- 1Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China
- 2National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
- 3Department of Toxicology and Sanitary Chemistry, School of Public Health, Tianjin Medical University, Tianjin, China
- 4School of Public Health, Inner Mongolia Medical University, Hohhot, China
- 5Key Laboratory of Liaoning Tumor Clinical Metabolomics, Jinzhou, Liaoning, China
Aim: Evidence linking dietary patterns and the risk of gastric cancer was limited, especially in Chinese populations. This study aimed to explore the association between dietary patterns and the risk of gastric cancer in residents of the Huaihe River Basin, China.
Methods: The association between dietary patterns and the risk of gastric cancer was investigated through a case-control study. Dietary patterns were identified with factor analysis based on responses to a food frequency questionnaire (FFQ). Gastric cancer was diagnosed according to the International Classification of Diseases, 10th Revision (ICD 10). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated across the tertiles of dietary pattern scores using unconditional logistic regression models.
Results: A total of 2,468 participants were included in this study. Six main dietary patterns were extracted, and those patterns explained 57.09% of the total variation in food intake. After adjusting for demographic characteristics, lifestyle factors, individual disease history, family history of cancer and Helicobacter. Pylori (H. pylori) infection, comparing the highest with the lowest tertiles of dietary pattern scores, the multivariable ORs (95% CIs) were 0.786 (0.488, 1.265; Ptrend < 0.001) for the flavors, garlic and protein pattern, 2.133 (1.299, 3.502; Ptrend < 0.001) for the fast food pattern, 1.050 (0.682, 1.617; Ptrend < 0.001) for the vegetable and fruit pattern, 0.919 (0.659, 1.282; Ptrend < 0.001) for the pickled food, processed meat products and soy products pattern, 1.149 (0.804, 1.642; Ptrend < 0.001) for the non-staple food pattern and 0.690 (0.481, 0.989; Ptrend < 0.001) for the coffee and dairy pattern.
Conclusions: The specific dietary patterns were associated with the risk of gastric cancer. This study has implications for the prevention of gastric cancer.
Introduction
As the fifth most frequently diagnosed cancer and the third leading cause of cancer deaths worldwide, gastric cancer has sparked concern (1). Although gastric cancer rates were significantly declining worldwide, the pace has been variable in different regions (1). China has the largest number of incident gastric cancer cases in 2020 (2). The disease burden remained heavy in China (3–5). The incident gastric cases in China accounted for 48.26% of the global burden in 2019 (4).
Infectious factors, environmental risk factors and lifestyle were all linked with an increased risk of gastric cancer (1). Specifically, dietary factors were associated with an increased risk of gastric cancer, such as specific food consumption (such as meat, alcohol and so on), a high-salt diet, a low-vitamin A and C diet and consuming large amounts of smoked or cured food (6–9).
Food factors of diet are correlated with each other, making them difficult to appear in isolation (10). The dietary pattern could provide a more comprehensive assessment of the collective importance of different dietary factors (10–12). The dietary pattern has been applied to summarize and capture the overall dietary exposure and variations (13). The results of the association between dietary patterns and gastric cancer were mixed (10, 14–17). Given that, the association between dietary patterns and the risk of gastric cancer was examined in residents of the Huaihe River Basin, China, in the present study. The focus should be on modifiable factors as early as possible, which could show high effectiveness in preventing gastric cancer at a low cost.
Materials and methods
Study population
The field investigation was performed in 14 counties or districts of the Huaihe River Basin in 2021. All participants were chosen from local residents. We used data from the local cancer registry to select gastric cancer cases. Controls were randomly selected from local residents. The study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. In addition, all participants were aware of the research purpose and provided written informed consent.
Inclusion criteria and exclusion criteria
Cases were included according to the following criteria: (1) cases were diagnosed with gastric cancer from January 1, 2020, to July 31, 2021; (2) the absence of mental disorders or cognition dysfunction; (3) Subjects who have lived in the local area for more than 10 years; (4) Subjects were not to be given a diagnosis of other malignant tumors; (5) The diagnostic criteria were consistent with the 2019 Chinese Cancer Registry Annual Report (ICD-10/C16). Cases were excluded according to the following criteria: (1) cases were diagnosed as gastric cancer before January 1, 2020; (2) Subjects who were unable to participate in the investigation due to health problems; (3) Subjects who were unable to cooperate in the investigation due to serious illness or other reasons; (4) having a medical disease that influenced language function or movement; (5) Subjects who have not lived in the local area for more than 10 years, or could not return to the local area for other reasons such as migrant work; (6) Subjects were to be given a diagnosis of other types of cancers; (7) The diagnostic criteria were different from the 2019 Chinese Cancer Registry Annual Report.
Controls were included according to the following criteria: (1) The age difference between cases and controls was within 5 years; (2) the absence of mental disorders or cognition dysfunction; (3) Subjects who have lived in the local area for more than 10 years; (4) Subjects were not to be given a diagnosis of malignant tumors. Controls were excluded according to the following criteria: (1) The age difference between cases and controls was over 5 years; (2) Subjects who were unable to participate in the investigation due to health problems; (3) Subjects who were unable to cooperate in the investigation due to serious illness or other reasons; (4) having a medical disease that influenced language function or movement; (5) Subjects who have not lived in the local area for more than 10 years, or could not return to the local area for other reasons such as migrant work; (6) Subjects were to be given a diagnosis of malignant tumors.
Data collection
Information on demography, lifestyle factors and medical history was collected by standard questionnaires. Dietary intake was assessed using a food frequency questionnaire (FFQ) including 16 food groups. The FFQ recorded about the specific mean consumption frequency (times/day, times/week, times/month, and times/year) and the specific mean consumption amount of selected food items during the previous months. Education was categorized into middle school or less, high school and college or more. Occupation was categorized into four categories: professional and administrative, office, sales and service, laborer and agriculture, unemployed, and others. Smoking status was categorized into never smoker, former smoker and current smoker (every day and sometimes). Drinking status was categorized into drinker and non-drinker. Health conditions including medical history, family history of cancers and so on were examined by clinicians. Height and weight were measured and recorded by a trained investigator. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Assessment of dietary patterns
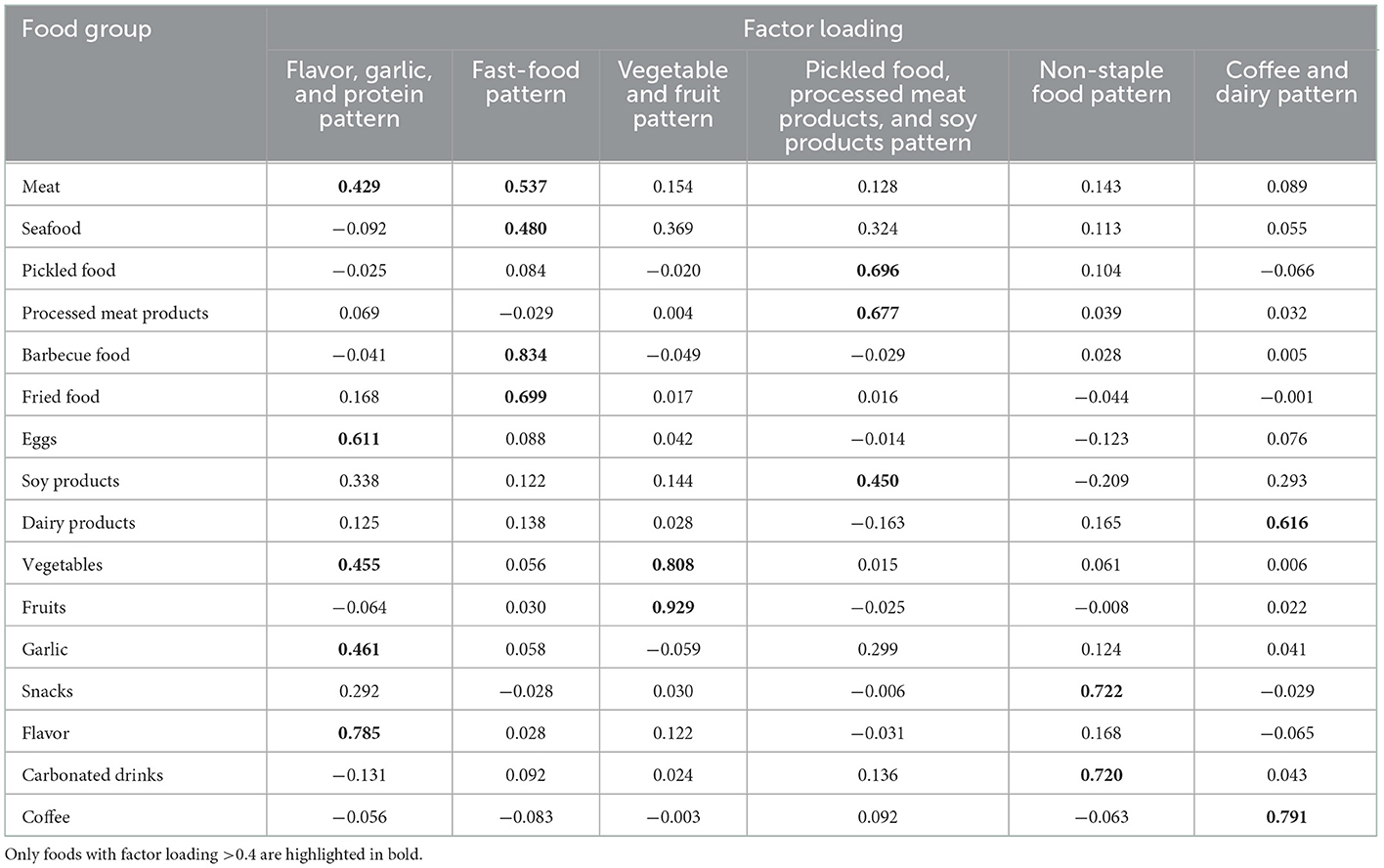
Factor analysis (principal components analysis) was used to derive major dietary patterns and to determine factor loadings for each food subgroups (in g/d). Evaluation of the eigenvalues and scree plot test was used to determine the number of retained factors. Varimax rotation was used to maintain uncorrelated factors and enhance interpretability. Six factors (eigenvalues >1.0) could describe distinctive dietary patterns of the study population. Food groups with absolute factor loadings >0.4 were the main contributors to the dietary patterns. Factors were named descriptively according to the food groups showing high loading (absolute value) with respect to each dietary pattern as follows: flavor, garlic and protein pattern (factor 1), fast-food pattern (factor 2), vegetable and fruit pattern (factor 3), pickled food, processed meat products and soy products pattern (factor 4), non-staple food pattern (factor 5), and coffee and dairy pattern (factor 6, Table 2), which totally explained 57.09% of the variance in food intake. For each participant, the factor scores for each dietary pattern were calculated by summing the consumption of the food groups weighted by their factor loadings and a higher score indicated stricter adherence to that dietary pattern.
Assessment of Helicobacter. pylori (H. pylori) infection
All participants completed the H. pylori questionnaire. It consisted of four main modules: (1) knowledge of H. pylori; (2) infection conditions of H. pylori; (3) treatment of H. pylori; (4) infection conditions of H. pylori in the family. All questionnaires were completed by specialized medical personnel to ensure their reliability, authenticity, and completeness. Then, all participants received the H. pylori test. H. pylori infection was detected by the C13 urea breath testing (UBT), which was administered by trained investigators following the instructions. If subjects were not aware own infection condition, the detection result was used to indicate their H. pylori infection. Additionally, participants were categorized as positive or negative.
Statistical analysis
SAS version 9.4 for windows (SAS, Inc.) was used to analyze all data. All continuous variables were described by mean and standard deviation, whilst categorical variables were described by number of cases and percentage. The general characteristics between the case and control subjects were compared by using Student's t-test for continuous variables and the chi-square test for categorical variables. The factor scores for each dietary pattern were categorized into tertiles. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated across the tertiles of the dietary pattern scores using the unconditional logistic regression model. The lowest tertile of each pattern score was used as the reference. Five models were developed. Model 1 was a crude model. Model 2 was adjusted for age (continuous: y), gender (male or female), and BMI (continuous: kg/m2). Model 3 was additionally adjusted for education lever (middle school or less, high school, college, or more), occupation (professional and administrative, office, sales and service, laborer and agriculture, unemployed, and others), drinking status (drinker or non-drinker), smoking status (every-day smoker, sometime smoker, ex-smoker, or non-smoker) and mood. Model 4 was adjusted for hypertension, diabetes, hyperlipidemia, coronary heart disease, stroke, chronic digestive disorders (gastric ulcer, duodenal ulcer, superficial gastritis, atrophic gastritis, gastric polyps, hepatitis, cirrhosis, and reflux), and family history of cancers (each yes or no) in addition to the variables included in model 3. Model 5 was adjusted for H. pylori infection in addition to the variables included in model 4. The linear trend test was performed by including the median value of each tertile category of the dietary pattern scores as a continuous variable in the model. Tests of statistical significance were two sided and p < 0.05 was considered to be statistically significant for all tests.
Results
Characteristics of the study population
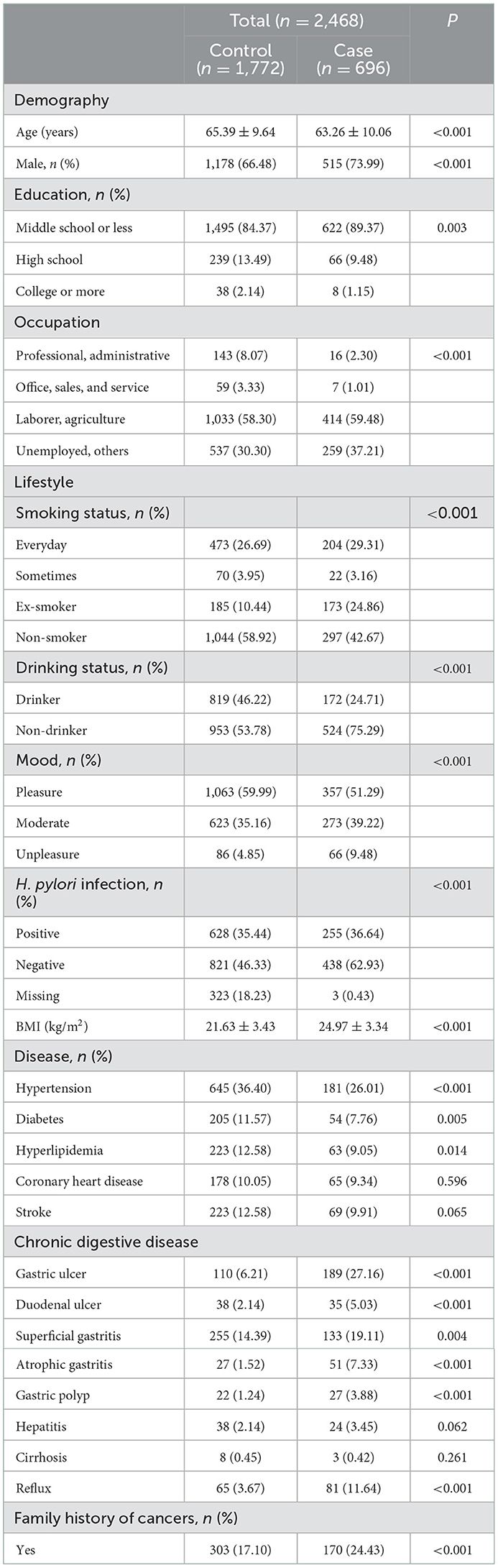
A total of 2,468 participants were included in this study. Table 1 gives the distribution of 696 cases of gastric cancer and 1,772 controls according to demography, lifestyle and other characteristics. There were differences between cases and controls. Compared with the control subjects, cases were more likely to be younger, male, laborer, smokers, non-drinker and to be given a diagnosis of H. pylori-positive, gastric ulcer, duodenal ulcer, superficial gastritis, atrophic gastritis, gastric polyp, reflux, and also were slightly more likely to have a family history of cancers. Additionally, controls were more pleasure, leaner than cases. Furthermore, the case subjects had a significantly higher proportion of individuals with a lower educational status, whereas the proportion of coronary heart disease, stroke, hepatitis, and cirrhosis was similar in both groups (results were shown in Table 1).
Major dietary patterns
Table 2 shows factor loadings of the principal component analysis-extracted dietary patterns. The first pattern was characterized by a high intake of flavors, eggs, garlic, vegetables and red meat and therefore was named the flavor, garlic, and protein pattern. The second pattern had high factor loadings for barbecue food, fried food, red meat and seafood and was named the fast-food pattern. The third pattern consisted of high consumption of fruits and vegetables and was named the vegetable and fruit pattern. The fourth pattern was characterized by a high intake of pickled food, processed meat products and soy products and therefore was named the pickled food, processed meat products and soy products pattern. The fifth pattern had high factor loadings for snacks and carbonated drinks and was named the non-staple food pattern. The last pattern consisted of high consumption of coffee and dairy products and was named the coffee and dairy pattern.
Association between dietary patterns and risk of gastric cancer
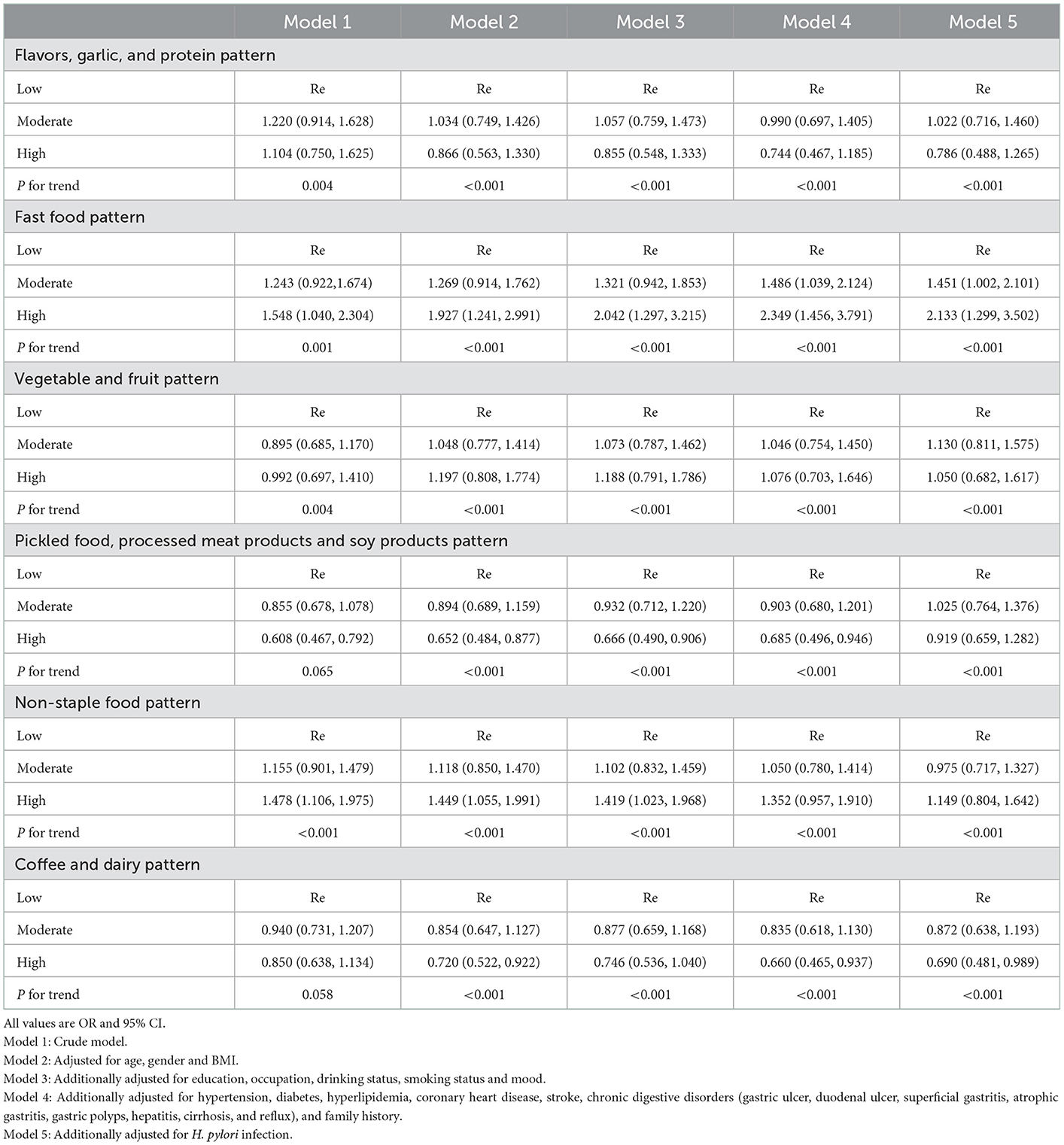
Table 3 described the ORs and 95% CIs according to the factor score tertiles for each pattern. Five models were used in this analysis. In all five models, the fast-food pattern was associated with an increased risk of gastric cancer [ORs (95% Cis) for the highest vs. lowest tertiles: 1.548 (1.040–2.304), 1.927 (1.241–2.991), 2.042 (1.297–3.215), 2.349 (1.456–3.791), 2.133 (1.299–3.502), p for trend < 0.01]. The non-staple food pattern was associated with an increased risk of gastric cancer in model 1, model 2, and model 3 [ORs (95% Cis) for the highest vs. lowest tertiles: 1.478 (1.106–1.975), 1.449 (1.055–1.991), 1.419 (1.023–1.968), p for trend < 0.01]. However, this association was eliminated in model 4 and model 5 [ORs (95% Cis) for the highest vs. lowest tertiles: 1.352 (0.957–1.910), 1.149 (0.804–1.642), p for trend < 0.01]. The pickled food, processed meat products and soy products pattern was associated with decreased risk of gastric cancer in model 1, model 2, model 3, and model 4 [ORs (95% Cis) for the highest vs. lowest tertiles: 0.608 (0.467–0.792), 0.652 (0.484–0.877), 0.666 (0.490–0.906), 0.685 (0.496–0.946), p for trend were 0.065 and others all < 0.001, respectively]. However, this association was eliminated in model 5 [ORs (95% CIs) for the highest vs. lowest tertiles: 0.919 (0.659–1.282), p for trend < 0.01]. The coffee and dairy pattern was associated with decreased risk of gastric cancer in model 2, model 4 and model 5 [ORs (95% CIs) for the highest vs. lowest tertiles: 0.720 (0.522–0.922), 0.660 (0.465–0.937), 0.690 (0.481–0.989), p for trend < 0.001]. However, this association was eliminated in model 1 and model 3 [ORs (95% CIs) for the highest vs. lowest tertiles: 0.850 (0.638–1.134), 0.746 (0.536–1.040), p for trend were 0.058 and <0.01, respectively]. On the other hand, no significant association was observed between other patterns and gastric cancer risk.
Discussion
In this case-control study, it was found that (1) the fast-food pattern and the non-staple food pattern may be associated with an increased risk of gastric cancer; (2) the pickled food, processed meat products and soy products pattern and the coffee and dairy products pattern may be associated with decreased risk of gastric cancer; (3) no significant association was observed between other patterns and risk of gastric cancer; (4) associations between dietary patterns and gastric cancer existed the linear tendency. The above findings could be summarized as a higher intake of specific foods might be associated with the risk of gastric cancer.
Our research has shown that the fast-food pattern and the non-staple food pattern could increase the risk of gastric cancer. The first had a higher consumption of barbecue food, fried food, red meat and seafood. The latter had a higher consumption of snacks and carbonated drinks. The results of ours were consistent with many studies (14, 18–20). There were several mechanisms to explain this association: (1) when red meat was cooked at high temperatures, genotoxic N-nitroso compounds, heterocyclic amines and polycyclic aromatic hydrocarbons could be produced, which were well-known carcinogens (10, 14, 19, 20). (2) Heme iron, which was contained in red meat might play a role in gastric carcinogenesis through the endogenous formation of carcinogenic N-nitroso compounds, DNA damage and oxidative stress (20, 21). Furthermore, iron was a critical factor for bacterial growth in H. pylori, which was a well-known risk factor for gastric cancer (20, 21). (3) Snacks usually contained saturated fats, which could induce the expression of certain inflammatory mediators associated with carcinogenesis (14). (4) Participants who consumed more carbonated drinks tended to have reflux symptoms (22). (5) Carbonated drink usually contained more sugar, which could lead to obesity (7). Obesity was conducive to chronic low-grade inflammation, hyperinsulinemia, hyperleptinemia, and an elevated production of endogenous sex steroid hormones, all of which might contribute to tumor growth (7).
Our research has shown that the pickled food, processed meat products and soy products pattern and the coffee and dairy pattern could decrease the risk of gastric cancer. The first had a high intake of pickled food, processed meat products and soy products. The latter had a high intake of coffee and dairy products. Different from other studies, our research has shown that a higher consumption of pickled food, processed meat products and soy products could decrease the risk of gastric cancer (20, 23). Pickled food and processed meat products usually were recognized as foods that could increase the risk of gastric cancer (20, 23). One side was attributed to salt that was added in pickled food and the other side was attributed to formation of carcinogens (23, 24). The different results may be explained by this important factor. Although pickled food and processed meat products had the largest factor loading, soy products also accounted for a large proportion. The actual intake amount of soy products was higher than that of pickled food and processed meat products. Many studies found that a higher intake of soy products could decrease the risk of gastric cancer (25, 26). Therefore, the promoting effect of pickled food and processed meat products on the risk of gastric cancer may be covered by the protective effect of soy products on the risk of gastric cancer in this dietary pattern. The benefits of soybean intake could be due to two factors. First, soybean and soy products contained isoflavones, which had anti-inflammatory and anti-oxidative effects (26, 27). Furthermore, some experimental studies suggested that genistein, a kind of isoflavones, could induce apoptosis of human gastric carcinoma cells and inhibit H. pylori growth (26). Second, soybean and soy products also included saponins, which were reported as an antitumor ingredient (27). Similar to other studies, our research has shown that a higher consumption of coffee and dairy products could decrease the risk of gastric cancer (28, 29). There were several mechanisms to explain this association: (1) coffee was a mix of bioactive compounds. It contained phenolic compounds and two lipids: cafestol and kahweol. These compounds could suppress cancer growth through antioxidant, anti-genotoxic activity, mitochondrial toxicity and anti-inflammatory environmental regulation (28). (2) Dairy products contained several components, including vitamin D, minerals, calcium and conjugated linoleic acid. The protective effects of these components on gastric cancer may be due to antitumor effects (30, 31). (3) Fermented dairy products such as cheese and yogurt contain lactic acid bacteria, which could suppress the growth of H. pylori by producing inhibitory substances, including lactic acid and bacteriocin (30, 31).
Strengthen and limitations
This study has some strengths. First, we used dietary patterns in our study. Compared to a single food, the dietary pattern could provide a more comprehensive assessment of the collective importance of different dietary factors. Second, all potential confounding factors were adjusted in our study. Last, this was a case-control study based on community. There also existed several potential limitations in this study. First, although our questionnaire requested detailed information regarding the consumption of food and food groups, it was a short version that included only 16 food groups. Second, the actual intake of various foods could not be estimated through the questionnaire, and recall bias was inevitable (32). Recalled diet was found to be more closely associated with past diet than with current diet, recall over many years contained errors due to failures in memory. Third, prevalence cases were included in this study. The prevalence-incidence bias was inevitable. Fourth, the data on total energy intake were not available in this study and it was not adjusted as a confounder. Fifth, confounding would distort the strength of the association (33). Although some potential confounders in this analysis were adjusted for, it was likely that some residual confounding remained due to imperfect statistical adjustment, imperfect confounder measurement, and the existence of confounders that were unknown, unmeasured or otherwise unadjusted for (33). Sixth, there existed the missing value of H. pylori in our study, which was a great contributor to increase the risk of gastric cancer. Seventh, participants were from the Huaihe River Basin, the applicability was limited. Last, due to the limitation of the observational study, causation could not be detected in this study.
Conclusions
In conclusion, it was indicated that specific dietary patterns may be associated with the risk of gastric cancer. This study had considerable public health implications. The quality of diet was important to health and longevity (34). Actually, the dietary pattern was associated with health behaviors, lifestyle and sociodemographic factors, it reflected individual awareness and attitudes toward health (34, 35). Participants who preferred an unhealthy diet need to receive dietary education and diet management for the prevention of gastric cancer. Societies and individuals might success in lowering their risk for gastric cancer by changing preferences in diet. Additional prospective studies with more participants were needed.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. In addition, all participants were aware of the research purpose and provided written informed consent. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XW: data analysis, writing—original draft, and writing—review and editing. QZ, WZ, and WW: writing—review and editing. HG: writing—original draft and writing—review and editing. NW: conceptualization, investigation, project administration, and supervision. XF and BZ: investigation and supervision. ZF: conceptualization and writing—review and editing. JW: conceptualization, methodology, project administration, supervision, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Chinese Academy of Engineering 2022 major strategic research and consulting project National Health Management Project Research (2022-XBZD-21-02), National Natural Science Foundation of China (grant number: 82273676), the National Key Research and Development Program of China (grant number: 2021YFA1301202), and Liaoning Province Scientific and Technological Project (2021JH2/10300039).
Acknowledgments
We thank the participants included in our study for their involvement and enthusiasm.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mukkamalla S, Recio-Boiles A, Babiker HM. Gastric Cancer. Treasure Island, FL: StatPearls Publishing (2022).
2. Wu Y, Li Y, Giovannucci E. Potential impact of time trend of lifestyle risk factors on burden of major gastrointestinal cancers in China. Gastroenterology. (2021) 161:1830–41. doi: 10.1053/j.gastro.2021.08.006
3. Zhou J, Zheng R, Zhang S, Chen R, Wang S, Sun K, et al. Gastric and esophageal cancer in China 2000 to 2030: Recent trends and short-term predictions of the future burden. Cancer Med. (2022) 11:1902–12. doi: 10.1002/cam4.4586
4. Yang X, Zhang T, Zhang H, Sang S, Chen H, Zuo X. Temporal trend of gastric cancer burden along with its risk factors in China from 1990 to 2019, and projections until 2030: comparison with Japan, South Korea, and Mongolia. Biomark Res. (2021) 9:84. doi: 10.1186/s40364-021-00340-6
5. Zhang T, Chen H, Yin X, He Q, Man J, Yang X, et al. Changing trends of disease burden of gastric cancer in China from 1990 to 2019 and its predictions: Findings from Global Burden of Disease Study. Chin J Cancer Res. (2021) 33:11–26. doi: 10.21147/j.issn.1000-9604.2021.01.02
6. Buckland G, Travier N, Huerta JM, Bueno-de-Mesquita HB, Siersema PD, Skeie G, et al. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int J Cancer. (2015) 137:598–606. doi: 10.1002/ijc.29411
7. Bouras E, Tsilidis KK, Triggi M, Siargkas A, Chourdakis M, Haidich AB. Diet and risk of gastric cancer: An umbrella review. Nutrients. (2022) 14:1764. doi: 10.3390/nu14091764
8. Ferro A, Rosato V, Rota M, Costa AR, Morais S, Pelucchi C, et al. Meat intake and risk of gastric cancer in the Stomach cancer Pooling (StoP) project. Int J Cancer. (2020) 147:45–55. doi: 10.1002/ijc.32707
9. Wu X, Chen L, Cheng J, Qian J, Fang Z, Wu J. Effect of dietary salt intake on risk of gastric cancer: A systematic review and meta-analysis of case-control studies. Nutrients. (2022) 14:4260. doi: 10.3390/nu14204260
10. Kim JH, Lee J, Choi IJ, Kim YI, Kim J. Dietary patterns and gastric cancer risk in a Korean population: A case-control study. Eur J Nutr. (2021) 60:389–97. doi: 10.1007/s00394-020-02253-w
11. Zhang T, Rayamajhi S, Meng G, Zhang Q, Liu L, Wu H, et al. Dietary patterns and risk for hyperuricemia in the general population: Results from the TCLSIH cohort study. Nutrition. (2022) 93:111501. doi: 10.1016/j.nut.2021.111501
12. Pou SA, Del PDM, De La Quintana AG, Forte CA, Aballay LR. Identification of dietary patterns in urban population of Argentina: Study on diet-obesity relation in population-based prevalence study. Nutr Res Pract. (2016) 10:616–22. doi: 10.4162/nrp.2016.10.6.616
13. Bertuccio P, Rosato V, Andreano A, Ferraroni M, Decarli A, Edefonti V, et al. Dietary patterns and gastric cancer risk: A systematic review and meta-analysis. Ann Oncol. (2013) 24:1450–8. doi: 10.1093/annonc/mdt108
14. Castello A, Fernandez DLN, Martin V, Davila-Batista V, Boldo E, Guevara M, et al. High adherence to the Western, Prudent, and Mediterranean dietary patterns and risk of gastric adenocarcinoma: MCC-Spain study. Gastric Cancer. (2018) 21:372–82. doi: 10.1007/s10120-017-0774-x
15. Kim MK, Sasaki S, Sasazuki S, Tsugane S. Prospective study of three major dietary patterns and risk of gastric cancer in Japan. Int J Cancer. (2004) 110:435–42. doi: 10.1002/ijc.20132
16. Masaki M, Sugimori H, Nakamura K, Tadera M. Dietary patterns and stomach cancer among middle-aged male workers in Tokyo. Asian Pac J Cancer Prev. (2003) 4:61–6.
17. Chen H, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, et al. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. (2002) 75:137–44. doi: 10.1093/ajcn/75.1.137
18. Karagulle M, Fidan E, Kavgaci H, Ozdemir F. The effects of environmental and dietary factors on the development of gastric cancer. J BUON. (2014) 19:1076–82.
19. Collatuzzo G, Etemadi A, Sotoudeh M, Nikmanesh A, Poustchi H, Khoshnia M, et al. Meat consumption and risk of esophageal and gastric cancer in the Golestan Cohort Study, Iran. Int J Cancer. (2022) 151:1005–12. doi: 10.1002/ijc.34056
20. Wilunda C, Yamaji T, Iwasaki M, Inoue M, Tsugane S, Sawada N. Meat consumption and gastric cancer risk: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr. (2022) 115:652–61. doi: 10.1093/ajcn/nqab367
21. Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: An overall and dose-response meta-analysis. Nutrients. (2019) 11:826. doi: 10.3390/nu11040826
22. Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, et al. Carbonated soft drink consumption and risk of esophageal adenocarcinoma. J Natl Cancer Inst. (2006) 98:72–5. doi: 10.1093/jnci/djj007
23. Ren JS, Kamangar F, Forman D, Islami F. Pickled food and risk of gastric cancer–A systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol Biomarkers Prev. (2012) 21:905–15. doi: 10.1158/1055-9965.EPI-12-0202
24. Zhao Z, Yin Z, Zhao Q. Red and processed meat consumption and gastric cancer risk: A systematic review and meta-analysis. Oncotarget. (2017) 8:30563–75. doi: 10.18632/oncotarget.15699
25. Yip C, Yip YC, Chan W. The associations of soy intakes with non-communicable diseases: A scoping review of meta-analyses. Br J Nutr. (2022) 2022:1–33. doi: 10.1017/S0007114522000691
26. Ko KP, Park SK, Yang JJ, Ma SH, Gwack J, Shin A, et al. Intake of soy products and other foods and gastric cancer risk: A prospective study. J Epidemiol. (2013) 23:337–43. doi: 10.2188/jea.JE20120232
27. Kim J. Protective effects of Asian dietary items on cancers - soy and ginseng. Asian Pac J Cancer Prev. (2008) 9:543–8.
28. Parra-Lara LG, Mendoza-Urbano DM, Bravo JC, Salamanca CH, Zambrano AR. Coffee consumption and its inverse relationship with gastric cancer: An ecological study. Nutrients. (2020) 12:3028. doi: 10.3390/nu12103028
29. Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, Astrup A. Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr Res. (2016) 60:32527. doi: 10.3402/fnr.v60.32527
30. Wang S, Zhou M, Ji A, Zhang D, He J. Milk/dairy products consumption and gastric cancer: An update meta-analysis of epidemiological studies. Oncotarget. (2018) 9:7126–35. doi: 10.18632/oncotarget.23496
31. Tian SB, Yu JC, Kang WM, Ma ZQ, Ye X, Cao ZJ. Association between dairy intake and gastric cancer: A meta-analysis of observational studies. PLoS ONE. (2014) 9:e101728. doi: 10.1371/journal.pone.0101728
32. Barry D, Livingstone V. The investigation and correction of recall bias for an ordinal response in a case-control study. Stat Med. (2006) 25:965–75. doi: 10.1002/sim.2238
33. Stanaway JD, Afshin A, Ashbaugh C, Bisignano C, Brauer M, Ferrara G, et al. Health effects associated with vegetable consumption: a Burden of Proof study. Nat Med. (2022) 28:2066–74. doi: 10.1038/s41591-022-01970-5
34. Ledikwe JH, Smiciklas-Wright H, Mitchell DC, Miller CK, Jensen GL. Dietary patterns of rural older adults are associated with weight and nutritional status. J Am Geriatr Soc. (2004) 52:589–95. doi: 10.1111/j.1532-5415.2004.52167.x
35. Sanchez-Villegas A, Delgado-Rodriguez M, Martinez-Gonzalez MA, De Irala-Estevez J. Gender, age, socio-demographic and lifestyle factors associated with major dietary patterns in the Spanish Project SUN (Seguimiento Universidad de Navarra). Eur J Clin Nutr. (2003) 57:285–92. doi: 10.1038/sj.ejcn.1601528
Keywords: dietary pattern, gastric cancer, factor analysis, unconditional logistic regression, case-control study
Citation: Wu X, Zhang Q, Guo H, Wang N, Fan X, Zhang B, Zhang W, Wang W, Fang Z and Wu J (2023) Dietary patterns and risk for gastric cancer: A case-control study in residents of the Huaihe River Basin, China. Front. Nutr. 10:1118113. doi: 10.3389/fnut.2023.1118113
Received: 07 December 2022; Accepted: 05 January 2023;
Published: 23 January 2023.
Edited by:
Roberta Zupo, National Institute of Gastroenterology S. de Bellis Research Hospital (IRCCS), ItalyReviewed by:
Yunping Zhou, Qingdao University, ChinaMengmeng Song, Beijing Shijitan Hospital, Capital Medical University, China
Copyright © 2023 Wu, Zhang, Guo, Wang, Fan, Zhang, Zhang, Wang, Fang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongze Fang,  ZmFuZ3pob25nemVAdG11LmVkdS5jbg==; Jing Wu,
ZmFuZ3pob25nemVAdG11LmVkdS5jbg==; Jing Wu,  d3VqaW5nQG5jbmNkLmNoaW5hY2RjLmNu
d3VqaW5nQG5jbmNkLmNoaW5hY2RjLmNu
 Xiaomin Wu
Xiaomin Wu Qian Zhang1,3
Qian Zhang1,3 Zhongze Fang
Zhongze Fang Jing Wu
Jing Wu