- 1Student Research Committee, Faculty of Nutrition and Food Science, Tabriz University of Medical Sciences, Tabriz, Iran
- 2Department of Emergency Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran
- 4Department of Community Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
- 5Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
- 6Nutrition Research Center, Faculty of Nutrition and Food Science, Tabriz University of Medical Sciences, Tabriz, Iran
Introduction: Several systematic reviews and meta-analyses have been carried out to assess the impact of synbiotics on lipid profiles and anthropometric parameters. In this regard, an umbrella meta-analysis was performed to provide a more accurate view of the overall impacts of synbiotic supplementation on lipid profile and anthropometric parameters.
Methods: Databases such as PubMed, Scopus, Embase, Web of Science, and Google Scholar were searched for this study from inception to January 2022. A random-effects model was applied to evaluate the effects of synbiotic supplementation on lipid profile and anthropometric parameters. The methodological quality of eligible articles was evaluated using the AMSTAR2 questionnaire. The GRADE approach was used to evaluate the overall certainty of the evidence in the meta-analyses.
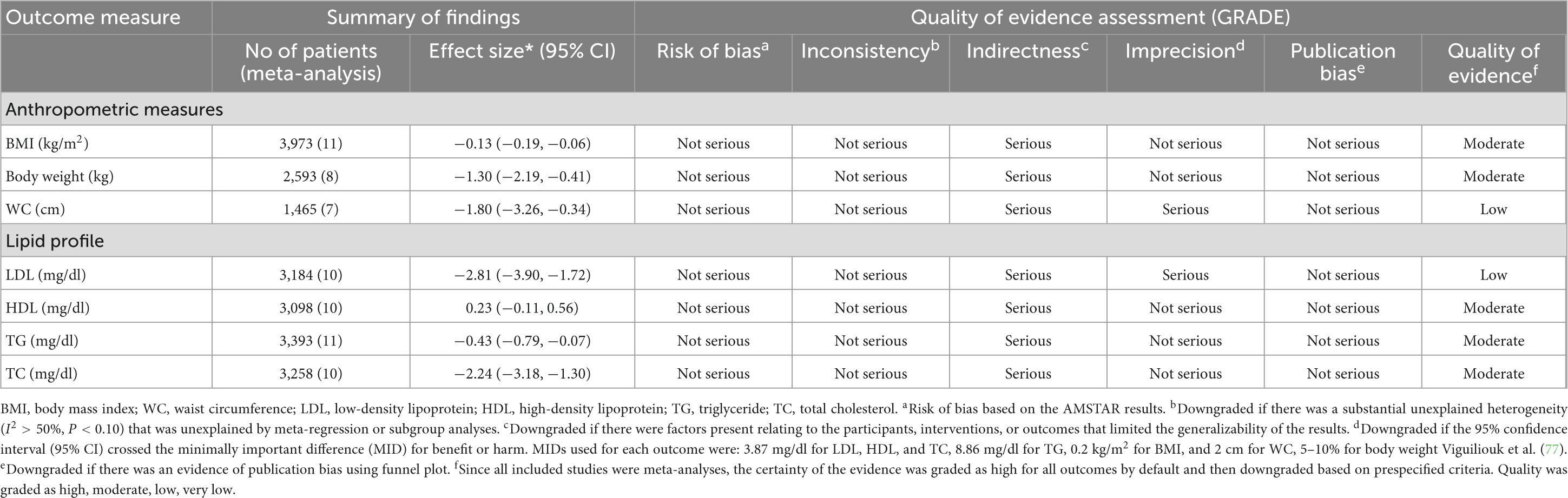
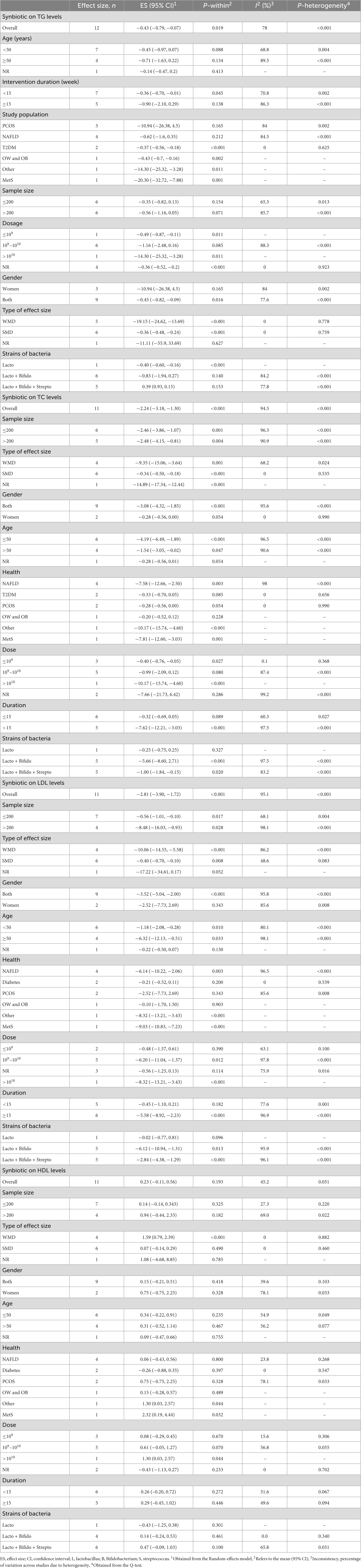
Results: Meta-analyses of 17 studies revealed significant decreases in body mass index (BMI) (ES: −0.13 kg/m2; 95% CI: −0.19, −0.06, p < 0.001, I2 = 0.0%, p = 0.870), BW (ES: −1.30 kg; 95% CI: −2.19, −0.41, p = 0.004, I2 = 88.9%, p < 0.001), waist circumference (WC) (ES: −1.80 cm; 95% CI: −3.26, −0.34, p = 0.016, I2 = 94.1%, p < 0.001), low-density lipoprotein cholesterol (LDL-C) (ES: −2.81 mg/dl; 95% CI: −3.90, −1.72, p < 0.001, I2 = 95.1%, p < 0.001), total cholesterol (TC) (ES = −2.24 mg/dl; 95% CI: −3.18, −1.30, p < 0.001, I2 = 94.5%, p < 0.001), and triglyceride (TG) (ES: −0.43 mg/dl; 95% CI: −0.79, −0.07, p = 0.019, I2 = 78.0%, p < 0.001) but not high-density lipoprotein cholesterol (HDL-C) (ES: 0.23 mg/dl; 95% CI: −0.11, 0.56, p = 0.193, I2 = 45.2%, p = 0.051) following synbiotic supplementation.
Discussion: The present umbrella meta-analysis suggests synbiotic supplementation can slightly improve lipid profile and anthropometric indices and might be a therapeutic option for obesity and its related disorders.
Systematic review registration: www.crd.york.ac.uk/prospero, identifier CRD42022304376.
Introduction
It is established that obesity is a multi-factorial, chronic, treatable, and neurobehavioral condition in which excess body fat mass results in adipose tissue dysfunction and abnormal physical forces of fat mass that lead to a variety of metabolic diseases (1). The increasing outbreak of obesity is one of the most important health concerns worldwide since being overweight increases the risk of several diseases, in particular, hyperlipidemia, diabetes, hypertension, cardiovascular disease (CVD), and cancer (2). Lifestyle interacts with local environmental and genetic factors to diversify the prevalence of obesity among populations.
Several studies have revealed that overweight and obesity by 2030 in women and men will reach 85 and 89%, respectively (1). This increases the risk of obesity-related risks such as coronary heart disease (CHD) by 97%, cancer by 61%, and types 2 diabetes by 21% (2, 3). Dyslipidemia is defined by one or more abnormal lipid concentrations in serum lipids [total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C)] (4). This problem can be caused by hereditary factors, but in most cases, obesity and overweight lead to this condition. The pathophysiology of typical dyslipidemia apperceived, in obesity is multi-factorial and includes, reduction of circulating TG lipolysis, hepatic overproduction of very-low-density lipoprotein (VLDL), disorder peripheral free fatty acids (FFA) trapping, enhanced FFA fluxes from adipocytes to the liver and other tissues, and the constitution of small dense LDL-C (5, 6). In addition, disruption of the ASP/C3 adesArg pathway may contribute to typical dyslipidemia. Therefore, the management and prevention of dyslipidemia have been considered in recent decades (7). The aim of treatment should be to increase physical activity and improve dietary habits by reducing total calorie intake and decreasing saturated fatty acid (SFA) consumption. Currently, several treatment options target each aspect of dyslipidemia but recent guidelines recommend complex therapies to treat the multiple lipid abnormalities (8–10). In recent years, it is proved that gut dysbiosis (imbalance between pathogenic and beneficial gut microbiome) is linked with diabetes, metabolic syndrome (MetS), dyslipidemia, and obesity by extra energy production altering the metabolism of energy in host and pro-inflammatory signals (11–13). Therefore, balancing the gut microbial flora plays a significant role in human health (14, 15). Synbiotics are nutritional supplements that are a mixture of probiotics and prebiotics in a synergic form (16). Synbiotics include both substrates and advantageous microorganisms, which might have synergic effects on the intestinal tract (17). Synbiotics have a beneficial effect on the host by improving survival and increasing the dose of live microbes in the gastrointestinal tract (18, 19). Numerous studies have indicated that the use of synbiotics could improve the glycemic status, lipid metabolism, markers of liver enzymes, inflammatory mediators, and the function of intestinal microbiota. However, there are differences between studies examining the effect of synbiotics on weight loss (17, 20, 21). Meta-analyses have examined the therapeutic impacts of synbiotics on lipid profile (20, 22) and obesity indices (23, 24); nevertheless, the findings are still inconsistent (25–28). Thus, the current umbrella meta-analysis study was designed to assess the effects of supplementation with synbiotics on TC, HDL-C, TG, and LDL-C levels and anthropometric indices, including body mass index (BMI), body weight (BW), and waist circumference (WC).
Materials and methods
The research protocol has been registered on PROSPERO (registration number: CRD42022304376). We conducted the current investigation in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (29).
The search strategy of literature
International scientific databases, such as Web of Science, Embase, PubMed, Scopus, and Google Scholar, were searched for relevant published papers till January 2022. The search technique for MeSH and the keywords utilized in this research are as follows: [(“Synbiotics” OR “Symbiotics” OR “Synbiotic”) AND (lipids OR High density lipoprotein cholesterol OR HDL-C OR Total cholesterol OR TC OR “Low density lipoprotein cholesterol” OR “LDL-C” OR Triglyceride OR TG OR “bodyweight” OR “body weight changes” OR “body mass index” OR “weight loss” OR “obesity” OR OR “BMI” OR “waist circumference” OR “WC”) AND (“meta-analysis” OR “systematic review”)]. The “*” keyword was used to improve the sensitivity of our study methodology. Also, to prevent the loss of research, a thorough search of references to relevant studies was conducted.
Inclusion and exclusion criteria
We followed these PICO criteria: Population/Patients (P: adults aged 18>), Intervention (I: treated with synbiotic), Comparison (C: control group), Outcome (O: anthropometric and lipid profile parameters), and Study design (S: meta-analysis). The previous meta-analyses, reviewed in the present study, investigated the effects of synbiotic supplementation on anthropometric and lipid profile parameters by using their effect size (ES) values and their corresponding confidence intervals (CI). In addition, other typologies of research studies including in vivo, in vitro, and ex vivo studies, observational studies, case reports, controlled clinical trials, and quasi-experimental studies were excluded from the present study.
Assessment of outcomes
The outcomes that were investigated in this umbrella meta-analysis included the effects of synbiotic supplementation on anthropometric indices and lipid profile. Among the anthropometric indices, BMI, BW, and WC were investigated and regarding the lipid profile, four indices of LDL-c, HDL-c, TG, and TC were investigated.
Data extraction and study selection
According to the qualifying requirements, two distinct reviewers evaluated the articles (SSA and MMA). We began by reviewing abstracts and titles. To establish if a research was appropriate for a meta-analysis, the entire texts of relevant papers were analyzed. Disputes were resolved by reaching an agreement with the senior reviewer (MV). The following information was retrieved from the chosen papers: the names of the first authors, the sample size, the publication year, dose of synbiotics, gender, health condition, length of the intervention, and ESs and their CIs.
Quality assessment
Two reviewers (VM and SSA) independently assessed the methodological quality of the qualifying articles using the AMSTAR2 questionnaire (30). The questionnaire has 16 questions to which reviewers must respond “Yes” or “Partial Yes” or “No” or “No Meta-analysis.” Critically poor quality, low quality, moderate quality, and excellent quality were assigned to the AMSTAR2 checklist. The third reviewer (MV) was also accountable for settling any conflicts.
Synthesis of data and statistical analysis
To estimate the pooled effect size, reported effect sizes ESs and CIs were used. The I2 statistic and Cochrane’s Q test were used to detect heterogeneity. When the I2 value >50% or the Q-test had p < 0.1, we considered between-study heterogeneity significant. When considerable heterogeneity existed across studies, we adopted the random-effects model. Subgroup analyses were conducted to identify potential sources of heterogeneity according to a number of variables, including dosage of synbiotic, type of ESs, duration of intervention, and the sample size, age of participants, health condition, bacteria strain type. A sensitivity analysis was performed to detect whether the total ES was associated with a single study. Egger (31) and Begg’s (32) tests were used to determine the small-study effect. Visual analysis of the funnel plot revealed publication bias (33). If publication bias was obvious, trim and fill methods are done. Version 16.0 of STATA was used for all statistical analyses (Stata Corporation, College Station, TX). When p < 0.05, values were deemed statistically significant.
Quality of evidence
GRADE (Standards for the Development, Evaluation, and Evaluation Working Group) criteria were used to evaluate the overall certainty of the evidence in the meta-analyses. The quality of the evidence was categorized according to four assessment criteria: high, moderate, low, and very low (34).
Results
Study selection and study characteristics
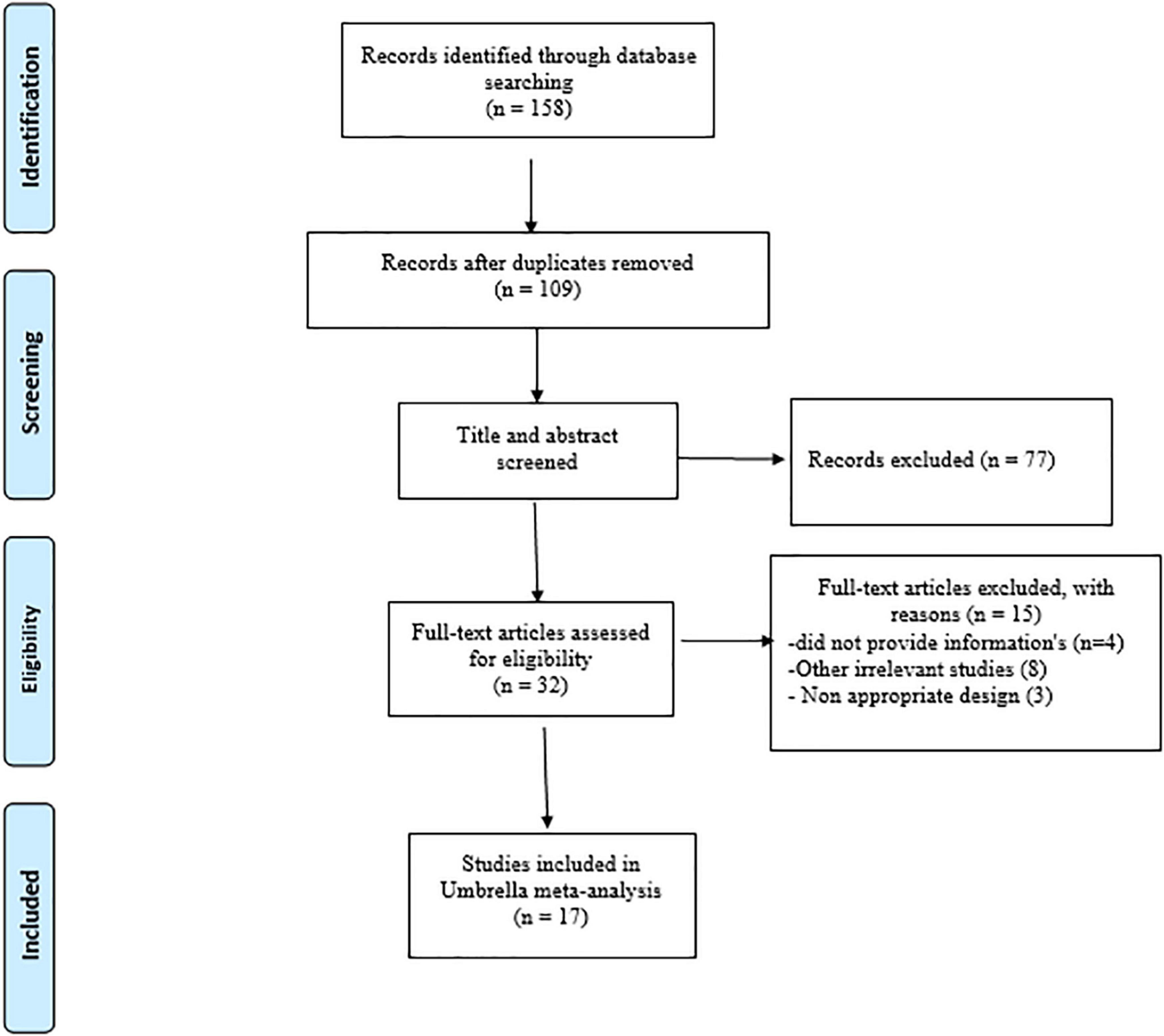
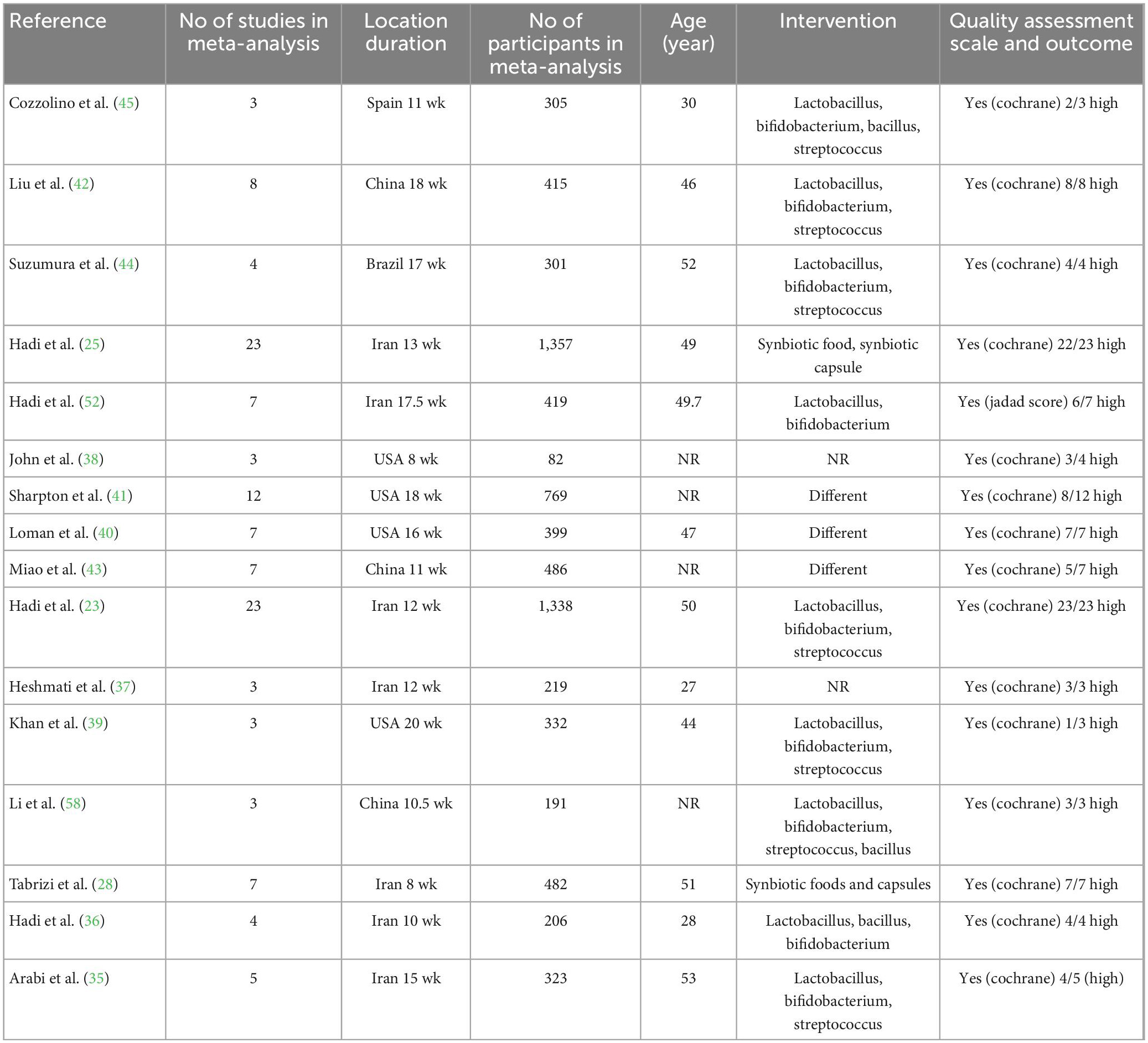
A total of 158 papers were found after a thorough search of internet databases. After removing 49 duplicate papers, the titles and abstracts of 109 papers were thoroughly examined, with 32 papers being chosen for full-text evaluation. Finally, 17 papers matched our inclusion criteria and were qualified for the umbrella meta-analyses. A flow chart of the PRISMA study and a study trend is given in Figure 1. The ES measures for studied variables are eight for weight, 11 for BMI, seven for WC, 12 for TG, 11 for TC, LDL-C, and HDL-C. In addition, seven studies were performed in Iran (20, 23, 25, 28, 35–37), four in the USA (38–41), three in China (26, 42, 43), two in Brazil (27, 44), and one in Spain (45). A total of 124 articles involving 7,772 participants were included in the present umbrella meta-analysis. Included studies were performed between 2014 and 2022. The number of subjects in studies ranged between 168 and 2,629. The participants’ average age ranged between 27 and 53 years. In the studies, the intervention lasted between 8 and 20 weeks. Administered synbiotics dosages ranged between 3.4 × 108 and 1.3 × 1010 CFU. The quality assessment process was performed in almost all meta-analyses included in the present study, except for one study (25), which did not report the quality assessment. Except for two studies by Hadi et al. (23) and Brasserie et al. (27), which utilized the Jadad score and CONSORT-based checklist, respectively, others applied Cochrane Collaboration’s tool to perform the quality assessment. Table 1 shows the characteristics of the studies that were included.
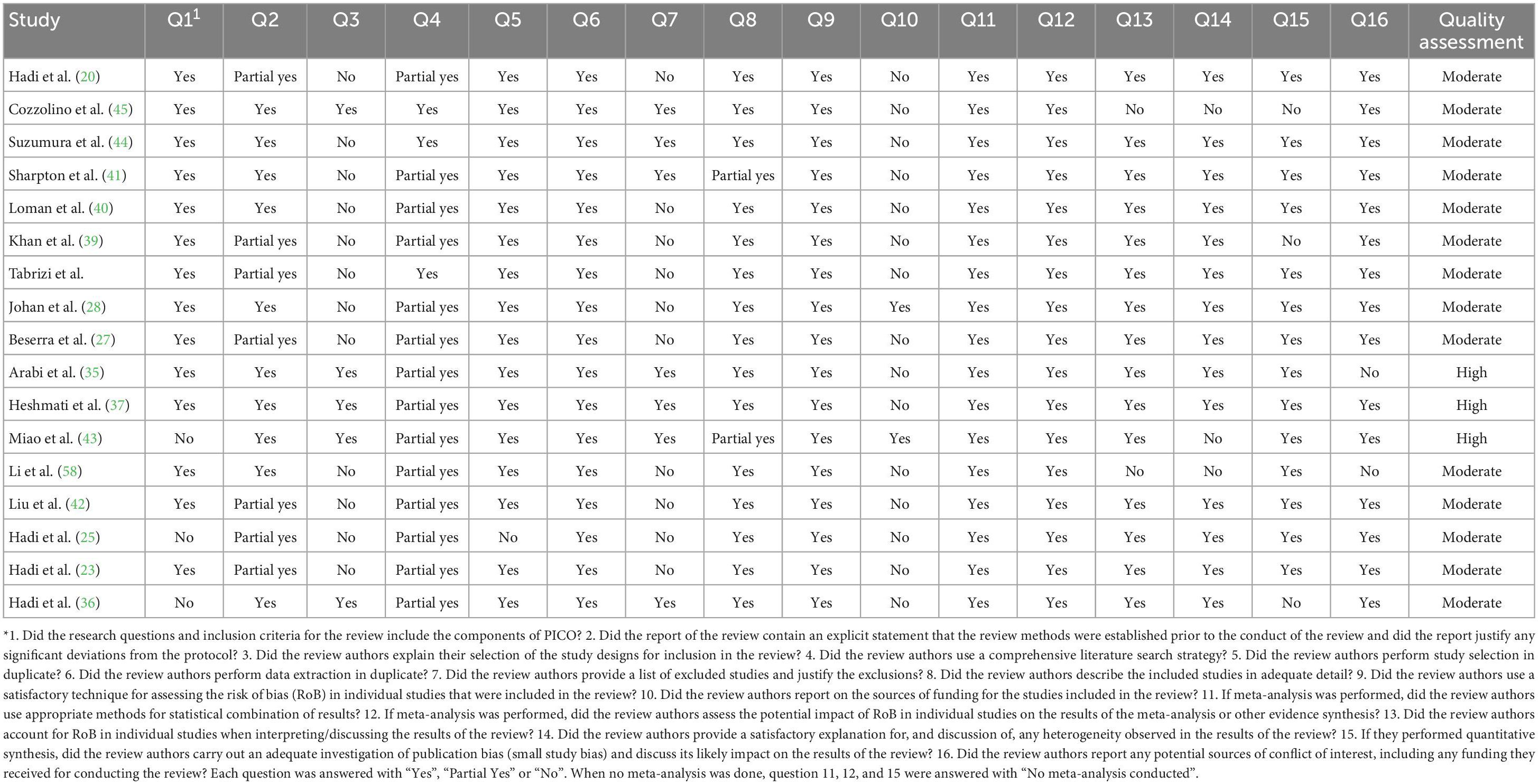
Evaluation of methodological quality
The methodological quality assessment details using the AMSTAR2 checklist are outlined in Table 2. Three meta-analyses out of 17 meta-analyses included high quality and 14 articles of moderate quality.
Synbiotic on BMI
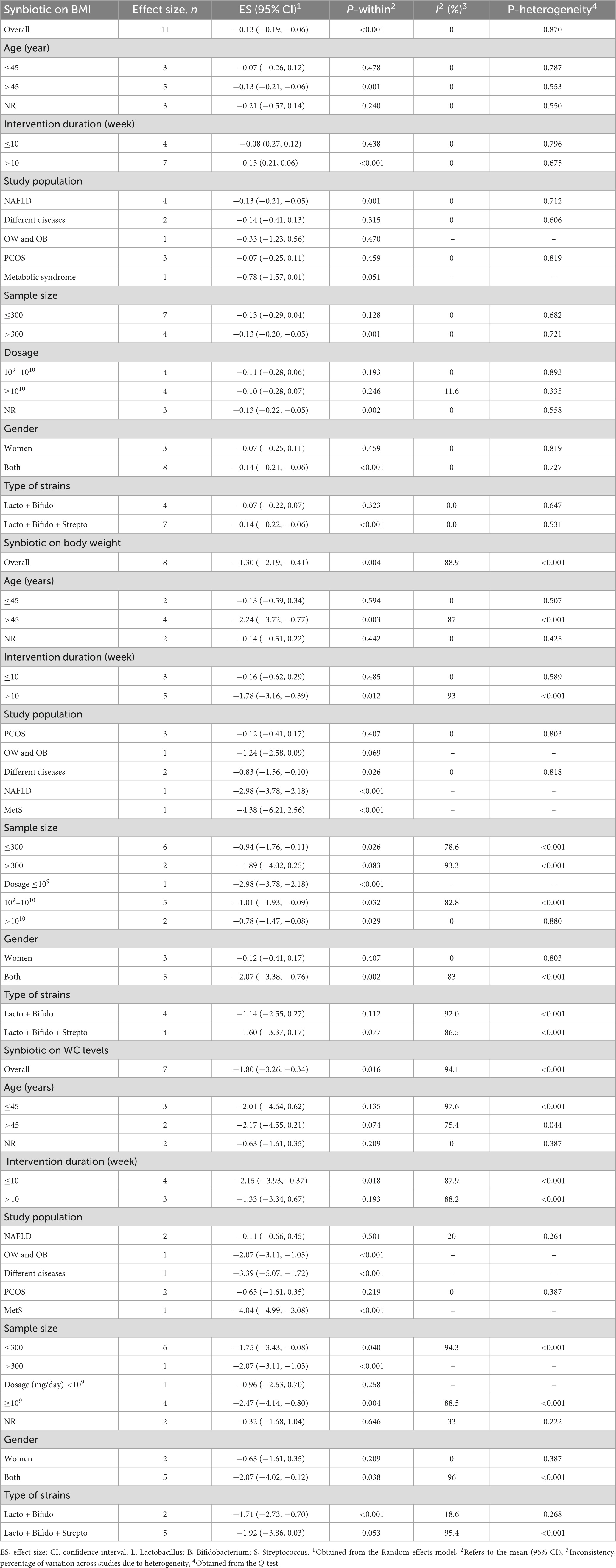
Synbiotic supplementation significantly decreased BMI (ES: −0.13 kg/m2; 95% CI: −0.19, −0.06, p < 0.001), according to a pooled analysis of 11 meta-analyses (Figure 2), without heterogeneity between-study (I2 = 0.0%, p = 0.870). Synbiotic supplementation in subjects with non-alcoholic fatty liver disease (NAFLD) with intervention >10 weeks, in studies with multi strains (Bifidobacteria, Lactobacilli plus Streptococcus) and a sample size of 200 individuals or over led to a remarkable reduction in BMI levels (Table 3). Also, the overall effects of synbiotics on BMI changed to non-significant after removing the Loman et al. (40) study by sensitivity analysis (ES: −0.12 kg/m2; 95% CI: −0.23, 0.001, p > 0.05). A significant small-study effect was detected using Begg’s (p = 0.002) unlike Egger’s (p = 0.076) tests. Visual inspection of the funnel plot revealed an uneven distribution of meta-analyses (Supplementary Figure 1). Thus, trim and fill methods was performed with 11 studies without imputed study (ES: −0.13 kg/m2; 95% CI: −0.19, −0.06, p < 0.05). The BMI quality of evidence was estimated as moderate performing the GRADE system (based on the indirectness) (Table 4).
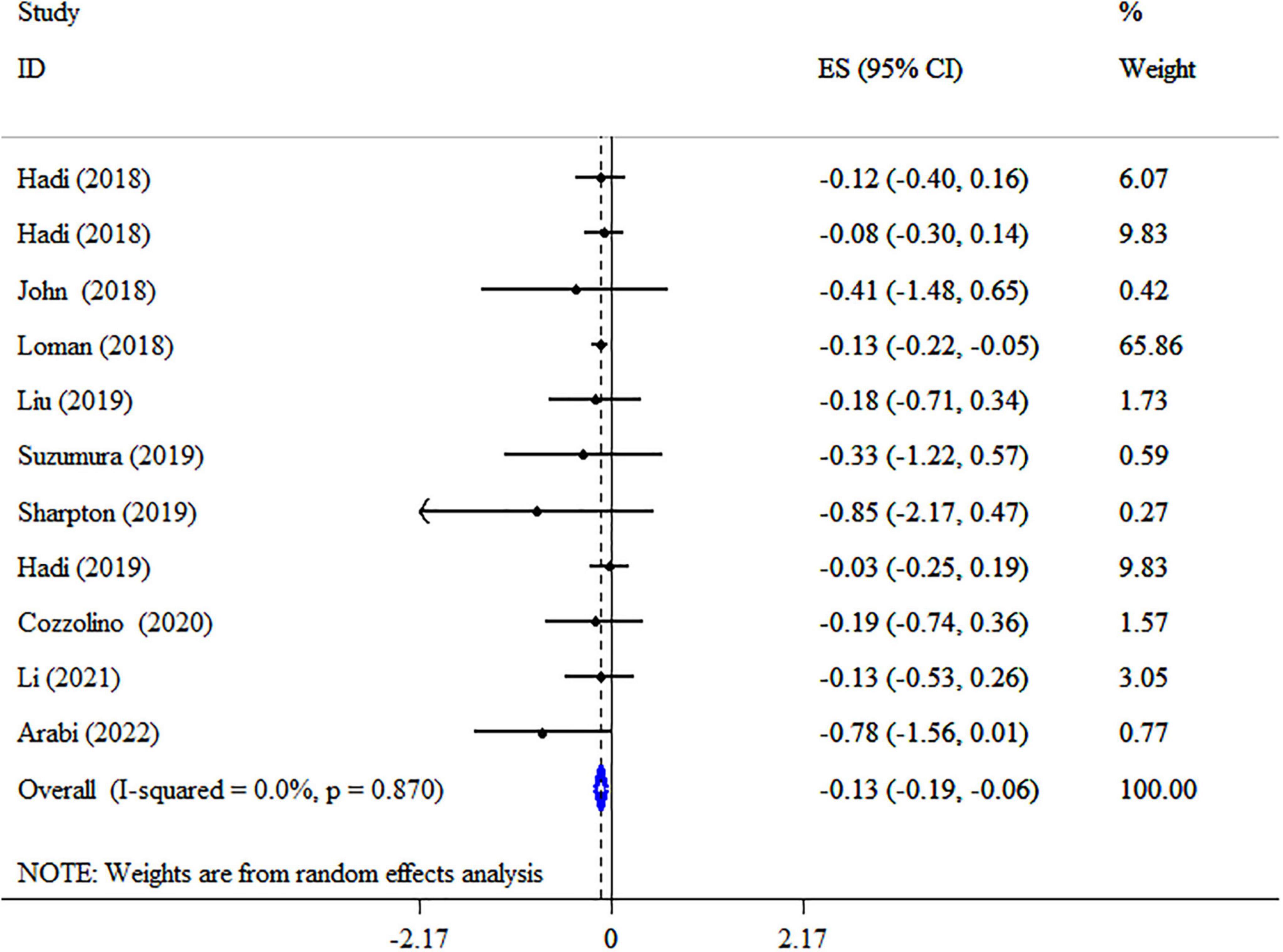
Figure 2. Forest plot with a mean difference and 95% confidence intervals (CIs) the impacts of synbiotic supplementation on BMI levels.
Synbiotics on BW
A pooled analysis of eight meta-analyses revealed that synbiotic supplementation reduced BW significantly (ES: −1.30 kg; 95% CI: −2.19, −0.41, p = 0.004) (Figure 3). However, there was high heterogeneity between studies (I2 = 88.9%, p < 0.001), which was decreased after subgroup analysis based on the dosage of synbiotic, duration of intervention, mean age, gender, and health condition (Table 3). Subgroup analysis demonstrated that synbiotic supplementation with a dosage of ≤109 CFU and subjects with a mean age of >45 years in the duration of intervention >10 weeks contributes to a more effective in reducing BW (Table 3). The sensitivity analysis showed that calculated overall ESs for BW alterations were not significantly changed after omitting each study. Based on Begg’s test, no evidence of publication bias was found (p = 0.266). The quality of evidence related to BW was downgraded to moderate due to serious limitations in indirectness (Table 4).
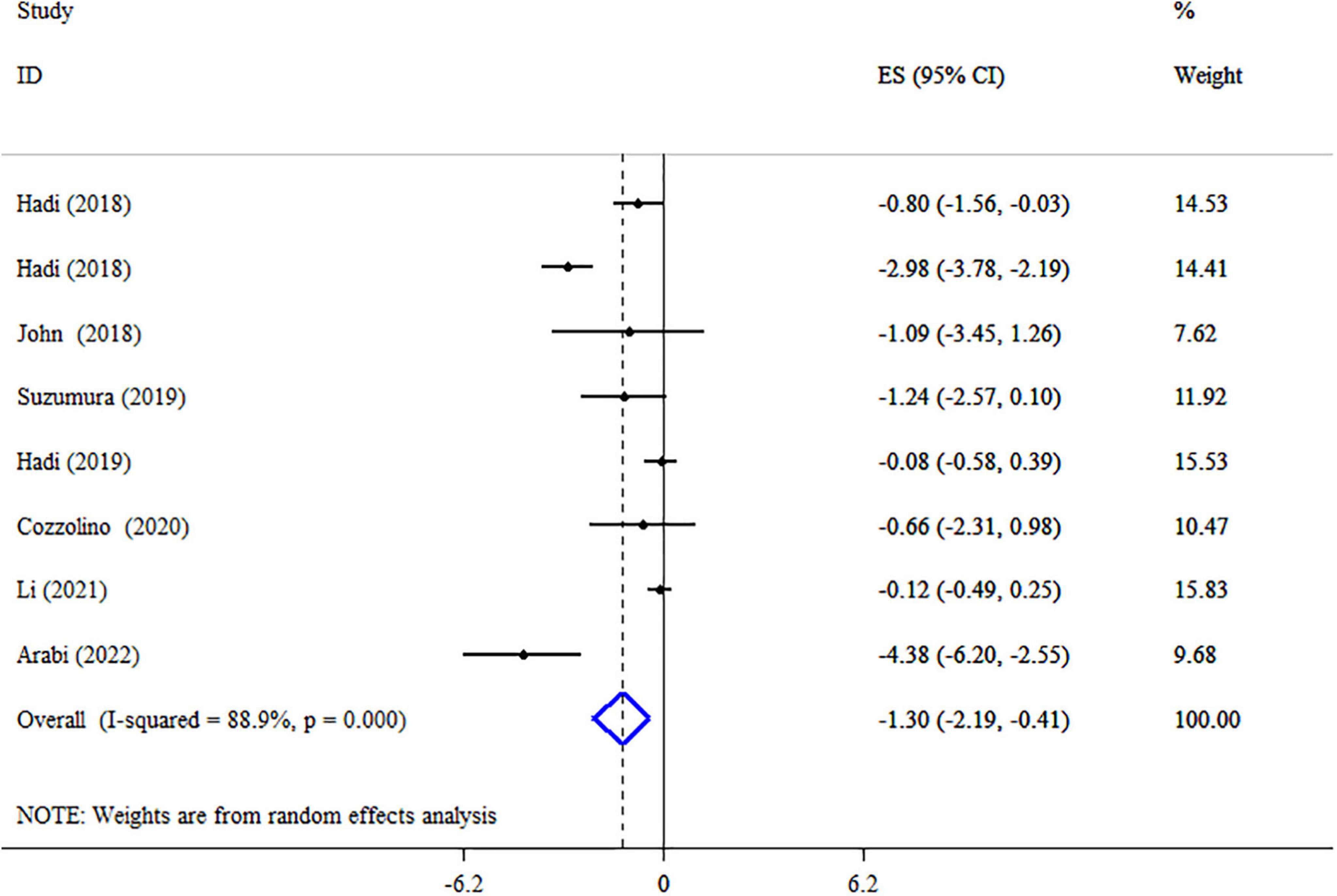
Figure 3. Forest plot detailing mean difference and 95% confidence intervals (CIs), the impacts of synbiotic supplementation on body weight.
Synbiotic on WC
A pooled analysis of seven studies including 1,465 participants indicated that synbiotic supplementation causes a significant reduction in WC (ES: −1.80 cm; 95% CI: −3.26, −0.34, p = 0.016) (Figure 4). There was a significant between-study heterogeneity (I2 = 94.1%, p < 0.001). Dosage, duration of intervention, strains of bacteria, and health conditions were detected as sources of heterogeneity in the subgroup analysis (Table 3). Synbiotic supplementation in subjects aged >45 years, duration of intervention ≤10 weeks, and intervention doses of ≥109 CFU led to a substantial reduction of WC levels. Also, we found a significant reduction in WC levels when used in studies with Bifidobacteria plus Lactobacilli strains (Table 3). Performing sensitivity analysis, there was no significant change when one particular study was removed. Begg’s test indicated no significant publication bias (p = 0.649). Based on the GRADE approach, the overall quality of the evidence for WC was considered low due to serious indirectness and imprecision (Table 4).
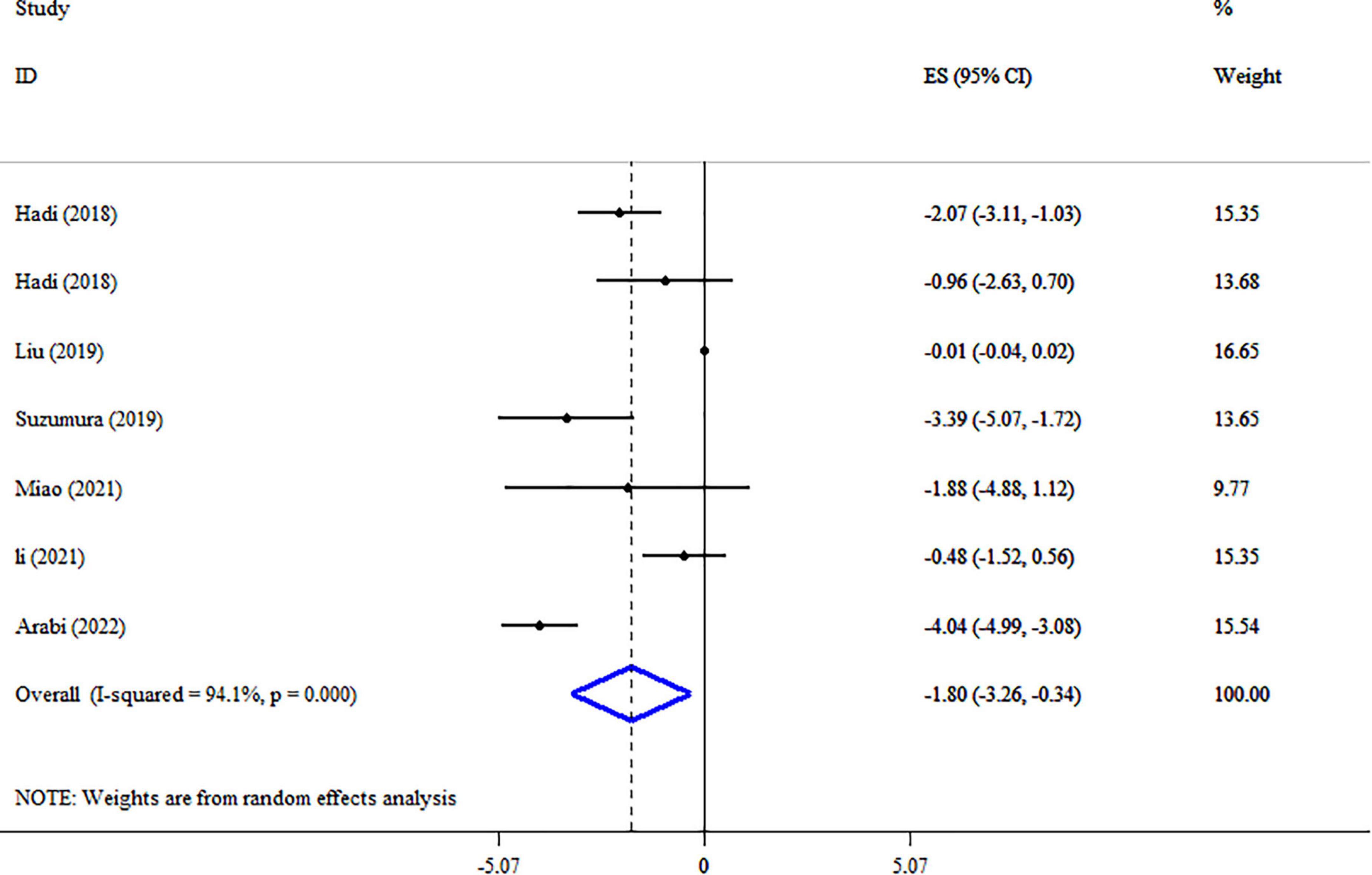
Figure 4. Forest plot detailing mean difference and 95% confidence intervals (CIs), the impacts of synbiotic supplementation on WC.
Synbiotic on LDL-c
Synbiotic supplementation meaningfully reduced LDL-C level based on the 10 meta-analyses with 11 ESs (ES: −2.81 mg/dl; 95% CI: −3.90, −1.72, p < 0.001) (Figure 5). Significant between-study heterogeneity was detected (I2 = 95.1%, p < 0.001). The dosage of synbiotics, mean age, and health condition were identified as sources of heterogeneity after subgroup analysis (Table 5). Subgroup analysis indicated that synbiotic supplementation in subjects with NAFLD with a dosage of 109–1010 CFU, intervention duration of ≥15-weeks, and type of ES weighted mean difference (WMD) contributes to a greater impact in the lowering LDL-C concentrations (Table 5). The following analysis indicated there was a significant reduction in LDL levels when using Bifidobacteria plus Lactobacilli strains (Table 5). A sensitivity analysis found that no special study affected the overall ES. Egger’s and Begg’s tests identified a small-study effect (p = 0.018 and p = 0.029, respectively), also a visual inspection of the funnel plot revealed the presence of publication bias (Supplementary Figure 2). Therefore, Trim and fill analysis was carried out with 11 studies (ES: −2.81 mg/dl; 95% CI: −3.90, −1.72, p < 0.05). As shown in Table 4, the LDL-C quality of evidence was rated as low using the GRADE system (based on the indirectness and imprecision).
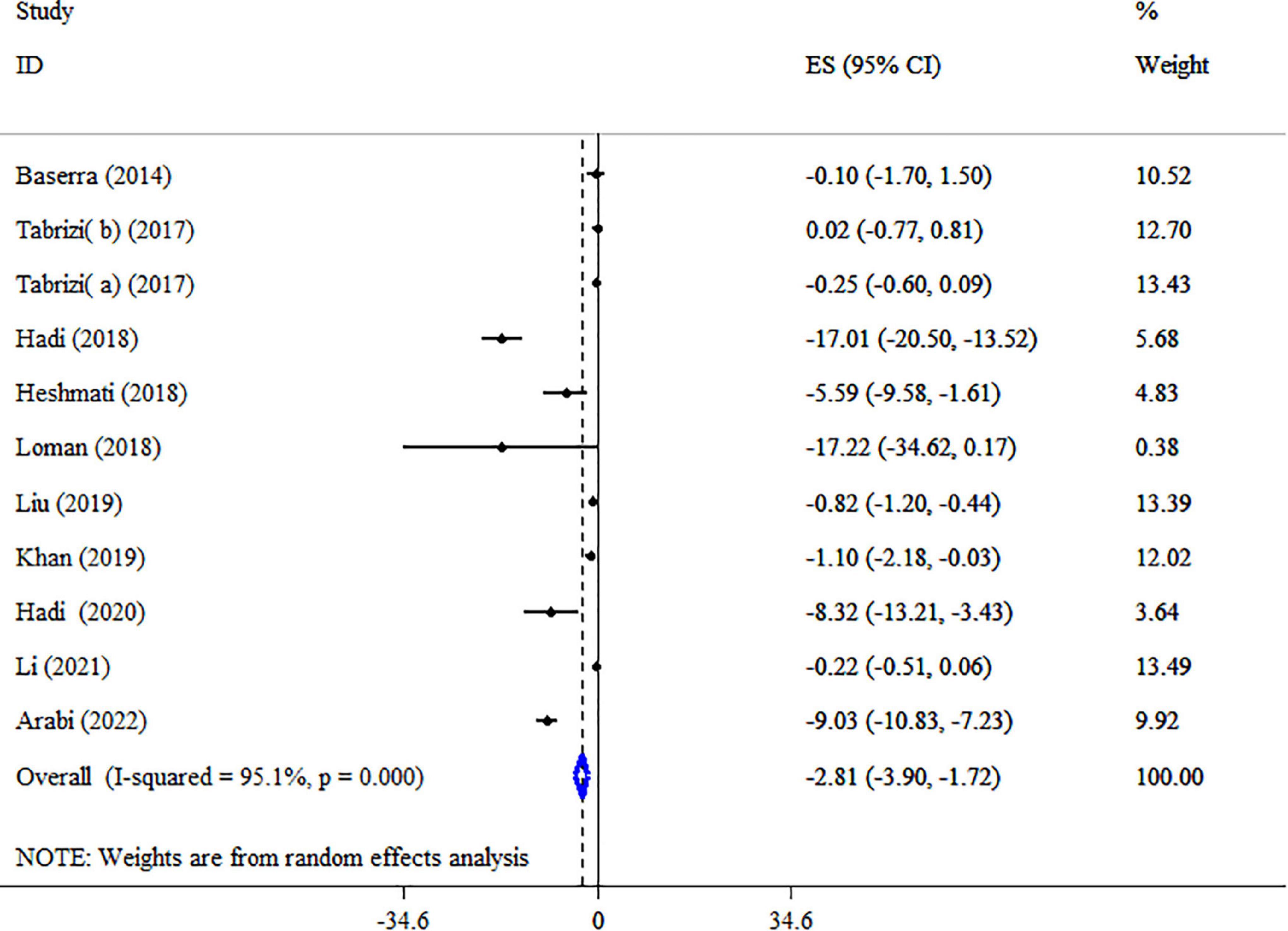
Figure 5. Forest plot detailing mean difference and 95% confidence intervals (CIs), the impacts of synbiotic supplementation on LDL-C levels.
Synbiotic on TC
The effect of synbiotics on TC level was reported in 10 studies with 11 ESs (Figure 6). Our analysis revealed a significant reduction in TC levels after synbiotic administration (ES = −2.24 mg/dl, 95% CI: −3.18, −1.30, p < 0.001), with high heterogeneity between-study (I2 = 94.5%, p < 0.001), which was decreased after subgroup analysis based on the dosage of synbiotic, duration of intervention, strains of bacteria, type of ESs and health condition. Subgroup analysis showed that the impacts of synbiotic reduction on TC were more robust in studies with participants with NAFLD, age ≤50 years, the sample size of ≤200, type of ES (WMD), and multi strains of bacteria (lactobacillus, bifidobacterium, plus streptococcus) than the entire sample (Table 5). Sensitivity analysis for TC concentrations did not show evidence of sensitivity. A considerable small-study effect was indicated using Egger’s and Begg’s tests (p = 0.015 and 0.029, respectively). Also, a visual inspection of the funnel plot found a significant publication bias among included studies (Supplementary Figure 3). Therefore, trim and fill analysis was conducted with 11 studies (no imputed studies) (ES = −2.24 mg/dl, 95% CI: −3.18, −1.30, p < 0.05). The quality of evidence related to TC was downgraded to moderate due to serious limitations in indirectness (Table 4).
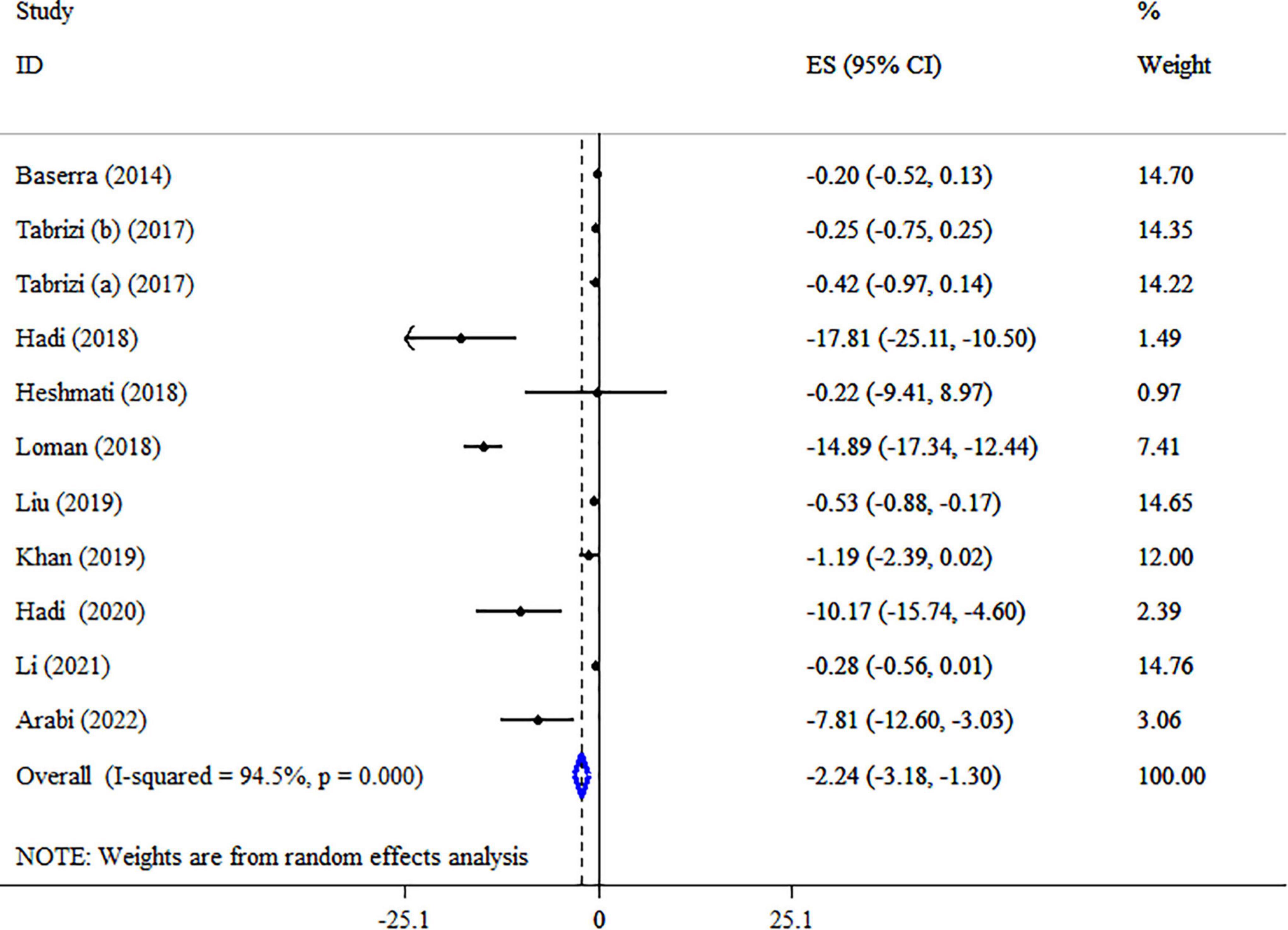
Figure 6. Forest plot detailing mean difference and 95% confidence intervals (CIs), the impacts of synbiotic supplementation on TC levels.
Synbiotic on TG
Eleven meta-analyses with 12 ESs, including 3,393 participants, have evaluated the effect of synbiotics on TG levels (Figure 7). The analysis indicated a significant decrease of TG by synbiotic supplementation (ES: −0.43 mg/dl; 95% CI: −0.79, −0.07, p = 0.019). However, significant heterogeneity was detected among studies (I2 = 78.0%, p < 0.001). The dosage of synbiotics, sample size, health status, and type of ES was identified as sources of heterogeneity in the subgroup analysis (Table 5). The effects of the synbiotics on TG in the type of ES (WMD), and participants with T2DM were more robust than the entire sample (Table 5). No evidence of the effect of a single study on the overall ES was detected using sensitivity analysis. A substantial small-study effect was shown using Egger’s unlike Begg’s tests (p < 0.001 and 0.244, respectively). After a visual inspection of the funnel plot, publication bias was observed (Supplementary Figure 4). Following trim and fill analysis, the overall ES did not alter (ES: −0.43 mg/dl; 95% CI: −0.79, −0.07, p < 0.05). According to the GRADE approach, TG was considered to have a moderate quality of evidence due to indirectness (Table 4).
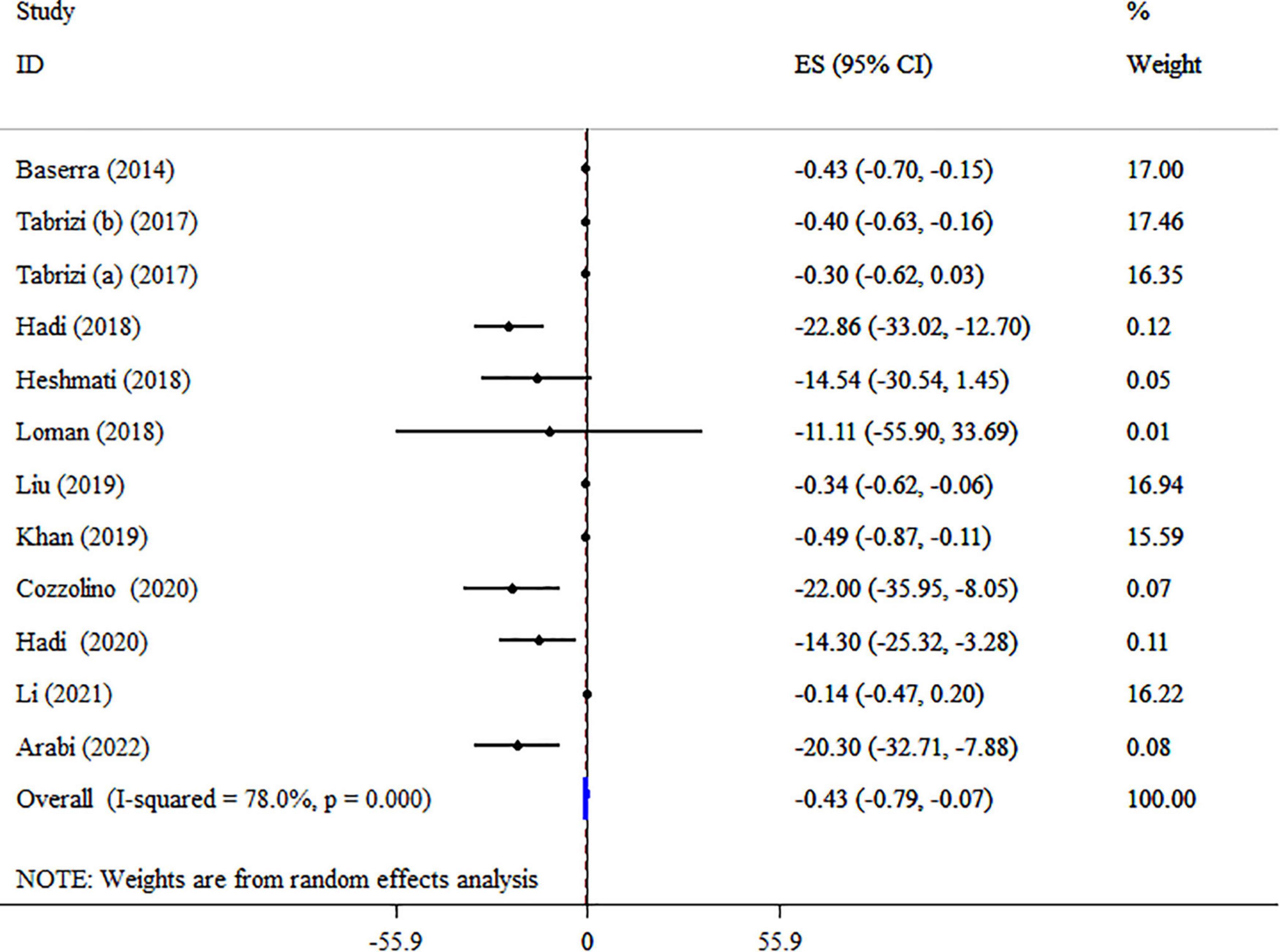
Figure 7. Forest plot detailing mean difference and 95% confidence intervals (CIs), the impacts of synbiotic supplementation on TG levels.
Synbiotic on HDL-c
Overall result from 10 meta-analyses containing 11 total ESs did not reveal significant alterations in HDL-C (ES: 0.23 mg/dl; 95% CI: −0.11, 0.56, p = 0.193; I2 = 45.2%, p = 0.051) (Figure 8). Also, in studies with type ES (WMD), a significant increase was observed in HDL-C levels (Table 5). Performing sensitivity analysis, there was no significant change when one single study was removed. There were no small-study effects using Egger’s and Begg’s tests (P = 0.189 and 0.350, respectively). In addition, a visual inspection of the funnel plot revealed asymmetry (Supplementary Figure 5). Therefore, the trim and fill test was carried out, and with imputing three fictitious studies, the result was still non-significance (ES: 0.08 mg/dl, 95% CI: −0.3, 0.5, p > 0.05). The HDL-C quality of evidence was rated as moderate using the GRADE system (based on the indirectness) (Table 4).
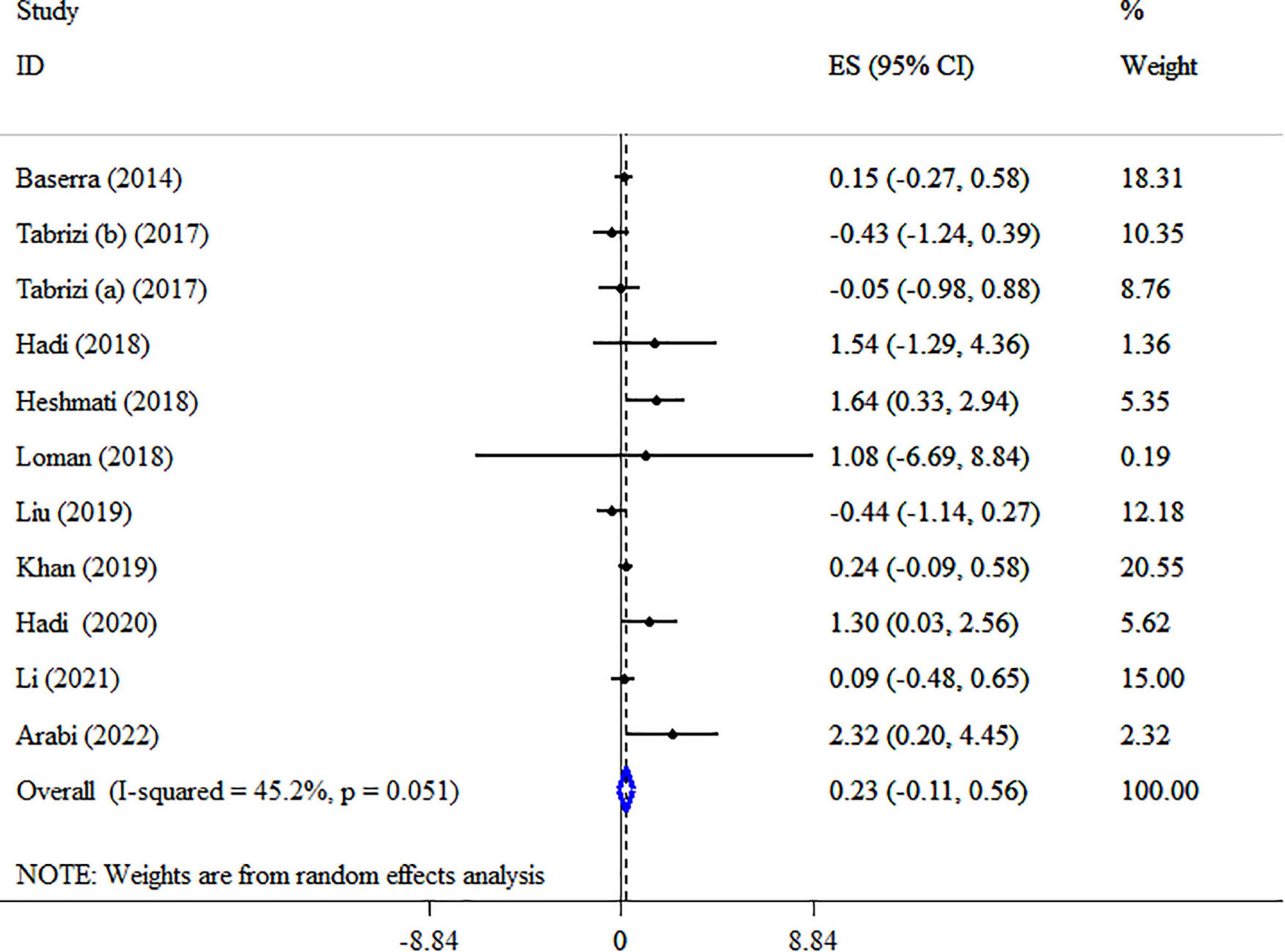
Figure 8. Forest plot detailing mean difference and 95% confidence intervals (CIs), the impacts of synbiotic supplementation on HDL-C levels.
Discussion
The current umbrella review and meta-analysis summarized the results of 17 meta-analyses, involving a total of 7,772 participants, and demonstrated that synbiotic supplementation could lead to a significant decrease in TG, TC, LDL-C, BW, BMI, and WC, nevertheless, no meaningful change was observed in HDL-C levels.
Stratifying the studies in different subgroups demonstrates different features of the effects of supplementation with synbiotics on lipid profile and anthropometric parameters and could help with gaining a conclusive result. For instance, stratifying the studies by the mean age of participants revealed that synbiotics had stronger effects on anthropometric parameters and LDL-C among older individuals (>45) in comparison with younger people. In contrast, the supplementation of synbiotics had a weaker impact on TC among individuals ≥50 years old in comparison with young adults, which is in line with a previous umbrella meta-analysis (46). The inconsistent findings might be due to the menopausal status of women. Menopause could affect lipid profile and the metabolism of lipoprotein namely LDL-C since the production of estrogen from ovaries reduces after menopause (47). According to the findings of a recent meta-analysis, in post-menopausal individuals, the levels of TG, TC, and LDL-C were higher than in women in pre-menopausal status. Thus, gender could be a potential factor in changing the final result, hence, most studies were conducted on both sex. Of note, almost all studies conducted on both genders showed more considerable effects of supplementation with synbiotics on lipid profile and anthropometric parameters than studies performed on women alone. Moreover, the present study shown that the impacts of synbiotic on lipid profile, BW, and WC were in a dose-dependent manner.
It should be mentioned that different health conditions could also affect the efficacy of synbiotic supplementation on the studied outcomes substantially. As an example, the administration of synbiotics had more promising impacts on BMI, body weight, TC, and LDL-C in NAFLD patients. On the other hand, among overweight and obese participants, synbiotics had a more substantial lowering effect on WC. Regarding TG levels, type 2 diabetic patients demonstrated more amending change following synbiotic administration. The lipid profile-lowering property of synbiotics could be due to its effect on insulin sensitivity, especially in NAFLD and diabetic patients (48, 49). As a large sample size reflects the higher power of the studies, studies with large sample sizes (more than 200 in lipid profile and 300 in anthropometric indices) exhibit more considerable results than studies with small ones. Nonetheless, synbiotic supplementation showed more promising effects on TC and BW with small sample sizes (≤200 and ≤300, respectively). Therefore, it could be concluded that sample size is not an important effective factor in the association between synbiotics and the aforementioned outcomes.
Another possible factor, which might affect the overall findings, is treatment duration. Short-term administration of synbiotics (≤10 weeks for anthropometric indices, ≤15 weeks for TC, and <15 weeks for LDL-C) resulted in smaller effects on BMI, body weight, TC, and LDL-C compared with long-term supplementation. The synbiotic effect on TG was not time-dependent. Also, WC was reduced significantly only in the subgroup of ≤10-week.
It needs to be mentioned that, in addition to the aforementioned factors like sample size and intervention duration, the different types of synbiotics could be of great importance. For instance, after subgroup analysis for strains of bacteria, synbiotic supplementation with two strains of Lactobacillus and Bifidobacterium significantly decreased WC by 1.71 cm and LDL-C by 6.12 mg/dl. Also, synbiotic supplementation with three strains of Lactobacillus, Bifidobacterium, and Streptococcus significantly decreased BMI by 0.14 kg/m2, TC by 1.00 mg/dl, and TG by 0.39 mg/dl. Numerous animal and human studies have demonstrated the beneficial effects of different strains of synbiotics, particularly those belonging to lactic acid bacteria and bifidobacteria, on lipid profile and anthropometric indices. In a study on healthy rats, Hosseinfard et al. (50) revealed that Lactobacillus. Plantarum significantly amended lipid profile levels namely TG, LDL-C, HDL-C, and TC. In another study, the weight-lowering property of L. Plantarum and L. gasseri on obese humans and animals was approved (51). An additional human study confirmed the effectiveness of special strains of synbiotics such as L. acidophilus, L. casei, and Bifidobacterium bifidum in reducing the levels of lipid profile (52). Other species of lactic acid bacteria and bifidobacteria, which could amend lipid profile levels in humans are as follows: Bifidobacterium animalis (53), Bifidobacterium infantis, Bifidobacterium breve, etc. (54), and L. acidophilus, L. casei; L. lactis, etc (55). The mechanism of action of different strains of synbiotic is through various ways indicating that weight-lowering and lipid profile-lowering characteristic of synbiotics is strain-specific. For example, in a study by Nabhani et al. (56), a combination of several strains namely L. fermentum, L. plantarum, L. acidophilus, L. Gasseri did not lead to a significant decrease in lipid profiles. In contrast, another study showed the administration of L. acidophilus, L. casei, and B. bifidum resulted in substantial alterations in LDL-C and HDL-C levels (57). Meanwhile, it has been demonstrated that the L. casei has a stronger effect on attenuating obesity than B. animalis VKB and VKL strains (58).
Because nearly all included studies had a low risk of bias, the findings of the present study could be dependable. In one study conducted by Hadi et al. (15), the population of the included studies was patients who had different health conditions, which might affect the generalizability of their results. It should be noted that the population of the included studies in the present research, in most cases, were patients with some health conditions namely NAFLD, MetS, overweight, and obesity, who were more likely to suffer from hyperlipidemia. Therefore, the findings of the current umbrella meta-analysis could be generalizable. Of note, regarding the assessed outcomes, we did not find any considerable unexplained heterogeneity, thus, the results of the study have consistency.
The mechanism of anti-obesity properties of synbiotics has been examined in several studies. The following mechanisms have been proposed: modulating lipid absorption and excretion (59), the activation of FXR receptor leading to a decrease in gluconeogenesis and mediating insulin production and glucose detonation (60, 61), attenuating hunger via elevating the levels of glucagon-like-peptides (GLP-1 and GLP-2) (62), and inhibiting lipogenesis and stimulating B-oxidation of fatty acids via modulating the expression of PPAR-a, ACAT, FAS, and SREBP-1 (63, 64). Although the exact mechanism of the action of synbiotics in modulating lipid profiles has remained unclear, numerous studies have suggested several mechanisms. It has been suggested that synbiotics could lower TC levels via cholesterol assimilation or deconjugation of bile salts (65–69). Besides, synbiotics could increase HDL-C levels through increased bile salt hydrolase activity (70). Probiotics might affect the levels of lipid profile via alleviating the activation of Toll-like receptor-4 (TLR-4) and the production of inflammatory cytokines, which consequently leads to a reduction in lipid profile (71). On the other hand, probiotics can modulate cholesterol metabolism via producing short-chain fatty acids (SCFAs) resulting in the prevention of the activation of hydroxymethyl glutaryl CoA reductase (HMG-CoA reductase), a rate-limiting enzyme in the cholesterol synthesis pathway (72). Other possible mechanisms regarding the cholesterol-lowering property of synbiotics include the following: conversion of cholesterol into coprostanol (73) and integrating cholesterol in cellular membranes (74). Besides, synbiotics could alleviate lipid profile levels via decreasing the secretion of VLDL, insulin resistance, inflammation, and de novo lipogenesis mediated by carbohydrate-responsive element-binding protein (ChREBP)/sterol regulatory element-binding protein (SREBP). Synbiotics also could reduce the accumulation of TG in adipose tissues and the liver by increasing the serum levels of the fasting-induced adipose factor (FIAF) following the inhibition of hepatic lipogenic enzymes mediated by ChREBP and SREBP-1c (75). On the other hand, increased levels of FIAF prohibit the release of TG from VLDL and chylomicrons via inhibiting endothelial lipoprotein lipase (LPS). Moreover, the increased levels of GLP-1 restrain the activity of gut lipases, which in turn leads to a decrease in TG absorption from the intestine (76).
Limitations
On the whole, in the present umbrella meta-analysis, all of the performed meta-analyses of RCTs, which addressed the impacts of synbiotics on lipid profile and anthropometric indices were included. The evaluation of possible biases was carried out. The results were assessed based on different subgroups. However, one of the important and instinctive limitations of the umbrella meta-analysis is that some similar RCTs from various meta-analyses would be re-analyzed in the umbrella meta-analysis, and as a result, these similar RCTs experienced an increased weight in the analysis; therefore, actual results may be even weaker than those acquired.
Conclusion
According to the results of the current study, synbiotic supplementation can attenuate TG, LDL-C, and TC levels as well as BW, BMI, and WC. Therefore, synbiotics might be a therapeutic option for obesity and its related disorders. However, it must be noted that several factors namely the intervention duration, synbiotic strains, and different health conditions could vary the beneficial effects of synbiotics.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
VM, MV, and MMA wrote the original manuscript and contributed to the conception of the manuscript. SSA and VM contributed to data collection, analysis, and manuscript drafting. MV and AK provided the advice and consultation. PD and VM contributed to the final revision of the manuscript. All authors read and approved the final manuscript.
Funding
This research protocol was approved and supported by the Student Research Committee, Tabriz University of Medical Sciences (Registration code: 69601).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1121541/full#supplementary-material
References
1. Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. (2017) 960:1–17. doi: 10.1007/978-3-319-48382-5_1
2. Fitch AK, Bays HE. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. (2022) 1:100004.
3. Rtveladze K, Marsh T, Barquera S, Sanchez Romero L, Levy D, Melendez G, et al. Obesity prevalence in Mexico: impact on health and economic burden. Public Health Nutr. (2014) 17:233–9. doi: 10.1017/S1368980013000086
4. Arsenault B, Rana J, Stroes E, Després J, Shah P, Kastelein J, et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol. (2009) 55:35–41. doi: 10.1016/j.jacc.2009.07.057
5. Klop B, Elte J, Cabezas M. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. (2013) 5:1218–40. doi: 10.3390/nu5041218
6. Sánchez-Moreno C, Ordovás J, Smith C, Baraza J, Lee Y, Garaulet M. APOA5 gene variation interacts with dietary fat intake to modulate obesity and circulating triglycerides in a Mediterranean population. J Nutr. (2011) 141:380–5. doi: 10.3945/jn.110.130344
7. Abumrad N, Davidson N. Role of the gut in lipid homeostasis. Physiol Rev. (2012) 92:1061–85. doi: 10.1152/physrev.00019.2011
8. Buse J, Ginsberg H, Bakris G, Clark N, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. (2007) 115:114–26. doi: 10.1161/CIRCULATIONAHA.106.179294
9. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. (2002) 106:3143–421.
10. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. (2019) 290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014
11. Carding S, Verbeke K, Vipond D, Corfe B, Owen L. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. (2015) 26:26191. doi: 10.3402/mehd.v26.26191
12. Chang C, Lin H. Dysbiosis in gastrointestinal disorders. Best Pract Res Clin Gastroenterol. (2016) 30:3–15. doi: 10.1016/j.bpg.2016.02.001
13. Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. (2016) 73:147–62. doi: 10.1007/s00018-015-2061-5
14. Targher G, Day C, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. (2010) 363:1341–50. doi: 10.1056/NEJMra0912063
15. Sayari S, Neishaboori H, Jameshorani M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin Mol Hepatol. (2018) 24:331–8. doi: 10.3350/cmh.2018.0006
17. Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. (2014) 99:535–42. doi: 10.3945/ajcn.113.068890
18. Abdel-Raheem SM, Abd-Allah SM, Hassanein KM. The effects of prebiotic, probiotic and synbiotic supplementation on intestinal microbial ecology and histomorphology of broiler chickens. Int J Agro Vet Med Sci. (2012) 6:277–89.
19. Musazadeh V, Faghfouri A, Kavyani Z, Dehghan P. Synbiotic as an adjunctive agent can be useful in the management of hyperglycemia in adults: an umbrella review and meta-research of meta-analysis studies. J Funct Foods. (2022) 99:105355.
20. Hadi A, Mohammadi H, Miraghajani M, Ghaedi E. Efficacy of synbiotic supplementation in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis of clinical trials: synbiotic supplementation and NAFLD. Crit Rev Food Sci Nutr. (2019) 59:2494–505. doi: 10.1080/10408398.2018.1458021
21. Mirmiranpour H, Huseini H, Derakhshanian H, Khodaii Z, Tavakoli-Far B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J Diabetes Metab Disord. (2019) 19:53–60. doi: 10.1007/s40200-019-00474-3
22. Mahboobi S, Rahimi F, Jafarnejad S. Effects of prebiotic and synbiotic supplementation on glycaemia and lipid profile in type 2 diabetes: a meta-analysis of randomized controlled trials. Adv Pharm Bull. (2018) 8:565–74. doi: 10.15171/apb.2018.065
23. Hadi A, Alizadeh K, Hajianfar H, Mohammadi H, Miraghajani M. Efficacy of synbiotic supplementation in obesity treatment: a systematic review and meta-analysis of clinical trials. Crit Rev Food Sci Nutr. (2020) 60:584–96. doi: 10.1080/10408398.2018.1545218
24. Mohammadi H, Ghavami A, Hadi A, Askari G, Symonds M, Miraghajani M. Effects of pro-/synbiotic supplementation on anthropometric and metabolic indices in overweight or obese children and adolescents: a systematic review and meta-analysis. Complement Ther Med. (2019) 44:269–76. doi: 10.1016/j.ctim.2019.05.008
25. Hadi A, Ghaedi E, Khalesi S, Pourmasoumi M, Arab A. Effects of synbiotic consumption on lipid profile: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Nutr. (2020) 59:2857–74. doi: 10.1007/s00394-020-02248-7
26. Li Y, Tan Y, Xia G, Shuai J. Effects of probiotics, prebiotics, and synbiotics on polycystic ovary syndrome: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2023) 63:522–38. doi: 10.1080/10408398.2021.1951155
27. Beserra B, Fernandes R, do Rosario V, Mocellin M, Kuntz M, Trindade EB. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr. (2015) 34:845–58. doi: 10.1016/j.clnu.2014.10.004
28. Tabrizi R, Moosazadeh M, Lankarani K, Akbari M, Heydari S, Kolahdooz F, et al. The effects of synbiotic supplementation on glucose metabolism and lipid profiles in patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins. (2018) 10:329–42. doi: 10.1007/s12602-017-9299-1
29. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
30. Shea B, Reeves B, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj. (2017) 358:j4008.
31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
32. Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101.
33. Musazadeh V, Zarezadeh M, Faghfouri A, Keramati M, Ghoreishi Z, Farnam A. Saffron, as an adjunct therapy, contributes to relieve depression symptoms: an umbrella meta-analysis. Pharmacol Res. (2022) 175:105963. doi: 10.1016/j.phrs.2021.105963
34. Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
35. Arabi S, Bahrami L, Rahnama I, Sahebkar A. Impact of synbiotic supplementation on cardiometabolic and anthropometric indices in patients with metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2022) 176:106061. doi: 10.1016/j.phrs.2022.106061
36. Hadi A, Arab A, Khalesi S, Rafie N, Kafeshani M, Kazemi M. Effects of probiotic supplementation on anthropometric and metabolic characteristics in adults with metabolic syndrome: a systematic review and meta-analysis of randomized clinical trials. Clin Nutr. (2021) 40:4662–73. doi: 10.1016/j.clnu.2021.05.027
37. Heshmati J, Farsi F, Yosaee S, Razavi M, Rezaeinejad M, Karimie E, et al. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiotics Antimicrob Proteins. (2019) 11:1236–47.
38. John G, Wang L, Nanavati J, Twose C, Singh R, Mullin G. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes. (2018) 9:167. doi: 10.3390/genes9030167
39. Khan M, Mihali A, Rawala M, Aslam A, Siddiqui W. The promising role of probiotic and synbiotic therapy in aminotransferase levels and inflammatory markers in patients with nonalcoholic fatty liver disease - a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. (2019) 31:703–15. doi: 10.1097/MEG.0000000000001371
40. Loman B, Hernández-Saavedra D, An R, Rector R. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. (2018) 76:822–39. doi: 10.1093/nutrit/nuy031
41. Sharpton S, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault N. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. (2019) 110:139–49. doi: 10.1093/ajcn/nqz042
42. Liu L, Li P, Liu Y, Zhang Y. Efficacy of probiotics and synbiotics in patients with nonalcoholic fatty liver disease: a meta-analysis. Dig Dis Sci. (2019) 64:3402–12. doi: 10.1007/s10620-019-05699-z
43. Miao C, Guo Q, Fang X, Chen Y, Zhao Y, Zhang Q. Effects of probiotic and synbiotic supplementation on insulin resistance in women with polycystic ovary syndrome: a meta-analysis. J Int Med Res. (2021) 49:3000605211031758. doi: 10.1177/03000605211031758
44. Suzumura E, Bersch-Ferreira Â, Torreglosa C, da Silva J, Coqueiro A, Kuntz M, et al. Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: a systematic review and meta-analyses of randomized trials. Nutr Rev. (2019) 77:430–50. doi: 10.1093/nutrit/nuz001
45. Cozzolino M, Vitagliano A, Pellegrini L, Chiurazzi M, Andriasani A, Ambrosini G, et al. Therapy with probiotics and synbiotics for polycystic ovarian syndrome: a systematic review and meta-analysis. Eur J Nutr. (2020) 59:2841–56. doi: 10.1007/s00394-020-02233-0
46. Zarezadeh M, Musazadeh V, Faghfouri A, Roshanravan N, Dehghan P. Probiotics act as a potent intervention in improving lipid profile: an umbrella systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2023) 63:145–58. doi: 10.1080/10408398.2021.2004578
47. Palit P, Mukhopadhyay A, Chattopadhyay D. Phyto-pharmacological perspective of Silymarin: a potential prophylactic or therapeutic agent for COVID-19, based on its promising immunomodulatory, anti-coagulant and anti-viral property. Phytother Res. (2021) 35:4246–57. doi: 10.1002/ptr.7084
48. Tang Y, Huang J, Zhang W, Qin S, Yang Y, Ren H, et al. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Therap Adv Gastroenterol. (2019) 12:1756284819878046. doi: 10.1177/1756284819878046
49. Mazloom Z, Yousefinejad A, Dabbaghmanesh M. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. (2013) 38:38–43.
50. Hosseinifard E, Bavafa-Valenlia K, Saghafi-Asl M, Morshedi M. Antioxidative and metabolic effects of Lactobacillus plantarum, inulin, and their synbiotic on the hypothalamus and serum of healthy rats. Nutr Metab Insights. (2020) 13:1178638820925092. doi: 10.1177/1178638820925092
51. Wu C, Weng W, Lai W, Tsai H, Liu W, Lee M, et al. Effect of Lactobacillus plantarum strain K21 on high-fat diet-fed obese mice. Evid Based Complement Alternat Med. (2015) 2015:391767. doi: 10.1155/2015/391767
52. Hadi A, Sepandi M, Marx W, Moradi S, Parastouei K. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: a randomized clinical trial. Complement Ther Med. (2019) 47:102216. doi: 10.1016/j.ctim.2019.102216
53. Childs C, Röytiö H, Alhoniemi E, Fekete A, Forssten S, Hudjec N, et al. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br J Nutr. (2014) 111:1945–56. doi: 10.1017/S0007114513004261
54. Rajkumar H, Mahmood N, Kumar M, Varikuti S, Challa H, Myakala S. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm. (2014) 2014:348959.
55. Gomes A, de Sousa R, Botelho P, Gomes T, Prada P, Mota J. The additional effects of a probiotic mix on abdominal adiposity and antioxidant Status: a double-blind, randomized trial. Obesity. (2017) 25:30–8. doi: 10.1002/oby.21671
56. Nabhani Z, Hezaveh S, Razmpoosh E, Asghari-Jafarabadi M, Gargari B. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: a randomized double blind placebo controlled clinical trial. Diabetes Res Clin Pract. (2018) 138:149–57. doi: 10.1016/j.diabres.2018.02.008
57. Ahmadi S, Jamilian M, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. Br J Nutr. (2016) 116:1394–401. doi: 10.1017/S0007114516003457
58. Li H, Zhou D, Gan R, Huang S, Zhao C, Shang A, et al. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: a narrative review. Nutrients. (2021) 13:3211. doi: 10.3390/nu13093211
59. Hamad E, Sato M, Uzu K, Yoshida T, Higashi S, Kawakami H, et al. Milk fermented by Lactobacillus gasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats. Br J Nutr. (2009) 101:716–24. doi: 10.1017/S0007114508043808
60. Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. (2010) 1802:363–72. doi: 10.1016/j.bbadis.2010.01.002
61. Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. (2015) 5:135–44.
62. Shyangdan D, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. (2011) 2011:CD006423. doi: 10.1002/14651858.CD006423.pub2
63. Yoo S, Kim Y, Park D, Jung U, Jeon S, Ahn Y, et al. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity. (2013) 21:2571–8. doi: 10.1002/oby.20428
64. Mei L, Tang Y, Li M, Yang P, Liu Z, Yuan J, et al. Co-administration of cholesterol-lowering probiotics and anthraquinone from Cassia obtusifolia L. Ameliorate non-alcoholic fatty liver. PLoS One. (2015) 10:e0138078. doi: 10.1371/journal.pone.0138078
65. Ziarno M, Sêkul E, Lafraya ÁA. Cholesterol Assimilation by Commercial Yoghurt Starter Cultures. Warszawa: Warsaw agricultural university (2007).
66. Lye H-S, Rahmat-Ali G, Liong M-T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J. (2010) 20:169–75.
67. Liong M, Dunshea F, Shah N. Effects of a synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolaemic pigs on high- and low-fat diets. Br J Nutr. (2007) 98:736–44. doi: 10.1017/S0007114507747803
68. Larkin T, Astheimer L, Price W. Dietary combination of soy with a probiotic or prebiotic food significantly reduces total and LDL cholesterol in mildly hypercholesterolaemic subjects. Eur J Clin Nutr. (2009) 63:238–45. doi: 10.1038/sj.ejcn.1602910
69. Klein A, Friedrich U, Vogelsang H, Jahreis G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur J Clin Nutr. (2008) 62:584–93. doi: 10.1038/sj.ejcn.1602761
70. Xiao J, Kondo S, Takahashi N, Miyaji K, Oshida K, Hiramatsu A, et al. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J Dairy Sci. (2003) 86:2452–61. doi: 10.3168/jds.S0022-0302(03)73839-9
71. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. (2013) 500:541–6. doi: 10.1038/nature12506
72. Saulnier D, Ringel Y, Heyman M, Foster J, Bercik P, Shulman R, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. (2013) 4:17–27. doi: 10.4161/gmic.22973
73. Barczynska R, Bandurska K, Slizewska K, Litwin M, Szalecki M, Libudzisz Z, et al. Intestinal microbiota, obesity and prebiotics. Pol J Microbiol. (2015) 64:93–100.
74. Ghorbani Z, Nazari S, Etesam F, Nourimajd S, Ahmadpanah M, et al. The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: a randomized multicenter trial. Arch Neurosci. (2018) 5:e60507.
75. Ghaderi A, Banafshe H, Mirhosseini N, Moradi M, Karimi M, Mehrzad F, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. (2019) 19:77. doi: 10.1186/s12888-019-2059-x
Keywords: synbiotic, lipid profile, anthropometric indices, obesity, meta-analysis
Citation: Musazadeh V, Mohammadi Anilou M, Vajdi M, Karimi A, Sedgh Ahrabi S and Dehghan P (2023) Effects of synbiotics supplementation on anthropometric and lipid profile parameters: Finding from an umbrella meta-analysis. Front. Nutr. 10:1121541. doi: 10.3389/fnut.2023.1121541
Received: 11 December 2022; Accepted: 06 February 2023;
Published: 23 February 2023.
Edited by:
Guiju Sun, Southeast University, ChinaReviewed by:
Stefan Kabisch, Charité – Universitätsmedizin Berlin, GermanyAzam Doustmohammadian, Iran University of Medical Sciences, Iran
Copyright © 2023 Musazadeh, Mohammadi Anilou, Vajdi, Karimi, Sedgh Ahrabi and Dehghan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parvin Dehghan,  RGVoZ2hhbi5udXRAZ21haWwuY29t
RGVoZ2hhbi5udXRAZ21haWwuY29t
 Vali Musazadeh
Vali Musazadeh Maryam Mohammadi Anilou2
Maryam Mohammadi Anilou2 Mahdi Vajdi
Mahdi Vajdi Sana Sedgh Ahrabi
Sana Sedgh Ahrabi