- 1Clincal Nutrition Department, Ningbo Medical Center Li Huili Hospital, Ningbo, Zhejiang, China
- 2Department of Hepatobiliary-Pancreatic Surgery, Ningbo Medical Center Li Huili Hospital, Ningbo, Zhejiang, China
- 3Nursing Department, Ningbo Medical Center Li Huili Hospital, Ningbo, Zhejiang, China
Objective: To compare the diagnostic value of four tools—the Global Leadership Initiative on Malnutrition (GLIM) criteria, the subjective global assessment (SGA), patient-generated subjective global assessment (PG-SGA), and prognostic nutritional index (PNI) in malnutrition among hospitalized patients undergoing hepatobiliary-pancreatic surgery. Meanwhile, to observe the nutritional intervention of these patients.
Methods: Present study was a cross-sectional study, including 506 hospitalized patients who underwent hepatobiliary-pancreatic surgery between December 2020 and February 2022 at Ningbo Medical Center Lihuili Hospital, China. The incidence rate of malnutrition was diagnosed using the four tools. The consistency of the four tools was analyzed by Cohen's kappa statistic. Data, including nutritional characteristics and nutritional interventions, were collected. The nutritional intervention was observed according to the principles of Five Steps Nutritional Treatment.
Results: The prevalence was 36.75, 44.58, and 60.24%, as diagnosed by the GLIM, PG-SGA, and PNI, respectively, among 332 tumor patients. Among the 174 non-tumor patients, the prevalence was 9.77, 10.92, and 32.18% as diagnosed by the GLIM, SGA, and PNI. The diagnostic concordance of PG-SGA and GLIM was higher (Kappa = 0.814, <0.001) than SGA vs. GLIM (Kappa = 0.752, P < 0.001) and PNI vs. GLIM (Kappa = 0.265, P < 0.001). The univariate analysis revealed that older age, lower BMI and tumorous were significantly associated with nutritional risks and malnutrition. Among 170 patients with nutritional risk, most of patients (118/170, 69.41%) did not meet the nutritional support standard.
Conclusion: The incidence of nutritional risk and malnutrition is high among patients with hepatobiliary and pancreatic diseases, specifically those with tumors. The GLIM showed the lowest prevalence of malnutrition among the four tools. The PG-SGA and GLIM had a relative high level of agreement. There was a low proportion of nutritional support in patients. More prospective and well-designed cohort studies are needed to confirm the relevance of these criteria in clinical practice in the future.
1. Introduction
Malnutrition has been diagnosed as a disease in the complication or comorbidity (CC)/major complication or comorbidity (MCC) catalog of China Healthcare Security Diagnosis Related Groups (CHS-DRGS) with disease codes E40-E46 (1). Nutritional intervention was an important part of multidisciplinary treatment to improve the prognosis of patients, including a shorter hospital stay, less post-operative complications, lower mortality and reduced medical costs (2–4). There were several tools available for the assessment of malnutrition, such as the subjective global assessment (SGA), patient-generated subjective global assessment (PG-SGA), and prognostic nutritional index (PNI). However, the diagnostic criteria have not been harmonized in recent years. In September 2018, the Global Leadership Initiative on Malnutrition (GLIM) was issued to reach a global consensus on the clinical diagnosis of malnutrition (5). It made a progress that the nutritional assessment developed from the subjective methods such as SGA and PG-SGA to diagnosis malnutrition by phenotypic and etiological indicators.
Hospitalized patients undergoing hepatobiliary-pancreatic surgery have a high risk of malnutrition (6). Complex changes in nutrient metabolism and varying degrees of malnutrition may occur in hepatobiliary and pancreatic diseases. Nutritional status in turn affects the occurrence, development and prognosis of the diseases to form a vicious circle. Therefore, it was vital to early diagnosis malnutrition and timely implement nutritional intervention in the multidisciplinary. The applicability and effectiveness of the GLIM criteria have been verified among hospitalized patients with stroke (7), chronic pulmonary obstructive diseases (COPD) (8), gastrointestinal surgery (9), and heart failure (10). However, malnutrition prevalence, as determined by GLIM, among hospitalized patients undergoing hepatobiliary-pancreatic surgery remains largely unknown. Therefore, we aimed to (1) assess its prevalence, as defined by the GLIM, among hospitalized patients undergoing hepatobiliary-pancreatic surgery; (2) compare the PG-SGA, SGA, and PNI with the GLIM criteria in diagnosing malnutrition among these patients; (3) observe the nutritional intervention in hospitalized patients; (4) analyze the factors influencing nutritional risk and malnutrition among these hospitalized patients. In summary, we hope that our research can provide a reference for the more accurate nutritional diagnosis and treatment management of this group.
2. Methods
2.1. Study design and participants
Present study was a single-center, cross-sectional study. It included hospitalized patients who underwent hepatobiliary surgery between December 2020 and February 2022 at Ningbo Medical Center Lihuili Hospital, China. patients included in this study must meet these criteria as follow: (1) aged 18 to 90 years; (2) hospitalized for more than 5 days; (3) conscious; (4) cooperate with nutritional assessment; The exclusion criteria included: (1) length of stay (LOS) of <5 days; (2) refusal to cooperate; and (3) incomplete data required by the study. The study was approved by the ethics committee of the Ningbo Medical Center Lihuili Hospital.
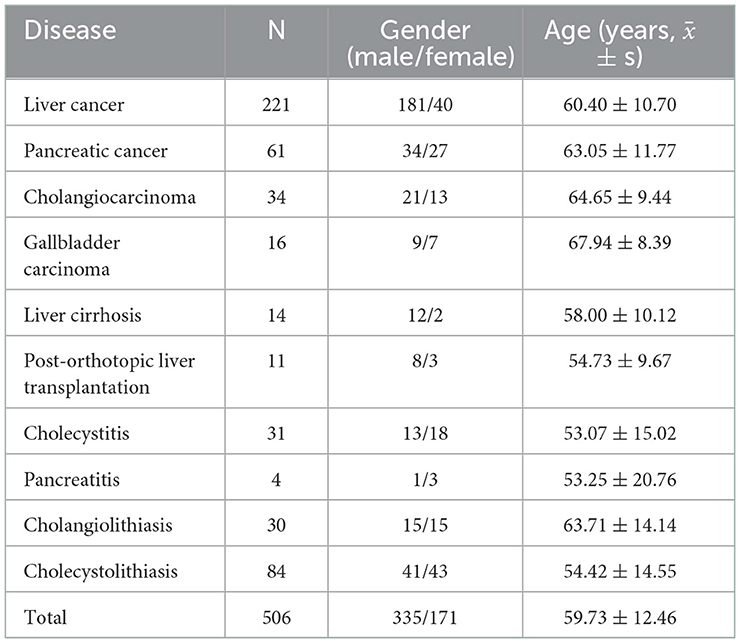
Finally, a total of 506 patients were finally enrolled, including 335 males (66.21%) and 171 females (33.79%). The mean age was 59.73 ± 12.46 years.
2.2. Data acquisition
Malnutrition risk was screened by a trained nutritionist using the Nutritional Risk Screening 2002 (NRS2002) score according to the ESPEN and CSPEN guidelines within the first day after admission (11). NRS2002 score was consisted of three parts including the nutritional status, the severity of illness and the age assessment. The total score of NRS2002 ranges from 0 to 7. NRS2002 score ≥3 indicated that the patient was nutritionally at-risk. Malnutrition was further defined using the GLIM, PNI, PG-SGA, and SGA criteria among participants with nutritional risk.
Clinical data including disease diagnosis, age, sex, height, body weight, body mass index (BMI), length of stay (LOS) and other regular laboratory data were collected. BMI is an evaluation index of overweight and obesity, while the cut-off points defining overweight and obesity are different for Eastern Asian and Western populations. Because our study participants were all Chinese individuals,BMI was categorized as underweight (<18.5 kg/m2), normal weight (18.5 kg/m2 ≤ BMI <24.0 kg/m2), overweight (24.0 kg/m2 ≤ BMI <28.0 kg/m2) and obese (BMI ≥ 28.0 kg/m2) according to the Chinese Working Group on Obesity (WGOC) categories (12). Laboratory test results included total protein, albumin, hemoglobin, leukocyte count, triglycerides (TG), and total cholesterol (TC).
2.3. Diagnostic tools for malnutrition (GLIM, PG-SGA, SGA, and PNI)
The GLIM assessment criteria includes two parts:
• Phenotypic criteria (at least one of three characteristics):
① Unintentional weight loss: >5% within past 6 months or >10% beyond 6 months;
② Low body mass index (kg/m2): <20 if <70 years, or <22 if >70 years; Asia: <18.5 if <70 years, or <20 if >70 years;
③ Reduced muscle mass: reduced by validated body composition measuring techniques.
However, we excluded measurements of total body muscle mass because there was no cut-off point based on clinical studies for the fat-free mass index (FFMI) in China (13).
• Etiologic (presence of at least one criterion):
① Reduced food intake or assimilation: ≤ 50% of ER >1 week, or any reduction for >2 weeks, or any chronic GI condition that adversely impacts food assimilation or absorption;
② Inflammation: Acute disease/injury or chronic disease-related.
The PG-SGA includes seven boxes. Boxes 1–4 are designed to be completed by the patient. Boxes 5–7 are completed by the medical staff. The final score was obtained by adding each individual items. Among tumor patients in our study, a total score ≥4 was defined as malnutrition (14).
Subjective global assessment (SGA) is commonly used as a reliable and valid nutritional assessment tool to diagnose malnutrition (15, 16). It is a subjective assessment based on medical history and physical examination. The grading scale is divided into 3 levels, level A defines as well-nourished, level B defines as moderately malnourished, level C defines as severely malnourished. SGA was assessed among patients with non-tumor diseases.
The computational formula of PNI was: serum albumin (ALB) (g/L) + 5 * total lymphocyte count (109/L), which was firstly proposed by Onodera et al. in 1984 (17). It was a comprehensive nutritional inflammatory indicator which can predict the prognostic of various solid tumors and cardiovascular diseases (18, 19), even the COVID-19 (20). According to the standard formulated by Onodera et al. a PNI <45 was considered malnutrition.
2.4. Concordance between the GLIM, SGA, PG-SGA, and PNI
We calculated the sensitivity, specificity, accuracy, and Cohen kappa coefficient (Kappa) to assess the diagnostic agreement. The value of kappa coefficient >0.8 defines as very good level of agreement, between 0.61 and 0.8 defines as good level of agreement, between 0.41 and 0.6 defines as moderate diagnostic agreement, between 0.21 and 0.4 defines as fair diagnostic agreement, and <0.2 defines as poor diagnostic agreement.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard deviation. The difference between groups were measured by t-test. Categorical variables were expressed as numbers (percentages) and difference between groups were measured by chi-square test. All statistical analyses were performed using SPSS 25.0, A P < 0.05 was treated as statistically significant.
3. Results
3.1. Study characteristics
According to the first diagnosis at admission, there were 10 types of diseases in hepatobiliary and pancreatic surgery section in our study, with the highest number of cases accounting for 43.68% (221/506) of liver cancer. The baseline characteristics of the study population are presented in Table 1.
3.2. Prevalence of nutritional risk and malnutrition diagnosed by the four methods
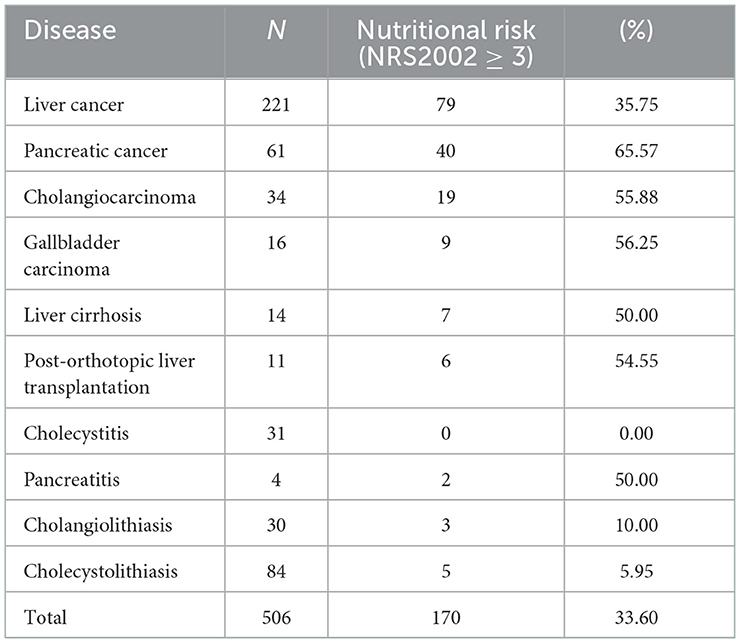
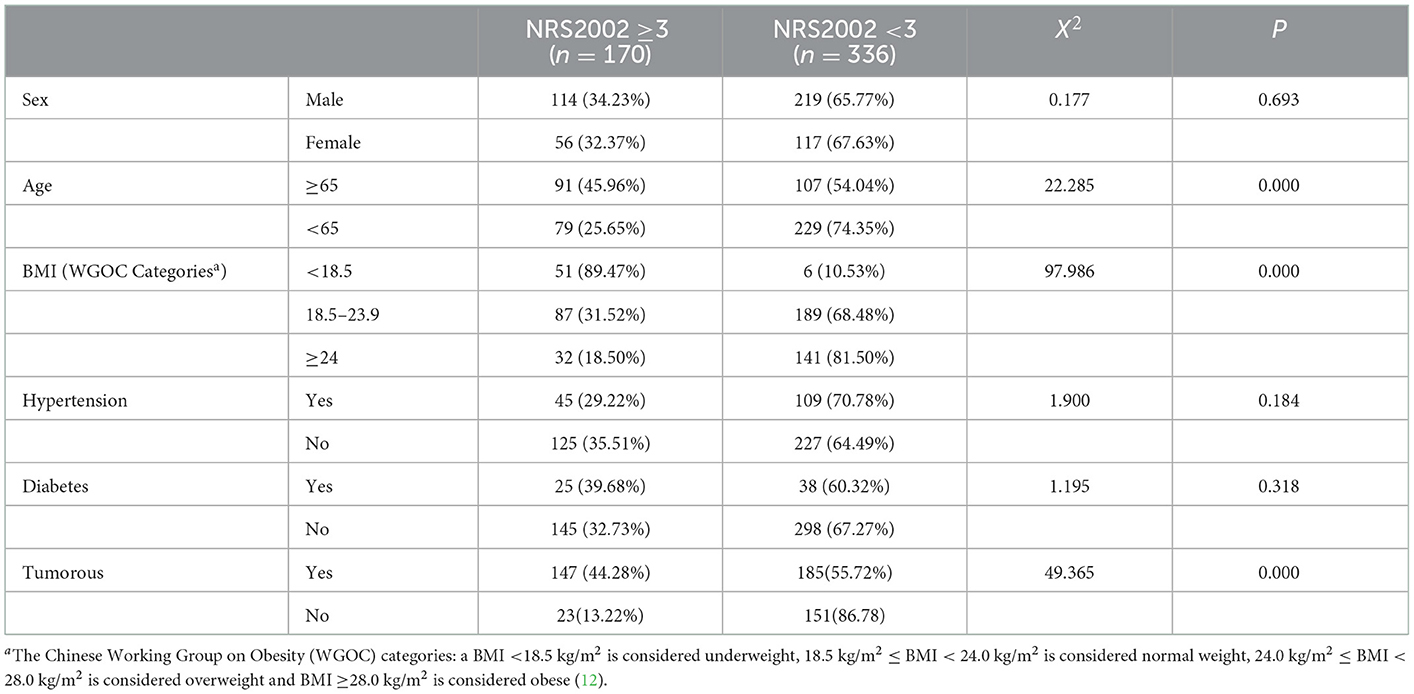
Among the 506 participants, 170 patients with nutritional risk were diagnosed by the NRS2002, the nutritional risk incidence rate of 33.60% at admission. Patients with pancreatic cancer had the highest proportion of nutritional risks (65.57%), and that of nutritional risks among the 31 patients with cholecystitis was 0.00% (Table 2).
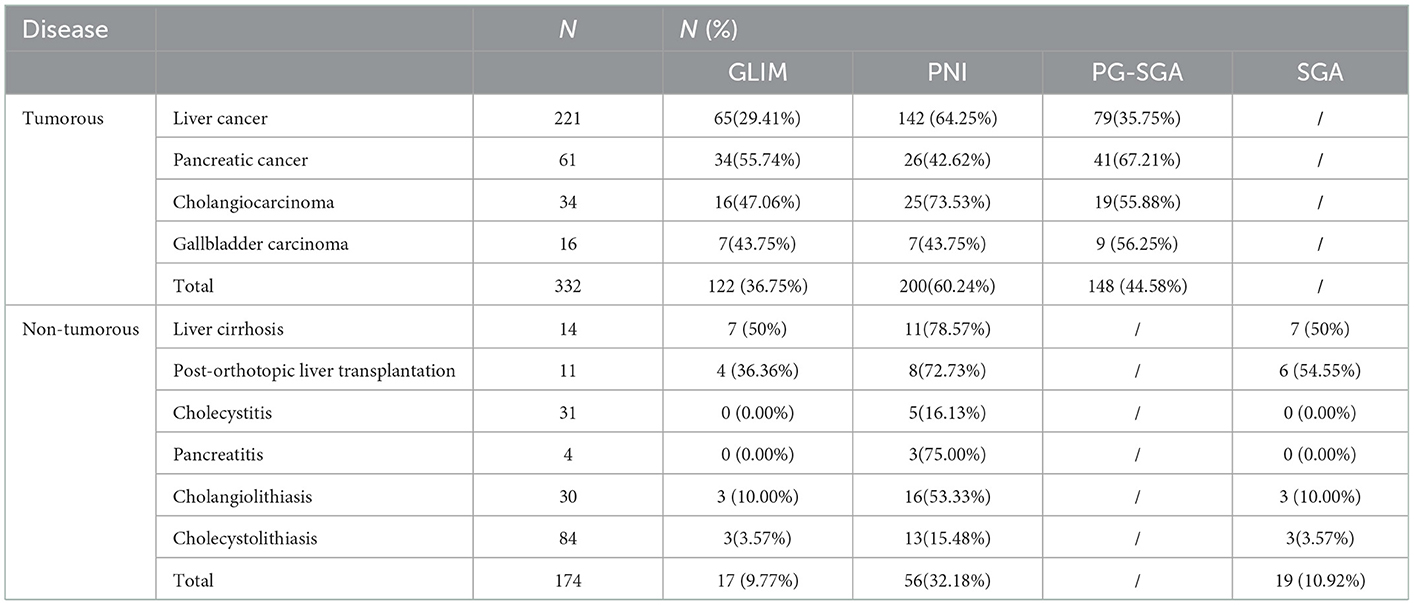
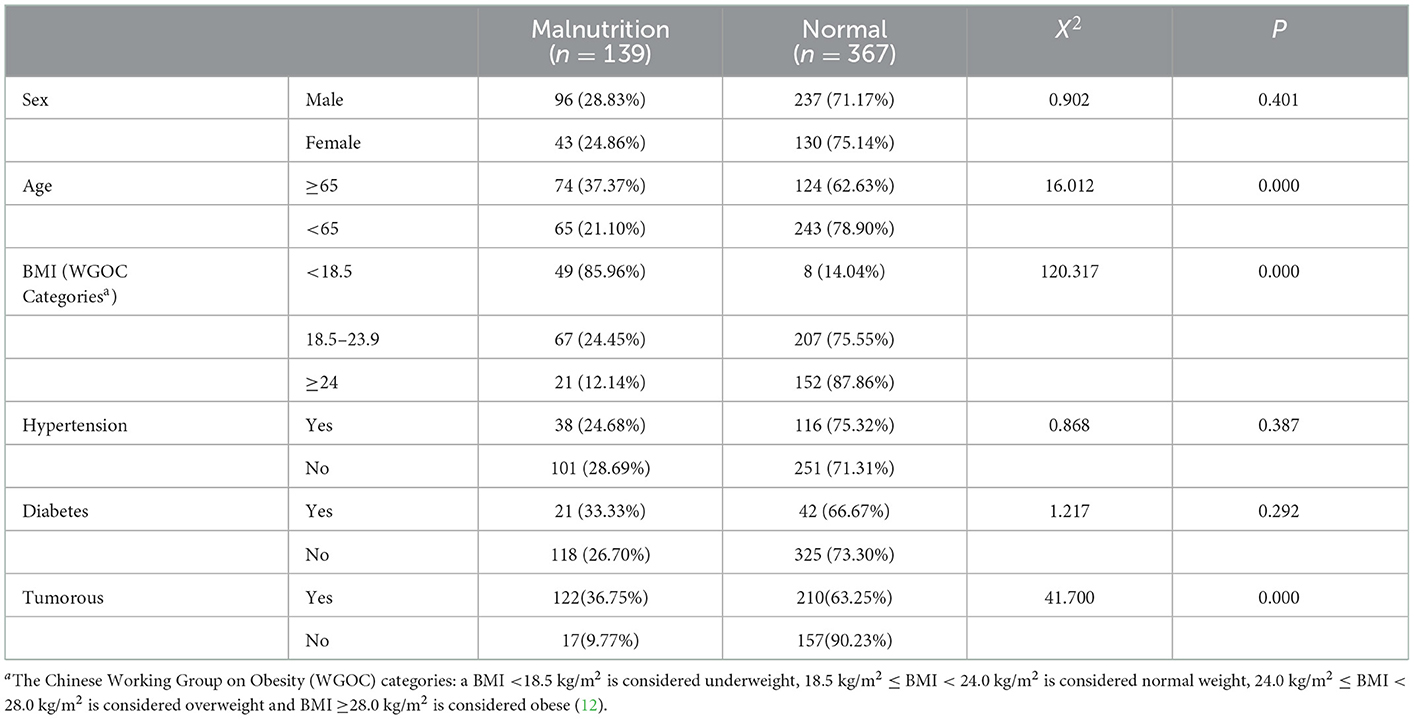
Table 3 shows the prevalence of malnutrition diagnosed by GLIM, PNI, PG-SGA, and SGA. Among 332 tumor patients, malnutrition was diagnosed in 122 (36.75%), 200 (60.24%), and 148(44.58%) patients using the GLIM, PNI, and PG-SGA, respectively. Among 174 non-tumor patients, it was diagnosed in 17 (9.77%), 56 (32.18%), and 19 (10.92%) using the GLIM, PNI, and SGA, respectively.
3.3. The observation of nutritional interventions in hepatobiliary-pancreatic surgery
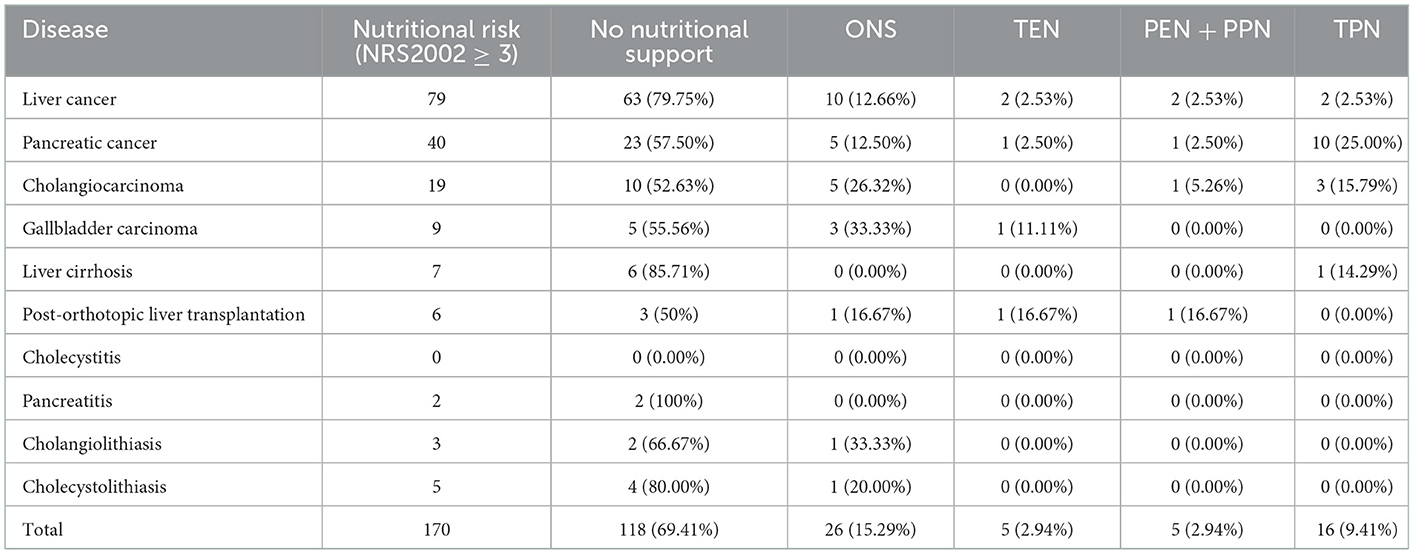
We also evaluated nutritional interventions in hepatobiliary-pancreatic surgery. Among 170 patients with nutritional risk, 26 received oral nutritional supplements (ONS), five received total enteral nutrition support (TEN), two received partial enteral and parenteral nutrition support (PEN + PPN), 16 patients received total parenteral nutrition support (TPN). Most (118/170, 69.41%) did not receive standard nutritional support (Table 4). Of the only 52 cases which received nutritional support, 46 were the patients with tumorous including 16 liver cancer patients, 17 pancreatic cancer patients, 9 cholangiocarcinoma patients and 4 gallbladder carcinoma patients. The other 6 patients without tumorous included 1 liver cirrhosis patient, 3 post-orthotopic liver transplantation patients, 1 cholangiolithiasis patient, and 1 cholecystolithiasis patient. There were 33% patients (17/52) who received the nutritional support during pre-surgery hospitalization and 67% patients (35/52) who received the nutritional support after surgery. We observed that clinical doctors were insufficiently aware of nutritional support and they did not follow the principles of nutritional interventions strictly. The bedside physicians mainly focused on the body weight and the serum albumin level instead of timely nutrition screening and assessment.
3.4. The relevance between the nutrition related indicators and the four methods
The data in Table 5 showed the relevance between the nutrition related indicators and the four methods. Of all the indicators, the level of serum albumin and hemoglobin in malnutrition group patients diagnosed by all the four tools were significantly lower than well-nourished group patients. The hs-CRP, an inflammation related indicator, was significantly higher in malnutrition group diagnosed by the GLIM, PG-SGA and PNI.
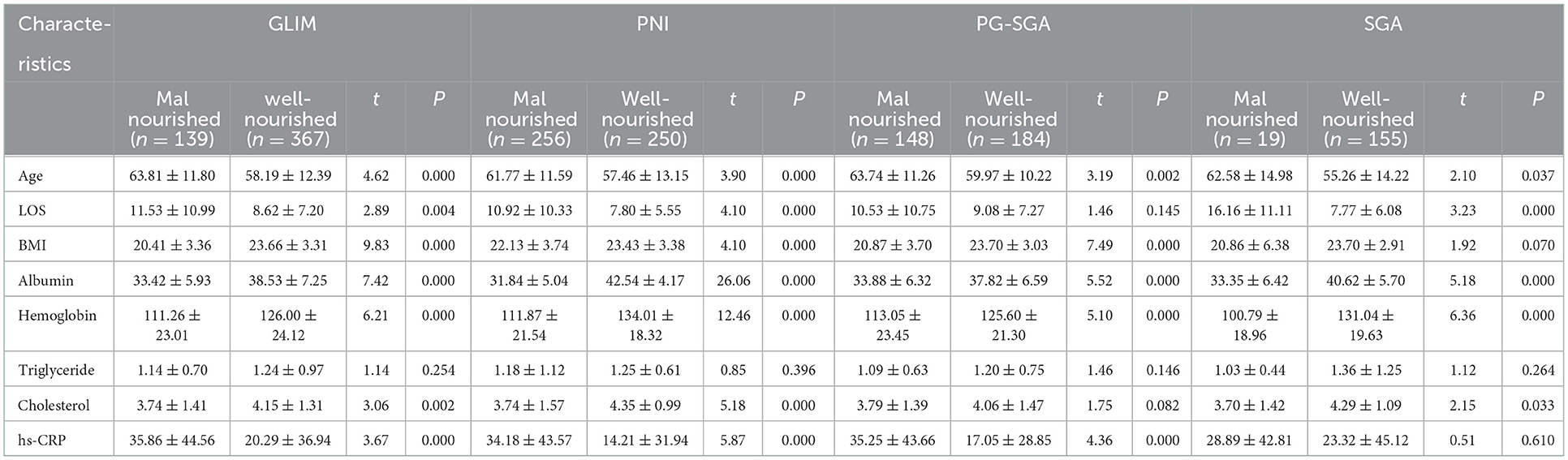
Table 5. Nutritional characteristics of the study population stratified by the GLIM, PNI, PG-SGA, and SGA.
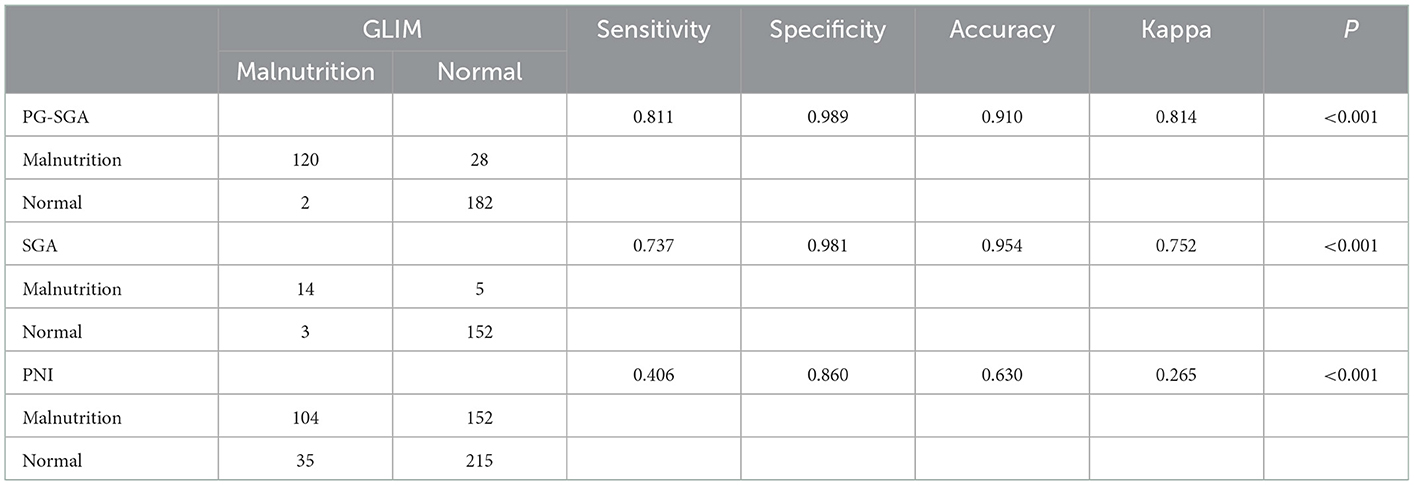
3.5. The diagnostic agreement among the GLIM, PG-SGA, SGA, and PNI
We also evaluated the diagnostic agreement among the GLIM, PG-SGA, SGA, and PNI. When compared with the other three methods, the GLIM and PG-SGA were highly consistent in diagnosing malnutrition, with a kappa value of 0.814 (Table 6).
3.6. The Univariate analysis of risk factors for nutritional risk and malnutrition
We analyzed the proportion of nutritional risk and malnutrition risk in different sociodemographic and disease treatment-related characteristics by the chi-square test (Tables 7, 8). The result showed that older age, lower BMI and tumorous were risk factors for nutritional risk and malnutrition.
4. Discussion
The prevalence of malnutrition among hospitalized patients undergoing hepatobiliary-pancreatic surgery is high (6), but the diagnosis has been controversial (21). In 2018, the American Society for Parenteral and Enteral Nutrition (ASPEN) and the Parenteral and Enteral Nutrition Society of Asia (PENSA) proposed the new GLIM criteria. These criteria provide new insights for the diagnosis of malnutrition. While, its varied prevalence in the same group may occur when diagnosed using different criteria or methods.
In this cross-sectional study, the GLIM criteria showed the lowest prevalence, and PNI defined the highest among the four methods. The prevalence was 36.75, 44.58, and 60.24%, as diagnosed by the GLIM, PG-SGA, and PNI, among 332 tumor patients. Among the 174 non-tumor patients, the prevalence was 9.77, 10.92, and 32.18% as determined by the GLIM, SGA, and PNI, respectively. A similar conclusion was reached by Miwa' study (22), the GLIM also showed the lowest prevalence (21%) among chronic liver disease patients when compared with SGA(35%), and RFH-GA (26%). As we know, the incidence of malnutrition varies relay on how estimator used the assessment tools, but regardless of the assessment tool, clinical outcomes in hospitalized patients with or without malnutrition are quite different.
The results of the Cohen's kappa statistic showed that the PG-SGA and GLIM had a relative high level of agreement (Kappa = 0.814, P < 0.001), the SGA and GLIM had a good level of agreement (Kappa = 0.752, P < 0.001), while the PNI and GLIM had fair level of agreement (Kappa = 0.265, P < 0.001). In the previous study, the PG-SGA and GLIM had a moderate level of agreement (kappa = 0.519, P < 0.001) in 360 esophageal cancer (EC) patients after esophagectomy (8). A possible explanation of the fair level of agreement between the GLIM and PNI may be that the PNI was a completely objective tool, including the level of serum albumin and the total number of peripheral leukocytes, while it did not consider BMI or reduced food intake. Meanwhile, the cutoff value of the PNI was different for different diseases. The widely used PNI cutoff value in gastrointestinal surgery is 45, and previous studies suggested that PNI ≤ 45 was significantly associated with poor prognosis in patients (23, 24). Moreover, Wang et al. (25) excluded patients with PNI ≤ 45, and the results showed that those with PNI≥ 55 had more difficulty obtaining a pathological complete response than patients with PNI >45 and <55. In Li Chen's study (26), the optimal cutoff value of PNI was 51. Therefore, the range of PNI cut-off values should be explored in clinical studies with large samples.
The possible reasons that the GLIM showed the lowest prevalence of malnutrition may be as follows: (1) in our study, most cases met the etiologic criteria because of the apparent reduced food intake, while did not meet the phenotypic one, mainly because that their weight loss did not meet the diagnostic criteria interval; (2) The GLIM criteria recommended the use of the dual-energy X-ray absorptiometry (DEXA), the computed tomography (CT) and the magnetic resonance imaging (MRI) to get the fat free mass index (FFMI), some other measurement of body composition such as bioelectrical impedance analysis (BIA) can be used as a reference. The normal values of the calculated muscle quality and the cut-point standards were still lack in China. So we excluded its measurement in our study, which may cause the lower prevalence of malnutrition diagnosed by the GLIM. In the present, some researchers have attempted to use the 15th percentile (p15) of the CC (A value <p15, male, 30 cm; female, 29 cm) as a positive for reduced muscle mass (8, 27). However, the optimal cut-off values for reduced muscle mass are debated. Other researchers discovered that using body composition measurements can further improve the GLIM criteria performance compared to using anthropometric measurements alone (28). There are racial and gender differences in normal values of the muscle mass and cut-off criteria, so the threshold of the related index of muscle mass reduction requires lager samples from different ethnic groups (e.g., Chinese, European) and higher quality research data.
Nutritional screening, nutritional assessment, nutritional intervention and monitoring were consisted of the standardized nutritional support therapy strategy. Among them, nutritional intervention plays a significant role (6). In our study, a low proportion of nutritional support was provided during hepatobiliary surgery. This may be because of the lack of recommendations for patients with nutritional risk by bedside physicians, and that of high-quality literature reports proving that nutritional support impacts the improvement in outcomes. So the medical staff should focus on the nutritional status and the nutritional intervention of hospitalized patients.
We found that the mean age of malnourished group was significantly higher than the well-nourished group according to all the four diagnostic tools. Moreover, other nutritional characteristics, including albumin and hemoglobin, were significantly lower in the malnourished group. These results are consistent with those of domestic and foreign studies (29, 30). Furthermore, the univariable analysis showed that older age, lower BMI and tumorous were risk factors for nutritional risk and malnutrition. The presence of benign or malignant disease due to the typical characteristics of malignancies may alter nutritional status (e.g., inflammation, central anorexia, metabolic disturbance worsen food intake, muscular status and energy reserve, which allow to define good nutritional status or malnutrition, are affected by tumorous).
Poulter (31) compared the GLIM, ESPEN, and ICD-10 criteria and identified 23.0%, 5.5%, and 12.6% of the cohort as malnourished, respectively. Xiaolin et al. compared the GLIM and PG-SGA criteria among cancer patients. They also found a lower prevalence of malnutrition diagnosed by the GLIM criteria (36.8 vs. 42.5%) (32). Regarding study strengths, this study is one of the first attempts to assess the diagnostic performance of four screening tools for malnutrition diagnosis, including the GLIM, PG-SGA, SGA, and PNI criteria, among hospitalized patients undergoing hepatobiliary-pancreatic surgery. Thus, our results show an important gap in this field.
However, our study has some limitations. First, it excluded reduction in muscle mass, which may have led to bias. Due to the short-staffed and the lack of equipment such as the dual-energy X-ray absorptiometry and bioelectrical impedance analysis in our study, we could not obtain the precise data about the muscle mass. Second, the GLIM calls for the use of the criteria in prospective and retrospective cohort studies to validate their application for clinical practice. However, our study design was retrospective and we used medical records, which inevitable had missing data, and the risk of selection bias was not negligible. Third, many studies observed the relationship between malnutrition and the adverse clinical outcomes of patients (33, 34). We mainly focused on short-term nutritional characteristics, and long-term clinical outcomes, such as complications, hospital readmission and mortality were not observed or reported. In the future, high-quality prospective clinical validation reports and prospective multi-center clinical application data analysis will be the basis for the improvement of the GLIM. The association between nutritional status and clinical outcome of patients assessed by various tools needs to be further explored.
Our study has practical implications. We assessed the prevalence of malnutrition, as defined by the GLIM, among hospitalized patients in hepatobiliary-pancreatic surgery and compared it with the other three criteria, which will help to reduce the bias of early clinical application of GLIM. Our findings provided some guidance and reference for the diagnosis of malnutrition, which may improve scientific and standardized nutrition management in hepatobiliary and pancreatic diseases. Meanwhile, we attempted to lay the foundation for further GLIM prospective clinical validation and health economics research. It is recommended to fill in the first page of the medical record with malnutrition information for disease codes E40-E46 and hopefully our findings can provide objective and valuable information for the diagnosis of malnutrition.
5. Conclusion
In conclusion, the incidence of nutritional risk and malnutrition was high among patients with hepatobiliary and pancreatic diseases, specifically in those with tumors. Among the four tools, the GLIM defines the lowest prevalence of malnutrition. The PG-SGA and GLIM had a relative high level of agreement. We should pay attention that there was only small part of patients received nutritional support in our current study. Further prospective and well-designed cohort studies were needed to validate the relevance of the GLIM criteria to explore the “gold standard” for clinical validity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Ningbo Medical Center Lihuili Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LZ contributed to conception, administration, writing, and editing of the manuscript. KJ, ZD, and RW contributed to nutritional risk screening and assessment of malnutrition. JF and LY contributed to data collection including disease diagnosis, age, sex, height, BMI, LOS, and laboratory data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2022KY1092).
Acknowledgments
We thank all members for their support in data collection. We acknowledge all the people who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhuming J, Xianna Z, Wang Y, Heshui W, Naishi L, Bin Z, et al. Three steps of global leadership (nutritional field) initiative on malnutrition (GLIM) and fill the diseases name (Chinese version ICD-10 listed) “nutritional risk” and “malnutrition” on front page of discharge medical record for letting big-data to find it to design the DRG contents for China. Chin J Clin Nutr. (2020) 28:257–67. doi: 10.3760/cma.j.cn115822-20200920-00216
2. Shen Y, Zhou Y, He T, Zhuang X. Effect of preoperative nutritional risk screening and enteral nutrition support in accelerated recovery after resection for esophageal cancer. Nutr Cancer. (2021) 73:596–601. doi: 10.1080/01635581.2020.1764981
3. Mudge L, Isenring E, Jamieson GG. Immunonutrition in patients undergoing esophageal cancer resection. Dis Esophagus. (2011) 24:160–5. doi: 10.1111/j.1442-2050.2010.01117.x
4. Mingliang W, Zhangyan K, Fangfang F, Huizhen W, Yongxiang L. Perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy: the first meta-analysis of randomized clinical trials. Dis Esophagus. (2020) 33:doz111. doi: 10.1093/dote/doz111
5. Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2019.02.033
6. Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. (2020) 39:3533–62. doi: 10.1016/j.clnu.2020.09.001
7. Chunman H, Xiaomeng L, Zhenshui L, Yingxia X, Xiang L, Wang Y, et al. Pevalence of nutritional risk and malnutrition and observation of nutritional intervention in hospitalized patients with stroke in a teaching hospital in Beijing. Chin J Clin Nutr. (2019) 27:331–7. doi: 10.3760/cma.j.issn.1674-635X.2019.06.001
8. Dávalos-Yerovi V, Marco E, Sánchez-Rodríguez D, Duran X, Meza-Valderrama D, Rodríguez DA, et al. Malnutrition according to GLIM criteria is associated with mortality and hospitalizations in rehabilitation patients with stable chronic obstructive pulmonary disease. Nutrients. (2021) 13:369. doi: 10.3390/nu13020369
9. Haines KL, Lao W, Nguyen BP, Krishnamoorthy V, Williams D, Gallagher S, et al. Evaluation of malnutrition via modified GLIM criteria for in patients undergoing emergent gastrointestinal surgery. Clin Nutr. (2021) 40:1367–75. doi: 10.1016/j.clnu.2020.08.026
10. Oguri M, Ishii H, Yasuda K, Sumi T, Takahashi H, Murohara T. Combined prognostic value of malnutrition using GLIM criteria and renal insufficiency in elderly heart failure. ESC Heart Fail. (2022) 9:704–11. doi: 10.1002/ehf2.13685
11. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. (2003) 22:415–21. doi: 10.1016/S0261-5614(03)00098-0
12. Chen CM Joint Joint Data Collection and Analysis Cooperation Group of China Obesity Working Group of China Office of the International Society of Life Science. A brief introduction to the recommendations on the classification of Chinese adult body mass index. Chin J Prev Med. (2001) 35:349–350. doi: 10.3760/j:issn:0253-9624.2001.05.019
13. Zhang X, Jiang Z, Wu H, Lu Q, Yang J, Yu K, et al. NRS 2002 nutritional risk screening and GLIM step 2 for diagnosis of malnutrition (without FFMI currently). Chin J Clin Nutr. (2020) 28:1–5. doi: 10.3760/cma.j.cn115822-20190923-00141
14. Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. (2002) 56:779–85. doi: 10.1038/sj.ejcn.1601412
15. Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? J Parenter Enter Nutr. (1987) 11:8–13. doi: 10.1177/014860718701100108
16. Mueller C Compher C Ellen DM American American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. ASPEN Clinical guidelines: nutrition screening, assessment, and intervention in adults. J Parenter Enter Nutr. (2011) 35:16–24. doi: 10.1177/0148607110389335
17. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
18. Sun J, Wang D, Mei Y, Jin H, Zhu K, Liu X, et al. Value of the prognostic nutritional index in advanced gastric cancer treated with preoperative chemotherapy. J Surg Res. (2017) 209:37–44. doi: 10.1016/j.jss.2016.09.050
19. Nakatani M, Migita K, Matsumoto S, Wakatsuki K, Ito M, Nakade H, et al. Prognostic significance of the prognostic nutritional index in esophageal cancer patients undergoing neoadjuvant chemotherapy. Dis Esophagus. (2017) 30:1–7. doi: 10.1093/dote/dox020
20. Mungmunpuntipantip R, Wiwanitkit V. Prognostic nutritional index, immune nutritional status and COVID-19. Int J Vitam Nutr Res. (2022) 92:3. doi: 10.1024/0300-9831/a000737
21. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Singer. Diagnostic criteria for malnutrition–an ESPEN consensus [statement]. Clin Nutr. (2015) 34:335–40. doi: 10.1016/j.clnu.2015.03.001
22. Miwa T, Hanai T, Nishimura K, Unome S, Maeda T, Ogiso Y, et al. Usefulness of the Global Leadership Initiative on Malnutrition criteria to predict sarcopenia and mortality in patients with chronic liver disease. Hepatol Res. (2022) 52:928–36. doi: 10.1111/hepr.13816
23. Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. (2013) 20:2647–54. doi: 10.1245/s10434-013-2926-5
24. Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. (2020) 271:693–700. doi: 10.1097/SLA.0000000000002985
25. Wang Y, Battseren B, Yin W, Lin Y, Zhou L, Yang F, et al. Predictive and prognostic value of prognostic nutritional index for locally advanced breast cancer. Gland Surg. (2019) 8:618–26. doi: 10.21037/gs.2019.10.08
26. Chen L, Bai P, Kong X, Huang S, Wang Z, Wang X, et al. Prognostic nutritional index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front Cell Dev Biol. (2021) 9:656741. doi: 10.3389/fcell.2021.656741
27. Yin L, Lin X, Li N, Zhang M, He X, Liu J, et al. Evaluation of the global leadership initiative on malnutrition criteria using different muscle mass indices for diagnosing malnutrition and predicting survival in lung cancer patients. J Parenter Enter Nutr. (2020) 45:607–17. doi: 10.1002/jpen.1873
28. Wang Y, Chen X, Wang Y, Liu Z, Fang Y, Peng Z, et al. Body composition measurement improved performance of GLIM criteria in diagnosing malnutrition compared to PG-SGA in ambulatory cancer patients: a prospective cross-sectional study. Nutrients. (2021) 13:2744. doi: 10.3390/nu13082744
29. Qian LU, Lichuan Z, Yujie W, Bing Z, Yan S. Analysis of nutritional risk and malnutrition in patients with head and neck cancer before radiotherapy. Med Res Educ. (2019) 36:54–62. doi: 10.3969/j.issn.1674-490X.2019.06.010
30. Righini CA, Timi N, Junet P, Bertolo A, Reyt E, Atallah I. Assessment of nutritional status at the time of diagnosis in patients treated for head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis. (2013) 130:8–14. doi: 10.1016/j.anorl.2012.10.001
31. Poulter S, Steer B, Baguley B, Edbrooke L, Kiss N. Comparison of the GLIM, ESPEN and ICD-10 criteria to diagnose malnutrition and predict 30-day outcomes: an observational study in an oncology population. Nutrients. (2021) 13:2602. doi: 10.3390/nu13082602
32. Xiaolin W, Dan H, Hongxia G, Xiaoming H. Application of global leadership initiative on malnutrition and patient generated subjective global assessment in diagnosing malnutrition among cancer patients. Electron J Metab. Nutr Cancer. (2021) 8:403–7.
33. Abizanda P, Sinclair A, Barcons N, Lizán L, Rodríguez-Mañas L. Costs of malnutrition in institutionalized and community-dwelling older adults: a systematic review. J Am Med Dir Assoc. (2016) 17:17–23. doi: 10.1016/j.jamda.2015.07.005
Keywords: GLIM, PG-SGA, SGA, PNI, malnutrition, hepatobiliary-pancreatic surgery, hospitalized patients
Citation: Zhou L, Fu J, Ding Z, Jin K, Wu R and Ye LX (2023) Comparison of GLIM, SGA, PG-SGA, and PNI in diagnosing malnutrition among hepatobiliary-pancreatic surgery patients. Front. Nutr. 10:1116243. doi: 10.3389/fnut.2023.1116243
Received: 05 December 2022; Accepted: 09 January 2023;
Published: 24 January 2023.
Edited by:
Akio Shimizu, The University of Nagano, JapanReviewed by:
Michał Czapla, Wroclaw Medical University, PolandAmanda Casirati, Fondazione IRCCS Policlinico San Matteo, Italy
Copyright © 2023 Zhou, Fu, Ding, Jin, Wu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Xiao Ye,  aDIwMTF5bHhAMTYzLmNvbQ==
aDIwMTF5bHhAMTYzLmNvbQ==
 Lingmei Zhou
Lingmei Zhou Jianying Fu2
Jianying Fu2