
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 21 February 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1135854
This article is part of the Research Topic Body Composition Changes and Nutrition Therapy in Surgical Oncology Patients View all 14 articles
 Lijuan Wang1
Lijuan Wang1 Pengxue Li2,3
Pengxue Li2,3 Yifu Hu2,3,4
Yifu Hu2,3,4 Bo Cheng1
Bo Cheng1 Lili Ding1
Lili Ding1 Lei Li2,3
Lei Li2,3 Jinghai Song2,3
Jinghai Song2,3 Junmin Wei2,3*
Junmin Wei2,3* Jingyong Xu2,3,4*
Jingyong Xu2,3,4*Objective: To analyze the correlation between preoperative nutritional status, frailty, sarcopenia, body composition, and anthropometry in geriatric inpatients undergoing major pancreatic and biliary surgery.
Methods: This is a cross-sectional study of the database from December 2020 to September 2022 in the department of hepatopancreatobiliary surgery, Beijing Hospital. Basal data, anthropometry, and body composition were recorded. NRS 2002, GLIM, FFP 2001, and AWGS 2019 criteria were performed. The incidence, overlap, and correlation of malnutrition, frailty, sarcopenia, and other nutrition-related variables were investigated. Group comparisons were implemented by stratification of age and malignancy. The present study adhered to the STROBE guidelines for cross-sectional study.
Results: A total of 140 consecutive cases were included. The prevalence of nutritional risk, malnutrition, frailty, and sarcopenia was 70.0, 67.1, 20.7, and 36.4%, respectively. The overlaps of malnutrition with sarcopenia, malnutrition with frailty, and sarcopenia with frailty were 36.4, 19.3, and 15.0%. There is a positive correlation between every two of the four diagnostic tools, and all six p-values were below 0.002. Albumin, prealbumin, CC, GS, 6MTW, ASMI, and FFMI showed a significantly negative correlation with the diagnoses of the four tools. Participants with frailty or sarcopenia were significantly more likely to suffer from malnutrition than their control groups with a 5.037 and 3.267 times higher risk, respectively (for frailty, 95% CI: 1.715–14.794, p = 0.003 and for sarcopenia, 95% CI: 2.151–4.963, p<0.001). Summarizing from stratification analysis, most body composition and function variables were worsen in the ≥70 years group than in the younger group, and malignant patients tended to experience more intake reduction and weight loss than the benign group, which affected the nutrition diagnosis.
Conclusion: Elderly inpatients undergoing major pancreatic and biliary surgery possessed high prevalence and overlap rates of malnutrition, frailty, and sarcopenia. Body composition and function deteriorated obviously with aging.
The geriatric syndrome refers to a range of multifactorial health conditions representing the accumulation of multiple system impairments in older adults. Malnutrition, frailty, and sarcopenia are three common geriatric syndromes, which can substantially lead to poor outcomes, such as disability, dysfunction, falls, and perioperative complications, and thereby increase the length of hospital stay (LOS) and the cost of hospitalization, and result in long-term care or even mortality (1–5).
Malnutrition or undernutrition refers to deficiencies in nutritional intake resulting in altered body composition, and approximately 1/3 of Chinese geriatric inpatients experience malnutrition (6). In the department of hepatopancreatobiliary surgery, the prevalence of nutritional risk and malnutrition are as high as 69.7 and 56.6% in our former study (7). Frailty is characterized by a cumulative decline in the physiological capacity of multiple organ systems and increased vulnerability to endogenous and exogenous stressors, with an estimated prevalence ranging from 18.8 to 41.9% in geriatric surgical patients and from 10.4 to 37.0% in general surgical patients (8, 9). Sarcopenia is an age-related syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, which accounts for 17.4% of Chinese community-dwelling and hospitalized elderly (10). In pancreatic surgery, the prevalence is 38.8% determined by the total psoas area index in CT scan (7).
Frailty, sarcopenia, and malnutrition have independent diagnostic criteria, but share many components, such as weight loss, muscle mass, or strength loss, and often coexist or overlap in elderly inpatients (11). In a recent systematic review, it was concluded that about half of the hospitalized older patients suffer from 2 or perhaps 3 of these debilitating conditions, and standardized screening for these conditions is highly controversial to guide nutritional and physical interventions (12). Due to the significant influence of these three clinical problems on outcomes, respectively, it is important to understand the current situation and provide basal data for further cohort study. So our study aims to investigate the prevalence and overlap of these conditions in the elderly who are going to receive major pancreatic and biliary surgery.
This study is a cross-sectional study analyzing the daily database of the Department of hepatopancreatobiliary surgery, Beijing Hospital. From December 2020 to September 2022, 205 consecutive patients undergoing major pancreatic and biliary surgery were screened, and then, 140 elderly patients were recruited in this study.
The inclusion criteria of this study are as follows: (1) age ≥60 years old, which is the age cut-off of older adults defined by the Nation Health Commission of China (13); (2) major pancreatic and biliary surgery, containing pancreatectomy (Whipple procedure, distal pancreatectomy, and local pancreatectomy), bile-enteral bypass due to malignant obstructive, and bile duct exploration; (3) voluntary enrollment and signed informed consent. Exclusion criteria contain (1) emergency operation; (2) cancer patients who underwent adjuvant therapy before operation; (3) severe disability or dementia, inability to cooperate with frailty and sarcopenia assessment or effective communication; (4) refusal of informed consent. The Ethics Committee of Beijing Hospital approved the study protocol and written informed consents were obtained from all participants. (Approval letter No. 2020BJYYEC-218-01). The present study adhered to the STROBE guidelines for cross-sectional study. Figure 1 shows the flowchart of this study.
The basal data include sex, age, height, weight, body mass index (BMI), co-morbidities, and serum examination (complete blood count, liver function, renal function, albumin, glucose, et al.). According to the standard of the guidelines for prevention and control of overweight and obesity in Chinese adults, a BMI < 18.5 kg/m2 was defined as underweight, 18.5 kg/m2 ≤ BMI < 24 kg/m2 was normal weight, 24 kg/m2 ≤ BMI < 28 kg/m2 was considered overweight, and BMI ≥ 28 kg/m2 was considered obesity (14).
A diet survey was conducted after admission. We recorded the change of diet before and after the diagnosis of the original disease, and calculated the contents composition, containing protein, carbohydrate, fat, and total energy.
Anthropometry was done 1 to 2 days after admission, including calf circumference (CC) and grip strength (GS), both of which, we used the average value of the left and right sides. To assess the functional status, 15-foot and 6-meter timed walk speed (6MTW) was conducted to get the walking speed. Bioelectrical impedance analysis (BIA) was applied with the InBody 720 bioimpedance body composition analyzer (Biospace Co., Ltd., Korea). Appendicular skeletal muscle mass index (ASMI) was calculated, which was the sum of the lean muscle mass of the upper and lower extremities adjusted with height. Also, the fat-free mass index (FFMI) was recorded. Visceral fat area (VFA), waist-hip ratio (WHR), and body fat percentage (BTP) were included to reflect fat metabolism.
We used Nutritional Risk Screening 2002 (NRS 2002) for nutritional screening for each patient within 24 h after admission, which was recommended by the European Society of Parenteral Enteral Nutrition (ESPEN) (15). NRS2002 contains three aspects: nutritional impairment: weight loss, intake reduction, and lower BMI (score 0–3), the severity of disease (score 0–3), and age [(score 0–1) (< 70 years: 0 scores and ≥ 70 years: 1 score)]. Scores for the final screening take into account all these three sections range from 0 to 7 and classify patients into one of two nutritional risk stages (or groups): at low nutritional risk group (NRS 2002 score < 3), and (moderate/high) risk of malnutrition group (NRS 2002 score ≥ 3). In pancreatic surgery, an NRS2002 score of more and equal to 5 was considered at high nutritional risk with remarkable clinical meaning (16).
The Global Leadership Initiative on Malnutrition (GLIM) criteria were implemented for malnutrition diagnosis and grading among patients with nutritional risk determined by NRS2002 (17). The framework of GLIM criteria includes three phenotypic criteria and two etiologic criteria, and the detailed items and cut-off values could be determined and modified in different centers and populations (18). In this study, we used GLIM criteria in a traditional way with the original criteria. Phenotypic criteria include (1) unintentional weight loss (WT): WT > 5% within the past 6 months, or WT > 10% beyond 6 months; (2) low BMI: BMI < 18.5 kg/m2 if age < 70 years, BMI < 20 kg/m2 if age ≥ 70 years; (3) reduced muscle mass: in our study, we used AMMI and FFMI assessed by BIA. AMMI < 7 kg/m2 or FFMI < 17 kg/m2 in men were considered patients with reduced muscle mass, and AMMI < 5.7 kg/m2 or FFMI < 15 kg/m2 in women were considered positive. Etiologic criteria include: (1) Reduced food intake: ≤50% of needs from 1 to 2 weeks, or any reduction for >2 weeks; (2) Disease burden or inflammation: in this study, most of the patients were suffering from malignancies and the co-morbidities were also taken into account. If at least one criterion was fulfilled in each section, malnutrition can be diagnosed.
The grading of malnutrition also followed the GLIM criteria. Unintentional weight loss (WT) > 10% within the past 6 months or WT > 20% beyond 6 months or low BMI (BMI < 17.0 kg/m2 if age < 70 years or BMI < 17.8 kg/m2 if age ≥ 70 years) or severe muscle deficit were defined as severe malnutrition. 5–10% Unintentional weight loss (WT) within the past 6 months or 10–20% WT beyond 6 months or low BMI (17.0 ≤ BMI < 20.0 kg/m2 if age < 70 years or 17.8 ≤ BMI < 22.0 kg/m2 if age ≥ 70 years) or Mild-to-Moderate muscle deficit were the grading criteria for moderate malnutrition.
In this study, we used the criteria for sarcopenia diagnosis recommended by the Asian Working Group for Sarcopenia (AWGS) (19). For patients in acute to chronic health care or clinical research settings, a two-step protocol was used: finding cases and diagnosis. In the first step, we tended to use objective criterion, so calf circumference (CC) (<34 cm in male, <33 cm in female) was facilitated to find cases at risk of sarcopenia, based on which, in the second step, sarcopenia can be diagnosed as follows: (1) Muscle strength: men with grip strength (GS) < 28 kg, women with GS < 18 kg; (2) Physical performance: 6-meter walk < 1 m/s; (3) AMMI: men with AMMI < 7 kg/m2, women with AMMI < 5.7 kg/m2. The result containing low ASMI and low muscle strength or low physical performance was sarcopenia, and the result containing all three criteria was severe sarcopenia.
The Fried Frailty Phenotype (FFP) is a recommended assessment tool for frailty in geriatric patients by Chinese expert group consensus (20). FFP criteria include five physical items: (1) Shrinking: Unintentional weight loss: ≥5% of body weight in the prior year; (2) Poor endurance and energy: self-reported exhaustion; (3) Weakness: poorer GS; (4) Slowness: lower walk speed; (5) Low physical activity. Patients who fulfilled none of these five criteria were classified as the non-frailty group, who fulfilled 1 or 2 criteria were classified as the pre-frailty group, and who fulfilled ≥3 criteria were considered as the frailty group. The thresholds of GS and gait speed were referred to the AWGS criteria. Table 1 shows the comparison of all the above diagnostic tools we used in this study.
The sample size was calculated by PASS software 11.0 (NCSS LLC., Kaysville, UT, USA). The confidence level was set at 0.8. According to our former study, the prevalence of malnutrition was 56.6% and we set the proportion at 60% (7). The tolerance error was set at 10%, so the two-sided confidential interval width was 0.12. The final sample size was 125. All statistical analysis was performed by IBM SPSS Statistics for Windows, version 27.0 (IBMCorp., Armonk, NY, USA). Measurement data that correspond to normal distribution were presented as mean with standard deviation (SD) and analyzed by Student’s t-test. Measurement data that did not correspond to normal distribution were presented as median with interquartile range (IQR) and analyzed by Mann–Whitney U test. Categorical data were presented as counts and percentages, and compared by chi-square (χ2) test. Correlations were analyzed by Spearman’s correlation coefficient analysis according to the classification of variables. Multivariate analysis was performed by binary logistic regression to identify potential associated factors of malnutrition. A p-value < 0.05 were declared as statistically significant. All figures including flowchart, overlap bubble chart, and correlation heatmap were designed and drawn by Microsoft Office (Version 2016), and the regression analysis figure was drawn by GraphPad Prism version 7.0.0 for Windows (GraphPad Software, San Diego, CA, USA).
A total of 140 participants were included with a mean age of 70.0 ± 7.3 years. 58.6% (82/140) were male. 75% (105/140) of cases were malignancies, of which, 71 cases were pancreatic duct adenocarcinoma. The details of the history and blood test at admission are shown in Table 2.
Table 3 shows the data for nutrition assessment. The mean BMI was 23.5 ± 3.6 kg/m2. 83 cases (59.3%) experienced weight loss to varying degrees, in which, 66 cases exceeded 5%. According to NRS 2002, 70.0% (n = 98) of cases were at risk of nutrition. 94 cases (67.1%) were malnutrition and 49 cases (35.0%) were severe malnutrition according to GLIM criteria. Based on FFP criteria, 53.6% (n = 75) participants were pre-frailty, and 20.7% (n = 29) were frailty. According to the AWGS 2019 consensus, at the step of finding cases, 52.9% (n = 74) cases were at risk of sarcopenia determined by reduced calf circumference, among which, in the second step, 36.4% (n = 31) participants were diagnosed as sarcopenia, 24.2% (n = 34) fulfilled the criteria of severe sarcopenia. We also reported every diagnostic criterion in each tool in Table 3 to reflect the composition of every diagnosis.
Figure 2 displays the overlap of these three conditions, besides which, 21 (15.0%) cases fulfilled all three criteria, and 26 (18.6%) cases were considered normal by all three criteria. Furthermore, we did a stratification analysis between the patients who fulfilled three criteria and healthy patients. The results showed that there were more patients with malignant diseases (54.3% vs. 16.7%, p = 0,024) and older age (70.8% vs. 17.4%, p < 0.001) in the fulfill-three-criteria group.
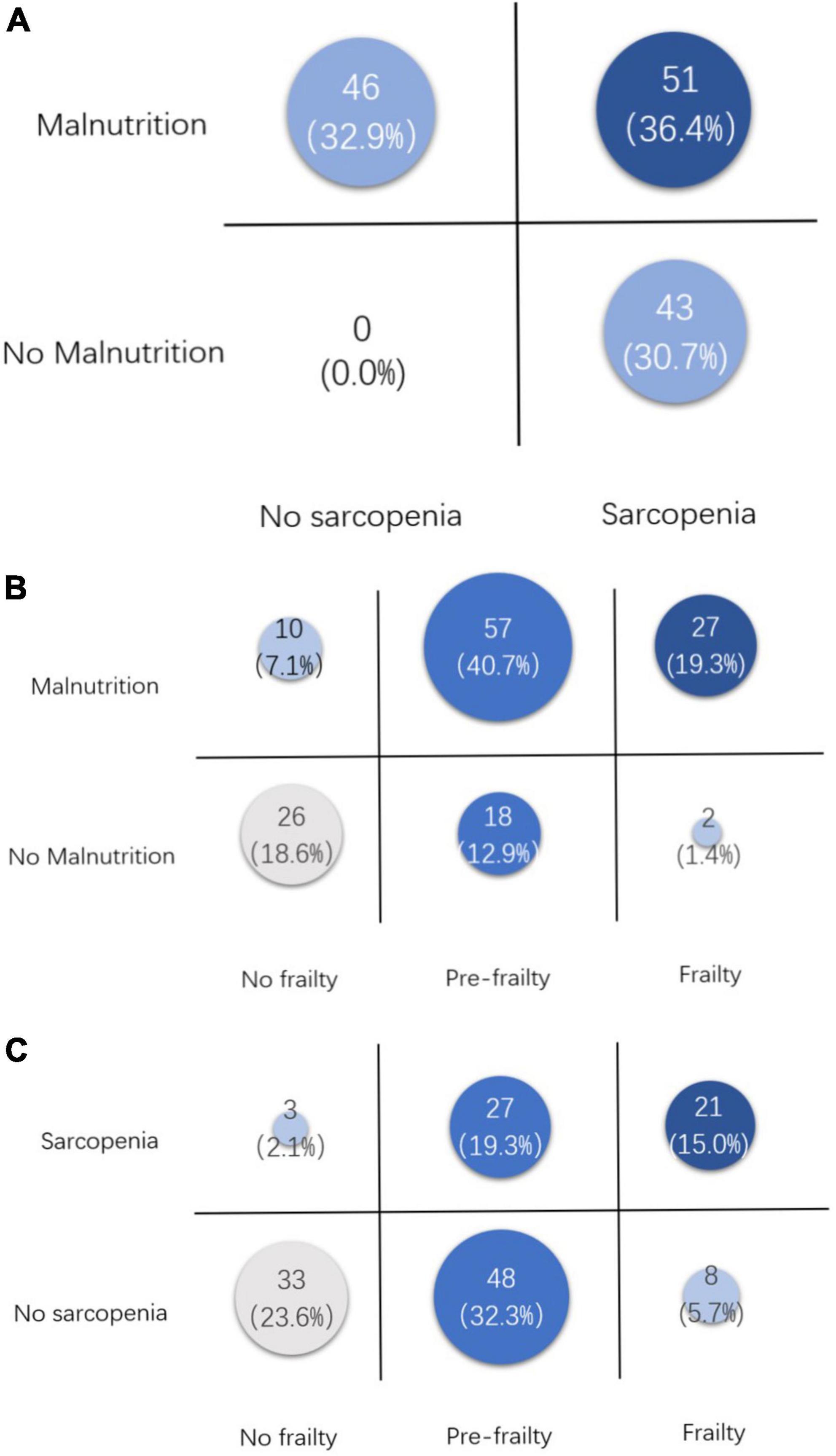
Figure 2. Overlaps between malnutrition, frailty, and sarcopenia. (A) Overlap between malnutrition and sarcopenia. (B) Overlap between malnutrition and frailty. (C) Overlap between sarcopenia and frailty.
A diet survey showed 71 cases had a decline in the intake of total energy, protein, and fat before and after the diagnosis of the disease. In Table 3, the amounts and percentages of reduction of energy, protein, and fat were shown in detail. Results of anthropometry and body composition analysis are also recorded in Table 3 and more men than women suffered from a decline in muscle mass and muscle-related function variables such as CC, GS, ASMI, and FFMI.
The patients were stratified by age and divided into the <70 years group and ≥70 years group. In Table 4, results show that the prevalence of nutritional risk, severe malnutrition, frailty, and sarcopenia were all significantly higher in the older group. Though there was no difference in the change in daily diet and weight, an obvious decline was found in both body composition and function. The changes in body composition appeared not only on the protein-related blood tests like hemoglobin, total protein, albumin, and pre-albumin, but also on the reduction of muscle mass (CC, ASMI, and FFMI), which logically affected the muscle function (e.g., GS and 6MTW).
When the patients were divided into malignant and benign groups, the results were completely different from the results in the groups stratified by age as above (Table 4). The main differences between malignant and benign groups were the changes in daily diet and weight, and no difference was found in body composition and function. Only prealbumin showed a significant decline in malignant disease in the benign disease group (16.6 ± 6.0 vs. 21.3 ± 10.2, p = 008), which was a sensitive variable to indicate recent nutrition changes. Both nutritional risk and severe nutritional risk were significantly higher in the malignant group, but no difference was found in the prevalence of GLIM-defined malnutrition. The malignant group possessed higher rates of frailty and sarcopenia.
Figure 3 is a heatmap showing the correlation between variables. From the perspective of overall color composition, the blue area shows a negative correlation between variables of serum test, body composition, and anthropometry with the four diagnostic tools. The red area could be divided into two parts: the part on the upper left of the blue area shows a positive correlation between every two of the four tools, and all six p-values were below 0.05; the part on the lower right of the blue area shows a positive correlation between variables of serum test, body composition, and anthropometry. In serum tests, hemoglobin, albumin, and prealbumin show a significant correlation with body composition and anthropometry. Body composition (BMI, ASMI, and FFMI) are well correlated with anthropometry (CC, GS, and 6MTW) with statistical significance.
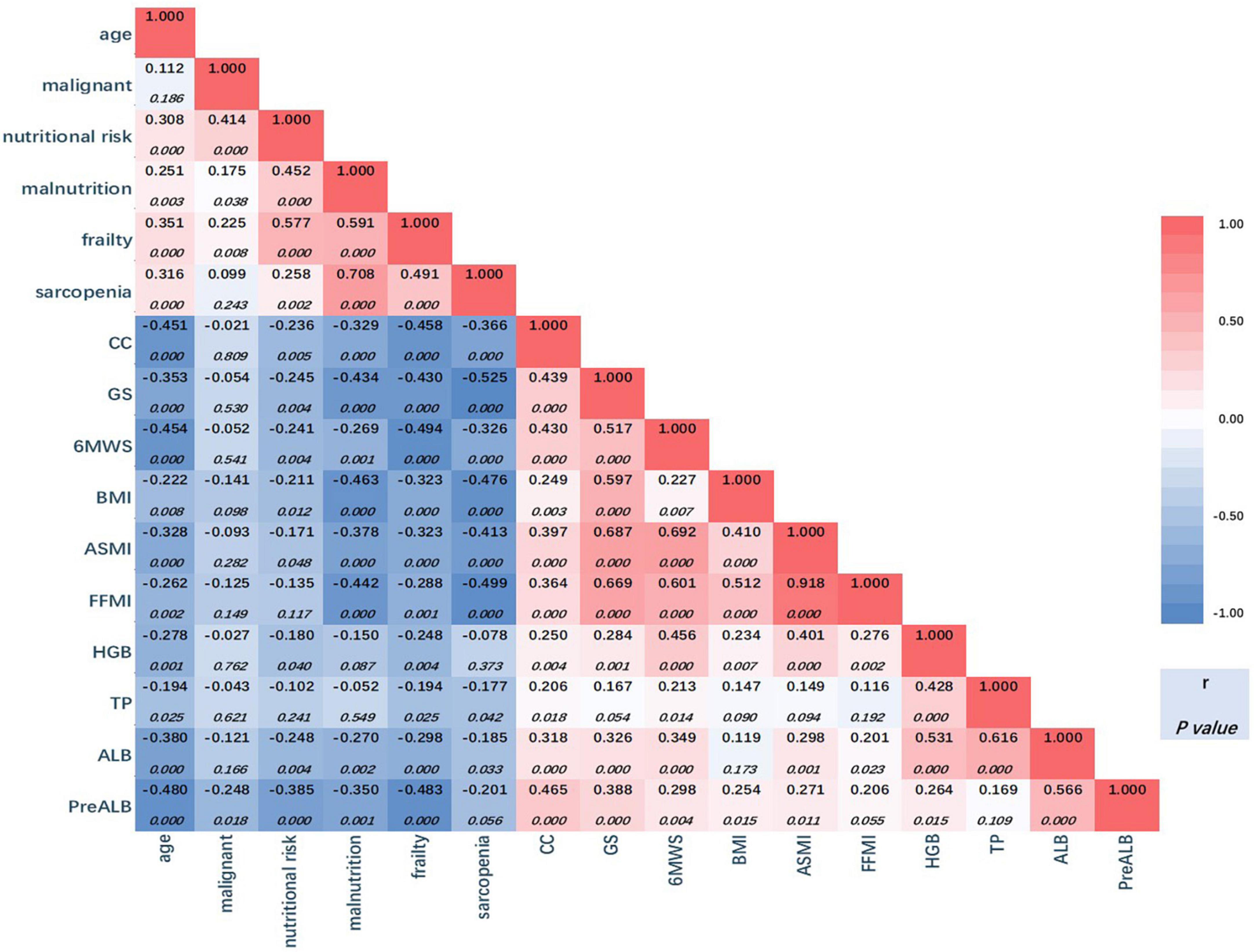
Figure 3. Correlation heatmap. The correlation coefficient numbers (r) are presented in the triangle, red for positive association, and blue for negative association. Darker colors indicate stronger associations (larger coefficient numbers). The significance levels for coefficients are presented below the r. CC, calf circumference; GS, grip strength; 6MWS, 6-meter walking speed; BMI, body mass index; ASMI, appendicular skeleton muscle index; FFMI, fat free mass index; HGB, hemoglobin; TP, total protein; ALB, albumin; PreALB, prealbumin.
The first column shows the correlation between age and other variables. The prevalence of nutritional risk, malnutrition, frailty, and sarcopenia were all positively correlated with age with significance, and all body composition and anthropometry variables were negatively correlated with age. Meanwhile, in the second column, nutritional risk, malnutrition, and frailty were proved to positively correlate with malignant diseases with statistical significance. However, a significant negative correlation was only found in prealbumin in all body composition and anthropometry variables (r = −0.248, p = 0.018).
After adjustment for age, malignant diseases, frailty, and sarcopenia with malnutrition as the dependent variables, multivariate logistic regression analysis showed that participants with frailty or sarcopenia were significantly more likely to suffer from malnutrition than their control groups with a 5.037 and 3.267 times higher risk, respectively (for frailty, 95% CI: 1.715–14.794, p = 0.003 and for sarcopenia, 95% CI: 2.151–4.963, p < 0.001) (Figure 4).
With increasing global aging problems, aging-related debilitating disorders, so-called geriatric syndrome, are becoming the hotspots of geriatric research. All frailty, sarcopenia, and malnutrition are components of geriatric syndrome and are closely interrelated and interdependent. Surgical patients suffer from a double attack of disease and aging. In our study, the prevalence of nutritional risk, and malnutrition are 70.0 and 67.1%, respectively, which are higher than in elderly patients with other gastrointestinal diseases (21). The prevalence of frailty is 20.7%, which is familiar to former articles and at a relatively higher proportion (9). The prevalence of sarcopenia is 36.4%, which is nearly the same as our data collected in pancreatic surgery diagnosed by a CT scan (7).
Diagnosis of malnutrition, frailty, and sarcopenia depend on the diagnostic tools, different tools might lead to different prevalence (22). In a recent systemic review, 18 tools of frailty diagnosis were reported, in which, FFP was the most commonly used one. Meanwhile, EWGSOP (European Working Group on Sarcopenia in Older People) criteria was the most commonly used in all and AWGS was the most commonly implemented in Asia. And for malnutrition, about thirteen tools were mentioned, besides which, BMI only and BMI with albumin were considered to be diagnostic criteria in three articles (12). However, it is difficult to avoid bias when calculating overlap data between different tools and it is still a controversy in this field. So in our study, we chose FFP, WGS, NRS2002, and GLIM to avoid selection bias.
Table 1 displays the comparison of FFP, WGS, NRS2002, and GLIM, which reflect the commonality and individuality of the tools. Weight loss was the only criterion shared by the four tools, which is not only for nutrition assessment but also a sensitive precursor for tumor diagnosis, especially for pancreatic cancer (23). Besides weight loss, NRS2002 and GLIM contain age, BMI, intake reduction, and assessment of disease (inflammation burden), which are relatively more comprehensive to assess the nutrition status. But no muscle assessment was contained in NRS2002, and GLIM contains the evaluation of muscle, but with a large range of measuring techniques. AWGS2019 and FFP2001 criteria are based on muscle assessment. The overlaps between frailty or sarcopenia and malnutrition were 19.3 and 36.4%, which are higher than was reported before (12). AWFS2019 criteria focus on both muscle mass and muscle function to diagnose sarcopenia, meanwhile, FFP2001 criteria only focus on muscle function and function-related symptoms like cognitive and behavioral impairment. So the overlap of sarcopenia and frailty (15.0%) was not as large as expected. Moreover, in this study, 21 (15.0%) cases fulfilled all three criteria, and 26 (18.6%) cases were considered normal or no risk by all three criteria. Therefore, due to different clinical values and low overlap rates, these diagnostic criteria would still coexist, and more comprehensive tools may be created and validated in the future.
According to the guidelines of ESPEN, malnutrition, sarcopenia, and frailty were treated as parallel definitions (24). The links between malnutrition and sarcopenia or frailty have already been explored in several cross-sectional studies, especially in older patients with chronic disease (25–27). In our study, in surgical patients, the correlations between these conditions were proved to be statistically significant, which were shown in Figure 3. However, it is difficult to judge the causal relationship between any two of these three statuses. Theoretically speaking, in this population, original surgical diseases lead to intake reduction and weight loss, which affected nutrients digestion and absorption, and then gave rise to the change in body composition, especially the change of muscle mass, sequentially muscular dysfunction. Nutrition risk or malnutrition seems to be the initiating factor (28, 29). Our results indicated that sarcopenia and frailty seemed to be risk factors for malnutrition, however, longitudinal studies are needed.
From the perspective of body composition in the criteria, BMI may not be sensitive enough to be used in the surgical population, only 5.7% of cases were lower than 18.5 kg/m2, and nearly 40% of patients suffered from overweight and obesity, in which, nearly 20% were sarcopenia (7). Even in pancreatic surgery, higher BMI was treated as a risk factor for a fatty pancreas and postoperative pancreatic fistula rather than a nutrition parameter (30). FFMI and ASMI, which reflect the real change in muscle mass, had become the focus of diagnostic criteria. In this study, FFMI accounted for 44.3% of phenotype criteria in GLIM, second only to weight loss. And it was proved to be well consistent in GLIM-defined malnutrition in this study and other reports (31). ASMI was the sum of the lean muscle mass of the upper and lower extremities adjusted with height, which was reported to be used in GLIM and well related to sarcopenia and frailty (32, 33). So with the improvement of availability and simplification of the examination method, ASMI and FFMI will become more popular in clinical practice.
In this study, we did stratification analyses by age and malignancy. Like reports from other centers, it was no doubt that nutritional status became worse with aging and malignant diagnosis (34). However, from Table 4, by comparing the data from the two stratifications, an interesting phenomenon was notable. In the age stratification, the significant differences were mainly in the changes in body composition and its related parameters, including basic metabolic rate (Harris-Benedict equation), muscle mass (CC, ASMI, and FFMI), muscle function (GS and 6MWS), BMI, and serum test (hemoglobin, total protein, albumin, and prealbumin), all of which reflected the long-term changes of the body due to aging rather than disease. Meanwhile, in the stratification of malignant diseases, the significant differences between malignant and benign groups were only weight loss and intake reduction, which were short-term changes due to the pathophysiologic characteristics of cancer, but no change in body composition existed. In the serum test, only pre-albumin was significantly lower in the malignant group, which has been proposed to be a useful nutritional biomarker due to its shorter half-life than albumin and correlated with different nutritional markers and higher mortality risk (35). So when referring to preoperative therapy, for patients with advanced age, we must pay attention to both nutrition support and function exercise, to improve long-term nutrition and function problems caused by aging, and increase preoperative reservation, which was defined as “prehabilitation” and needed a relatively longer period (36). And for cancer patients with nutritional risk or malnutrition, we should commit to increasing intake and improving nutrition status by different support routes even for a short period (16, 37). Prealbumin might be a biomarker to monitor the effectiveness of preoperative nutrition support but needs further study.
As we know, few researchers have been reported to study the effect of two of the three conditions in older adults, but the three conditions are rarely studied together (38). This study is the first one to study the overlap of these three conditions in pancreatic and biliary surgery. Since this is a cross-sectional study, we tried our best to follow the STROBE statement, but there must be some limitations that are difficult to avoid. First, we used a relatively lower confidence level (0.8) and prevalence of malnutrition to determine the sample size, which may underestimate the sample size, especially when we did the stratification analysis; second, a cross-sectional study could not verify the causal relationship. Although we used multivariate regression analysis, the aim was to explore the possible relevance and provide the necessary direction for future cohort studies. Third, the sample population is elderly, so whether the tangent point value can represent other populations should be a deeper study field and need more work.
Elderly inpatients undergoing major pancreatic and biliary surgery had a high prevalence and overlap rates of malnutrition, frailty, and sarcopenia. Body composition and function deteriorated obviously with aging. Patients with malignant diseases often suffer from short-term nutrition changes like intake reduction. Simple and effective biomarker needs to be explored and validated. Rational preoperative prehabilitation containing nutrition support and exercise should be considered in this population to reduce postoperative complications and mortality.
The data supporting this study’s findings are available from the corresponding author upon reasonable request. Requests to access the datasets should be directed to JX, eHVqaW5neW9uZ0Biamhtb2guY24=.
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Hospital. The patients/participants provided their written informed consent to participate in this study.
JX and JW: conception, design, and administrative support. JX, LL, JS, and JW: provision of study materials or patients. JX, YH, PL, LW, LD, and BC: collection, assembly of data, data analysis, and interpretation. LW, YH, and JX: manuscript writing. LW, PL, YH, BC, LD, LL, JS, JW, and JX: final approval of manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National High-Level Hospital Clinical Research Funding (No. BJ-2022-075), Beijing Hospital Nova Project (No. BJ-2020-082), and the Food Science and Technology Fund of the Chinese Institute of Food Science and Technology (No. 2021-M01).
We thank all the members of the Department of Hepato-Bilio-Pancreatic Surgery and Clinical Nutrition who help us to finish this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bieniek J, Wilczyński K, Szewieczek J. Fried frailty phenotype assessment components as applied to geriatric inpatients. Clin Interv Aging. (2016) 11:453–9. doi: 10.2147/CIA.S101369
2. Walston J, Buta B, Xue Q. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. (2018) 34:25–38. doi: 10.1016/j.cger.2017.09.004
3. Morley J, Vellas B, van Kan G, Anker S, Bauer J, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. (2013) 14:392–7.
4. Chen R, Zhao W, Zhang X, Liang H, Song N, Liu Z, et al. Relationship between frailty and long-term care needs in Chinese community-dwelling older adults: a cross-sectional study. BMJ Open. (2022) 12:e051801. doi: 10.1136/bmjopen-2021-051801
5. Ruiz A, Buitrago G, Rodríguez N, Gómez G, Sulo S, Gómez C, et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin Nutr. (2019) 38:1310–6.
6. Liang Y, Zhang Y, Li Y, Chen Y, Xu J, Liu M, et al. Identification of frailty and its risk factors in elderly hospitalized patients from different wards: a cross-sectional study in China. Clin Interv Aging. (2019) 14:2249–59. doi: 10.2147/CIA.S225149
7. Xu J, Li C, Zhang H, Liu Y, Wei J. Total psoas area index is valuable to assess sarcopenia, sarcopenic overweight/obesity and predict outcomes in patients undergoing open pancreatoduodenectomy. Risk Manag Healthc Policy. (2020) 13:761–70. doi: 10.2147/RMHP.S257677
8. Darvall J, Gregorevic K, Story D, Hubbard R, Lim W. Frailty indexes in perioperative and critical care: a systematic review. Arch Gerontol Geriatr. (2018) 79:88–96. doi: 10.1016/j.archger.2018.08.006
9. Hewitt J, Long S, Carter B, Bach S, McCarthy K, Clegg A. The prevalence of frailty and its association with clinical outcomes in general surgery: a systematic review and meta-analysis. Age Ageing. (2018) 47:793–800. doi: 10.1093/ageing/afy110
10. Ren X, Zhang X, He Q, Du L, Chen K, Chen S, et al. Prevalence of sarcopenia in Chinese community-dwelling elderly: a systematic review. BMC Public Health. (2022) 22:1702. doi: 10.1186/s12889-022-13909-z
11. Jeejeebhoy K. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: overlap of clinical features. Curr Opin Clin Nutr Metab Care. (2012) 15:213–9. doi: 10.1097/MCO.0b013e328352694f
12. Ligthart-Melis G, Luiking Y, Kakourou A, Cederholm T, Maier A, de van der Schueren M. Frailty, sarcopenia, and malnutrition frequently (Co-)occur in hospitalized older adults: a systematic review and meta-analysis. J Am Med Dir Assoc. (2020) 21:1216–28. doi: 10.1016/j.jamda.2020.03.006
13. Nation Health Commission of the People’s Republic of China. Standard for Healthy Chinese Older Adults (WS/T 802—2022). Beijing: Nation Health Commission of the People’s Republic of China (2022).
14. Chen C, Lu F, Department of Disease Control Ministry of Health, Pr China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. (2004) 17(Suppl.):1–36.
15. Kondrup J, Allison S, Elia M, Vellas B, Plauth M, Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (Espen). ESPEN guidelines for nutrition screening 2002. Clin Nutr. (2003) 22:415–21.
16. Xu J, Tian X, Song J, Chen J, Yang Y, Wei J. Preoperative nutrition support may reduce the prevalence of postoperative pancreatic fistula after open pancreaticoduodenectomy in patients with high nutritional risk determined by NRS2002. Biomed Res Int. (2021) 2021:6691966. doi: 10.1155/2021/6691966
17. Cederholm T, Jensen G, Correia M, Gonzalez M, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. (2019) 10:207–17.
18. Correia M, Tappenden K, Malone A, Prado C, Evans D, Sauer A, et al. Utilization and validation of the Global Leadership Initiative on Malnutrition (GLIM): a scoping review. Clin Nutr. (2022) 41:687–97. doi: 10.1016/j.clnu.2022.01.018
19. Chen L, Woo J, Assantachai P, Auyeung T, Chou M, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
20. Fried L, Tangen C, Walston J, Newman A, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–56.
21. Liu C, Lu Z, Li Z, Xu J, Cui H, Zhu M. Influence of malnutrition according to the GLIM criteria on the clinical outcomes of hospitalized patients with cancer. Front Nutr. (2021) 8:774636. doi: 10.3389/fnut.2021.774636
22. Xu J, Zhu M, Zhang H, Li L, Tang P, Chen W, et al. A cross-sectional study of GLIM-defined malnutrition based on new validated calf circumference cut-off values and different screening tools in hospitalised patients over 70 years old. J Nutr Health Aging. (2020) 24:832–8. doi: 10.1007/s12603-020-1386-4
23. Nicholson B, Thompson M, Hobbs F, Nguyen M, McLellan J, Green B, et al. Measured weight loss as a precursor to cancer diagnosis: retrospective cohort analysis of 43 302 primary care patients. J Cachexia Sarcopenia Muscle. (2022) 13:2492–503. doi: 10.1002/jcsm.13051
24. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff S, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64.
25. Vettoretti S, Caldiroli L, Armelloni S, Ferrari C, Cesari M, Messa P. Sarcopenia is associated with malnutrition but not with systemic inflammation in older persons with advanced CKD. Nutrients. (2019) 11:1378. doi: 10.3390/nu11061378
26. Marco E, Sánchez-Rodríguez D, Dávalos-Yerovi V, Duran X, Pascual E, Muniesa J, et al. Malnutrition according to ESPEN consensus predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. Clin Nutr. (2019) 38:2180–6. doi: 10.1016/j.clnu.2018.09.014
27. Laur C, McNicholl T, Valaitis R, Keller H. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl Physiol Nutr Metab. (2017) 42:449–58.
28. Scott D, Jones G. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int. (2014) 25:791–2.
29. Beaudart C, Sanchez-Rodriguez D, Locquet M, Reginster J, Lengelé L, Bruyère O. Malnutrition as a strong predictor of the onset of sarcopenia. Nutrients. (2019) 11:2883. doi: 10.3390/nu11122883
30. Zhou L, Xiao W, Li C, Gao Y, Gong W, Lu G. Impact of fatty pancreas on postoperative pancreatic fistulae: a meta-analysis. Front Oncol. (2021) 11:622282. doi: 10.3389/fonc.2021.622282
31. Gao X, Liu H, Zhang L, Tian H, Zhou D, Li G, et al. The application value of preoperative fat-free mass index within global leadership Initiative on Malnutrition-defined malnutrition criteria for postoperative outcomes in patients with esophagogastric cancer. Nutrition. (2022) 102:111748. doi: 10.1016/j.nut.2022.111748
32. Liu H, Gao X, Zhang L, Zhang Y, Wang X. Application of the GLIM criteria in patients with intestinal insufficiency and intestinal failure at nutritional risk on admission. Eur J Clin Nutr. (2022) 76:1003–9. doi: 10.1038/s41430-022-01084-8
33. Gillis C, Fenton T, Gramlich L, Sajobi T, Culos-Reed S, Bousquet-Dion G. Older frail prehabilitated patients who cannot attain a 400 m 6-min walking distance before colorectal surgery suffer more postoperative complications. Eur J Surg Oncol. (2021) 47:874–81. doi: 10.1016/j.ejso.2020.09.041
34. Wang J, Zhuang Q, Tan S, Xu J, Zhang Y, Yan M, et al. Loss of body weight and skeletal muscle negatively affect postoperative outcomes after major abdominal surgery in geriatric patients with cancer. Nutrition. (2022) 106:111907. doi: 10.1016/j.nut.2022.111907
35. Bretscher C, Buergin M, Gurzeler G, Kägi-Braun N, Gressies C, Tribolet P, et al. The association between prealbumin, all-cause mortality and response to nutritional treatment in patients at nutritional risk. Secondary analysis of a randomized-controlled trial. JPEN J Parenter Enteral Nutr. (2023). doi: 10.1002/jpen.2470 [Epub ahead of print].
36. Baimas-George M, Watson M, Elhage S, Parala-Metz A, Vrochides D, Davis B. Prehabilitation in frail surgical patients: a systematic review. World J Surg. (2020) 44:3668–78. doi: 10.1007/s00268-020-05658-0
37. Weimann A, Wobith M. ESPEN Guidelines on Clinical nutrition in surgery – special issues to be revisited. Eur J Surg Oncol. (2022) S0748-7983(22)00694-1. doi: 10.1016/j.ejso.2022.10.002
Keywords: malnutrition, frailty, sarcopenia, body composition, surgery
Citation: Wang L, Li P, Hu Y, Cheng B, Ding L, Li L, Song J, Wei J and Xu J (2023) Relationship between preoperative malnutrition, frailty, sarcopenia, body composition, and anthropometry in elderly patients undergoing major pancreatic and biliary surgery. Front. Nutr. 10:1135854. doi: 10.3389/fnut.2023.1135854
Received: 02 January 2023; Accepted: 07 February 2023;
Published: 21 February 2023.
Edited by:
Shanjun Tan, Fudan University, ChinaReviewed by:
Xuejin Gao, Nanjing General Hospital of Nanjing Military Command, ChinaCopyright © 2023 Wang, Li, Hu, Cheng, Ding, Li, Song, Wei and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingyong Xu,  eHVqaW5neW9uZ0Biamhtb2guY24=; Junmin Wei,
eHVqaW5neW9uZ0Biamhtb2guY24=; Junmin Wei,  d2VpanVubWluc2NpQDE2My5jb20=
d2VpanVubWluc2NpQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.