
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 11 August 2023
Sec. Nutrition, Psychology and Brain Health
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1115727
This article is part of the Research TopicNeurological, Psychological and Endocrine Markers of Eating Disorders and ObesityView all 6 articles
 Lieneke K. Janssen1,2*†
Lieneke K. Janssen1,2*† Iris Duif1†
Iris Duif1† Anne E. M. Speckens3
Anne E. M. Speckens3 Ilke van Loon1
Ilke van Loon1 Joost Wegman1
Joost Wegman1 Jeanne H. M. de Vries4
Jeanne H. M. de Vries4 Roshan Cools1,3
Roshan Cools1,3 Esther Aarts1*
Esther Aarts1*Introduction: Accumulating evidence suggests that increased neural responses during the anticipation of high-calorie food play an important role in the tendency to overeat. A promising method for counteracting enhanced food anticipation in overeating might be mindfulness-based interventions (MBIs). However, the neural mechanisms by which MBIs can affect food reward anticipation are unclear. In this randomized, actively controlled study, the primary objective was to investigate the effect of an 8-week mindful eating intervention on reward anticipation. We hypothesized that mindful eating would decrease striatal reward anticipation responses. Additionally, responses in the midbrain—from which the reward pathways originate—were explored.
Methods: Using functional magnetic resonance imaging (fMRI), we tested 58 healthy participants with a wide body mass index range (BMI: 19–35 kg/m2), motivated to change their eating behavior. During scanning they performed an incentive delay task, measuring neural reward anticipation responses to caloric and monetary cues before and after 8 weeks of mindful eating or educational cooking (active control).
Results: Compared with the educational cooking intervention, mindful eating affected neural reward anticipation responses, with reduced caloric relative to monetary reward responses. This effect was, however, not seen in the striatum, but only in the midbrain. The secondary objective was to assess temporary and long-lasting (1 year follow-up) intervention effects on self-reported eating behavior and anthropometric measures [BMI, waist circumference, waist-to-hip-ratio (WHR)]. We did not observe effects of the mindful eating intervention on eating behavior. Instead, the control intervention showed temporary beneficial effects on BMI, waist circumference, and diet quality, but not on WHR or self-reported eating behavior, as well as long-lasting increases in knowledge about healthy eating.
Discussion: These results suggest that an 8-week mindful eating intervention may have decreased the relative salience of food cues by affecting midbrain but not striatal reward responses, without necessarily affecting regular eating behavior. However, these exploratory results should be verified in confirmatory research.
The primary and secondary objectives of the study were registered in the Dutch Trial Register (NTR): NL4923 (NTR5025).
Reward-related disorders such as addiction, binge-eating disorder and obesity, are characterized by altered responses to reward cues related to the target of abuse (1–3). Mesolimbic regions in the brain, including the striatum and the midbrain—with its dopaminergic projections to the striatum (4, 5)—respond to increases in appetitive motivation induced by reward cues (6). Responses of these subcortical reward regions have been related to eating behavior. For example, greater ventral striatal responses to reward cues have been associated with subsequent food intake (7) and future weight gain in healthy- and overweight individuals (7–9) [for a review, (see 3)]. Reductions in striatal food-cue responses after a weight loss intervention were even predictive of the later outcome of the weight loss intervention (10). Moreover, increases in BMI—from lean to obese—were associated with increased midbrain responses to high-calorie food cues in women (11) and to anticipating rewards during risky choices in adolescents (12). Interventions targeted at diminishing subcortical responses to food reward cues may therefore be promising for addressing people’s tendency to overeat.
Mindfulness-based interventions are aimed at cultivating attention to present-moment experience, without judgment (13). Protocolized mindfulness interventions, such as mindfulness-based stress reduction (MBSR) have shown to be effective in reducing subcortical responses to emotional stimuli in anxiety (14) as well as in healthy individuals (15). Furthermore, mindfulness meditation training can improve executive control processes such as conflict monitoring and response inhibition (16), as well as alter functional connectivity of brain networks involved in attention, cognitive processing, awareness, sensory integration, and reward processing (17). Importantly, mindfulness-based interventions aimed at changing eating behavior have been shown to reduce binge eating in clinical populations (18, 19), to reduce abdominal fat (20, 21), to increase self-reported mindful eating (22) and to reduce reward-driven eating in obese individuals (23). However, only two of these trials were actively controlled (18, 22, 23). It is therefore unclear whether these beneficial effects can be attributed to mindfulness per se. In fact, Kristeller et al. (18) found that both mindfulness-based eating awareness training (MB-EAT) and a psycho-educational/cognitive-behavioral (i.e., active control) intervention decreased binge-eating symptoms relative to a waitlist control group to a similar degree. Given the different nature of these interventions, it is possible that reduced symptomatology was mediated by dissociable brain mechanisms, as has previously been observed in an actively controlled clinical trial on social anxiety (14). In this fMRI study, reduced social anxiety symptoms were observed for both the mindfulness and the active control intervention, but the interventions had opposite effects on neural responses in the posterior cingulate and the dorsomedial prefrontal cortex during self-referential processing Studies investigating the neurocognitive mechanism underlying mindful eating are required to assess whether a mindful eating intervention can diminish neural responses to food reward cues.
Kirk et al. (24) performed three studies on neurocognitive reward mechanisms underlying mindfulness. They found that meditators, relative to controls, showed lower neural responses in striatum during reward anticipation (24), as well as diminished BOLD responses in putamen during positive and negative prediction errors (25). In addition, they found that mindfulness training modulated value signals in vmPFC to primary reward (juice) delivery (26). However, these studies do not yet address the question how mindfulness training affects neural responses for food reward anticipation. Specifically, the first two studies were performed in experienced meditators vs. controls instead of in a randomized controlled design, and the third study investigated reward responses at the moment of reward delivery, instead of anticipation. Reward anticipation is particularly interesting to investigate in light of eating behavior, as increases in reward anticipation have been shown to have predictive value for weight gain or overeating-related behavior (1–3, 7–9, 12).
Here, we present an actively controlled randomized study investigating the effects of mindfulness on reward anticipation in the brain of healthy adults who wanted to change their eating behavior for various reasons. We studied the effects of an 8-week mindful eating intervention aimed at changing undesired eating habits vs. a carefully matched educational cooking intervention (active control). To assess reward anticipation, we used an incentive delay task (27) during fMRI, which has been shown to produce reliable mesolimbic responses to reward cues (5). We hypothesized that the mindful eating intervention would reduce reward cue responses in the striatum (primary objective), and also explored these effects in the dopaminergic midbrain as part of the mesolimbic reward circuit. We included both monetary and caloric rewards in the task, which enabled us to assess whether the effect on anticipatory reward responses is specific to the caloric domain, or generalizes to the monetary domain. As a secondary objective, we assessed the effects of mindful eating on anthropometric measures (BMI, waist-to-hip ratio (WHR), and waist circumference) and on self-reported questionnaires related to eating behavior and knowledge of healthy eating.
The participants that were included in the current sample (n = 58; see Table 1 for group characteristics) largely overlap with those from the sample reported previously (28). Only right-handed adults in the age between 18 and 55 years old, with a BMI between 19 and 35 kg/m2, who had an adequate demand of the Dutch language (i.e., who could understand the task instructions, questionnaires, and intervention materials) and who, importantly, were highly motivated to change their eating behavior (not to lose weight per se) were included in the study. The exclusion criteria were as follows: MRI-incompatibility; diabetes mellitus; self-reported (history of) hepatic, cardiac, respiratory, renal, cerebro-vascular, endocrine, metabolic, pulmonary, or cardiovascular diseases or of taste or smell impairments; food allergies relevant to the study; uncontrolled hypertension (in rest: diastolic >90 mmHg; systolic >160 mmHg); self-reported (history of) eating, neurological, or psychiatric disorders; a score > 11 on the Hospital Anxiety and Depression Scale (29) (HADS); extremely high restrained eating scores [≥4.00 for women, ≥3.60 for men, based on a normative Dutch sample (30)] on the Dutch Eating Behaviour Questionnaire (31) (DEBQ); self-reported drug or alcohol addiction in the past 6 months; self-reported use of neuroleptic or other psychotropic medication; deafness, blindness, or sensorimotor handicaps; current strict dieting, under treatment with a dietitian, or a change in body weight of more than 5 kg in the past 2 months (self-report). Crucially, subjects with previous MBSR (Mindfulness-Based Stress Reduction) or MBCT (Mindfulness-Based Cognitive Therapy) experience were excluded from the study (self-report).
The study was conducted between October 2013 and February 2016. Participants were recruited from Nijmegen and surroundings through advertisement. From 523 initial registrations (see Figure 1 for a flow diagram), 118 participants were assessed for their eligibility to take part in the study during a separate screening session (Section 2.2). Ninety-two participants were included in the study and randomly allocated to the mindful eating (n = 45) or educational cooking (n = 47) intervention. Of the included participants, 83 completed a pre-intervention test session and started the allocated intervention (mindful eating: n = 43; educational cooking: n = 40). Fifteen participants did not return for the post-intervention test session, resulting in a total of 68 complete datasets (mindful eating: n = 36; educational cooking: n = 32). An additional 10 participants were excluded from the analyses following testing because of technical problems (n = 6), excessive movement during fMRI scanning (n = 1), an incidental finding after the post-test session (n = 1), or because of poor task performance (n = 2) (for details see Section 2.6.2). Despite a numerical difference in dropouts between groups, the number of people excluded from analysis was not significantly different [mindful eating: 28.8%, educational cooking: 44.7%, χ2(1, N = 92) = 2.461, p = 0.117]. The relatively high dropout rate is addressed in Section 4. The reasons for discontinuation of the intervention or study were queried by the experimenters and categorized as “dissatisfied,” “personal circumstances” or “unknown” (see Figure 1). Note that the number of included participants was chosen based on typical neuroimaging studies and anticipating dropout due to the interventions. No power calculation was conducted at the time because of a lack of similar studies to extract relevant information from.
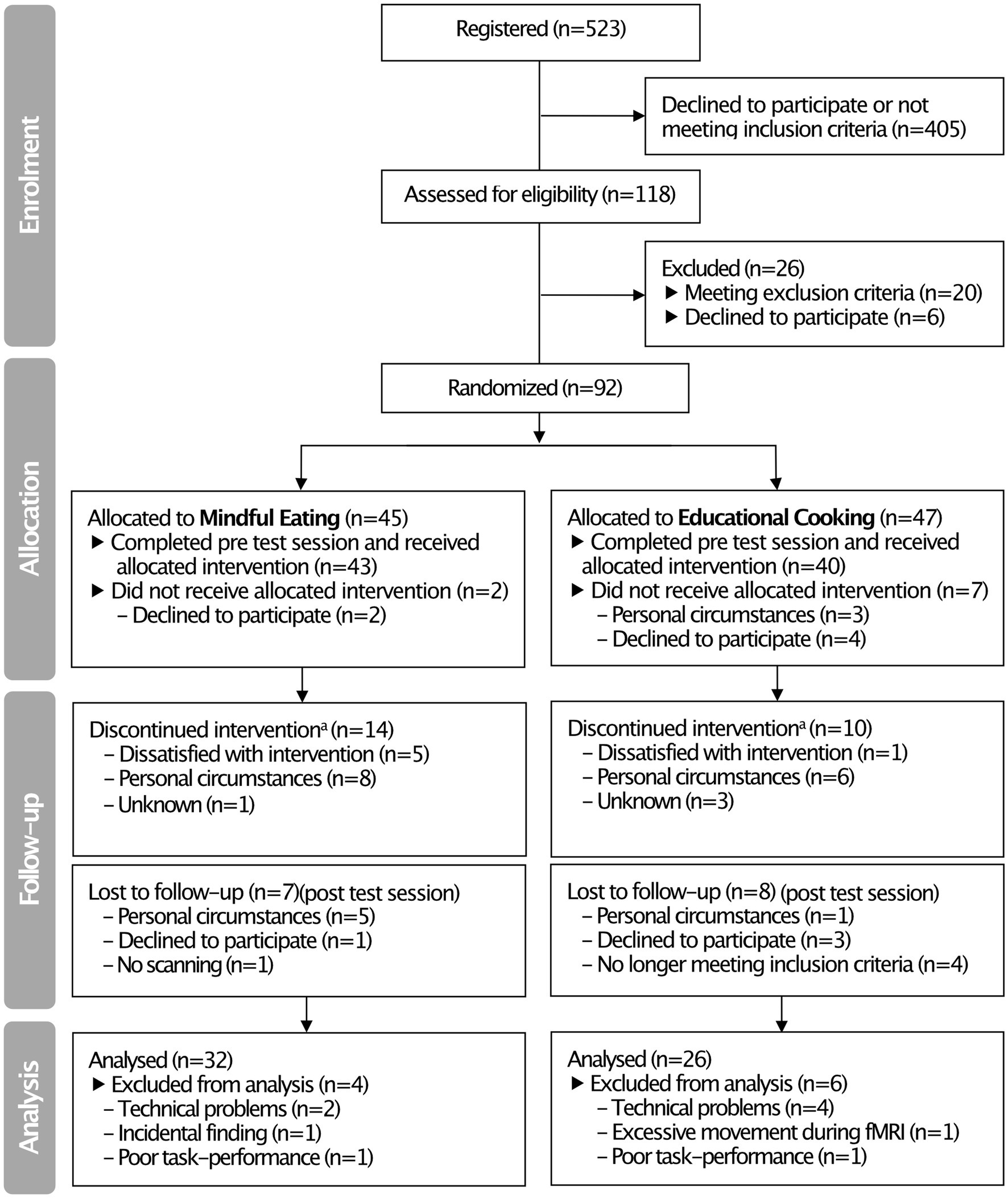
Figure 1. CONSORT flow diagram summarizing the inclusion and exclusion of participants in the pre-intervention (“Allocation”) and immediate post-intervention (“Follow-up”) test sessions. aAttended <4 sessions of the intervention program. Note that these participants were invited back to the laboratory for the post-intervention test session.
All participants who were assessed for their eligibility to take part in the study gave written informed consent and were reimbursed for participation according to the local institutional guidelines (i.e., 8 Euros per hour for behavioral testing, 10 Euros per hour for scanning). The study protocol was approved by the local ethics committee (CMO region Arnhem-Nijmegen, the Netherlands, 2013-188) and was in accordance with the Declaration of Helsinki. The trial was registered at the Dutch trial register [NL4923 (NTR5025)].
In the separate screening interview, all participants were assessed for inclusion and exclusion criteria (listed under Section 2.1) and the matching criteria: age, gender, BMI, and experience with meditation and yoga. Anthropometric measures (weight, height, waist and hip circumference) and physical measures (blood pressure) were collected and a number of self-report questionnaires used for exclusion (listed with the exclusion criteria below) were administered.
After inclusion, participants came to the MRI laboratory twice—before and after the intervention—and a third time to the behavioral lab 1 year later. Participants were instructed to abstain from eating foods and drinking anything else than water 4 h prior to the start of the test sessions. Participants were also instructed to abstain from drinking alcohol 24 h before the test session. As secondary outcome measures, anthropometric measurements were taken again (weight, height, waist and hip circumference) before scanning and participants completed self-reported measures of diet quality and eating behavior: the Dutch Healthy Diet - Food Frequency Questionnaire (32) (DHD-FFQ) on food intake; a shortened version of the Food Behavior Questionnaire (FBQ) with subscales on “knowledge of healthy eating” and “temptation”; and the Dutch Eating Behaviour Questionnaire (31) (DEBQ) with subscales on restraint, emotional, and external eating behaviors. To further characterize the sample, to account for between-group differences at baseline that could occur by chance, and to further explore the effectiveness of the intervention programs, the following self-report questionnaires and scales were administered: the Five Facet Mindfulness Questionnaire – Short Form (33) (FFMQ-SF) to assess degree of mindfulness; a Treatment Credibility Questionnaire (TCQ) to assess how much participants believed the intervention would work for them; the Positive And Negative Affect Scale (34) (PANAS) to assess positive and negative affect before scanning; the Behavioral Inhibition System/Behavioral Approach System questionnaire (35) (BIS-BAS) to assess punishment and reward sensitivity; the Hospital Anxiety and Depression Scale (29) (HADS) to assess levels of anxiety and depression; the Fagerstrom Test for Nicotine Dependence (36) (FTND) to assess smoking and nicotine dependence; the Barratt Impulsiveness Scale-11 (37) (BIS-11) to assess impulsivity; the Kirby monetary choice questionnaire (38) to assess delayed reward discounting; and the neuropsychological digit span test (39) to assess working memory capacity. Note that the pre-training TCQ was filled out at the first training session, not on the pre-training test session, as participants were unaware of the contents of their training at that time. After completing the questionnaires, participants underwent a 1-h MR scanning session in which they performed an incentive delay task, reported here. Participants also performed a food Stroop task inside the scanner, followed by a reversal learning and outcome devaluation task outside the scanner. Data from these three experimental paradigms are reported elsewhere (28, 40, 41).
One year after the intervention, participants were re-invited to the laboratory to reassess anthropometric measurements, i.e., weight, waist and hip circumference, and the self-report questionnaires as administered on pre- and post-test sessions. Reward anticipation was not re-assessed at one-year follow-up.
Participants were randomly assigned to one of two intervention programs: mindful eating (ME) or educational cooking (EC; active control). Participants were assigned by a computer through minimization (42), which guarantees that groups are balanced in terms of certain a priori determined minimization factors: age (categories: 18–25y, 26–35y, 36–45y, 46–55y), gender (categories: male, female), BMI (categories: 19–24.9 kg/m2 normal weight, 25–29.9 kg/m2 overweight, 30–35 kg/m2 moderately obese) and experience with meditation and yoga (categories: never, 0–2 years, 2–5 years, 5–10 years, >10 years).
The intervention programs were matched in terms of time, effort, and group contact, but differed significantly in terms of content. Both programs consisted of 8 weekly, 2.5 h group sessions plus 1 day (6 h) dedicated to the intervention goals. Participants were asked to spend 45 min per day on homework assignments and to record the amount of time spent on homework forms. In the information letters, the intervention programs were described as “eating with attention” (ME) and “eating with knowledge” (EC) to prevent a selection bias of participants interested in mindfulness. Only after the first test session, participants were informed about the intervention to which they were randomized, to ensure that baseline measurements were not influenced by intervention expectations. Because group size was set to 10–15 participants per round, included participants were divided across three rounds for each intervention (3xME, 3xEC).
The aim of the ME intervention was to increase experiential awareness of food and eating. The ME program was based on the original MBSR program developed by Kabat-Zinn et al. (43). Participants performed formal mindfulness practices (i.e., body scan, sitting meditation, walking meditation and mindful movement), aimed at increasing general mindfulness skills, which were similar to the original program. In addition, participants performed informal mindfulness practices based on the Mindful Eating, Conscious Living program (44), which were mainly directed to mindful eating and not part of the original MBSR program. Sessions focused on the automatic pilot, perception of hunger and satiation, creating awareness of boundaries in eating behavior, stress-related eating, coping with stress, coping with (negative) thoughts, self-compassion, and how to incorporate mindfulness in daily life. Toward the end of the program, participants had a “silent day.” During this day, the whole group performed formal mindfulness exercises and ate a meal together in complete silence. Homework consisted of a formal mindfulness practice and an informal mindfulness practice directed at one moment (e.g., a meal) a day. The ME intervention was developed and delivered by qualified mindfulness teachers from the Radboud University Medical Centre for Mindfulness.
The aim of the EC intervention was to increase informational awareness of healthy food and eating. The EC program was based on the Dutch healthy food-based dietary guidelines.1 To establish similar (active) group activities as in the ME, participants were enrolled in cooking workshops during the group meetings of the EC. Sessions focused on healthy eating, healthy cooking of vegetables and fruit, use of different types of fat and salt for cooking, reading of nutrition labels on food products, healthy snacking, guidelines for making healthy choices when eating in restaurants, and how to incorporate healthy eating and cooking in daily life. Toward the end of the program, participants had a “balance day,” during which the participants adhered to all nutritional health guidelines for every snack and meal. Homework assignments entailed practicing cooking techniques, or grocery shopping with informational awareness, and counting the amount of calorie intake for one meal a day. The EC intervention was developed and delivered by a qualified dietitian from Wageningen University and a professional chef of the Nutrition and Dietetics faculty of the University of Applied Sciences of Arnhem-Nijmegen guided the cooking sessions.
We adapted the original incentive delay task (27) to assess neural and behavioral responses to reward anticipation following monetary as well as caloric cues (Figure 2). In short, on each trial, participants were cued about which of four rewards they could win (monetary: 1 or 50 cents; caloric: a sip of water or of a high-calorie drink of their choice [orange juice, whole chocolate milk or regular cola)], typically referred to as “reward anticipation.” As soon as a white star (target) appeared on the screen, participants had to press a button with their right index finger as fast as possible. If participants responded within an individually determined time-window, they won and the reward was added to their cumulative gain as shown on the screen. Note that rewards were not actually received during scanning. Nonetheless this phase of the task is typically referred to as “reward receipt.” On average, 59.6% (SD: 10.0) of the trials were hit trials. Participants performed 4 blocks of 25 trials (a total of a 100 trials) resulting in a task duration of 20–25 min. A block contained either high/low monetary or high/low-calorie trials. Each trial type was repeated approximately 25 times (M: 24.4, SD: 2.78). Block-presentation was pseudo-randomly distributed and counterbalanced across participants (randomization scheme: ABBA or BAAB). All stimuli were presented with a digital projector at the back end of the MRI scanner bore, which was visible via a mirror mounted on the head coil around the participant’s head. Responses were made using an MRI-compatible button box.
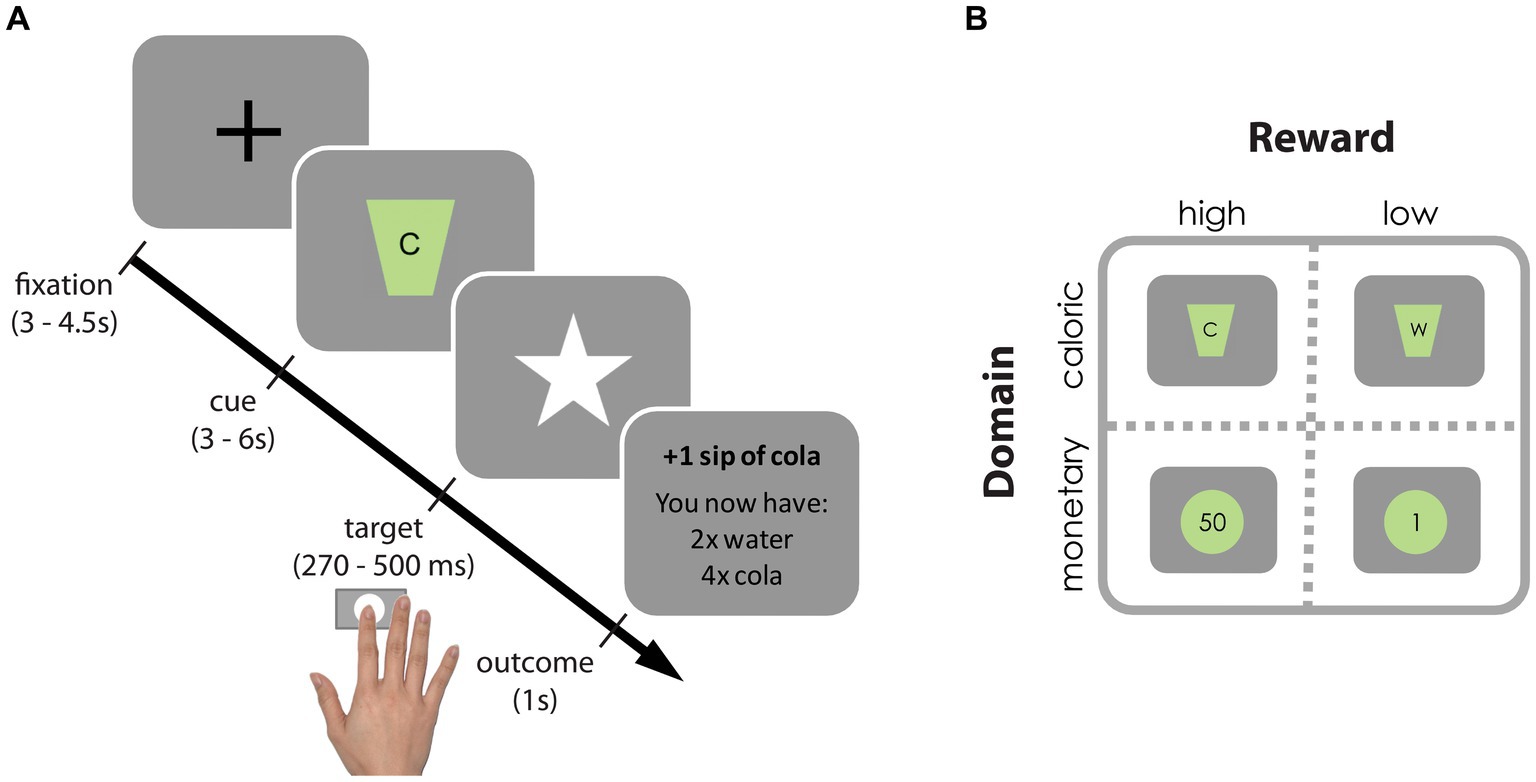
Figure 2. Incentive delay task. (A) Each trial started with a fixation cross, followed by a cue signaling which reward could be earned on that trial. Subsequently, a white star (i.e., target) appeared for a brief period and participants were instructed to press a button as fast as possible upon detection using their right index finger. If participants pressed before the response deadline (hit trial), the target remained on the screen, informing participants of the successful registration of their key press. Subsequently, a brief feedback image informing the participants about the total gain was presented. If participants pressed too late or failed to press at all (too late or miss trial, respectively), they were presented with the text message “you win nothing” plus the total gain so far. To ensure participants won similar amounts of each reward (in ±2/3 of the trials), target presentation times were determined individually and adaptively: following hit trials the response deadline for that reward cue was decreased with 10 ms, following too late or miss trials it increased with 10 ms. (B) Reward cues for high- and low-calorie cues [(C) Participant’s choice from cola, orange juice or chocolate milk vs. water (W)] and high and low monetary cues (50 cents vs. 1 cent).
After scanning, participants received and drank their total caloric gain. Their total monetary gain was added to their financial reimbursement. Participants received instructions for the incentive delay task before going into the scanner and were aware they would receive their gain following scanning. Before scanning, participants rated how much they wanted and liked each reward on a Visual Analogue Scale (VAS, 100 mm). To expose participants to the reward outcomes, they were provided with the actual coins, and one sip (5 mL) of water and one of the chosen drink while rating the VAS.
Participants performed the incentive delay task while whole-brain functional images were acquired on a Siemens 3 T Skyra MRI scanner (Siemens Medical system, Erlangen, Germany) using a 32-channel head coil to measure blood oxygen level dependent (BOLD) contrast. A multi-echo echo-planar imaging (EPI) sequence was used to acquire 34 axial slices per functional volume in ascending direction (voxel size 3.5 × 3.5 × 3 mm; repetition time (TR) 2,070 ms; TE 9 ms, 19.25 ms, 29.5 ms, and 39.75 ms; flip angle 90°; field of view 224 mm). This is a method that uses accelerated parallel imaging to reduce image artifacts (in plane acceleration 3) and acquire images at multiple TEs following a single excitation (45). Before the acquisition of functional images, a high-resolution anatomical scan was acquired (T1-weighted MPRAGE, voxel size 1 × 1 × 1 mm, TR 2,300 ms, TE 3.03 ms, 192 sagittal slices, flip angle 8°, field of view 256 mm).
Between-group comparisons of pre-intervention measures were analyzed using independent-samples t-tests, Fisher’s Exact Tests, or Mann–Whitney U tests using two-tailed tests in SPSS (version 23.0, Chicago, IL). The significance level was set at an alpha of p = 0.05.
Mean latencies of the manual responses in the incentive delay task were analyzed using repeated-measures ANOVA with within-participant factors Reward (high, low), Domain (caloric, monetary), Time (pre, post session), and the between-participant factor Intervention (ME, EC). Specific effects were tested with subsequent F-tests. All analyses were performed using two-tailed tests in SPSS (version 23.0, Chicago, IL). The significance level was set at an alpha of p = 0.05, partial eta squared (ηp2) was reported to indicate effect sizes in the repeated measures ANOVAs.
Data were pre-processed and analyzed using FSL version 5.0.112 and SPM8.3
To decide on the optimal approach for pre-processing for analysis of the mesoblimbic regions of interest, we performed three approaches, which differed in how motion-related noise was accounted for. The final approach was determined based on the strength of the main task effect (i.e., the t-value of the high minus low reward anticipation contrast) independent of training, i.e., across all participants and sessions. For the first approach, we accounted for motion-related noise by adding 12 rigid-body transformation parameters (three translations and rotations, and their linear derivatives) obtained during realignment to the first level model. As a second approach, we used non-aggressive ICA-AROMA (46) to reduce motion-induced signal variations in the fMRI data. Because ICA-AROMA takes out noise components, the 12 rigid-body transformation parameters obtained during realignment were not included in the model. For our third approach, we again used ICA-AROMA. However, rather than reducing motion-related noise in the fMRI data directly, we added the time courses of the independent components that accounted for less than 5% of task-related variance to the first level model. To achieve this, we used the components identified as motion by ICA-AROMA in a multiple regression analysis with the task regressors as predictors and the motion-related time courses as dependent variables. From this analysis, the adjusted R2 was obtained to identify how much of the total variance in a time course was captured by the task’s design. If the adjusted R2 of a component was higher than 5%, it was not included in the first level model as noise regressor. The 12 rigid-body transformation parameters obtained during realignment were also included in the model. The third approach showed the strongest main task effect (brain responses to high minus low reward cues) and was therefore used as our final pre-processing approach. In the next Section 2.6.3.2, we describe this approach in more detail.
The volumes for each echo time were realigned to correct for motion artifacts (estimation of the realignment parameters is done for the first echo and then copied to the other echoes). The four echo images were combined into a single MR volume based on 31 volumes acquired before the actual experiment started using an optimized echo weighting method (45). Combined functional images were slice-time corrected by realigning the time-series for each voxel temporally to acquisition of the middle slice. The images were subsequently spatially smoothed using an isotropic 6 mm full-width at half-maximum Gaussian kernel. Non-aggressive ICA-AROMA (46) was used to identify motion-induced signal variations in the fMRI data. Participant-specific structural and functional data were then coregistered to a standard structural or functional stereotactic space, respectively, (Montreal Neurological Institute (MNI) template). After segmentation of the structural images using a unified segmentation approach, structural images were spatially coregistered to the mean of the functional images. The resulting transformation matrix of the segmentation step was then used to normalize the anatomical and functional images into Montreal Neurological Institute space. The functional images were resampled at voxel size 2 × 2 × 2 mm.
Statistical analyses of fMRI data at the individual participant (first) level were performed using an event-related approach and included 13 regressors of interest: four regressors for cue presentation (high- and low-calorie cues, high and low monetary cues), one regressor for target presentation, four outcome regressors for hits (high- and low-calorie hits, high and low monetary hits), and four outcome regressors for trials on which participants responded too late (high- and low- calorie too late, high and low monetary too late). If participants failed to respond on a trial (i.e., a miss), the trial was excluded from analyses. Onsets of the regressors were modeled as a stick function (duration = 0 s) convolved with a canonical hemodynamic response function (47). Furthermore, we only added time courses of the independent noise components that accounted for less than 5% of task-related variance to the first level model as regressors of non-interest. Note that the number of these regressors varied per subject and session. In addition, 12 rigid-body parameters, a constant term, and two regressors that reflected signal variation in white matter and cerebrospinal fluid regions were included as regressors of non-interest. High pass filtering (128 s) was applied to the time series of the functional images to remove low-frequency drifts and correction for serial correlations was done using an autoregressive AR(1) model.
We ran two general linear models (GLMs) at the second level: one for reward anticipation with high minus low reward cue contrast images, and one for reward receipt with hit minus too late contrast images. Analysis of variance (ANOVA) was performed in a full-factorial design, with between-subject factor Intervention and within-subject factors Time and Domain, resulting in 8 cells. Effects were considered statistically significant when reaching a threshold of p < 0.05, family wise error (FWE) corrected for multiple comparisons at the peak level, whole brain or in the a priori defined regions of interest (see below). We report whole-brain and small volume corrected (pFWE < 0.05) effects, and show the statistical maps at p < 0.001 and p < 0.005 uncorrected thresholds in Figure 3 for exploratory purposes.
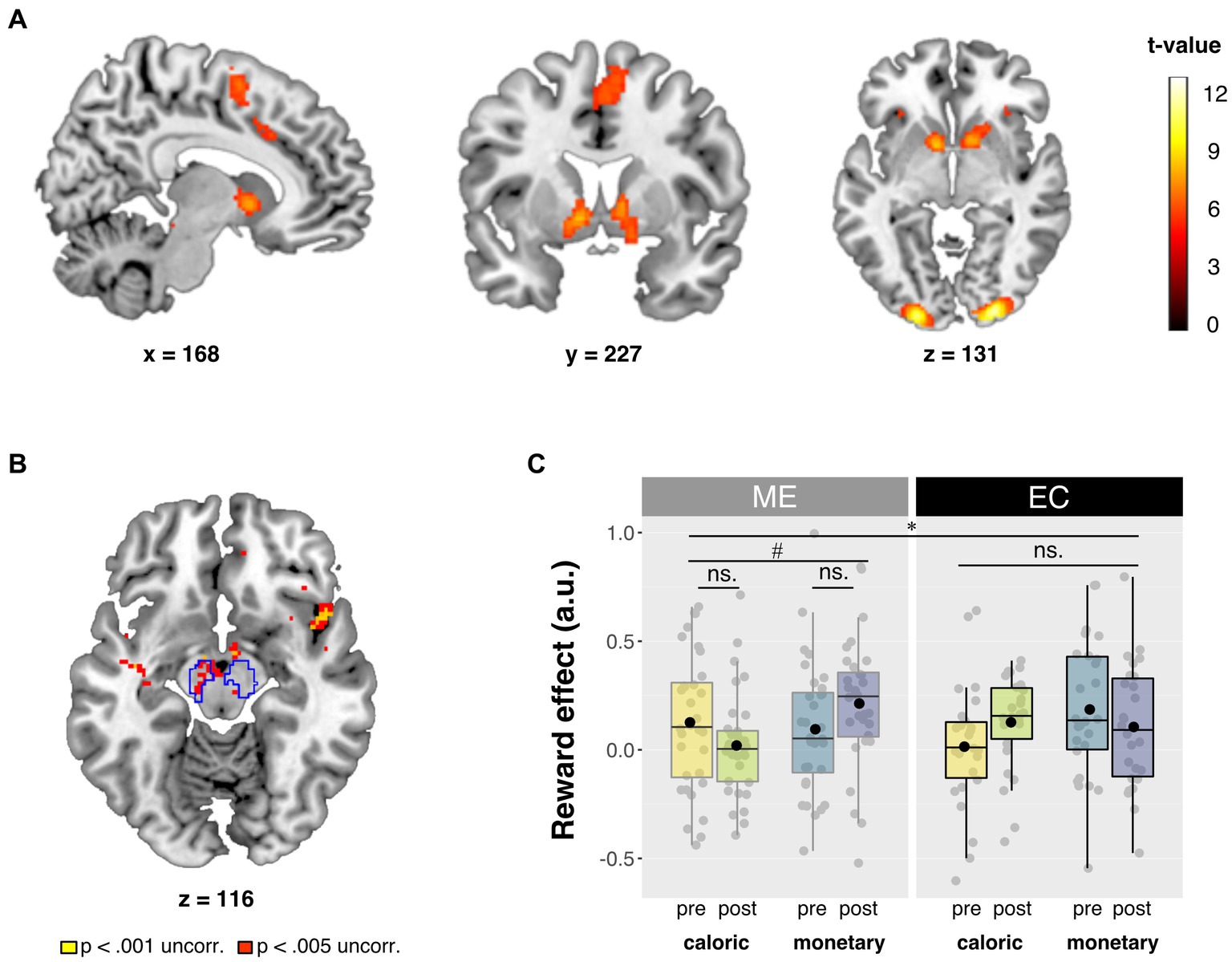
Figure 3. Summary of neuroimaging results. (A) Main effect of reward. Contrast of high vs. low reward cue trials (high > low). Full brain statistical parametric maps were thresholded at p < 0.05 (FWE-corrected, peak-level). (B) Axial slice of whole brain interaction effect of Domain × Time × Intervention for the Reward contrast (high > low). Statistical parametric maps were thresholded at p < 0.001 (yellow) and p < 0.005 (red) uncorrected for visualization purposes. Outlined regions are corrected for multiple comparisons within our small search volume, at peak pFWE < 0.05. (C) Betas from the bilateral probabilistic midbrain ROI [outlined in blue in panel (B)]. Post-minus pre-intervention mean betas based on the high minus low reward contrast are presented for each domain (caloric, monetary) and for each intervention group (ME, EC) in arbitrary units (a.u.). Box plots show the median and interquartile range, with the black dot denoting the mean. All statistical parametric maps are overlaid onto a T1-weighted canonical image. Slice coordinates are defined in MNI152 space and images are shown in neurological convention (left = left). *p < 0.025 (Bonferroni corrected for two ROIs) and #p < 0.05.
To further investigate the effects of intervention on reward anticipation and receipt, region-of-interest (ROI) analyses were performed using a priori defined ROIs for midbrain and striatum. ROIs were anatomically defined based on a high-resolution probabilistic in vivo atlas that included midbrain and striatal nuclei (48): bilateral substantia nigra (atlas: region 7), and ventral tegmental area (region 11) for midbrain, and bilateral caudate nucleus (region 2), nucleus accumbens (region 3) and putamen (region 1) for striatum at 100% overlap. Probabilistically weighted mean beta weights were extracted from all voxels in both ROIs separately using MarsBar (49). The probabilistically weighted averaged beta-weights were analyzed per region using ANOVA with the same factors as in the whole-brain analyses. As two ROIs were tested (striatum and midbrain), effects for each total region were considered significant when reaching a threshold of p < 0.025 (Bonferroni corrected for multiple comparisons). Post hoc, the same effects were tested in the striatal sub-regions (bilateral caudate nucleus, nucleus accumbens, and putamen) because striatal sub-regions have been associated with distinct neurocognitive mechanisms.
Effects of training on anthropometric, neuropsychological and self-report measurements were analyzed using two-tailed repeated-measures ANOVA with Time (pre, post) as within-participant factor and Intervention (ME, EC) as between-participant factor. In case of violation of the assumption of sphericity as indicated by Mauchly’s test, the Huyhn-Feldt correction was used to adjust the degrees of freedom accordingly (see Section 3).
To assess the longevity of the anthropometric and self-report measures that exhibited a significant Time x Intervention interaction, we ran post hoc ANOVAs adding the one-year follow-up data as a third level in factor Time for BMI, waist, DHD-FFQ, and FBQ knowledge. One-year follow-up data was available of 26 participants in the ME group and 21 participants in the EC group. In case of violation of the assumption of sphericity as indicated by Mauchly’s test, the Huyhn-Feldt correction was used to adjust the degrees of freedom accordingly (see Section 3). Planned post hoc comparisons were performed to statistically compare follow-up data to data from both the pre- and post-test sessions separately. All analyses were performed using two-tailed tests in SPSS (version 23.0, Chicago, IL). The significance level was set at an alpha of p = 0.05, partial eta squared (ηp2) was reported to indicate effect sizes in the repeated measures ANOVAs.
The results reported in this study are based on data from 58 healthy, right-handed participants (48 women; mean age: 31.6, SD: 11.0, range: 19–52 years; mean body mass index (BMI): 26.0, SD: 3.68, range: 19.7–34.7 kg/m2). We first confirmed that the mindful eating (ME) and educational cooking (EC) groups were well matched in terms of the minimization factors age, gender, body mass index (BMI) and experience with meditation and yoga (Table 1). Note that the average education level of the ME group was numerically, but not significantly higher than that of the EC group (p = 0.053). Post hoc correlation analyses revealed no correlations between educational level and the neural effects described below and is therefore unlikely to drive these effects. The total time participants spent on the intervention, and the number of sessions participants attended did not differ significantly between the two groups (Table 1).
As a primary objective, we assessed the effects of the intervention on reward anticipation during the incentive delay task. We start with the behavioral responses during the task (Table 2). Across sessions and intervention groups, participants responded faster on high than on low reward trials [main Reward: F (1, 56)=25.0, p < 0.001, ηp2 = 0.309], thus revealing a reward benefit (Table 2). In addition, participants across sessions and intervention groups responded faster to monetary relative to caloric reward cues [main Domain: F (1, 56) = 17.4, p < 0.001, ηp2 = 0.237]. We observed a reward benefit for both caloric [F (1, 56) = 4.5, p = 0.038, ηp2 = 0.074] and monetary trials [F (1, 56) = 25.6, p < 0.001, ηp2 = 0.314], which was, however, larger in the monetary trials [Reward × Domain interaction: F (1, 56) = 9.0, p = 0.004, ηp2 = 0.139]. Participants responded faster on post- relative to pre-intervention test sessions [pre: 310.66 (SD: 21.3), post: 304.60 ms (SD: 20.8); main Time: F (1, 56) = 4.4, p < 0.041, ηp2 = 0.072]. However, there was no evidence for effects of intervention type [4-way interaction between Intervention × Time × Reward × Domain [F (1, 56)<1], indicating that the speeding of responding on the second vs. the first session was not qualified by reward magnitude, reward type or intervention type.
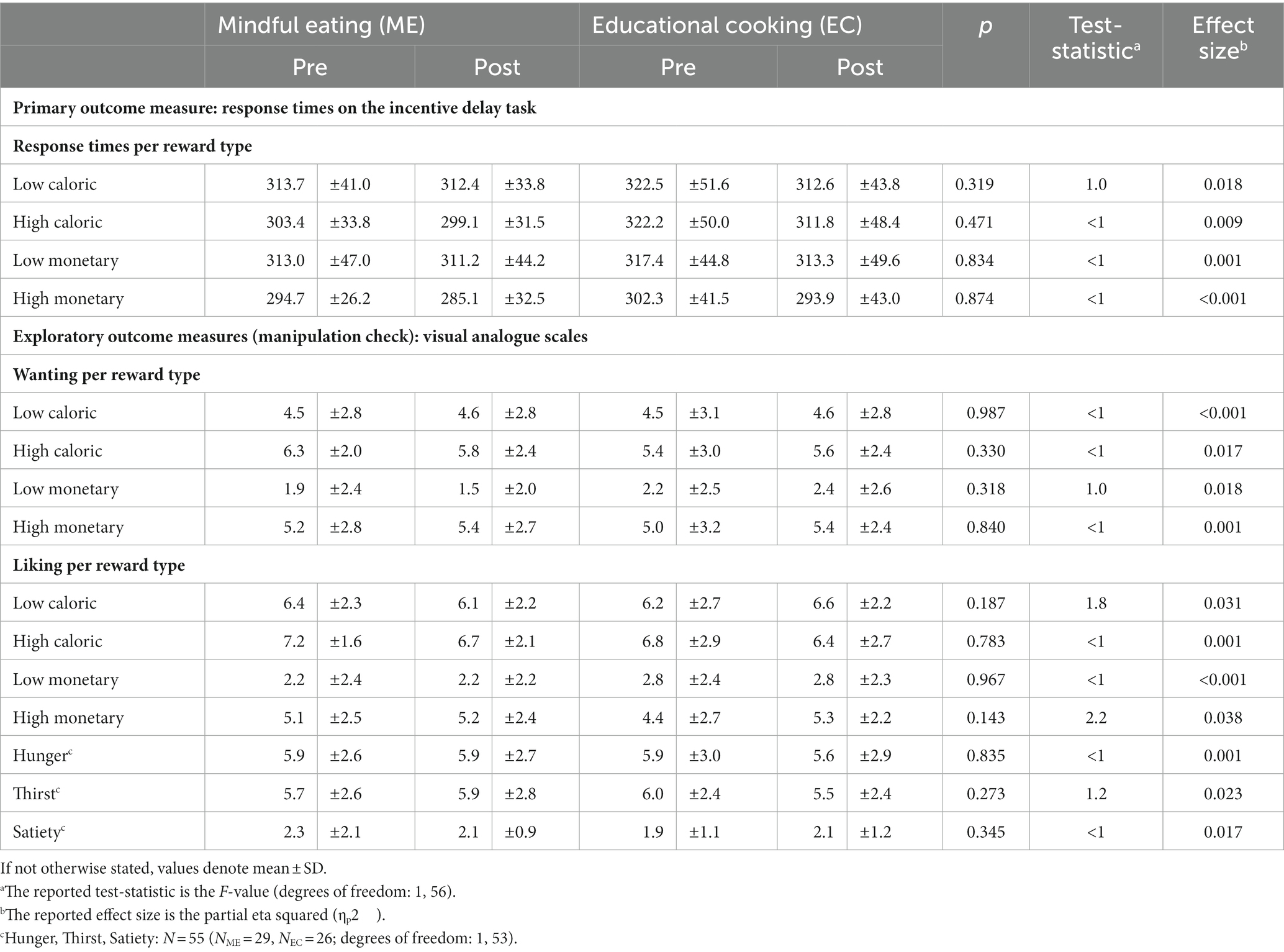
Table 2. Task-related outcomes pre- and post-training, for each group separately, and Time (pre, post) × Intervention (ME, EC) statistics (ME, mindful eating; EC, educational cooking).
There were also no effects of the intervention on any other behavioral task-related measures that we included to control for potentially unexpected group-differences in wanting and liking of the included rewards, or hunger, thirst, and satiety VAS ratings during the task [no Time x Intervention interactions (Table 2)].
An additional main effect of Time (pre vs. post) was only observed for response times on high reward trials, with faster responses following the interventions. No main effects of Intervention were found (Supplementary Table S1).
Before assessing the intervention effects on the neural responses during reward anticipation (primary outcome), we identified brain regions that responded to reward anticipation across sessions and intervention groups (main effect of Reward condition: high>low). At our whole-brain corrected threshold (FWE < 0.05, peak-level), this contrast yielded significant responses in striatum (right caudate nucleus, right nucleus accumbens, right putamen, and left pallidum) and two right midbrain regions, as well as in occipital, motor and frontal regions (Figure 3A). Note that the optimal preprocessing pipeline was selected based on maximal main effects of reward anticipation (see Section 2.6.3.1), so no inference can be made on the magnitude of these main effects. Reward anticipation differed in mostly posterior regions for monetary vs. caloric reward cues (i.e., interaction of Domain × Reward), independent of sessions and intervention groups. For all contrasts, see Table 3.
We were primarily interested in the effects of mindful eating on reward anticipation in our a priori defined, anatomical region-of-interest (ROI): the striatum. We explored the same effects in an anatomical midbrain ROI. First, we explored these effects using our probabilistic ROIs as small search volumes. We found five peaks for the Reward × Domain × Time × Intervention interaction in the striatum (three regions in caudate nucleus, one in putamen, and one in pallidum), as well as one peak in the midbrain. However, these peaks were not significant when correcting for multiple comparisons across the two search volumes (i.e., midbrain and striatum), i.e., all pFWE > 0.025 (Figure 3B).
Based on our hypotheses, we also performed ROI analyses (Figure 3C) using a bilateral probabilistic structural ROI for the striatum (primary) and the midbrain (see Section 2.6.3.6). No four-way interaction effect (Intervention × Time × Domain × Reward) was found for the striatum. Post hoc analyses of the separate striatal regions also showed no effect of intervention [Intervention × Time × Domain × Reward: putamen: F (1, 56) < 1, p = 0.385, ηp2 = 0.014, caudate nucleus: F (1, 56) = 1.3, p = 0.255, ηp2 = 0.023, nucleus accumbens: F (1, 56) = 2.2, p = 0.142, ηp2 = 0.038]. Interestingly, for the midbrain ROI, we did observe a significant four-way interaction in the ROI betas, and—in contrast to the observed midbrain effect in the small volume analysis mentioned above—this effect did survive correction for multiple comparisons [Intervention × Time × Domain × Reward: F (1, 56) = 7.9, p = 0.007, ηp2 = 0.123, α = 0.025]. Post hoc analyses showed a significant relative reduction in caloric vs. monetary reward anticipation in midbrain after the mindful eating training [Time × Domain × Reward for ME: F (1, 31) = 4.4, p = 0.043, ηp2 = 0.125]. This effect was not significant in the educational cooking group [Time × Domain × Reward for EC: F (1, 25) = 3.7, p = 0.065, ηp2 = 0.130] and, if anything, showed the opposite effect. When further breaking down the interaction in the mindfulness group, we found no significant training effect in the caloric domain [Time × Reward: F (1, 31) = 2.1, p = 0.156, ηp2 = 0.064], or in the monetary domain [Time × Reward: F (1, 31) = 2.8, p = 0.104, ηp2 = 0.083] separately. This means that we can only interpret the ME effect on midbrain reward anticipation responses as a relative decrease for caloric vs. monetary reward (see above-mentioned significant Time × Domain × Reward effect for ME). Pre-intervention Reward differences could not explain the observed interaction in the midbrain [caloric: t (56) = 1.4, p = 0.169, cohen’s d = 0.370, monetary: t (56) = 1.1, p = 0.272, Cohen’s d = 0.292].
To explore whether the time spent on training affected anticipatory reward processing in the midbrain, we ran a post hoc analysis with total time spent on training for each participant as a covariate. Adding this covariate (and interaction terms) did not change the results [Reward × Domain × Time × Intervention: F (1, 55) = 7.4, p = 0.009, ηp2 = 0.118]. Because BMI and waist showed effects of the intervention (see secondary Results: Anthropometric measures), we added BMI and waist as covariates to the analysis. This also did not change the results qualitatively [Reward × Domain × Time × Intervention interaction with BMI covariate: F (1, 55)=5.93, p = 0.018, ηp2 = 0.097; with waist circumference covariate: F (1, 55) = 5.94, p = 0.018, ηp2 = 0.097].
The intervention did not affect neural responses during the receipt of reward. Specifically, no significant main effects of Intervention or interactions with Intervention were found for BOLD responses to reward receipt in whole-brain analyses, nor in ROI analyses using a priori defined ROIs for striatum and midbrain. For main effects and other interaction effects of reward receipt see Table 4.
As a secondary outcome, we analyzed the effects of the two interventions on the anthropometric measures. Although we initially intended to assess only BMI and waist-to-hip ratio (WHR), we have added the analysis of waist circumference as an additional exploratory measure to assess abdominal obesity (50). Changes in abdominal obesity as measured with WHR may be masked because of the relative nature of the measure [i.e., if the interventions affect waist and hip circumference similarly, especially in women (51)]. The interventions had differential effects on the anthropometric measures as indicated by a significant Time x Intervention interaction. Specifically, the active control, EC, intervention resulted in both decreased BMI and waist circumference [main Time: BMI: F (1, 25) = 6.2, p = 0.020, ηp2 = 0.198; waist circumference: F (1, 25) = 17.9, p < 0.001, ηp2 = 0.418], whereas the ME intervention did not affect either of them [main Time: BMI: F (1, 31) < 1, p = 0.648, ηp2 = 0.007; waist circumference: F (1, 31) < 1, p = 0.504, ηp2 = 0.015]. Waist-to-hip ratio was not affected by either of the interventions [Time × Intervention: F (1, 56) < 1, p = 0.379, ηp2 = 0.014]. For all interactions see Table 5. Main effects of Time were also observed for waist circumference and for WHR, but not for BMI. No main effects of Intervention were observed for these measures (Supplementary Table S2).
As another secondary outcome, we assessed intervention effects on eating-related self-reported measures. We found that EC participants reported closer compliance to the Dutch food-based guidelines for healthy eating [main Time: F (1, 25) = 12.8, p = 0.001, ηp2 = 0.339] than ME participants following their intervention [main Time: F (1, 31) = 1.4, p = 0.244, ηp2 = 0.044], as substantiated by a significant Time × Intervention interaction for DHD-FFQ scores. EC participants also showed a significant increase in knowledge on healthy eating following the intervention [main Time: F (1, 25) = 48.8, p < 0.001, ηp2 = 0.661], whereas ME participants did not [main Time: F (1, 31) < 1, p = 0.394, ηp2 = 0.024], as evidenced by a significant Time × Intervention interaction for FBQ scores. For both these measures, we also observed a main effect of Time, but not Intervention (Supplementary Table S2). The other sub-scale of the FBQ (temptation) did not show any differential intervention effects; neither did any of the sub-scales of the DEBQ (restraint, emotional, and external eating). For all interactions see Table 5. Analysis of the other self-reported and neuropsychological measurements—including those related to the intervention (FFMQ-SF, TCQ), affect (PANAS, BIS-BAS, HADS), impulsivity (FTND, BIS-11, Kirby), and working memory (digit span) revealed no significant interactions between Time and Intervention (Table 5). A main effect of Time was observed for positive affect, as assessed on the PANAS, and for people’s anxiety score on the HADS. Across groups, positive affect was lower following the interventions, whereas the anxiety score had increased (Supplementary Table S2).
To establish whether the observed differential intervention effects (Time × Intervention interactions) in the anthropometric and eating-related self-report measures were long-lasting, we ran post hoc analyses by adding the one-year follow-up data as an extra level of factor Time (pre, post, follow-up) in the ANOVAs for all participants from the reported sample that returned for the follow-up (ME: n = 26, EC: n = 20) (Figure 4). For BMI and waist circumference, degrees of freedom were corrected using Huynh-Feldt estimates of sphericity due to violation of the sphericity assumption. BMI, WHR, and waist circumference did not show any long-term intervention-related changes [Intervention × Time, BMI: F (1.550, 69.77) < 1, p = 0.468, ηp2 = 0.015; WHR: F (2, 44) < 1, p = 0.589, ηp2 = 0.024; waist: F (1.742, 78.384) = 2.213, p = 0.123, ηp2 = 0.047]. BMI, WHR, and waist circumference changed over time irrespective of the intervention [main Time, BMI: F (1.550, 69.77) = 3.730, p = 0.039, ηp2 = 0.077; WHR: F (2, 44) = 5.099, p = 0.010, ηp2 = 0.188; waist: F (1.742, 78.384) = 4.837, p = 0.014, ηp2 = 0.097]. Planned post hoc comparisons revealed that the non-significant intervention effects on the anthropometric measures—after including the follow-up time point—were caused by a lack of significant differences between pre-intervention measurements and one-year follow-up measurements for either group (all p > 0.1). This means that the BMI- and waist circumference-reducing effects of the active control (EC) intervention (vs. the mindful eating intervention) were no longer visible at one-year follow-up.
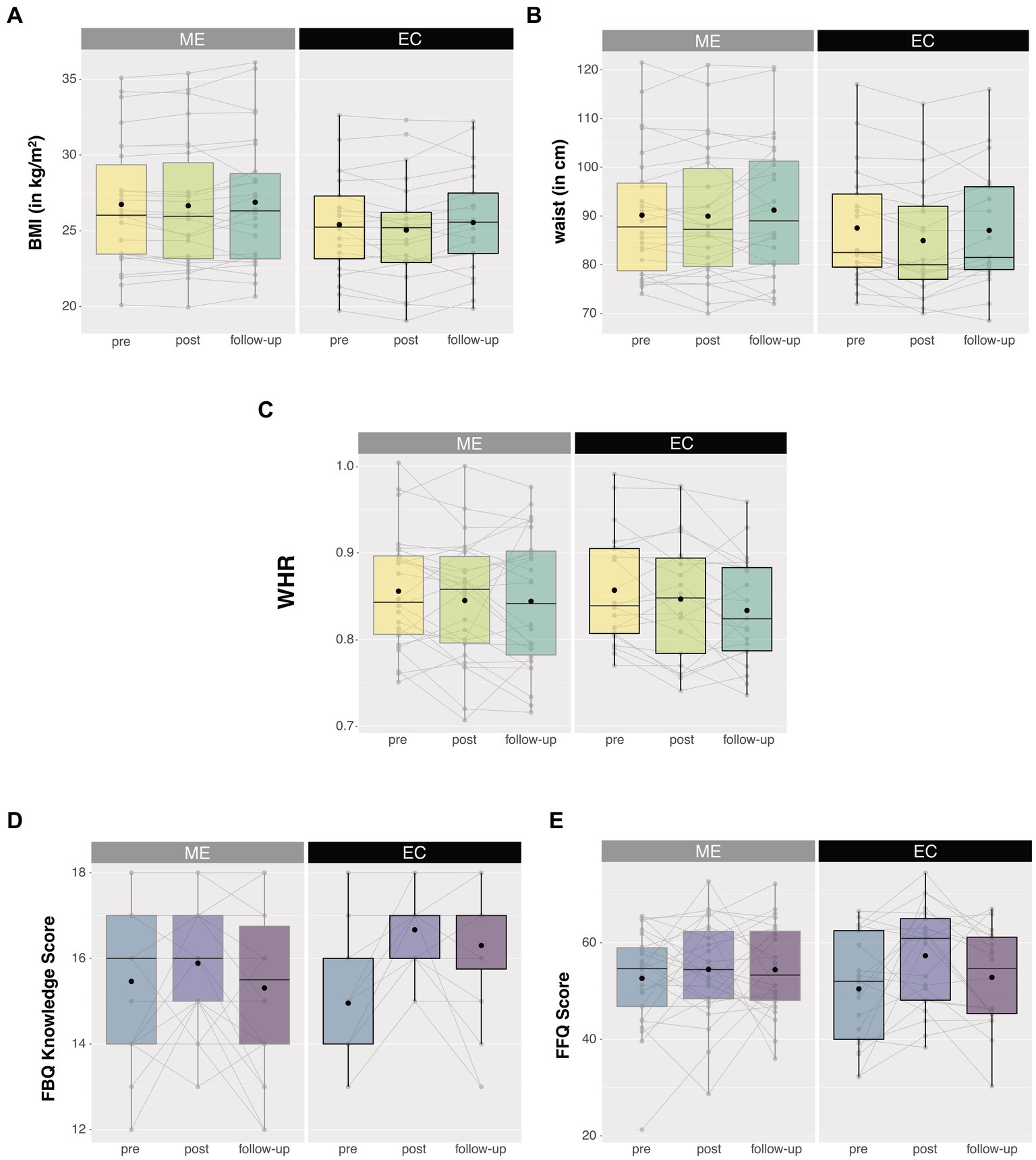
Figure 4. Anthropometric (upper panels) and eating-related self-report measures (lower panels) 1 year after the intervention. No long-lasting intervention effects were observed for (A) BMI, (B) waist circumference, (C) waist-to-hip ratio (WHR), and (E) compliance to the Dutch guidelines for healthy diet (DHD-FFQ). Only knowledge on healthy eating (D) remained high following the educational cooking (EC) intervention. No intervention effects were observed for the mindful eating (ME) group. Box plots show the median and interquartile range, with the black dot denoting the mean. Note that the medians of the EC group in figure (D) do not fall in the interquartile range. Individual data points at the different test sessions are connected for illustrative purpose. **Asterisks denote a significant Time × Intervention interaction with p < 0.01.
The EC-related increase in knowledge on healthy eating remained significant after including the one-year follow-up time point [Intervention × Time, FBQ knowledge: F (2, 44) = 7.4, p = 0.002, ηp2 = 0.253], caused by a lingering increase in knowledge on healthy eating for EC participants at one-year follow-up relative to pre-intervention measurements [F (1, 20) = 17.06, p = 0.001, ηp2 = 0.460]. In contrast, the effects of the active control (EC) intervention on self-reported compliance to the Dutch guidelines for healthy diet were not long lasting (Intervention × Time, DHD-FFQ: F (2, 43) = 2.121, p = 0.132, ηp2 = 0.090). Similar to the anthropometric measures, planned post-hoc comparisons revealed that these DHD-FFQ scores were comparable between the pre-intervention and one-year follow-up measures for either group (all p > 0.1), meaning that the previously observed post-pre effects of the active control (EC) intervention were only short lasting.
The primary objective of this study was to investigate the effects of an 8-week mindful eating intervention on striatal reward anticipation responses as well as response times during an incentive delay task in healthy adults who are highly motivated to change their eating habits. In addition to the striatum, we explored these effects in the midbrain—as part of the mesolimbic reward circuit with its dopaminergic projections to the striatum (4, 5)—as regions of interests (ROIs). We observed that mindful eating training significantly impacted reward anticipation in the midbrain relative to the active control intervention (i.e., educational cooking training), with relatively reduced caloric vs. monetary reward responses in this region after the intervention. This effect was small and did not reach significance in the whole-brain corrected analysis. We found no effect of the interventions in the striatum or on response times during the incentive delay task. Previous studies have shown that greater subcortical reward responses to caloric cues, particularly in striatum, are associated with obesity (52, 53), with weight gain (9), and with increased snack food intake in healthy-weight to overweight individuals (7). Despite this clear involvement of striatum in food reward anticipation and its relationship with eating behavior, we found no effects of mindful eating training on striatal BOLD responses. Below, we interpret these null results. Anthropometric measures of adiposity (i.e., secondary outcome: BMI) temporarily decreased and self-reported (knowledge of) healthy food intake (i.e., secondary outcome: eating behavior questionnaires) increased following the educational cooking intervention, but not following the mindful eating intervention.
Although not a priori hypothesized, the relatively reduced anticipatory responses to the caloric (i.e., high-calorie drink vs. water) compared with the monetary (50 ct. vs. 1 ct.) cues in midbrain following the mindful eating intervention may not be surprising. Dopaminergic midbrain neurons are crucial for processing predicted reward value (5, 54) and, in concert with striatum, modulate motivated behavior such as eating (55). In line with this, Small et al. (56) showed that midbrain activity, as measured with positron emission tomography (H215O), decreased with reduced self-reported reward value of chocolate in a sample of healthy individuals consuming chocolate beyond satiety. In another study, midbrain BOLD responses to sips of palatable milkshake were found to positively correlate with subsequent ad libitum milkshake intake in a group of healthy-weight to moderately obese individuals (57). Moreover, overweight and obese compared with normal weight adolescents showed increased activations in midbrain during anticipation of decisions involving risk and reward (12). Furthermore, both midbrain and striatal BOLD responses to palatable food pictures were found to correlate positively with self-reported reward drive in healthy individuals (58). These (indirect) measures of motivated eating behavior are thus associated with greater mesolimbic responses when processing food reward value. The specificity of our results for midbrain, not striatum, finds resonance in a study in healthy individuals by O’Doherty et al. (59). This study found significant responses to cues predicting the receipt of a glucose solution vs. a neutral taste in midbrain only, whereas both midbrain and striatum were responsive to cues predicting the receipt of a sweet vs. an aversive salty taste. The latter contrast may be a more sensitive, given its valence contrast, than our caloric vs. water contrast, which was perhaps not sufficiently sensitive to show intervention effects in the striatum—despite showing main task effects of reward anticipation. Given the coding of predicted reward in the midbrain, we speculate that the currently observed relative effect of the mindful eating intervention on anticipatory midbrain responses to caloric vs. monetary cues suggests that mindful eating practice may be able to reduce the impact of food cues on reward processing.
Our finding that anticipatory midbrain responses were relatively reduced in the caloric vs. monetary domain is in line with a previous study showing that only a brief 50-min mindful eating workshop (vs. an educational video) reduced subsequent impulsive choice patterns for food-, but not money-related outcomes (60). However, in studies comparing meditators with non-meditating controls, meditators exhibited reduced striatal BOLD responses to primary reward prediction errors (25) as well as monetary reward anticipation (24). In the latter study, Kirk et al. (24) compared meditators to non-meditators without a baseline measurement. The observed decrease in striatal reward processing could thus be due to pre-existing between-group differences (61). Since the present study was actively controlled including pre and post measurements, the current effects can be more reliably ascribed to the mindfulness intervention. Kirk et al. (26) also performed a similar randomized actively controlled study including pre and post measurements and found that vmPFC value signals were modulated by the mindfulness intervention for both primary (juice) and secondary (monetary) rewards. These general reward effects vs. our relative caloric vs. monetary effects might be due to both the type of intervention [general MBSR in Kirk et al. (26) vs. mindful eating presently] as well as the study sample. Specifically, in our study, participants were highly motivated to change undesired eating habits and their mindfulness practice was targeted at overcoming those – including homework practices such as resisting impulsive eating behaviors. Moreover, note that we did not observe any effects of either the ME or the EC intervention on neural responses at the time of caloric or monetary reward receipt. One might have expected reductions in vmPFC BOLD responses following the mindfulness-based intervention as was reported by Kirk et al. (26) for juice delivery (i.e., reward receipt), which we did not observe in whole-brain analysis. Other important differences with the current study are that we used promised (i.e., delivered after scanning) instead of actual rewards (delivered during scanning), and our design was optimized for investigating reward anticipation, with perhaps not enough successful reward receipt trials (i.e., approximately 33% of all anticipated rewards were missed). A meta-analysis of BOLD responses in incentive delay tasks shows consistent activation of vmPFC for reward receipt, but not anticipation (62). Together, our results suggest that a targeted mindful eating—instead of general mindfulness—intervention may have more specific effects on caloric vs. monetary reward anticipation.
The question arises whether mindfulness affects midbrain responses through top-down or bottom-up processes. Current theories on mindfulness-based interventions emphasize that improvements in emotion regulation occur through increased prefrontal cortex-mediated top-down control of regions processing affect, such as the amygdala (63, 64). An alternative way to reducing incentive motivation is through extinction during mindfulness practice, akin to exposure therapy (63, 64), which would rather be a bottom-up process. Practicing mindful eating requires one to actively withhold or interrupt cue-triggered eating, a process that may lead to extinction of conditioned responses to highly caloric stimuli (63, 65, 66) as well as the formation of new memories related to those stimuli (i.e., not reacting to them). As a result, choices for high caloric foods may be further reduced (67, 68). However, incentive motivation could also be reduced through other bottom-up effects on, for example, physiological state rather than through extinction. Increased awareness of states like hunger or satiety (69) are known to modulate conditioned responses to reward-related cues (70). Future confirmatory studies are needed to verify the exploratory midbrain findings and investigate the underlying bottom-up vs. top-down mechanisms, for instance by employing tasks manipulating top-down control on food reward processes, addressing the effects of physiological state and interoception, and by employing connectivity analyses between cortical and mesolimbic regions.
The present mindful eating effects on caloric vs. monetary reward anticipation in the midbrain were not accompanied by changes in our secondary outcome measures related to real-life eating behavior, i.e., reductions in weight, waist-hip ratio or waist circumference, or changes in self-reported eating behavior. Several other studies have found that an intensive mindful eating intervention did lead to reduced measures associated with overeating such as consumption of sweets (22), binges, externally and emotionally driven eating (71) and reductions in BMI (20) in non-clinical populations, as well as number of binges in binge-eating disorder (18). On the other hand, a more recent review by Warren et al. (72) concludes that there is a lack of compelling evidence of mindfulness and mindful eating interventions leading to a reduction in weight. The current lack of mindful eating intervention-related reductions in our secondary measures of adiposity might well reflect the heterogeneity of the included sample. People differed widely in their motivation to take part in the intervention program, which was not necessarily a desire to lose weight. Also, people’s weight status ranged from normal-weight to moderately obese, with an average BMI of only slightly overweight, whereas larger mindfulness-related reductions in food intake have been seen in overweight and obese populations in previous studies (72). Moreover, the study design—including sample size—was optimized for the primary outcome measure (i.e., neural effects) and plausibly underpowered for showing these food behavior-related effects after the mindful eating intervention. We were also not able to show increased self-reported mindfulness after the intervention on the established short version of the Five Facet Mindfulness Questionnaire (33), but this questionnaire could only be employed in a sub group (in n = 22 of the total n = 32 ME vs. n = 26 EC). However, ineffectiveness of our mindful eating intervention is highly unlikely given the observed midbrain findings in the hypothesized direction here (although exploratory) and our previously published effects on behavioral flexibility (28). Sampling a greater and more homogeneous population in terms of BMI and people’s motivation to change their eating habits is advised for future studies to be able to confirm reduced mesolimbic reward responses and demonstrate its link with altered eating behavior following a mindful eating intervention. For now, it is unclear how mindfulness-induced reductions in midbrain responses to caloric vs. monetary reward anticipation contribute to changes in real-life eating behavior.
In contrast, we did observe beneficial effects of the educational cooking intervention on anthropometric measures of obesity and self-reported eating behavior, whereas this group did not demonstrate any intervention effects on mesolimbic reward anticipatory responses. The beneficial effects might not be surprising for this group, since the educational cooking intervention was explicitly aimed at promoting healthy food intake, with reduced intake of sugar, fats and salt as part of the homework assignments. This led to increased self-reported adherence to the Dutch healthy diet (DHD-FFQ) and short-term reductions in weight and waist circumference. Given those health benefits of the educational cooking intervention and the relatively reduced food reward anticipation responses of the mindful eating intervention, it might be fruitful to develop a combined program for therapeutic practice or for preventive strategies. Although weight control and diet interventions are often successful in producing significant weight loss on the short term, they often fail to produce long-term weight maintenance (73). This is supported by our analyses of BMI, waist circumference, and self-reported compliance to the Dutch healthy diet guidelines (DHD-FFQ) at one-year follow-up in the present study. These secondary measures returned to baseline 1 year after the educational cooking intervention, despite the fact that knowledge of healthy eating remained significantly higher compared with baseline in the educational cooking group. Previous studies investigating factors contributing to successful weight maintenance have shown that reductions in subcortical responses to food reward cues may be beneficial for prevention or treatment of obesity (7–9). We therefore speculate that a combination of the two interventions with a focus on both information and behavior might lead to longer-lasting health benefits than either intervention on its own.
Although a strength of this study, by enabling observed effects to be actually attributed to mindfulness practice (64), we note that the lack of a—likely, very subtle—effect of mindful eating, e.g., on striatal reward anticipation, might well reflect the inclusion of a well-matched active control intervention. The contrast between the impact of the mindful eating and control intervention on people is undoubtedly smaller than when a mindful eating intervention had been compared with a waitlist control group. Although the exercises and contents of the two interventions did not overlap, we cannot exclude the possibility that the cooking workshops offered in the active control intervention implicitly led to some increase in experiential awareness as was the aim of the mindful eating intervention. Furthermore, we were not able to offer participants the opportunity to take part in the other intervention program after completion of the study, which is commonly done for mindfulness studies that include a waitlist control group, and which, based on anecdotal evidence reported by the experimenters diminished some people’s motivation to complete the intervention program they had been assigned to in this study. As such, the design of our randomized, active-controlled nature of the study was probably also the reason for a high dropout rate and lower than anticipated sample size. To address differences in results of previous mindfulness or meditation studies without active control condition, future mindfulness intervention studies, especially those aimed at unraveling subtle mechanistic effects, are recommended to not only include a well-matched active control intervention but also a waitlist control group.
In conclusion, we found that an intensive mindful eating intervention reduced midbrain food, relative to monetary, reward anticipation. These results have to be confirmed in future studies, as we primarily hypothesized striatal effects, and the midbrain findings are the result of exploratory ROI analyses, which did not reach significance in the whole-brain corrected analysis. Future studies are also required to demonstrate the clinical relevance of mindfulness-mediated reductions in food anticipation for counteracting reward cue-driven overeating, particularly given that we did not observe mindfulness-related changes in anthropometric or eating behavior measures. Given the success of mindfulness-based programs in reducing symptoms of other reward-related disorders such as substance use (74, 75) and problem gambling (76), our findings of relatively specific reduced anticipatory reward responses may also be relevant for these other targets of abuse.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Mendeley Data, http://dx.doi.org/10.17632/fthcv3kns9.1.
The studies involving human participants were reviewed and approved by Commissie Mensgebonden Onderzoek regio Arnhem-Nijmegen. The patients/participants provided their written informed consent to participate in this study.
EA acquired funding for the study. EA, LJ, RC, AS, and JV designed the study. LJ, ID, IL, and JW acquired and analyzed the data, supervised by EA and RC. AS and JV supervised the execution of the interventions. LJ, ID, and EA wrote the first version of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by a VENI grant (016.135.023) of The Netherlands Organization for Scientific Research (NWO) and an AXA Research Fund fellowship (Ref: 2011) to EA. RC was supported by the James S. McDonnell Foundation (220020328) and a VICI grant (453-14-005) of NWO.
The authors wish to thank the participants and all people who were involved in developing, teaching, and arranging the logistics of the intervention programs. Specifically, the authors would like to thank Ellen Jansen and Nicole Schoonbrood of the Radboud university medical center for Mindfulness for developing and teaching the mindful eating intervention. The authors would also like to thank Desiree Lucassen for developing the educational cooking intervention in collaboration with the Division of Human Nutrition and Health of Wageningen University, and for teaching it. She was assisted by chef Pieter Paul de Grood and bachelor student Hanne de Jong. In addition, the authors are thankful to Susanne Leij-Halfwerk and Suzan de Bruijn of the department of Nutrition and Dietetics at HAN University of Applied Sciences for access to their cooking classrooms. Furthermore, the authors are grateful to Marcel Zwiers for helpful comments regarding ICA AROMA, and to Vishnu Murty and Ian Ballard for advice on the use of a probabilistic midbrain ROI.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1115727/full#supplementary-material
1. García-García, I, Horstmann, A, Jurado, MA, Garolera, M, Chaudhry, SJ, Margulies, DS, et al. Reward processing in obesity, substance addiction and non-substance addiction. Obes Rev. (2014) 15:853–69. doi: 10.1111/obr.12221
2. Volkow, ND, Wang, GJ, Fowler, JS, and Telang, F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. (2008) 363:3191–200. doi: 10.1098/rstb.2008.0107
3. Val-Laillet, D, Aarts, E, Weber, B, Ferrari, M, Quaresima, V, Stoeckel, LE, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage. (2015) 8:1–31. doi: 10.1016/j.nicl.2015.03.016
4. Alexander, GE, DeLong, MR, and Strick, PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. (1986) 9:357–81. doi: 10.1146/annurev.ne.09.030186.002041
5. Haber, SN, and Knutson, B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. (2010) 35:4–26. doi: 10.1038/npp.2009.129
6. Knutson, B, Taylor, J, Kaufman, M, Peterson, R, and Glover, G. Distributed neural representation of expected value. J Neurosci. (2005) 25:4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005
7. Lawrence, NS, Hinton, EC, Parkinson, JA, and Lawrence, AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage. (2012) 63:415–22. doi: 10.1016/j.neuroimage.2012.06.070
8. Stice, E, Yokum, S, Burger, KS, Epstein, LH, and Small, DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. (2011) 31:4360–6. doi: 10.1523/JNEUROSCI.6604-10.2011
9. Demos, KE, Heatherton, TF, and Kelley, WM. Individual differences in nucleus Accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. (2012) 32:5549–52. doi: 10.1523/JNEUROSCI.5958-11.2012
10. Hermann, P, Gál, V, Kóbor, I, Kirwan, CB, Kovács, P, Kitka, T, et al. Efficacy of weight loss intervention can be predicted based on early alterations of fMRI food cue reactivity in the striatum. NeuroImage Clin. (2019) 23:101803. doi: 10.1016/j.nicl.2019.101803
11. Carnell, S, Benson, L, Pantazatos, SP, Hirsch, J, and Geliebter, A. Amodal brain activation and functional connectivity in response to high-energy-density food cues in obesity. Obesity (Silver Spring, Md). (2014) 22:2370–8. doi: 10.1002/oby.20859
12. Delgado-Rico, E, Soriano-Mas, C, Verdejo-Román, J, Río-Valle, JS, and Verdejo-García, A. Decreased insular and increased midbrain activations during decision-making under risk in adolescents with excess weight. Obesity. (2013) 21:1662–8. doi: 10.1002/oby.20375
13. Kabat-Zinn, J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Revision. (1984) 7:71–2.
14. Goldin, P, Ziv, M, Jazaieri, H, and Gross, JJ. Randomized controlled trial of mindfulness-based stress reduction versus aerobic exercise: effects on the self-referential brain network in social anxiety disorder. Front Hum Neurosci. (2012) 6:6. doi: 10.3389/fnhum.2012.00295
15. Lutz, J, Herwig, U, Opialla, S, Hittmeyer, A, Jäncke, L, Rufer, M, et al. Mindfulness and emotion regulation—an fMRI study. Soc Cogn Affect Neurosci. (2013) 9:776–85. doi: 10.1093/scan/nst043
16. Pozuelos, JP, Mead, BR, Rueda, MR, and Malinowski, P. Short-term mindful breath awareness training improves inhibitory control and response monitoring. Prog Brain Res. (2019) 244:137–63. doi: 10.1016/bs.pbr.2018.10.019
17. Tang, YY, Tang, Y, Tang, R, and Lewis-Peacock, JA. Brief mental training reorganizes large-scale brain networks. Front Syst Neurosci. (2017) 11:6. doi: 10.3389/fnsys.2017.00006
18. Kristeller, J, Wolever, R, and Sheets, V. Mindfulness-based eating awareness training (MB-EAT) for binge eating: a randomized clinical trial. Mindfulness. (2013) 5:282–97. doi: 10.1007/s12671-012-0179-1
19. Leahey, TM, Crowther, JH, and Irwin, SR. A cognitive-behavioral mindfulness group therapy intervention for the treatment of binge eating in bariatric surgery patients. Cogn Behav Pract. (2008) 15:364–75. doi: 10.1016/j.cbpra.2008.01.004
20. Tapper, K, Shaw, C, Ilsley, J, Hill, AJ, Bond, FW, and Moore, L. Exploratory randomised controlled trial of a mindfulness-based weight loss intervention for women. Appetite. (2009) 52:396–404. doi: 10.1016/j.appet.2008.11.012
21. Daubenmier, J, Kristeller, J, Hecht, FM, Maninger, N, Kuwata, M, Jhaveri, K, et al. Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: an exploratory randomized controlled study. J Obes. (2011) 2011:1–13. doi: 10.1155/2011/651936
22. Mason, AE, Epel, ES, Kristeller, J, Moran, PJ, Dallman, M, Lustig, RH, et al. Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: data from the SHINE randomized controlled trial. J Behav Med. (2015) 39:201–13. doi: 10.1007/s10865-015-9692-8
23. Mason, AE, Epel, ES, Aschbacher, K, Lustig, RH, Acree, M, Kristeller, J, et al. Reduced reward-driven eating accounts for the impact of a mindfulness-based diet and exercise intervention on weight loss: data from the SHINE randomized controlled trial. Appetite. (2016) 100:86–93. doi: 10.1016/j.appet.2016.02.009
24. Kirk, U, Brown, KW, and Downar, J. Adaptive neural reward processing during anticipation and receipt of monetary rewards in mindfulness meditators. Soc Cogn Affect Neurosci. (2014) 10:752–9. doi: 10.1093/scan/nsu112
25. Kirk, U, and Montague, PR. Mindfulness meditation modulates reward prediction errors in a passive conditioning task. Front Psychol. (2015) 6:90. doi: 10.3389/fpsyg.2015.00090
26. Kirk, U, Gu, X, Harvey, AH, Fonagy, P, and Montague, PR. Mindfulness training modulates value signals in ventromedial prefrontal cortex through input from insular cortex. NeuroImage. (2014) 100:254–62. doi: 10.1016/j.neuroimage.2014.06.035
27. Knutson, B, Fong, GW, Adams, CM, Varner, JL, and Hommer, D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. (2001) 12:3683–7. doi: 10.1097/00001756-200112040-00016
28. Janssen, LK, Duif, I, van Loon, I, de Vries, JHM, Speckens, AEM, Cools, R, et al. Greater mindful eating practice is associated with better reversal learning. Sci Rep. (2018) 8:5702. doi: 10.1038/s41598-018-24001-1
29. Zigmond, AS, and Snaith, RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
30. van Strien, T. Nederlandse Vragenlijst voor eetgedrag (NVE). Handleiding [Dutch eating behaviour questionnaire. Manual]. Stress Int J Biol Stress. (2005)
31. van Strien, T, Frijters, JER, Bergers, GPA, and Defares, PB. The Dutch eating behavior questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Nternational journal of. Eat Disord. (1986) 5:295–315. doi: 10.1002/1098-108X(198602)5:2<295::AID-EAT2260050209>3.0.CO;2-T
32. van Lee, L, Feskens, EJM, Hooft van Huysduynen, EJC, de Vries, JHM, van 't Veer, P, and Geelen, A. The Dutch healthy diet index as assessed by 24 h recalls and FFQ: associations with biomarkers from a cross-sectional study. Journal of nutritional. Science. (2013) 2:e40. doi: 10.1017/jns.2013.28
33. Baer, R, Smith, GT, Hopkins, J, Krietemeyer, J, and Toney, L. Using self-report assessment methods to explore facets of mindfulness. Assessment. (2006) 13:27–45. doi: 10.1177/1073191105283504
34. Watson, D, Clark, LA, and Tellegen, A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. (1988) 54:1063–70. doi: 10.1037/0022-3514.54.6.1063
35. Carver, CS, and White, TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. (1994) 67:319–33. doi: 10.1037/0022-3514.67.2.319
36. Heatherton, TF, Kozlowski, LT, and Frecker, RC. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. (1991) 86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x
37. Patton, JH, Stanford, MS, and Barratt, ES, (1995), Factor structure of the barratt impulsiveness scale. J Clin Psychol. 51, 768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1
38. Kirby, KN. One-year temporal stability of delay-discount rates. Psychon Bull Rev. (2009) 16:457–62. doi: 10.3758/PBR.16.3.457
39. Groth-Marnat, G. Handbook of psychological assessment. Hoboken, New Jersey, USA: John Wiley & Sons (2009).
40. Janssen, LK, Duif, I, Van, LI, Wegman, J, De, VJHM, Cools, R, et al. NeuroImage loss of lateral prefrontal cortex control in food-directed attention and goal- directed food choice in obesity. NeuroImage. (2016) 146:148–56. doi: 10.1016/j.neuroimage.2016.11.015
41. Janssen, LK. Breaking bad habits - a meditation on the neurocognitive mechanisms of compulsive behavior. Hoboken, New Jersey, USA: Radboud University (2017). Available at: https://hdl.handle.net/2066/173246
42. Scott, NW, McPherson, GC, Ramsay, CR, and Campbell, MK. The method of minimization for allocation to clinical trials: a review. Control Clin Trials. (2002) 23:662–74. doi: 10.1016/S0197-2456(02)00242-8
43. Kabat-Zinn, J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain and illness. New York: Delacorte (1990).
44. Bays, JC. Mindful eating: A guide to rediscovering a healthy and joyful relationship with food. Boulder, Colorado, USA: Shambhala Publications (2009).
45. Poser, BA, Versluis, MJ, Hoogduin, JM, and Norris, DG. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: parallel-acquired inhomogeneity-desensitized fMRI. Magn Reson Med. (2006) 55:1227–35. doi: 10.1002/mrm.20900
46. Pruim, RHR, Mennes, M, Van, RD, Llera, A, Buitelaar, JK, and Beckmann, CF. NeuroImage ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. (2015) 112:267–77. doi: 10.1016/j.neuroimage.2015.02.064
47. Friston, KJ, Fletcher, P, Josephs, O, Holmes, A, Rugg, MD, and Turner, R. Event-related fMRI: characterizing differential responses. NeuroImage. (1998) 7:30–40. doi: 10.1006/nimg.1997.0306
48. Pauli, WM, Nili, AN, and Tyszka, JM. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data. (2018) 5:180063. doi: 10.1038/sdata.2018.63
49. Brett, M, Anton, JL, Valabregue, R, and Poline, JB. Region of interest analysis using an SPM toolbox. NeuroImage. (2002) 16:497. doi: 10.1016/S1053-8119(02)90013-3
50. de Koning, L, Merchant, AT, Pogue, J, and Anand, SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. (2007) 28:850–6. doi: 10.1093/eurheartj/ehm026
51. Wing, RR, Jeffery, RW, Burton, LR, Thorson, C, Kuller, LH, and Folsom, AR. Change in waist-hip ratio with weight loss and its association with change in cardiovascular risk factors. Am J Clin Nutr. (1992) 55:1086–92. doi: 10.1093/ajcn/55.6.1086
52. Stoeckel, LE, Weller, RE, Cook, EW 3rd, Twieg, DB, Knowlton, RC, and Cox, JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. (2008) 41:636–47. doi: 10.1016/j.neuroimage.2008.02.031
53. Rothemund, Y, Preuschhof, C, Bohner, G, Bauknecht, HC, Klingebiel, R, Flor, H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. (2007) 37:410–21. doi: 10.1016/j.neuroimage.2007.05.008
54. Schultz, W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. (2006) 57:87–115. doi: 10.1146/annurev.psych.56.091103.070229
55. Berridge, KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. (2009) 97:537–50. doi: 10.1016/j.physbeh.2009.02.044
56. Small, DM, Zatorre, RJ, Dagher, A, Evans, AC, and Jones-Gotman, M. Changes in brain activity related to eating chocolate. Brain. (2001) 124:1720–33. doi: 10.1093/brain/124.9.1720
57. Nolan-Poupart, S, Veldhuizen, MG, Geha, P, and Small, DM. Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite. (2013) 60:168–74. doi: 10.1016/j.appet.2012.09.032
58. Beaver, JD, Lawrence, AD, van Ditzhuijzen, J, Davis, MH, Woods, A, and Calder, AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. (2006) 26:5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006
59. O’Doherty, JP, Deichmann, R, Critchley, HD, and Dolan, RJ. Neural responses during anticipation of a primary taste reward. Neuron. (2002) 33:815–26. doi: 10.1016/S0896-6273(02)00603-7
60. Hendrickson, KL, and Rasmussen, EB. Effects of mindful eating training on delay and probability discounting for food and money in obese and healthy-weight individuals. Behav Res Ther. (2013) 51:399–409. doi: 10.1016/j.brat.2013.04.002
61. Mascaro, JS, Rilling, JK, Negi, LT, and Raison, CL. Pre-existing brain function predicts subsequent practice of mindfulness and compassion meditation. NeuroImage. (2013) 69:35–42. doi: 10.1016/j.neuroimage.2012.12.021
62. Oldham, S, Murawski, C, Fornito, A, Youssef, G, Yücel, M, and Lorenzetti, V. The anticipation and outcome phases of reward and loss processing: a neuroimaging meta-analysis of the monetary incentive delay task. Hum Brain Mapp. (2018) 39:3398–418. doi: 10.1002/hbm.24184
63. Hölzel, BK, Lazar, SW, Gard, T, Schuman-Olivier, Z, Vago, DR, and Ott, U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. (2011) 6:537–59. doi: 10.1177/1745691611419671
64. Tang, YY, Holzel, BK, and Posner, MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. (2015) 16:213–25. doi: 10.1038/nrn3916
65. McClure, SM, Daw, ND, and Read, MP. A computational substrate for incentive salience. Trends Neurosci. (2003) 26:423–8. doi: 10.1016/S0166-2236(03)00177-2
66. Schyns, G, Roefs, A, Mulkens, S, and Jansen, A. Expectancy violation, reduction of food cue reactivity and less eating in the absence of hunger after one food cue exposure session for overweight and obese women. Behav Res Ther. (2016) 76:57–64. doi: 10.1016/j.brat.2015.11.007
67. Veling, H, Aarts, H, and Stroebe, W. Using stop signals to reduce impulsive choices for palatable unhealthy foods. Br J Health Psychol. (2013) 18:354–68. doi: 10.1111/j.2044-8287.2012.02092.x
68. Veling, H, Aarts, H, and Stroebe, W. Stop signals decrease choices for palatable foods through decreased food evaluation. Front Psychol. (2013) 4:875. doi: 10.3389/fpsyg.2013.00875
69. Van De Veer, E, Van Herpen, E, and Van Trijp, HCM. Body and mind: mindfulness helps consumers to compensate for prior food intake by enhancing the responsiveness to physiological cues. J Consum Res. (2015) 42:783–803. doi: 10.1093/jcr/ucv058
70. Dagher, A. Functional brain imaging of appetite. Trends Endocrinol Metab. (2012) 23:250–60. doi: 10.1016/j.tem.2012.02.009
71. Alberts, HJEM, Thewissen, R, and Raes, L. Dealing with problematic eating behaviour. The effects of a mindfulness-based intervention on eating behaviour, food cravings, dichotomous thinking and body image concern. Appetite. (2012) 58:847–51. doi: 10.1016/j.appet.2012.01.009
72. Warren, JM, Smith, N, and Ashwell, M. A structured literature review on the role of mindfulness, mindful eating and intuitive eating in changing eating behaviours: effectiveness and associated potential mechanisms. Nutr Res Rev. (2017) 30:272–83. doi: 10.1017/S0954422417000154
73. Wilson, GT. Behavioral treatment of obesity: thirty years and counting. Adv Behav Res Ther. (1994) 16:31–75. doi: 10.1016/0146-6402(94)90002-7
74. Brewer, JA, Mallik, S, Babuscio, TA, Nich, C, Johnson, HE, Deleone, CM, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. (2011) 119:72–80. doi: 10.1016/j.drugalcdep.2011.05.027
75. Witkiewitz, K, Warner, K, Sully, B, Barricks, A, Stauffer, C, Thompson, BL, et al. Randomized trial comparing mindfulness-based relapse prevention with relapse prevention for women offenders at a residential addiction treatment center. Subst Use Misuse. (2014) 49:536–46. doi: 10.3109/10826084.2013.856922
Keywords: reward anticipation, mindful eating, striatum, midbrain, fMRI
Citation: Janssen LK, Duif I, Speckens AEM, van Loon I, Wegman J, de Vries JHM, Cools R and Aarts E (2023) The effects of an 8-week mindful eating intervention on anticipatory reward responses in striatum and midbrain. Front. Nutr. 10:1115727. doi: 10.3389/fnut.2023.1115727
Received: 04 December 2022; Accepted: 31 July 2023;
Published: 11 August 2023.
Edited by:
Roser Granero, Autonomous University of Barcelona, SpainReviewed by:
Laura Martin, University of Kansas Medical Center, United StatesCopyright © 2023 Janssen, Duif, Speckens, van Loon, Wegman, de Vries, Cools and Aarts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lieneke K. Janssen, bGllbmVrZS5qYW5zc2VuQGdtYWlsLmNvbQ==; Esther Aarts, ZXN0aGVyLmFhcnRzQGRvbmRlcnMucnUubmw=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.