
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Nutr. , 21 February 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1121213
Breast milk jaundice (BMJ) is one of the main factors leading to interruption or early termination of breastfeeding. Interrupting breastfeeding to treat BMJ may increase the adverse consequences for infant growth and disease prevention. The Intestinal flora and metabolites are increasingly recognized as a potential therapeutic target in BMJ. First, dysbacteriosis can lead to a decrease in the metabolite short-chain fatty acids. At the same time, SCFA can act on specific G protein-coupled receptors 41 and 43 (GPR41/43), and a decrease in SCFA downregulates the GPR41/43 pathway, leading to a diminished inhibition of intestinal inflammation. In addition, intestinal inflammation leads to a decrease in intestinal motility and a large amount of bilirubin enters the enterohepatic circulation. Ultimately, these changes will result in the development of BMJ. In this review, we will describe the underlying pathogenetic mechanism of the intestinal flora effects on BMJ.
Breastfeeding is considered as an optimal way to feed infants during the neonatal period, providing them with the developmental nutrients needed and shaping their immune systems (1). Most studies have shown that exclusive breastfeeding provides potential long-term benefits to neurodevelopment (2, 3). Interrupting breastfeeding to treat breast milk jaundice (BMJ) has long been controversial and may increase the risk of early termination of breastfeeding (4). In the community, the impact of neonatal BMJ on reduced breastfeeding and vaccination rates has been widely observed, which may resulting in adverse consequences for infant growth and disease prevention, even though clinicians generally advise continuing exclusive breastfeeding during BMJ (5). Therefore, clarifying the pathogenesis of BMJ is of great importance to prevent the occurrence of these adverse effects (Figure 1).
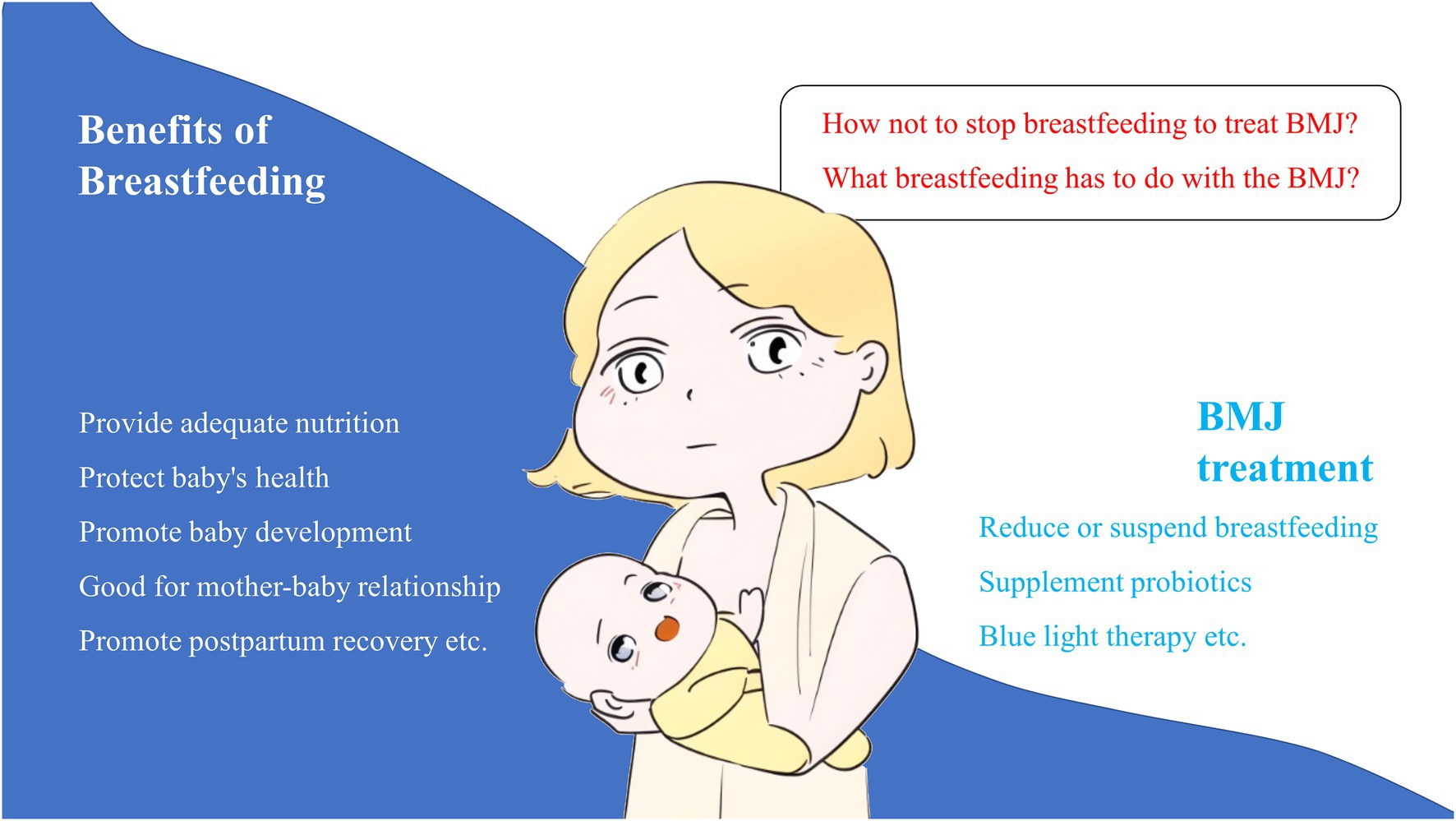
Figure 1. The benefits of breastfeeding and the main modalities for treating BMJ, as well as issues to consider.
Breastfeeding jaundice and breast milk jaundice are two patterns of jaundice in newborns, which can occur with breastfeeding (6). The former frequently show excessive initial weight loss and be dehydrated in newborns who due to not receive sufficient breast milk causing early unconjugated hyperbilirubinemia between 4 and 7 days postpartum (7, 8). The latter usually can be appeared to infants who are well suckling, with satisfactory weight gain, and mild jaundice which typically occurs 1 week after birth, and possibly last up to 12 weeks, in addition, hyperbilirubinemia can be observed decreases when breast milk is replaced with infant formula (9).
The etiological mechanism of BMJ is not completely clear, and the increase in enterohepatic circulation is the usually compelling theory, although it may be caused by several factors. Notably, recent studies have reported that dysbacteriosis is one of the causative factors for BMJ and played an important role in the pathogenesis of BMJ (10, 11). In addition, studies have shown that the metabolites of microbiota also played an important role in physiological functions and have been regarded as a bridge between microbiota and diseases, such as bile acids, branched-chain amino acids, short-chain fatty acids, tryptophan, and indole derivatives (11, 12). Among these metabolites, short-chain fatty acids (SCFA) has become a focal point in recent years. Studies have shown that SCFA played an important role in maintaining the integrity of the intestinal epithelium and repairing the mucosa after injury (13, 14). However, few studies have focused on the role of SCFA in the pathogenesis of BMJ. The purpose of this review was to explore the value of intestinal flora and SCFA in the pathogenesis of BMJ. Reviewing and analyzing these mechanisms will help prevent and control BMJ, thereby ensuring the rate of breastfeeding.
The World Health Organization (WHO) recommends exclusive breastfeeding of infants for the first 6 months of life, but among the factors leading to interruption or early termination of breastfeeding, BMJ is one of the main factors (5). Notably, in recent years, with the popularization of breastfeeding in China, more and more infants are found to develop BMJ in the clinic, and BMJ is gradually becoming the leading cause of neonatal jaundice (15). Neonatal jaundice is a common condition that affects up to 80% of newborn babies and has become an important public health problem (16, 17). Accumulating evidence noted that breast-fed infants are at higher risk of jaundice than formula-fed infants (18, 19). Newman et al. (20) first reported that breastmilk was a contributing factor to the development of neonatal jaundice. Breastmilk contains the substance beta-glucuronidase, which, in the intestine, decouples glucuronic acid from conjugated bilirubin and allows bilirubin to be reabsorbed by the enterohepatic circulation (21). In addition, bilirubin undergoes selective metabolism by UDP-glucuronosyltransferase (UGT) 1A1, that UGT1A1 gene mutation is closely related to BMJ (22, 23). BMJ is usually harmless and a self-limiting disease with a good prognosis for infants. However, few newborns with high levels of serum unconjugated bilirubin may damage the child’s central nervous system, leaving severe neurological sequelae (24, 25). Therefore, exploring the possible pathogenesis of BMJ can help relieve some of the anxiety of breastfeeding in pregnant women and help the health of newborns.
The human intestinal flora is composed of microbial communities that is known as a “microbial organ” within the host, a new human physiological system, and a “superorganism” with human tissues and organs (26, 27). These microbial communities are interconnected, engaged in a continuous exchange of information with the host cell, and regulated the transformation of important chemicals (28).
Studies have shown that dysbacteriosis is closely related to the occurrence of BMJ: Under normal conditions, certain bacteria in the intestinal tract can transform bilirubin into stercobilin and affect bilirubin excretion by promoting Intestinal motility during bilirubin metabolism (8). However, dysbacteriosis can lead to a decrease in bilirubin secretion, which result in higher enterohepatic circulation and thus induce BMJ (10, 29, 30). Moreover, Lactobacillus rhamnosus GG were used to treat jaundice without stopping breastfeeding, and more satisfactory results were achieved (31). As mentioned above, dysbacteriosis is closely related to the occurrence of BMJ, but the exact mechanism of how dysbacteriosis lead to BMJ remains to be further investigated.
The intestinal flora effect on the host is mainly through metabolites, among them, SCFA has received extensive attention in intestinal diseases in recent years (32, 33). Interestingly, SCFA may be the key between dysbacteriosis and the occurrence of BMJ. SCFA are mainly produced by the fermentation of dietary fiber by microbiota, which is produced in the upper part of the colon near the ileocecal junction (34, 35). Coincidentally, the enterohepatic circulation of bilirubin that induced BMJ also happens to be hydrolytically separated at the end of the ileum, and then forms unconjugated bilirubin that is absorbed by the intestine (36). Therefore, the former and the latter are very close to the site of production and are relevant. In addition, Yang et al. found that bacteria reduction in bilirubin encephalopathy is associated with SCFA metabolism (37). Chen et al. showed that the abundance of some bacteria that mainly produced SCFA in the bilirubin encephalopathy group were significantly lower than that in the control group. Our study has also found that a significant difference in SCFA between the BMJ group and the control group (38). The decrease in SCFA due to dysbacteriosis may be an important reason for the occurrence of BMJ.
The healthy intestinal tract is basically maintained in anaerobic or low-oxygen conditions, resulting in the composition of the intestinal flora being dominated by anaerobic bacteria (39, 40). Studies showed that compared to infants with formula feeding, a decrease in anaerobic bacteria represented by Veronococcus spp. and Clostridium spp. in infants with breastfeeding (41). Another study reported that an increased abundance of facultative anaerobe (predominantly Proteobacteria) in the intestinal flora of infants with BMJ (10). In infants with BMJ, there was an increase in aerobic Streptococcus spp. and a decrease in anaerobic Enterococcus spp. (42). This suggests that the composition of intestinal flora in infants with BMJ is mainly characterized by a change in anaerobic bacteria transform into facultative anaerobes and aerobes. This may be due to the alteration of intestinal flora induced by intestinal inflammation (43), which results in a decrease of metabolites represented by SCFA. SCFA can act on specific G protein-coupled receptors 41 and 43 (GPR41/43), thereby modulating the immune response (the main response presented is inhibition of inflammation) (44). A decrease in SCFA due to dysbacteriosis can aggravate the intestinal inflammatory response, which leads to a decrease in intestinal motility, resulting in a large of bilirubin entering the enterohepatic circulation and ultimately causing the development of BMJ (45, 46). Thus, dysbacteriosis affect bilirubin excretion may by regulating the SCFA-GPR41/43 pathway (Figure 2).
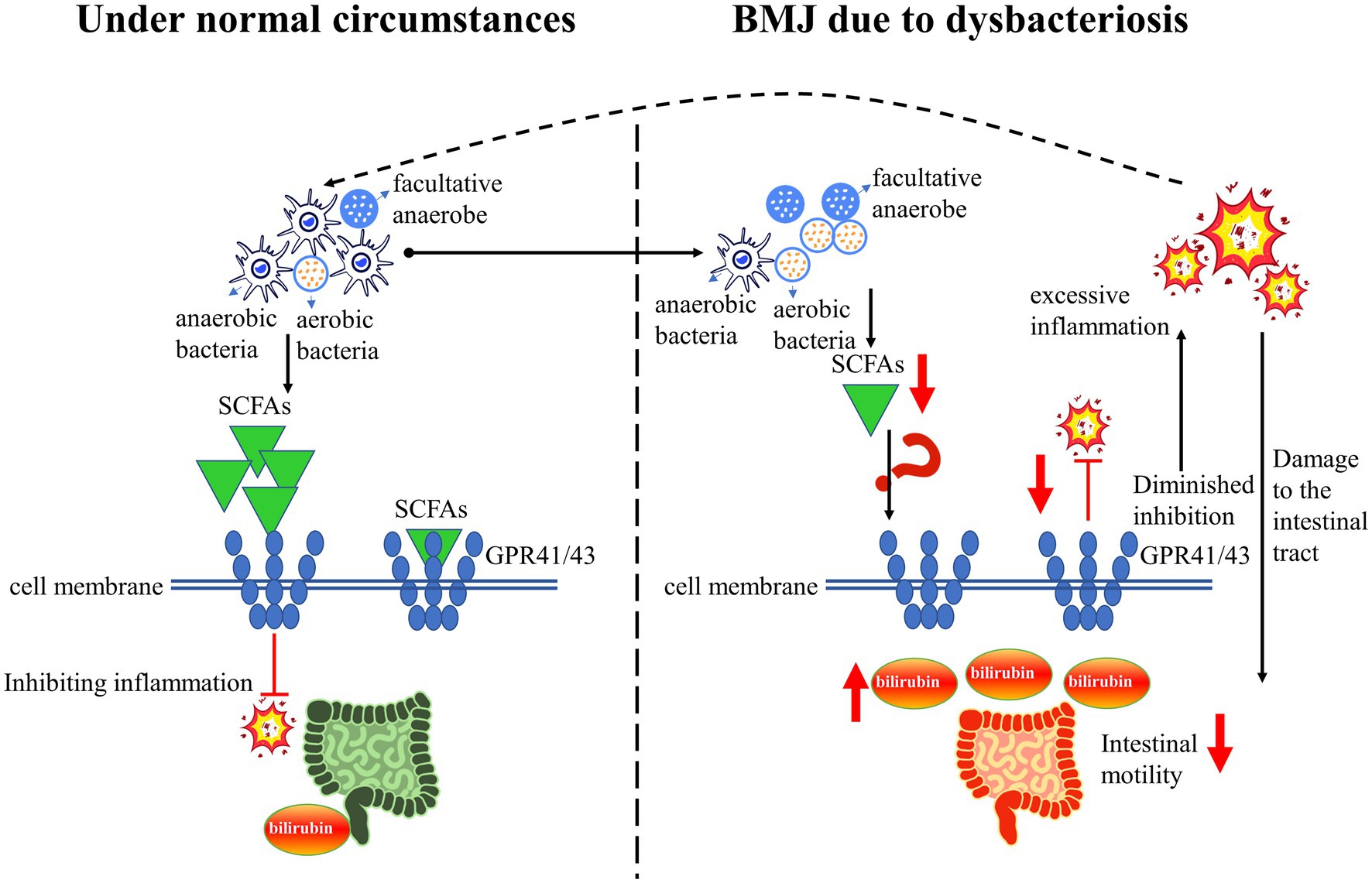
Figure 2. A simplified schematic diagram: dysbacteriosis leads to a decrease in SCFA, and downregulate the GPR41/43 pathway, resulting in the occurrence of BMJ.
An increasing large of studies have demonstrated that gut microbiota regulates bilirubin metabolism in enterohepatic circulation. Probiotics can affect neonatal hyperbilirubinemia via various potential mechanisms. A study demonstrated that Bifidobacterium can inhibit bilirubin enterohepatic circulation by reducing the activity of β-glucuronidase (47). Saccharomyces boulardii has been found a potential mechanism for decreasing enterohepatic circulation to, namely increase intestinal polyamines to promote intestinal maturityis (48). Prebiotics are non-digestible food supplement which decreased serum bilirubin via regulate intestinal microflora to remove the regulation of β-glucuronidase in the middle colon to reduce the enterohepatic circulation of bilirubin (49, 50). The relationship between intestinal microbiota and encephalopathy is an attractive topic, the Microbiota-gut-brain axis is a relatively new concept. Studies have demonstrated that the dysbiosis of gut microbiota can indirectly affects the blood brain barrier and brain regions via short chain fatty acid (51).
Dysbacteriosis can lead to a decrease in SCFA, and downregulate the GPR41/43 pathway, leading to a diminished inhibition of intestinal inflammation, thereby a decrease in intestinal motility and a large amount of bilirubin entering the enterohepatic circulation. Ultimately, these changes will lead to the occurrence and development of BMJ (Figure 2). Thus, modulating dysbacteriosis in BMJ is an effective strategic target for reducing serum bilirubin, but detailed information on the mechanisms remains unknown and this is an area where more research is needed. Although probiotics and prebiotics have a significant impact on the treatment of hyperbilirubinemia, the efficacy remains controversial. It is necessary to investigate the mechanisms in detail in order to provide compelling new therapeutic strategies for BMJ. In addition, more multicenter randomized clinical trial studies are needed to further elucidate the long-term benefits or risks of probiotics and prebiotics on BMJ. Overall, understanding the mechanisms of intestinal flora in the role of BMJ may increase the promotion and support of breastfeeding, and achieve the United Nations Sustainable Development Goal of maternal and child health.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by the National Natural Science Foundation of China (82260317), funds of the Zunyi Science and Technology Bureau (2018–10, 2018–190, and 2020–12), and Science and Technology Department of Guizhou Province (ZK [2021] 371).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lokossou, GAG, Kouakanou, L, Schumacher, A, and Zenclussen, AC. Human breast Milk: from food to active immune response with disease protection in infants and mothers. Front Immunol. (2022) 13:849012. doi: 10.3389/fimmu.2022.849012
2. Belfort, MB, Knight, E, Chandarana, S, Ikem, E, Gould, JF, Collins, CT, et al. Associations of maternal Milk feeding with neurodevelopmental outcomes at 7 years of age in former preterm infants. JAMA Netw Open. (2022) 5:e2221608. doi: 10.1001/jamanetworkopen.2022.21608
3. Dimitroglou, M, Iliodromiti, Z, Christou, E, Volaki, P, Petropoulou, C, Sokou, R, et al. Human breast Milk: the key role in the maturation of immune, gastrointestinal and central nervous systems: a narrative review. Diagn. (2022) 12:2208. doi: 10.3390/diagnostics12092208
4. Soldi, A, Tonetto, P, Varalda, A, and Bertino, E. Neonatal jaundice and human milk. J Matern Fetal Neonatal Med. (2011) 24:85–7. doi: 10.3109/14767058.2011.607612
5. Guo, Q, Cui, M, Liu, X, Zhao, S, Liu, P, and Wang, L. Effect of epidermal growth factor in human Milk and maternal diet on late-onset breast Milk jaundice: a case-control study in Beijing. Nutrients. (2022) 14:4587. doi: 10.3390/nu14214587
6. Leung, AK, and Sauve, RS. Breastfeeding and breast milk jaundice. J R Soc Health. (1989) 109:213–7. doi: 10.1177/146642408910900615
7. Noel-Weiss, J, Courant, G, and Woodend, AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. (2008) 2:e99–e110.
8. Preer, GL, and Philipp, BL. Understanding and managing breast milk jaundice. Arch Dis Child Fetal Neonatal Ed. (2011) 96:F461–6. doi: 10.1136/adc.2010.184416
9. Prameela, KK. Breastfeeding during breast milk jaundice -a pathophysiological perspective. Med J Malays. (2019) 74:527–33.
10. Li, Y, Shen, N, Li, J, Hu, R, Mo, X, and Xu, L. Changes in intestinal Flora and Metabolites in neonates with breast Milk jaundice. Front Pediatr. (2020) 8:177. doi: 10.3389/fped.2020.00177
11. McCarville, JL, Chen, GY, Cuevas, VD, Troha, K, and Ayres, JS. Microbiota metabolites in health and disease. Annu Rev Immunol. (2020) 38:147–70. doi: 10.1146/annurev-immunol-071219-125715
12. Agus, A, Clément, K, and Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. (2021) 70:1174–82. doi: 10.1136/gutjnl-2020-323071
13. Gonçalves, P, Araújo, JR, and Di Santo, JP. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. (2018) 24:558–72. doi: 10.1093/ibd/izx029
14. Kayama, H, Okumura, R, and Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu Rev Immunol. (2020) 38:23–48. doi: 10.1146/annurev-immunol-070119-115104
15. Yang, LF, Li, J, Hu, R, Xu, LQ, Li, YX, and Sheng, WT. Association of fatty acid composition in human milk with breast milk jaundice in neonates. Zhongguo Dang Dai Er Ke Za Zhi. (2020) 22:1256–60. doi: 10.7499/j.issn.1008-8830.2007012
16. Akagawa, S, Akagawa, Y, Yamanouchi, S, Teramoto, Y, Yasuda, M, Fujishiro, S, et al. Association of Neonatal Jaundice with gut Dysbiosis characterized by decreased Bifidobacteriales. Meta. (2021) 11:887. doi: 10.3390/metabo11120887
17. Schwartz, HP, Haberman, BE, and Ruddy, RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. (2011) 27:884–9. doi: 10.1097/PEC.0b013e31822c9b4c
18. Bhutani, VK, Donn, SM, and Johnson, LH. Risk management of severe neonatal hyperbilirubinemia to prevent kernicterus. Clin Perinatol. (2005) 32:125–39, vii. doi: 10.1016/j.clp.2004.11.002
19. Gourley, GR. Breast-feeding, neonatal jaundice and kernicterus. Semin Neonatol. (2002) 7:135–41. doi: 10.1053/siny.2002.0101
20. Newman, AJ, and Gross, S. Hyperbilirubinemia in breast-fed infants. Pediatrics. (1963) 32:995–1001. doi: 10.1542/peds.32.6.995
21. Ma, XW, and Fan, WQ. Earlier nutrient fortification of Breastmilk fed LBW infants improves jaundice related outcomes. Nutrients. (2020) 12:2116. doi: 10.3390/nu12072116
22. Fujiwara, R, Maruo, Y, Chen, S, and Tukey, RH. Role of extrahepatic UDP-glucuronosyltransferase 1A1: advances in understanding breast milk-induced neonatal hyperbilirubinemia. Toxicol Appl Pharmacol. (2015) 289:124–32. doi: 10.1016/j.taap.2015.08.018
23. Maruo, Y, Morioka, Y, Fujito, H, Nakahara, S, Yanagi, T, Matsui, K, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. (2014) 165:36–41.e1. doi: 10.1016/j.jpeds.2014.01.060
24. Altuntaş, N. Is there any effect of Hyperbilirubinemia on breastfeeding? If any, at which level? Breastfeed Med. (2020) 15:29–34. doi: 10.1089/bfm.2019.0176
25. Riordan, SM, and Shapiro, SM. Review of bilirubin neurotoxicity I: molecular biology and neuropathology of disease. Pediatr Res. (2020) 87:327–31. doi: 10.1038/s41390-019-0608-0
26. Kundu, P, Blacher, E, Elinav, E, and Pettersson, S. Our gut microbiome: the evolving inner self. Cells. (2017) 171:1481–93. doi: 10.1016/j.cell.2017.11.024
27. O’Hara, AM, and Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. (2006) 7:688–93. doi: 10.1038/sj.embor.7400731
28. Schmidt, TSB, Raes, J, and Bork, P. The human gut microbiome: from association to modulation. Cells. (2018) 172:1198–215. doi: 10.1016/j.cell.2018.02.044
29. Tuzun, F, Kumral, A, Duman, N, and Ozkan, H. Breast milk jaundice: effect of bacteria present in breast milk and infant feces. J Pediatr Gastroenterol Nutr. (2013) 56:328–32. doi: 10.1097/MPG.0b013e31827a964b
30. Zhou, S, Wang, Z, He, F, Qiu, H, Wang, Y, Wang, H, et al. Association of serum bilirubin in newborns affected by jaundice with gut microbiota dysbiosis. J Nutr Biochem. (2019) 63:54–61. doi: 10.1016/j.jnutbio.2018.09.016
31. Mutlu, M, Aslan, Y, Kader, Ş, and Aktürk Acar, F. Preventive effects of probiotic supplementation on neonatal Hyperbilirubinemia caused by Isoimmunization. Am J Perinatol. (2020) 37:1173–6. doi: 10.1055/s-0039-1692690
32. Shen, G, Wu, J, Ye, BC, and Qi, N. Gut microbiota-derived metabolites in the development of diseases. Can J Infect Dis Med Microbiol. (2021) 2021:6658674. doi: 10.1155/2021/6658674
33. Wang, W, Fang, D, Zhang, H, Xue, J, Wangchuk, D, Du, J, et al. Sodium butyrate selectively kills cancer cells and inhibits migration in colorectal cancer by targeting Thioredoxin-1. Onco Targets Ther. (2020) 13:4691–704. doi: 10.2147/ott.S235575
34. Ghonimy, A, Zhang, DM, Farouk, MH, and Wang, Q. The impact of Carnitine on dietary fiber and gut bacteria metabolism and their mutual interaction in Monogastrics. Int J Mol Sci. (2018) 19:1008. doi: 10.3390/ijms19041008
35. McNabney, SM, and Henagan, TM. Short chain fatty acids in the colon and Peripheral tissues: a focus on butyrate, colon cancer. Obes Insulin Resist Nutr. (2017) 9:1384. doi: 10.3390/nu9121348
36. Hansen, TWR, Wong, RJ, and Stevenson, DK. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol Rev. (2020) 100:1291–346. doi: 10.1152/physrev.00004.2019
37. Yang, N, Yang, RX, Wang, AH, and Zhang, YQ. The effect of intestinal flora on the neural development of severe hyperbilirubinemia neonates. Eur Rev Med Pharmacol Sci. (2019) 23:1291–5. doi: 10.26355/eurrev_201902_17024
38. Duan, M, Han, ZH, Huang, T, Yang, Y, and Huang, B. Characterization of gut microbiota and short-chain fatty acid in breastfed infants with or without breast milk jaundice. Lett Appl Microbiol. (2021) 72:60–7. doi: 10.1111/lam.13382
39. Byndloss, MX, and Bäumler, AJ. The germ-organ theory of non-communicable diseases. Nat Rev Microbiol. (2018) 16:103–10. doi: 10.1038/nrmicro.2017.158
40. Litvak, Y, Byndloss, MX, Tsolis, RM, and Bäumler, AJ. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr Opin Microbiol. (2017) 39:1–6. doi: 10.1016/j.mib.2017.07.003
41. Ma, J, Li, Z, Zhang, W, Zhang, C, Zhang, Y, Mei, H, et al. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci Rep. (2020) 10:15792. doi: 10.1038/s41598-020-72635-x
42. Duan, M, Yu, J, Feng, J, He, Y, Xiao, S, Zhu, D, et al. 16S ribosomal RNA-based gut microbiome composition analysis in infants with breast milk jaundice. Open Life Sci. (2018) 13:208–16. doi: 10.1515/biol-2018-0025
43. Vítek, L, and Tiribelli, C. Bilirubin, intestinal integrity, the microbiome, and inflammation. N Engl J Med. (2020) 383:684–6. doi: 10.1056/NEJMcibr2013250
44. Kim, MH, Kang, SG, Park, JH, Yanagisawa, M, and Kim, CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. (2013) 145:396–406.e391-310. doi: 10.1053/j.gastro.2013.04.056
45. Chen, K, and Yuan, T. The role of microbiota in neonatal hyperbilirubinemia. Am J Transl Res. (2020) 12:7459–74.
46. Novák, P, Jackson, AO, Zhao, GJ, and Yin, K. Bilirubin in metabolic syndrome and associated inflammatory diseases: new perspectives. Life Sci. (2020) 257:118032. doi: 10.1016/j.lfs.2020.118032
47. Rocha Martin, VN, Lacroix, C, Killer, J, Bunesova, V, Voney, E, Braegger, C, et al. Cutibacterium avidum is phylogenetically diverse with a subpopulation being adapted to the infant gut. Syst Appl Microbiol. (2019) 42:506–16. doi: 10.1016/j.syapm.2019.05.001
48. Serce, O, Gursoy, T, Ovali, F, and Karatekin, G. Effects of saccharomyces boulardii on neonatal hyperbilirubinemia: a randomized controlled trial. Am J Perinatol. (2015) 30:137–42. doi: 10.1055/s-0034-1376390
49. Armanian, AM, Barekatain, B, Hoseinzadeh, M, and Salehimehr, N. Prebiotics for the management of hyperbilirubinemia in preterm neonates. J Matern Fetal Neonatal Med. (2016) 29:3009–13. doi: 10.3109/14767058.2015.1113520
50. Barszcz, M, Taciak, M, and Skomiał, J. The effects of inulin, dried Jerusalem artichoke tuber and a multispecies probiotic preparation on microbiota ecology and immune status of the large intestine in young pigs. Arch Anim Nutr. (2016) 70:278–92. doi: 10.1080/1745039x.2016.1184368
Keywords: breast milk jaundice, intestinal flora, short-chain fatty acids, G protein-coupled receptors 41 and 43, neonate
Citation: Huang H, Huang J, Huang W, Huang N and Duan M (2023) Breast milk jaundice affects breastfeeding: From the perspective of intestinal flora and SCFAs-GPR41/43. Front. Nutr. 10:1121213. doi: 10.3389/fnut.2023.1121213
Received: 11 December 2022; Accepted: 03 February 2023;
Published: 21 February 2023.
Edited by:
Cheng Guoqiang, Fudan University, ChinaReviewed by:
Phillipp Hartmann, University of California, San Diego, United StatesCopyright © 2023 Huang, Huang, Huang, Huang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanqu Huang, ✉ aHVhbmduYW5xdUAxNjMuY29t; Miao Duan, ✉ d2VpbWlhbzEyM0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.