
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 16 February 2023
Sec. Nutrition, Psychology and Brain Health
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1142035
This article is part of the Research TopicNutrition and Mood Disorders: Exploring their Relationship, and Nutritional Strategies for AlleviationView all 8 articles
 Kuo-Chuan Hung1,2†
Kuo-Chuan Hung1,2† Jheng-Yan Wu3†
Jheng-Yan Wu3† Amina M. Illias4,5
Amina M. Illias4,5 Chong-Chi Chiu6,7,8
Chong-Chi Chiu6,7,8 Ying-Jen Chang1
Ying-Jen Chang1 Shu-Wei Liao1
Shu-Wei Liao1 Kuei-Fen Wang1
Kuei-Fen Wang1 I-Wen Chen9*
I-Wen Chen9* Cheuk-Kwan Sun7,10*
Cheuk-Kwan Sun7,10*Background: Although post-stroke depression (PSD) affects one-third of patients following an acute stroke, pooled evidence addressing the correlation between a low vitamin D status and the risk of PSD remains inconclusive.
Methods: Comprehensive database search of Medline, EMBASE, Cochrane library, and Google Scholar was performed from inception to December 2022. The primary outcome was the association of PSD risk with a low vitamin D status, while the secondary outcomes included the relationship between PSD and other risk factors.
Results: Analysis of seven observational studies published between 2014 and 2022 with 1,580 patients showed pooled incidences of vitamin D deficiency (defined as 25[OH] D levels < 50 nmol/L) and PSD of 60.1 and 26.1%, respectively. Patients with PSD had a lower circulating vitamin D concentration compared to those without [mean difference (MD) =−13.94 nmol/L, 95% CI: −21.83 to −6.05, p = 0.0005, I2 = 91%, six studies, 1,414 patients]. Meta-analysis also demonstrated a correlation between a low vitamin D level and an increased PSD risk [odd ratio (OR) = 3.25, 95% CI: 1.57–6.69, p = 0.001, I2 = 78.7%, 1,108 patients], the heterogeneity of which was found to be associated with the incidence of vitamin D deficiency but not female proportion on meta-regression. Besides, female gender (OR = 1.78, 95% CI: 1.3–2.44, p = 0.003, I2 = 31%, five studies, 1,220 patients), hyperlipidemia (OR = 1.55, 95% CI: 1.01–2.36, p = 0.04, I2 = 0%, four studies, 976 patients), and high National Institutes of Health Stroke Scale (NIHSS) scores (MD = 1.45, 95% CI: 0.58–2.32, p = 0.001, I2 = 82%, five studies, 1,220 patients) were potential risk factors for PSD. For the primary outcome, the certainty of evidence was very low. Regarding secondary outcomes, the certainty of evidence was low for BMI, female gender, hypertension, diabetes, and stroke history, and very low for age, level of education, hyperlipidemia, cardiovascular disease, and NIHSS scores.
Conclusion: The results suggested an association of a low circulating vitamin D level with an increased risk of PSD. Besides, female gender, hyperlipidemia, high NIHSS score were related to an increased risk or occurrence of PSD. The current study may imply the necessity of routine circulating vitamin D screening in this population.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022381580.
Post-stroke depression (PSD), which is frequently observed weeks and months following an acute stroke, is a common neuropsychiatric sequela (1, 2). In addition to psychosocial factors, the etiology of PSD is multifactorial involving a myriad of pathophysiological changes including dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, abnormal neurotrophic response, reduced levels of monoamines, glutamate-mediated excitotoxicity, and increased inflammation (3, 4). Prior investigations have hypothesized that focal brain injury-induced hyperactivation of the HPA axis could cause an increased production of HPA hormones, thereby changing the expressions of corticoid receptors primarily in the limbic system. The resulting alterations in the negative feedback mechanism then contribute to both morphological and functional impairments of key brain areas with a high density of glucocorticoid receptors, in particular the hippocampus (5), which is pivotal in governing memory and emotions. The damage may predispose to the development of cognitive and depressive disorders (6). Inflammation in the brain has also been reported to play a role in the occurrence of depression (7). Pro-inflammatory cytokines have been found to affect the concentrations of serotonin (5-hydroxytryptamine, 5-HT) and norepinephrine, thereby altering transmission in the neural circuits (8). Moreover, inflammation is known to activate the response system to stress (9). In concert with this finding, compared with individuals without depression, the stress response of those with depression has been shown to be over-activated (10). Indeed, a previous meta-analysis of over 15 thousand patients for a mean of 6.87 months after stroke reported an incidence of depressive disorder up to 33.5% (11). For early prevention of PSD, previous studies have attempted to identify the risk factors (e.g., female gender) (12–14). Nevertheless, the non-modifiable nature of the reported predictors has limited their clinical use.
Vitamin D is a fat-soluble vitamin vital for skeletal and extraskeletal health such as the maintenance of immune function, cancer prevention, and integrity of the cardiovascular system (15–18). Besides, vitamin D deficiency has been linked to an increased risk of postoperative delirium or depression (19, 20), suggesting its neuropsychiatric and neurocognitive function. Consistently, a growing body of evidence has shown a low circulating vitamin D level in a variety of populations with depressive disorder (21–23). Focusing on stroke survivors, several studies have also reported similar findings (24–26). As the incidence of low circulating vitamin D level was not uncommon in patients with stroke (24, 27, 28), it is of utmost importance to identify whether vitamin D level is a modifiable factor in the development of PSD. Nevertheless, pooled evidence through a systematic approach addressing the correlation between PSD and vitamin D level is scarce. Therefore, the primary outcome of the current meta-analysis aimed at investigating the association of PSD with the circulating vitamin D level, while the secondary outcomes included the relationships between PSD and other risk factors.
The protocol of the current meta-analysis was registered on PROSPERO (registration no.: CRD42022381580). This study was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
We performed a comprehensive search of the Medline (ovid), EMBASE (ovid), Cochrane library, and Google Scholar databases from inception to December 2022 without placing restrictions on the publication year, language, or sample size. The following search terms were used: (”Stroke” or “CVA” or “Cerebrovascular Accident” or “Cerebrovascular Stroke*” or “Brain Infarction” or “Cerebral Infarction” or “Brain Stem Infarctions” or “Brain Ischemia” or “Brain infarction” or “Ischemic stroke” or “Stroke survivors”) and (”Depressi*” or “post-stroke depression” or “depressive symptoms”) and (”Vitamin D” or “Vitamin D deficienc*” or “vitamin D2” or “vitamin D3” or “25OHD” or “25(OH)D” or “25-hydroxyvitamin D” or “Hydroxycholecalciferols” or “hypovitaminosis D” OR “plasma/serum 25 (OH) Vitamin D”). Supplementary Table 1 summarizes the search strategy for one of these databases (i.e., Medline). Using the reference lists of relevant systematic reviews and those within the included articles, we manually searched for studies possibly missed on the initial search.
The following criteria were used to determine if a peer-reviewed study was eligible for inclusion: (a) adults (i.e., 18 years of age or above) with stroke regardless of its mechanism (i.e., infarction or hemorrhage); (b) available information regarding serum vitamin D level before the diagnosis of PSD; and (c) randomized controlled studies or observational studies. The exclusion criteria were (1) studies that focused on patients with depression before stroke attack; (2) those in which details regarding vitamin D level or outcome were unavailable; (3) those presented as conference abstracts, reviews, letters, case reports; and (4) non-peer-reviewed articles.
Three steps were taken to determine study eligibility: (1) Duplicated records were removed by using the EndNote software; (2) Two authors independently screened the titles/abstracts of the retrieved records to identify articles for full-text reading; and (3) Studies were included if they fulfilled the inclusion criteria after full-text reading. All discrepancies in opinions were resolved through consultation with a third author.
Relevant information was retrieved from each study, including first author/publication year, characteristics of the study population (e.g., gender), number of patients, body mass index (BMI), comorbidities [e.g., hypertension, diabetes mellitus (DM)], National Institutes of Health stroke scale (NIHSS), circulating vitamin D levels, follow-up period, incidence of PSD, and country of publication. Two authors retrieved data independently using a specific data extraction sheet. Any disagreements were resolved via discussion. Whenever the relevant information was unclear or missing, we emailed the corresponding author for clarification.
The primary outcome of the current meta-analysis was the relationship between the risk of PSD and a low vitamin D status, which was defined based on individual studies. The secondary outcomes were the associations of other factors (e.g., hypertension) with the risk of PSD. The definition of PSD was in accordance with that of each study. Vitamin D deficiency was defined as a circulating 25[OH] D level < 50 nmol/L (27).
The quality of individual studies was scrutinized based on their risks of bias using the Newcastle-Ottawa Scale (NOS) that comprises eight items contained in three domains, namely selection, comparability, and outcome. A certain number of stars can be assigned to each item; while a maximum of two stars can be given to an item in the selection and outcome domains, each item in the comparability domain can receive a maximum of only one star. A study is regarded as “low-risk” if it has been assigned seven or more stars.
Two independent authors used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (29) for assessing the certainty of evidence for the study outcomes. Inconsistencies in opinions were settled through arbitration involving a third author.
All data were analyzed to calculate the pooled mean difference (MD) and odd ratio (OR) by using the random-effects model. The 95% confidence interval (CI) was also reported for each outcome. The heterogeneity was examined using I2 statistics with I2 values ≥50% representing substantial heterogeneity as previously reported (30, 31). To examine the reliability of the primary and secondary outcomes, sensitivity analysis was conducted using a leave-one-out approach. For an outcome shared by 10 or more studies, the existence of potential publication bias was discerned through visual inspection of a funnel plot. For primary outcome, meta-regression was conducted to identify the origin of heterogeneity by using the proportion of females and incidence of vitamin D deficiency as covariates. The Review Manager (RevMan) or comprehensive Meta-Analysis (CMA) V3 software (Biostat, Englewood, NJ, USA) were applied for statistical analyses. A probability value, p, less than 0.05 was deemed statistically significant.
A total of 332 records were identified through literature search. Following a review of the titles and abstracts, 310 were excluded because of duplications or failure to meet the inclusion criteria. After a full-text review of the remaining 22 articles, 15 were further excluded for a variety of reasons shown in Figure 1. Finally, seven observational studies published between 2014 and 2022 were included in the current meta-analysis (24–26, 32–35).
The characteristics of the 1,580 included patients diagnosed with stroke (mean or median age: 59.5–73.2 years; BMI: 23–25 kg/kg2) are summarized in Table 1. Six studies recruited mixed gender populations with the proportion of males ranging from 47.3 to 72.4%, while one study only focused on male patients (35). The number of patients were between 126 and 442. The diagnosis of PSD was based on DSM-IV criteria in three studies (26, 32, 33), Hamilton Depression Scale (HAMD) ≥ 7 in another three studies (24, 25, 35), and the Beck Depression Inventory II in one study (34). The follow-up time was one month in four studies (24, 25, 32, 33) and up to six months in another study (26), while two studies did not provide relevant information (34, 35). The incidence of vitamin D deficiency was available in five (24, 26, 32–34) out of the seven studies (range: 41.9–79.5%) with a pooled incidence of 60.1% (95% CI: 43.8–74.4%) (Figure 2A). The incidence of PSD was reported in six studies (range: 18.6–29.6%) (24–26, 32, 33, 35), while this information was not available in one study (34). Overall, the pooled incidence of PSD was 26.1% (95% CI: 23.3–29.2%) (Figure 2B). The studies were conducted in two countries including China (n = 6) (24–26, 32, 33, 35) and Korea (n = 1) (34). The quality of each study is shown in Table 1. All studies with a total number of stars ranging from 7 to 9 are regarded as “low-risk.”
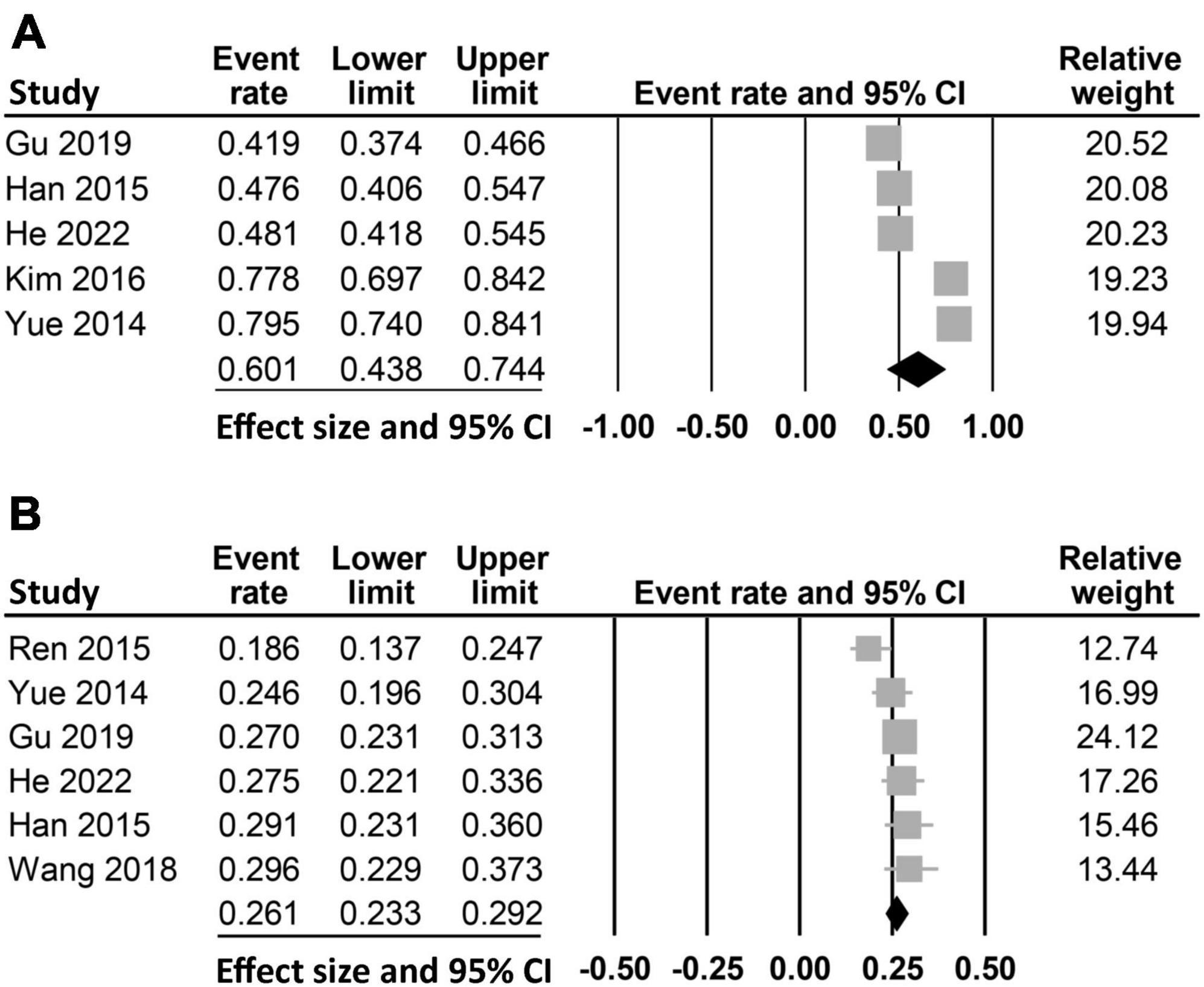
Figure 2. Pooled incidence of (A) vitamin D deficiency (pooled incidence: 60.1%, 95% CI: 43.8–74.4%, five studies) and (B) post-stroke depression (PSD) (pooled incidence: 26.1%, 95% CI: 23.3–29.2%, six studies). CI, confidence interval.
Six studies reported the circulating vitamin D level in patients with (n = 367) or without (n = 1,047) PSD (Figure 3A). Meta-analysis of these data revealed a lower vitamin D level in patients with PSD compared to those without (MD: −13.94 nmol/L, 95% CI: −21.83 to −6.05, p = 0.0005, I2 = 91%, six studies, 1,414 patients) (24–26, 32, 33, 35). Sensitivity analysis showed consistent findings, suggesting robustness of evidence. The circulating vitamin D levels were not available in one study (34), in which the authors reported a higher Beck depression Inventory II score in patients with vitamin D deficiency compared to that in those with a normal vitamin D level.
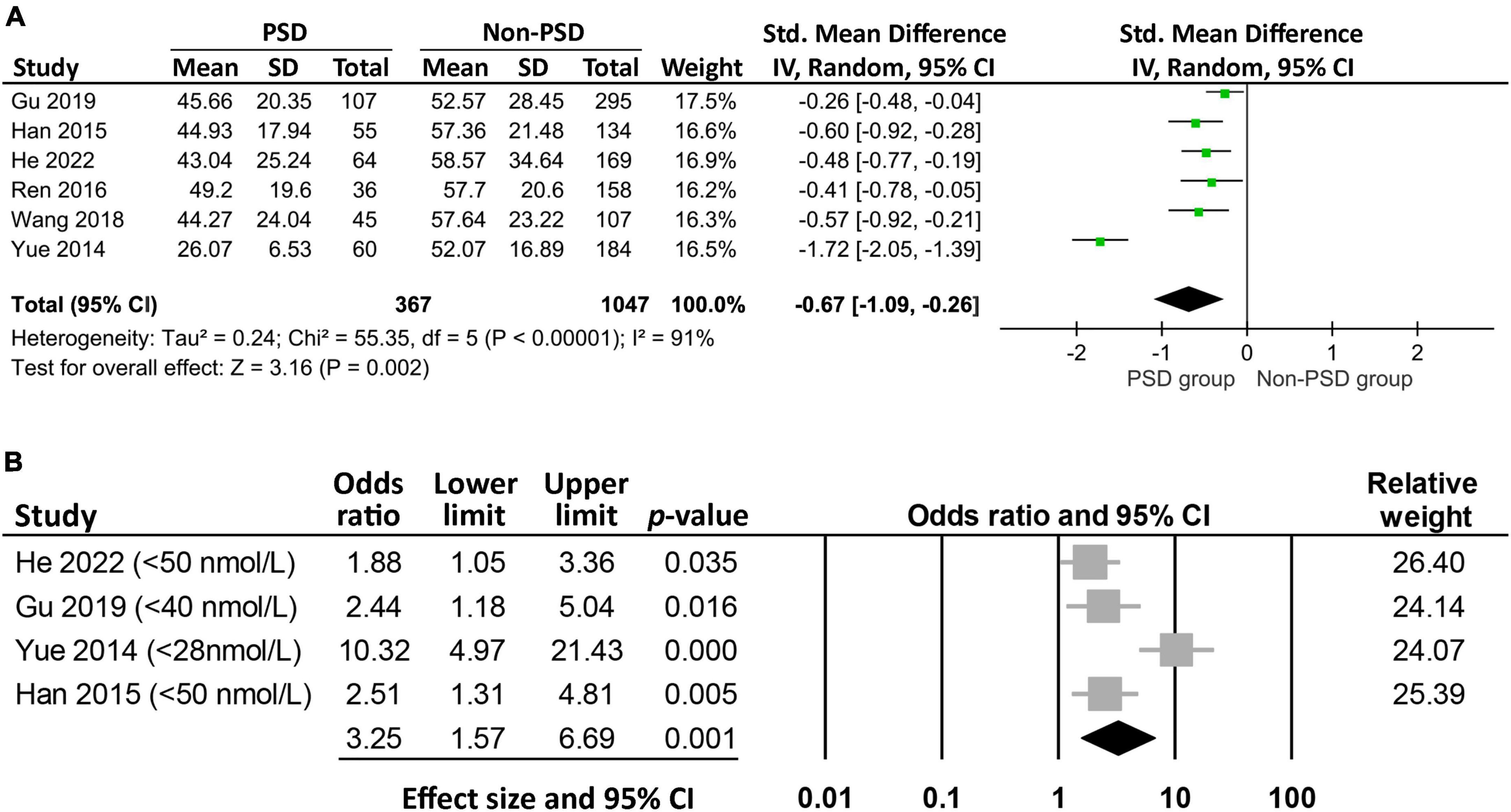
Figure 3. Forest plot comparing (A) the circulating vitamin D level between patients with and those without post-stroke depression (PSD), showing a lower circulating vitamin D level (mean difference: −13.94 nmol/L, 95% CI: −21.83 to −6.05, p = 0.0005, I2 = 91%, six studies, 1,414 patients) in patients with PSD; (B) the risk of PSD between patients with and those without low vitamin D levels, indicating a higher risk of PSD in patients with a lower level of vitamin D (odds ratio = 3.25, 95% CI: 1.57–6.69, p = 0.001, I2 = 78.7%, 1,108 patients). CI, confidence interval, SD, standard deviation; IV, inverse variance.
Analysis of the four studies that reported the event/total number or odds ratio on the relationship between low vitamin D status and PSD revealed a higher risk of PSD in patients with a lower circulating level of vitamin D (OR = 3.25, 95% CI:1.57–6.69, p = 0.001, I2 = 78.7%, 1,108 patients) (Figure 3B) (24, 26, 32, 33). The finding remained consistent on sensitivity analysis. Meta-regression revealed an association of the heterogeneity of this finding with the incidence of vitamin D deficiency (coefficient: 0.04, p = 0.0004) (Figure 4A), but not the proportion of female gender (coefficient: 0.036, p = 0.467) (Figure 4B).
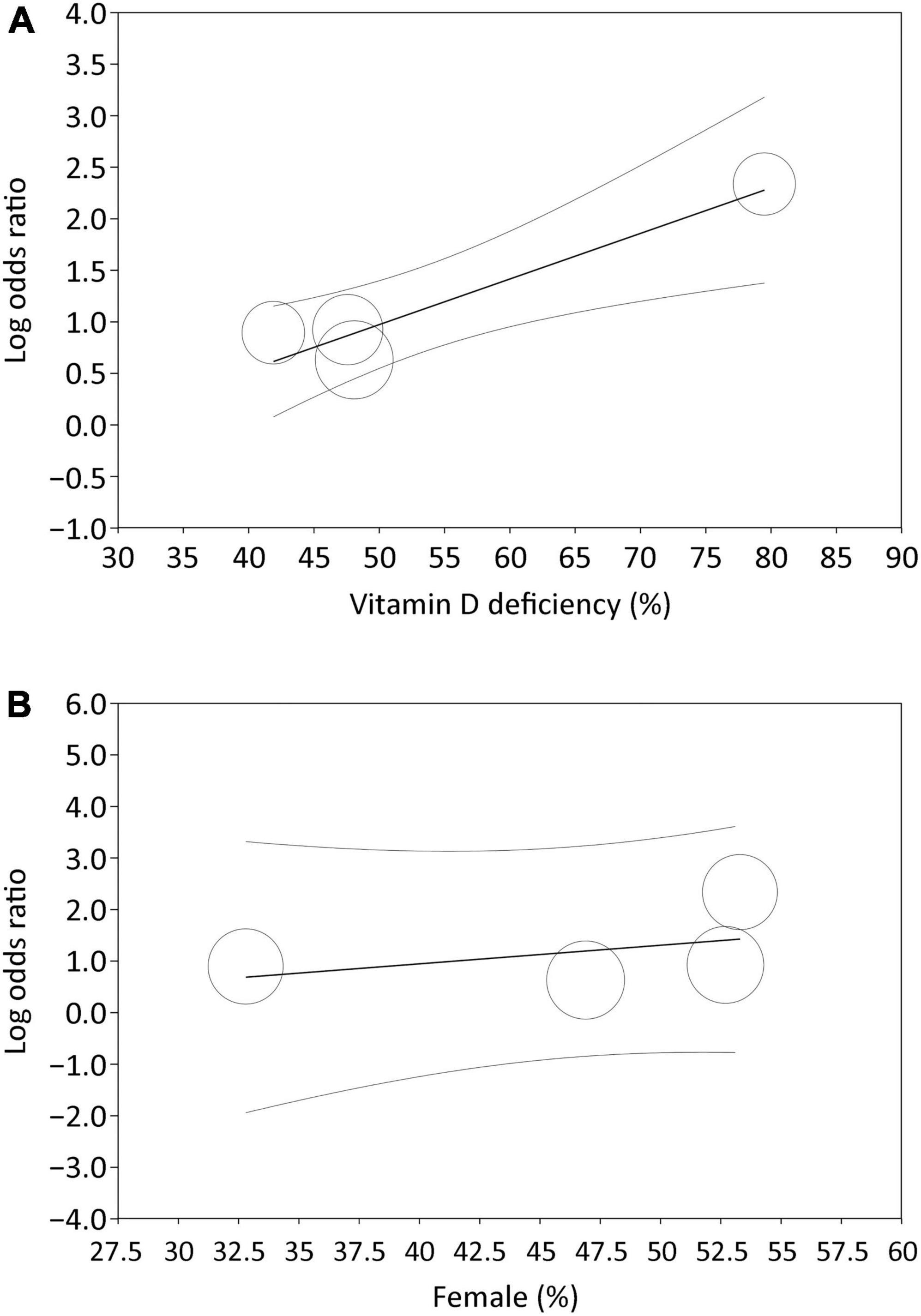
Figure 4. Meta-regression showing impact of (A) incidence of vitamin D deficiency (coefficient: 0.04, p = 0.0004), and (B) proportion of female gender (coefficient: 0.036, p = 0.467) on the association of low vitamin D status with risk of post-stroke depression, identifying incidence of vitamin D deficiency as a potential covariant affecting the relationship between a low vitamin D status and risk of post-stroke depression.
The associations of age, female gender, BMI, and level of education with PSD are summarized in Figure 5. There were no differences in age (MD:1.61 years, 95% CI: −3.16 to 6.38, p = 0.51, I2 = 92%, five studies, 1,220 patients) (Figure 5A) (24–26, 32, 33), BMI (MD: 0.25 kg/m2, 95% CI: −0.2 to 0.71, p = 0.28, I2 = 10%, five studies, 1,220 patients) (Figure 5B) (24–26, 32, 33), and level of education (MD =−0.47, 96% CI: −1.89 to 0.95, p = 0.52, I2 = 76%, four studies, 976 patients) (Figure 5C) (24, 25, 32, 33) in patients with or without PSD. However, women showed a higher risk of PSD compared to that in men (OR = 1.78, 95% CI: 1.3–2.44, p = 0.003, I2 = 31%, five studies, 1,220 patients) (Figure 5D) (24–26, 32, 33).
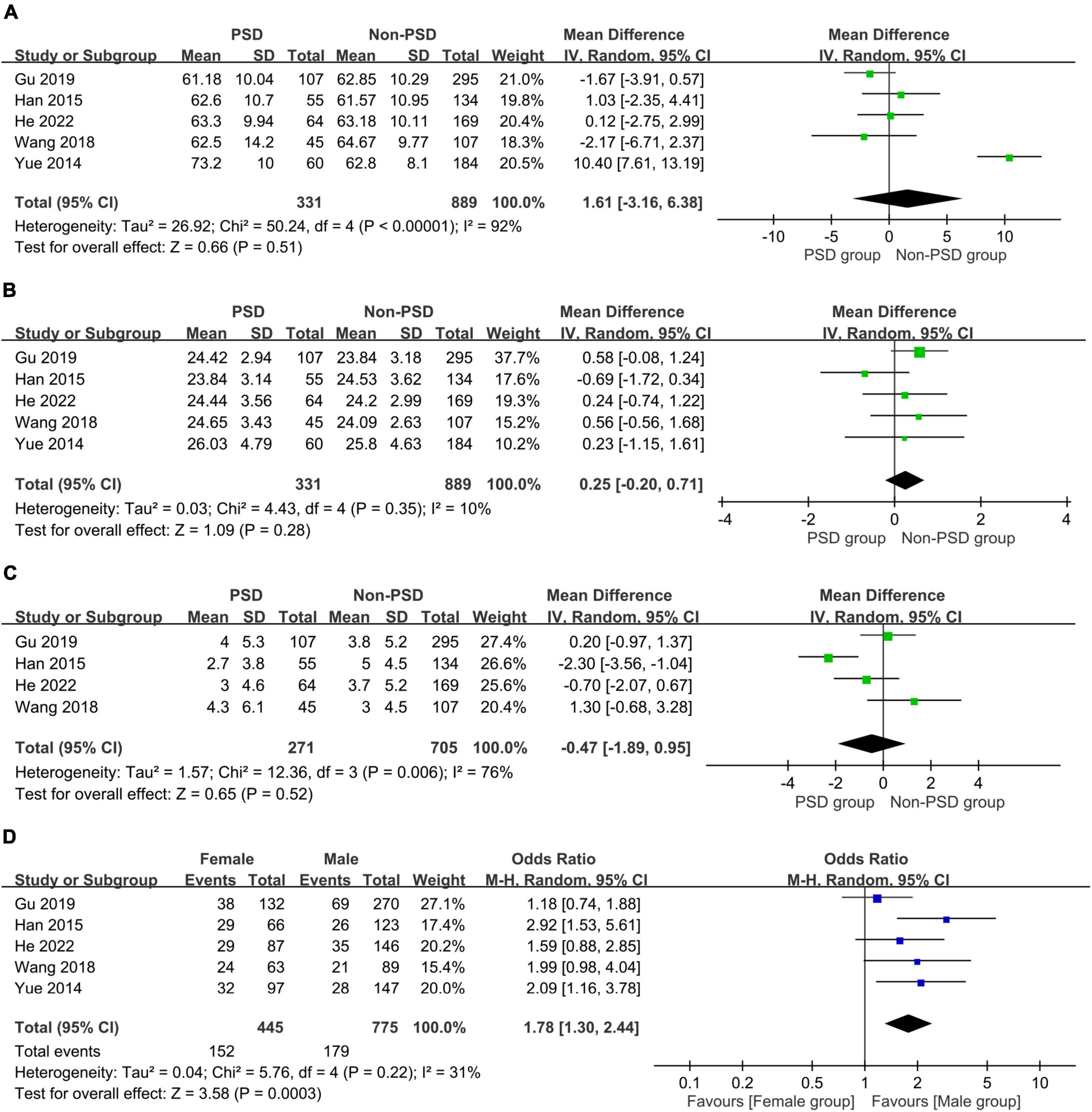
Figure 5. Forest plot showing the associations of post-stroke depression with (A) age (mean difference:1.61 years, 95% CI: −3.16 to 6.38, p = 0.51, I2 = 92%, five studies, 1,220 patients); (B) body mass index (BMI) (mean difference: 0.25 kg/m2, 95% CI: −0.2 to 0.71, p = 0.28, I2 = 10%, five studies, 1,220 patients); (C) education level (mean difference =−0.47, 96% CI: −1.89 to 0.95, p = 0.52, I2 = 76%, four studies, 976 patients); and (D) female gender (odds ratio = 1.78, 95% CI: 1.3–2.44, p = 0.003, I2 = 31%, five studies, 1,220 patients), indicating an correlation of female gender with the risk of post-stroke depression. MH, Mantel-Haenszel; IV, inverse variance; CI, confidence interval.
The associations of PSD with comorbidities including systemic and vascular diseases are shown in Figures 6, 7, respectively. The presence of hypertension (OR = 1.11, 95% CI: 0.74–1.65, p = 0.62, I2 = 44%, five studies, 1,220 patients) (Figure 6A) (24–26, 32, 33) or diabetes mellitus (OR = 1.15, 95% CI: 0.87–1.52, p = 0.33, I2 = 0%, five studies, 1,220 patients) (Figure 6B) (24–26, 32, 33) did not correlate with a higher risk of PSD, while patients with hyperlipidemia were at risk of PSD compared to those without (OR = 1.55, 95% CI: 1.01–2.36, p = 0.04, I2 = 0%, four studies, 976 patients) (Figure 6C) (24, 25, 32, 33). There was no increased risk of PSD in patients with stroke history (OR = 1.39, 95% CI: 0.88–2.2, p = 0.16, I2 = 0%, four studies, 976 patients) (Figure 7A) (24, 25, 32, 33) or cardiovascular disease (OR = 0.64, 95% CI: 0.26–1.6, p = 0.34, I2 = 41%, four studies, 976 patients) (Figure 7B) (24, 25, 32, 33). However, patients with PSD had a higher NIHSS score at stroke onset than that in those without PSD (MD: 1.45, 95% CI: 0.58–2.32, p = 0.001, I2 = 82%, five studies, 1,220 patients) (Figure 7C) (24–26, 32, 33).
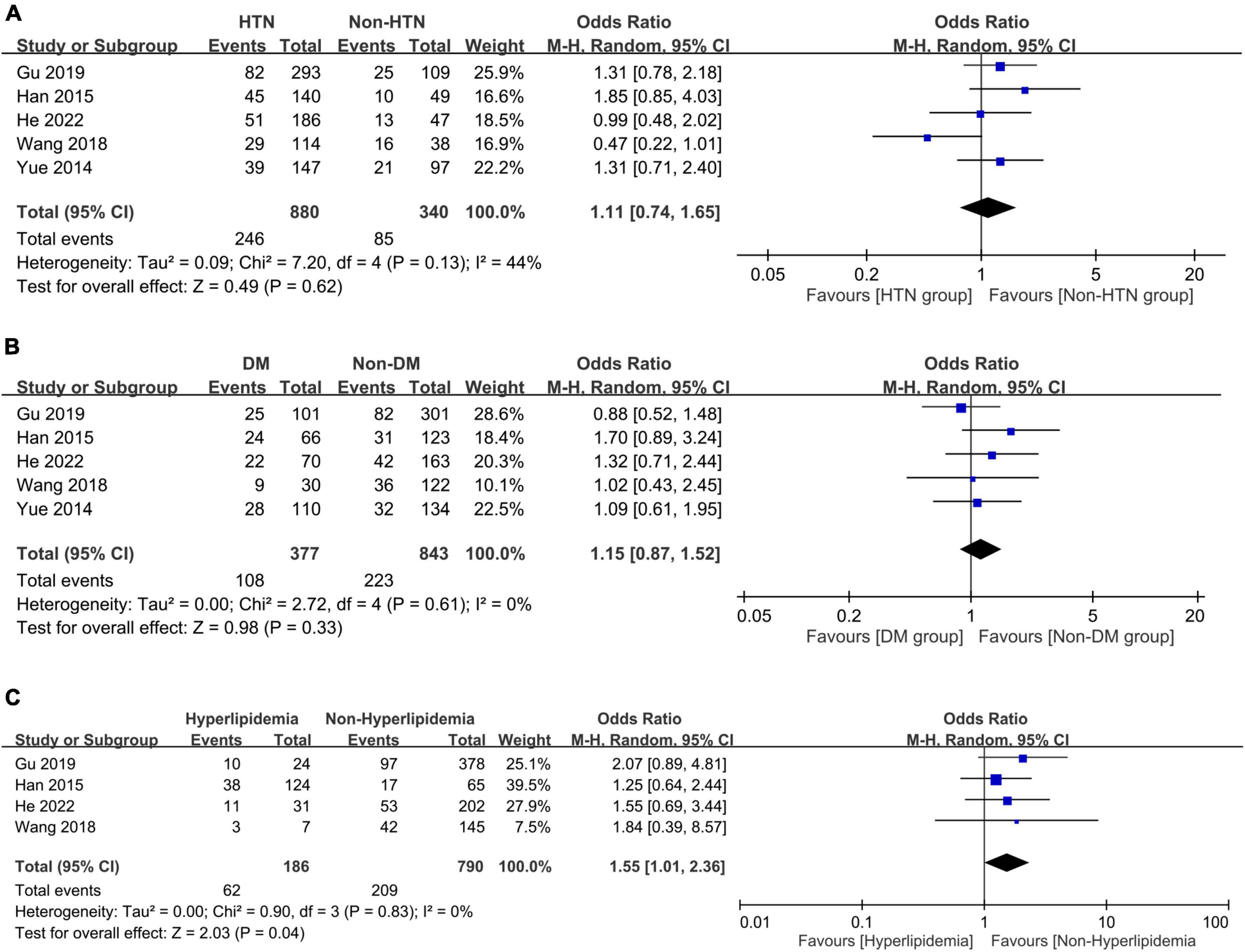
Figure 6. Forest plot showing the associations of post-stroke depression with (A) hypertension (HTN) (odds ratio = 1.11, 95% CI: 0.74–1.65, p = 0.62, I2 = 44%, five studies, 1,220 patients); (B) diabetes mellitus (DM) (odds ratio = 1.15, 95% CI: 0.87–1.52, p = 0.33, I2 = 0%, five studies, 1,220 patients); and (C) hyperlipidemia (odds ratio = 1.55, 95% CI: 1.01–2.36, p = 0.04, I2 = 0%, four studies, 976 patients), demonstrating a link between hyperlipidemia and the risk of post-stroke depression. CI, confidence interval; MH, Mantel-Haenszel; CI, confidence interval.
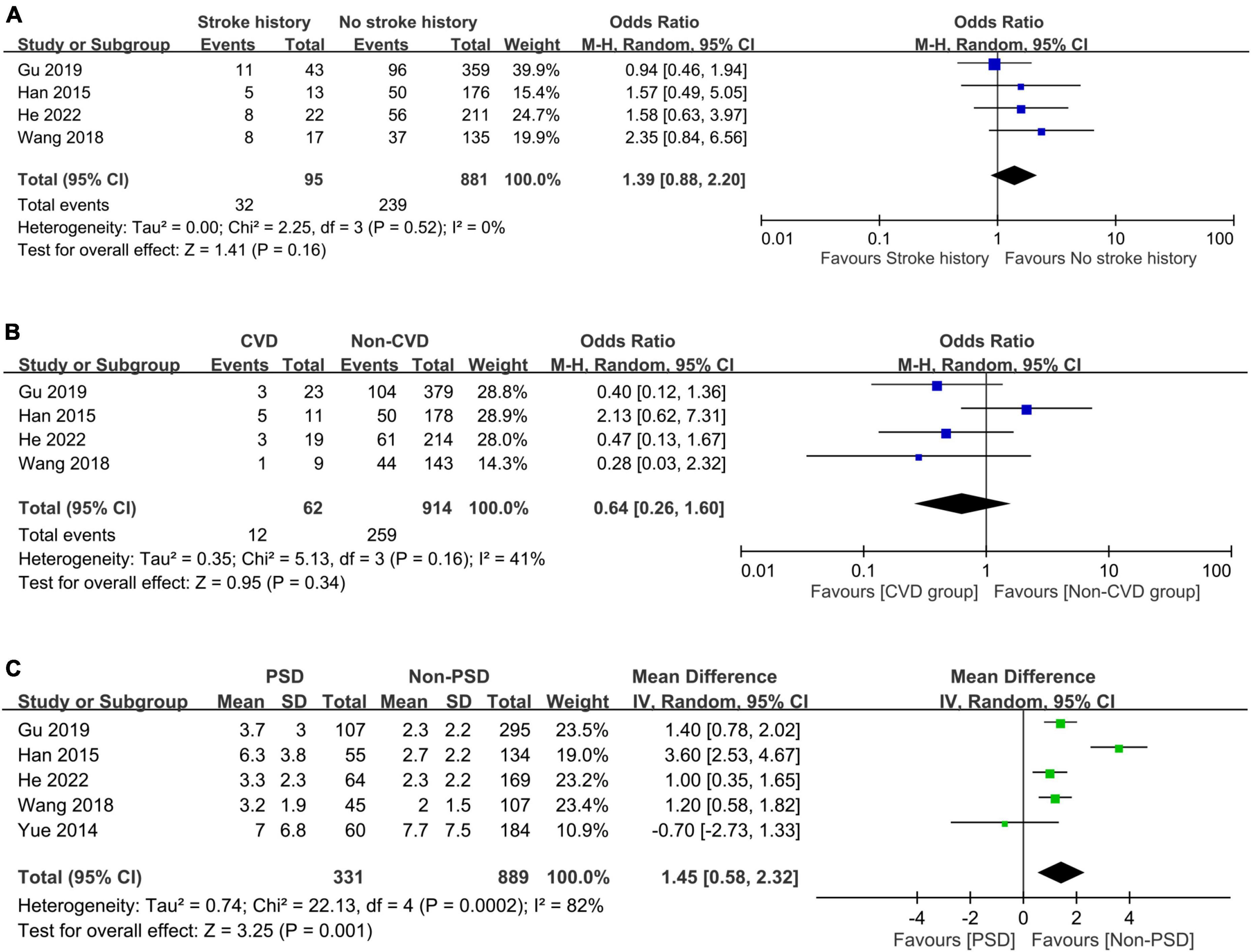
Figure 7. Forest plot showing the association of post-stroke depression (PSD) with (A) stroke history (odds ratio = 1.39, 95% CI: 0.88–2.2, p = 0.16, I2 = 0%, four studies, 976 patients); (B) cardiovascular disease (CVD) (odds ratio = 0.64, 95% CI: 0.26–1.6, p = 0.34, I2 = 41%, four studies, 976 patients); and (C) score on the National Institute of Health stroke scale (NIHSS) (mean difference: 1.45, 95% CI: 0.58–2.32, p = 0.001, I2 = 82%, five studies, 1,220 patients), revealing a correlation between NIHSS score and the occurrence of post-stroke depression. CI, confidence interval; MH, Mantel-Haenszel.
The certainty of evidence is demonstrated in Supplementary Table 2. For the primary outcome of the associations of circulating vitamin D concentration with the occurrence and risk of PSD, the certainty of evidence was very low. Regarding the secondary outcomes of the impacts of other factors on the occurrence and risk of PSD, the certainty of evidence was low for BMI, female gender, hypertension, DM, stroke history. On the other hand, the certainty of evidence was very low for age, level of education, hyperlipidemia, cardiovascular disease, and NIHSS scores.
Despite reports from observational studies of a connection between a low circulating vitamin D level and PSD (24–26) that affects one-third of patients following acute stroke (14), there was no pooled evidence showing their correlation or identifying the influences of other potential confounders. Through focusing on patients with stroke, the current meta-analysis demonstrated that the pooled incidences of vitamin D deficiency and PSD were 60.1 and 26.1%, respectively. Stroke survivors with PSD had a lower circulating vitamin D concentration compared to those without (MD: −13.94 nmol/L). Consistently, a lower level of circulating vitamin D was associated with a three-fold increase in the risk of PSD (OR: 3.25). In addition, examining the relationship between ten other variables and PSD identified three potential predictors including female gender, hyperlipidemia, and a high NIHSS score. There were no associations of PSD with age, BMI, level of education, hypertension, DM, stroke history, and cardiovascular disease (all p > 0.05).
Although previous studies have linked a low vitamin D level to an increased risk of depression (36–38), the exact mechanisms underlying the correlation are not fully understood (39, 40). One possibility is that vitamin D plays a role in the synthesis of neurotransmitters (e.g., serotonin, and norepinephrine, dopamine) associated with mood regulation (40). Vitamin D may also affect the immune system and inflammation, which have been reported to contribute to the development of depression (41, 42). A previous review suggested that a low vitamin D level may disturb the HPA axis (43), thereby causing a dysregulation of stress and mood. This finding was further supported by the result of a previous experimental study that demonstrated antidepressant-like effect of vitamin D that acts through the brain-derived neurotrophic factor (BDNF) and NT-3/NT-4 signaling pathways in the hippocampus (44). In addition, gender may have a role to play in the development of PSD. A prior animal study demonstrated significant improvement in the depressive behaviors of PSD rats through estrogen administration, possibly involving tyrosine kinase B (TrkB)/brain-derived neurotrophic factor (BDNF)/cAMP response element-binding protein (CREB) signaling in the hippocampus (45, 46). Consistent finding was noted in a cross-sectional multi-ethnic cohort clinical investigation including 3,017 men and 2,929 women that reported an association between a low vitamin D level and a lower estradiol level in women (47). Besides, a large cohort study involving 139,128 middle-aged adults found an association of low vitamin D levels with the development of new-onset depression as well as sustained depressive symptoms (48), supporting the role of vitamin D in the psychiatric disorder. Despite the inconsistent findings from two previous meta-analytic studies (49), evidence from several meta-analyses focusing on non-stroke individuals still reported a potential relationship between the development of depression and low serum vitamin D concentrations (22, 23, 50).
The variation in the prevalence of PSD between 18 and 33% among individual studies (4) may be attributed to differences in study designs and methods of depression evaluation (51). Despite advances in diagnosis and treatment of PSD, our finding of a pooled incidence of PSD of 26.1% from six studies published between 2014 and 2022 was comparable to that of a previous large-scale meta-analysis of 51 studies conducted between 1977 and 2002 that reported a 33% pooled prevalence of the condition (51). Therefore, the current study suggests that the development of PSD was not effectively prevented in recent decades. PSD has been shown to be associated with poor short- and long-term outcomes, including a prolonged hospital stay, profound cognitive deficits, serious functional disability, a poor life quality, impaired rehabilitation outcomes, and even mortality (4, 52–57). The urgency of the need for PSD prophylaxis has highlighted the utmost importance of identifying the risk factors for PSD. The well-known predictors of PSD include female gender, stroke severity, physical disability, cognitive impairment, depression before stroke, as well as a lack of family and social support (12–14). In the current meta-analysis, we also found that female gender and stroke severity measured with NIHSS were both predictors of PSD. Despite these findings, most risk factors were non-modifiable, underscoring the difficulty in PSD prophylaxis. Although a low circulating vitamin D has been reported to be a risk factor for depression (21–23), our report is the first meta-analysis addressing this modifiable factor in the post-stroke setting.
According to a large-scale study involving 14,302 adults aged from 18 to 65 years, the prevalence of vitamin D insufficiency and deficiency were 32.7 and 41.9%, respectively (58). One of the interesting findings of that study was a lower circulating vitamin D level in women than in men (58), which may partially explain an elevated risk of PSD associated with the female gender in the current study. With regard to the effect of age, a previous study of elderly patients in a rehabilitation unit revealed a prevalence of vitamin D deficiency up to 44% (59). Focusing on disease status, previous studies have also shown an equally high prevalence of vitamin D deficiency in patients with stroke, ranging from 30 to 62.9% (24, 27, 28). The current meta-analysis revealed a pooled incidence of 60.1% of vitamin D deficiency, which was consistent with that reported in current literature. Therefore, our findings and those from previous studies suggest a ubiquity of a low circulating vitamin D level in both the general and elderly population, especially in those with stroke (58, 59), for whom a routine assessment of circulating vitamin D levels may be necessary.
Current pharmacological and non-pharmacological therapies for PSD include cognitive behavior therapy (60), transcranial magnetic stimulation (61), hyperbaric oxygen therapy (62), selective serotonin reuptake inhibitors (SSRIs) (63, 64), and acupuncture combined with antidepressants (65). Although SSRIs are recommended as the first-line treatment, their effectiveness and tolerability remain controversial (64). A previous meta-analysis of 10 trials including a total of 5,370 patients supported the efficacy of early SSRI therapy for PSD prophylaxis despite a lack of improvement in the patient’s functional independence (63). However, adverse side effects from SSRIs (e.g., seizure and nausea) are another clinical concern (63). On the other hand, although previous meta-analyses reported the beneficial effects of non-pharmacological approaches including cognitive behavior therapy (23 studies with 1,972 patients), hyperbaric oxygen therapy (27 randomized controlled trials with 2,250 participants), and transcranial magnetic stimulation (7 randomized controlled trials with 351 participants) against the depressive symptoms of PSD (60–62), the pooled evidence remained inconclusive because of the limitations of the included studies (e.g., poor methodological quality or limited number of patients). In respect of the impact of vitamin D supplementation on symptoms of depression, the findings of previous studies in the non-stroke population remained inconsistent (66–68) and there were no relevant studies focusing on PSD. Nevertheless, there was evidence showing an association of vitamin D supplementation with better rehabilitation outcomes in patients diagnosed with stroke (69). Accordingly, this approach may still be recommended for this patient population despite the lack of evidence supporting the beneficial effects of vitamin D supplementation on PSD.
Our finding of an association of a low vitamin D status with PSD may partly be explained by the link between the former and poor sleep quality (70). A previous retrospective study on 1,619 individuals diagnosed with acute ischemic stroke reported an increased risk of subsequent depression and anxiety in those with persistent poor baseline sleep quality (71). Another study further supported the correlation by showing an improved sleep quality in patients with chronic pain by using vitamin D supplements (72), underscoring the possibility that vitamin D-related impairment of sleep quality may contribute to the risk of PSD. Further clinical investigations are needed to address this issue.
Despite our provision of pooled evidence on the relationship between PSD and circulating vitamin D level through analyzing multiple studies, there were several limitations in the current meta-analysis. First, previous investigations have suggested that certain factors, including racial/ethnic differences, may have an impact on the risk of PSD (73–75). As all of the included studies were conducted in Asian countries, our findings may not be applicable to populations of other ethnic backgrounds. Besides, the possibility of publication bias may exist. Second, the impacts of other confounding factors such as socioeconomic status (76), obesity (77), and nutrient deficiency/diet (78, 79), which were not evaluated in the current study, may bias our results. Third, our finding should be interpreted with caution because meta-analysis of observational studies is unable to establish a causal relationship. Fourth, there was a high heterogeneity in our primary outcome probably attributable to the variations in follow-up time and the diagnostic approach to depression. In fact, because the diagnosis of depression (e.g., DSM-IV criteria, and HAMD Score) was based on self-reported assessment in the included studies, the possibility of reporting bias could not be ruled out. Fifth, we were unable to investigate a dose-response relationship between the risk of PSD and circulating vitamin D concentration due to a lack of relevant data. Sixth, despite the previous identification of a low circulating vitamin D level as a risk factor for stroke (80), no data on serum vitamin D concentration before stroke occurrence were available in the included studies so that the relationship between vitamin D level, stroke, and depression could not be fully elucidated. Finally, the impacts of low vitamin D levels on other prognostic outcomes in this population were not assessed. Further studies are needed to address these issues.
The results of the current meta-analysis suggested an association of a low circulating vitamin D level with an over three-fold increase in the risk of post-stroke depression. Moreover, female gender, hyperlipidemia, high NIHSS score were linked to an increased risk or occurrence of post-stroke depression. Our findings may indicate the need for routine screening of circulating vitamin D concentration in patients with stroke and in other high-risk populations. Taking into consideration the limited number of studies available and their observational nature, further well-controlled prospective investigations are warranted to verify the correlation between vitamin D level and the development of post-stroke depression as well as to explore the efficacy of potential intervention strategies (e.g., vitamin D supplementation).
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
K-CH and J-YW: conceptualization. AI: methodology and software. C-CC and Y-JC: validation. K-CH and S-WL: formal analysis. K-FW and I-WC: investigation. I-WC: resources. I-WC and K-CH: data curation. K-CH, J-YW, I-WC, and C-KS: writing—original draft preparation. K-CH, I-WC, and C-KS: writing—review and editing. C-KS: visualization and supervision. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1142035/full#supplementary-material
1. Nickel A, Thomalla G. Post-stroke depression: impact of lesion location and methodological limitations-A topical review. Front Neurol. (2017) 8:498. doi: 10.3389/fneur.2017.00498
2. Robinson R, Jorge R. Post-stroke depression: a review. Am J Psychiatry. (2016) 173:221–31. doi: 10.1176/appi.ajp.2015.15030363
3. Towfighi A, Ovbiagele B, El Husseini N, Hackett M, Jorge R, Kissela B, et al. Poststroke depression: a scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2017) 48:e30–43. doi: 10.1161/STR.0000000000000113
4. Medeiros G, Roy D, Kontos N, Beach S. Post-stroke depression: a 2020 updated review. Gen Hosp Psychiatry. (2020) 66:70–80. doi: 10.1016/j.genhosppsych.2020.06.011
5. Bolshakov A, Tret’yakova L, Kvichansky A, Gulyaeva N. Glucocorticoids: Dr. Jekyll and Mr. Hyde of hippocampal neuroinflammation. Biochemistry. (2021) 86:156–67. doi: 10.1134/S0006297921020048
6. Gulyaeva N. Functional neurochemistry of the ventral and dorsal hippocampus: stress, depression, dementia and remote hippocampal damage. Neurochem Res. (2019) 44:1306–22. doi: 10.1007/s11064-018-2662-0
7. Ayerbe L, Ayis S, Crichton S, Wolfe C, Rudd A. The natural history of depression up to 15 years after stroke: the South London stroke register. Stroke. (2013) 44:1105–10. doi: 10.1161/STROKEAHA.111.679340
8. Ménard C, Hodes G, Russo S. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. (2016) 321:138–62. doi: 10.1016/j.neuroscience.2015.05.053
9. Shi K, Tian D, Li Z, Ducruet A, Lawton M, Shi F. Global brain inflammation in stroke. Lancet Neurol. (2019) 18:1058–66. doi: 10.1016/S1474-4422(19)30078-X
10. Tang Y, Xu H, Du X, Lit L, Walker W, Lu A, et al. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: a microarray study. J Cereb Blood Flow Metab. (2006) 26:1089–102. doi: 10.1038/sj.jcbfm.9600264
11. Mitchell A, Sheth B, Gill J, Yadegarfar M, Stubbs B, Yadegarfar M, et al. Prevalence and predictors of post-stroke mood disorders: a meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen Hosp Psychiatry. (2017) 47:48–60. doi: 10.1016/j.genhosppsych.2017.04.001
12. Hackett M, Anderson C. Predictors of depression after stroke: a systematic review of observational studies. Stroke. (2005) 36:2296–301. doi: 10.1161/01.STR.0000183622.75135.a4
13. Babkair L. Risk factors for poststroke depression: an integrative review. J Neurosci Nurs. (2017) 49:73–84. doi: 10.1097/JNN.0000000000000271
14. De Ryck A, Brouns R, Geurden M, Elseviers M, De Deyn P, Engelborghs S. Risk factors for poststroke depression: identification of inconsistencies based on a systematic review. J Geriatr Psychiatry Neurol. (2014) 27:147–58. doi: 10.1177/0891988714527514
15. Khazai N, Judd S, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. (2008) 10:110–7. doi: 10.1007/s11926-008-0020-y
16. Martens P, Gysemans C, Verstuyf A, Mathieu A. Vitamin D’s effect on immune function. Nutrients. (2020) 12:1248. doi: 10.3390/nu12051248
17. Muñoz A, Grant W. Vitamin D and cancer: an historical overview of the epidemiology and mechanisms. Nutrients. (2022) 14:1448. doi: 10.3390/nu14071448
18. de la Guía-Galipienso F, Martínez-Ferran M, Vallecillo N, Lavie C, Sanchis-Gomar F, Pareja-Galeano H. Vitamin D and cardiovascular health. Clin Nutr. (2021) 40:2946–57. doi: 10.1016/j.clnu.2020.12.025
19. Hung K, Wang L, Lin Y, Yu C, Chang C, Sun C, et al. Association of preoperative vitamin D deficiency with the risk of postoperative delirium and cognitive dysfunction: a meta-analysis. J Clin Anesth. (2022) 79:110681. doi: 10.1016/j.jclinane.2022.110681
20. de Oliveira C, Hirani V, Biddulph J. Associations between vitamin D levels and depressive symptoms in later life: evidence from the English longitudinal study of ageing (ELSA). J Gerontol A Biol Sci Med Sci. (2018) 73:1377–82. doi: 10.1093/gerona/glx130
21. Tan Q, Liu S, Chen D. Poor vitamin D status and the risk of maternal depression: a dose-response meta-analysis of observational studies. Public Health Nutr. (2021) 24:2161–70. doi: 10.1017/S1368980019004919
22. Vellekkatt F, Menon V. Efficacy of vitamin D supplementation in major depression: a meta-analysis of randomized controlled trials. J Postgrad Med. (2019) 65:74–80.
23. Anglin R, Samaan Z, Walter S, McDonald S. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:100–7. doi: 10.1192/bjp.bp.111.106666
24. He W, Ruan Y. Poor sleep quality, vitamin D deficiency and depression in the stroke population: a cohort study. J Affect Disord. (2022) 308:199–204. doi: 10.1016/j.jad.2022.04.031
25. Wang Q, Zhu Z, Liu Y, Tu X, He J. Relationship between serum vitamin D levels and inflammatory markers in acute stroke patients. Brain Behav. (2018) 8:e00885. doi: 10.1002/brb3.885
26. Yue W, Xiang L, Zhang Y, Ji Y, Li X. Association of serum 25-hydroxyvitamin D with symptoms of depression after 6 months in stroke patients. Neurochem Res. (2014) 39:2218–24. doi: 10.1007/s11064-014-1423-y
27. Miao H, Zhu H, Luan X, Huang G, Chen M, Yuan Z, et al. Risk factors of vitamin D deficiency in Chinese ischemic stroke patients: a cross-sectional study. Front Aging Neurosci. (2020) 12:613498. doi: 10.3389/fnagi.2020.613498
28. Rad R, Zarbakhsh M, Sarabi S. The relationship of vitamin D deficiency with severity and outcome of acute stroke. Rom J Intern Med. (2021) 59:351–8. doi: 10.2478/rjim-2021-0013
29. Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
30. Hung K, Chang Y, Chen I, Soong T, Ho C, Hsing C, et al. Efficacy of high flow nasal oxygenation against hypoxemia in sedated patients receiving gastrointestinal endoscopic procedures: a systematic review and meta-analysis. J Clin Anesth. (2022) 77:110651. doi: 10.1016/j.jclinane.2022.110651
31. Hung K, Wu S, Hsu C, Ko C, Chen J, Huang P, et al. Efficacy of laryngeal mask airway against postoperative pharyngolaryngeal complications following thyroid surgery: a systematic review and meta-analysis of randomized controlled studies. Sci Rep. (2022) 12:18210. doi: 10.1038/s41598-022-21989-5
32. Gu Y, Zhu Z, Luan X, He J. Vitamin D status and its association with season, depression in stroke. Neurosci Lett. (2019) 690:99–105. doi: 10.1016/j.neulet.2018.09.046
33. Han B, Lyu Y, Sun H, Wei Y, He J. Low serum levels of vitamin D are associated with post-stroke depression. Eur J Neurol. (2015) 22:1269–74. doi: 10.1111/ene.12607
34. Kim S, Seok H, Kim D. Relationship between serum vitamin D levels and symptoms of depression in stroke patients. Ann Rehabil Med. (2016) 40:120–5. doi: 10.5535/arm.2016.40.1.120
35. Ren W, Gu Y, Zhu L, Wang L, Chang Y, Yan M, et al. The effect of cigarette smoking on vitamin D level and depression in male patients with acute ischemic stroke. Compr Psychiatry. (2016) 65:9–14. doi: 10.1016/j.comppsych.2015.09.006
36. Khan B, Shafiq H, Abbas S, Jabeen S, Khan S, Afsar T, et al. Vitamin D status and its correlation to depression. Ann Gen Psychiatry. (2022) 21:32. doi: 10.1186/s12991-022-00406-1
37. El-Salem K, Khalil H, Al-Sharman A, Al-Mistarehi A, Yassin A, Alhayk K, et al. Serum vitamin d inversely correlates with depression scores in people with multiple sclerosis. Mult Scler Relat Disord. (2021) 48:102732. doi: 10.1016/j.msard.2020.102732
38. Kim G, Jeon G. Correlation between serum 25-hydroxyvitamin D level and depression among Korean women with secondary amenorrhea: a cross-sectional observational study. Nutrients. (2022) 14:2835. doi: 10.3390/nu14142835
39. Geng C, Shaikh A, Han W, Chen D, Guo Y, Jiang P. Vitamin D and depression: mechanisms, determination and application. Asia Pac J Clin Nutr. (2019) 28:689–94.
40. Akpınar Ş, Karadağ M. Is vitamin D important in anxiety or depression? What is the truth? Curr Nutr Rep. (2022) 11:675–81. doi: 10.1007/s13668-022-00441-0
41. Grudet C, Wolkowitz O, Mellon S, Malm J, Reus V, Brundin L, et al. Vitamin D and inflammation in major depressive disorder. J Affect Disord. (2020) 267:33–41. doi: 10.1016/j.jad.2020.01.168
42. Dogan-Sander E, Mergl R, Willenberg A, Baber R, Wirkner K, Riedel-Heller S, et al. Inflammation and the association of vitamin D and depressive symptomatology. Nutrients. (2021) 13:1972. doi: 10.3390/nu13061972
43. Amini S, Jafarirad S, Amani R. Postpartum depression and vitamin D: a systematic review. Crit Rev Food Sci Nutr. (2019) 59:1514–20. doi: 10.1080/10408398.2017.1423276
44. Koshkina A, Dudnichenko T, Baranenko D, Fedotova J, Drago F. Effects of vitamin D(3) in long-term ovariectomized rats subjected to chronic unpredictable mild stress: BDNF, NT-3, and NT-4 implications. Nutrients. (2019) 11:1726. doi: 10.3390/nu11081726
45. Jiang H, Xiao L, Jin K, Shao B. Estrogen administration attenuates post-stroke depression by enhancing CREB/BDNF/TrkB signaling in the rat hippocampus. Exp Ther Med. (2021) 21:433. doi: 10.3892/etm.2021.9850
46. Su Q, Cheng Y, Jin K, Cheng J, Lin Y, Lin Z, et al. Estrogen therapy increases BDNF expression and improves post-stroke depression in ovariectomy-treated rats. Exp Ther Med. (2016) 12:1843–8. doi: 10.3892/etm.2016.3531
47. Zhao D, Ouyang P, de Boer I, Lutsey P, Farag Y, Guallar E, et al. Serum vitamin D and sex hormones levels in men and women: the multi-ethnic study of atherosclerosis (MESA). Maturitas. (2017) 96:95–102. doi: 10.1016/j.maturitas.2016.11.017
48. Ronaldson A, Arias de la Torre J, Gaughran F, Bakolis I, Hatch S, Hotopf M, et al. Prospective associations between vitamin D and depression in middle-aged adults: findings from the UK Biobank cohort. Psychol Med. (2022) 52:1866–74. doi: 10.1017/S0033291720003657
49. Shaffer J, Edmondson D, Wasson L, Falzon L, Homma K, Ezeokoli N, et al. Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosom Med. (2014) 76:190–6. doi: 10.1097/PSY.0000000000000044
50. Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. (2014) 6:1501–18. doi: 10.3390/nu6041501
51. Hackett M, Yapa C, Parag V, Anderson C. Frequency of depression after stroke: a systematic review of observational studies. Stroke. (2005) 36:1330–40. doi: 10.1161/01.STR.0000165928.19135.35
52. Brodaty H, Withall A, Altendorf A, Sachdev P. Rates of depression at 3 and 15 months poststroke and their relationship with cognitive decline: the Sydney stroke study. Am J Geriatr Psychiatry. (2007) 15:477–86. doi: 10.1097/JGP.0b013e3180590bca
53. Ezema C, Akusoba P, Nweke M, Uchewoke C, Agono J, Usoro G. Influence of post-stroke depression on functional independence in activities of daily living. Ethiop J Health Sci. (2019) 29:841–6. doi: 10.4314/ejhs.v29i1.5
54. Hadidi N, Treat-Jacobson D, Lindquist R. Poststroke depression and functional outcome: a critical review of literature. Heart Lung. (2009) 38:151–62. doi: 10.1016/j.hrtlng.2008.05.002
55. Das J, G K. Post stroke depression: the sequelae of cerebral stroke. Neurosci Biobehav Rev. (2018) 90:104–14. doi: 10.1016/j.neubiorev.2018.04.005
56. Ghose S, Williams L, Swindle R. Depression and other mental health diagnoses after stroke increase inpatient and outpatient medical utilization three years poststroke. Med Care. (2005) 43:1259–64. doi: 10.1097/01.mlr.0000185711.50480.13
57. Wang Y, Wang Y, Zhang B, Lin Y, Tan S, Lu Z. Depressed serum 25-hydroxyvitamin D levels increase hospital stay and alter glucose homeostasis in first-ever ischemic stroke. Curr Neurovasc Res. (2019) 16:340–7. doi: 10.2174/1567202616666190924161947
58. Jiang W, Wu D, Xiao G, Ding B, Chen E. An epidemiology survey of vitamin D deficiency and its influencing factors. Med Clin. (2020) 154:7–12. doi: 10.1016/j.medcli.2019.03.019
59. Neo J, Kong K. Prevalence of vitamin D deficiency in elderly patients admitted to an inpatient rehabilitation unit in tropical Singapore. Rehabil Res Pract. (2016) 2016:9689760. doi: 10.1155/2016/9689760
60. Wang S, Wang Y, Zhang Q, Wu S, Ng C, Ungvari G, et al. Cognitive behavioral therapy for post-stroke depression: a meta-analysis. J Affect Disord. (2018) 235:589–96. doi: 10.1016/j.jad.2018.04.011
61. Shao D, Zhao Z, Zhang Y, Zhou X, Zhao L, Dong M, et al. Efficacy of repetitive transcranial magnetic stimulation for post-stroke depression: a systematic review and meta-analysis of randomized clinical trials. Braz J Med Biol Res. (2021) 54:e10010. doi: 10.1590/1414-431x202010010
62. Liang X, Hao Y, Duan X, Han X, Cai X. Hyperbaric oxygen therapy for post-stroke depression: a systematic review and meta-analysis. Clin Neurol Neurosurg. (2020) 195:105910. doi: 10.1016/j.clineuro.2020.105910
63. Zhou S, Liu S, Liu X, Zhuang W. Selective serotonin reuptake inhibitors for functional independence and depression prevention in early stage of post-stroke: a meta-analysis. Medicine. (2020) 99:e19062. doi: 10.1097/MD.0000000000019062
64. Mortensen J, Andersen G. Pharmacological management of post-stroke depression: an update of the evidence and clinical guidance. Expert Opin Pharmacother. (2021) 22:1157–66. doi: 10.1080/14656566.2021.1880566
65. Zhang K, Cui G, Gao Y, Shen W. Does acupuncture combined with antidepressants have a better therapeutic effect on post-stroke depression? A systematic review and meta-analysis. Acupunct Med. (2021) 39:432–40. doi: 10.1177/0964528420967675
66. Kaviani M, Nikooyeh B, Zand H, Yaghmaei P, Neyestani T. Effects of vitamin D supplementation on depression and some involved neurotransmitters. J Affect Disord. (2020) 269:28–35. doi: 10.1016/j.jad.2020.03.029
67. de Koning E, Lips P, Penninx B, Elders P, Heijboer A, den Heijer M, et al. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. Am J Clin Nutr. (2019) 110:1119–30. doi: 10.1093/ajcn/nqz141
68. Zhu C, Zhang Y, Wang T, Lin Y, Yu J, Xia Q, et al. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. (2020) 10:e01760. doi: 10.1002/brb3.1760
69. Marek K, Cichoń N, Saluk-Bijak J, Bijak M, Miller E. The role of vitamin D in stroke prevention and the effects of its supplementation for post-stroke rehabilitation: a narrative review. Nutrients. (2022) 14:2761. doi: 10.3390/nu14132761
70. Al-Khudhairy M, AlOtaibi A, AbdulRahman L, Al-Garni M, Yaslam R, Fatani R. The association of self-reported iron and vitamin D levels on sleep quality and pain perception in a subset of Saudi population. Risk Manag Healthc Policy. (2021) 14:4853–65. doi: 10.2147/RMHP.S318698
71. Fan X, Yang Y, Wang S, Zhang Y, Wang A, Liao X, et al. Impact of persistent poor sleep quality on post-stroke anxiety and depression: a national prospective clinical registry study. Nat Sci Sleep. (2022) 14:1125–35. doi: 10.2147/NSS.S357536
72. Huang W, Shah S, Long Q, Crankshaw A, Tangpricha V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin J Pain. (2013) 29:341–7. doi: 10.1097/AJP.0b013e318255655d
73. Dong L, Sánchez B, Skolarus L, Morgenstern L, Lisabeth L. Ethnic differences in prevalence of post-stroke depression. Circ Cardiovasc Qual Outcomes. (2018) 11:e004222. doi: 10.1161/CIRCOUTCOMES.117.004222
74. Goldmann E, Roberts E, Parikh N, Lord A, Boden-Albala B. Race/ethnic differences in post-stroke depression (PSD): findings from the stroke warning information and faster treatment (SWIFT) study. Ethn Dis. (2016) 26:1–8. doi: 10.18865/ed.26.1.1
75. Poynter B, Hon M, Diaz-Granados N, Kapral M, Grace S, Stewart D. Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics. (2009) 50:563–9. doi: 10.1016/S0033-3182(09)70857-6
76. Paprocka-Borowicz M, Wiatr M, Ciałowicz M, Borowicz W, Kaczmarek A, Marques A, et al. Influence of physical activity and socio-economic status on depression and anxiety symptoms in patients after stroke. Int J Environ Res Public Health. (2021) 18:8058. doi: 10.3390/ijerph18158058
77. Milaneschi Y, Simmons W, van Rossum E, Penninx B. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
78. Hua J, Lu J, Tang X, Fang Q. Association between geriatric nutritional risk index and depression after ischemic stroke. Nutrients. (2022) 14:2698. doi: 10.3390/nu14132698
79. Cherian L, Agarwal P, Holland T, Schneider J, Aggarwal N. Western diet associated with increased post-stroke depressive symptoms. J Nutr Sci. (2022) 11:e44. doi: 10.1017/jns.2022.38
Keywords: vitamin D, stroke, depression, nutrition, post-stroke depression
Citation: Hung K-C, Wu J-Y, Illias AM, Chiu C-C, Chang Y-J, Liao S-W, Wang K-F, Chen I-W and Sun C-K (2023) Association of a low vitamin D status with risk of post-stroke depression: A meta-analysis and systematic review. Front. Nutr. 10:1142035. doi: 10.3389/fnut.2023.1142035
Received: 11 January 2023; Accepted: 02 February 2023;
Published: 16 February 2023.
Edited by:
Yashi Mi, University of Arizona, United StatesReviewed by:
Shaowei Wang, University of Southern California, United StatesCopyright © 2023 Hung, Wu, Illias, Chiu, Chang, Liao, Wang, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheuk-Kwan Sun,  cmVzZWFyY2hnYXRlMDAwQGdtYWlsLmNvbQ==; I-Wen Chen,
cmVzZWFyY2hnYXRlMDAwQGdtYWlsLmNvbQ==; I-Wen Chen,  bWF2aXNpbmdAZ21haWwuY29t
bWF2aXNpbmdAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.