruit and its functional properties: a review of recent research In: M Emeje , editor. Chem Properties of Starch. Rijeka:IntechOpen (2020).
117. de la Rosa-Millan, J, Agama-Acevedo, E, Osorio-Díaz, P, and Bello-Pérez, LA. Effect of cooking, annealing and storage on starch digestibility and physicochemical characteristics of unripe banana flour. Revista mexicana de ingeniería química. (2014) 13:371–8.
118. Rodríguez-Damian, AR, De La Rosa-Millán, J, Agama-Acevedo, E, Osorio-Díaz, P, and Bello-Pérez, LA. Effect of different thermal treatments and storage on starch digestibility and physicochemical characteristics of unripe banana flour. J. Food Process. Preserv. (2013) 37:987–98. doi: 10.1111/j.1745-4549.2012.00737.x
119. Bede, D, and Zaixiang, L. Recent developments in resistant starch as a functional food. Starch-Stärke. (2021) 73:2000139. doi: 10.1002/star.202000139
120. Lauková, M, Karovičová, J, Minarovičová, L, and Kohajdová, Z. Wheat bran stabilization and its effect on cookies quality. Potravinarstvo Slovak J Food Sci. (2019) 13:109–15. doi: 10.5219/1021
121. Suma, K, and Nandini, PV. Physical and sensory attributes of fibre enriched cookies. Food Sci Res J. (2015) 6:207–14. doi: 10.15740/HAS/FSRJ/6.2/207-214
122. Raigond, P, Dutt, S, and Singh, B. Resistant Starch in Food In: JM Mérillon and K Ramawat, editors. Bioactive Molecules in Food, Reference Series in Phytochemistry. Cham: Springer (2019). 815–46.
123. Champ, M . Resistant starch In: A-C Eliasson , editor. Starch in Food. Salt Lake City: Woodland Publishing (2004). 560–74.
124. Cui, SW, Wu, Y, and Ding, H. The range of dietary fibre ingredients and a comparison of their technical functionality In: JA Delcour and K Poutanen, editors. Fibre-rich and Wholegrain Foods: Improving Quality. Sawston: Woodhead Publishing (2013). 96–119.
125. Jiang, F, Du, C, Jiang, W, Wang, L, and Du, SK. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. (2020) 150:1155–61. doi: 10.1016/j.ijbiomac.2019.10.124
126. Kahraman, K, Aktas-Akyildiz, E, Ozturk, S, and Koksel, H. Effect of different resistant starch sources and wheat bran on dietary fibre content and in vitro glycaemic index values of cookies. J. Cereal Sci. (2019) 90:102851. doi: 10.1016/j.jcs.2019.102851
127. Zhang, W, Cheng, B, Li, J, Shu, Z, Wang, P, and Zeng, X. Structure and properties of octenyl succinic anhydride-modified high-amylose. Japonica rice starches. Polymers. (2021) 13:1325. doi: 10.3390/polym13081325
128. Dupuis, JH, Liu, Q, and Yada, RY. Methodologies for increasing the resistant starch content of food starches: A review. Compr. Rev. Food Sci. Food Saf. (2014) 13:1219–34. doi: 10.1111/1541-4337.12104
129. Zheng, MZ, Xiao, Y, Yang, S, Liu, HM, Liu, MH, Yaqoob, S, et al. Effects of heat-moisture, autoclaving, and microwave treatments on physicochemical properties of proso millet starch. Food Sci Nutr. (2006) 8:735–43. doi: 10.1002/fsn3.1295
130. Raigond, P, Ezekiel, R, and Raigond, B. Resistant starch in food: a review. J. Sci. Food Agric. (2015) 95:1968–78. doi: 10.1002/jsfa.6966
131. Tamimi, EKT, Seib, PA, Snyder, BS, and Haub, MD. Consumption of cross-linked resistant starch on glucose and insulin responses in humans. J Nutr Metab. (2010) 2010:1–6. doi: 10.1155/2010/651063
132. Urubkov, SA, Khovanskaya, S, and Smirnov, S. Comparative analysis of the glycemic index of amaranth and other gluten-free products. Food Process Tech Technol. (2019):629–34. doi: 10.21603/2074-9414-2019-4-629-634
133. Ojedokun, FO, Ikujenlola, AV, and Abiose, SH. Nutritional evaluation, glycemic index and sensory property of breakfast cereals developed from malted amaranth and roasted sesame blends. Sci J Food Sci Nutr. (2020) 6:12–9. doi: 10.37871/sjfsn.id28
134. Olagunju, AI, Omoba, OS, Oluwajuyitan, TD, and Akinrinlola, OF. Physicochemical composition, antioxidant properties and glycemic index of optimized extruded snacks from blends of amaranth, soycake and shallot flours. SSRN. 1–19. (2022). doi: 10.2139/ssrn.4062550,
135. Bastidas, EG, Roura, R, Rizzolo, DAD, Massanés, T, and Gomis, R. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: an integrative review. J. Nutr. Food Sci. (2016) 6. doi: 10.4172/2155-9600.1000497
136. Lutz, M, and Bascuñán-Godoy, L. The revival of quinoa: a crop for health In: V Waisundara and N Shiomi, editors. Superfood and Functional Food—An Overview of Their Processing and Utilization. Rijeka: InTech (2017). 37–54.
137. Miranda-Ramos, K, Millán-Linares, M, and Haros, CM. effect of chia as breadmaking ingredient on nutritional quality, mineral availability, and glycemic index of bread. Foods. (2020) 9:663. doi: 10.3390/foods9050663
138. Di Cairano, M, Condelli, N, Caruso, MC, Cela, N, Tolve, R, and Galgano, F. Use of underexploited flours for the reduction of glycaemic index of gluten-free biscuits: Physicochemical and sensory characterization. Food Bioprocess Technol. (2021) 14:1490–502. doi: 10.1007/s11947-021-02650-x
139. Annor, GA, Marcone, M, Bertoft, E, and Seetharaman, K. In vitro starch digestibility and expected glycemic index of kodo millet (Paspalum scrobiculatum) as affected by starch–protein–lipid interactions. Cereal Chem. (2013) 90:211–7. doi: 10.1094/CCHEM-06-12-0074-R
140. Anitha, S, Kane-Potaka, J, Tsusaka, TW, Botha, R, Rajendran, A, Givens, DI, et al. A systematic review and meta-analysis of the potential of millets for managing and reducing the risk of developing diabetes mellitus. Front Nutr. (2021) 8:687428. doi: 10.3389/fnut.2021.687428
141. Mcsweeney, M . Proso Millet as an Ingredient in Foods Common to North Americans. Guelph, ON: University of Guelph (2014).
142. McSweeney, MB, Seetharaman, K, Dan Ramdath, D, and Duizer, LM. Chemical and physical characteristics of proso millet (Panicum miliaceum)-based products. Cereal Chem. (2017) 94:357–62. doi: 10.1094/CCHEM-07-16-0185-R
143. Ren, X, Chen, J, Molla, MM, Wang, C, Diao, X, and Shen, Q. In vitro starch digestibility and in vivo glycemic response of foxtail millet and its products. Food Funct. (2016) 7:372–9. doi: 10.1039/C5FO01074H
144. Narayanan, J, Sanjeevi, V, Rohini, U, Trueman, P, and Viswanathan, V. Postprandial glycaemic response of foxtail millet dosa in comparison to a rice dosa in patients with type 2 diabetes. Indian J. Med. Res. (2016) 144:712–7. doi: 10.4103/ijmr.IJMR_551_15
145. Lestari, LA, Huriyati, E, and Marsono, Y. The development of low glycemic index cookie bars from foxtail millet (Setaria italica), arrowroot (Maranta arundinacea) flour, and kidney beans (Phaseolus vulgaris). J. Food Sci. Technol. (2017) 54:1406–13. doi: 10.1007/s13197-017-2552-5
146. Ugare, R, Chimmad, B, Naik, R, Bharati, P, and Itagi, S. Glycemic index and significance of barnyard millet (Echinochloa frumentacae) in type II diabetics. J. Food Sci. Technol. (2014) 51:392–5. doi: 10.1007/s13197-011-0516-8
147. Eashwarage, IS, Herath, HMT, and Gunathilake, KGT. Dietary fibre, resistant starch and eleven commonly consumed legumes Horse Gram. Res J Chem. (2017) 7:1–7.
148. Nnadi, IM, and Keshinro, OO. The effect of the glycaemic response of three commonly consumed meals on postprandial plasma glucose in type 2 diabetics at the University of Nigeria Teaching Hospital, Enugu. South Afr J Clin Nutr. (2016) 29:90–4. doi: 10.1080/16070658.2016.1216512
149. Singh, M, Manickavasagan, A, Shobana, S, and Mohan, V. Glycemic index of pulses and pulse-based products: a review. Crit. Rev. Food Sci. Nutr. (2021) 61:1567–88. doi: 10.1080/10408398.2020.1762162
150. Oyeyinka, SA, Singh, S, and Amonsou, EO. Physicochemical properties of starches extracted from bambara groundnut landraces. Starch-Stärke. (2017) 69:1600089. doi: 10.1002/star.201600089
151. Oboh, HA, and Agu, K. The effects of various traditional processing methods on the glycemic index and glycemic load of cowpeas (Vigna unguiculata). J. Food Biochem. (2010) 34:1332–42. doi: 10.1111/j.1745-4514.2010.00423.x
152. Oboh, H, Osagie, A, and Omotosho, A. Glycemic response of some boiled legumes commonly eaten in Nigeria. Diabetol. Croat. (2010) 39:113–38.
153. Akinlua, O, Sedodo, NS, and Victoria, AJ. Glycemic index of selected Nigerian foods for apparently healthy people. J. Obes. Weight Loss Ther. (2013) 3:160–4. doi: 10.4172/21657904.1000160
154. Schnell, M, de Delahaye, EP, and Mezones, Y. Metabolic responses to Venezuelan corn meal and rice bran supplemented Arepas (breads). Cereal Chem. (2005) 82:77–80. doi: 10.1094/CC-82-0077
155. Premakumari, S, Balasasirekha, R, Gomathi, K, Supriya, S, Alagusundram, K, and Mohan, RJ. Evaluation of organoleptic properties and glycemic index of recipes with rice bran. Int J Curr Res Rev. (2013) 5:01–8.
156. Camps, S, Lim, J, Ishikado, A, Inaba, Y, Suwa, M, Matsumoto, M, et al. Co-ingestion of rice bran soymilk or plain soymilk with white bread: effects on the glycemic and insulinemic response. Nutrients. (2018) 10:449. doi: 10.3390/nu10040449
157. Rico, D, Villaverde, A, Martinez-Villaluenga, C, Gutierrez, AL, Caballero, PA, Ronda, F, et al. Application of autoclave treatment for development of a natural wheat bran antioxidant ingredient. Foods. (2020) 9:781. doi: 10.3390/foods9060781
158. Naji-Tabasi, S, Niazmand, R, and Modiri-Dovom, A. Application of mucilaginous seeds (Alyssum homolocarpum and Salvia macrosiphon Boiss) and wheat bran in improving technological and nutritional properties of pasta. J. Food Sci. (2021) 86:2288–99. doi: 10.1111/1750-3841.15762
159. Jimenez-Pulido, IJ, Daniel, R, Perez, J, Martínez-Villaluenga, C, De Luis, D, and Martín Diana, AB. Impact of protein content on the antioxidants, anti-inflammatory properties and glycemic index of wheat and wheat bran. Foods. (2022) 11, 11:2049. doi: 10.3390/foods11142049
160. Agama-Acevedo, E, Islas-Hernández, JJ, Pacheco-Vargas, G, Osorio-Díaz, P, and Bello-Pérez, LA. Starch digestibility and glycemic index of cookies partially substituted with unripe banana flour. LWT-Food Sci Technol. (2012) 46:177–82. doi: 10.1016/j.lwt.2011.10.010
161. Sandhu, KS, and Lim, ST. Structural characteristics and in vitro digestibility of mango kernel starches (Mangifera indica L.). Food Chem. (2008) 107:92–7. doi: 10.1016/j.foodchem.2007.07.046
162. Wang, Q, Li, L, Wang, T, and Zheng, X. A review of extrusion-modified underutilized cereal flour: chemical composition, functionality, and its modulation on starchy food quality. Food Chem. (2022) 370:131361. doi: 10.1016/j.foodchem.2021.131361
163. Lawal, OM, Fogliano, V, Rotte, I, Fagbemi, TN, Dekker, M, and Linnemann, AR. Leafy vegetables fortification enhanced the nutritional profile and reduced the glycemic index of yellow cassava pasta. Food Funct. (2022) 13:6118–28. doi: 10.1039/D2FO00072E
164. de Toledo, NMV, Nunes, LP, da Silva, PPM, Spoto, MHF, and Canniatti-Brazaca, SG. Influence of pineapple, apple and melon by-products on cookies: physicochemical and sensory aspects. Int. J. Food Sci. Technol. (2017) 52:1185–92. doi: 10.1111/ijfs.13383
165. Theagarajan, R, Malur Narayanaswamy, L, Dutta, S, Moses, JA, and Chinnaswamy, A. Valorisation of grape pomace (cv. Muscat) for development of functional cookies. Int. J. Food Sci. Technol. (2019) 54:1299–305. doi: 10.1111/ijfs.14119
166. FAO/IZiNCG , (2018). FAO/INFOODS/IZiNCG Global food composition database for phytate Version 1.0—PhyFoodComp 1.0. Rome, Italy.
167. Gupta, RK, Gangoliya, SS, and Singh, NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. (2015) 52:676–84. doi: 10.1007/s13197-013-0978-y
168. Naseer, B, Naik, HR, Hussain, SZ, Qadri, T, Dar, BN, Amin, T, et al. Development of low glycemic index instant Phirni (pudding) mix-its visco-thermal, morphological and rheological characterization. Sci. Rep. (2022) 12:10710. doi: 10.1038/s41598-022-15060-6
169. Fu, J, Wang, Y, Tan, S, and Wang, J. Effects of banana resistant starch on the biochemical indexes and intestinal flora of obese rats induced by a high-fat diet and their correlation analysis. Front Bioeng Biotechnol. (2021) 9:575724. doi: 10.3389/fbioe.2021.575724
170. Nichenametla, SN, Weidauer, LA, Wey, HE, Beare, TM, Specker, BL, and Dey, M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double-blind controlled cross-over intervention. Mol. Nutr. Food Res. (2014) 58:1365–9. doi: 10.1002/mnfr.201300829
171. Eleazu, CO . The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus; prospects, challenges and solutions. Afr. Health Sci. (2016) 16:468–79. doi: 10.4314/ahs.v16i2.15
172. Weickert, MO, Möhlig, M, Schöfl, C, Arafat, AM, Otto, B, Viehoff, H, et al. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care. (2006) 29:775–80. doi: 10.2337/diacare.29.04.06.dc05-2374
173. Ajila, CM, Leelavathi, K, and Prasada, UJSR. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. (2008) 48:319–26. doi: 10.1016/j.jcs.2007.10.001
Keywords: low glycemic index, nutraceutical starch, processing by-products, underutilized, therapeutic
Citation: Dega V and Barbhai MD (2023) Exploring the underutilized novel foods and starches for formulation of low glycemic therapeutic foods: a review. Front. Nutr. 10:1162462. doi: 10.3389/fnut.2023.1162462
Edited by:
Syed Zameer Hussain, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, IndiaReviewed by:
Paucean Adriana, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaAmira M. Galal Darwish, Borg Al Arab Technological University, Egypt
Copyright © 2023 Dega and Barbhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vijayalakshmi Dega, dmlqaS5kZWdhQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Vijayalakshmi Dega
Vijayalakshmi Dega Mrunal Deepak Barbhai
Mrunal Deepak Barbhai