- 1Department of Nutrition and Food Hygiene, School of Public Health, Medical College of Soochow University, Suzhou, China
- 2Department of Obstetrics and Gynecology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 3Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Soochow University, Suzhou, China
- 4Department of Occupational and Environmental Health, School of Public Health, Medical College of Soochow University, Suzhou, China
Although numerous epidemiological studies investigated the association between dietary fat intakes or serum lipid levels and ovarian cancer risk, a consistent and explicit conclusion for specific dietary fats or serum lipids that increase the risk of ovarian cancer is not available. In this study, a systematic review and meta-analysis were conducted to assess the key dietary fats and serum lipids that increased the risk of ovarian cancer. Databases such as PubMed, Web of Science, and EMBASE were searched for observational studies. A total of 41 studies met the inclusion criteria, including 18 cohort and 23 case–control studies (109,507 patients with ovarian cancer and 2,558,182 control/non-ovarian cancer participants). Higher dietary intakes of total fat (RR = 1.19, 95% CI = 1.06–1.33, I2 = 60.3%), cholesterol (RR = 1.14, 95% CI = 1.03–1.26, I2 = 19.4%), saturated fat (RR = 1.13, 95% CI = 1.04–1.22, I2 = 13.4%), and animal fat (RR = 1.21, 95% CI = 1.01–1.43, I2 = 70.5%) were significantly associated with a higher risk of ovarian cancer. A higher level of serum triglycerides was accompanied by a higher risk of ovarian cancer (RR = 1.33, 95% CI = 1.02–1.72, I2 = 89.3%). This meta-analysis indicated that a higher daily intake of total fat, saturated fat, animal fat, and cholesterol and higher levels of serum triglycerides were significantly associated with an increased risk of ovarian cancer.
1. Introduction
Ovarian cancer is the most lethal gynecological malignancy, with limited screening modalities due to its low anatomical location, and lack of early symptoms and specific biomarkers. Additionally, 90% of reported ovarian cancer cases are epithelial ovarian cancer, which is already in advanced stages (stages III–IV) at the time of diagnosis and has a poor prognosis (1, 2).
Numbers of factors are suggested to impact the reproduction of ovarian cancer. High parity, oral contraceptive use, high lactation duration, tubal ligation, hysterectomy, oophorectomy, and salpingectomy are beneficial factors for ovarian cancer (3, 4). On the contrary, environmental endocrine disruptors, pelvic inflammation, and lipids are harmful factors in ovarian cancer (5–7), and a relationship between early adulthood and a high mortality rate in ovarian cancer patients may exist (8, 9). Particularly, omental metastasis, in which ovarian cancer cells lay over the omentum, an apron-like layer of adipose tissue in the peritoneal cavity, leads to significant consequences for patient morbidity and mortality (6).
Epidemiological evidence has gradually accrued that links obesity and dyslipidemia with higher rates of cancer and a higher risk of cancer-related mortality (9, 10). Specifically, diet fats might be a key modifiable factor, and serum lipids may be a potential predictor for diseases including cancer, both of which were associated with ovarian cancer risk and progression through altering systemic inflammation (11–13). A randomized controlled trial of 48,835 postmenopausal women revealed a 40% decrease in the incidence of ovarian cancer in individuals with a low-fat diet compared to a traditional diet (14). Moreover, some studies have reported that dietary polyunsaturated fat is a beneficial factor for ovarian cancer. For example, an Italian case–control study showed that n-3 polyunsaturated fatty acid was associated with a lower risk of ovarian cancer (15). Nevertheless, others suggested a lack of evidence for associations between dietary fat intake and ovarian cancer risk. A case–control study from Australia indicated no evidence for a protective role of n-3 polyunsaturated fatty acids in ovarian cancer (16). Meanwhile, higher concentrations of serum total cholesterol and high-density lipoprotein cholesterol (HDL-C) were reported to be associated with a lower overall risk of cancer (17). Higher serum cholesterol levels, however, were associated with a higher risk of ovarian cancer, according to nested case–control research from a serum bank (18).
Interpretation of epidemiological findings evaluating the relation between dietary fat and serum lipids and ovarian cancer may be hampered by differences in geographic dietary patterns and different characteristics of participants, as well as by adjustment for confounding factors such as total energy intake, BMI, or reproductive history. Therefore, quantitative analysis of the relationship between dietary fat, serum lipids, and ovarian cancer is essential and may also provide insight into the etiology of this complicating disease. Previously, epidemiological studies and meta-analyses have mostly focused on the association of single factors such as dietary fat or serum lipid levels with ovarian cancer risk (19–21). Currently, there are fewer meta-analyses that also summarize the association between dietary fat, serum lipid levels, and ovarian cancer risk. In this study, we performed an up-to-date and expanding meta-analysis to summarize epidemiological evidence from observational studies on both associations of dietary fat intakes and serum lipid levels with ovarian cancer risk by considering potential confounding factors using stratified sub-analyses and meta-regression analysis.
2. Materials and methods
The present study was conducted following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (22). This systematic review is registered in the PROSPERO International Prospective Register of Systematic Reviews with the identification code CRD42022349731.
2.1. Search strategy
An electronic database search was conducted to determine peer-reviewed articles published until 1 October 2022. Databases included PubMed, Web of Science, and Embase. The key search terms are dietary fat or fat intake, serum lipid, and ovarian cancer. The search strategy in the Supplementary Table S1. The bibliographies in the full retrieved articles and previously meta-analyses and related reviews were also searched for additional studies (9, 19, 21, 23).
2.2. Study selection
The Population, Intervention, Comparison, Outcome, and Study Design (PICOS) (24) criteria were used for the studies screened, as shown in Supplementary Table S2.
Studies that met the criteria below were included if they: (1) observational studies with cohort, case–control or nested case–control, (2) reported the outcome of interest as the occurrence of ovarian cancer or the incidence of tumors including ovarian cancer, (3) reported the exposure of interest as dietary fat intake (total fat, saturated fat, unsaturated fat, trans-fat, animal fat, plant fat, dairy fat, and cholesterol) or its subtypes or serum cholesterol level, and (4) reported odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) along with 95% CI to calculate.
2.3. Data extraction
Data extraction was completed independently by two authors, with any discrepancies resolved by discussion. Information extracted from each study included study characteristics (author, publication year and aria, study name, and sample size), amount or frequency of exposures (type and source of dietary fat, and serum lipids), RRs and 95% CI, and variable adjusted factors.
2.4. Quality and level of evidence assessments
The methodological quality of included studies was evaluated by the 9-star Newcastle-Ottawa Scale (NOS) scoring criteria (25). Literature selection, data extraction, and quality assessment were reworked and rechecked for accuracy by co-authors (Supplementary Tables S3, S4).
2.5. Analyses
The incidence of ovarian cancer was the outcome variable. We estimated the summary effect size, including RRs, ORs, or HRs and their corresponding 95% CIs for ovarian cancer among participants in the highest category of intake (dietary total fat and cholesterol, saturated fat, monounsaturated fat, polyunsaturated fat, trans-fat, animal fat, plant fat, and dairy fat) compared with the lowest category. Serum cholesterol, triglycerides, HDL-C, low-density lipoprotein cholesterol (LDL-C), and dyslipidemia, which reflect serum lipid levels, were also extracted for this study simultaneously. If there are multiple associated estimates, the most adjusted risk estimate was extracted. Of the included studies, ORs, RRs, and HRs were calculated using logistic regression analysis and Cox proportional hazard models. Because of the low absolute risk of ovarian cancer, all results of associations in these studies were reported as RRs, and the estimates of ORs or HRs from case–control studies and cohort studies were all assumed to be valid estimates of the RR.
Heterogeneity was evaluated by the Q-test and the I2 statistic. The significance level of the Q-test was defined as p < 0.10 and I2 values ≥ 50%, which indicated significant heterogeneity (26). Pooled effect sizes and their 95% CIs were estimated using a random effects model. Subgroup analyses from a pre-determined list of factors known to influence ovarian cancer risk (family history, oral contraceptive use, menopausal status, hormone use, pregnancy times, and body mass index), dietary assessment (total energy intake), and study characteristics (cohort vs. case–control studies, geographic location) were also conducted. The effects of lifestyle factors (smoking, alcohol consumption, and physical activity) were additionally synthesized, and fasting status were also incorporated. Heterogeneities between subgroups were evaluated by meta-regression analysis once again. The influence of individual studies was assessed by sensitivity analysis.
Funnel plots and Egger's test were used to assess publication bias (27), and “trim and fill” was also used to correct a bias (28). All statistical analyses were performed using STATA software, version 15.0 (STATA Corp, College Station, TX, USA).
3. Results
3.1. Study selection and characteristics
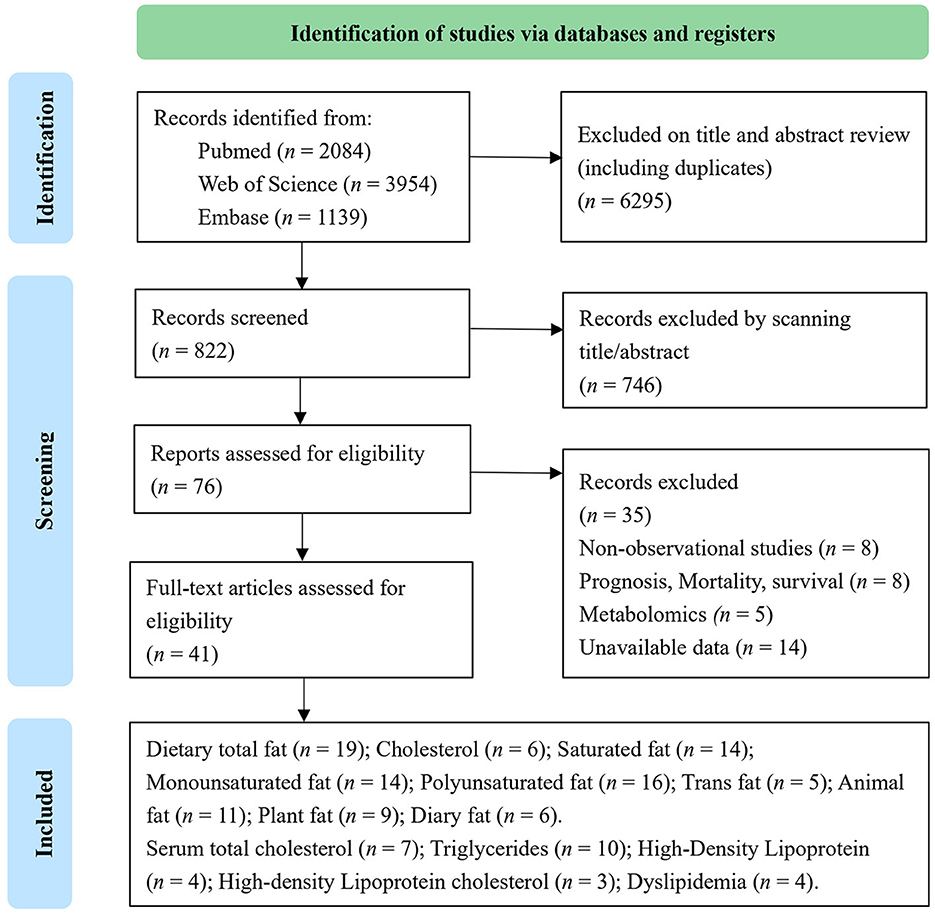
Out of 7,117 publications collected from the electronic and manual literature searches, 7,041 articles were excluded through browsing titles or abstracts. In the next step, 76 potentially relevant publications that appeared to meet the specified inclusion criteria were reviewed further. After the assessment of these full texts, 35 records were excluded due to insufficient data or the use of duplicate samples in these studies. Finally, 41 studies were considered eligible and underwent quantitative evaluation (Figure 1). Among selected research studies, nine cohort studies (29–37) and 17 case–control studies (15, 16, 38–52) focused on the types and sources of dietary fat, including dietary total fat, cholesterol, saturated, monounsaturated, polyunsaturated, trans-fats, animal, plant, and dairy fats (Table 1). Meanwhile, 18 cohort studies (18, 53–60) and six case–control studies (62–67) focused on serum lipids, including total cholesterol, triglycerides, HDL-C, LDL-C, and dyslipidemia (Table 2). A flow chart of study selection is displayed in Figure 1.
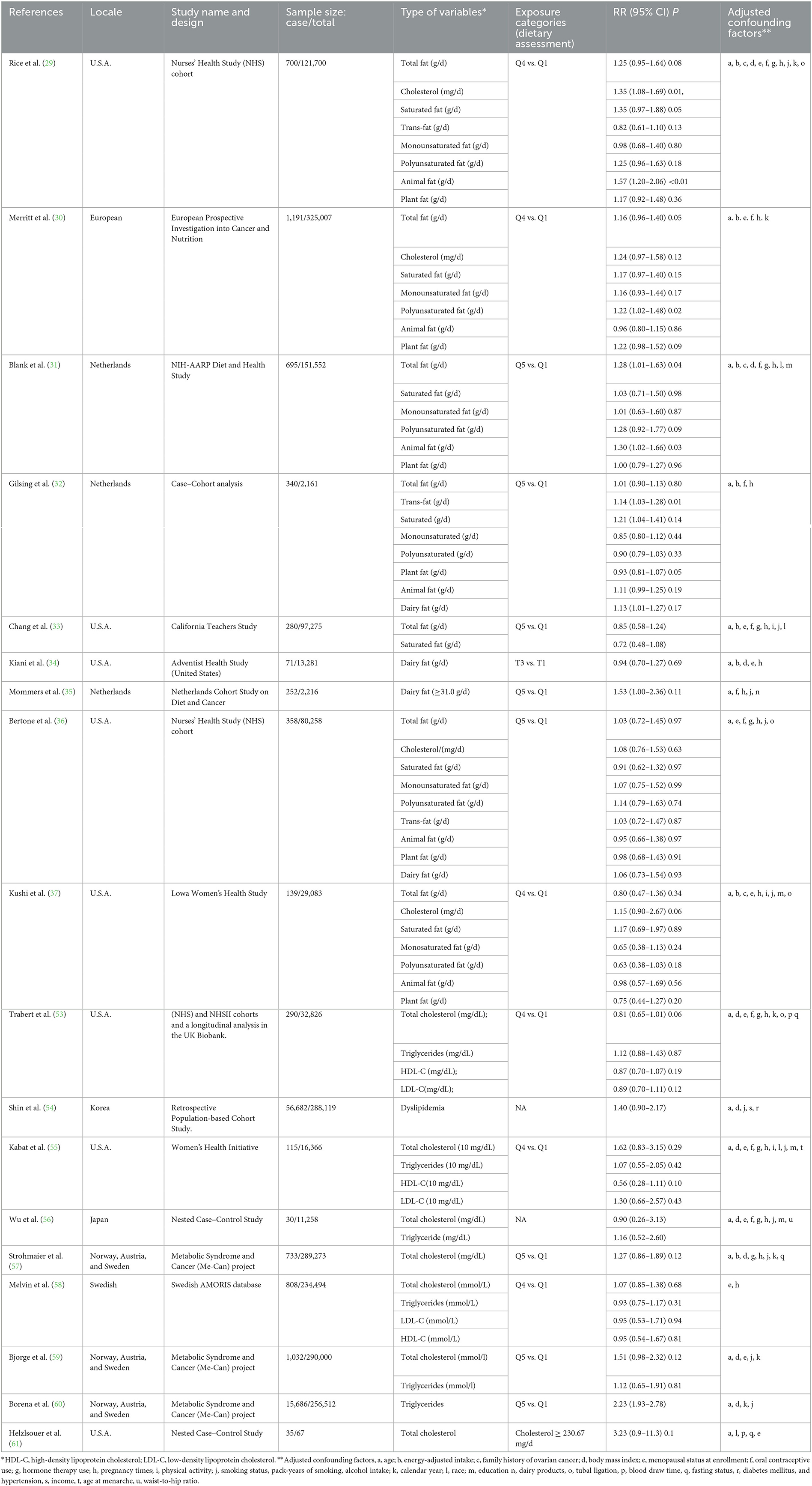
Table 1. Characteristics of 18 cohort studies included in the meta-analysis of dietary fat intake and serum lipid levels to the risk of ovarian cancer.
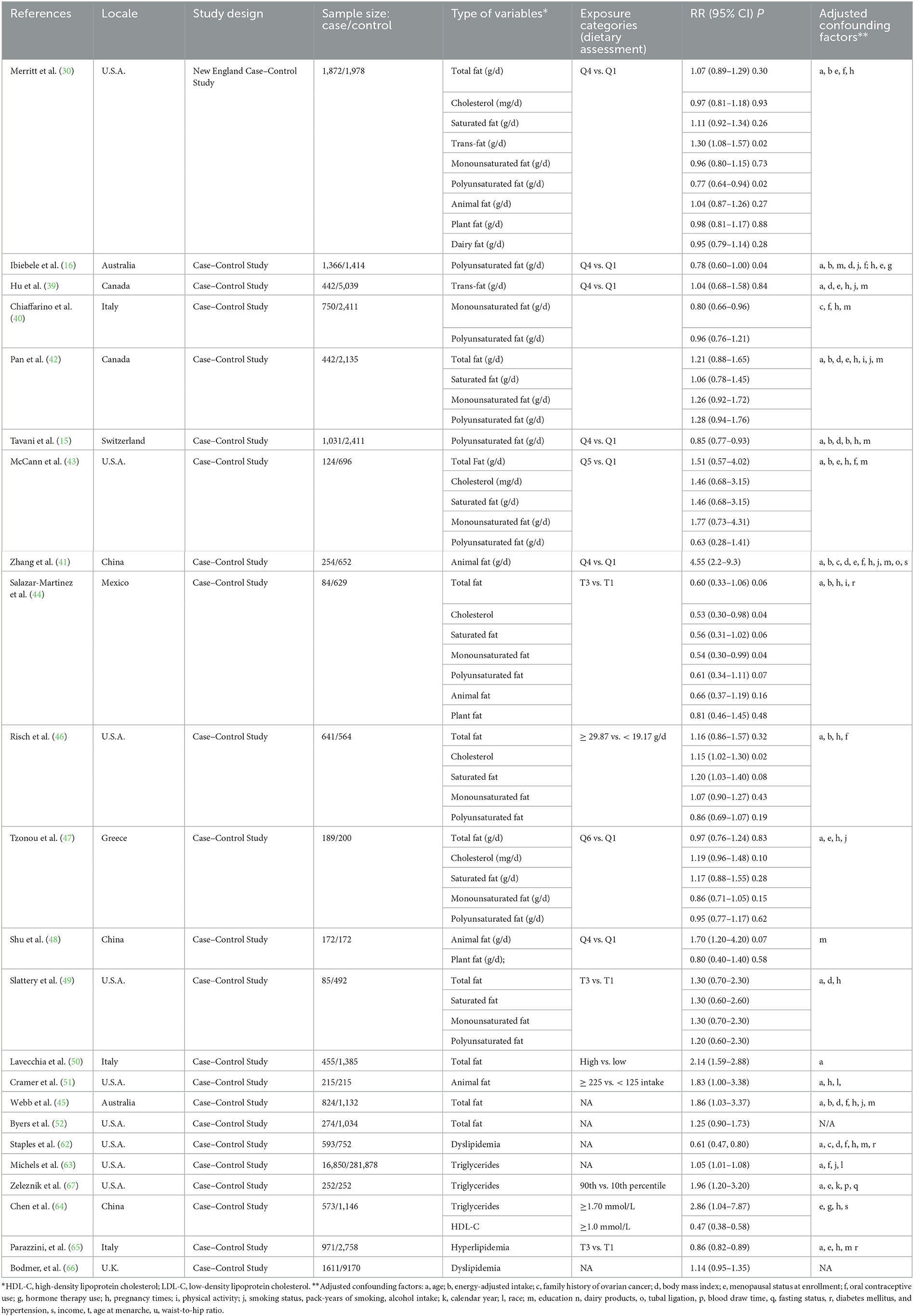
Table 2. Characteristics of 23 case–control studies included in the meta-analysis of dietary fat intake and serum lipid levels to the risk of ovarian cancer.
Eligible studies were published from 1989 to 2021 in North America (n = 20), Europe (n = 14), East Asia (n = 5), and Australia (n = 2). In these studies, a total of 2,667,689 individual data (109,507 patients with ovarian cancer and 2,558,182 control/non-ovarian cancer participants) were reviewed.
3.2. Dietary fat intake and ovarian cancer risk
A meta-analysis of 26 articles (29–33, 36, 37, 41–44, 46, 63) including 856,557 individuals was performed to assess the association between dietary fat intake and the risk of ovarian cancer. The results showed that individuals with higher total dietary fat (RR = 1.19, 95% CI = 1.06–1.33) (Figure 2) and cholesterol (RR = 1.14, 95% CI = 1.03–1.26, I2 = 19.4%) intake experienced a significantly higher risk of developing ovarian cancer (Figure 3). There was moderate heterogeneity across these studies for total fat (I2 = 60.3%, p = 0.009).
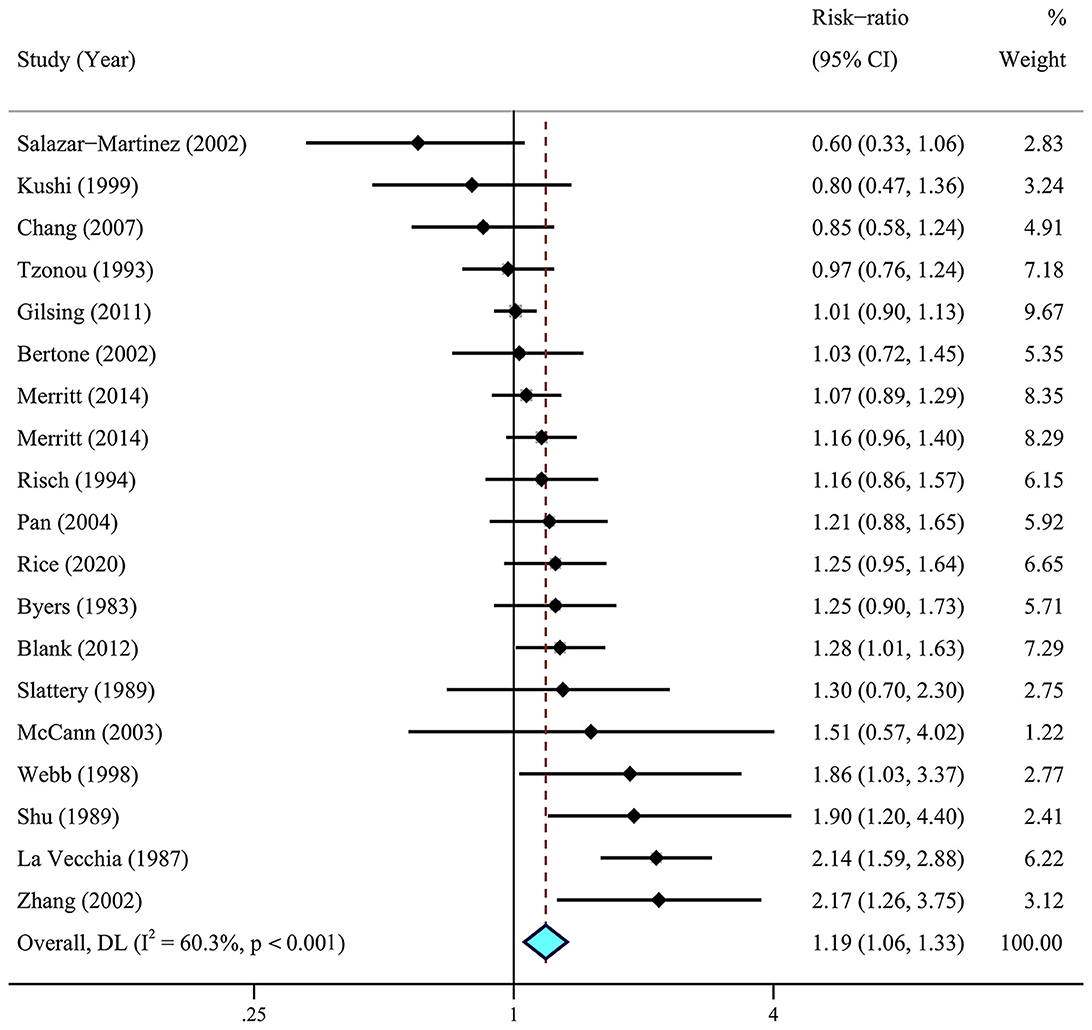
Figure 2. Relationship between dietary total fat intake and ovarian cancer risk. Effect estimate for random-effect analysis; p-value is for Cochran's Q statistics for heterogeneity; I2 is the proportion of total variation in study estimates from heterogeneity rather than sampling error.
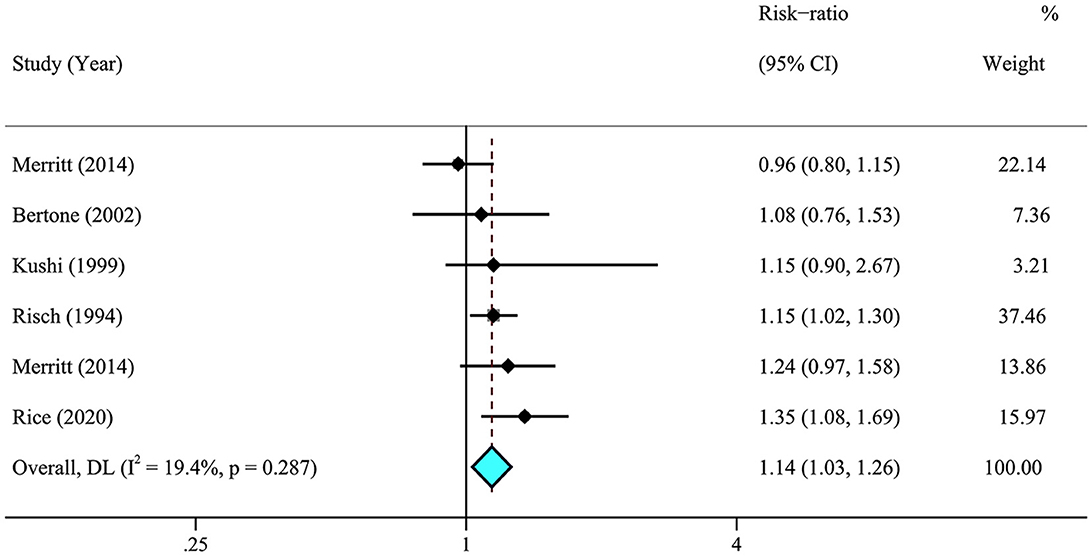
Figure 3. Relationship between dietary cholesterol intake and ovarian cancer risk. Effect estimate for random-effect analysis; p-value is for Cochran's Q statistics for heterogeneity; I2 is the proportion of total variation in study estimates from heterogeneity rather than sampling error.
A further meta-analysis for the impact of specific types of dietary fat on ovarian cancer was conducted using data from six cohort studies (29–33, 36) and 12 case–control studies (15, 16, 39, 40, 42–44, 46, 47, 49). The results showed that higher dietary intake of saturated fat (RR = 1.13, 95% CI = 1.04–1.22, I2 = 13.4%) was statistically significantly associated with a higher risk of ovarian cancer. No significant association was observed for the dietary intake of monounsaturated fat (RR = 0.96, 95% CI = 0.87–1.06, I2 = 43.2%), polyunsaturated fat (RR = 0.95, 95% CI = 0.86–1.05, I2 = 60.3%), and trans-fat (RR = 1.10, 95% CI = 0.96–1.26, I2 = 43.2%) (Figure 3). Of note, the results from case–control studies showed that individuals who experienced a lower risk of ovarian cancer were significantly associated with higher intake of polyunsaturated fat (RR = 0.88, 95% CI = 0.80–0.97, I2 = 33.0%) and trans-fat (RR = 1.25, 95% CI = 1.06–1.49, I2 = 0.00%) with little heterogeneity (Figure 4).
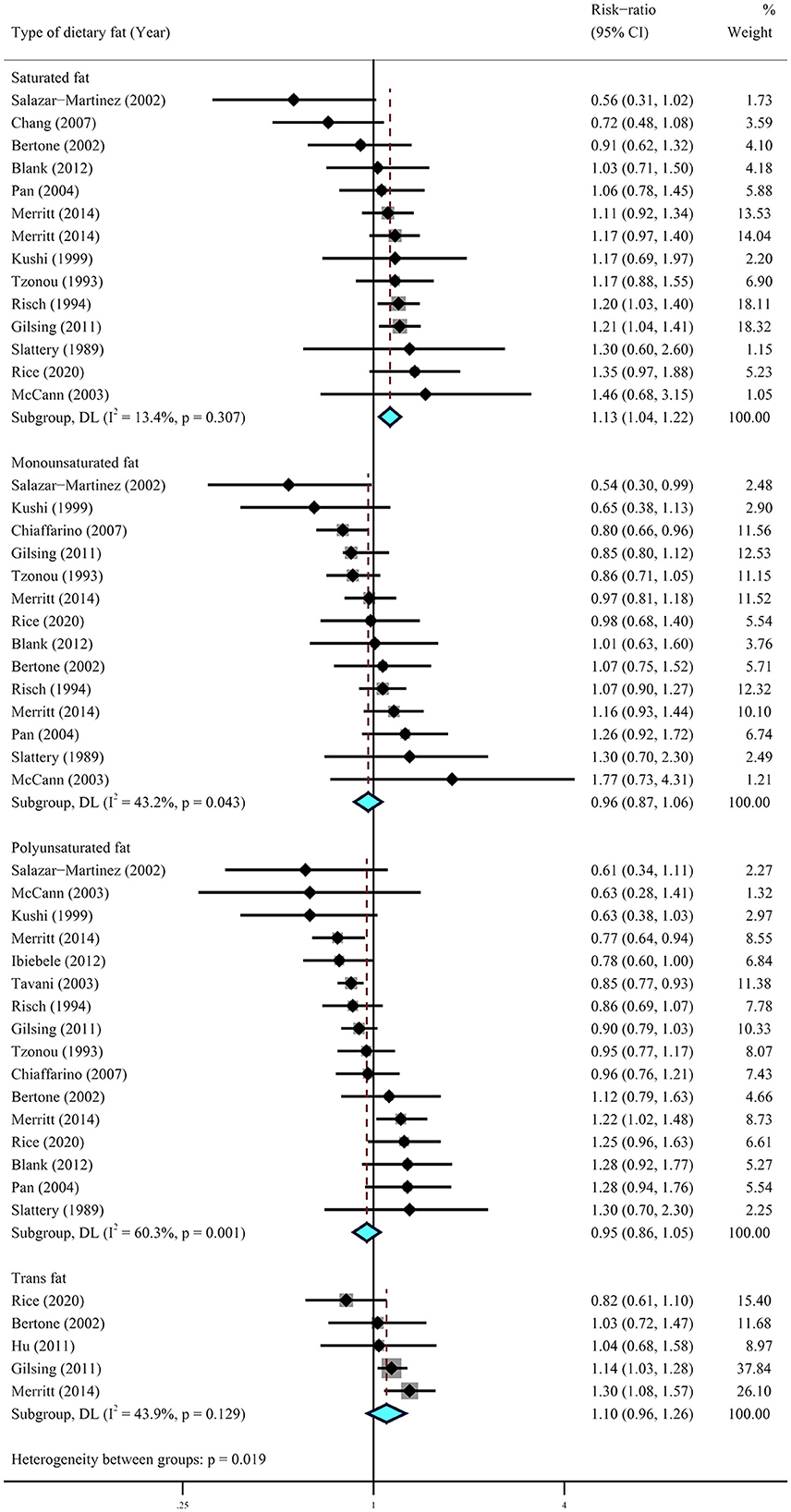
Figure 4. Summary most adjusted relative risks of saturated fat, monounsaturated fat, polyunsaturated fat, and trans-fat and ovarian cancer. Effect estimate for random-effect analysis; p-value is for Cochran's Q statistics for heterogeneity; I2 is the proportion of total variation in study estimates from heterogeneity rather than sampling error.
We then conducted a meta-analysis for the sources of dietary fats, using data from eight cohorts (29–32, 34–37) and five case–control (38, 41, 44, 48, 51) studies. The results suggested that higher dietary intake of animal fat (RR = 1.21, 95% CI = 1.01–1.43, I2 = 70.5%) was significantly associated with a higher risk of ovarian cancer, while no association was observed for plant fat (RR = 1.00, 95% CI = 0.92–1.09, I2 = 0.8%) and dairy fats (RR = 1.07, 95% CI = 0.95–1.20, I2 = 19.2%) (Figure 5).
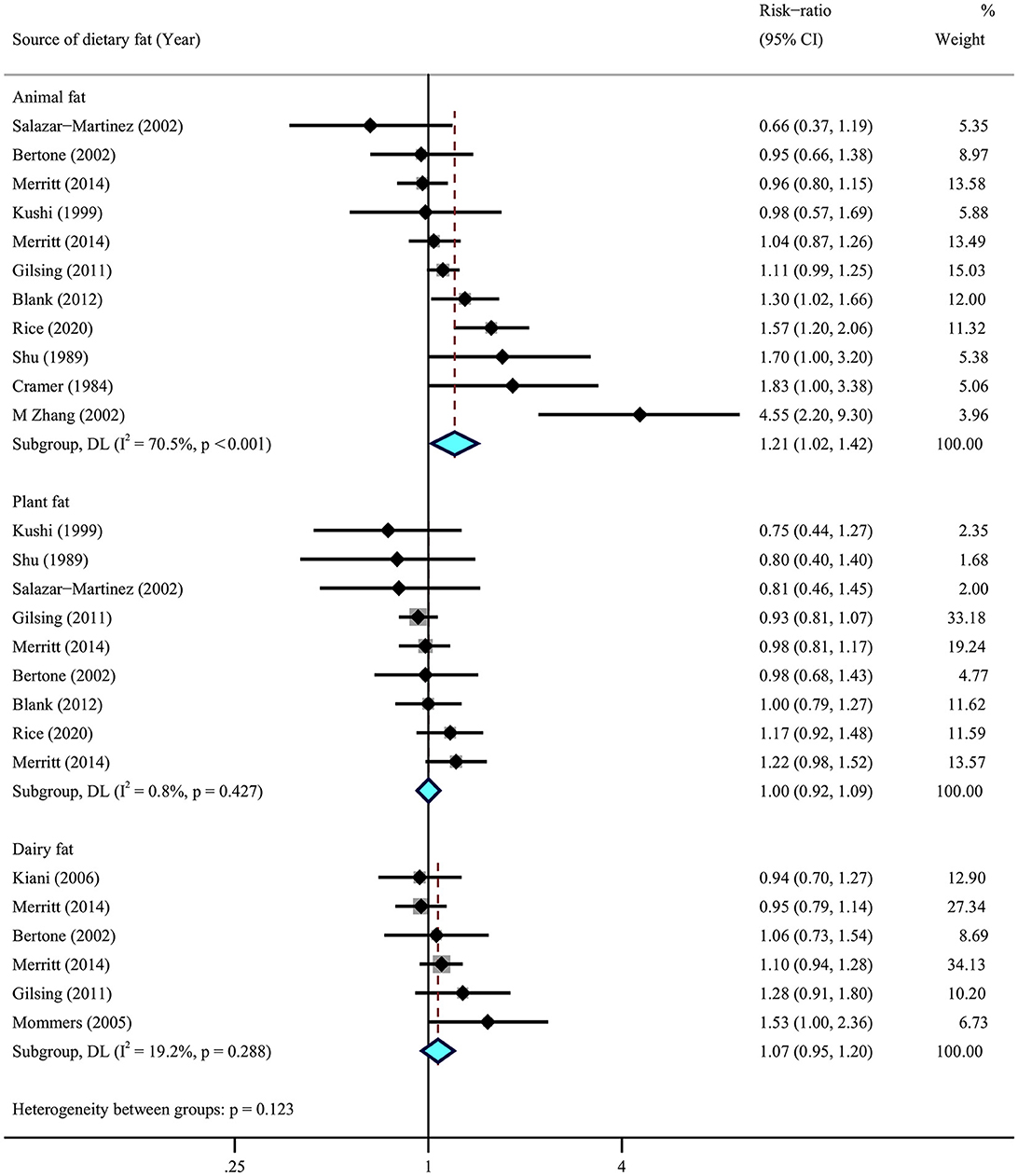
Figure 5. Summary most adjusted relative risks of animal fat, plant fat, and dairy fat and ovarian cancer. Effect estimate for random-effect analysis; p-value is for Cochran's Q statistics for heterogeneity; I2 is the proportion of total variation in study estimates from heterogeneity rather than sampling error.
3.3. Serum lipid levels
Among selected nine cohort (18, 53–60) and six case–control (62, 63, 65–68) studies including 1,811,132 individuals, only seven studies showed a statistical association with the risk of ovarian cancer (Figure 6). The meta-analysis suggested a significant association between higher levels of serum triglycerides and a higher risk of ovarian cancer (RR = 1.33, 95% CI = 1.02–1.72, I2 = 89.3%), whereas there were insignificant correlations between serum cholesterol (RR = 1.17, 95% CI = 0.91–1.50, I2 = 56.2%), LDL-C (RR = 0.93, 95% CI = 0.76–1.14, I2 = 0.0%), and ovarian cancer risk. There were no obvious correlations between dyslipidemia and the risk of ovarian cancer (RR = 0.88, 95% CI = 0.59–1.30, I2 = 86.9%). In addition, there was a suggestive inverse association for HDL-C (RR = 0.68, 95% CI = 0.45–1.03, I2 = 83.5%) with ovarian cancer risk.
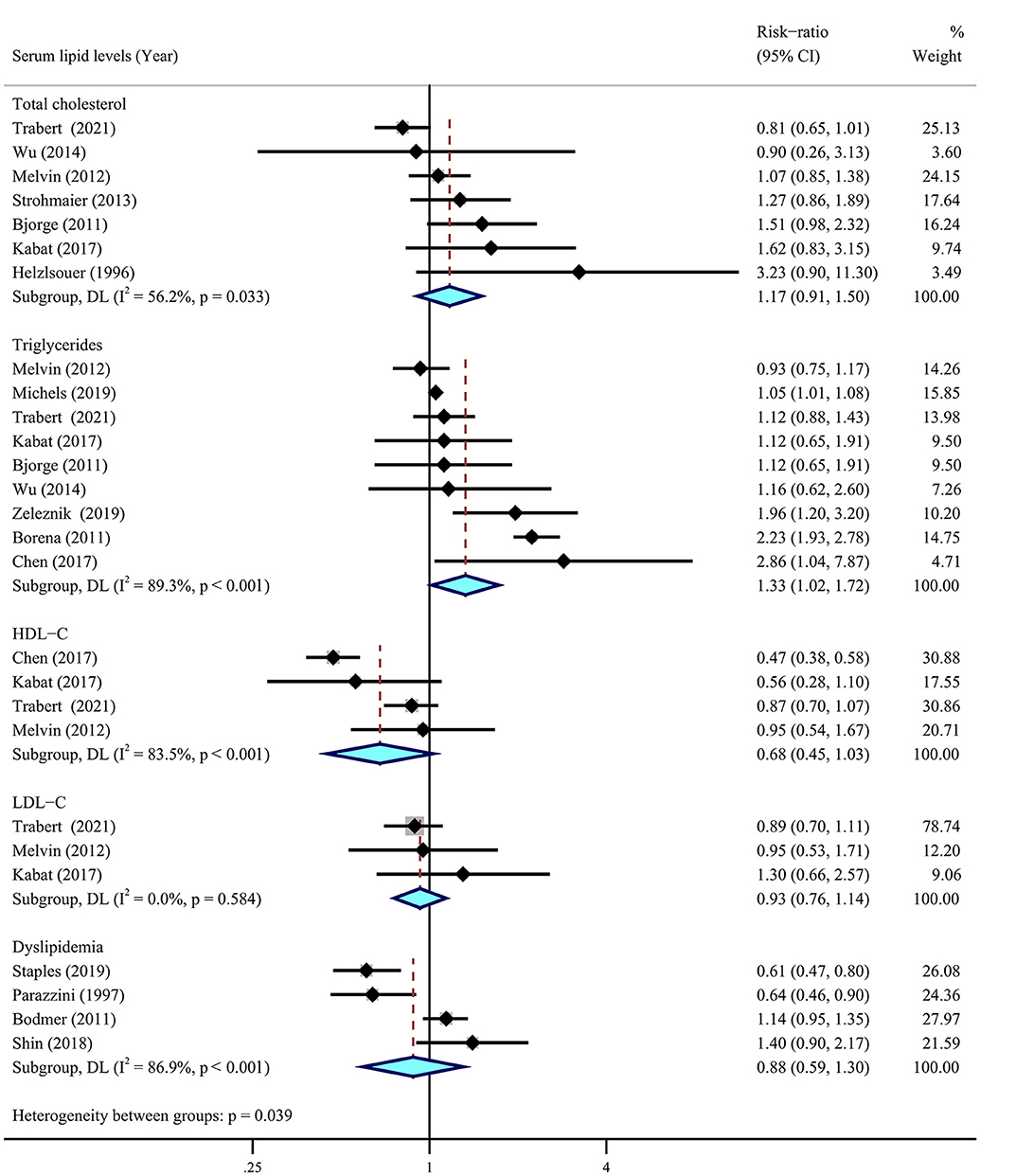
Figure 6. Relationship between serum lipid levels and ovarian cancer risk. Summary most adjusted risk-ratio of total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), dyslipidemia, and ovarian cancer risk. All effect estimate is random-effect analysis; p-value is for Cochran's Q statistics for heterogeneity; I2 is the proportion of total variation in study estimates from heterogeneity rather than sampling error.
3.4. Sensitivity and subgroup analysis
We further excluded individual studies for these five significant associations observed between dietary total fat, cholesterol, saturated fat, animal fat, and serum triglycerides, which showed no change in combined relative risk (Supplementary Tables S1–S5). Moreover, sensitivity analyses did not suggest any significant associations with other dietary fats and serum lipids.
Stratified sub-analyses showed that among five significant associations to ovarian cancer risk, the higher heterogeneity in dietary total fat (RR = 1.19, 95% CI = 1.06–1.33, I2 = 60.3%) may be explained by differences in study type (case–control study, RR = 1.31, 95% CI = 1.08–1.58, I2 = 66.7%), area (Europe, RR = 1.23, 95% CI = 0.98–1.54, I2 = 83.4%), and total energy intake (no adjusted, RR = 1.45, 95% CI = 1.14–1.85, I2 = 67.1%) (Supplementary Table S5). Otherwise, the results of subgroup analysis were hardly modified by area, or various confounding factors, such as adjustments for BMI, menopausal status, oral contraceptives, hormone therapy, pregnancy times, and lifestyle factors (Supplementary Table S6).
3.5. Publication bias
There was no publication bias by means of visual inspection of funnel plots and formal statistical tests. None of the studies had a significant effect on the pooled risk estimates and 95% CIs (Supplementary Figures S6–S10). We further used Egger's test to check for potential publication bias for specific fats. No evidence of publication bias for dietary total fat (p = 0.963), cholesterol (p = 0.451), monounsaturated fat (p = 0.396), polyunsaturated fat (p = 0.135), trans-fat (p = 0.168), animal fat (p = 0.898), plant fat (p = 0.590), and dairy fat (p = 0.627) was shown. However, there was publication bias for saturated fat according to Egger's test (p = 0.009), and we re-estimated the effect size using the meta-trim method. The adjusted pooled effects did not alter the combined relative risk (RR = 1.13, 95% CI = 1.06–1.22) (Supplementary Figure S11).
4. Discussion
There is a rapidly growing interest in the prevention of cancer by modifying dietary structure and lifestyle. In this expanding meta-analysis of 41 observational studies, we focused on ovarian cancer risk related to both dietary fat intakes and serum lipids. The current results suggest four significant associations for dietary fat, including dietary total fat and cholesterol, saturated fat, and animal fat. Moreover, we found that higher serum triglyceride levels increased the risk of ovarian cancer. Furthermore, the significant associations between polyunsaturated and trans-fat and ovarian cancer risk were only observed in a case–control study. Our meta-analysis, which covered only prospective studies, provides new evidence on the relationships between dietary fats, serum lipid levels, and the risk of ovarian cancer and could strengthen its internal validity.
Our study suggested that dietary saturated fatty acids, trans-fatty acids, and animal-derived fats may be a risk factor for increased ovarian cancer development and that this positive association is biologically plausible. Foods high in saturated fats, especially processed and red meats, have been related to a higher risk of cancer and mortality (69). Nutritional factors, including trans-fatty acids, may be implicated in peroxisome proliferator-activated receptor (PPAR) γ stimulator. Elaic acid, a major long-chain trans-fatty acid, has been shown to transform cancer by altering specific G protein and epidermal growth factor receptor signaling pathways (70). Co-culturing adipocytes and ovarian cancer cells lead to the direct transfer of lipids from adipocytes to ovarian cancer cells, which promotes the growth of tumors both in vitro and in vivo (5). Obesity also increased lipogenesis, improved vascularity, and reduced M1 macrophage infiltration, all of which assisted ovarian cancer metastatic success (7).
Dietary cholesterol has always been controversial for its health effects. The marginal relationships between dietary cholesterol intake and the risk of ovarian cancer in our meta-analysis may have some biological basis. This finding was consistent with a meta-analysis of dose–response from seven studies, which found a marginally positive association (per 50 mg/day: RR = 1.01, 95% CI = 1.00–1.03) between dietary cholesterol intake and ovarian cancer risk (20). Cholesterol is a special lipid that is required for membrane biogenesis, cell proliferation, and differentiation. In addition to the dietary source, cholesterol can be synthesized in humans by the liver and carried throughout the body via the transporters LDL and HDL. Some studies have reported a positive association between serum cholesterol, a mandatory precursor of steroid hormones involved in cancer promotion and death (71). Experimental studies showed that high-level cholesterol could activate oncogenic effects by promoting systemic inflammation or directly binding to smoothened receptors, which in turn activate Hedgehog signaling and the mevalonate pathway (72–74). According to the available evidence, lipid-lowering medicines, such as statins, PARP inhibition, fenofibrate, and PCSK9, have been implied to have anti-tumor effects in several human malignancies, including ovarian cancer (68, 75, 76). The current meta-analysis demonstrated a positive association between serum triglycerides, but not cholesterol, and the risk of ovarian cancer development, with higher heterogeneity. Even so, we did not exclude the possibility that total serum cholesterol was etiologically significant in ovarian cancer. Therefore, it is necessary to investigate if serum lipid biomarkers can predict the risk of ovarian cancer.
However, this study also had several limitations that must be considered when interpreting the results. First, diet data in most of the published study were collected from a food frequency questionnaire or 24-h retrospective method by dietary recall or self-reported, which may cause failures in terms of memory and data inaccuracy and result in a significant bias. Second, individuals are mostly from North America and Europe, and the results of the meta-analysis may have implications for extrapolation to other regions due to differences in geographic location, ethnicity, and dietary patterns. Third, another limitation concerns the direct relationship between intakes of dietary fat and serum lipid levels because higher intakes of dietary fat do not necessarily lead to hyperlipidemia. Studies have now shown that some foods have the potential to improve lipid profiles, such as synbiotics (77) and probiotics (78) from yogurt or dairy. Several randomized controlled trials have also reported that improved dietary structure or food items can effectively improve serum lipid levels in patients with cardiovascular disease (77–79). However, no study has yet assessed both dietary fat intakes and serum lipids with ovarian cancer risk. In addition, due to the limited number of studies included for synthesis, we could not identify the association of the ratio of LDL-C to HDL-C with ovarian cancer risk, even though some studies reported that LDL/HDL can predict the occurrence of cardiovascular diseases (80).
In conclusion, the present up-to-date and expanding meta-analysis indicated that higher intake of dietary total fat, cholesterol, saturated fat, and animal fat was associated with an increased risk of ovarian cancer. Moreover, our evidence suggested that higher levels of serum triglycerides also increased the risk of ovarian cancer. Extensive prospective studies with more authentic measurements of nutritional or dietary variables are needed to confirm the significant association between serum dyslipidemias and ovarian cancer risk.
5. Conclusion
This meta-analysis indicated that higher daily intaker of total fat, saturated fat, animal fat, and cholesterol and higher levels of serum triglycerides were significantly associated with an increased risk of ovarian cancer.
Author contributions
XZ, H-MD, and B-YL were all responsible for the conception and design of the manuscript. XZ and L-FD searched the literature, screened for final inclusion of the articles, completed the quality assessment, and planned the data analysis. XZ performed the meta-analysis. XZ, G-CC, and B-YL wrote the manuscript. XZ, G-CC, J-FL, LF, JL, Z-YH, FJ, Z-LZ, and B-YL revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (81872622, 81673151, U1832140, and 81703209) and a sub-project was funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1153986/full#supplementary-material
References
1. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up (vol 24, pg 24, 2013). Ann Oncol. (2018) 29:259. doi: 10.1093/annonc/mdy157
2. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. (2019) 35:151–6. doi: 10.1016/j.soncn.2019.02.001
3. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. (2019) 393:1240–53. doi: 10.1016/S0140-6736(18)32552-2
4. Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol. (2016) 34:2888. doi: 10.1200/JCO.2016.66.8178
5. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. (2011) 17:1498–503. doi: 10.1038/nm.2492
6. Ladanyi A, Mukherjee A, Kenny HA, Johnson A, Mitra AK, Sundaresan S, et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. (2018) 37:2285–301. doi: 10.1038/s41388-017-0093-z
7. Liu Y, Metzinger MN, Lewellen KA, Cripps SN, Carey KD, Harper EI, et al. Obesity contributes to ovarian cancer metastatic success through increased lipogenesis, enhanced vascularity, and decreased infiltration of M1 macrophages. Cancer Res. (2015) 75:5046–57. doi: 10.1158/0008-5472.CAN-15-0706
8. Schlumbrecht MP, Sun CC, Wong KN, Broaddus RR, Gershenson DM, Bodurka DC. Clinicodemographic factors influencing outcomes in patients with low-grade serous ovarian carcinoma. Cancer. (2011) 117:3741–9. doi: 10.1002/cncr.25929
9. Yang H-S, Yoon C, Myung S-K, Park SM. Effect of obesity on survival of women with epithelial ovarian cancer a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. (2011) 21:1525–32. doi: 10.1097/IGC.0b013e31822eb5f8
10. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
11. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc Nutr Soc. (2008) 67:253–6. doi: 10.1017/S002966510800712X
12. Merritt MA, Tzoulaki J, van den Brandt PA, Schouten LJ, Tsilidis KK, Weiderpass E, et al. Nutrient-wide association study of 57 foods/nutrients and epithelial ovarian cancer in the European Prospective Investigation into Cancer and Nutrition study and the Netherlands Cohort Study. Am J Clin Nutr. (2016) 103:161–7. doi: 10.3945/ajcn.115.118588
13. Saeidi J, Motaghipur R, Sepehrian A, Mohtashami M, Nia FF, Ghasemi A. Dietary fats promote inflammation in Wistar rats as well as induce proliferation, invasion of SKOV3 ovarian cancer cells. J Food Biochem. (2020) 44:e13177. doi: 10.1111/jfbc.13177
14. Gamba CS, Stefanick ML, Shikany JM, Larson J, Linos E, Sims ST, et al. Low-fat diet and skin cancer risk: the women's health initiative randomized controlled dietary modification trial. Cancer Epidemiol Biomark Prev. (2013) 22:1509–19. doi: 10.1158/1055-9965.EPI-13-0341
15. Tavani A, Pelucchi C, Parpinel M, Negri E, Franceschi S, Levi F, et al. n-3 polyunsaturated fatty acid intake and cancer risk in Italy and Switzerland. Int J Cancer. (2003) 105:113–6. doi: 10.1002/ijc.11018
16. Ibiebele TI, Nagle CM, Bain CJ, Webb PM. Intake of omega-3 and omega-6 fatty acids and risk of ovarian cancer. Cancer Causes Control. (2012) 23:1775–83. doi: 10.1007/s10552-012-0053-4
17. Vilchez JA, Martinez-Ruiz A, Sancho-Rodriguez N, Martinez-Hernandez P, Noguera-Velasco JA. The real role of prediagnostic high-density lipoprotein cholesterol and the cancer risk: a concise review. Eur J Clin Invest. (2014) 44:103–14. doi: 10.1111/eci.12185
18. Gerber M, Saintot M. Prospective study of serum micronutrients and ovarian cancer. J Natl Cancer Inst. (1997) 89:581–2. doi: 10.1093/jnci/89.8.581
19. Qiu WL, Lu H, Qi YN, Wang XW. Dietary fat intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Oncotarget. (2016) 7:37390–406. doi: 10.18632/oncotarget.8940
20. Alireza S, Sakineh S-B, Mohammad P, Kurosh D. Dietary fat intake and risk of ovarian cancer: a systematic review and dose-response meta-analysis of observational studies. Nutr Cancer. (2019) 71:939–53. doi: 10.1080/01635581.2019.1595049
21. Hou R, Wu QJ, Gong TT, Jiang L. Dietary fat and fatty acid intake and epithelial ovarian cancer risk: evidence from epidemiological studies. Oncotarget. (2015) 6:43099–119. doi: 10.18632/oncotarget.5525
22. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology - A proposal for reporting. J Am Med Assoc. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
23. Zhang DY Xi YZ, Feng YL. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: a systematic review and meta-analysis of observational epidemiologic studies. Eur J Cancer Prev. (2021) 30:161–70. doi: 10.1097/CEJ.0000000000000597
24. Higgins JPT, Salley G. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 Cochrane Database of Systematic Reviews. The Cochrane Collaboration (2011). Available online at: http://www.cochrane-handbook.org
25. Wells GA, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses (2013). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
26. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
27. Matthias E, George DS, Martin S, Minder C. Bias in meta analysis detected by a simple, graphical test. Br Med J. (19970. 315:629–34. doi: 10.1136/bmj.315.7109.629
28. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
29. Rice MS, Poole EM, Willett WC, Tworoger SS. Adult dietary fat intake and ovarian cancer risk. Int J Cancer. (2020) 146:2756–72. doi: 10.1002/ijc.32635
30. Merritt MA, Riboli E, Weiderpass E, Tsilidis KK, Overvad K, Tjonneland A, et al. Dietary fat intake and risk of epithelial ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. (2014) 38:528–37. doi: 10.1016/j.canep.2014.07.011
31. Blank MM, Wentzensen N, Murphy MA, Hollenbeck A, Park Y. Dietary fat intake and risk of ovarian cancer in the NIH-AARP Diet and Health Study. Br J Cancer. (2012) 106:596–602. doi: 10.1038/bjc.2011.572
32. Gilsing AMJ, Weijenberg MP, Goldbohm RA, van den Brandt PA, Schouten LJ. Consumption of dietary fat and meat and risk of ovarian cancer in the Netherlands Cohort Study. Am J Clin Nutr. (2011) 93:118–26. doi: 10.3945/ajcn.2010.29888
33. Chang ET, Lee VS, Canchola AJ, Dalvi TB, Clarke CA, Reynolds P, et al. Dietary patterns and risk of ovarian cancer in the California Teachers Study Cohort. Nutr Cancer. (2008) 60:285–91. doi: 10.1080/01635580701733091
34. Kiani F, Knutsen S, Singh P, Ursin G, Fraser G. Dietary risk factors for ovarian cancer: the Adventist Health Study (United States). Cancer Causes Control. (2006) 17:137–46. doi: 10.1007/s10552-005-5383-z
35. Mommers M, Schouten LJ, Goldbohm RA, van den Brandt PA. Dairy consumption and ovarian cancer risk in the Netherlands Cohort Study on Diet and Cancer. Br J Cancer. (2006) 94:165–70. doi: 10.1038/sj.bjc.6602890
36. Bertone ER, Rosner BA, Hunter DJ, Stampfer MJ, Speizer FE, Colditz GA, et al. Dietary fat intake and ovarian cancer in a cohort of US women. Am J Epidemiol. (2002) 156:22–31. doi: 10.1093/aje/kwf008
37. Kushi LH, Mink PJ, Folsom AR, Anderson KE, Zheng W, Lazovich D, et al. Prospective study of diet and ovarian cancer. Am J Epidemiol. (1999) 149:21–31. doi: 10.1093/oxfordjournals.aje.a009723
38. Merritt MA, Cramer DW, Missmer SA, Vitonis AF, Titus LJ, Terry KL. Dietary fat intake and risk of epithelial ovarian cancer by tumour histology. Br J Cancer. (2014) 110:1392–401. doi: 10.1038/bjc.2014.16
39. Hu JF, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L, et al. Dietary transfatty acids and cancer risk. Eur J Cancer Prev. (2011) 20:530–8. doi: 10.1097/CEJ.0b013e328348fbfb
40. Chiaffarino F, Parazzini F, Bosetti C, Franceschi S, Talamini R, Canzonieri V, et al. Risk factors for ovarian cancer histotypes. Eur J Cancer. (2007) 43:1208–13. doi: 10.1016/j.ejca.2007.01.035
41. Zhang M, Yang ZY, Binns CW, Lee AH. Diet and ovarian cancer risk: a case-control study in China. Br J Cancer. (2002) 86:712–7. doi: 10.1038/sj.bjc.6600085
42. Pan SY, Ugnat AM, Mao Y, Wen SW, Johnson KC, Canadian Canc Registries E, et al. case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol Biomark Prev. (2004) 13:1521–7. doi: 10.1158/1055-9965.1521.13.9
43. McCann SE, Freudenheim JL, Marshall JR, Graham S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr. (2003) 133:1937–42. doi: 10.1093/jn/133.6.1937
44. Salazar-Martinez E, Lazcano-Ponce EC, Lira-Lira GG, Escudero-De los Rios P, Hernandez-Avila M. Nutritional determinants of epithelial ovarian cancer risk: a case-control study in Mexico. Oncology. (2002) 63:151–7. doi: 10.1159/000063814
45. Webb PM, Bain CJ, Purdie DM, Harvey PWJ, Green A. Milk consumption, galactose metabolism and ovarian cancer (Australia). Cancer Causes Control. (1998) 9:637–44. doi: 10.1023/A:1008891714412
46. Risch HA, Jain M, Marrett LD, Howe GR. Dietary-fat intake and risk of epithelial. ovarian-cancer. J Natl Cancer Inst. (1994) 86:1409–15. doi: 10.1093/jnci/86.18.1409
47. Tzonou A, Hsieh CC, Polychronopoulou A, Kaprinis G, Toupadaki N, Trichopoulou A, et al. A case-control study in Greece. Int J Cancer. (1993) 55:411–4. doi: 10.1002/ijc.2910550314
48. Shu XO, Gao YT, Yuan JM, Ziegler RG, Brinton LA. Dietary factors and epithelial ovarian-cancer. Br J Cancer. (1989) 59:92–6. doi: 10.1038/bjc.1989.18
49. Slattery ML, Schuman KL, West DW, French TK, Robison LM. Nutrient intake and ovarian-cancer. Am J Epidemiol. (1989) 130:497–502. doi: 10.1093/oxfordjournals.aje.a115363
50. Lavecchia C, Decarli A, Negri E, Parazzini F, Gentile A, Cecchetti G, et al. Ovarian-cancer. J Natl Cancer Inst. (1987) 79:663–9.
51. Cramer DW, Welch WR, Hutchison GB, Willett W, Scully RE. Dietary animal fat in relation to ovarian-cancer risk. Obstet Gynecol. (1984) 63:833–8.
52. Byers T, Marshall J, Graham S, Mettlin C, Swanson M. A case control study of dietary and nondietary factors in ovarian-cancer. J Natl Cancer Inst. (1983) 71:681–6.
53. Trabert B, Hathaway CA, Rice MS, Rimm EB, Sluss PM, Terry KL, et al. Ovarian cancer risk in relation to blood cholesterol and triglycerides. Cancer Epidemiol Biomark Prev. (2021) 30:2044–51. doi: 10.1158/1055-9965.EPI-21-0443
54. Shin DW, Choi YJ, Kim HS, Han K-D, Yoon H, Park YS, et al. Secondary breast, ovarian, and uterine cancers after colorectal cancer: a nationwide population-based cohort study in Korea. Dis Colon Rectum. (2018) 61:1250–7. doi: 10.1097/DCR.0000000000001203
55. Kabat GC, Kim MY, Chlebowski RT, Vitolins MZ, Wassertheil-Smoller S, Rohan TE. Serum lipids and risk of obesity-related cancers in postmenopausal women. Cancer Causes Control. (2018) 29:13–24. doi: 10.1007/s10552-017-0991-y
56. Wu MM, Chen HC, Chen CL, You SL, Cheng WF, Chen CA, et al. Prospective study of gynecological cancer risk in relation to adiposity factors: cumulative incidence and association with plasma adipokine levels. PLoS ONE. (2014) 9:11. doi: 10.1371/journal.pone.0104630
57. Strohmaier S, Edlinger M, Manjer J, Stocks T, Bjorge T, Borena W, et al. Total serum cholesterol and cancer incidence in the metabolic syndrome and cancer project (Me-Can). PLoS ONE. (2013) 8:8. doi: 10.1371/journal.pone.0054242
58. Melvin JC, Seth D, Holmberg L, Garmo H, Hammar N, Jungner I, et al. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS Study. Cancer Epidemiol Biomark Prev. (2012) 21:1381–4. doi: 10.1158/1055-9965.EPI-12-0188
59. Bjorge T, Lukanova A, Tretli S, Manjer J, Ulmer H, Stocks T, et al. Metabolic risk factors and ovarian cancer in the Metabolic Syndrome and Cancer project. Int J Epidemiol. (2011) 40:1667–77. doi: 10.1093/ije/dyr130
60. Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjorge T, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control. (2011) 22:291–9. doi: 10.1007/s10552-010-9697-0
61. Helzlsouer KJ, Alberg AJ, Norkus EP, Morris JS, Hoffman SC, Comstock GW. Prospective study of serum micronutrients and ovarian cancer. J Natl Cancer Inst. (1996) 88:32–7. doi: 10.1093/jnci/88.1.32
62. Staples JN, Peres LC, Camacho F, Alberg AJ, Bandera EV, Barnholtz-Sloan J, et al. Cardiometabolic comorbidities and epithelial ovarian cancer risk among African-American women in the African-American Cancer Epidemiology Study (AACES). Gynecol Oncol. (2020) 158:123–9. doi: 10.1016/j.ygyno.2020.04.700
63. Michels KA, McNeel TS, Trabert B. Metabolic syndrome and risk of ovarian and fallopian tube cancer in the United States: an analysis of linked SEER-Medicare data. Gynecol Oncol. (2019) 155:294–300. doi: 10.1016/j.ygyno.2019.08.032
64. Chen Y, Zhang L, Liu W, Wang K. Case-control study of metabolic syndrome and ovarian cancer in Chinese population. Nutr Metab. (2017) 14:21. doi: 10.1186/s12986-017-0176-4
65. Parazzini F, Moroni S, LaVecchia C, Negri E, dalPino D, Bolis G. Ovarian cancer risk and history of selected medical conditions linked with female hormones. Eur J Cancer. (1997) 33:1634–7. doi: 10.1016/S0959-8049(97)00011-7
66. Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol. (2011) 123:200–4. doi: 10.1016/j.ygyno.2011.06.038
67. Zeleznik OA, Eliassen AH, Kraft P, Poole EM, Rosner BA, Jeanfavre S, et al. A prospective analysis of circulating plasma metabolites associated with ovarian cancer risk. Cancer Res. (2020) 80:1357–67. doi: 10.1158/0008-5472.CAN-19-2567
68. Chen C-W, Buj R, Dahl ES, Leon KE, Aird KM. ATM inhibition synergizes with fenofibrate in high grade serous ovarian cancer cells. Heliyon. (2020) 6:e05097. doi: 10.1101/2020.05.29.123919
69. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. Br Med J. (2015) 351:h3978. doi: 10.1136/bmj.h3978
70. Murtaugh MA, Ma KN, Caan BJ, Sweeney C, Wolff R, Samowitz WS, et al. Interactions of peroxisome proliferator-activated receptor gamma and diet in etiology of colorectal cancer. Cancer Epidemiol Biomark Prev. (2005) 14:1224–9. doi: 10.1158/1055-9965.EPI-04-0681
71. Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. In: Berstein LM, Santen RJ, editors. Innovative Endocrinology of Cancer. Vol. 630 (2008). p. 148–65.
72. Goebel A, Rauner M, Hofbauer LC, Rachner TD. Cholesterol and beyond - The role of the mevalonate pathway in cancer biology. Biochim Biophys Acta. (2020) 1873:188351. doi: 10.1016/j.bbcan.2020.188351
73. Huang PX, Nedelcu D, Watanabe M, Jao C, Kim Y, Liu J, Salic A. Cellular cholesterol directly activates smoothened in hedgehog signaling. Cell. (2016) 166:1176. doi: 10.1016/j.cell.2016.08.003
74. Riobo NA. Cholesterol and its derivatives in Sonic Hedgehog signaling and cancer. Curr Opin Pharmacol. (2012) 12:736–41. doi: 10.1016/j.coph.2012.07.002
75. Parmar K, Kochupurakkal BS, Lazaro J-B, Wang ZC, Palakurthi S, Kirschmeier PT, et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin Cancer Res. (2019) 25:6127–40. doi: 10.1158/1078-0432.CCR-19-0448
76. Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J, et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. (2020) 588:693. doi: 10.1038/s41586-020-2911-7
77. Musazadeh V, Anilou MM, Vajdi M, Karimi A, Ahrabi SS, Dehghan P. Effects of synbiotics supplementation on anthropometric and lipid profile parameters: Finding from an umbrella meta-analysis. Front Nutr. (2023) 10:1121541. doi: 10.3389/fnut.2023.1121541
78. Zarezadeh M, Musazadeh V, Faghfouri AH, Roshanravan N, Dehghan P. Probiotics act as a potent intervention in improving lipid profile: an umbrella systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2022) 63:145–58. doi: 10.1080/10408398.2021.2004578
79. Sellem L, Eichelmann F, Jackson KG, Wittenbecher C, Schulze MB, Lovegrove JA. Replacement of dietary saturated with unsaturated fatty acids is associated with beneficial effects on lipidome metabolites: a secondary analysis of a randomized trial. Am J Clin Nutr. (2023) 117:1248–61. doi: 10.1016/j.ajcnut.2023.03.024
Keywords: ovarian cancer, dietary fat, serum lipid levels, meta-analysis, observational study
Citation: Zhang X, Ding H-M, Deng L-F, Chen G-C, Li J, He Z-Y, Fu L, Li J-F, Jiang F, Zhang Z-L and Li B-Y (2023) Dietary fats and serum lipids in relation to the risk of ovarian cancer: a meta-analysis of observational studies. Front. Nutr. 10:1153986. doi: 10.3389/fnut.2023.1153986
Received: 24 March 2023; Accepted: 24 August 2023;
Published: 14 September 2023.
Edited by:
Gustavo Cernera, University of Naples Federico II, ItalyReviewed by:
Vali Musazadeh, Tabriz University of Medical Sciences, IranZhendong Mei, Brigham and Women's Hospital, United States
Copyright © 2023 Zhang, Ding, Deng, Chen, Li, He, Fu, Li, Jiang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing-Yan Li, YmluZ3lhbmxpQHN1ZGEuZWR1LmNu
†These authors have contributed equally to this work
 Xu Zhang
Xu Zhang Hong-Mei Ding
Hong-Mei Ding Li-Feng Deng3†
Li-Feng Deng3† Guo-Chong Chen
Guo-Chong Chen Jie Li
Jie Li Ze-Yin He
Ze-Yin He Li Fu
Li Fu Jia-Fu Li
Jia-Fu Li Fei Jiang
Fei Jiang Zeng-Li Zhang
Zeng-Li Zhang Bing-Yan Li
Bing-Yan Li