
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 30 June 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1145762
 Shakila Ansari1
Shakila Ansari1 Noushin Mohammadifard2
Noushin Mohammadifard2 Fahimeh Haghighatdoost3*
Fahimeh Haghighatdoost3* Ehsan Zarepur2,4
Ehsan Zarepur2,4 Shirin Mahmoudi3
Shirin Mahmoudi3 Fatemeh Nouri3
Fatemeh Nouri3 Fereidoon Nouhi5,6
Fereidoon Nouhi5,6 Hassan Alikhasi7
Hassan Alikhasi7 Fariborz Sharifianjazi8
Fariborz Sharifianjazi8 Ketevan Tavamaishvili9
Ketevan Tavamaishvili9 Shahin Shirani10
Shahin Shirani10 Tooba Kazemi11
Tooba Kazemi11 Nahid Azdaki12
Nahid Azdaki12 Nahid Salehi13
Nahid Salehi13 Masoud Lotfizadeh14
Masoud Lotfizadeh14 Kamal Solati15
Kamal Solati15 Samad Ghaffari16
Samad Ghaffari16 Elmira Javanmardi17
Elmira Javanmardi17 Arsalan Salari18
Arsalan Salari18 Mostafa Dehghani19
Mostafa Dehghani19 Mostafa Cheraghi19
Mostafa Cheraghi19 Ahmadreza Assareh20
Ahmadreza Assareh20 Habib Haybar20
Habib Haybar20 Seyedeh M. Namayandeh21,22
Seyedeh M. Namayandeh21,22 Reza Madadi23
Reza Madadi23 Nizal Sarrafzadegan3,24
Nizal Sarrafzadegan3,24Background: Ultra-processed foods (UPF) consumption may affect the risk of PCAD through affecting cardio metabolic risk factors. This study aimed to evaluate the association between UPFs consumption and premature coronary artery disease (PCAD).
Methods: A case–control study was conducted on 2,354 Iranian adults (≥ 19 years). Dietary intake was assessed using a validated 110-item food frequency questionnaire (FFQ) and foods were classified based on the NOVA system, which groups all foods according to the nature, extent and purposes of the industrial processes they undergo. PCAD was defined as having an stenosis of at least single coronary artery equal and above 75% or left main coronary of equal or more than 50% in women less than 70 and men less than 60 years, determined by angiography. The odds of PCAD across the tertiles of UPFs consumption were assessed by binary logistic regression.
Results: After adjustment for potential confounders, participants in the top tertile of UPFs were twice as likely to have PCAD compared with those in the bottom tertile (OR: 2.52; 95% CI: 1.97–3.23). Moreover, those in the highest tertile of the UPFs consumption had more than two times higher risk for having severe PCAD than those in the first tertile (OR: 2.64; 95% CI: 2.16–3.22). In addition, there was a significant upward trend in PCAD risk and PCAD severity as tertiles increased (P-trend < 0.001 for all models).
Conclusion: Higher consumption of UPFs was related to increased risk of PCAD and higher chance of having severe PCAD in Iranian adults. Although, future cohort studies are needed to confirm the results of this study, these findings indicated the necessity of reducing UPFs intake.
Coronary artery disease (CAD) is the most frequent cardiac complication of chronic diseases in developing countries (1). In general, CAD occurs in old ages, but some studies have reported that almost 4–10% of the patients are young (2). Premature coronary artery disease (PCAD) affects women younger than 45 and men younger than 55 years, but these cut-offs vary from 45 to 70 years in different studies (3–5). CAD causes around 350,000 deaths per year globally (6) and accounts for approximately 50% of all deaths each year in Iran (7). A healthy eating pattern may reduce the risk of heart disease by 50% (8), which is mediated by the effect of dietary habits on multiple risk factors of cardiovascular disease (CVD). Ultra-processed foods (UPF) consumption is one of the important factors which considerably affect diet quality and nutrients intake (9) and influence the risk of various cardio metabolic risk factors (10, 11).
There are various food classifications based on the level of processing amongst them the NOVA system is the most common (12). Accordingly, UPFs group refers to industrial manufactured foods like, breakfast cereals, biscuits, fast foods, sodas, ice-cream, and frozen meals (13). Most of the UPFs contain modified starch, sodium, hydrogenated oils, colors and classes of additives which all lead to production of compounds with potential cardio-metabolic effects (14). Phosphate which is widely used in UPFs in order to enhance appearance, and shelf life, can lead to atherosclerosis by promoting vascular calcification (15). While UPFs are high dense calorie products, they are poor in nutrients (9). Providing a major daily energy intake from UPFs deprives the intake of fresh and less processed foods, which indirectly endangers health (16). Finally, UPFs underlie obesity (11), hypertension, dyslipidemia and hyperglycemia (17) which all are risk factors for CVD (18). The NutriNet-Santé cohort study on French adult found that every 10% increment in UPFs consumption was linked with 12% increase in the risk of CVD (19). Consistently, the Moli-sani study on a large sample of the Italian adult population revealed that higher amount of dietary UPFs increased the risk of CVD and all-cause mortality (20). In contrast, a Brazilian study found that the higher UPFs intake was in line with the decreased odds of the simultaneous presence of CAD, peripheral arterial diseases, and stroke (21).
Due to the fact that UPFs have a long shelf life, are tasty, ready to use, and most importantly are not expensive (22) the tendency toward UPFs consumption is increasing worldwide (23). The individual consumption data revealed that UPFs consumption accounted for 60% and up to 36% of daily energy intake in the United State and European countries, respectively, (20). In contrast, due to the low consumption of UPFs reported in previous studies in Iran [8–20%; (9, 11) of daily energy intake], it is possible to obtain different relations compared to previous studies.
Although there are several studies on the relationship between UPFs and CVD, so far, no study has been done specifically on PCAD. Also, due to differences among lifestyle, dietary habits and the average consumption of UPFs in each population, there may be contradictions between the results of different studies. Therefore, this study aims to investigate the association between UPFs consumption and the PCAD in a multi-centric study of Iranians.
This case–control study was conducted using the data from IRAN Premature Coronary Artery Disease study (IPAD) (24). IPAD is a multi-center case-control study with the primary objective of determining the frequency of PCAD and its related risk factors in different ethnicities of Iranians including Fars, Azari, Kurd, Arab, Lor, Gilak, Balouch, Turkman, Qashqaei, and Bakhtiari. The inclusion criteria were females less than 70 and males less than 60 years who underwent coronary an giography and had an stenosis of at least single coronary artery equal and above 75% or left main coronary of equal or more than 50% in the cases and normal coronary artery in control group. Previous history of documented CAD, such as coronary artery bypass surgery, balloon angioplasty, or percutaneous coronary intervention was considered exclusion criteria. A total of 2,351 eligible patients were selected via convenient sampling from reference hospitals in 16 cities of Iran. This study was reviewed and approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1396.2.055). We obtained a written informed consent from all patients and the Declaration of Helsinki was considered.
Trained healthcare professionals completed questionnaires and collected data regarding demographics characteristics, personal and family history of illnesses and medications. A demographic questionnaire was used to collect marital status and level of education. The height of patients was measured by a wall-fixed stadiometer with a sensitivity of 0.1 cm while participants were barefoot, and weight was obtained while participants were minimally clothing and recorded to the nearest 0.1 kg. Body mass index (BMI) was calculated by dividing weight in kilogram by height squared in meter. Waist circumference (WC) was measured with a tape measure between the lowest rib and iliac crest to the nearest 0.1 cm (25). Systolic and diastolic blood pressures (SBP and DBP) were measured by a digital sphygmomanometer (BC 08, Beurer, Germany) after being seated for at least 5 min. Blood pressure was measured twice per person and the average value was recorded.
Data regarding usual dietary intakes of participants over the past year were obtained via a validated and reliable 110-item semi-quantitative food frequency questionnaire (FFQ) (26). The FFQ provided the frequency in ten-option categories (‘seldom/never, once per month, 2–3 per month, once per week, 2–3 per week, 4–6 per week, 1 per day, 2–3 per day, 4–5 per day, and 6 or more per day). In addition, they were asked to record the portion sizes of each food and beverage items. Using household measurements, the frequency was converted to daily intake and multiplied by the portion sizes were changed to gram. Portion sizes of foods and beverages were also obtained. Subsequently, the daily intake of each nutrient, UPFs and daily energy intake were determined by using Iranian Food Consumption Processor and food composition table (RE) (27, 28).
Ultra-processed foods were determined according to the NOVA classification system (8). NOVA classifies foods into four groups based on the amount of processing done on the foods. The first group consists of unprocessed or minimally processed foods like vegetables and fruits, the second group refers to processed culinary ingredients, like sugar, and salt, the third group includes processed foods, like canned foods, and cheeses, and the last group are UPFs which are industrial compounds without or with the least amount of whole foods and with notable amounts of colors, flavors and preservatives. Accordingly, foods with specific formulations of ingredients which are produced by a series of industrial processes were regarded as ultra-processed foods in our study These foods include pre-prepared dishes, industrial packaged breads, breakfast cereals, confectionery, biscuits, pastries, buns, and cakes, fast food dishes, reconstituted meat products, sweetened milk-based products chips, crackers, and other salty snacks, margarine and other spreads, sauces, dressing and gravies, industrial desserts, soft drinks, sweetened fruit juices, and drinks (29). Pre-prepared pasta, stuffed pasta, frozen (mixed) dishes, and alcoholic beverages (wine, beer, spirits) were not included in the UPFs list of this study since their consumption is not common in the Iranian diet and do not substantially contribute to daily energy and nutrient intake.
Blood samples were collected after a fasting period of 12 h to measure fasting blood sugar (FBS), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Serum lipids and glucose were measured using the enzymatic method, based on calorimetry, utilizing commercial kits (Pars Azmoun, Iran) with an automatic device (Selecta E, Vitalab, Netherlands). Based on the definition of the National Cholesterol Education Program (NCEP) TG ≥ 150 mg/dl, TC ≥ 240 mg/dl, LDL ≥ 160 mg/dl, and HDL-C < 40 in men and < 50 mg/dl in women, were considered as hypertriglyceridemia, hypercholesterolemia, raised LDL-C and low HDL-C, respectively (30).
All analyses were performed using SPSS Statistics 26.0 software (IBM, Armonk, NY, United States). Two-tailed p-values were reported and statistical significance was considered p < 0.05. Kolmogorov Smirnov test was used to explore the normality of the distribution for quantitative variables. The tertiles of UPFs were determined based on the overall range of UPFs consumption in the whole population. General characteristics of participants across the tertiles of UPFs were compared by one-way analysis of variance (ANOVA) for continuous variables such as BMI, age and WC and Chi-square test for categorical variables. The independent-sample Student t-test was used to distinguish means of continuous variables between cases and controls. For comparing the distribution of participants in categorical variable levels between cases and controls, the chi-square test was run. The association between UPFs consumption and PCAD was assessed by binary logistic regression in different models. The crude model remained unadjusted. Model 1 was adjusted for age, sex, and daily energy intake. Model 2 was additionally adjusted for smoking, level of education, different ethnicities and family history of CVD. Model 3 was additionally adjusted for the Mediterranean dietary score. BMI in model 4, and WC in model 5 were additionally and separately adjusted as mediators. Due to the small sample size of some subgroups of ethnicities, the association between UPFs consumption and PCAD in different ethnicities was assessed by binary logistic regression in just one adjusted model for age, sex, and daily energy intake.
General characteristics of cases and controls across the tertiles of UPFs consumption are presented in Table 1. On average, the contribution of UPFs to daily energy intake was 6.55% kcal/day (SD: 5.71). Ceases were older, more likely to have family history of CVD (p-value < 0·001), hypertriglyceridemia (p-value < 0·001) and hypertension (p-value: 0.308). The average of FBS and SBP levels were significantly higher in cases than that of in controls (p-value < 0·001 and 0.044 respectively). In comparison to controls, cases showed lower LDL-C, HDL-C and TC mean values (p-value < 0·001). Assessing participants’ characteristics across the tertiles of UPFs consumption revealed that individuals in the top tertile of UPFs consumption were younger (p-value: < 0·001), more educated (p-value: < 0·001) more frequently current smokers (p-value: < 0·001) and had lower TC levels (p-value: 0.033).
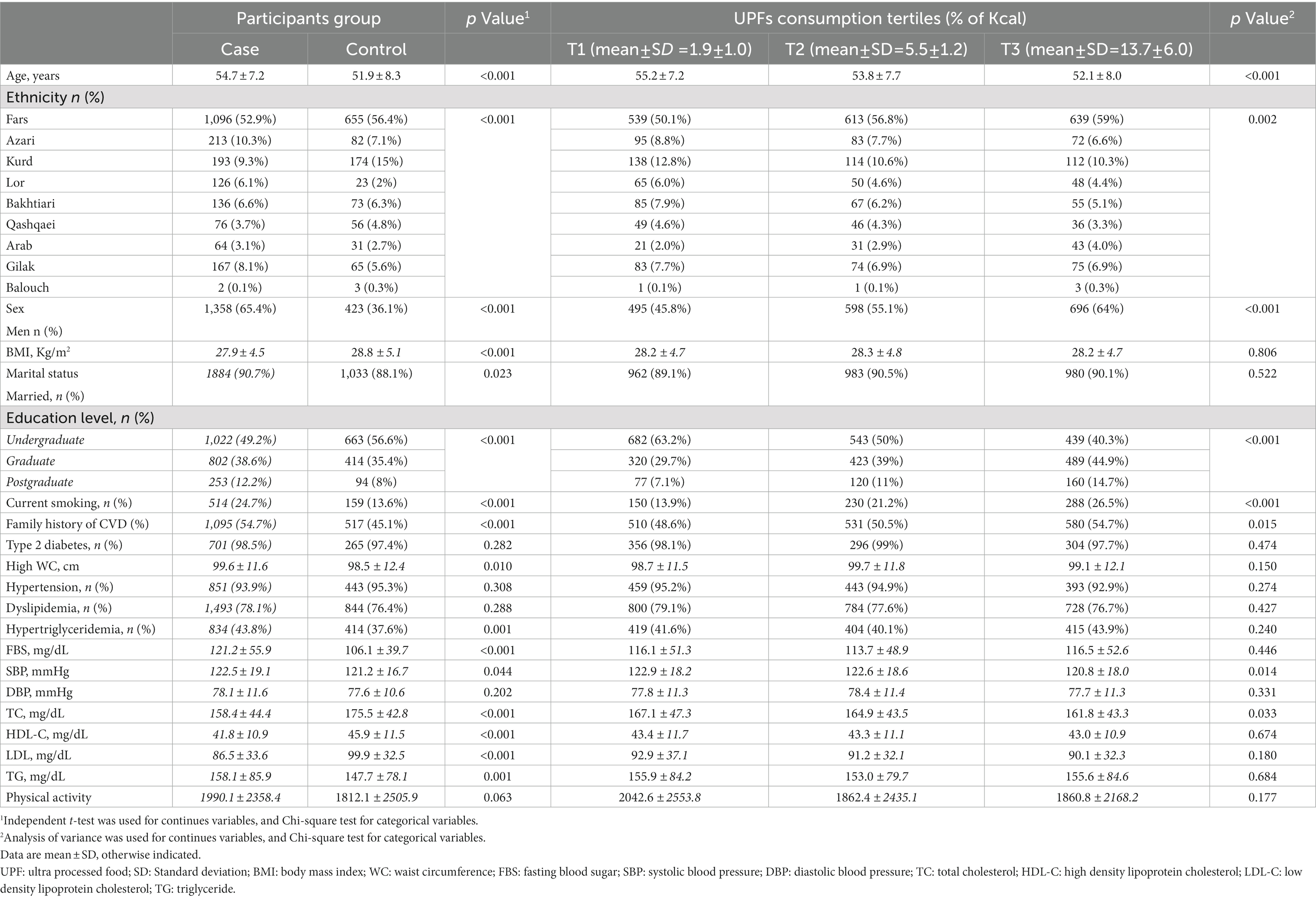
Table 1. General characteristics of participants based on case and control and across tertiles of ultra-processed foods consumption.
Dietary intakes of cases and controls across the tertiles of UPFs consumption are indicated in Table 2. Cases had higher intake of saturated fatty acids (SFAs; p-value: < 0·001), cholesterol (p-value: < 0·001), dairy products (p-value: < 0·001), cake and cookies, soft drinks, sweets, processed meats and mayonnaise and dressings (p-value: < 0·001) than that of in control group. Total fat (p-value: < 0·001), protein (p-value: < 0·001), Fruits and Vegetables (p-value: < 0·001), monounsaturated fatty acids (MUFAs; p-value: 0.024) and polyunsaturated fatty acids (PUFAs; p-value: < 0·001) dietary intakes of controls were significantly higher than cases. Evaluating dietary intakes of participants across the tertiles of UPFs consumption showed that individuals in the highest tertile of UPFs consumption had higher intake of total energy, SFAs, PUFAs (p-value: 0.012), cholesterol, cake and cookies, soft drinks, sweets, processed meats and mayonnaise and dressings (p for all < 0·001) than participants in the first tertile. Bread, sodium (sodium content of table salt and cooking salt were not measured), protein and fiber intake of those in the third tertile of UPFs consumption was significantly lower than whom in the first tertile (p-value: < 0·001 for all).
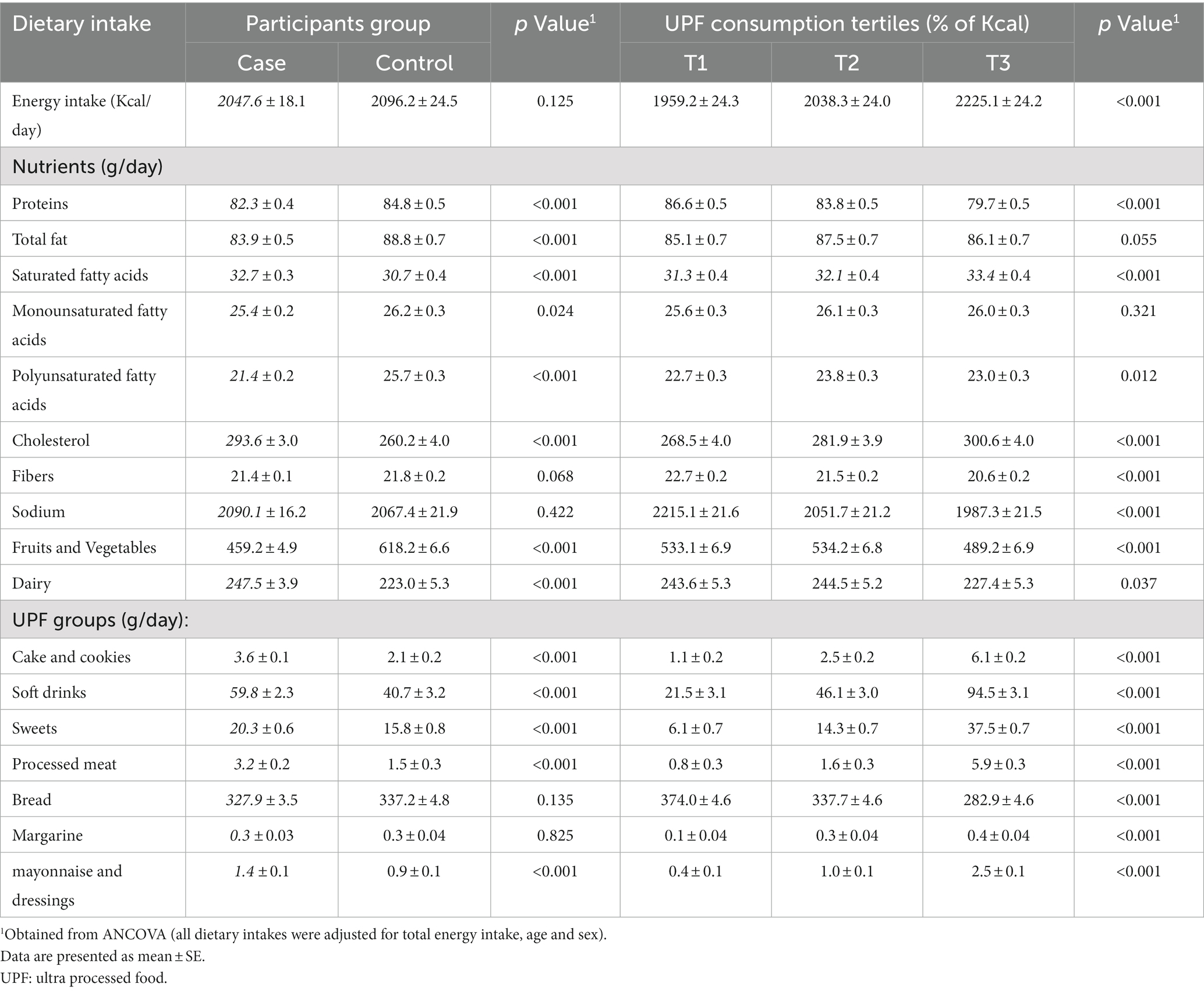
Table 2. Dietary intakes of participants based on case and control and across tertiles of ultra-processed food (UPFs) consumption.
Multivariable-adjusted ORs (95% CI) for PCAD across the tertiles of UPFs consumption are provided in Table 3. Crud model showed that those in the highest tertile of the UPFs consumption had higher chance of PCAD in comparison with those in the lowest tertile (OR: 2.14; 95% CI: 1.78–2.57). Adjustment for confounding variables strengthened this relationship (OR: 2.52; 95% CI: 1.97–3.23). In addition, there was a significant upward trend in PCAD risk as tertiles of UPFs consumption increased (p trend < 0.001 for all models).
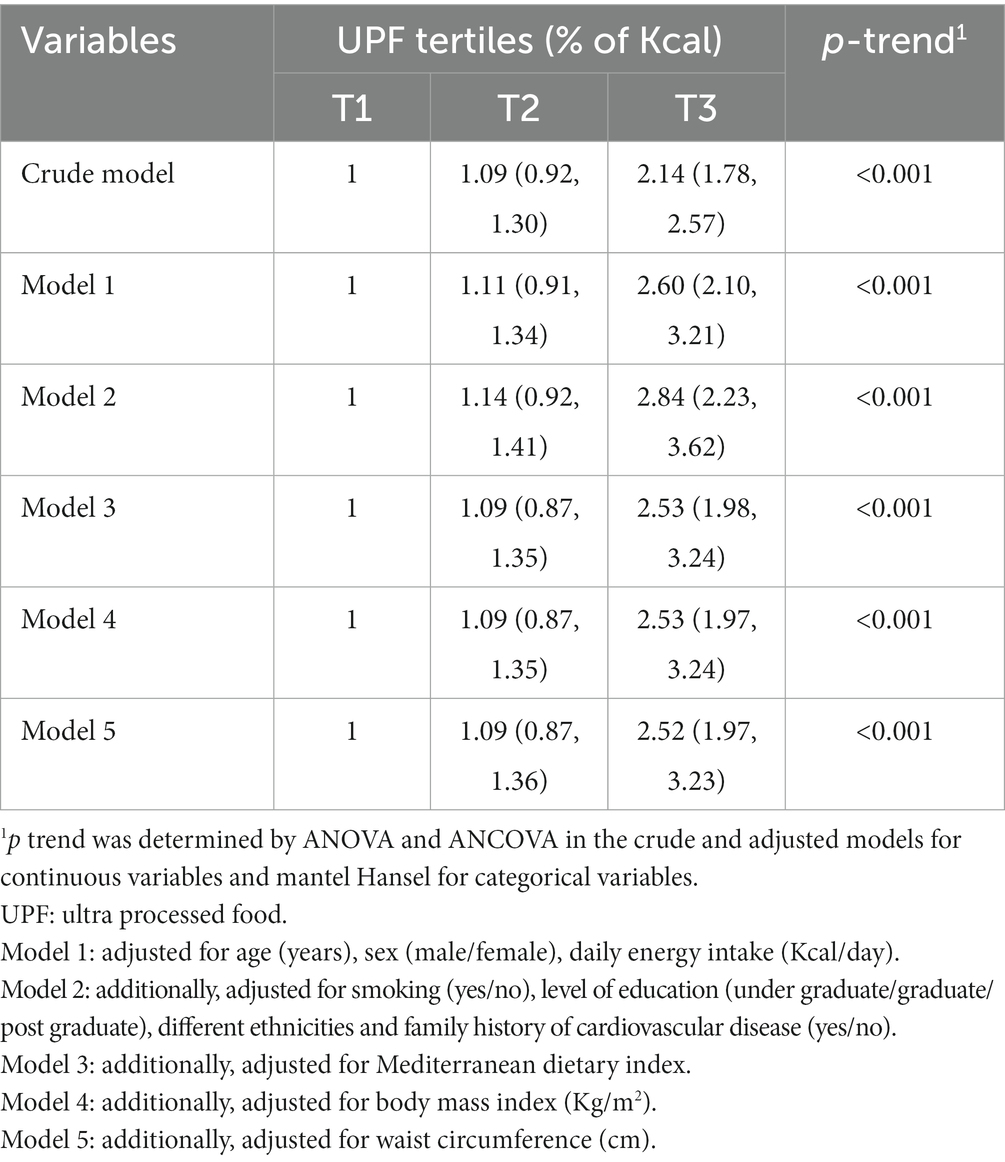
Table 3. ORs (95%CI) of premature coronary artery disease across tertiles of ultra-processed foods consumption.
Multivariable-adjusted ORs (95% CI) for PCAD severity across the tertiles of UPFs consumption are provided in Table 4. Crud model showed that those in the highest tertile of the UPFs consumption had approximately two times higher chance for having severe PCAD than those in the first tertile (OR: 2.34; 95% CI: 1.97–2.74). In the fully adjusted model, we found a stronger relationship between UPFs consumption and PCAD severity (OR: 2.64; 95% CI: 2.16–3.22). In addition, there was a significant upward trend in odds of PCAD severity, as tertiles of UPFs consumption increased (p trend < 0.001 for all models).
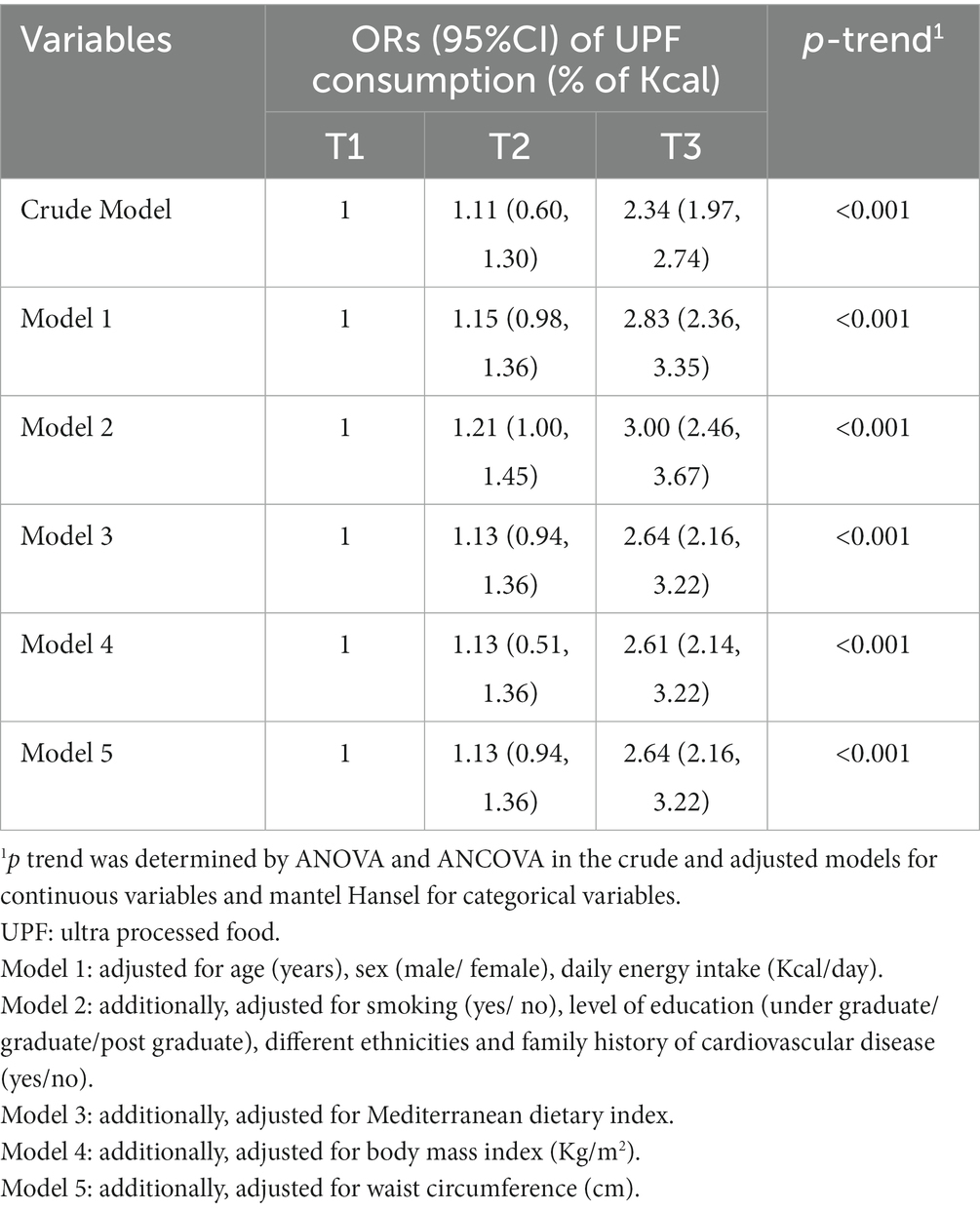
Table 4. Odds ratio (OR) (95%CI) of premature coronary artery disease severity across tertiles of ultra-processed foods consumption.
Multivariable-adjusted ORs (95% CI) for PCAD severity in different ethnicity across the tertiles of UPFs consumption are provided in Table 5. We found that in the highest tertile of the UPFs consumption, participants of three ethnicities (1-Balouch and Arab and Qashqaei, 2-Fars and 3-Kord) had statistically significant higher chance of PCAD than those in the lowest tertile (OR: 2.56; 95% CI: 1.18–5.57, OR: 2.78; 95% CI: 2.08–3.71 and OR: 6.12; 95% CI: 3.19–11.75, respectively).
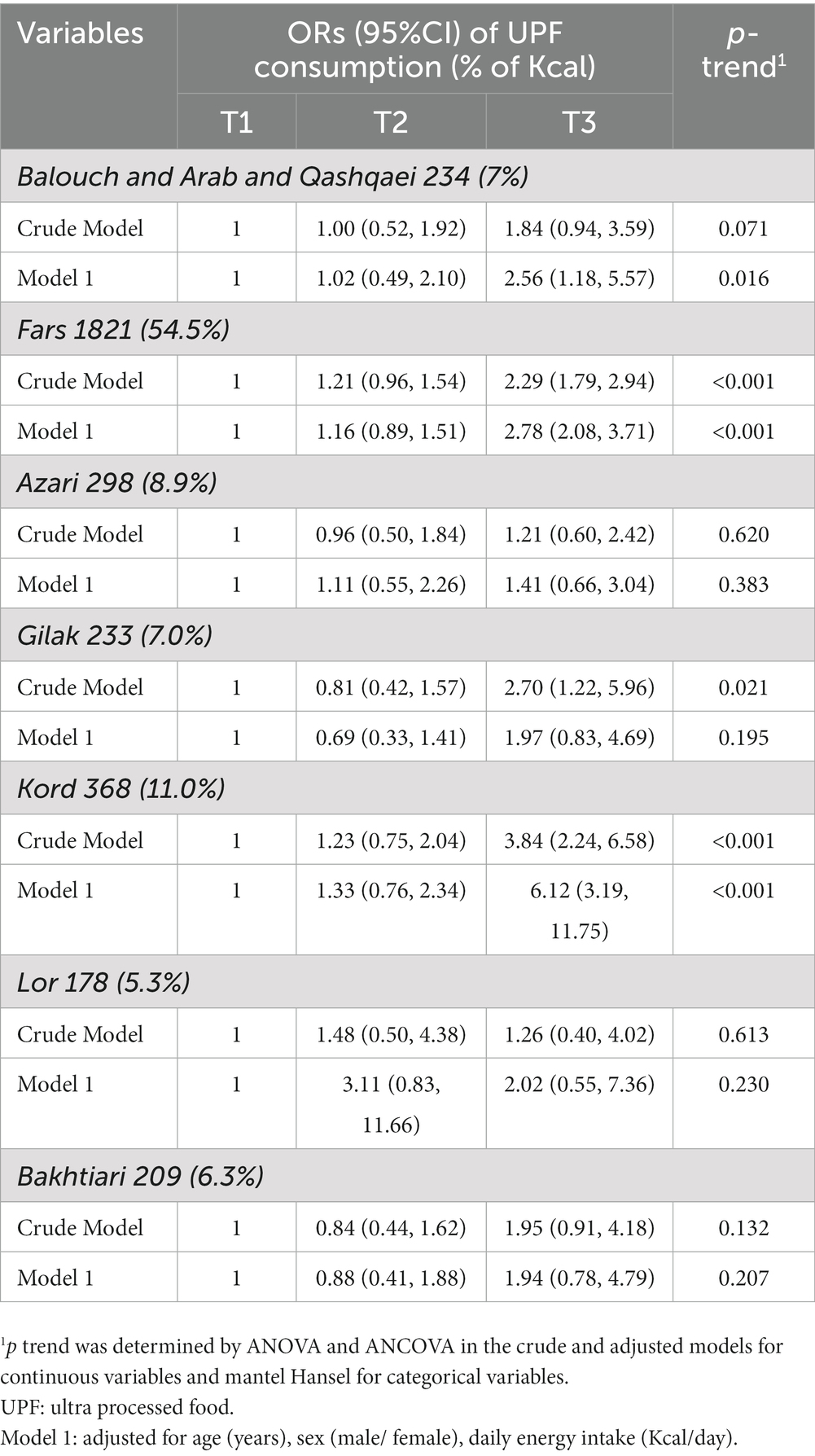
Table 5. ORs (95%CI) of premature coronary artery disease in different ethnicity across tertiles of ultra-processed foods consumption.
In this multi-centric case-control study, a significant direct relationship was obtained between UPFs consumption and the risk of PCAD. This is the first study that examined the relation between UPFs and PCAD in a population with different ethnicities. We found 2.3 times higher risk of PCAD in the 3rd tertile of UPFs consumption. Although the share of UPFs in daily energy intake in this population was substantially lower than Western populations [6.5 vs. 60% in United Kingdom (31) and in the United States (32)], our results were similar with earlier ones in the magnitude. For instance, the Aragon Workers’ Health Study on middle-aged Spanish worker’s demonstrated that approximately 500 g/day of UPFs consumption was associated with a 2-fold higher prevalence of coronary atherosclerosis compared to the consumption of only 100 g/day (16). In addition, a Brazilian study by da Silva et al. (21) declared that higher UPF consumption was related to increased risk of peripheral arterial disease in the whole sample and women, but this association was not seen in men. A cohort study on nearly 155,000 American adults aged 55–74 also revealed that participants in the highest quintile of UPFs consumption had 68% higher risk of heart disease than those in the first quintile (33). A recent meta-analysis on prospective cohort studies also revealed that highest UPF consumption was associated with a 29% increase in the risk of CVD.
Global interest for consuming UPFs is rapidly increasing. UPFs are high-calorie dense products which are usually consumed in large portion sizes. Due to this fact, higher consumption of UPFs is associated with lower intake of fruits, vegetables, olive oil, nuts and legumes which are high in fiber, PUFAs, anti-oxidants and anti-inflammatory properties which all are cardio-protective (34, 35). Similarly, refined sugars which are numerously used in UPFs products, specifically sugar-sweetened beverages, may cause a delay in satiety signal (36). This, in turn, leads to excessive calorie, fats and sugar intake and consequently excess weight, which are strong risk factor for CVD (37). Beslay et al. (38) declared that each 10% increase in UPFs consumption in 110,260 adult participants from the French prospective population-based NutriNet-Santé cohort study, was associated with an 11% and a 10% increase in the risk of overweight and obesity, respectively. In addition an observational study in Iranian adults declared that men with higher UPFs consumption were twice as likely to be overweight compared with men with the lowest intake, but this association was not observed in women (11).
High amount of salt (39), trans fatty acids (TFAs), SFAs (40) and low amount of fiber of UPFs products might be other contributors to CVD in UPFs. Processed meats, cakes and cereals, crackers and pre-prepared foods contain high amount of salt, which are linked to hypertension, endothelial damages and CVD (41). In a large Spanish cohort study, university educated middle aged in the top tertile of UPFs consumption had 21% greater risk of hypertension than those in the bottom tertile (39). In addition, hydrogenated vegetable oils used in the production of UPFs (40) raises LDL-C and Lp(a) lipoprotein, reduces HDL-C, and increases the ratio of total cholesterol to HDL-C, which altogether increase the risk of CVD (42). Beside this, low amount of fiber and food additives alter the composition of gut microbiota, leading to inflammatory disease, weight gain and CVD (43).
Beyond the nutritional composition of UPFs, other harmful components and mechanisms may play a role. Additives, stabilizers and colors used in UPFs are special concerns for CVD. Phosphate, a known UPFs additive, is usually used in order to enhance appearance, and shelf life (15). Inorganic phosphate of UPFs are absorbed by approximately 90% in gastro intestinal tract (44) which causes calcium deposition in blood vessels at a faster rate (15). The production of acrylamide and acrolein, two compounds which produced during heat treated procedures, have been related to CVD risk (45, 46). Plastic packaging of UPFs products is also a risk factor. Bisphenol A, an industrial substance used in plastic packaging, plays a role in CVD process (47).
This study comes with some limitations. Due to the nature of case–control study, establishing a causal relationship between UPFs and PCAD was not possible. In addition, in the case of using FFQ, recall bias and misclassification of individuals across tertiles of the UPFs are likely. This study also presents important strengths. First, this is the first study which examined the relationship between UPFs and PCAD. Second, using a relatively large sample size from different parts of the country with multiple ethnicities increases the external validity of our findings and therefore our results can be generalized to different populations of Iranians. Third, using the NOVA food classification which is a valid tool for nutrition epidemiologic studies.
In conclusion, we found that higher consumption of UPFs was related to increased risk of PCAD and higher chance for having severe PCAD in different ethnicities of Iranian adults. Although, future cohort studies are warranted to confirm the results of this study, our findings can increase people’s awareness about the risk of UPFs consumption on cardiovascular health and the necessity to reduce UPFs intake.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Ethics Committee in Isfahan, Iran. The patients/participants provided their written informed consent to participate in this study.
ShA and FH contributed to the concept. FaN contributed to data entry, managing, and cleaning. KT, NM, and FSh contributed to data interpreting. ShA, FH, and NM contributed to manuscript drafting. NM and NiS contributed to study design. SM analyzed the data. EZ, SM, FeN, HA, TK, NA, NS, KS, ML, SG, EJ, AS, MD, MC, AA, HH, SN, SS, ShSh, and RM contributed to the data collection. All authors contributed to the article and approved the submitted version.
This study has been funded by the Research and Technology Department, Iran Ministry of Health and Medical Education and the Iranian Network of Cardiovascular Research (296055).
The authors would like to thank the Isfahan Cardiovascular Research Institute and Isfahan University of Medical Sciences for their support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
UPF, ultra processed food; PCAD, premature coronary artery disease; CVD, cardio vascular disease; FFQ, food frequency questioner; FBS, fasting blood sugar; TG, triglyceride; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; MUFA, mono unsaturated fatty acids; PUFA, poly unsaturated fatty acids; SFA, saturated fatty acid; TFA, trans fatty acid; SBP, systolic blood pressure; DBP, diastolic blood pressure.
1. Poorzand, H, Tsarouhas, K, Hozhabrossadati, SA, Khorrampazhouh, N, Bondarsahebi, Y, Bacopoulou, F, et al. Risk factors of premature coronary artery disease in Iran: a systematic review and meta-analysis. Eur J Clin Investig. (2019) 49:e13124. doi: 10.1111/eci.13124
2. Mohammad, AM, Jehangeer, HI, and Shaikhow, SK. Prevalence and risk factors of premature coronary artery disease in patients undergoing coronary angiography in Kurdistan, Iraq. Cardiovasc Disord. (2015) 15:1–6. doi: 10.1186/s12872-015-0145-7
3. Achari, V, and Thakur, A. Association of major modifiable risk factors among patients with coronary artery disease–a retrospective analysis. J Assoc Physicians India. (2004) 52:103–8.
4. Khot, UN, Khot, MB, Bajzer, CT, Sapp, SK, Ohman, EM, Brener, SJ, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. (2003) 290:898–904. doi: 10.1001/jama.290.7.898
5. Anderson, JL, Horne, BD, Camp, NJ, Muhlestein, JB, Hopkins, PN, Cannon-Albright, LA, et al. Joint effects of common genetic variants from multiple genes and pathways on the risk of premature coronary artery disease. Am Heart J. (2010) 160:250–6.e3. doi: 10.1016/j.ahj.2010.05.031
6. Benjamin, EJ, Muntner, P, Alonso, A, Bittencourt, MS, Callaway, CW, Carson, AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–e528. doi: 10.1161/CIR.0000000000000659
7. Hatmi, Z, Tahvildari, S, Gafarzadeh Motlag, A, and Sabouri, KA. Prevalence of coronary artery disease risk factors in Iran: a population based survey. BMC Cardiovasc Disord. (2007) 7:1–5. doi: 10.1186/1471-2261-7-32
8. Monteiro, CA, Cannon, G, Moubarac, J-C, Levy, RB, Louzada, MLC, and Jaime, PC. The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. (2018) 21:5–17. doi: 10.1017/S1368980017000234
9. Haghighatdoost, F, Hajihashemi, P, Mohammadifard, N, Najafi, F, Farshidi, H, Lotfizadeh, M, et al. Association between ultra-processed foods consumption and micronutrient intake and diet quality in Iranian adults: a multicentric study. Public Health Nutr. (2022) 26:1–26. doi: 10.1017/S1368980022002038
10. Monteiro, CA, Cannon, G, Lawrence, M, Costa Louzada, M, and Pereira, MP. Ultra-processed Foods, Diet Quality, and Health Using the NOVA Classification System. Rome: FAO (2019). 49 p.
11. Haghighatdoost, F, Atefi, M, Mohammadifard, N, Daryabeygi-Khotbehsara, R, Khosravi, A, and Mansourian, M. The relationship between ultra-processed foods consumption and obesity indicators in Iranian adults. Nutr Metab Cardiovasc Dis. (2022). doi: 10.1016/j.numecd.2022.05.019
12. Martinez-Perez, C, San-Cristobal, R, Guallar-Castillon, P, Martínez-González, MÁ, Salas-Salvadó, J, Corella, D, et al. Use of different food classification systems to assess the association between ultra-processed food consumption and cardiometabolic health in an elderly population with metabolic syndrome (PREDIMED-plus cohort). Nutrients. (2021) 13:2471. doi: 10.3390/nu13072471
13. Monteiro, CA, Cannon, G, Levy, RB, Moubarac, J-C, Louzada, ML, Rauber, F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. (2019) 22:936–41. doi: 10.1017/S1368980018003762
14. Pagliai, G, Dinu, M, Madarena, M, Bonaccio, M, Iacoviello, L, and FJBJON, S. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. (2021) 125:308–18. doi: 10.1017/S0007114520002688
15. Giachelli, CM. The emerging role of phosphate in vascular calcification. Kidney Int. (2009) 75:890–7. doi: 10.1038/ki.2008.644
16. Montero-Salazar, H, Donat-Vargas, C, Moreno-Franco, B, Sandoval-Insausti, H, Civeira, F, Laclaustra, M, et al. High consumption of ultra-processed food may double the risk of subclinical coronary atherosclerosis: the Aragon workers’ health study (AWHS). BMC Med. (2020) 18:1–11. doi: 10.1186/s12916-020-01678-8
17. Silva Meneguelli, T, Viana Hinkelmann, J, Hermsdorff, HHM, Zulet, MÁ, Martínez, JA, and Bressan, J. Food consumption by degree of processing and cardiometabolic risk: a systematic review. Int J Food Sci Nutr. (2020) 71:678–92. doi: 10.1080/09637486.2020.1725961
18. Hajar, R. Risk factors for coronary artery disease: historical perspectives. Heart Views. (2017) 18:109–14. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_106_17
19. Srour, B, Fezeu, LK, Kesse-Guyot, E, Allès, B, Méjean, C, Andrianasolo, RM, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ Open. (2019):1451. doi: 10.1136/bmj.l1451
20. Bonaccio, M, Di Castelnuovo, A, Costanzo, S, De Curtis, A, Persichillo, M, Sofi, F, et al. Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani study. Am J Clin Nutr. (2021) 113:446–55. doi: 10.1093/ajcn/nqaa299
21. da Silva, A, Brum Felício, M, Caldas, APS, Hermsdorff, HH, Torreglosa, CR, Bersch-Ferreira, ÂC, et al. Ultra-processed foods consumption is associated with cardiovascular disease and cardiometabolic risk factors in Brazilians with established cardiovascular events. Int J Food Sci Nutr. (2021) 72:1128–37. doi: 10.1080/09637486.2021.1908963
22. Monteiro, CA, Cannon, G, Levy, R, Moubarac, J-C, Jaime, P, Martins, AP, et al. NOVA. The star shines bright. World Nutr. (2016) 7:28–38.
23. Moubarac, J-C. Ultra-processed Food and Drink Products in Latin America: Trends, Impact on Obesity, Policy Implications. Washington, DC: Pan American Health Organization, World Health Organization (2015).
24. Zarepur, E, Mohammadifard, N, Mansourian, M, Roohafza, H, Sadeghi, M, Khosravi, A, et al. Rationale, design, and preliminary results of the Iran-premature coronary artery disease study (I-PAD): a multi-center case-control study of different Iranian ethnicities. ARYA Atherosclerosis. (2020) 16:295–300. doi: 10.22122/arya.v16i6.2241
25. World Health Organization. Measuring obesityclassification and description of anthropometric data. Report on a WHO consultation of the epidemiology of obesity. Warsaw 21–23 October 1987. Copenhagen: WHO, (1989). Nutrition Unit document, EUR/ICP/NUT. 1987;123.
26. Mohammadifard, N, Khosravi, A, Esmaillzadeh, A, Feizi, A, Abdollahi, Z, Salehi, F, et al. Validation of simplified tools for assessment of sodium intake in iranian population: rationale, design and initial findings. Arch Iran Med. (2016) 19, 652–658.
27. Rafiei, M, Boshtam, M, Marandi, A, Jalali, A, and Vakili, R. The Iranian food consumption program (IFCP), a unique nutritional software in Iran. Iran J Public Health. (2002) 31:105–7.
28. Dorosty Motlagh, A, and Tabatabaei, M In: D Taghzie, editor. Food Composition Tables, Doniaie Taghzie (2007)
30. Expert Panel on Detection E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
31. Rauber, F, Louzada, MLC, Steele, EM, Millett, C, Monteiro, CA, and Levy, RB. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014). Nutrients. (2018) 10, 587–600. doi: 10.3390/nu10050587
32. Baraldi, LG, Steele, EM, Canella, DS, and Monteiro, CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. (2018) 8:e020574. doi: 10.1136/bmjopen-2017-020574
33. Zhong, G-C, Gu, H-T, Peng, Y, Wang, K, Wu, Y-Q-L, Hu, T-Y, et al. Association of ultra-processed food consumption with cardiovascular mortality in the US population: long-term results from a large prospective multicenter study. Int J Behav Nutr Phys Act. (2021) 18. doi: 10.1186/s12966-021-01081-3
34. Esposito, K, Marfella, R, Ciotola, M, Di Palo, C, Giugliano, F, Giugliano, G, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. (2004) 292:1440–6. doi: 10.1001/jama.292.12.1440
35. Estruch, R, Ros, E, Salas-Salvadó, J, Covas, M-I, Corella, D, Arós, F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. (2018) 378:e34. doi: 10.1056/NEJMoa1800389
36. Benelam, B. Satiety and the anorexia of ageing. Br J Community Nurs. (2009) 14:332–5. doi: 10.12968/bjcn.2009.14.8.43512
37. Mendonça, RD, Pimenta, AM, Gea, A, de la Fuente-Arrillaga, C, Martinez-Gonzalez, MA, Lopes, ACS, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-up (SUN) cohort study. Am J Clin Nutr. (2016) 104:1433–40. doi: 10.3945/ajcn.116.135004
38. Beslay, M, Srour, B, Méjean, C, Allès, B, Fiolet, T, Debras, C, et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Santé cohort. PLoS Med. (2020) 17:e1003256. doi: 10.1371/journal.pmed.1003256
39. Mendonça, RD, Lopes, ACS, Pimenta, AM, Gea, A, Martinez-Gonzalez, MA, and Bes-Rastrollo, M. Ultra-processed food consumption and the incidence of hypertension in a Mediterranean cohort: the Seguimiento Universidad de Navarra project. Am J Hypertens. (2017) 30:358–66. doi: 10.1093/ajh/hpw137
40. Kummerow, FA. The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis. (2009) 205:458–65. doi: 10.1016/j.atherosclerosis.2009.03.009
41. Wang, M, Du, X, Huang, W, and Xu, Y. Ultra-processed foods consumption increases the risk of hypertension in adults: a systematic review and meta-analysis. Am J Hypertens. (2022) 35:892–901. doi: 10.1093/ajh/hpac069
42. Mozaffarian, D, Katan, MB, Ascherio, A, Stampfer, MJ, and Willett, WC. Trans fatty acids and cardiovascular disease. N Engl J Med. (2006) 354:1601–13. doi: 10.1056/NEJMra054035
43. Myhrstad, MC, Tunsjø, H, Charnock, C, and Telle-Hansen, VH. Dietary fiber, gut microbiota, and metabolic regulation—current status in human randomized trials. Nutrients. (2020) 12:859. doi: 10.3390/nu12030859
44. Uribarri, J, Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Seminars in dialysis; (2007).
45. Zhang, Y, Huang, M, Zhuang, P, Jiao, J, Chen, X, Wang, J, et al. Exposure to acrylamide and the risk of cardiovascular diseases in the National Health and nutrition examination survey 2003–2006. Environ Int. (2018) 117:154–63. doi: 10.1016/j.envint.2018.04.047
46. DeJarnett, N, Conklin, D, Riggs, D, Myers, J, O’Toole, T, and Hamzeh, I. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. (2014) 3:e000934. doi: 10.1161/JAHA.114.000934
Keywords: ultra-processed food, premature coronary artery disease, coronary artery diseases, cardiovascular disease, processed food
Citation: Ansari S, Mohammadifard N, Haghighatdoost F, Zarepur E, Mahmoudi S, Nouri F, Nouhi F, Alikhasi H, Sharifianjazi F, Tavamaishvili K, Shirani S, Kazemi T, Azdaki N, Salehi N, Lotfizadeh M, Solati K, Ghaffari S, Javanmardi E, Salari A, Dehghani M, Cheraghi M, Assareh A, Haybar H, Namayandeh SM, Madadi R and Sarrafzadegan N (2023) The relationship between ultra processed food consumption and premature coronary artery disease: Iran premature coronary artery disease study (IPAD). Front. Nutr. 10:1145762. doi: 10.3389/fnut.2023.1145762
Received: 16 January 2023; Accepted: 27 March 2023;
Published: 30 June 2023.
Edited by:
Alessandro Di Cerbo, University of Camerino, ItalyReviewed by:
Corinna May Walsh, University of the Free State, South AfricaCopyright © 2023 Ansari, Mohammadifard, Haghighatdoost, Zarepur, Mahmoudi, Nouri, Nouhi, Alikhasi, Sharifianjazi, Tavamaishvili, Shirani, Kazemi, Azdaki, Salehi, Lotfizadeh, Solati, Ghaffari, Javanmardi, Salari, Dehghani, Cheraghi, Assareh, Haybar, Namayandeh, Madadi and Sarrafzadegan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahimeh Haghighatdoost, Zl9oYWdoaWdoYXRkb29zdEB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.